Abstract
Objective
To evaluate the associations between intakes of vitamins A, C, and E and risk of colon cancer.
Methods
Using the primary data from 13 cohort studies, we estimated study- and sex-specific relative risks (RR) with Cox proportional hazards models and subsequently pooled RRs using a random effects model.
Results
Among 676,141 men and women, 5,454 colon cancer cases were identified (7–20 years of follow-up across studies). Vitamin A, C, and E intakes from food only were not associated with colon cancer risk. For intakes from food and supplements (total), the pooled multivariate RRs (95% CI) were 0.88 (0.76–1.02, >4,000 vs. ≤1,000 μg/day) for vitamin A, 0.81 (0.71–0.92, >600 vs. ≤100 mg/day) for vitamin C, and 0.78 (0.66–0.92, >200 vs. ≤6 mg/day) for vitamin E. Adjustment for total folate intake attenuated these associations, but the inverse associations with vitamins C and E remained significant. Multivitamin use was significantly inversely associated with colon cancer risk (RR = 0.88, 95% CI: 0.81–0.96).
Conclusions
Modest inverse associations with vitamin C and E intakes may be due to high correlations with folate intake, which had a similar inverse association with colon cancer. An inverse association with multivitamin use, a major source of folate and other vitamins, deserves further study.
Keywords: Vitamin A, Vitamin C, Vitamin E, Multivitamin, Colon cancer, Cohort study, Pooled analysis
Introduction
Diet and lifestyle factors play an important role in the etiology of colon cancer, and a large proportion of colon cancer incidence might be prevented by a healthy lifestyle [1]. Among dietary factors, vitamins A, C, and E have been hypothesized to reduce the risk of colon cancer because of their anticarcinogenic properties: vitamin A regulates nuclear receptors that suppress tumor formation, induces cell apoptosis [2], and enhances immune function [3]. Vitamin C has antioxidant properties and enhances the immune system [4]. Vitamin E inhibits lipid peroxidation in cell membranes, prevents oxidative damage of DNA by scavenging free radicals, and inhibits carcinogen production [5, 6].
A limited number of observational studies have investigated the associations between intakes of vitamins A, C, and E and risk of colon cancer, and the results for each vitamin have been inconsistent [7–14]. A pooled analysis of nested case–control studies from five cohorts reported a modestly lower risk of colorectal cancer [15] comparing the highest quartile of serum alpha-tocopherol (vitamin E) concentration to the lowest. On the other hand, a meta-analysis of seven clinical trials that tested effects of different combinations of β-carotene and vitamins A, C, and E on recurrence of colorectal adenomas (precursors of colorectal cancer) found no significant beneficial effects for supplementation with these nutrients [16]. Two recent clinical trials of vitamin E supplementation that were not included in the meta-analysis also found no beneficial effects of vitamin E supplementation compared with a placebo on colorectal cancer incidence [17, 18]. Some studies [19, 20], but not all [21], have found that use of multivitamin supplements, a good source of vitamins A, C, and E, and others, was associated with lower risk of colorectal cancer.
Whether vitamins A, C, and E provide protection from colon cancer is of considerable public health importance; however, this remains as an open question because of inconsistent findings from previous studies, and randomized trials may have been of insufficient duration to reach definitive conclusions. In this analysis, we examined whether intakes of vitamins A, C, and E from foods and supplemental sources and use of multiple vitamin supplements were associated with risk of colon cancer by reanalyzing the primary data from 13 prospective cohort studies from Europe and North America.
Materials and methods
Study population
The Pooling Project of Prospective Studies of Diet and Cancer (Pooling Project), an international consortium of cohort studies, was established to summarize the associations between dietary factors and risks of several cancers by analyzing and combining the individual data from each participating cohort study. The details of the Pooling Project have been described previously [22]. Thirteen prospective cohort studies from North America and Europe that met the following inclusion criteria for the colorectal cancer analyses were identified [13, 14, 23–32]: 1) at least 50 incident colorectal cancer cases; 2) assessment of usual dietary intake; 3) a validation study of the dietary assessment method or a closely related instrument; and 4) assessment of intakes of vitamins A, C, and E.
Studies including both men and women were separated into sex-specific cohorts. The Nurses’ Health Study (NHS) was treated as two separate studies, NHSa (1980–1986) and NHSb (1986–2000) to take advantage of the more detailed dietary assessment in 1986. Following survival data analysis theory, blocks of person-time in different time periods are asymptotically uncorrelated, regardless of the extent to which they are derived from the same people [33], so pooling estimates from these two time periods is a statistically valid alternative to using a single time period. Because the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study and the Women’s Health Study were clinical trials of vitamin E and beta-carotene supplementation, we included participants in the placebo group only for these studies.
Dietary assessment
Each study assessed nutrient intakes by a self-administered food frequency questionnaire (FFQ) at baseline and provided intake data for vitamins A, C, and E from foods only (dietary intake) and from foods and supplements (total intake), if available. Information on use of vitamin supplements (multivitamins and/or individual vitamin A, C, or E supplements) at baseline was available in 10 studies. For the Netherlands Cohort Study and the New York State Cohort, which collected information on the use of vitamin supplements, but did not estimate supplemental intake, we derived an estimate of total intake for each vitamin. For the Netherlands Cohort Study, we estimated supplemental intake using the most common dose of vitamins A, C, and E in multivitamins and in individual vitamin A, C, and E supplements reported in their FFQ validation study [34]. For the New York State Cohort, we estimated supplemental intake using the dose for generic multivitamins and vitamin A, C, and E supplements used in the Nurses’ Health Study. For both studies, we assumed a frequency of one multivitamin or each individual supplement a day. Nutrient intakes from foods were energy-adjusted to 2,100 kcal/day for men and 1,600 kcal/day for women by the residual method [35].
In the validation studies (Wolk and Krogh, personal communication) [34, 36–42], the Pearson correlation coefficients between dietary vitamin intake estimated by the FFQ and the reference method were generally 0.4–0.7 for each vitamin. In additional validation studies using biomarkers, the Pearson correlation coefficients between dietary vitamin E intake estimated by the FFQ and its plasma concentration were 0.41 in the Nurses’ Health Study and 0.51 in the Health Professionals Follow-up Study [43].
We also had information on nondietary risk factors, which was collected by self-administered questionnaires at baseline in each study. We reformatted these variables to create standardized categories for each variable across studies.
Case ascertainment
Incident colon cancer cases were identified by each cohort through either self-report on the questionnaire with subsequent medical record review [14, 31]; linkage with a cancer registry [13, 26–28, 30, 32]; or both [23–25, 29]. Some studies also had an additional linkage with a death registry [13, 14, 23–25, 28–30, 32]. Each colon cancer case was further classified by tumor site: tumors from the cecum to the splenic flexure were considered to be proximal colon cancers, and tumors in the descending and sigmoid colon were defined as distal colon cancers. The follow-up rate of studies included in this report has generally been over 90%.
We excluded individuals who had a history of cancer other than nonmelanoma skin cancer at baseline and those who reported implausible energy intakes (beyond 3 standard deviations from the study-specific loge-transformed mean energy intake).
Statistical analysis
Data analyses were conducted using a two-stage method: (1) study- and sex-specific analyses and (2) subsequent pooled analysis of the study-specific results. Study- and sex-specific relative risks (RR) and two-sided 95% confidence intervals (CI) were estimated with the Cox proportional hazards model [44] using SAS PROC PHREG [45]. The Canadian National Breast Screening Study and the Netherlands Cohort Study were analyzed as case-cohort studies [46]. Age at baseline (in years) and the year the baseline questionnaire was returned were used as stratification variables, thereby creating a time metric which simultaneously accounted for age, calendar time, and time since entry into the study. Person-years of follow-up time were calculated from the date of the baseline questionnaire until the date of colon cancer diagnosis, death, loss to follow-up, if applicable, or end of follow-up, whichever came first.
Two different categorical analyses were conducted for each vitamin: RRs of colon cancer were estimated according to study-specific quintiles of intake and in separate analyses according to absolute intake cutpoints, which were identical across studies. Study- and sex-specific quintiles of vitamin intake were defined using the distributions of the baseline cohort in each study except for the Canadian National Breast Screening Study and the Netherlands Cohort Study where the distributions in the subcohorts were used. We excluded the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study and the Netherlands Cohort Study from the quintile analyses of total vitamin intake because their prevalence of multivitamin use was much lower (≤8%) than in the other studies (>30%), which resulted in their intake levels for the highest categories not being comparable to those in the other studies. However, we included the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study and the Netherlands Cohort Study in the categorical analyses in which identical absolute intake cutpoints were applied. The test for trend across categories of intake was performed by assigning participants the median value of their category and entering it as a continuous term in a regression model.
In multivariate analyses, we adjusted for height, body mass index, education, physical activity, smoking, alcohol consumption, family history of colorectal cancer, use of nonsteroidal anti-inflammatory drugs, use of oral contraceptives and postmenopausal hormone therapy use among women, and intakes of red meat, total milk, dietary folate, and total energy; multivitamin use was also adjusted for in analyses of dietary vitamin intake. In some multivariate models, further adjustment was made for intake of total folate or total vitamin C or E (see footnotes in the tables for the covariates in the specific models and for coding of the covariates). In the multivariate analyses, the proportion of missing values for each covariate measured in a study was generally less than 5%; an indicator variable for missing responses was created for each covariate in a study, if needed [47, 48].
The pooled estimates and 95% CIs were calculated using a random effects model which weighted the study-specific loge RRs by the inverse of the sum of their variance and the estimated between-studies variance; between-studies heterogeneity was tested by the Q statistic [49, 50]. A meta-regression model was used to test for effect modification by sex, study population, smoking status, and postmenopausal hormone therapy use in women because these interactions could only be assessed between studies (sex and study population) or because the potential effect modifier was a nominal variable with three levels (smoking status and postmenopausal hormone therapy use in women) [47]. We examined whether the associations for vitamin intakes differed by body mass index and alcohol consumption by including a cross-product term of the ordinal score of the level of each factor and intake of each vitamin expressed as a continuous variable in the model [50]. We tested for differences in the results between proximal and distal colon cancers using a contrast test [51].
Results
During follow-up of 209,263 men and 466,878 women (Total = 676,141) for up to seven to twenty years in thirteen cohort studies, we identified 5,454 incident colon cancer cases (1,695 cases in men and 3,759 cases in women; 2,850 proximal colon cancer cases, 2,151 distal colon cancer cases, and 453 colon cancer cases with unknown site information). Median dietary intakes (intake from food only) for each vitamin showed 2–4 fold differences across studies (Table 1). The wide range in dietary intakes across studies reflects both the differences in the FFQs used in the studies and the differences in true intake across the populations. The prevalence of multivitamin supplement use was higher for the studies from the United States (30–50%) compared with those from Europe (3–8%). The most commonly used individual vitamin supplements were vitamin C supplements (prevalence = 6–38% across studies). In the aggregated dataset of the ten studies (n = 608,348) with information on use of both multivitamins and individual vitamin supplements, the prevalence of vitamin supplement use was 0.5% for vitamin A supplements only, 1% for vitamin E supplements only, 4% for vitamin C supplements only, 4% for any combination of individual vitamin A, C, and E supplements without multivitamins, 14% for multivitamins only, and 17% for multivitamins with any combination of individual vitamin A, C, and E supplements. The prevalence of vitamin supplements use in the US-based cohort studies included in this pooled analysis was similar to that of the US population [52].
Table 1.
Description of studies in the analyses of intakes of vitamins A, C, and E and use of vitamin supplements and colon cancer in the Pooling Project
| Study | Follow-up period | Baseline cohortc (n) | Cancer cases (n) | Dietary intakea |
Total intakeb |
Prevalence of supplement use |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin A (μg/day) | Vitamin C (mg/day) | Vitamin E (mg/day) | Vitamin A (μg/day) | Vitamin C (mg/day) | Vitamin E (mg/day) | Multivitamins (%) | Vitamin A (%) | Vitamin C (%) | Vitamin E (%) | ||||
| Men | |||||||||||||
| Alpha-Tocopherol Beta-Carotene Cancer Prevention Study (ATBC)d | 1985–1999 | 6,784 | 44 | 1,243 (712–2,382)e | 73 (43–122) | 8 (6–15) | 1,319 (730–2,730) | 76 (44–156) | 8 (6–17) | 8 | – | – | – |
| Cancer Prevention Study II Nutrition Cohort (CPS II) | 1992–1999 | 66,071 | 467 | 1,325 (798–2,104) | 128 (58–220) | 11 (8–14) | 1,606 (870–3,344) | 166 (68–722) | 13 (8–251) | 33 | 5 | 24 | 17 |
| Health Professionals Follow-up Study (HPFS) | 1986–2000 | 47,766 | 456 | 1,555 (874–3,004) | 160 (82–276) | 8 (5–11) | 2,053 (972–4,919) | 235 (100–1,165) | 10 (6–413) | 43 | 9 | 38 | 21 |
| Netherlands Cohort Study (NLCS) | 1986–1993 | 58,279 | 393 | 937 (661–1,416) | 90 (53–148) | 13 (8–22) | 950 (668–1,451) | 94 (53–154) | 14 (8–23) | 3 | 2 | 6 | 2 |
| New York State Cohort (NYSC) | 1980–1987 | 30,363 | 335 | 1,650 (884–3,714) | 197 (107–321) | 7 (5–11) | 2,167 (988–5,386) | 240 (121–769) | 10 (5–311) | 38 | 7 | 24 | 19 |
| Women | |||||||||||||
| Breast Cancer Detection Demonstration Project Follow-up Cohort (BCDDP) | 1987–1998 | 41,987 | 349 | 1,270 (735–2,205) | 148 (64–273) | 9 (6–16) | 1,613 (803–3,422) | 194 (78–845) | 13 (7–278) | 33 | 3 | 20 | 15 |
| Canadian National Breast Screening Study (CNBSS) | 1980–2000 | 49,654 | 431 | 1,016 (613–1,739) | 131 (66–215) | 16 (10–23) | – | – | – | – | – | – | – |
| Cancer Prevention Study II Nutrition Cohort (CPS II) | 1992–1999 | 74,046 | 349 | 1,099 (683–1,705) | 128 (58–220) | 9 (6–16) | 1,459 (762–3,138) | 183 (72–759) | 19 (7–288) | 42 | 6 | 28 | 23 |
| Iowa Women’s Health Study (IWHS) | 1986–2001 | 34,588 | 799 | 1,479 (740–2,679) | 132 (71–217) | 8 (6–11) | 1,883 (820–4,064) | 177 (83–672) | 10 (7–232) | 33 | 8 | 29 | 15 |
| Netherlands Cohort Study (NLCS) | 1986–1993 | 62,573 | 353 | 814 (561–1,282) | 101 (59–161) | 11 (7–17) | 834 (569 –1,327) | 106 (62–167) | 11 (7–18) | 6 | 4 | 7 | 2 |
| New York State Cohort (NYSC) | 1980–1987 | 22,550 | 223 | 1,586 (833–3,500) | 182 (99–295) | 7 (4–10) | 2,275 (987–5,423) | 237 (119–769) | 11 (5–312) | 49 | 9 | 30 | 22 |
| New York University Women’s Health Study (NYUWHS) | 1985–1998 | 13,258 | 96 | 1,122 (641–2,024) | 164 (81–278) | 8 (6–11) | 2,031 (780–3,507) | 249 (108–1,194) | 17 (7–287) | 50 | 7 | 37 | 27 |
| Nurses’ Health Study (a) (NHSa) | 1980–1986 | 88,651 | 162 | 1,375 (734–2,712) | 120 (61–209) | 4 (3–6) | 1,767 (808–4,089) | 155 (71–674) | 5 (3–206) | 34 | 4 | 19 | 13 |
| Nurses’ Health Study (b) (NHSb)f | 1986–2000 | 68,502 | 429 | 1,331 (783–2,328) | 141 (76–234) | 6 (4–8) | 1,736 (870–3,982) | 198 (91–795) | 8 (5–407) | 43 | 7 | 36 | 20 |
| Prospective Study on Hormones, Diet and Breast Cancer (ORDET) | 1987–2001 | 9,027 | 43 | 1,025 (553–2,318) | 116 (71–182) | 6 (5–9) | – | – | – | – | – | – | – |
| Swedish Mammography Cohort (SMC) | 1987–2003 | 60,950 | 485 | 1,302 (721–2,314) | 66 (30–124) | 5 (4–7) | – | – | – | – | – | – | – |
| Women’s Health Study (WHS)d | 1993–2003 | 9,594 | 40 | 1,172 (677–2,081) | 128 (70–211) | 5 (4–8) | 1,440 (736–3,030) | 156 (78–415) | 7 (4–223) | 30 | 2 | 8 | 14 |
| Total | 676,141 | 5,454 | |||||||||||
Intake from food only. Unit is retinol equivalents μg/day for vitamin A and α-tocopherol equivalents mg/day for vitamin E
Intake from food and supplements
Cohort sizes after applying study-specific exclusion criteria and then excluding participants with loge-transformed energy intake values beyond three standard deviations from the study-specific mean and previous cancer diagnoses (other than nonmelanoma skin cancer); the Canadian National Breast Screening Study and the Netherlands Cohort Study are analyzed as case-cohort studies so their baseline cohort sizes do not reflect the above exclusions
Placebo group only
Calorie-adjusted median (10th–90th percentile) intake among baseline cohort or subcohort for the Canadian National Breast Screening Study and the Netherlands Cohort Study
These women are a subset of the women included in Nurses’ Health Study (a) and are not included in the total baseline cohort size
In the age-adjusted model, dietary vitamin A intake was inversely associated with risk of colon cancer (pooled RR = 0.86, 95% CI: 0.79–0.94 for comparison of the highest quintile versus the lowest, p-value, test for trend = 0.01, Table 2); however, after adjusting for other colon cancer risk factors, the association was attenuated and no longer statistically significant (pooled multivariate RR = 0.92, 95% CI: 0.81–1.05 for comparison of the highest quintile vs. the lowest, p-value, test for trend = 0.17). This attenuation in the RR was largely due to control of confounding by dietary folate intake (pooled RR from the model adjusting for age and dietary folate only = 0.92, 95% CI: 0.80–1.05 for comparison of the highest quintile versus the lowest). Dietary vitamin C and E intakes were not associated with risk of colon cancer in either the age-adjusted or multivariate models when modeled as quintiles. When more extreme categories of dietary vitamin intake were examined, we observed similar results. Comparing the highest versus the lowest decile of intake, the pooled multivariate RR was 0.89 (95% CI: 0.73–1.09) for dietary vitamin A, 0.99 (95% CI: 0.84–1.18) for dietary vitamin C, and 1.01 (95% CI: 0.89–1.15) for dietary vitamin E. For each vitamin, there was no significant between-studies heterogeneity and no effect modification by sex in the multivariate models. When we examined the associations between dietary intakes of vitamins A, C, and E and colon cancer risk in the studies that collected multivitamin and/or individual vitamin A, C or E supplement use data, we observed similar results (data not shown). In addition, when the associations were examined in only individuals who did not use multivitamins, the results for dietary intake of each vitamin were similar to those reported in Table 2 (data not shown).
Table 2.
Pooled relative risks of colon cancer for quintiles of dietary and total vitamin A, C, and E intakes
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | p-value, test for trend | p-value, test for between-studies heterogeneitya | p-value, test for between-studies heterogeneity due to sexa | |
|---|---|---|---|---|---|---|---|---|
| Dietary intake | ||||||||
| Vitamin A | ||||||||
| Age-adjusted | 1.00 | 0.98 (0.90–1.06) | 0.93 (0.85–1.01) | 0.84 (0.77–0.92) | 0.86 (0.79–0.94) | 0.01 | 0.48 | 0.68 |
| Multivariateb | 1.00 | 1.01 (0.92–1.11) | 1.00 (0.91–1.09) | 0.91 (0.81–1.02) | 0.92 (0.81–1.05) | 0.17 | 0.13 | 0.76 |
| Vitamin C | ||||||||
| Age-adjusted | 1.00 | 0.95 (0.87–1.04) | 0.85 (0.78–0.93) | 0.90 (0.82–0.98) | 0.95 (0.87–1.03) | 0.28 | 0.55 | 0.65 |
| Multivariate | 1.00 | 0.97 (0.89–1.07) | 0.90 (0.81–0.99) | 0.98 (0.88–1.08) | 1.06 (0.95–1.18) | 0.12 | 0.68 | 0.27 |
| Vitamin E | ||||||||
| Age-adjusted | 1.00 | 1.01 (0.91–1.12) | 0.91 (0.82–1.00) | 0.95 (0.86–1.06) | 0.95 (0.86–1.05) | 0.35 | 0.14 | 0.81 |
| Multivariate | 1.00 | 1.04 (0.93–1.16) | 0.94 (0.84–1.04) | 1.00 (0.89–1.13) | 0.99 (0.89–1.11) | 0.71 | 0.24 | 0.51 |
| Total intakec | ||||||||
| Vitamin A | ||||||||
| Age-adjusted | 1.00 | 0.84 (0.75–0.95) | 0.80 (0.73–0.89) | 0.84 (0.74–0.94) | 0.71 (0.64–0.79) | <0.001 | 0.54 | 0.25 |
| Multivariated | 1.00 | 0.89 (0.79–0.99) | 0.86 (0.77–0.95) | 0.90 (0.81–1.01) | 0.78 (0.69–0.87) | 0.001 | 0.79 | 0.38 |
| Vitamin C | ||||||||
| Age-adjusted | 1.00 | 0.88 (0.79–0.97) | 0.91 (0.82–1.00) | 0.83 (0.74–0.93) | 0.73 (0.66–0.81) | <0.0001 | 0.87 | 0.73 |
| Multivariate | 1.00 | 0.91 (0.82–1.01) | 0.96 (0.86–1.07) | 0.89 (0.79–0.99) | 0.80 (0.71–0.90) | <0.001 | 0.95 | 0.56 |
| Vitamin E | ||||||||
| Age-adjusted | 1.00 | 0.93 (0.84–1.02) | 0.88 (0.80–0.97) | 0.82 (0.74–0.91) | 0.76 (0.68–0.85) | 0.001 | 0.32 | 0.88 |
| Multivariate | 1.00 | 0.95 (0.86–1.05) | 0.91 (0.82–1.01) | 0.88 (0.79–0.97) | 0.82 (0.74–0.91) | 0.01 | 0.53 | 0.85 |
For quintile 5
Adjusted for body mass index (<23, 23–<25, 25–<30, ≥30 kg/m2), height (men: <1.70, 1.70–<1.75, 1.75–<1.80, 1.80–<1.85, ≥1.85 m; women: <1.60, 1.60–<1.65, 1.65–<1.70, 1.70–<1.75, ≥1.75 m), education (<high school graduate, high school graduate, >high school graduate), physical activity (low, medium, high), family history of colorectal cancer (no, yes), use of nonsteroidal anti-inflammatory drugs (no, yes), multivitamin use (no, yes<6 times/wk, yes ≥6 times/week, yes missing dose for BCDDP, HPFS, IWHS, NYUWHS, NHS a and b, and WHS; no, yes for ATBC, CPS II, NLCS and NYSC), smoking (never, past (<20, 20–<40, ≥40 years), current (<25 cigarettes/day and<40 years,<25 cigarettes/day and ≥40 years, ≥25 cigarettes/day and <40 years, ≥25 cigarettes/day and ≥40 years), alcohol consumption (0, >0–<5, 5–<15, 15–<30, ≥30 g/day), intakes of red meat (quintiles), total milk (quartiles), dietary folate (quintiles) and total energy (continuous), and use of postmenopausal hormone therapy (premenopausal, never, ever) and oral contraceptive use (never, ever) in women. Age in years and year of questionnaire return were included as stratification variables
The Alpha-Tocopherol Beta-Carotene Cancer Prevention Study and the Netherlands Cohort Study were excluded from these analyses because the prevalence of multivitamin and vitamin C and E supplement use in these studies was much lower than that in the other studies with supplement use data. Consequently, the total intakes in the highest quintiles of the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study and the Netherlands Cohort Study are not comparable to those in the other studies
Adjusted for same covariates in footnote b except multivitamin use
Total intakes of vitamins A, C, and E (sum of intakes from food, multivitamins and individual vitamin supplements) were associated with 24–30% lower risks of colon cancer for comparisons of the highest quintile versus the lowest in age-adjusted models (Table 2). After adjusting for other colon cancer risk factors, the inverse associations for total intakes of vitamins A, C, and E were attenuated slightly, but remained statistically significant. When we adjusted for total folate intake, the associations were further attenuated. Comparing the highest versus the lowest quintile of intake, the pooled multivariate RR was 0.81 (95% CI: 0.71–0.94, p-value, test for trend = 0.07) for total vitamin A, 0.84 (95% CI: 0.74–0.95, p-value, test for trend = 0.004) for total vitamin C, and 0.83 (95% CI: 0.73–0.94, p-value, test for trend = 0.04) for total vitamin E. When the associations were examined by age at diagnosis (≤65 vs. >65 years old), total vitamin A intake showed a marginally significant difference (p-value test for difference = 0.05), but total intakes of vitamins C and E did not show a significant difference (data not shown). In addition, the associations were not modified by body mass index, smoking habits, alcohol consumption, or postmenopausal hormone therapy use in women (data not shown). When we examined the associations by different periods of follow-up time, we observed stronger associations between total vitamin A, C, and E intake and risk of colon cancer during the first five years of follow-up than those observed after a 5-year lag. When follow-up time was limited to the first five years, the pooled multivariate RRs comparing the highest versus the lowest quintile of intake were 0.70 (95% CI: 0.59–0.84) for total vitamin A, 0.77 (95% CI: 0.65–0.92) for total vitamin C, and 0.73 (95% CI: 0.58–0.93) for total vitamin E. After a 5-year lag, the pooled multivariate RRs for the same comparisons were 0.84 (95% CI: 0.72–0.98) for total vitamin A, 0.84 (95% CI: 0.72–0.98) for total vitamin C and 0.90 (95% CI: 0.78–1.04) for total vitamin E. The associations for total vitamin A and C intake did not differ significantly (p > 0.15) by follow-up time, but the test for total vitamin E intake was of borderline significance (p = 0.05).
A major source of intake of vitamins was multivitamin supplements, many of which contain folate. Because total folate intake was also significantly inversely associated with risk of colon cancer in this study population (pooled multivariate RR = 0.85, 95% CI: 0.77–0.95 for comparison of the highest quintile of total folate intake versus the lowest; [60]), we performed categorical analyses using identical absolute intake cutpoints across studies to separate the effect of total vitamin A, C, and E intake on risk of colon cancer from that of total folate intake (Table 3). We chose cutpoints to differentiate nonusers of multivitamins, users of multivitamins only, and users of individual vitamin supplements (regardless of whether they also used multivitamins). In these analyses, for each vitamin, the prevalence of supplement use in the aggregated dataset for the reference category was ≤5% for multivitamins and ≤0.1% for the corresponding individual vitamin supplements; in the highest category, the prevalence was >70% for multivitamins in the analysis of each vitamin and 45% for vitamin A supplements, 94% for vitamin C supplements, and 100% for vitamin E supplements in the analyses for each corresponding vitamin. In the multivariate model, which adjusted for dietary folate intake and other risk factors for colon cancer (multivariate model 1), we found statistically significant inverse associations between total intakes of vitamins C and E and risk of colon cancer. The median correlation coefficient between intakes of each vitamin and total folate across studies was 0.56 for total vitamin C, and 0.51 for total vitamin E. When we adjusted for total folate intake rather than dietary folate intakes (multivariate model 2), we observed that total intakes of vitamin C and E retained statistically significant inverse associations with risk of colon cancer. Total vitamin A intake was not significantly associated with risk of colon cancer in either of the multivariate models. Mutual adjustment for total intakes of vitamins C and E (multivariate model 3) also attenuated the association for each vitamin (median correlation coefficient between intakes of vitamins C and E = 0.57). When we examined the association between supplemental intakes of vitamins A, C, and E and risk of colon cancer, we observed results similar to those for total intakes of each vitamin (data not shown).
Table 3.
Pooled relative risks of colon cancer for categories of total vitamin A, C, and E intakes
| Total vitamin A | Categoriesb |
p-value, test for trend | p-value, test for between-studies heterogeneitya | p-value, test for between-studies heterogeneity due to sexa | |||||
|---|---|---|---|---|---|---|---|---|---|
| ≤1,000 (μg/day) | >1,000–1,500 | >1,500–2,000 | >2,000–2,500 | >2,500–4,000 | >4,000 | ||||
| Case (n) | 1,152 | 1,072 | 613 | 492 | 794 | 372 | |||
| Age-adjusted | 1.00 | 0.89 (0.81–0.97) | 0.82 (0.74–0.92) | 0.84 (0.74–0.94) | 0.79 (0.70–0.88) | 0.78 (0.68–0.89) | <0.001 | 0.71 | 0.30 |
| Multivariate 1c | 1.00 | 0.92 (0.84–1.01) | 0.89 (0.79–1.00) | 0.91 (0.80–1.03) | 0.87 (0.77–0.98) | 0.88 (0.76–1.02) | 0.01 | 0.81 | 0.44 |
| Multivariate 2d | 1.00 | 0.93 (0.84–1.02) | 0.90 (0.80–1.02) | 0.93 (0.81–1.06) | 0.91 (0.78–1.08) | 0.93 (0.79–1.10) | 0.21 | 0.51 | 0.94 |
| Total vitamin C | Categoriesb |
p-value, test for trend | p-value, test for between-studies heterogeneitya | p-value, test for between-studies heterogeneity due to sexa | |||||
|---|---|---|---|---|---|---|---|---|---|
| ≤100 (mg/day) | >100–150 | >150–200 | >200–250 | >250–600 | >600 | ||||
| Case (n) | 1,006 | 934 | 781 | 497 | 675 | 602 | |||
| Age-adjusted | 1.00 | 0.85 (0.77–0.94) | 0.93 (0.83–1.05) | 0.94 (0.80–1.11) | 0.79 (0.70–0.88) | 0.73 (0.65–0.82) | <0.001 | 0.71 | 0.50 |
| Multivariate 1c | 1.00 | 0.88 (0.79–0.97) | 0.98 (0.87–1.10) | 1.00 (0.86–1.16) | 0.86 (0.76–0.98) | 0.81 (0.71–0.92) | 0.002 | 0.84 | 0.65 |
| Multivariate 2d | 1.00 | 0.89 (0.80–0.99) | 1.02 (0.89–1.16) | 1.02 (0.89–1.18) | 0.91 (0.79–1.03) | 0.86 (0.74–0.99) | 0.02 | 0.87 | 0.61 |
| Multivariate 3e | 1.00 | 0.88 (0.79–0.98) | 0.99 (0.88–1.11) | 1.01 (0.87–1.16) | 0.90 (0.78–1.03) | 0.86 (0.74–1.00) | 0.08 | 0.79 | 0.47 |
| Total vitamin E | Categoriesb |
p-value, test for trend | p-value, test for between-studies heterogeneitya | p-value, test for between-studies heterogeneity due to sexa | ||||
|---|---|---|---|---|---|---|---|---|
| ≤6 (mg/day) | >6–9 | >9–25 | >25–200 | >200 | ||||
| Case (n) | 568 | 1,208 | 1,679 | 553 | 487 | |||
| Age-adjusted | 1.00 | 0.90 (0.79–1.01) | 0.83 (0.74–0.93) | 0.84 (0.65–1.06) | 0.71 (0.59–0.83) | 0.001 | 0.18 | 0.61 |
| Multivariate 1c | 1.00 | 0.94 (0.83–1.06) | 0.89 (0.79–1.00) | 0.91 (0.72–1.13) | 0.78 (0.66–0.92) | 0.01 | 0.27 | 0.89 |
| Multivariate 2d | 1.00 | 0.93 (0.83–1.05) | 0.89 (0.78–1.01) | 0.93 (0.73–1.19) | 0.80 (0.65–0.97) | 0.06 | 0.13 | 0.79 |
| Multivariate 3f | 1.00 | 0.94 (0.82–1.07) | 0.89 (0.79–1.01) | 0.96 (0.76–1.21) | 0.83 (0.70–0.99) | 0.16 | 0.31 | 0.83 |
For the highest category
For vitamin A analyses, the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study and the Netherlands Cohort Study were excluded from the highest category because these studies did not have any cases in that category. For vitamin C analyses, the Netherlands Cohort Study was excluded from the highest category because the study did not have any cases in that category. For vitamin E analyses, the male cohort of the New York State Cohort was excluded from >25 to 200 mg/day category, and the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study and the Netherlands Cohort Study were excluded from the highest category because these studies did not have any cases in that category. The participants who were not cases who would have been in the highest category were included in the next highest category
Adjusted for same covariates listed in Table 2 footnote b except multivitamin use
Adjusted for covariates in multivariate model 1 except dietary folate intake (quintiles) was replaced with total folate intake (quintiles)
Adjusted for covariates in multivariate model 1 and intake of total vitamin E (≤6, >6–9, >9–25, >25–200, >200 mg/day)
Adjusted for covariates in multivariate model 1 and intake of total vitamin C (≤100, >100–150, >150–200, >200–250, >250–600, >600 mg/day)
The joint effect of total intakes of vitamins C and E on risk of colon cancer was also examined. Compared to the group with intakes of ≤200 mg/day vitamin C and ≤25 mg/day vitamin E (low intakes for both vitamins), the group with intakes of >600 mg/day of vitamin C and >200 mg/day of vitamin E (high intakes for both vitamins) had a significantly lower risk of colon cancer (pooled multivariate RR = 0.84, 95% CI: 0.72–0.98). The pooled multivariate RR was 1.09 (95% CI: 0.87–1.36) for the group with low vitamin C but high vitamin E intake (≤200 mg/day vitamin C and >200 mg/day vitamin E) and 0.88 (95% CI: 0.75–1.04) for the group with high vitamin C but low vitamin E intake (>600 mg/day vitamin C and ≤25 mg/day vitamin E).
We also examined the associations between vitamin intakes and risk of colon cancer by tumor site (Table 4). The associations with dietary vitamin A, C, and E intake did not differ by tumor site in the multivariate models (p-value, test for common effects by tumor site for quintile 5 ≥ 0.45, data not shown). For total vitamin A, C, and E intakes, the associations were slightly stronger in the distal colon than the proximal colon, but the differences were not statistically significant (p-value, test for common effects by tumor site for the highest category ≥0.34).
Table 4.
Pooled multivariate relative risksa of colon cancer for categories of total vitamin A, C, and E intakes by tumor site
| Total vitamin A | Categoriesb |
p-value, test for trend | p-value, test for between-studies heterogeneityc | p-value, test for between-studies heterogeneity due to sexc | p-value, test for common effects by tumor sitec | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤1,000 (μg/day) | >1,000–1,500 | >1,500–2,000 | >2,000–2,500 | >2,500–4,000 | >4,000 | |||||
| Case (n) | 598/497d | 580/414 | 347/235 | 270/183 | 405/326 | 192/150 | ||||
| Proximal | 1.00 | 0.96 (0.84–1.09) | 0.96 (0.82–1.13) | 0.94 (0.80–1.12) | 0.85 (0.72–0.99) | 0.87 (0.71–1.07) | 0.02 | 0.94 | 0.63 | 0.66 |
| Distal | 1.00 | 0.84 (0.72–0.97) | 0.80 (0.67–0.97) | 0.82 (0.67–1.00) | 0.85 (0.67–1.09) | 0.83 (0.65–1.05) | 0.15 | 0.80 | 0.56 | |
| Total vitamin C | Categoriesb |
p-value, test for trend | p-value, test for between-studies heterogeneityc | p-value, test for between-studies heterogeneity due to sexc | p-value, test for common effects by tumor sitec | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤100 (mg/day) | >100–150 | >150–200 | >200–250 | >250–600 | >600 | |||||
| Case (n) | 539/418 | 493/391 | 399/306 | 282/176 | 350/264 | 329/219 | ||||
| Proximal | 1.00 | 0.85 (0.71–1.00) | 0.94 (0.76–1.16) | 1.08 (0.82–1.41) | 0.85 (0.71–1.01) | 0.85 (0.70–1.05) | 0.09 | 0.31 | 0.74 | 0.34 |
| Distal | 1.00 | 0.92 (0.79–1.07) | 0.98 (0.76–1.25) | 0.89 (0.71–1.11) | 0.83 (0.68–1.02) | 0.72 (0.55–0.93) | 0.01 | 0.19 | 0.97 | |
| Total vitamin E | Categoriesb |
p-value, test for trend | p-value, test for between-studies heterogeneityc | p-value, test for between-studies heterogeneity due to sexc | p-value, test for common effects by tumor sitec | ||||
|---|---|---|---|---|---|---|---|---|---|
| ≤6 (mg/day) | >6–9 | >9–25 | >25–200 | >200 | |||||
| Case (n) | 287/241 | 656/459 | 872/690 | 301/214 | 276/170 | ||||
| Proximal | 1.00 | 0.96 (0.80–1.15) | 0.83 (0.70–0.97) | 0.81 (0.63–1.05) | 0.82 (0.67–0.99) | 0.14 | 0.49 | 0.24 | 0.58 |
| Distal | 1.00 | 0.90 (0.74–1.09) | 0.97 (0.80–1.16) | 0.99 (0.75–1.32) | 0.73 (0.58–0.92) | 0.02 | 0.63 | 0.46 | |
Adjusted for same covariates listed in Table 2 footnote c
The following studies were excluded from a category because these studies did not have any cases in that category; the participants who were not cases who would have been in that category were included in the next highest category. The Alpha-Tocopherol Beta-Carotene Cancer Prevention Study was excluded from the highest category of total vitamins A and E intake in both the proximal and the distal colon cancer analyses. The Alpha-Tocopherol Beta-Carotene Cancer Prevention Study was also excluded from the >250–600 mg/d category of total vitamin C intake in the proximal colon cancer analyses, the >25–200 mg/d category of total vitamin E intake in the proximal colon cancer analyses, and the >200–250 mg/d category of total vitamin C intake in the distal colon cancer analyses. Both the male and the female cohorts of the Netherlands Cohort Study were excluded from the highest category of total vitamins A, C, and E intake in both the proximal and distal colon cancer analyses. The male cohort of the Netherlands Cohort Study was also excluded from the >2,000–2,500 and >2,500–4,000 μg/day categories of total vitamin A intake in the distal colon cancer analyses. The Women’s Health Study was excluded from the highest category of total vitamin C intake in the proximal colon cancer analyses and of total vitamin A intake in the distal colon cancer analyses. The New York University Women’s Health Study was excluded from the distal colon cancer analyses for total vitamin C intake and total vitamin E intake because the study had no cases in the reference category. Both the male and the female cohorts of the New York State Cohort were excluded from the >25–200 mg/d category of total vitamin E intake in both the proximal and distal colon cancer analyses
For the highest category
Cases in proximal/distal colon
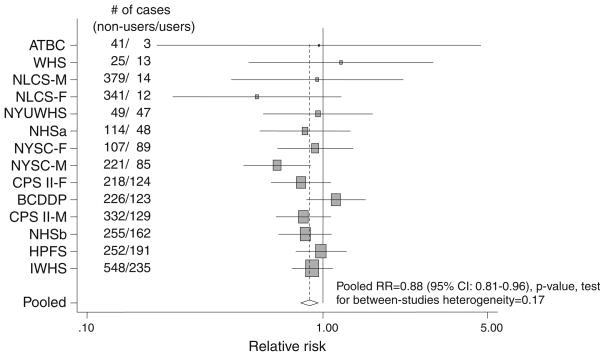
Because multivitamin supplements were an important source of supplemental vitamin intake, we analyzed the relation of multivitamin use to risk of colon cancer (Fig. 1). We found that multivitamin use was associated with a significantly lower risk of colon cancer; the pooled age-adjusted RR was 0.84 (95% CI: 0.77–0.92; p-value test for between-studies heterogeneity = 0.12) and the pooled multivariate RR was 0.88 (95% CI: 0.81–0.96; p-value test for between-studies heterogeneity = 0.17) comparing users versus nonusers. The association was not modified by sex, age at diagnosis, or alcohol intake (data not shown). The association also did not differ significantly by tumor site (p-value, test for common effects by tumor site = 0.50); the pooled multivariate RRs were 0.86 (95% CI: 0.78–0.94, p-value, test for between-studies heterogeneity = 0.45) for proximal colon cancers and 0.91 (95% CI: 0.80–1.02, p-value, test for between-studies heterogeneity = 0.34) for distal colon cancers comparing users versus nonusers. When we examined the association between multivitamin use and risk of colon cancer by different periods of follow-up time, the pooled multivariate RRs were 0.85 (95% CI: 0.75–0.97) when follow-up time was limited to the first five years and 0.91 (95% CI: 0.83–1.00) after a 5-year lag (p-value, test for difference by follow-up period = 0.41).
Fig. 1.
Study-specific and pooled multivariate RRs (adjusted for covariates listed in Table 2 legend c) of colon cancer in multivitamin users versus nonusers. The squares and horizontal lines correspond to the study-specific multivariate RR and 95% CIs, respectively. The size of a square reflects the study-specific weight (inverse of the variance), and the diamond represents the pooled multivariate RR and 95% CI. The vertical dotted line represents the pooled RR. The abbreviations of the studies are the same as in Table 1
We also examined the association between use of multivitamins and individual vitamin supplements and risk of colon cancer by mutually adjusting for combinations of vitamin supplement use. Compared to nonusers of supplements, the pooled multivariate RRs of colon cancer were 0.92 (95% CI: 0.82–1.04) for users of multivitamins only; and 0.77 (95% CI: 0.70–0.85) for those who used any combination of vitamin A, C and E supplements and used multivitamins. There was no statistically significant between-studies heterogeneity for these comparisons.
Discussion
We found no association between dietary intakes of vitamins A, C, and E and risk of colon cancer in this pooled analysis of thirteen prospective cohort studies. However, total intakes of vitamins A, C, and E were each inversely associated with risk of colon cancer. After adjusting for total folate intake, the inverse association between total vitamin A intake and risk of colon cancer in the categorical analysis was attenuated and no longer statistically significant, whereas the inverse associations between total intakes of vitamins C and E and risk of colon cancer were slightly weakened but remained statistically significant. Multivitamin use, particularly in combination with use of individual vitamin A, C and/or E supplements, was inversely associated with risk of colon cancer.
Because multivitamin supplements containing folate were one of the main sources of total vitamin intakes, total vitamin A, C, and E intakes were positively correlated with total folate intake. However, total vitamin A intake was more highly correlated with total folate intake than total vitamin C and E intakes. Therefore, the different degree of attenuation in the RRs for total intakes of vitamins A, C, and E may be due to differences in the correlations between total intake of each vitamin and total folate. The inverse association observed for total vitamin A intake in the age-adjusted analysis may be partially due to folate and other nutrients in multivitamins.
Plausible biologic mechanisms support our results, which suggest that total vitamin C and E intakes may be associated with a decreased risk of colon cancer independently of total folate intake. Vitamin C as an electron donor reduces reactive radicals and iron. Vitamin E (α-tocopherol), which acts as an antioxidant, breaks free radical chain reactions by transferring a phenolic hydrogen to a free radical leaving a less reactive α-tocopheroxy radical. The α-tocopheroxy radical then may react with vitamin C to regenerate α-tocopherol [53]. In this process, vitamin C acts as an electron donor to spare vitamin E. Thus, vitamins C and E may act jointly to have a beneficial effect on risk of colon cancer.
We observed that the associations between total vitamin C and E intake and risk of colon cancer were stronger in the distal colon than in the proximal colon although the differences were not statistically significant. The different strength of the associations may be partially due to physiologic differences between the proximal and distal colon. The proximal colon plays a major role in fermentation of undigested substances such as dietary fiber, whereas the distal colon is more involved in water absorption and electrolyte transport [54]. It has also been suggested that tumors in the proximal and distal colon might follow different molecular carcinogenic pathways [54].
Multivitamin use was associated with a significantly lower risk of colon cancer in our study. One may argue that multivitamin users may be more health conscious and have a healthier lifestyle than nonusers, which may confound the association observed between multivitamin use and risk of colon cancer. In our analysis, we adjusted for several colon cancer risk factors including lifestyle and other dietary factors and observed no substantial confounding by these factors. However, in most studies included in these analyses, information on colorectal cancer screening practices and nonsteroidal anti-inflammatory drug use was lacking, thus incomplete adjustment for healthy behaviors which may be related to supplement use may result in our observed associations being confounded.
In contrast to our findings, randomized clinical trials have failed to confirm beneficial effects of antioxidant vitamins on colorectal adenomas or cancer [16, 55]. However, the results of trials do not provide conclusive evidence against the protective effect of antioxidant vitamins because of alternative explanations related to the trials. The duration of the available trials has been relatively short, mostly 4–7 years, which may not be sufficiently long enough to observe preventive effects. In addition, some trials used small adenomas as an endpoint. If antioxidant vitamins play a role during the progression of small to advanced adenomas or the progression of advanced adenomas to invasive cancer, but not in the development of adenomas, the beneficial effects of vitamins would not be detected in those trials.
We observed stronger associations with vitamins and multivitamin use during the first 5 years of follow-up than those observed after a 5-year lag. Because most studies did not reassess diet during the follow-up, the different strength of the associations observed during different follow-up periods may partially be due to increasing misclassification of vitamin intakes over time. In the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study trial, compared to the nonvitamin E supplementation group, the vitamin E supplementation group had a modestly lowered risk of colorectal cancer during the approximately 6 years of the trial (RR = 0.78, 95% CI: 0.55–1.09). However, this suggestive inverse association disappeared during the post-trial follow-up period (RR = 1.02, 95% CI: 0.70–1.47, 3–6 years after the end of the trial) [56]. The stronger associations observed during the first 5 years of follow-up rather than after a 5-year lag also suggest that vitamin intakes may not have long-term effects on colon cancer risk. However, other studies have reported that past, but not recent, long-term multivitamin supplement use was associated with a lower risk of colorectal cancer [19, 20].
In our study, information on duration and past use of vitamin supplements at baseline was not available from most studies. This may induce misclassification of intakes of total vitamins A, C, and E because the reference group may include former vitamin supplement users whose usual intakes of vitamins A, C, and E may have been higher than that measured at baseline. Also, we could not examine the effect of duration of vitamin supplement use as has been done in some studies that found the beneficial effect of vitamin supplement use on colon cancer among only those individuals who had used vitamin supplements for a long duration [57, 58]. Selenium, which is another nutrient important in the antioxidant system suggested to have a protective effect on colon cancer risk [59], was not investigated in our study due to a lack of reliable food composition data for selenium.
Our study had several strengths. This study included over 5,000 colon cancer cases from thirteen studies of diverse populations, which provided a wide range of vitamin intakes and prevalence of vitamin supplement use. Moreover, as opposed to meta-analyses of the published literature, we were able to examine dose–response relationships in detail by reanalyzing the primary data from each study and we were able to adjust for other colon cancer risk factors by standardizing the format of the covariates across studies. In addition, due to the large sample size, we could examine whether associations with specific vitamin intakes and vitamin supplement use were modified by various factors.
In conclusion, we found null associations for dietary vitamin A, C, and E intakes and total vitamin A intake and inverse associations for total vitamin C and E intake and multivitamin use. These associations were generally consistent across studies, between men and women, and across subsites of the colon. Separating the effect of each vitamin from the effect of multivitamins containing folate and other vitamins which may protect against colon cancer was challenging. Although we made an effort to separate the effect of each vitamin independent from that of multivitamins in the categorical analyses and in analyses of vitamin supplement use, moderate to high correlations among these vitamins made it difficult to separate completely the effect of each vitamin on risk of colon cancer. As reported in our accompanying article [60], high folate intake, mostly from multivitamin supplements, was associated with a lower risk of colon cancer. Because modest inverse associations with vitamins C and E and multivitamin use were similar to that with folate, we cannot rule out the possibility that the apparent protective effects of total vitamin C and E intakes and of multivitamin supplement use against colon cancer were due to their positive correlations with total folate intake or intakes of other vitamins present in multivitamins such as vitamin B6.
Acknowledgments
Financial support The study was funded by research grants CA55075 from the National Institutes of Health and the National Colorectal Cancer Research Alliance of the Entertainment Industry Foundation.
Contributor Information
Yikyung Park, Department of Nutrition, Harvard School of Public Health, 665 Huntington Avenue, Boston, MA 02115, USA.
Donna Spiegelman, Department of Epidemiology, Harvard School of Public Health, 665 Huntington Ave, Boston, MA 02115, USA. Department of Biostatistics, Harvard School of Public Health, Boston, MA, USA.
David J. Hunter, Department of Nutrition, Harvard School of Public Health, 665 Huntington Avenue, Boston, MA 02115, USA. Department of Epidemiology, Harvard School of Public Health, 665 Huntington Ave, Boston, MA 02115, USA. Channing Laboratory, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA
Leif Bergkvist, Department of Surgery and Centre for Clinical Research, Central Hospital, Västerås, Sweden.
Julie E. Buring, Department of Epidemiology, Harvard School of Public Health, 665 Huntington Ave, Boston, MA 02115, USA. Division of Preventive Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA
Jo L. Freudenheim, Department of Social and Preventive Medicine, University at Buffalo, State University of New York, Buffalo, NY, USA
Edward Giovannucci, Department of Nutrition, Harvard School of Public Health, 665 Huntington Avenue, Boston, MA 02115, USA. Department of Epidemiology, Harvard School of Public Health, 665 Huntington Ave, Boston, MA 02115, USA. Channing Laboratory, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA.
R. Alexandra Goldbohm, Department of Prevention and Health, TNO Quality of Life, Leiden, The Netherlands.
Lisa Harnack, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, MN, USA.
Ikuko Kato, Department of Pathology, Karmanos Cancer Institute, Wayne State University, Detroit, MI, USA.
Vittorio Krogh, Nutritional Epidemiology Unit, National Cancer Institute, Milan, Italy.
Michael F. Leitzmann, Institute of Epidemiology and Preventive Medicine, University of Regensburg, Regensburg, Germany
Paul J. Limburg, Division of Gastroenterology and Hepatology, Mayo Clinic College of Medicine, Rochester, MN, USA
James R. Marshall, Department of Cancer Prevention and Population Science, Roswell Park Cancer Institute, Buffalo, NY, USA
Marjorie L. McCullough, Epidemiology Research Program, American Cancer Society, Atlanta, GA, USA
Anthony B. Miller, Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada
Thomas E. Rohan, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA
Roy Shore, Department of Environmental Medicine, New York University, New York, NY, USARadiation Effects Research Foundation, Hiroshima, Japan.
Sabina Sieri, Nutritional Epidemiology Unit, National Cancer Institute, Milan, Italy.
Meir J. Stampfer, Department of Nutrition, Harvard School of Public Health, 665 Huntington Avenue, Boston, MA 02115, USA. Department of Epidemiology, Harvard School of Public Health, 665 Huntington Ave, Boston, MA 02115, USA. Channing Laboratory, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA
Jarmo Virtamo, Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, Helsinki, Finland.
Matty Weijenberg, Department of Epidemiology, School of Oncology and Developmental Biology (GROW), Maastricht University, Maastricht, The Netherlands.
Walter C. Willett, Department of Nutrition, Harvard School of Public Health, 665 Huntington Avenue, Boston, MA 02115, USA. Department of Epidemiology, Harvard School of Public Health, 665 Huntington Ave, Boston, MA 02115, USA. Channing Laboratory, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA
Alicja Wolk, Division of Nutritional Epidemiology, The National Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden.
Shumin M. Zhang, Department of Epidemiology, Harvard School of Public Health, 665 Huntington Ave, Boston, MA 02115, USA. Division of Preventive Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA
Stephanie A. Smith-Warner, Email: pooling@hsphsun2.harvard.edu, Department of Nutrition, Harvard School of Public Health, 665 Huntington Avenue, Boston, MA 02115, USA. Department of Epidemiology, Harvard School of Public Health, 665 Huntington Ave, Boston, MA 02115, USA
References
- 1.Platz EA, Willett WC, Colditz GA, Rimm EB, Spiegelman D, Giovannucci E. Proportion of colon cancer risk that might be preventable in a cohort of middle-aged US men. Cancer Causes Control. 2000;11:579–588. doi: 10.1023/a:1008999232442. [DOI] [PubMed] [Google Scholar]
- 2.Niles RM. Vitamin A and cancer. Nutrition. 2000;16:573–576. doi: 10.1016/s0899-9007(00)00347-6. [DOI] [PubMed] [Google Scholar]
- 3.Semba RD. Vitamin A and immunity to viral, bacterial and protozoan infections. Proc Nutr Soc. 1999;58:719–727. doi: 10.1017/s0029665199000944. [DOI] [PubMed] [Google Scholar]
- 4.Padayatty SJ, Katz A, Wang Y, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22:18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 5.Packer L. Protective role of vitamin E in biological systems. Am J Clin Nutr. 1991;53:1050S–1055S. doi: 10.1093/ajcn/53.4.1050S. [DOI] [PubMed] [Google Scholar]
- 6.Kline K, Yu W, Sanders BG. Vitamin E: mechanisms of action as tumor cell growth inhibitors. J Nutr. 2001;131:161S–163S. doi: 10.1093/jn/131.1.161S. [DOI] [PubMed] [Google Scholar]
- 7.Potter JD, McMichael AJ. Diet and cancer of the colon and rectum: a case–control study. J Natl Cancer Inst. 1986;76:557–569. doi: 10.1093/jnci/76.4.557. [DOI] [PubMed] [Google Scholar]
- 8.Slattery ML, Edwards SL, Anderson K, Caan B. Vitamin E and colon cancer: is there an association? Nutr Cancer. 1998;30:201–206. doi: 10.1080/01635589809514664. [DOI] [PubMed] [Google Scholar]
- 9.La Vecchia C, Braga C, Negri E, et al. Intake of selected micronutrients and risk of colorectal cancer. Int J Cancer. 1997;73:525–530. doi: 10.1002/(sici)1097-0215(19971114)73:4<525::aid-ijc12>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Chiu BC, Ji BT, Dai Q, et al. Dietary factors and risk of colon cancer in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2003;12:201–208. [PubMed] [Google Scholar]
- 11.Levi F, Pasche C, Lucchini F, La Vecchia C. Selected micronutrients and colorectal cancer. A case–control study from the canton of Vaud, Switzerland. Eur J Cancer. 2000;36:2115–2119. doi: 10.1016/s0959-8049(00)00195-7. [DOI] [PubMed] [Google Scholar]
- 12.Shibata A, Paganini-Hill A, Ross RK, Henderson BE. Intake of vegetables, fruits, beta-carotene, vitamin C and vitamin supplements and cancer incidence among the elderly: a prospective study. Br J Cancer. 1992;66:673–679. doi: 10.1038/bjc.1992.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bostick RM, Potter JD, McKenzie DR, et al. Reduced risk of colon cancer with high intake of vitamin E: the Iowa Women’s Health Study. Cancer Res. 1993;53:4230–4237. [PubMed] [Google Scholar]
- 14.Wu K, Willett WC, Chan JM, et al. A prospective study on supplemental vitamin e intake and risk of colon cancer in women and men. Cancer Epidemiol Biomarkers Prev. 2002;11:1298–1304. [PubMed] [Google Scholar]
- 15.Longnecker MP, Martin-Moreno JM, Knekt P, et al. Serum alpha-tocopherol concentration in relation to subsequent colorectal cancer: pooled data from five cohorts. J Natl Cancer Inst. 1992;84:430–435. doi: 10.1093/jnci/84.6.430. [DOI] [PubMed] [Google Scholar]
- 16.Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Anti-oxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet. 2004;364:1219–1228. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- 17.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. Jama. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 18.Lonn E, Bosch J, Yusuf S, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. Jama. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 19.Giovannucci E, Stampfer MJ, Colditz GA, et al. Multivitamin use, folate, and colon cancer in women in the Nurses’ Health Study. Ann Intern Med. 1998;129:517–524. doi: 10.7326/0003-4819-129-7-199810010-00002. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs EJ, Connell CJ, Chao A, et al. Multivitamin use and colorectal cancer incidence in a US cohort: does timing matter? Am J Epidemiol. 2003;158:621–628. doi: 10.1093/aje/kwg190. [DOI] [PubMed] [Google Scholar]
- 21.Zhang SM, Moore SC, Lin J, et al. Folate, vitamin B6, multivitamin supplements, and colorectal cancer risk in women. Am J Epidemiol. 2006;163:108–115. doi: 10.1093/aje/kwj016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith-Warner SA, Spiegelman D, Ritz J, et al. Methods for pooling results of epidemiologic studies: the pooling project of prospective studies of diet and cancer. Am J Epidemiol. 2006;163:1053–1064. doi: 10.1093/aje/kwj127. [DOI] [PubMed] [Google Scholar]
- 23.Malila N, Virtamo J, Virtanen M, Pietinen P, Albanes D, Teppo L. Dietary and serum alpha-tocopherol, beta-carotene and retinol, and risk for colorectal cancer in male smokers. Eur J Clin Nutr. 2002;56:615–621. doi: 10.1038/sj.ejcn.1601366. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs EJ, Connell CJ, Patel AV, et al. Vitamin C and vitamin E supplement use and colorectal cancer mortality in a large American Cancer Society cohort. Cancer Epidemiol Biomarkers Prev. 2001;10:17–23. [PubMed] [Google Scholar]
- 25.Mai V, Flood A, Peters U, Lacey JV, Jr, Schairer C, Schatzkin A. Dietary fibre and risk of colorectal cancer in the Breast Cancer Detection Demonstration Project (BCDDP) follow-up cohort. Int J Epidemiol. 2003;32:234–239. doi: 10.1093/ije/dyg052. [DOI] [PubMed] [Google Scholar]
- 26.Terry P, Jain M, Miller AB, Howe GR, Rohan TE. Dietary intake of folic acid and colorectal cancer risk in a cohort of women. Int J Cancer. 2002;97:864–867. doi: 10.1002/ijc.10138. [DOI] [PubMed] [Google Scholar]
- 27.Verhoeven DTH, Assen N, Goldbohm RA, et al. Vitamins C and E, retinol, beta-carotene and dietary fibre in relation to breast cancer risk: a prospective cohort study. Br J Cancer. 1997;75:149–155. doi: 10.1038/bjc.1997.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandera EV, Freudenheim JL, Marshall JR, et al. Diet and alcohol consumption and lung cancer risk in the New York State Cohort (United States) Cancer Causes Control. 1997;8:828–840. doi: 10.1023/a:1018456127018. [DOI] [PubMed] [Google Scholar]
- 29.Kato I, Akhmedkhanov A, Koenig K, Toniolo PG, Shore RE, Riboli E. Prospective study of diet and female colorectal cancer: the New York University Women’s Health Study. Nutr Cancer. 1997;28:276–281. doi: 10.1080/01635589709514588. [DOI] [PubMed] [Google Scholar]
- 30.Terry P, Giovannucci E, Michels KB, et al. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J Natl Cancer Inst. 2001;93:525–533. doi: 10.1093/jnci/93.7.525. [DOI] [PubMed] [Google Scholar]
- 31.Higginbotham S, Zhang ZF, Lee IM, et al. Dietary glycemic load and risk of colorectal cancer in the Women’s Health Study. J Natl Cancer Inst. 2004;96:229–233. doi: 10.1093/jnci/djh020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sieri S, Krogh V, Muti P, et al. Fat and protein intake and subsequent breast cancer risk in postmenopausal women. Nutr Cancer. 2002;42:10–17. doi: 10.1207/S15327914NC421_2. [DOI] [PubMed] [Google Scholar]
- 33.Rothman KJ, Greenland S. Modern epidemiology. Lippincott Williams & Wilkins; Philadelphia: 1998. [Google Scholar]
- 34.Goldbohm RA, van den Brandt PA, Brants HAM, et al. Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. Eur J Clin Nutr. 1994;48:253–265. [PubMed] [Google Scholar]
- 35.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 36.Jain M, Howe GR, Rohan T. Dietary assessment in epidemiology: comparison of a food frequency and a dietary history questionnaire witih a 7-day food record. Am J Epidemiol. 1996;143:953–960. doi: 10.1093/oxfordjournals.aje.a008839. [DOI] [PubMed] [Google Scholar]
- 37.Pietinen P, Hartman AM, Haapa E, et al. Reproducibility and validity of dietary assessment instruments II. A qualitative food-frequency questionnaire. Am J Epidemiol. 1988;128:667–676. doi: 10.1093/oxfordjournals.aje.a115014. [DOI] [PubMed] [Google Scholar]
- 38.Flagg EW, Coates RJ, Calle EE, Potischman N, Thun MJ. Validation of the American Cancer Society Cancer Prevention Study II Nutrition Survey Cohort food frequency questionnaire. Epidemiology. 2000;11:462–468. doi: 10.1097/00001648-200007000-00017. [DOI] [PubMed] [Google Scholar]
- 39.Munger RG, Folsom AR, Kushi LH, Kaye SA, Sellers TA. Dietary assessment of older Iowa women with a food frequency questionnaire: nutrient intake, reproducibility, and comparison with 24-hour dietary recall interviews. Am J Epidemiol. 1992;136:192–200. doi: 10.1093/oxfordjournals.aje.a116485. [DOI] [PubMed] [Google Scholar]
- 40.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 41.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 42.Willett W. Nutritional epidemiology. Oxford University Press; New York: 1998. [Google Scholar]
- 43.Ascherio A, Stampfer MJ, Colditz GA, Rimm EB, Litin L, Willett WC. Correlations of vitamin A and E intakes with the plasma concentrations of carotenoids and tocopherols among American men and women. J Nutr. 1992;122:1792–1801. doi: 10.1093/jn/122.9.1792. [DOI] [PubMed] [Google Scholar]
- 44.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34(2):187–220. [Google Scholar]
- 45.SAS II. SAS/STAT user’s guide, version 8. SAS Institute Inc; Cary: 1999. [Google Scholar]
- 46.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 47.Miettinen O. Theoretical epidemiology. Oxford University Press; New York: 1985. [Google Scholar]
- 48.Huberman M, Langholz B. Application of the missing-indicator method in matched case–control studies with incomplete data. Am J Epidemiol. 1999;150:1340–1345. doi: 10.1093/oxfordjournals.aje.a009966. [DOI] [PubMed] [Google Scholar]
- 49.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 50.Stram DO. Meta-analysis of published data using a linear mixed-effects model. Biometrics. 1996;52:536–544. [PubMed] [Google Scholar]
- 51.Anderson T. Introduction to multivariate statistics. Wiley; New York: 1984. [Google Scholar]
- 52.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. Am J Epidemiol. 2004;160:339–349. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 53.Murray RK, Granner GD, Mayes PA, Rodwell VW. Harper’s biochemistry. McGraw-Hill; New York: 2000. [Google Scholar]
- 54.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–408. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 55.Davies AA, Davey Smith G, Harbord R, et al. Nutritional interventions and outcome in patients with cancer or preinvasive lesions: systematic review. J Natl Cancer Inst. 2006;98:961–973. doi: 10.1093/jnci/djj263. [DOI] [PubMed] [Google Scholar]
- 56.Virtamo J, Pietinen P, Huttunen JK, et al. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up. Jama. 2003;290:476–485. doi: 10.1001/jama.290.4.476. [DOI] [PubMed] [Google Scholar]
- 57.Giovannucci E, Stampfer MJ, Colditz GA, et al. Multivitamin use, folate and colon cancer in women in the nurses’ health study. Ann Intern Med. 1998;129:517–524. doi: 10.7326/0003-4819-129-7-199810010-00002. [DOI] [PubMed] [Google Scholar]
- 58.Jacobs EJ, Connell CJ, Patel AV, et al. Multivitamin use and colon cancer mortality in the Cancer Prevention Study II cohort (United States) Cancer Causes Control. 2001;12:927–934. doi: 10.1023/a:1013716323466. [DOI] [PubMed] [Google Scholar]
- 59.Dumas L. Lung cancer in women: rising epidemic, preventable disease. Nurs Clin North Am. 1992;27:859–869. [PubMed] [Google Scholar]
- 60.Kim D-H, Smith-Warner SA, Spiegelman D, Yaun S-S, Colditz GA, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Harnack L, Jacobs EJ, Leitzmann M, Mannisto S, Miller AB, Potter JD, Rohan TE, Schatzkin A, Speizer FE, Stevens VL, Stolzenberg-Solomon R, Terry P, Toniolo P, Weijenberg MP, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Hunter DJ. Pooled analyses of 13 prospective cohort studies on folate intake and colon cancer. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]