Abstract
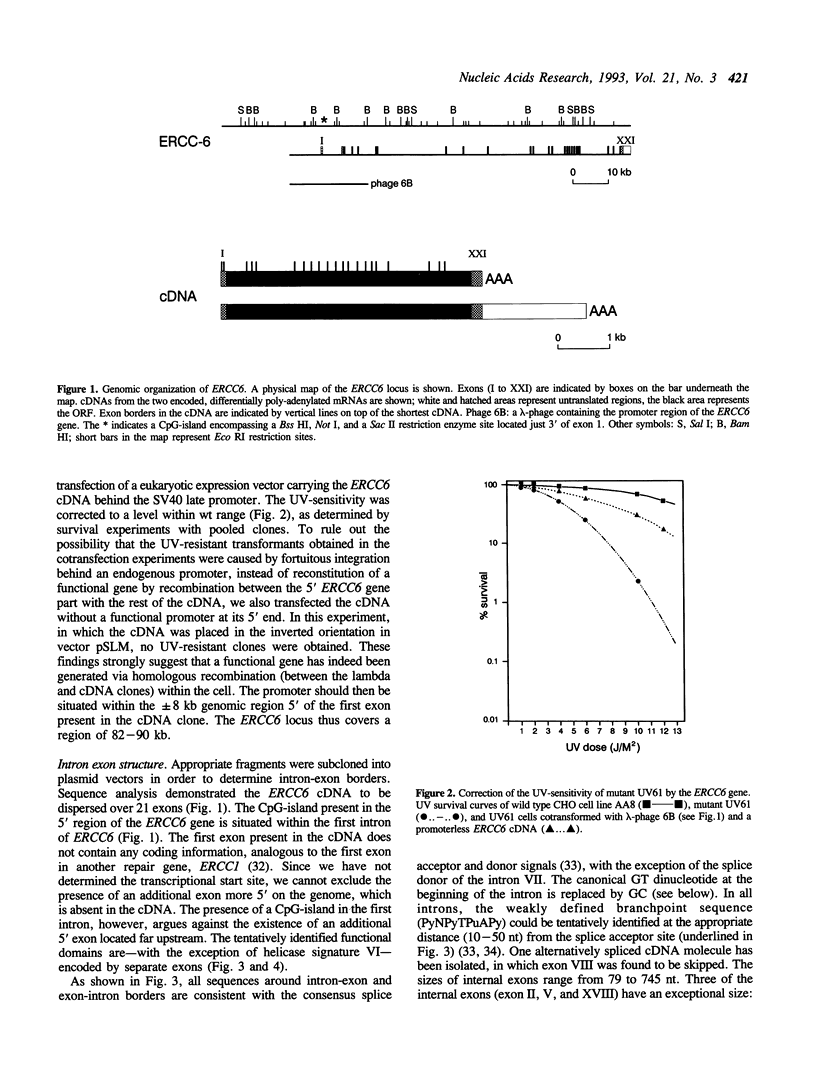
The human repair gene ERCC6--a presumed DNA (or RNA) helicase--has recently been found to function specifically in preferential nucleotide excision repair (NER). This NER subpathway is primarily directed towards repair of (the transcribed strand of) active genes. Mutations in the ERCC6 gene are responsible for the human hereditary repair disorder Cockayne's syndrome complementation group B, the most common form of the disease. In this report, the genomic organization and expression of this gene are described. It consists of at least 21 exons, together with the promoter covering a region of 82-90 kb on the genome. Postulated functional domains deduced from the predicted amino acid sequence, including 7 distinct helicase signatures, are--with one exception--encoded on separate exons. Consensus splice donor and acceptor sequences are present at all exon borders with the exception of the unusual splice donor at the end of exon VII. The 'invariable' GT dinucleotide in the consensus (C,A)AG/GTPuAGT is replaced by the exceptional GC. Based on 42 GC splice donor sequences identified by an extensive literature search we found a statistically highly significant better 'overall' match of the surrounding nucleotides to the consensus sequence compared to normal GT-sites. This confirms and extends the observation made recently by Jackson (Nucl. Acids Res., 19, 3795-3798 (1991)) derived from analysis of 26 cases. Analysis of ERCC6 cDNA clones revealed the occurrence of alternative polyadenylation, resulting in the (differential) expression of two mRNA molecules (which are barely detectable on Northern blots) of 5 and 7 kb in length.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson R. D., Barbosa P., Kalumuck K., O'Brien W. E. Characterization of the human argininosuccinate lyase gene and analysis of exon skipping. Genomics. 1991 May;10(1):126–132. doi: 10.1016/0888-7543(91)90492-w. [DOI] [PubMed] [Google Scholar]
- Barnum S. R., Amiguet P., Amiguet-Barras F., Fey G., Tack B. F. Complete intron/exon organization of DNA encoding the alpha' chain of human C3. J Biol Chem. 1989 May 25;264(15):8471–8474. [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Busch D., Greiner C., Lewis K., Ford R., Adair G., Thompson L. Summary of complementation groups of UV-sensitive CHO cell mutants isolated by large-scale screening. Mutagenesis. 1989 Sep;4(5):349–354. doi: 10.1093/mutage/4.5.349. [DOI] [PubMed] [Google Scholar]
- Byrd A. D., Schardl C. L., Songlin P. J., Mogen K. L., Siegel M. R. The beta-tubulin gene of Epichloë typhina from perennial ryegrass (Lolium perenne). Curr Genet. 1990 Nov;18(4):347–354. doi: 10.1007/BF00318216. [DOI] [PubMed] [Google Scholar]
- De Weerd-Kastelein E. A., Keijzer W., Bootsma D. Genetic heterogeneity of xeroderma pigmentosum demonstrated by somatic cell hybridization. Nat New Biol. 1972 Jul 19;238(81):80–83. doi: 10.1038/newbio238080a0. [DOI] [PubMed] [Google Scholar]
- Dorit R. L., Schoenbach L., Gilbert W. How big is the universe of exons? Science. 1990 Dec 7;250(4986):1377–1382. doi: 10.1126/science.2255907. [DOI] [PubMed] [Google Scholar]
- Espelund M., Stacy R. A., Jakobsen K. S. A simple method for generating single-stranded DNA probes labeled to high activities. Nucleic Acids Res. 1990 Oct 25;18(20):6157–6158. doi: 10.1093/nar/18.20.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flejter W. L., McDaniel L. D., Johns D., Friedberg E. C., Schultz R. A. Correction of xeroderma pigmentosum complementation group D mutant cell phenotypes by chromosome and gene transfer: involvement of the human ERCC2 DNA repair gene. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):261–265. doi: 10.1073/pnas.89.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotti M., Jones R., Emery D. C., Chia W., Hall L. Structure and expression of the rat epididymal secretory protein I gene. An androgen-regulated member of the lipocalin superfamily with a rare splice donor site. Biochem J. 1992 Jan 1;281(Pt 1):203–210. doi: 10.1042/bj2810203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haviland D. L., Haviland J. C., Fleischer D. T., Wetsel R. A. Structure of the murine fifth complement component (C5) gene. A large, highly interrupted gene with a variant donor splice site and organizational homology with the third and fourth complement component genes. J Biol Chem. 1991 Jun 25;266(18):11818–11825. [PubMed] [Google Scholar]
- Hawkins J. D. A survey on intron and exon lengths. Nucleic Acids Res. 1988 Nov 11;16(21):9893–9908. doi: 10.1093/nar/16.21.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich R., Eisman R., Surrey S., Delgrosso K., Bennett J. S., Schwartz E., Poncz M. Organization of the gene for platelet glycoprotein IIb. Biochemistry. 1990 Feb 6;29(5):1232–1244. doi: 10.1021/bi00457a020. [DOI] [PubMed] [Google Scholar]
- Hoefsloot L. H., Hoogeveen-Westerveld M., Reuser A. J., Oostra B. A. Characterization of the human lysosomal alpha-glucosidase gene. Biochem J. 1990 Dec 1;272(2):493–497. doi: 10.1042/bj2720493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Bootsma D. Molecular genetics of eukaryotic DNA excision repair. Cancer Cells. 1990 Oct;2(10):311–320. [PubMed] [Google Scholar]
- Iggo R. D., Jamieson D. J., MacNeill S. A., Southgate J., McPheat J., Lane D. P. p68 RNA helicase: identification of a nucleolar form and cloning of related genes containing a conserved intron in yeasts. Mol Cell Biol. 1991 Mar;11(3):1326–1333. doi: 10.1128/mcb.11.3.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson I. J. A reappraisal of non-consensus mRNA splice sites. Nucleic Acids Res. 1991 Jul 25;19(14):3795–3798. doi: 10.1093/nar/19.14.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongeneel C. V., Sahli R., McMaster G. K., Hirt B. A precise map of splice junctions in the mRNAs of minute virus of mice, an autonomous parvovirus. J Virol. 1986 Sep;59(3):564–573. doi: 10.1128/jvi.59.3.564-573.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor G. J., Barsalou L. S., Hanawalt P. C. Selective repair of specific chromatin domains in UV-irradiated cells from xeroderma pigmentosum complementation group C. Mutat Res. 1990 May;235(3):171–180. doi: 10.1016/0921-8777(90)90071-c. [DOI] [PubMed] [Google Scholar]
- Kotula L., Laury-Kleintop L. D., Showe L., Sahr K., Linnenbach A. J., Forget B., Curtis P. J. The exon-intron organization of the human erythrocyte alpha-spectrin gene. Genomics. 1991 Jan;9(1):131–140. doi: 10.1016/0888-7543(91)90230-c. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. Three complementation groups in Cockayne syndrome. Mutat Res. 1982 Dec;106(2):347–356. doi: 10.1016/0027-5107(82)90115-4. [DOI] [PubMed] [Google Scholar]
- Link C. J., Jr, Burt R. K., Bohr V. A. Gene-specific repair of DNA damage induced by UV irradiation and cancer chemotherapeutics. Cancer Cells. 1991 Nov;3(11):427–436. [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
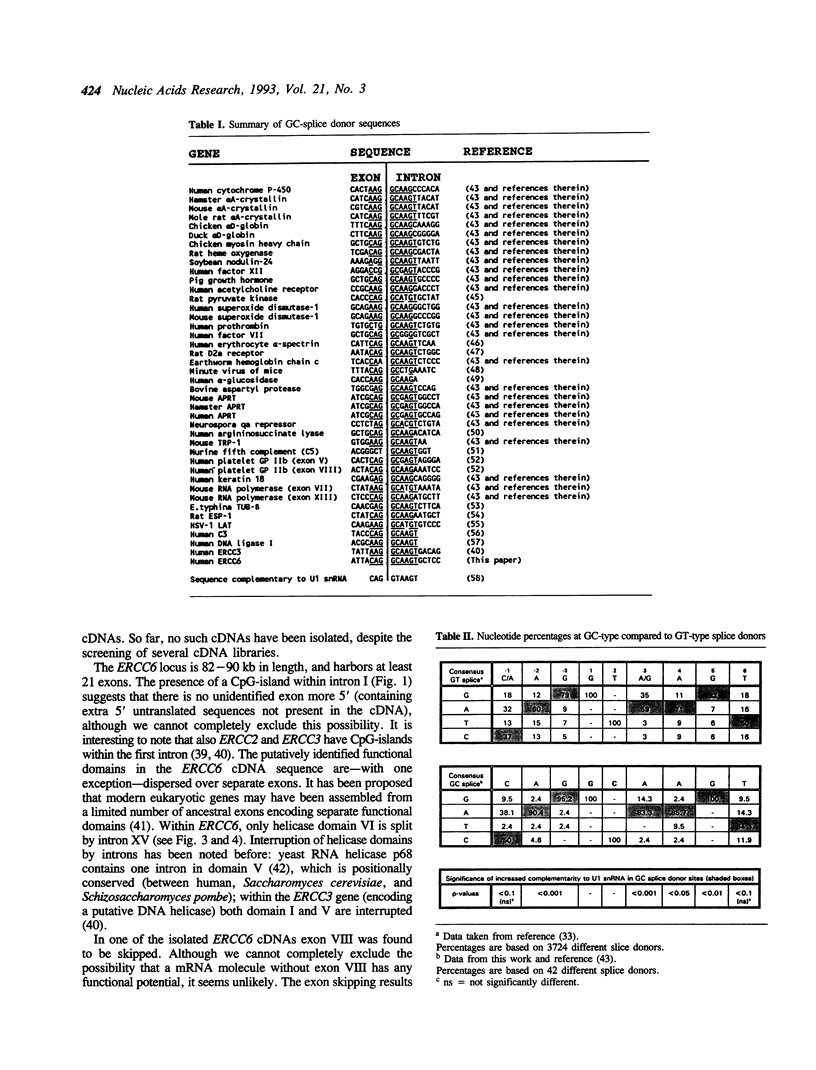
- Nance M. A., Berry S. A. Cockayne syndrome: review of 140 cases. Am J Med Genet. 1992 Jan 1;42(1):68–84. doi: 10.1002/ajmg.1320420115. [DOI] [PubMed] [Google Scholar]
- Noguiez P., Barnes D. E., Mohrenweiser H. W., Lindahl T. Structure of the human DNA ligase I gene. Nucleic Acids Res. 1992 Aug 11;20(15):3845–3850. doi: 10.1093/nar/20.15.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley K. L., Mack K. J., Gandelman K. Y., Todd R. D. Organization and expression of the rat D2A receptor gene: identification of alternative transcripts and a variant donor splice site. Biochemistry. 1990 Feb 13;29(6):1367–1371. doi: 10.1021/bi00458a003. [DOI] [PubMed] [Google Scholar]
- Robberson B. L., Cote G. J., Berget S. M. Exon definition may facilitate splice site selection in RNAs with multiple exons. Mol Cell Biol. 1990 Jan;10(1):84–94. doi: 10.1128/mcb.10.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. H., Kraemer K. H., Lutzner M. A., Festoff B. W., Coon H. G. Xeroderma pigmentosum. An inherited diseases with sun sensitivity, multiple cutaneous neoplasms, and abnormal DNA repair. Ann Intern Med. 1974 Feb;80(2):221–248. doi: 10.7326/0003-4819-80-2-221. [DOI] [PubMed] [Google Scholar]
- Rosbash M., Séraphin B. Who's on first? The U1 snRNP-5' splice site interaction and splicing. Trends Biochem Sci. 1991 May;16(5):187–190. doi: 10.1016/0968-0004(91)90073-5. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Krainer A. R., Maniatis T., Green M. R. Excision of an intact intron as a novel lariat structure during pre-mRNA splicing in vitro. Cell. 1984 Aug;38(1):317–331. doi: 10.1016/0092-8674(84)90553-1. [DOI] [PubMed] [Google Scholar]
- Senapathy P., Shapiro M. B., Harris N. L. Splice junctions, branch point sites, and exons: sequence statistics, identification, and applications to genome project. Methods Enzymol. 1990;183:252–278. doi: 10.1016/0076-6879(90)83018-5. [DOI] [PubMed] [Google Scholar]
- Shivji K. K., Kenny M. K., Wood R. D. Proliferating cell nuclear antigen is required for DNA excision repair. Cell. 1992 Apr 17;69(2):367–374. doi: 10.1016/0092-8674(92)90416-a. [DOI] [PubMed] [Google Scholar]
- Spivack J. G., Woods G. M., Fraser N. W. Identification of a novel latency-specific splice donor signal within the herpes simplex virus type 1 2.0-kilobase latency-associated transcript (LAT): translation inhibition of LAT open reading frames by the intron within the 2.0-kilobase LAT. J Virol. 1991 Dec;65(12):6800–6810. doi: 10.1128/jvi.65.12.6800-6810.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanini M., Collins A. R., Riboni R., Klaude M., Botta E., Mitchell D. L., Nuzzo F. Novel Chinese hamster ultraviolet-sensitive mutants for excision repair form complementation groups 9 and 10. Cancer Res. 1991 Aug 1;51(15):3965–3971. [PubMed] [Google Scholar]
- Takenaka M., Noguchi T., Inoue H., Yamada K., Matsuda T., Tanaka T. Rat pyruvate kinase M gene. Its complete structure and characterization of the 5'-flanking region. J Biol Chem. 1989 Feb 5;264(4):2363–2367. [PubMed] [Google Scholar]
- Tanaka K., Kawai K., Kumahara Y., Ikenaga M., Okada Y. Genetic complementation groups in cockayne syndrome. Somatic Cell Genet. 1981 Jul;7(4):445–455. doi: 10.1007/BF01542989. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Shiomi T., Salazar E. P., Stewart S. A. An eighth complementation group of rodent cells hypersensitive to ultraviolet radiation. Somat Cell Mol Genet. 1988 Nov;14(6):605–612. doi: 10.1007/BF01535314. [DOI] [PubMed] [Google Scholar]
- Troelstra C., Landsvater R. M., Wiegant J., van der Ploeg M., Viel G., Buys C. H., Hoeijmakers J. H. Localization of the nucleotide excision repair gene ERCC6 to human chromosome 10q11-q21. Genomics. 1992 Apr;12(4):745–749. doi: 10.1016/0888-7543(92)90304-b. [DOI] [PubMed] [Google Scholar]
- Troelstra C., Odijk H., de Wit J., Westerveld A., Thompson L. H., Bootsma D., Hoeijmakers J. H. Molecular cloning of the human DNA excision repair gene ERCC-6. Mol Cell Biol. 1990 Nov;10(11):5806–5813. doi: 10.1128/mcb.10.11.5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troelstra C., van Gool A., de Wit J., Vermeulen W., Bootsma D., Hoeijmakers J. H. ERCC6, a member of a subfamily of putative helicases, is involved in Cockayne's syndrome and preferential repair of active genes. Cell. 1992 Dec 11;71(6):939–953. doi: 10.1016/0092-8674(92)90390-x. [DOI] [PubMed] [Google Scholar]
- Venema J., Bartosová Z., Natarajan A. T., van Zeeland A. A., Mullenders L. H. Transcription affects the rate but not the extent of repair of cyclobutane pyrimidine dimers in the human adenosine deaminase gene. J Biol Chem. 1992 May 5;267(13):8852–8856. [PubMed] [Google Scholar]
- Venema J., Mullenders L. H., Natarajan A. T., van Zeeland A. A., Mayne L. V. The genetic defect in Cockayne syndrome is associated with a defect in repair of UV-induced DNA damage in transcriptionally active DNA. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4707–4711. doi: 10.1073/pnas.87.12.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema J., van Hoffen A., Karcagi V., Natarajan A. T., van Zeeland A. A., Mullenders L. H. Xeroderma pigmentosum complementation group C cells remove pyrimidine dimers selectively from the transcribed strand of active genes. Mol Cell Biol. 1991 Aug;11(8):4128–4134. doi: 10.1128/mcb.11.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema J., van Hoffen A., Natarajan A. T., van Zeeland A. A., Mullenders L. H. The residual repair capacity of xeroderma pigmentosum complementation group C fibroblasts is highly specific for transcriptionally active DNA. Nucleic Acids Res. 1990 Feb 11;18(3):443–448. doi: 10.1093/nar/18.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen W., Stefanini M., Giliani S., Hoeijmakers J. H., Bootsma D. Xeroderma pigmentosum complementation group H falls into complementation group D. Mutat Res. 1991 Sep;255(2):201–208. doi: 10.1016/0921-8777(91)90054-s. [DOI] [PubMed] [Google Scholar]
- Watson R. J. A transcriptional arrest mechanism involved in controlling constitutive levels of mouse c-myb mRNA. Oncogene. 1988 Mar;2(3):267–272. [PubMed] [Google Scholar]
- Weber C. A., Salazar E. P., Stewart S. A., Thompson L. H. ERCC2: cDNA cloning and molecular characterization of a human nucleotide excision repair gene with high homology to yeast RAD3. EMBO J. 1990 May;9(5):1437–1447. doi: 10.1002/j.1460-2075.1990.tb08260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeda G., Ma L. B., van Ham R. C., van der Eb A. J., Hoeijmakers J. H. Structure and expression of the human XPBC/ERCC-3 gene involved in DNA repair disorders xeroderma pigmentosum and Cockayne's syndrome. Nucleic Acids Res. 1991 Nov 25;19(22):6301–6308. doi: 10.1093/nar/19.22.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeda G., van Ham R. C., Vermeulen W., Bootsma D., van der Eb A. J., Hoeijmakers J. H. A presumed DNA helicase encoded by ERCC-3 is involved in the human repair disorders xeroderma pigmentosum and Cockayne's syndrome. Cell. 1990 Aug 24;62(4):777–791. doi: 10.1016/0092-8674(90)90122-u. [DOI] [PubMed] [Google Scholar]
- Wickens M. How the messenger got its tail: addition of poly(A) in the nucleus. Trends Biochem Sci. 1990 Jul;15(7):277–281. doi: 10.1016/0968-0004(90)90054-f. [DOI] [PubMed] [Google Scholar]
- Zdzienicka M. Z., van der Schans G. P., Simons J. W. Identification of a new seventh complementation group of UV-sensitive mutants in Chinese hamster cells. Mutat Res. 1988 Sep;194(2):165–170. doi: 10.1016/0167-8817(88)90018-1. [DOI] [PubMed] [Google Scholar]
- van Duin M., Koken M. H., van den Tol J., ten Dijke P., Odijk H., Westerveld A., Bootsma D., Hoeijmakers J. H. Genomic characterization of the human DNA excision repair gene ERCC-1. Nucleic Acids Res. 1987 Nov 25;15(22):9195–9213. doi: 10.1093/nar/15.22.9195. [DOI] [PMC free article] [PubMed] [Google Scholar]