Abstract
Renin synthesis and secretion by principal cells of the collecting duct (CD) is enhanced in angiotensin (Ang) II-dependent hypertension. The presence of renin/(pro)renin and its receptor, the (pro)renin receptor [(P)RR], in the CD may provide a pathway for Ang I generation with further conversion to Ang II. To assess if (P)RR activation occurs during Ang II-dependent hypertension, we examined renal (P)RR levels and soluble (P)RR (s(P)RR) excretion in the urine of chronic Ang II-infused rats (80 ng/min; for 2-weeks, n=10) and sham-operated rats (n=10). Systolic blood pressure and Ang II levels in the plasma and kidney were increased while plasma renin activity was suppressed in Ang II-infused rats. Renal (P)RR transcripts were upregulated in the cortex and medulla of Ang II-infused rats. (P)RR immunoreactivity in CD cells and the protein levels of the full-length form (37 kDa band) were significantly decreased in the medulla of Ang II-infused rats. The soluble (P)RR (28 kDa band) was detected in the renal medulla and urine samples of Ang II-infused rats which also showed increases in urinary renin content. To determine if the s(P)RR could stimulate Ang I formation, urine samples were incubated with recombinant human (pro)renin. Urine samples of Ang II-infused rats exhibited increased Ang I formation compared to sham-operated rats. Thus, in chronic Ang II-infused rats the catalytic activity of the augmented renin produced in the CD may be enhanced by the intraluminal s(P)RR and cell-surface located (P)RR, thus contributing to enhanced intratubular Ang II formation.
Keywords: Ang II-dependent hypertension, intrarenal renin-angiotensin system, intratubular RAS, collecting duct renin
Introduction
Intrarenal formation of angiotensin I (Ang I) occurs via actions of renal renin on angiotensinogen (AGT) delivered to the kidney, and produced by the proximal tubule (PT) cells.1 In addition to its localization in juxtaglomerular (JG) cells, renin mRNA and protein are expressed in connecting tubules (CNT) and cortical and medullary collecting ducts (CD). 2–5 In response to chronic Ang II infusions, the renin gene expression increases in principal cells of CNT and CD. 4 Stimulation of CD renin in Ang II-dependent hypertension is mediated by an Ang II type 1 receptor (AT1R) mechanism since treatment with AT1R blockers prevents this response. 6 The stimulation of CD renin in both kidneys of two-kidney one-clip (2K1C) Goldblatt hypertensive rats indicates that it occurs independently of changes in blood pressure. 7
Nguyen et al., cloned the (pro)renin receptor (P)RR; a 350-amino acid protein with a single transmembrane domain, that binds renin or (pro)renin thereby increasing their catalytic activity. 8 In the human and rat kidney, (P)RR has been localized in glomerular mesangial cells, the sub-endothelium of renal arteries, podocytes, and distal nephron cells.8–10 Recently, Advani et al.,11 demonstrated by immunohistochemistry and in-situ hybridization that (P)RR is predominantly expressed on the apical membranes of acid-secreting cells in the CD.11 Recent findings by Cousin et al.,12 have also demonstrated that the full-length form of (P)RR can be processed intracellularly by cleavage leading to a soluble form (s(P)RR) which can be secreted into the plasma and consequently bind renin. The discovery of the (P)RR has enhanced our understanding of the physiology of the tissue RAS and its contribution in cardiovascular and renal diseases; 13–15 however, little is known regarding the changes in (P)RR in hypertension.
The demonstrations that in Ang II-dependent hypertension there is augmentation of CD renin gene expression 4 and that most of the renin in medullary tissues of Ang II hypertensive rats is active, 7 suggest that (P)RR may be involved in the activation of (pro)renin and/or renin in the distal nephron segments, thus contributing to increased intrarenal and/or intratubular Ang I formation. In the present study, we tested the hypothesis that the (P)RR in the renal medulla and CD tubular fluid as reflected in the urine is augmented in chronic Ang II-infused rats.
Material and Methods
Experimental animals and sample collections
All experimental protocols were approved by the Tulane Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (150 to 175 g; Charles River Laboratories, Wilmington, MA) were cage-housed and maintained in a temperature-controlled room with 12-hours light/dark cycle, with free access to tap water and standard rat chow (Ralston Purina, St. Louis, MO). Ten rats had osmotic mini-pumps subcutaneously implanted to infuse Ang II (80 ng/min, 14 days). Ten rats were sham-operated. Following a training period, the systolic blood pressures (SBP) were monitored by tail-cuff plethysmography (IITC Instruments, Woodland Hills, CA). On subsequent days, the rats were placed in metabolic cages for collection of urine both with and without protease inhibitor cocktail. 16 At the end of the study, 5 rats were anesthetized with Pentobarbital Sodium (Ovation, Inc, Deerfield, IL) for left kidney excision after unilateral ligature and the right kidneys were sequentially perfused with saline solution (0.9% NaCl) and 4% paraformaldehyde (PFH). A similar set of rats were killed by conscious decapitation and kidneys were dissected into renal medulla and cortex for measurements of Ang II content, RNA and protein extractions, as described. 4,16 Trunk blood samples were collected for determination of plasma renin activity (PRA) and plasma Ang II concentration as previously described. 16
Urine Ang II and Renin Content (URC)
Urinary Ang II concentrations were determined by radioimmunoassay as previous described 16 by incubating the samples with rabbit anti–Ang II antiserum (Peninsula Laboratories, Torrance, CA) and 125I–radiolabeled Ang II (Perkin Elmer life and Analytical Sciences, Waltham, Massachusetts). Results are reported in fentomole per 24 hours of urine. To evaluate the contribution of the s(P)RR to enhance renin activity, we measured the Ang I formation following addition of human recombinant (pro)renin (hPR; Lee BioSolutions, Inc. Cat # 510-11PR; St Louis, MO) to urine samples of sham-operated and Ang II-infused rats collected with or without protease inhibitors. The procedure used was a modification of the Plasma Renin Activity (PRA; Diasorin Inc., Stillwater, MN) assay. Briefly, 250 ul of rat urine with maleate generation buffer and PMSF from the PRA kit was spiked with 250 μl of Renin Substrate Tetradecapeptide (RST; 4uM) (Sigma-Aldrich Inc., St. Louis, MO). Additionally, either 20 μl of WFML; a specific renin inhibitor (1mM; AnaSpec Inc.), or 10 μl of hPR (2.2 μM) were added to identical aliquots of the urine-RST mixture. Samples were incubated in a 37 °C water bath while replicate urines without the spike; being used to measure the background Ang I levels, were incubated at 4 °C for 15 min. Following the generation step, all of the samples were subjected to the remaining steps of the PRA assay. The measured values in ng Ang I formed/ml/h was converted into enzyme unit excreted in 24 h urines. The background Ang I levels were subtracted from all the samples. For the samples spiked with hPR, the background renin and non-specific enzyme activity were subtracted.
(P)RR expression studies
Quantitative real-time RT-PCR
Total RNA was isolated and quantified as previously described. 4 The (P)RR mRNA levels were quantified by real-time RT-PCR [Primers: 5′-ATCCTTGAGACGAAACAAGA-3′ (sense); 5′-AGCCAGTCATAATCCACAGT-3′ (antisense), and 5′-6-FAM-ACACCCAAAGTCCCTACAACCTTG-BHQ1-3′ (fluorogenic probe)] and normalized against the expression level of rat GAPDH mRNA [Primers: 5′-CAGAACATCATCCCTGCATC-3′ (sense); 5′-CTGCTTCACCACCTTCTTGA-3′ (antisense); and 5′-6-HEX-CCTGGAGAAACCTGCCAAGTATGATGA-BHQ2-3′ (fluorogenic probe)].
Immunohistochemistry
Rat kidney sections were stained by peroxidase techniques as previously described.17 The (P)RR immunostaining was performed using a rabbit anti-(P)RR antibody (No. 1623; Dr. Genevieve Nguyen, College of France, France) at a 1:4,000 dilution. For immunocolocalization, we used the rabbit anti (P)RR antibody; as well as a goat anti (P)RR (Abcam; Cambridge, MA) at 1:400 dilution that were detected with DAB reaction, and anti-anion exchanger 1 (AE1; (Alpha Diagnostic International, San Antonio, TX) for type A intercalated cells 18 detected with Bajoran Purple Chromogen (Biocare Medical, LLC).
Immunoblotting analysis
The renal (P)RR protein levels were examined using a polyclonal rabbit anti-(P)RR that recognizes the intracellular segment and the ectodomain (ATP6AP2, 1:400 dilution; Cat ID HPA003156; Sigma-Aldrich, St. Louis, MO) as previously described.19. In addition, (P)RR protein levels were examined in 10X concentrated urine samples, previously collected into a protease inhibitor cocktail, using similar protocol conditions. Because it has been recently reported that the soluble form of the (P)RR can be generated intracellularly by furin cleavage, 12 we also measured the protein levels of furin in renal medullary tissues of these rats using a mouse anti-furin as previously described, 20 at a dilution 1:100, overnight, followed by incubation with a anti-mouse secondary antibody at a 1:5,000 dilution. All analyses by Western blotting were performed using the Odyssey detection system (LI-COR Biosciences, Lincoln, NE).
Immunoprecipitation (IP) of the (P)RR
To assess if renin or (pro)renin could bind the s(P)RR, urine samples were tested. IP experiments were developed using Dynabeads M-280 (Invitrogen, Carlsbad, CA) covalently bound to purified sheep anti-rabbit IgG coated with the rabbit anti-(P)RR (ATP6AP2, 1:400 dilution; Cat ID HPA003156; Sigma-Aldrich, St. Louis, MO) and incubated with urine samples containing 100 μg of protein. Both supernatant (S) and immunoprecipitated (IP) fractions were resolved by immunoblotting against 1:100 rabbit polyclonal anti-renin H-105 antibody (Santa Cruz, Cat ID sc-22752; Santa Cruz, CA). Human recombinant renin (hR) and (pro)renin (hPR) proteins (1 μL) (Lee BioSolutions, Inc. Cat No. 510-11PR and 11R; St. Louis, MO) were used as positive controls.
Statistical Analysis
The statistical significance defined at a value of P<0.05 was determined by using paired and unpaired Student’s t test or by one-way ANOVA with Tukey post-test.
Results
Body Weight (BW), Systolic Blood Pressure (SBP), Plasma Renin Activity (PRA), Plasma Ang II levels, and Urine Ang II levels
Body weights were similar at the study outset (sham: 221 ± 5; Ang II-infused: 220 ± 5 grams, P=NS) and significantly increased in both sham-operated and Ang II-infused rats, with a greater gain in sham-operated rats (Table 1). SBP averages were similar in both groups when the study began (sham: 109 ± 4; Ang II-infused: 118 ± 13 mm Hg, P=NS); however, SBP significantly increased in the Ang II-infused rats compared to the sham-operated rats (Table 1). At day 14, PRA was suppressed, while plasma and renal cortical and medullary Ang II levels, as well as urinary Ang II and renin content were increased in the Ang II-infused rats compared to the sham-operated rats (Table 1).
Table 1.
Physiological parameters after 14 days of angiotensin II infusions.
| Variable | Sham | Ang II |
|---|---|---|
| Body weight (g) | 275 ± 8 | 238 ± 4* |
| Systolic blood pressure (mmHg) | 126 ± 8 | 228 ± 8† |
| PRA (ng Ang I·mL−1·h−1) | 5.5 ± 2.0 | 0.3 ± 0.2* |
| Plasma Ang II (fmol/mL) | 22 ± 9 | 179 ± 30* |
| Urine Ang II excretion (fmol/24 h) | 2,079 ± 361 | 3,813 ± 431* |
| Kidney cortex Ang II (fmol/g) | 229 ± 50 | 723 ± 7* |
| Kidney medulla Ang II (fmol/g) | 593 ± 10 | 1898 ± 34* |
| Urinary renin (enzymatic units × 10−6 in 24 h) | 2.06 ± 0.34 | 10.01 ± 2.18* |
Values are mean ± SEM.
P<0.05,
P<0.001 versus sham-operated rats. Ang II: angiotensin II; PRA: plasma renin activity.
(P)RR Immunohistochemistry studies
The (P)RR immunoreactivity was observed primarily in cells of the CNT and CD throughout the cortex, and in the outer and inner medulla (Figure 1). Negative control sections showed no (P)RR immunoreactivity (data not shown). Figure 1A shows bulging cells with intense apical (P)RR immunoreactivity (DAB; brown and arrows), which were positive for basolateral AE1 (Bajoran Purple and asterisks), confirming the expression of (P)RR in type A intercalated cells in accordance with previous findings reported by Advani et al. 11 The cells with specific apical positive staining for (P)RR were negative for aquaporin 2 (data not shown).
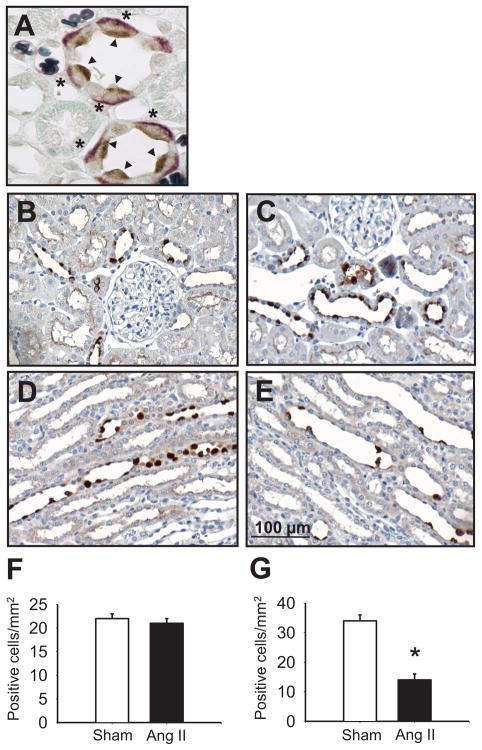
Figure 1. (P)RR immunoreactivity in kidneys from sham-operated and Ang II-infused rat.
A. paraffin-embedded kidney sections (3 μ) showing (P)RR specific staining (brown; arrow heads) on the apical membrane of the collecting duct cells co-localizing with basolateral AE1 immunoreactivity (Bajoran purple; asterisk) in type-A intercalated cells. (original magnification, × 1000). B. Densitometric analysis of the (P)RR intensity of sham-operated (B and D) and Ang II-infused rats (C and E) showing reduced number of positive cells in renal medullary tissues of Ang II-infused compared to sham-operated rats (F) but not in the renal cortex (G). Scale bar: 100 μm. Values are mean ± S.E. *P<0.05 vs. sham-operated rats.
Quantification of (P)RR expression in chronic Ang II-infused rats
Positive (P)RR immunoreactivity in CD cells was similar in the cortex of both the Ang II-infused and sham-operated rats (Ang II: 1.3 ± 0.2; sham: 1.0 ± 0.2 fold change compared to controls). This similarity was also reflected in the number of positive (P)RR stained cells (Ang II: 21 ± 1 vs. sham: 22 ± 1 positive cells/mm2; Figure 1B, C, F). In contrast, in the renal medulla, the (P)RR immunoreactivity was significantly decreased (Ang II: 0.7 ± 0.1 vs. sham: 1.0 ± 0.1 fold change; P<0.001), and the number of positive cells with specific immunoreactivity was significantly less (Ang II: 14 ± 2 vs. sham: 34 ± 2 positive cells/mm2; P<0.001; Figure 1, D, E, G).
The (P)RR transcript levels were significantly greater in the renal medulla compared to the cortex in both groups. For both cortex and medulla, the (P)RR mRNA levels [(P)RR/GAPDH ratio] were significantly increased in the Ang II-infused rats compared to sham-operated [Ang II (cortex: 1.0 ± 0.1; medulla: 1.6 ± 0.1); sham (cortex: 0.7 ± 0.1; medulla: 1.2 ± 0.1); P<0.001] (Figure 2A).
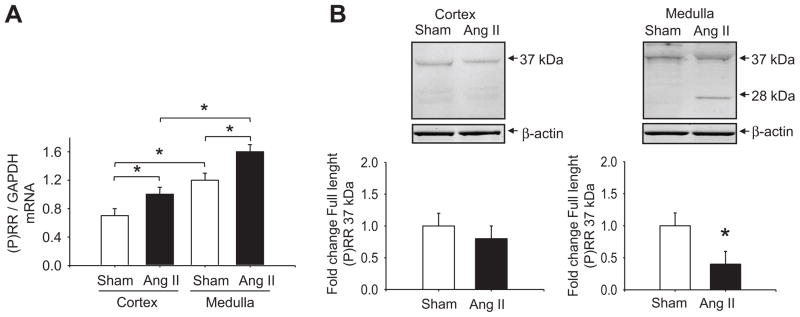
Figure 2. (P)RR real-time qRT-PCR and Western blot analyses in renal cortex and medulla.
A. The mRNA levels for (P)RR were up-regulated in the renal cortex and medulla in Ang II-infused rats. *P<0.001 (n=5). B. A representative blot of the (P)RR protein expression levels in the cortex and medulla as a fold change with respect to control rats showed a decrease in the full-length (P)RR and the presence of the soluble form (s(P)RR) in the renal medulla of Ang II-infused rats. *P<0.05 (n=5).
Kidney (P)RR protein immunoblots from Ang II-infused and sham-operated rats showed the specific (P)RR band of 37 kDa. This band; although unchanged in the cortex, was significantly decreased in the medulla of Ang II-infused rats (cortex: 0.8 ± 0.2 vs. 1.0 ± 0.2 fold change compared to control; P=NS; medulla: 0.4 ± 0.2 vs. 1.0 ± 0.2 fold change; P<0.05; Figure 2, B). Furthermore, the presence of the s(P)RR form (28 kDa band, Figure 2, B) became apparent while it was not detectable in the renal medulla of sham rats nor in the cortex of either group.
Evidence of the presence of furin in the renal medullary tissues of Ang II hypertensive rats
In an attempt to rule out if furin, a protease recently involved in the intracellular cleavage of (P)RR, 12 changes in the kidney of Ang II-hypertensive rats; we measured its protein levels in inner medullary tissues of the Ang II-infused and sham-operated rats. Furin/β-actin ratio was significantly augmented in Ang II-infused rats compared to sham-operated rats (Ang II-infused: 1.4 ± 0.2 vs. sham: 1.0 ± 0.1 fold change; P<0.05).
Evidence of functional s(P)RR in the urine of Ang II hypertensive rats
Based on the results of the (P)RR expression data, we assessed the presence of s(P)RR in the urine of Ang II-infused hypertensive rats. While urinary creatinine was not different between both groups (9,246 ± 1,109 vs. 10,201 ± 388 μg/day, P=NS), the (P)RR immunoblots using urines of Ang II-infused rats showed a transition from the 37 kDa band to the 28 kDa band, whereas in the sham-operated rats the s(P)RR form was not detectable (Figure 3A). These results suggest that secretion and urinary excretion of the s(P)RR form is induced in Ang II-infused rats. To determine if the soluble form of (P)RR was bound to renin and/or (pro)renin in the urine of these rats, we immunoprecipitated (IP) the (P)RR. As shown in Figure 3B, using human recombinant (pro)renin and renin as positive controls, rat renin was detected in the IP, and its absence in the supernatant (S) fractions indicate that most of the renin was bound to (P)RR.
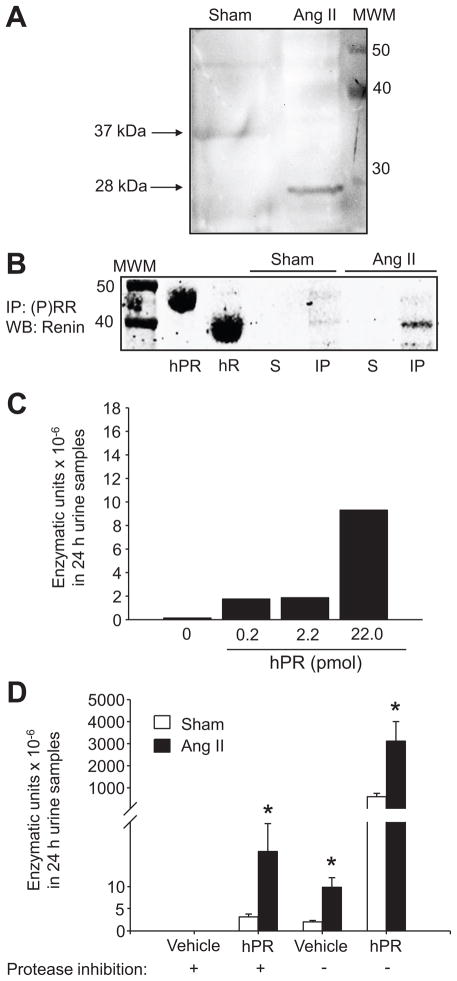
Figure 3.
Evidence of functional s(P)RR in the urine of Ang II hypertensive rats A. Detection of the soluble form of the (P)RR (28 kDa) in urine samples of sham-operated and Ang II infused rats. Eighty micrograms of protein from 10X concentrated urine samples were loaded and incubated with a rabbit anti-(P)RR. B. Immunoprecipitation of (P)RR in the urine samples of Ang II-infused and sham-operated rats demonstrated the absence of renin in the supernatant (S) fractions using the rabbit polyclonal anti-renin H-105 antibody (Santa Cruz, Cat ID sc-22752). C. A dose-response curve of hPR added in the experiment. D. Urines; initially collected with or without protease inhibitor cocktail, measured in the presence and absence of 10 μl of hPR (2.2 pmol/μl), resulted in increased amounts of Ang I forming-enzymatic units excreted per day × 10−6 in Ang II-infused rats compared with sham-operated rats. In samples spiked with hPR, the background renin and non-specific enzyme activity were subtracted. MWM: molecular weight marker; IP: immunoprecipitate; WB: Western blot; hR: recombinant human renin; hPR: recombinant human (pro)renin.
To examine if the presence of the s(P)RR had functional implications in the urine of Ang II-infused rats, we further determined the renin activity in the presence or absence of hPR. Ten μl of 2.2 picomol/μl of hPR added in the experiment was determined based upon a dose-response curve in urine samples of Ang II-infused rats (Figure 3C). In urines collected without protease inhibitors, the amount of Ang I forming-enzymatic units × 10−6 in 24 h urine samples from Ang II-infused rats increased fivefold compared with sham-operated rats (Table 1 and Figure 3D), and increased even further in the presence of hPR in both groups (3,118.33 ± 886.25 vs. 597.69 ± 151.48; P<0.05, Figure 3D). To assess non-specific enzymatic activation of the added hPR, the assay was repeated with urine samples collected into the same inhibitor cocktail used for plasma Ang II determination. 16 Under these conditions, urine samples from Ang II-infused rats still showed greater enzymatic activity than sham rat urine (18.21 ± 6.41 vs. 3.21 ± 0.65 enzymatic units × 10−6 in 24 h urine samples, P<0.05, Figure 3D); while no activity was detected in urine samples without hPR, indicating that the s(P)RR is functional and can enhance activity of (pro)renin leading to Ang I generation (Figure 3D).
Discussion
The present findings demonstrate that chronic infusion of Ang II for 14 days in Sprague-Dawley rats increased renal (P)RR transcript levels and augmented the soluble form of the (P)RR (28 kDa band) in renal inner medullary tissues and tubular fluid in distal nephron segments as reflected in the urine. Novel findings from this study suggest that the s(P)RR in the renal medulla is stimulated during Ang II-dependent hypertension, and that this form of the (P)RR is secreted into the tubular fluid, which may contribute to increased renin activity in the CD of Ang II hypertensive rats.
Using immunoblot analysis, we found that the protein levels of the full-length (P)RR (37 kDa band) were decreased in the renal medulla of chronic Ang II-infused rats. This finding was associated with increased protein levels of the s(P)RR in these tissues, suggesting that post transcriptional changes in the intracellular processing of the (P)RR can be induced by a mechanism dependent on Ang II. Recently, it has been revealed that s(P)RR can be generated subcellularly by a furin-mediated cleavage. 12 Moreover, it has been shown that CHO cells exhibit the 28 kDa form, the amino-terminal fragment of the (P)RR on the endoplasmic reticulum and in the vesicle-like structures, but not on the plasma membrane; and that this form can be secreted into the extracellular space.21 In the present study, we found that furin protein levels were upregulated in inner medullary tissues of Ang II-infused rats compared to sham-operated rats. However, it cannot be excluded that other intracellular proteases may also be involved in the processing of (P)RR12 in this model of experimental hypertension.
Schefe et al. 22 reported that the promyelocytic leukemia zinc-finger transcription factor interacts directly with the C-terminal domain of the (P)RR, which is then translocated into the nucleus and represses transcription of the (P)RR. 22 Accordingly, we found decreased protein levels of the full-length (P)RR (37 kDa band) and (P)RR immunoreactivity in the renal medulla of Ang II-infused rats. These findings contrasted with the augmentation of (P)RR mRNA levels in these tissues, further suggesting that post-transcriptional modifications of the full-length (P)RR occurs to promote the formation of the s(P)RR in the renal medulla with its augmented secretion into the tubular fluid. It has been proposed that some soluble receptors behave like agonists, in which the complexes formed by the ligand and the soluble receptor target a second receptor on specific cells. 23 The present study supports the notion that the (P)RR in chronic Ang II-dependent hypertensive rats contributes to increased intratubular Ang II formation by binding renin or (pro)renin produced and secreted by the neighboring principal cell, 3–5 thus anchoring renin and/or (pro)renin at the cell surface which may help to prevent or minimize renin washout into the urine. 1 Furthermore, the secretion by the intercalated cells of the s(P)RR form into the tubular fluid may also increase binding to renin or (pro)renin, thereby enhancing even further the intraluminal conversion of AGT to Ang I, and ultimately to Ang II since ACE is also present in the CD. 24, 25 Indeed, we found that the chronic Ang II-infused rats exhibited higher urinary renin activity than sham-operated rats. Shao et al.16 recently demonstrated that the increases in intrarenal Ang II levels during chronic Ang II infusions involve substantial stimulation of endogenous Ang II formation which contributes to overall augmentation of intrarenal and intratubular Ang II content. 16 Increased urinary excretion of endogenous Ang II in Val(5)-Ang II-infused rats has been primarily due to an AT1R-dependent mechanism and/or de novo formation of Ang II within the tubular lumen since co-treatment with candesartan significantly prevented this response. 26 Gonzalez-Villalobos et al, 27, 28 reported that ACE inhibition with lisinopril (100 mg/L) in mice chronically infused with Ang II at a dose of 400 ng/kg/min ameliorated mean arterial pressure (MAP) and plasma and intrarenal Ang II concentrations. Importantly, these mice exhibited down-regulation of renin expression in the CD cells. 28 Taken together, the demonstration of the presence of s(P)RR and increased renin activity in the urine of Ang II-infused rats supports a functional role for the soluble (P)RR in the tubular fluid facilitating the generation of Ang I from AGT delivered to distal nephron segments from the proximal tubule during Ang II-dependent hypertension.
It has been shown that in contrast to the species-specificity for AGT cleavage, the binding of renin and (pro)renin to (P)RR shows an unpredicted low species-specificity. 29 Feldt et al. 29 showed that mouse (P)RR is able to bind human renin and (pro)renin. In the present study, we added 2.2 picomol/μl of hPR to urine samples from Ang II-infused and sham-operated rats to examine if the soluble form of (P)RR was able to increase renin activity in presence of renin substrate tetradecapeptide. Indeed, the renin activity in urine samples from Ang II-infused rats; in the absence of protease inhibitors, markedly increased. It has been reported that binding of renin to the (P)RR induced a four-fold increase of the catalytic efficiency of AGT conversion to Ang I. 30 The concentration of hPR used in these assays was markedly higher than the value of equilibrium dissociation constant (KD) reported for rat (P)RR and human (pro)renin which is 3.7 nM. 31 These observations are of great relevance in light of recent demonstrations of renin up-regulation in distal nephron segments of Ang II-dependent hypertensive rats, 4, 7 and renin and/or (pro)renin secretion by CD cells; 3, 5 altogether providing a mechanistic basis for enhanced tubular Ang II formation in hypertension. The combined action of these RAS components on AGT delivered to the distal nephron segments might be key factors to explain the increases in distal tubular formation of Ang II during Ang II-dependent hypertension. 16, 32
Perspectives
The results of the current study provide evidence of a functional role for the soluble form of the (P)RR to enhance intrarenal and intratubular Ang II generation. Because the s(P)RR in the urine is primarily derived from the collecting duct segments, the findings support an increased intraluminal renin activity, which may contribute to increase Ang II formation. The existence of an inducible and functional form of the s(P)RR in the renal medullary tissues and in the urine of chronic Ang II-infused rats reflects increased s(P)RR in collecting duct tubular fluid and further supports the potential role of this soluble form to enhance intratubular renin activity in hypertension. The recent in vivo demonstration that increased urinary Ang II concentrations in mice infused chronically with Ang II enhance distal sodium reabsorption 32 emphasizes further the importance that renin and (P)RR may have in the distal nephron segments contributing to the increases in intratubular Ang II formation, thus allowing for a greater distal tubular sodium reabsorption leading to progression of hypertension.
Acknowledgments
We extend thanks to Dr. Genevieve Nguyen (College of France; France) for generously providing the (P)RR antibody. Also, we want to thank Victoria L. Martin, BS and Kimberly Kavanagh, BS for their excellent technical assistance.
Sources of Funding
Institutional Developmental Award Program of the National Center for Research Resources (P20RR-017659), American Heart Association (AHA; 09BGIA2280440), and Eunice Kennedy Shriver National Institute of Child Health & Human Development (K12HD043451). A. A. G. is a recipient of CONICYT Postdoctoral fellowship from Chile. L. S. L. is a recipient of a CAPES Postdoctoral Fellowship from Brazil.
Footnotes
Disclosures
The authors report no conflicts.
References
- 1.Prieto-Carrasquero MC, Botros FT, Kobori H, Navar LG. Collecting Duct Renin: A major player in Angiotensin II-dependent Hypertension. J Am Soc Hypertens. 2009;3:96–104. doi: 10.1016/j.jash.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, Physiology, and Molecular Biology of Renin Secretion. Physiol Rev. 1990;70:1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 3.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 4.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The Collecting Duct Is the Major Source of Prorenin in Diabetes. Hypertension. 2008;51:1597–1604. doi: 10.1161/HYPERTENSIONAHA.107.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F632–F637. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prieto-Carrasquero MC, Botros FT, Pagan J, Kobori H, Seth DM, Casarini DE, Navar LG. Collecting Duct Renin Is Upregulated in Both Kidneys of 2-Kidney, 1-Clip Goldblatt Hypertensive Rats. Hypertension. 2008;51:1590–1596. doi: 10.1161/HYPERTENSIONAHA.108.110916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen G. Increased cyclooxygenase-2, hyperfiltration, glomerulosclerosis, and diabetic nephropathy: put the blame on the (pro)renin receptor? Kidney Int. 2006;70:618–620. doi: 10.1038/sj.ki.5001723. [DOI] [PubMed] [Google Scholar]
- 10.Ichihara A, Sakoda M, Kurauchi-Mito A, Kaneshiro Y, Itoh H. Involvement of (pro)renin receptor in the glomerular filtration barrier. J Mol Med. 2008;86:629–635. doi: 10.1007/s00109-008-0327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, Kuliszewski MA, Leong-Poi H, Gilbert RE. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension. 2009;54:261–269. doi: 10.1161/HYPERTENSIONAHA.109.128645. [DOI] [PubMed] [Google Scholar]
- 12.Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G. Soluble Form of the (Pro)Renin Receptor Generated by Intracellular Cleavage by Furin Is Secreted in Plasma. Hypertension. 2009;53:1077–1082. doi: 10.1161/HYPERTENSIONAHA.108.127258. [DOI] [PubMed] [Google Scholar]
- 13.Kaneshiro Y, Ichihara A, Sakoda M, Takemitsu T, Nabi AHM, Uddin MN, Nakagawa T, Nishiyama A, Suzuki F, Inagami T, Itoh H. Slowly Progressive, Angiotensin II-Independent Glomerulosclerosis in Human (Pro)renin Receptor-Transgenic Rats. J Am Soc Nephrol. 2007;18:1789–1795. doi: 10.1681/ASN.2006091062. [DOI] [PubMed] [Google Scholar]
- 14.Ichihara A, Suzuki F, Nakagawa T, Kaneshiro Y, Takemitsu T, Sakoda M, Nabi AHM, Nishiyama A, Sugaya T, Hayashi M, Inagami T. Prorenin Receptor Blockade Inhibits Development of Glomerulosclerosis in Diabetic Angiotensin II Type 1a Receptor-Deficient Mice. J Am Soc Nephrol. 2006;17:1950–1961. doi: 10.1681/ASN.2006010029. [DOI] [PubMed] [Google Scholar]
- 15.Burckle CA, Jan Danser AH, Muller DN, Garrelds IM, Gasc JM, Popova E, Plehm R, Peters J, Bader M, Nguyen G. Elevated blood pressure and heart rate in human renin receptor transgenic rats. Hypertension. 2006;47:552–556. doi: 10.1161/01.HYP.0000199912.47657.04. [DOI] [PubMed] [Google Scholar]
- 16.Shao W, Seth DM, Navar LG. Augmentation of endogenous intrarenal angiotensin II levels in Val5-ANG II-infused rats. Am J Physiol Renal Physiol. 2009;296:F1067–F1071. doi: 10.1152/ajprenal.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Botros FT, Prieto-Carrasquero MC, Martin VL, Navar LG. Heme oxygenase induction attenuates afferent arteriolar autoregulatory responses. Am J Physiol Renal Physiol. 2008;295:F904–F911. doi: 10.1152/ajprenal.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins RhBG and RhCG in mouse kidney. Am J Physiol Renal Physiol. 2003;284:F323–F337. doi: 10.1152/ajprenal.00050.2002. [DOI] [PubMed] [Google Scholar]
- 19.Prieto MC, Williams DE, Liu L, Kavanagh KL, Mullins JJ, Mitchell KD. Enhancement of Renin and Prorenin Receptor in the Collecting Ducts of Cyp1a1-Ren2 Rats Contribute to Development and Progression of Malignant Hypertension. Am J Physiol Renal Physiol. 2010 doi: 10.1152/ajprenal.00433.2010. [Epub ahead of print]. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cameron A, Appel J, Houghten RA, Lindberg I. Polyarginines are potent furin inhibitors. J Biol Chem. 2000;275:36741–36749. doi: 10.1074/jbc.M003848200. [DOI] [PubMed] [Google Scholar]
- 21.Senbonmatsu T, Iida S, Yoshikawa A, Aizaki Y, Xiao S, Nishimura S, Inagami T. New perspectives on secretion of (pro)renin receptor into extracellular space. Front Biosci (Elite Ed) 2010;2:1362–1367. doi: 10.2741/e196. [DOI] [PubMed] [Google Scholar]
- 22.Schefe JH, Menk M, Reinemund J, Effertz K, Hobbs RM, Pandolfi PP, Ruiz P, Unger T, Funke-Kaiser H. A Novel Signal Transduction Cascade Involving Direct Physical Interaction of the Renin/Prorenin Receptor With the Transcription Factor Promyelocytic Zinc Finger Protein. Circ Res. 2006;99:1355–1366. doi: 10.1161/01.RES.0000251700.00994.0d. [DOI] [PubMed] [Google Scholar]
- 23.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80:227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 24.Casarini DE, Boim MA, Stella RCR, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol Renal Physiol. 1997;272:F405–F409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 25.Redublo Quinto BM, Camargo de Andrade MC, Ronchi FA, Santos EL, ves Correa SA, Shimuta SI, Pesquero JB, Mortara RA, Casarini DE. Expression of angiotensin I-converting enzymes and bradykinin B2 receptors in mouse inner medullary-collecting duct cells. International Immunopharmacology. 2008;8:254–260. doi: 10.1016/j.intimp.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Shao W, Seth DM, Navar LG. Angiotensin II Type 1 Receptor-Mediated Augmentation of Urinary Excretion of Endogenous Angiotensin II in Val5-Angiotensin II-Infused Rats. Hypertension. 2010;56:378–383. doi: 10.1161/HYPERTENSIONAHA.110.153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez-Villalobos RA, Satou R, Ohashi N, Semprun-Prieto LC, Katsurada A, Kim C, Upchurch GM, Prieto MC, Kobori H, Navar LG. Intrarenal mouse renin-angiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol Renal Physiol. 2010;298:F150–F157. doi: 10.1152/ajprenal.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez-Villalobos RA, Satou R, Seth DM, Semprun-Prieto LC, Katsurada A, Kobori H, Navar LG. Angiotensin-converting enzyme-derived angiotensin II formation during angiotensin II-induced hypertension. Hypertension. 2009;53:351–355. doi: 10.1161/HYPERTENSIONAHA.108.124511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feldt S, Maschke U, Dechend R, Luft FC, Muller DN. The putative (pro)renin receptor blocker HRP fails to prevent (pro)renin signaling. J Am Soc Nephrol. 2008;19:743–748. doi: 10.1681/ASN.2007091030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danser AH, Deinum J. Renin, prorenin and the putative (pro)renin receptor. Hypertension. 2005;46:1069–1076. doi: 10.1161/01.HYP.0000186329.92187.2e. [DOI] [PubMed] [Google Scholar]
- 31.Biswas KB, Nabi AH, Nakagawa T, Ebihara A, Suzuki F. Species specificity of prorenin binding to the (pro)renin receptor in vitro. Front Biosci. 2010;2:1234–1240. doi: 10.2741/e183. [DOI] [PubMed] [Google Scholar]
- 32.Zhao D, Seth DM, Navar LG. Enhanced distal nephron sodium reabsorption in chronic angiotensin II-infused mice. Hypertension. 2009;54:120–126. doi: 10.1161/HYPERTENSIONAHA.109.133785. [DOI] [PMC free article] [PubMed] [Google Scholar]