Abstract
BACKGROUND
The role played by coronary perfusion in the maintenance of AF electrical sources that anchor to the posterior wall of the left atrium (PLA) has been incompletely investigated.
OBJECTIVE
We hypothesized that the PLA-pulmonary vein region is perfused by branches originating from both the right and left coronary arteries, and evaluated whether such branches could serve as conduits to chemically ablate restricted PLA regions.
METHODS
In Langendorff-perfused sheep hearts, we identified the right and left anterior atrial arteries (RAAA and LAAA), and branches of the left circumflex artery (LCX) as main coronary artery branches perfusing the atria. During sustained AF, we injected 20 ml boluses of cold Tyrode’s solution (4°C) into each artery to determine changes in dominant frequency (DF). The injection that yielded the largest DF decrease indicated the coronary branch to be subsequently perfused with ethanol. We selectively injected ethanol into the LAAA (n=4), the LCX (n=4) or the RAAA (n=1).
RESULTS
Six out of 9 AF cases rapidly terminated upon ethanol perfusion. In those hearts and in 8 additional preparations (n=17), Congo Red or Evans Blue were subsequently perfused into the remaining atrial branches. The perfusion territories were classified as follows: Triple vessel PLA perfusion (n=4), LAAA dominant PLA perfusion (n=5), balanced double vessel PLA perfusion (n=5) and LCX or RAAA dominant (n=3).
CONCLUSIONS
The PLA coronary perfusion relies on a variable contribution of right and left coronary branches. Regional irrigation of ethanol in well-delineated PLA perfusion territories enabled ablation of high frequency sites during AF.
Keywords: atrial fibrillation, chemical ablation, atrial coronary artery, coronary perfusion territory
Introduction
While the mechanisms of atrial fibrillation (AF) are still debated, a growing body of evidence suggests that specific anatomical regions of the atria such as the posterior left atrium (PLA) and the pulmonary veins (PVs) are critical for AF initiation and maintenance. Interventional ablation techniques have been developed to isolate electrically such areas from the remainder of the atria. 1,2 These strategies have demonstrated substantial efficacy but long procedure times and interventional risks have limited their widespread availability. 3,4 Therefore, interventions that would enable ablating atrial regions with significantly shorter procedure times could prove useful. For instance, it was recently shown that retrograde ethanol delivery via the vein of Marshall could be adjunctive to radio-frequency in patients with AF. This work presented a novel paradigm of employing chemicals to ablate limited atrial regions. 5,6 Similarly, the feasibility of using coronary branches as conduits to perfuse chemicals to the atria has not been investigated. More generally, the role of atrial coronary perfusion in AF initiation and maintenance is incompletely understood. One of the main limitations of our current understanding is that the origin of PLA coronary irrigation is unknown. Previous studies 7–9 have mainly described three coronary branches supplying blood to the atria: 1- The right anterior atrial artery (RAAA) also described as the sinus node artery and other small branches arising from the RCA such as the right intermediate atrial artery (RCA-D). 2- The ramutis ostii cavae superioris also described as the left anterior atrial artery (LAAA) which arises from the left main coronary artery or the initial portion of the left circumflex. 3- The branches of left circumflex artery (LCX). In isolated sheep hearts, we evaluated the PLA irrigation by atrial coronary branches and we attempted to delineate PLA coronary perfusion beds. Also, we catheterized small atrial coronary branches to deliver ablative substances during AF. It should be noted that our objectives were exclusively limited to an anatomical observation of left atrial coronary perfusion territories as they pertain to atrial fibrillation maintenance in the ovine heart. This work cannot be considered as a basis for clinical implementation of chemical intra-coronary atrial fibrillation ablation.
Methods
Langendorff-perfused Sheep Heart and stretch-induced AF model
All animal experiments were carried out according to National Institutes of Health guidelines. Seventeen sheep (45–50 kg) were anesthetized with propofol (0.4 mg/kg), then heparinized (200U/kg, IP) with warm oxygenated Tyrode’s solution (pH 7.4; 95% O2, 5% CO2, 36 to 38 °C). We implemented a model of isolated sheep heart enabling the modulation of intra-atrial pressure as described previously. 10–12 AF was initiated by burst pacing at a cycle length of 10 Hz (10 seconds, 5-ms pulse duration, twice threshold) after having set the intra-atrial pressure at 12 cm H2O. This model is characterized by the inducibility of sustained episodes of AF (>1 hour) in each experiment. Bipolar electrodes were positioned at the left and right atrial appendages (RAA and LAA), on the left atrial roof (Roof), in the left superior, left inferior, right superior and right inferior PVs (LSPV, LIPV, RSPV, RIPV, respectively). In addition, a quadripolar catheter was inserted in the coronary sinus to record bipolar electrograms at the proximal (CS-P) and distal (CS-D) coronary sinus. During AF, the bipolar electrograms were acquired at a sample rate of 200 points/sec. Segments of 4096 points - approximately 20 sec.- were analyzed with fast-Fourier transformation as previously reported13 and the highest dominant frequency location (DFMax) was determined. Dominant frequency (DF) dispersion among perfusion territories was assessed by selecting the highest DF in each of the atrial perfusion territories, which were delineated as detailed below.
Experimental Protocols
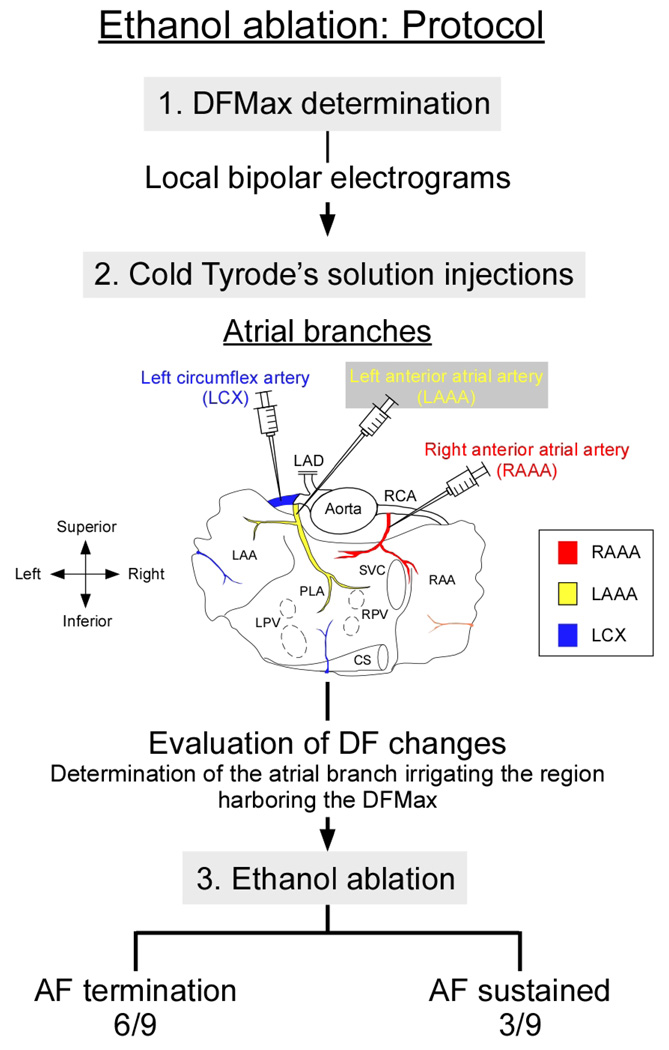
In Figure 1, we present our experimental protocol. In all experiments we obtained sustained episodes of stretch-related AF. In 9 hearts, we retrogradely guided a PTCA Dilation balloon (1.5 mm × 8 mm, Boston Scientific, Natick, MA) or a metal catheter into the RAAA, LCX, and LAAA through the RCA or left anterior descending artery (LAD), respectively. Thereafter, we made iterative test bolus injections (20 ml) of cold Tyrode’s solution (4°C) to assess regional and global changes in DF. The injection that yielded the largest DF decrease indicated the coronary branch to be subsequently injected with ethanol. In those 9 hearts as well as in 8 additional preparations-utilized for the sole purpose of further anatomical description- we delineated the atrial coronary perfusion territories as follows: Congo red (1–5 ml, 4 mg/ml, Sigma, Inc.) was selectively perfused into the RAAA and Evan’s blue (1–5 ml, 2 mg/ml, Sigma, Inc.) into the LAAA or LCX. Both atria including the PLA and PVs were then dissected for delineation of atrial perfusion territories and acquisition of photographic snapshots.
Figure 1. Experimental protocol.
AF: Atrial fibrillation, DFMax: highest dominant frequency, LAD: left anterior descending artery, RCA: right coronary artery, LAA: left atrial appendage, RAA: right atrial appendage, LPV: left pulmonary vein, RPV: right pulmonary artery, PLA: posterior left atrium, CS: coronary sinus, SVC: superior vena cava
Statistical Analysis
Group data were expressed as mean±SD. Statistical comparisons were performed by two-way ANOVA with Boneferroni's test (figure 2A), t-test, or χ2 test when appropriate. Differences were considered significant when p <0.05.
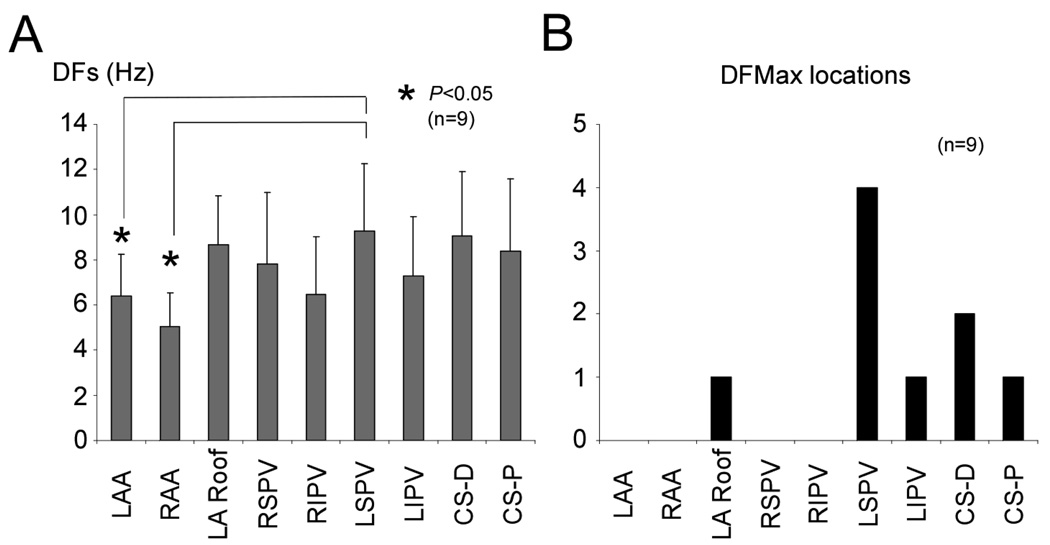
Figure 2. Spatial DFs distribution during AF.
A, Average DFs at the LAA, RAA, LA Roof, RSPV, RIPV, LSPV, LIPV, CS-D, and CS-P during AF. B, Histogram of DFMax atrial locations. DF: dominant frequency, LA Roof: left atrial roof, RSPV: right superior pulmonary vein, RIPV: right inferior pulmonary vein, LSPV: left superior pulmonary vein, LIPV: left inferior pulmonary vein, CS-D: distal of coronary sinus, CS-P: proximal of coronary sinus
Results
AF termination
As illustrated at the bottom of Figure 1, six out of nine AF episodes terminated after perfusion of ethanol into the atrial branch perfusing the region harboring the DFMax.
AF dynamics and DFs distribution
Figure 2A presents the DF distribution at several atrial locations during AF. DFs at the LSPV were substantially higher than at the right and left atrial appendages (RAA and LAA), 9.3±2.9 vs 5.9±1.2 and 4.9±1.2 Hz, respectively, p<0.05. Figure 2B is an histogram of DFMax atrial locations in 9 experiments. DFMax was consistently found within or near the left atrium, including the LA roof, the left pulmonary veins and the coronary sinus.
Chemical ablation of restricted atrial regions during AF
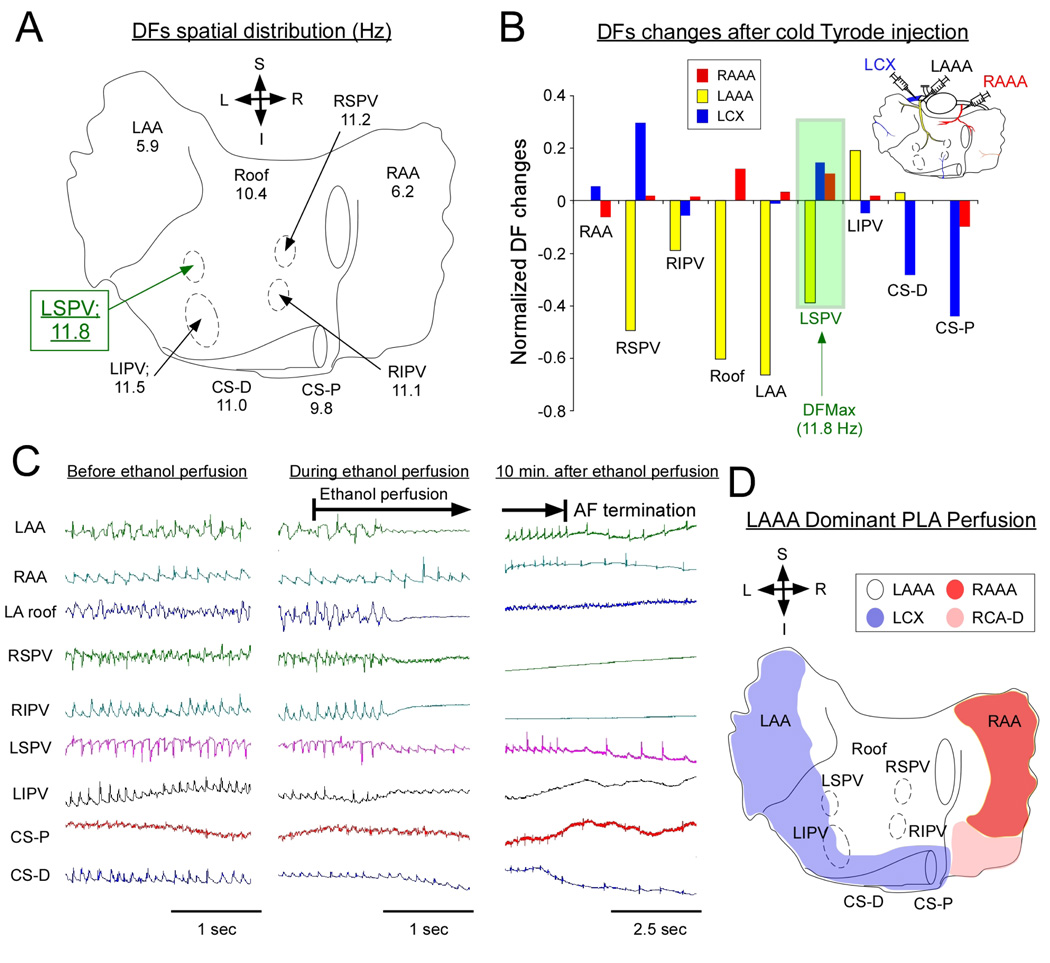
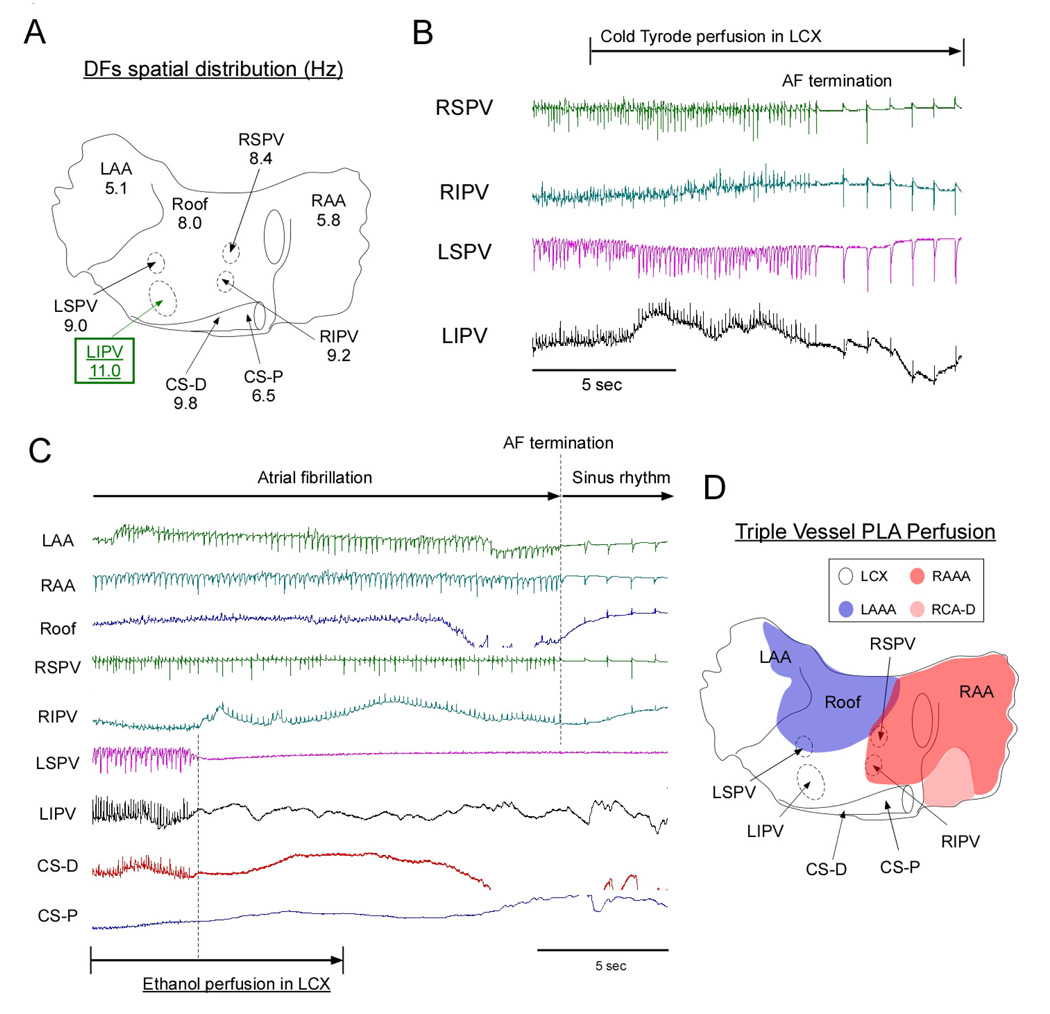
Figure 3A illustrates the spatial distribution of DFs in a representative experiment. The highest frequency of excitation localized at the LSPV. Figure 3B is an example of the DF changes that were elicited upon selective sequential perfusion of cold Tyrode’s solution to the LAAA, RAAA and LCX branches. The largest DFMax decreases were seen when the LAAA branch was used for perfusion with cold Tyrode’s solution (yellow in figure 3B). This resulted in up to 40% reduction in the LSPV, which corresponded to the location of the DFMax. In contrast, perfusion of Tyrode’s solution through the LCX (blue) induced little change at the DFMax location (LSPV) but substantially decreased coronary sinus DFs, which were initially slower than at the LSPV (see panel A). We thus decided to perfuse ethanol (5ml) into the LAAA to assess whether major changes in AF dynamics or frequency would be observed. In figure 3C, atrial electrograms before, during and after LAAA ethanol injection are presented. Upon LAAA ethanol perfusion, an isoelectric line established the absence of electrical activity at the LA roof, RSPV and RIPV while a decrease in electrograms amplitude was also present at other atrial locations including the LSPV and LIPV. Ten minutes later, AF terminated (Figure 3C, right panel). Figure 3D illustrates the atrial coronary perfusion territories in the same heart after perfusion of Evans blue and Congo red to the LCX and RAAA respectively. The area chemically ablated spanned over the LA roof, a relatively large portion of the PLA, the inter-atrial septum and the medial portion of the RAA. This corresponded to a common anatomical variant whereby most of the PLA was irrigated by the LAAA (see below LAAA dominant PLA perfusion). In figure 4, we present an example of another anatomical variant. In this heart, the DFMax localized at the LIPV (figure 4A). In only one case, Cold Tyrode’s solution perfusion through the LCX led to rapid AF termination (figure 4B). However, when the LAAA was perfused with cold Tyrode’s solution, little changes in DFMax was noted (data not shown). This indicated that the branches irrigating the LIPV region in this heart emanated from the LCX and not from the LAAA. We thus decided to perfuse ethanol (20 ml) into the LCX. As illustrated in figure 4C, the electrical activity became abruptly undetectable in a limited region that included the CS and both left PVs. In comparison no major electrogram changes were seen at any other atrial locations following ethanol injection. Strikingly, AF terminated and sinus rhythm resumed after a few seconds. Evans blue and Congo red injections in the LAAA and RAAA respectively confirmed that the chemically ablated region included the CS, the LIPV and a large portion of the LAA. Altogether, we concluded that in this heart the PLA was perfused by branches emanating from the LCX, LAAA and RAAA (see below Triple Vessel Perfusion anatomical variant).
Figure 3. Ethanol ablation: Representative example. LAAA dominant PLA perfusion anatomical variant.
A, DFs spatial distribution during AF in one heart. B, DF changes after perfusion of cold Tyrode’s solution (4 C°) to the LAAA, RAAA and LCX. In this heart, the DFMax located at the LSPV (11.8 Hz, highlighted in green). LSPV DF significantly decreased when cold Tyrode’s solution was perfused into the LAAA. In contrast, after perfusion of cold Tyrode’s solution into the RAAA or the LCX, LSPV DF slightly increased. C, Bipolar electrograms before and after ethanol perfusion. D, Composite schematic of atrial perfusion territory in the same heart (LAAA dominant PLA perfusion). RAAA: right anterior atrial artery, RCA-D: right intermediate atrial artery, LAAA: left anterior atrial artery, LCX: left circumflex artery
Figure 4. Ethanol ablation: Representative example. Triple vessel PLA perfusion anatomical variant.
A, DFs spatial distribution during AF in one heart. B, AF termination after selective perfusion of cold Tyrode’s solution into the LCX. C, AF termination after selective perfusion of ethanol (100 %; 20 ml) into the LCX. D, Composite schematic of atrial perfusion territories in the same heart (Triple vessel PLA perfusion).
To evaluate the effect of cold Tyrode’s perfusion on the subsequent ethanol injection, we have first compared AF dynamics and DFmax values before cold Tyrode perfusion, after cold Tyrode perfusion and 15 min and before ethanol perfusion. The results are presented in supplemental figure 1A and show that DFmax values returned to baseline after an initial sharp decrease caused by cold Tyrode’s perfusion.
Outcome and AF inducibility after chemical ablation
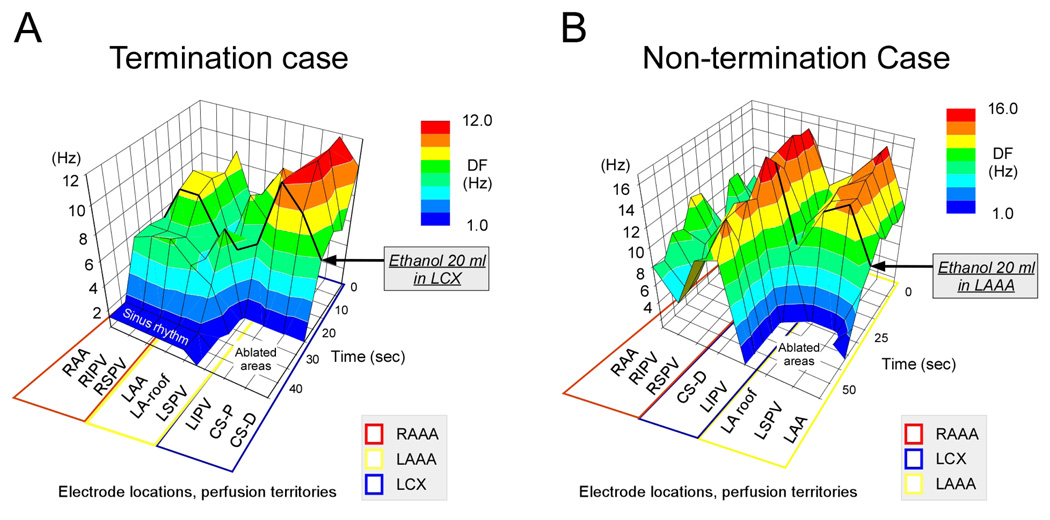
As indicated in figure 1, AF terminated in 6/9 hearts after ethanol perfusion into the coronary branch irrigating the DFMax region. In all hearts in which termination was observed, a re-induction protocol (10Hz burst stimulation) yielded the following results: In 2 hearts, sustained AF proved to be re-inducible and AF persisted for more than 10 minutes. However, AF was not sustained in the rest of the experiments (40 episodes in 4 hearts); it lasted 13.0±23.4 sec. (range: 0–106 sec) before terminating. Interestingly, in those 3 hearts in which AF did not terminate, the DF dispersion among perfusion territories was substantially smaller than in the hearts in which AF terminated (0.5±0.1 vs 3.3±0.4 Hz, p<0.05). This is illustrated in Figure 5, in which we have plotted the time course of DF changes in the different perfusion territories before and after a bolus of ethanol in two representative examples, one in which AF terminated (A) and another in which AF did not terminate (B). In figure 5A ethanol was injected in the LCX because the highest DF was recorded at the CS (10.7 Hz) in the region perfused by the LCX. The second highest DF was found in the region perfused by the RAAA (RSPV: 8 Hz) but was substantially slower. AF terminated readily after ethanol injection. By comparison in Figure 5B, two locations in two separate perfusion territories (LSPV, 13.5 Hz, LAAA territory; CS-D, 14.3 Hz in LCX territory) closely competed for the DFMax before ethanol injection. AF persisted in this case despite ethanol injection. Importantly, the percentage of PLA ablated areas was not significantly different between termination cases (n=6) and non-termination cases (n=3): 61.2±23.2 and 66.6±11.2 %, respectively, NS. Also, the percentage of left and right atria ablated area was not significant between non-termination cases vs termination cases, 38.1±23.4 vs 43.7±25.0 %, respectively, NS. In addition, there was no ventricular area infarcted when the LAAA or RAAA were perfused with ethanol. As expected, the perfusion of ethanol in the LCX yielded a lateral ventricular infarction corresponding to 29.9±6.8% of the total ventricular area. Furthermore, we have compared volumes of ethanol injected between termination and non-termination hearts. The results are presented in supplemental figure 1B. The graph indicates an absence of relationship between the ethanol volume and the propensity of AF to terminate. Oppositely, the volumes of ethanol perfused in non-termination hearts tended to be larger than in hearts in which AF terminated.
Figure 5. DF changes after ethanol perfusion.
A, Representative example of AF termination before and after ethanol injection. Before ethanol injection, a single DFMax region at the CS was perfused the LCX (blue). After ethanol ablation, AF terminated and sinus rhythm resumed. B, Representative example of a non-termination experiment before and after ethanol injection. Before ethanol ablation, two individual DFMax regions, at the LSPV and at the CS-D, were perfused respectively by the LAAA (yellow) and the LCX (blue). After ethanol perfusion in the LAAA in this example, the electrical activity became abruptly undetectable at the LAA, LSPV and LA roof. However, no major electrograms changes were seen at other atrial locations including the CS-D. Black solid line indicates the time at which ethanol was injected.
Atrial coronary perfusion territories
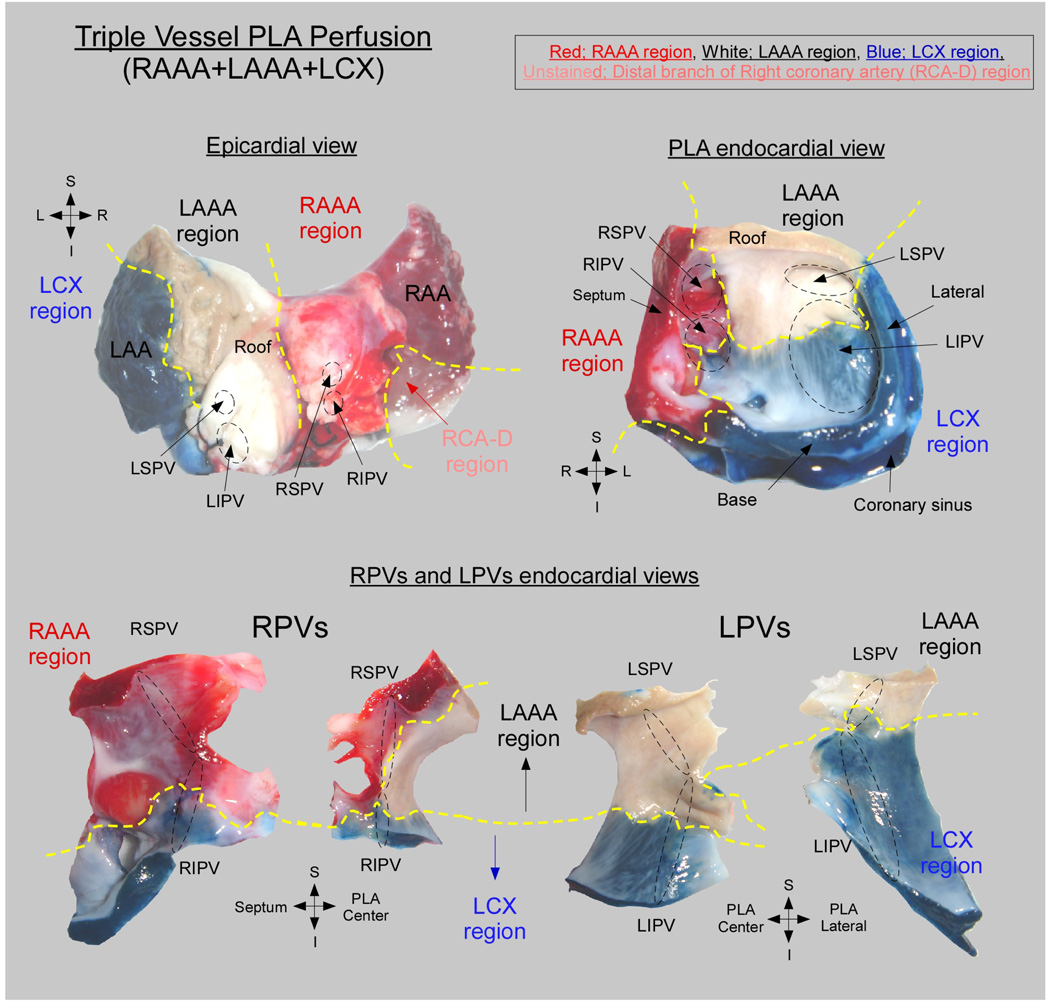
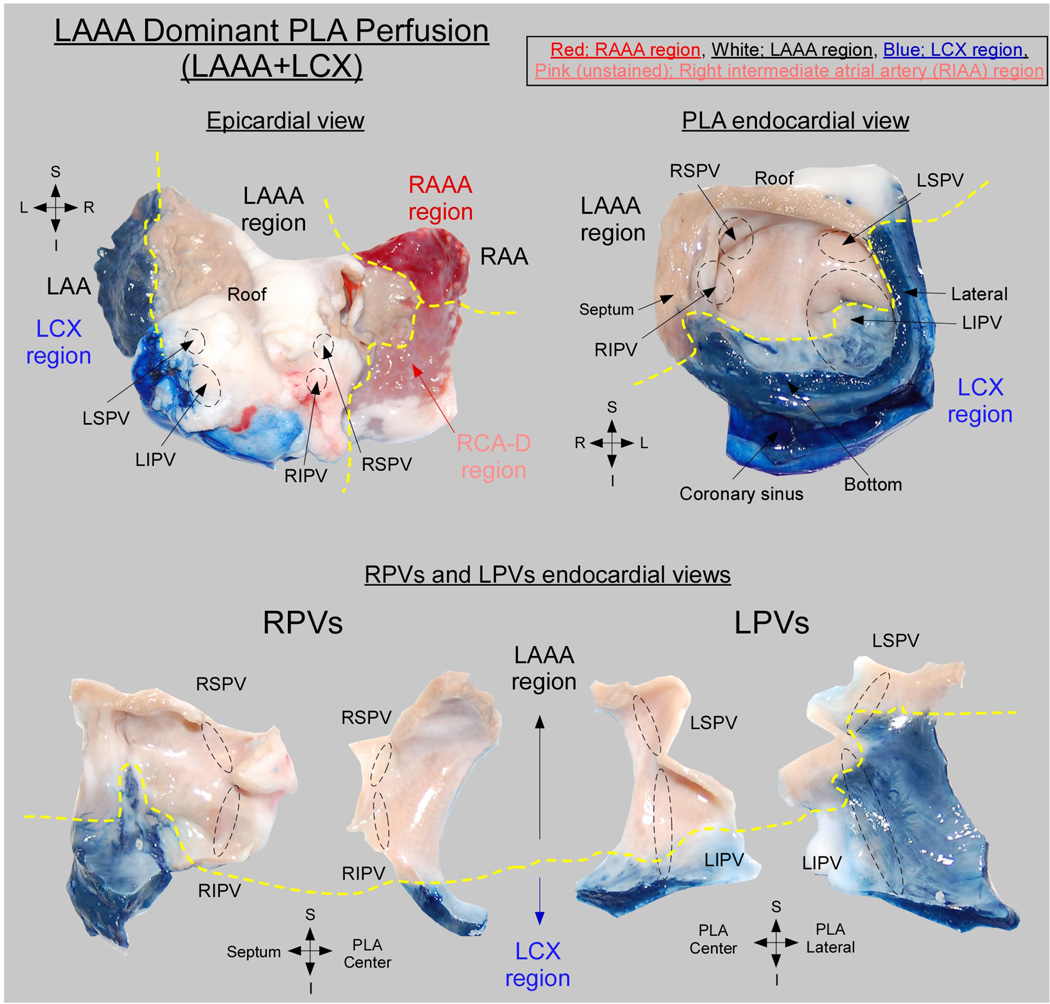
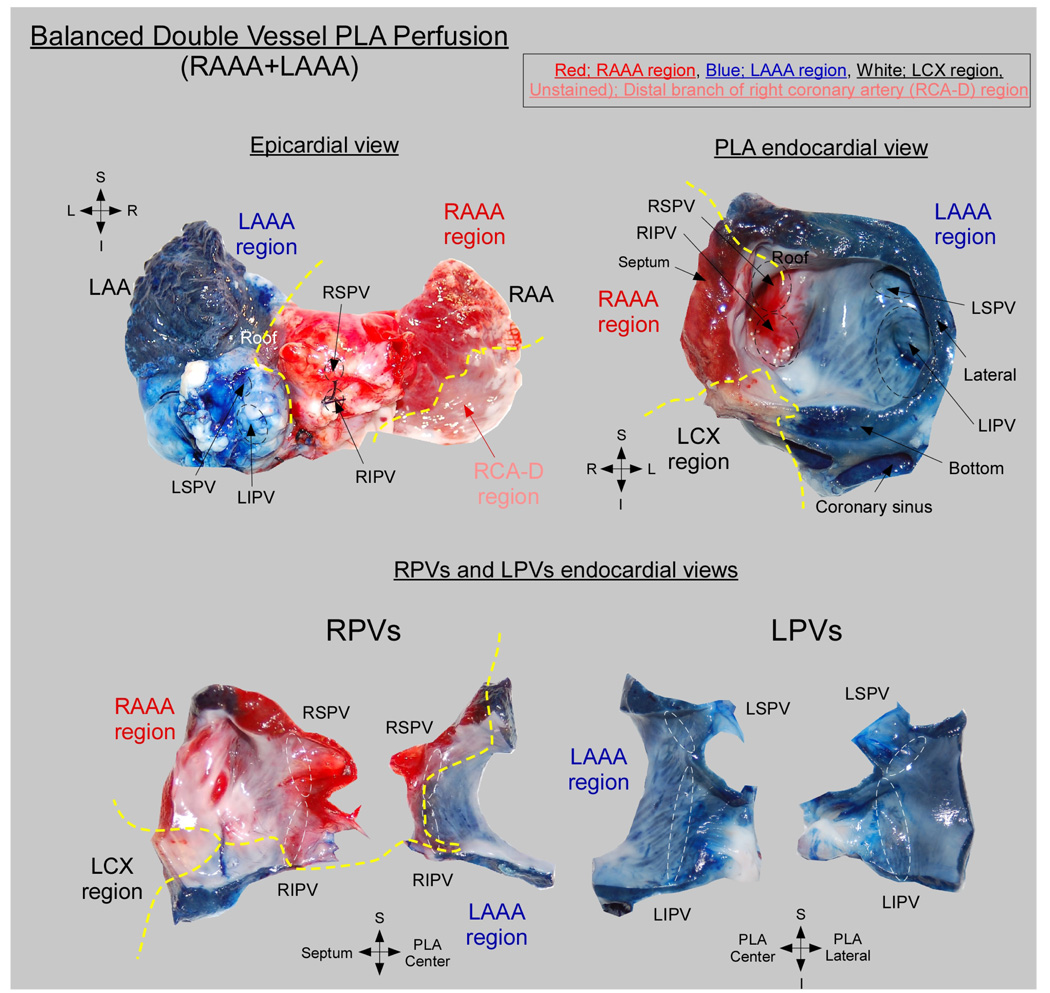
The PLA-PVs myocardium was found to be irrigated by coronary branches of both the RCA and the left coronary artery. In all hearts (n=17), 3 atrial coronary branches-the LAAA, RAAA and atrial branches of the LCX contributed to the perfusion of the PLA-PVs myocardium. Each of those atrial branches irrigated a well-defined perfusion territory at the PLA. However, the contribution of the individual LAAA, RAAA and LCX branches to the perfusion of the PLA-PVs myocardium varied widely between specimens. Specifically, 3 equally frequent anatomical variants were observed: triple vessel PLA perfusion (Figure 6), LAAA dominant PLA perfusion (Figure 7) and balanced double vessel PLA perfusion (Figure 8). The color photographs in figure 6, figure 7 and figure 8 show the different anatomical variants and the clear distinction among perfusion territories. Figure 6 is an example of the triple vessel PLA perfusion variant. In this heart the LA roof, the LSPV and a portion of the LIPV were chemically ablated by ethanol perfusion. In addition, the RSPV, a portion of the RIPV and atrial septum were supplied by the RAAA branch (red) while portions of the RIPV and LIPV were supplied by LCX branches (blue). Interestingly, as illustrated by the lower panel of figure 6, in this heart separate contributions from 2 or more different arterial branches were also observed in the pulmonary veins. Here the RPVs were irrigated by the LCX, RAAA and LAAA branches, whereas the LPVs were supplied by the LCX and LAAA branches. In the example of figure 7, the perfusion of the PLA and PVs was dominated by the LAAA (LAAA dominant variant). On the other hand, in Figure 8, both RAAA and LAAA were predominant (balanced double vessel variant). In all specimens, the 3 aforementioned variants were equally represented: 23.5% of the hearts presented with a triple vessel perfusion, 29.5% with a LAAA dominant and 29.5% with a balanced double vessel perfusion. Also, 2 hearts were found to have LCX dominant PLA perfusion and in one heart the PLA was irrigated by the RAAA exclusively as no visible LAAA was found.
Figure 6. Triple vessel PLA perfusion.
(Upper left) Posterior aspect of a biatrial preparation showing the relationship between regional perfusion territories and anatomical landmarks. Perfusion territories are indicated as follows: Red, RAAA; White, LAAA; Blue, LCX; Unstained, RCA-D. (Upper right) PLA perfusion territories. (Lower) PVs sagittal sections showing the LA-PV junction. Yellow doted line delineates regional perfusion territories.
Figure 7. LAAA dominant PLA perfusion.
Abbreviations and color code as in Figure 6.
Figure 8. Balanced double vessel PLA perfusion.
Perfusion territories are indicated as follows: Red, RAAA; Blue, LAAA; White, LCX; Unstained, RCA-D.
Discussion
We focused our attention on atrial coronary perfusion to assess whether regional delivery of ablative chemicals through atrial coronary branches could lead to AF termination. The results are as follows:
-
-
In sheep hearts the PLA is irrigated by a variable contribution of atrial branches arising from the LCX, LAAA and RAAA. In most specimens the PLA received blood from both right and left coronary branches.
-
-
During AF, a selective catheterization of atrial branches in isolated hearts enabled chemical ablation of PLA regions activated at the highest frequency of excitation. In 6/9 hearts, this resulted in AF termination.
Perfusion territories of the atrial coronary branches
Some investigators have attempted to describe atrial coronary perfusion territories in the region of the PLAPV. It was shown that the right and left coronaries irrigate the atrial myocardium. 7,9 James and Burch 7 indicated that in 6/10 human hearts the major atrial branch arises from the RCA, while in 4/10 hearts it emanates from the LCA. Yalcin et al. 9 showed that, alike in the human heart, the sheep main atrial branches arise from the LCA or the initial segment of the LCX, and/or from the initial segment of the RCA. To the best of our knowledge, however, the extent of atrial perfusion territories at the PLA has not been investigated. Our results provide evidence that in the mammalian heart the PLA-PVs myocardium is irrigated by branches of varying origin. In most hearts, coronary branches arising from both the initial portion of the right coronary and the initial portion of the LCX or left main coronaries provided blood to the PLA. Besides, the contribution of right coronary branches was highly variable. In total, 3 main anatomical variants were found in 17 hearts. The first corresponded to a “triple vessel PLA perfusion” (see figure 6) in which the RCA, the proximal and distal LCX were equally contributing. In the second and third variants of PLA perfusion one (LAAA) or 2 (LAAA and RAAA) large coronary branches (see figure 7 and figure 8) predominated. Altogether, we confirm a large inter-specimen variability in the extent of atrial coronary perfusion territories. 7,14 These data also suggest that advanced coronary artery disease may associate with atrial infarctions involving variable regions of the PLA-PV.
Chemical atrial regional ablation: feasibility in isolated hearts
Previously, coronary sinus injection of ethanol was presented for patients with AF.5 Valderrabano et al. proposed a retrograde ethanol injection in the distal coronary sinus which enabled the ablation of a limited PLA region in the vicinity of the LIPV. Besides, the use of atrial coronary artery branches as conduits for the delivery of chemicals was previously introduced for the ventricles in patients with severe hypertrophic cardiomyopathy.15 In those patients, selective catheterization of a septal artery enabled injection of small volumes of ethanol that ablated a portion of the hypertrophied myocardium. Here, we demonstrated that intra-coronary delivery of ethanol to the PLA-PV region activated at the highest frequency resulted in drastic changes in the frequency of excitation, and resulted in AF termination in most hearts. However, it should be noted AF did not terminate in 2/4 LAAA injections and in 1/4 LCX injections that corresponded to triple vessel or LAAA dominant perfusion anatomical variants. In comparison, AF terminated in all instances of RAAA injection corresponding to balanced double vessel or RAAA dominant perfusion. A limited sample size makes difficult an educated speculation on the relative success of chemical ablation based upon the branch injected or the perfusion territory anatomical variant. This question will have to be investigated in future works.
Relevance to Clinical Implementation
These experiments in isolated hearts are no indication of the feasibility of this approach in the whole animal, let alone in humans. Various factors would significantly hamper the implementation of chemical ablation in humans. First, in a large number of hearts the coronary supplying the PLA is the same as that supplying the sinus node. Hence, any attempts to deliver ablating chemicals to the PLA would be associated with sinus node damage. The ovine atrial coronary anatomy might present significant differences with the human one and cannot be used to guide therapy in humans. Thus, extensive feasibility and safety evaluation needs to be undertaken before any use in the clinical scenario.
Limitations
We were technically unable to selectively catheterize small atrial branches arising from the distal LCX segment. Thus we chose to deliver variable volumes of ethanol depending on the LCX size in each experiment. Also, the cold Tyrode or ethanol flow rate was likely to vary based upon the perfusion pressure, the artery diameter and the overall area perfused. Those parameters were not controlled during our experiments. Besides, a decrease in atrial contractility following ethanol injection might have contributed to AF termination. An evaluation of atrial contractility will have to be performed in subsequent investigations. Finally, a full evaluation of the electrophysiological effects of ethanol perfusion will need to be evaluated in a larger number of experiments.
Supplementary Material
Acknowledgments
We thank Dr José Jalife for his support and for fruitful discussions.
Funding Sources:
This work was supported by National Heart Lung and Blood Institute grants PO1 HL039707, PO1 HL087226 and RO1 HL070074 supporting M.K and K.C; RO1-HL087055 and ACCF/GE Healthcare Career Development Award to JK; Heart Rhythm Society fellowship award and the Fellowship of Japan Heart Foundation/The Japanese Society of Electrocardiology to MY.
Abbreviations
- AF
atrial fibrillation
- PLA
posterior left atrium
- PV
pulmonary vein
- RAAA
right anterior atrial artery
- RCAD
right intermediate atrial artery
- LAAA
left anterior atrial artery
- LCX
left circumflex artery
- RAA
right atrial appendage
- LAA
left atrial appendage Roof: atrial roof
- LSPV
left superior pulmonary vein
- LIPV
left inferior pulmonary vein
- RSPV
right superior pulmonary vein
- RIPV
right inferior pulmonary vein
- CS-P
proximal of coronary sinus
- CS-D
distal of coronary sinus
- DF
dominant frequency
- DFMax
highest dominant frequency
- LAD
left anterior descending artery
- SVC
superior vena cava
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:None
References
- 1.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Oral H, Pappone C, Chugh A, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354:934–941. doi: 10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- 3.Pappone C, Oral H, Santinelli V, et al. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation. 2004;109:2724–2726. doi: 10.1161/01.CIR.0000131866.44650.46. [DOI] [PubMed] [Google Scholar]
- 4.Sacher F, Monahan KH, Thomas SP, et al. Phrenic nerve injury after atrial fibrillation catheter ablation: characterization and outcome in a multicenter study. J Am Coll Cardiol. 2006;47:2498–2503. doi: 10.1016/j.jacc.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 5.Valderrabano M, Chen HR, Sidhu J, et al. Retrograde ethanol infusion in the vein of Marshall: regional left atrial ablation, vagal denervation and feasibility in humans. Circ Arrhythm Electrophysiol. 2009;2:50–56. doi: 10.1161/CIRCEP.108.818427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valderrabano M, Liu X, Sasaridis C, et al. Ethanol infusion in the vein of Marshall: Adjunctive effects during ablation of atrial fibrillation. Heart Rhythm. 2009;6:1552–1558. doi: 10.1016/j.hrthm.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James TN, Burch GE. The atrial coronary arteries in man. Circulation. 1958;17:90–98. doi: 10.1161/01.cir.17.1.90. [DOI] [PubMed] [Google Scholar]
- 8.Saremi F, Abolhoda A, Ashikyan O, et al. Arterial supply to sinuatrial and atrioventricular nodes: imaging with multidetector CT. Radiology. 2008;246:99–107. doi: 10.1148/radiol.2461070030. discussion 108–109. [DOI] [PubMed] [Google Scholar]
- 9.Yalcin B, Kirici Y, Ozan H. The sinus node artery: anatomic investigations based on injection-corrosion of 60 sheep hearts. Interact Cardiovasc Thorac Surg. 2004;3:249–253. doi: 10.1016/j.icvts.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Ravelli F, Allessie M. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff-perfused rabbit heart. Circulation. 1997;96:1686–1695. doi: 10.1161/01.cir.96.5.1686. [DOI] [PubMed] [Google Scholar]
- 11.Kalifa J, Jalife J, Zaitsev AV, et al. Intra-atrial pressure increases rate and organization of waves emanating from the superior pulmonary veins during atrial fibrillation. Circulation. 2003;108:668–671. doi: 10.1161/01.CIR.0000086979.39843.7B. [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki M, Vaquero LM, Hou L, et al. Mechanisms of stretch-induced atrial fibrillation in the presence and the absence of adrenocholinergic stimulation: interplay between rotors and focal discharges. Heart Rhythm. 2009;6:1009–1017. doi: 10.1016/j.hrthm.2009.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samie FH, Berenfeld O, Anumonwo J, et al. Rectification of the background potassium current: a determinant of rotor dynamics in ventricular fibrillation. Circ Res. 2001;89:1216–1223. doi: 10.1161/hh2401.100818. [DOI] [PubMed] [Google Scholar]
- 14.Kugel M. Anatomical studies on the coronary arteries and their branches: I. Arteria anastomotica auricularis magna. Am Heart J. 1927;3:260–270. [Google Scholar]
- 15.Sigwart U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet. 1995;346:211–214. doi: 10.1016/s0140-6736(95)91267-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.