Abstract
Previous findings in liver transplantation patients have raised the concept that HLA plays a dualistic role. HLA matching will reduce rejection but may augment MHC restricted cellular immune mechanisms of liver allograft injury. To evaluate this concept, we studied CMV hepatitis in 399 FK506-treated liver transplant patients, including 355 cases for which complete HLA-A,B,DR,DQ typing information was available. CMV hepatitis developed in 25 patients, and 17 of them (or 68%) showed a one or two HLA-DR antigen match with the donor. In contrast, HLA-DR matches were found in only 35% of 330 patients without CMV hepatitis (P=0.005). No significant associations were seen for HLA-A, HLA-B, and HLA-DQ antigens. In pretransplant CMV-seronegative patients with seropositive grafts (n=39), the frequency of CMV hepatitis was 44% for HLA-DR-matched livers but 14% for HLA-DR-unmatched livers. In seropositive recipients (n=187), these frequencies were 12% and 2% for HLA-DR-matched and unmatched liver grafts. Chronic rejection developed in 29 patients (or 8%) during a follow-up between 10 and 24 months after transplantation. Its incidence was higher in the CMV hepatitis group (24% vs. 6%) (P=0.007). Although no associations were found between HLA matching and the incidence of chronic rejection, there was an earlier onset of chronic rejection of HLA-DR-matched livers irrespective of CMV hepatitis.
These findings suggest that an HLA-DR match between donor and recipient increases the incidence of CMV hepatitis in both primary and secondary CMV infections. Although HLA compatibility leads to less acute cellular rejection, it is suggested that DR matching may accelerate chronic rejection of liver transplants, perhaps through HLA-DR-restricted immunological mechanisms toward viral antigens, including CMV.
Liver allografts matched for HLA, especially HLA-DR, have lower survival rates, not only in cyclosporine-treated patients (1, 2) but also in patients on FK506 immunosuppression (3). Since HLA mismatching contributes to liver allograft rejection, we have proposed a dualistic role of HLA in liver transplantation (1). HLA compatibility will reduce rejection but may augment other cellular immune mechanisms of liver allograft injury, especially those mediated by major histocompatibility complex–restricted lymphocytes. These mechanisms could be related to immune responses to viral infections and underlying autoimmune disease.
Cytomegalovirus hepatitis in liver transplant recipients has offered an opportunity to investigate the dualistic role of HLA. CMV-specific immune mechanisms are believed to be the primary mediators of liver allopaft injury during CMV infection (4). Several in vitro studies have demonstrated MHC-restricted lymphocyte responsiveness to CMV (5–11). According to the MHC restriction concept, CMV antigen presentation by “self” HLA antigens will augment CMV-specific cellular immunity and cell-mediated injury. HLA matching of CMV-infected transplants patients will permit CMV antigen presentation by “self” HLA antigens, and this would promote T cell mediated effector mechanisms of allograft damage. In this report we present evidence that an HLA-DR compatibility is associated with more CMV hepatitis in liver transplant patients.
A significant complication of CMV infection is chronic rejection. Higher frequencies of graft coronary artery disease have been reported for CMV-infected heart transplant recipients (12, 13). In lung transplant patients, CMV infection is associated with bronchiolitis obliterans due to chronic rejection (14, 15). In vitro proliferation assays have demonstrated a persistence of primed CMV-specific lymphocytes in bronchoalveolar lavages several months after symptomatic CMV infection, and this is associated with a high incidence of subsequent bronchiolitis obliterans (16). Kidney transplant patients with CMV infection experience more rejection, and no differences have been noted in survivals of HLA-matched and mismatched kidney transplants (17, 18). Moreover, a higher incidence of CMV infection has been reported for patients with HLA-DR-matched kidney transplants (18, 19). This suggests that CMV infection can override the beneficial effect of HLA matching on kidney transplant outcome.
In liver transplant patients, CMV infection is associated with the development of the vanishing bile duct syndrome, a manifestation of chronic rejection (20). The incidence of vanishing bile duct syndrome appears to be higher for liver transplants from donors with HLA-DR matches (2). The studies described in this report show an association between chronic rejection and CMV hepatitis and also that HLA-DR matching accelerates the development of chronic rejection in liver transplant recipients.
MATERIALS AND METHODS
Study population and immunosuppression
This study was conducted on 399 adult patients who underwent orthotopic liver transplantation at the University of Pittsburgh between August 1989 and December 1990 and who had survived for at least 3 months after surgery. Liver allograft biopsies were performed when clinically indicated by an elevation of liver function tests, changes in the color or quantity of bile production, or by any clinical suspicion of graft dysfunction.
All patients received FK506 as the primary immunosuppressive agent. The protocol of FK506 treatment is described elsewhere (21). Briefly, FK506 was initially given in a continuous infusion at 0.1 mg/kg/day, which was converted to an oral dose of 0.15 mg/kg every 12 hr with the return of bowel function. Subsequent dosage adjustments were guided by the quality of the graft, the presence of rejection, toxicity, and FK506 plasma trough level (normal value: <2 ng/ml). Rejection episodes were treated with a 1 g bolus of methylprednisolone or a “recycling” of high-dose steroids starting at 200 mg and tapering to 20 mg over five days. Steroid-resistant rejection episodes were treated with a 5-day course of OKT3.
Serology
Complete donor-recipient HLA typing was done for 355 liver transplant cases. HLA-A,B typing was done by standard Amos-modified lymphocytotoxicity assays with local and commercial typing trays. HLA-DR,DQ typing was done by two-color fluorescence. Pretransplant CMV serological status of donor and recipient was available for 262 transplant cases.
Diagnosis of CMV hepatitis
This study focused on CMV hepatitis rather than CMV infection of liver transplant patients. The diagnosis of CMV hepatitis was based on typical inclusion bodies or direct immunoperoxidase detection of CMV early antigen in a liver specimen obtained by (percutaneous) liver biopsy.
Diagnosis of chronic rejection
Histological criteria for chronic rejection are described elsewhere (22) and include lymphocytic bile duct damage in 50% or more of the portal triads, with evidence of bile duct loss and hepatocanalicular cholestasis.
Statistical analysis
Associations between HLA matching and CMV hepatitis and chronic rejection were assessed by chi-square analysis. Odds ratios were calculated for the estimation of the relative risks. The Mann Whitney U nonparametric test was used to analyze differences in the time of onset of chronic rejection between groups.
RESULTS
CMV hepatitis developed in 25 of 355 liver transplant patients (7%); the median time of diagnosis was 33.5 days after transplantation (range: 13–278 days). The incidence of CMV hepatitis was significantly higher for HLA-DR-compatible liver transplants (Table 1). Seventeen of the 25 CMV hepatitis patients (68%) shared at least one HLA-DR antigen with the donor (Table 1). In contrast, HLA-DR sharing was found in 123 of 330 patients (35%) without CMV hepatitis (P=0.005). An HLA-DR match increased the relative risk of CMV HLA by a factor of 3.6. There seemed no preferential sharing of any particular HLA-DR antigen in the CMV hepatitis group. Also, the incidence of other symptomatic CMV infections appeared unaffected by HLA-DR sharing. There were no differences between DR matched and unmatched hepatitis patients in terms of severity of liver dysfunction and responsiveness to ganciclovir therapy.
Table 1.
Association between CMV hepatitis and HLA matching in liver transplantation
| Donor with Match | Frequency of HLA-matched liver transplants |
Significance | Relative riska | |
|---|---|---|---|---|
| CMV hepatitis (n=25) | No CMV hepatitis (n=330) | |||
| For HLA-A | 40% | 37% | P=NS | |
| For HLA-B | 16% | 24% | P=NS | |
| For HLA-DR | 68% | 35% | P=0.005 | 3.6 |
| For HLA-DQ | 72% | 64% | P=NS | |
Relative risk of CMV hepatitis in matched versus unmatched liver transplants.
No significant associations were found between CMV hepatitis and donor-recipient sharing of HLA-A, HLA-B, and HLA-DQ antigens (Table 1). In this group of FK506-treated patients, a similar incidence of CMV hepatitis was seen in patients who received OKT3 (4/57 or 7%) and those without OKT3 treatment (21/298 or 7%).
Pretransplant CMV serological status was known for 262 HLA-DR-typed donor-recipient pairs (Table 2). Thirty-nine of the CMV-seronegative patients received CMV-seropositive grafts and 11 patients (28%) developed CMV hepatitis. In this group, the incidence of CMV hepatitis was about three times higher in livers with a shared HLA-DR antigen than in HLA-DR unmatched grafts (44% vs. 14%) (P=0.07). Of the other 36 seronegative recipients, only 3 (8%) who received livers from seronegative donors developed CMV hepatitis. Only one had HLA-DR sharing.
Table 2.
Incidence of primary and secondary CMV hepatitis in HLA-DR-matched and HLA-DR-unmatched liver transplants
| Pretransplant CMV status | HLA-DR-matched liver transplants |
HLA-DR-unmatched liver transplants |
Significance | Relative riska | ||
|---|---|---|---|---|---|---|
| n | CMV hepatitis | n | CMV hepatitis | |||
| Recipient−, Donor+ | 18 | 44% | 21 | 14% | P=0.07 | 4.8 |
| Recipient+, Donor+ or−b | 69 | 12% | 118 | 2% | P=0.006 | 7.6 |
| Recipient−, Donor− | 15 | 7% | 21 | 10% | NS | — |
Relative risk of CMV hepatitis for HLA-DR matching.
In this group, 110 donors were CMV+ and 77 donors were CMV−.
CMV hepatitis was observed in 10 (5%) of 187 pretransplant seropositive patients, and 8 of these cases involved an HLA-DR match. Table 2 shows that in this group the frequency of hepatitis due to secondary CMV infection was 12% in HLA-DR-matched livers but only 2% in the HLA-DR-unmatched liver transplants (P=0.006). These data suggest that HLA-DR sharing between recipient arid donor increases the risk for both primary and secondary CMV hepatitis after liver transplantation.
During a follow-up of between 10 and 24 months after transplantation, 29 patients (8%) developed chronic rejection. The incidence of chronic rejection was four-fold higher in the CMV hepatitis group (24%) than in patients without CMV hepatitis (6%; P<0.005) [Table 3]). Moreover, chronic rejection occurred sooner in the CMV hepatitis group. The median posttransplant time of onset was 60 days (range: 43–501 days) in the CMV hepatitis group and 245 days (range: 35–660 days) in patients without CMV hepatitis (P=0.07). Five of the 7 patients (or 71%) with chronic rejection preceded by CMV hepatitis shared HLA-DR with the liver transplant donor.
Table 3.
Associations of chronic rejection with CMV hepatitis and HLA matching of liver transplants
| Allograft status | Chronic rejection (n=29) | No chronic rejection (n=326) | Significance |
|---|---|---|---|
| CMV hepatitis | 24% | 6% | P=0.0007 |
| HLA-A match | 28% | 38% | NS |
| HLA-B match | 10% | 25% | NS |
| HLA-DR match | 45% | 39% | NS |
| HLA-DQ match | 82% | 66% | NS |
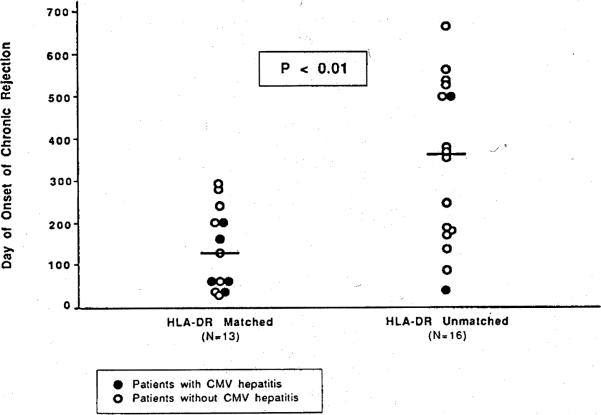
In the overall group of liver transplant patients, we did not find significant differences in the incidence of chronic rejection of liver transplants matched or mismatched for HLA-A,B,DR or DQ (Table 3). However, HLA-DR sharing was associated with an earlier onset of chronic rejection in patients irrespective of CMV hepatitis status (Fig. 1). The median onset of chronic rejection was 130 days (range: 35–284 days) for livers with an HLA-DR match and 356 days (range: 43–660 days) for HLA-DR-unmatched livers (P<0.01). Thus, HLA-DR matching appears to accelerate the development of chronic rejection after liver transplantation.
Figure 1.
Accelerated onset of chronic rejection in HLA-DR-matched liver transplants.
DISCUSSION
These observations suggest that HLA-DR sharing between donor and recipient promotes the development of CMV hepatitis in liver transplant patients. They are consistent with the concept of a dualistic role of HLA that predicts that HLA compatibility decreases graft rejection but may augment other cellular immune mechanisms of transplant injury, especially those mediated by MHC-restricted lymphocytes (1). CMV disease appears to be mediated by cellular immune reactivity (4), and several in vitro studies have demonstrated HLA restricted lymphocyte reactivity toward CMV (5–11). The present data suggest that HLA-DR antigen sharing between donor and recipient leads to higher incidence of CMV hepatitis in liver transplant patients. No association of CMV hepatitis with HLA-A, HLA-B, and HLA-DQ sharing was observed.
The incidence of CMV hepatitis was lower in this group of FK506-treated patients (7%) than that reported from our center in cyclosporine-treated liver transplant recipients (12%) (25). Similarly, the incidence and severity of enteric CMV infection was less in FK506-treated than in cyclosporine-treated patients (26).
In cyclosporine-treated patients OKT3 therapy is associated with a higher incidence of CMV infection (23). Whereas patients on FK506 require less OKT3 therapy (21), it also appears that in FK506-treated patients, OKT3 does not promote CMV enteritis (26)—or, as shown in this study, the development of CMV hepatitis. Because the pathogenesis of CMV infection is thought to involve cell-mediated immune mechanisms, it is possible that this lower incidence of CMV infection is related to the immunosuppressive efficacy of FK506. Whatever the explanation, HLA-DR-matched liver transplants in FK506-treated patients are at increased risk for hepatitis caused by primary and secondary CMV infection. HLA-DR matching status seems germane in the consideration of CMV prophylaxis after liver transplantation.
Although structural similarities have been reported between CMV and HLA gene sequences (27–29), the most likely explanation for the increased incidence of CMV hepatitis in HLA-DR-matched transplanted livers is the phenomenon of MHC restriction of antigen-specific lymphocyte reactivity. CMV infection of a transplanted liver leads to the expression of CMV-derived antigens—which, in context with MHC molecules on the cell surface, are presented by infected cells recognized by recipient T lymphocytes. Both class I (5,6) and class II (8–11) HLA-restricted CMV-speeific T cell responses have been reported. HLA matching will permit MHC-restricted antigen presentation, thereby augmenting cell-mediated immune responses toward CMV-infected liver allografts.
This study did not reveal significant associations between CMV hepatitis and liver transplant matching for class I antigens encoded by the HLA-A and HLA-B loci. In vitro studies of human and murine lymphocytes have demonstrated class I MHC-restricted and CD8-positive CMV-specific cytotoxic T cells (5, 6, 30). These cells are believed to play a major role in the development of protective immunity to CMV infection (7, 30). Conversely, class II MHC-restricted CMV-specific T cells generally have the CD4 phenotype and can be expected to produce lymphokines that mediate inflammatory processes leading to hepatic injury. Such lymphokines could augment cellular rejection mechanisms. For instance, CMV-activated T cells could release interleukin-2 which causes expansion of graft-infiltrating alloreactive lymphocytes, and interferon-gamma, which further upregulates the expression of HLA antigens on target cells. Recent studies in lung transplant patients have demonstrated that the persistence of proliferative CMV-specific lymphocytes in bronchoalveolar lavages correlates with increased donor ailoreactivity within the lung allograft (16). CMV infection also increases the risk of chronic rejection of lung allografts (15).
This study has also shown a higher incidence of chronic rejection in liver transplant recipients who experienced CMV hepatitis. O'Grady et al. (20) have reported an association between CMV infection and the vanishing bile duct syndrome in liver transplant patients, and that HLA-DR matching of the liver donor represented an additional risk factor. These investigators concluded that HLA-DR status is not a predisposing factor for CMV infection. Our data suggest, instead, that HLA-DR matching increases the risk of CMV hepatitis, which then leads to a higher incidence of chronic rejection.
We have found that HLA-DR matching is associated with an earlier onset, but not a higher frequency, of chronic rejection. Since this HLA-DR matching effect was also seen in patients without CMV hepatitis, it seems that additional HLA-DR-restricted lymphocyte responses to as-yet-undefined antigens may contribute to accelerated chronic rejection of the liver allograft. Donaldson et al. (2) have proposed a mechanism of HLA-DR-restricted T cell response to class I HLA antigens to explain the higher risk of vanishing bile duct syndrome. This mechanism requires the generation of peptides from endogenous class I HLA molecules that would be presented by class II HLA molecules on the surface of the bile duct epithelium of the donor liver. At present, we are unaware of experimental support of this model. Moreover, class I HLA antigens are readily secreted by the transplanted liver (31), and it seems possible that such soluble antigens are taken up and processed primarily by antigen-presenting cells of the recipient. Therefore, we believe that the accelerated chronic rejection of an HLA-DR-matched liver transplant is more likely related to HLA-DR-restricted immune responses during viral infection, and perhaps even autoimmune disease.
In summary, the findings described in this report expand the concept of the dualistic role of HLA in liver transplantation and help explain why survival is poorer with better matches. Although HLA-DR matching reduces acute cellular rejection, it is apparent that HLA-DR matching will not only increase the risk of CMV hepatitis but also accelerate chronic rejection. A better understanding of the different HLA-associated immune mechanisms within the liver allograft may lead to improved management strategies in hepatic transplantation.
Acknowledgments
We acknowledge Marian Vanek and the staff of the tissue typing laboratory for the HLA typing of the patients and donors studied in this analysis. The assistance of Carolyn Nolte in the preparation of the manuscript is gratefully acknowledged.
Footnotes
This work was supported by Grants AI-23467 and DK-29961 from the National Institutes of Health, and by the Pathology Education Research Foundation.
REFERENCES
- 1.Markus BH, Duquesnoy RJ, Gordon RD, et al. Histocompatibility and liver transplant outcome: does HLA exert a dualistic effect? Transplantation. 1988;46:372. [PMC free article] [PubMed] [Google Scholar]
- 2.Donaldson PT, O'Grady J, Portmann B, et al. Evidence for an immune response to HLA class I antigens in the vanishing bile duct syndrome after liver transplantation. Lancet. 1987;1:945. doi: 10.1016/s0140-6736(87)90293-5. [DOI] [PubMed] [Google Scholar]
- 3.Iwaki Y, Kobayashi M, Starzl TE. Effect of HLA matching on orthotopic liver transplantation. Abstracts of the 11th Annual Meeting of American Society of Transplant Physicians; Chicago, IL. May 26–27, 1992. [Google Scholar]
- 4.Ho M. Role of specific cytotoxic lymphocytes in cellular immunity against murine cytomegalovirus. Infect Immun. 1980;27:767. doi: 10.1128/iai.27.3.767-776.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinnan GV, Kirmani N, Esber E. HLA-restrieted cytotoxic T lymphocyte and nonthymic cytotoxic lymphocyte responses to cytomegalovirus infection of bone marrow transplant recipients. J Immunol. 1981;126:2036. [PubMed] [Google Scholar]
- 6.Quinnan GV, Kirmani N, Rook AH, et al. Cytotoxic T cells in cytomegalovirus infection: HLA-restricted T lymphocyte and non T-lymphocyte cytotoxic responses correlated with recovery from cytomegalovirus infection in bone marrow transplant recipients. N Engl J Med. 1982;307:7. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- 7.Quinnan GV, Bums WH, Kirmani N, et al. HLA-restricted cyto-toxic T lymphocytes are an early immune response and important defense mechanisms in cytomegalovirus infections. Rev Infect Dis. 1984;6:156. doi: 10.1093/clinids/6.2.156. [DOI] [PubMed] [Google Scholar]
- 8.Lindsay MD, Torpey DJ, Rinaldo CR., Jr. HLA-DR restricted cytotoxicity of cytomegalovirus infected monocytes mediated by Leu-3-positive T cells. J Immunol. 1986;136:3045. [PubMed] [Google Scholar]
- 9.Linner KM, Monroy C, Bach FH, Gehrz RC. Dw subtypes of serologically defined DR-DQ specificities restrict recognition of cytomegalovirus. Hum Immunol. 1986;17:79. doi: 10.1016/0198-8859(86)90077-7. [DOI] [PubMed] [Google Scholar]
- 10.Gehrz RC, Fuad S, Young-Nan C, Bach FH. HLA class II restriction of T helper cell response to cytomegalovirus (CMV). I:Immunogenetic control of restriction. J Immunol. 1987;138:3145. [PubMed] [Google Scholar]
- 11.Gehrz RC, Liu NC, Eckhardt J, Klaus A. Relevance of immune responses to pathogenesis of cytomegalovims-associated diseases. Transplant Proc. 1991;23:75. [PubMed] [Google Scholar]
- 12.Grattan MT, Moreno-Cabal CE, Starnes VA, Oyer PE, Stinson EB, Shumway NE. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA. 1988;261:3561. [PubMed] [Google Scholar]
- 13.McDonald K, Rector T, Barnlin E, Olivari MT. Cytomegalovirus infection in cardiac transplant recipients predicts the incidence of allograft atherosclerosis. J Am Coll Cardiol. 1989;13:213A. [Google Scholar]
- 14.Burke CM, Glanville AR, Macovial, et al. The specification of cytomegalovirus infection following human heart-lung transplantation. J Heart Transplant. 1986;5:267. [PubMed] [Google Scholar]
- 15.Keenan RJ, Lega ME, Dummer JS, et al. Cytomegalovirus serologic status and postoperative infection correlates with risk of developing chronic rejection after pulmonary transplantation. Transplantation. 1991;51:433. doi: 10.1097/00007890-199102000-00032. [DOI] [PubMed] [Google Scholar]
- 16.Zeevi A, Uknis ME, Spichty KH, et al. Proliferation of cytomegalovirus primed lymphocytes in bronchoalveolar lavages from lung transplant patients. Transplantation. 1992;54:635. doi: 10.1097/00007890-199210000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Waltzer WC, Arnold AN, Anaise D, et al. Impact of cytomegalovirus infection and HLA matching on outcome of renal transplantation. Transplant Proc. 1987;19:4077. [PubMed] [Google Scholar]
- 18.May AG, Betts RF, Freeman RB, Andrus CH. An analysis of cytomegalovirus infection and HLA antigen matching on the outcome of renal transplantation. Ann Surg. 1978;187:110. doi: 10.1097/00000658-197802000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pouteil-Noble C, Betuel H, Raffaele P, et al. Influence de la compatibilite HLA sur l'infection a cytomegalovirus en transplantation renale. Presse Med. 1991;27:20. [PubMed] [Google Scholar]
- 20.O'Grady JG, Sutherland S, Harvey F, et al. Cytomegalovirus infection and donor/recipient HLA antigens: interdependent co-factors in pathogenesis of vanishing bile duct syndrome after liver transplantation. Lancet. 1988;2:302. doi: 10.1016/s0140-6736(88)92356-2. [DOI] [PubMed] [Google Scholar]
- 21.Fung J, Abu-Elmagd K, Jain A, et al. A randomized trial of primary liver transplantation under immunosuppression with FK506 vs. cyclosporine. Transplant Proc. 1991;23:2977. [PMC free article] [PubMed] [Google Scholar]
- 22.Demetris AJ, Fung JJ, Todo S, et al. Conversion of liver allograft recipients from cyclosporine to FK506 immunosuppressive therapy: a clinicopathologic study of 96 patients. Transplantation. 1992;53:1056. doi: 10.1097/00007890-199205000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh N, Dummer JS, Kusne S, et al. Infections with cytomegalovirus and other herpesvirus in 121 liver transplant recipients: transmission by donated organ and the effect of OKT3 antibodies. J Infect Dis. 1988;158:124. doi: 10.1093/infdis/158.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis WD, Jenkins RL, Burke PA, et al. FK506 rescue therapy in liver transplant recipients with drug-resistant rejection. Transplant Proc. 1991;23:2989. [PubMed] [Google Scholar]
- 25.Dummer JS. Cytomegalovirus infection after liver transplantation: clinical manifestations and strategies for prevention. Rev Infect Dis. 1990;12(suppl. 7):S767. doi: 10.1093/clinids/12.supplement_7.s767. [DOI] [PubMed] [Google Scholar]
- 26.Sakr M, Hassanein T, Gavaler J, et al. Cytomegalovirus infection of the upper gastrointestinal tract following liver transplantation—incidence, location, and severity in cyclosporine- and FK506-treated patients. Transplantation. 1992;53:786. doi: 10.1097/00007890-199204000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujinami RS, Nelson JA, Walker L, Oldstone MB. Sequence homology and immunologic cross reactivity of human cytomegalovirus with HLA-DR B chain: a means for graft rejection and immunosuppression. J Virol. 1988;62:100. doi: 10.1128/jvi.62.1.100-105.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beck S, Barrell BG. Human cytomegalovirus encodes a glycopro-tein homologous to MHC class I antigens. Nature. 1988;331:269. doi: 10.1038/331269a0. [DOI] [PubMed] [Google Scholar]
- 29.Wiley D. MHC gene in cytomegalovirus. Nature. 1988;331:209. doi: 10.1038/331209a0. [DOI] [PubMed] [Google Scholar]
- 30.Reddehase MJ, Mutter W, Munch IX, Burning KH, Koszinowisk CD8-positive T lymphocytes specific for murine cytomegalovirus immediate early antigens mediate protective immunity. J Virol. 1987;61:3102. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies HS, Pollard SG, Calne RY. Soluble HLA antigens in the circulation of liver graft recipients. Transplantation. 1989;47:524. doi: 10.1097/00007890-198903000-00025. [DOI] [PubMed] [Google Scholar]