Abstract
Dendritic cell (DC) progenitors were propagated in liquid culture from nonparenchymal cells resident in normal mouse (B10.BR; H-2k, I-E+) liver in response to granulocyte-macrophage colony stimulating factor (GM-CSF). The liver-derived DC progenitors were MHC class H−/dim and did not express counter receptors for CTLA-4, a structural homologue of the T cell activation molecule CD28. Following subcutaneous or intravenous injection, these liver-derived cells migrated to T cell-dependent areas of lymph nodes and spleen of unmodified, allogeneic (B10; H-2b; I-E−) recipients, where they were identified 1-5 days, and 1 and 2 months after injection by their strong surface expression of donor MHC class II (I-Ek) and their dendritic morphology. Maximal numbers of liver-derived DC in the spleen were recorded 5 days after injection. Both clusters of strongly donor MHC class II+ cells— and (more rarely) dividing cells—could also be identified, suggesting cell replication in situ. Using the same techniques employed to generate DC progenitors from normal liver, GM-CSF-stimulated cells were propagated for 10 days from the bone marrow and spleen of nonimmunosuppressed mice sacrificed 14 days after orthotopic liver transplantation (B10;H-2b → C3H;H-2k). Immunocytochemical staining for recipient and donor MHC class II phenotype revealed the growth both of host cells with DC characteristics, and of cells expressing donor alloantigens (I-Ab. These results are consistent with the growth, in response to GM-CSF, of donor-derived DC from progenitors seeded from the liver allograft to recipient lymphoid tissue. The functional activity of the progenitors of chimeric DC and the possible role of these cells in the establishment and maintenance of donor-specific tolerance following liver transplantation remain to be determined.
It is now thought that the comparative ease of “acceptance” of different transplanted organs may reflect their endowment with “passenger” leukocytes (1, 2). Of these, bone marrow–derived dendritic cells (DC)* are thought to be the most important, because of their capacity both to present antigens and to deliver activation signals to T cells (3-5). The liver, which is the most tolerogenic whole organ (2, 6-10), has a comparatively heavy content of DC (11-15), and can be transplanted across major histocompatibility barriers in most mouse strain combinations, without immunosuppression (2). Further insight into the tolerogenicity of the liver may emerge from studies of the functional properties of liver DC which, to date, have been the subject of very few investigations.
Recently, we have shown that DC with potent allostimulatory activity for naive T cells can be isolated from normal mouse liver (16). In addition, we have succeeded in propagating DC progenitors from liver non-parenchymal cells (NPC) in response to the cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF) (17). Although these nonadherent, low-buoyant-density cells express DC-restricted surface phenotypic markers, they are avidly phagocytic (a characteristic of immature DC) and are resistant to the induction by cytokines of either cell surface major histocompatibility complex (MHC) class II antigen expression or allostimulatory activity.
An important function of DC lineage cells is their capacity to migrate or “home” to secondary lymphoid tissue (18, 19) and therein to present antigen to T cells. The nature and the effectiveness of this cellular interaction may determine the immunogenicity/tolerogenicity of graft alloantigens. In this study, we have examined the in vivo migratory activity, fate, and growth potential of mouse liver–derived DC progenitors.
MATERIALS AND METHODS
Animals
Adult 8–12 week old male B10.BR (H-2k I-E+), C57BL/10SnJ (B10, H-2b, I-A+) and C3H/HeJ (C3H, H-2k, I-E+) mice were purchased from The Jackson Laboratory, Bar Harbor, ME. They were maintained in the specific pathogen–free facility of the University of Pittsburgh Medical Center.
Isolation of nonparenchymal cells from liver
Nonparenchymal cells (NPC) were isolated from normal B10BR mouse liver following in situ perfusion, digestion in collagenase solution, and Percoll centrifugation, as described in detail elsewhere (17). Hepatocyte contamination was consistently <5%. Fresh spleen cell populations were prepared using the same protocol, to control for possible enzyme-mediated effects.
Culture of liver, spleen, or bone marrow–derived DC lineage cells with GM-CSF
Liver NPC or spleen cells, or bone marrow cells isolated using conventional procedures (2.5×l06) were placed in wells of 24-well plates containing 2 ml of RPMI-1640 complete medium (Gibco, Grand Island, NY) supplemented with 10% v/v fetal calf serum and 0.4 ng/ml recombinant mouse GM-CSF (R&D Systems, Minneapolis, MN). Nonadherent, low buoyant density DC-lineage cells released from developing clusters were propagated as described recently (17), using a modification of the method of Inaba et al. (20). The cells were harvested routinely for study after 7–10 days of culture. In some experiments, GM-CSF-stimulated liver-derived populations were depleted of all Ia-rich cells by complement-dependent lysis, using mouse anti-I-Ek monoclonal antibody (mAb) (PharMingen, San Diego, CA) and low-toxicity rabbit complement (Accurate Chemical & Scientific, Westbury, NY). Ia+ cell depletion was confirmed by both flow cytometry and immunocytochemistry.
Flow cytometric analysis
Immunophenotypic analysis of liver or spleen-derived cells was performed by either direct or indirect immunofluorescence staining as described elsewhere, using an extensive panel of mAbs (17). These included antibodies directed against mouse lymphoid, myeloid and DC-restricted markers (33D1, TIB227; ATCC, NLDC-145; and CDllc, N418; kindly provided by Dr. R.M. Steinman, Rockefeller University, New York, NY). In addition, counter-receptors of CTLA-4 (a structural homologue of CD28) (CTLA-4CR), which include CD80 family members (B7/BB1) and B70/B7-2, were identified using the CTLA-4Ig fusion protein (kindly provided by Dr. P S. Linsley, Bristol Myers Squibb Pharmaceutical Research Institute, Seattle, WA) (21), with human Ig (Sigma) as a negative control. Flow cytometric analysis was performed using a FACSTAR flow cytometer (Becton Dickinson, San Jose, CA), and five thousand events were acquired for each sample.
Mixed leukocyte cultures
To test the immunogenicity of cultured liver or spleen-derived cells, one-way mixed leukocyte cultures were performed in 96-well, round-bottomed microculture plates with variable numbers of GM-CSF-stimulated, γ-irradiated (20 Gy) allogeneic (B10.BR) or syngeneic (BIO) liver or spleen cells as stimulators. B10 spleen cells (4×l05 per well) enriched for T cells by passage (1 hr) through a nylon wool column were used as responders. Cultures were maintained for 72 hr; [3H]TdR was added 18 hr before harvesting and the extent of DNA synthesis determined by liquid scintillation counting.
Dendritic cell homing
Nondepleted or Ia-depleted cultured B10.BR liver- or spleen-derived cells were washed in Hanks’ balanced salt solution and injected s.c. (1 or 2.5×105 cells in 50 μl) into one hind footpad or i.v. (106 in 200 μl) via the lateral tail vein of normal B10 mice. One day to 2 months later, groups of 3 animals were killed and the draining popliteal lymph node (where appropriate), thymus, and spleen were removed, embedded in Tissue-Tek (O.C.T. Compound, Miles, Elkhart, IN) and frozen at −70°C. Cryostat sections (5 μm) were air-dried at room temperature (RT) overnight, then stored at −70°C until used.
Immunocytochemistry
Cryostat sections (equilibrated at RT) or cytocentrifuge preparations of cultured cells were fixed in acetone, then stained for donor MHC class II using biotinylated mouse IgG2a anti-mouse I-Ek,d,p,r or anti I-Ab mAbs (PharMingen) as appropriate, in an avidin-biotin-peroxidase complex (ABC) staining procedure. Controls included sections or cytospin preparations of normal donor or recipient strain tissues or cells. The incidence of donor MHC class II+ cells in sections was determined by the mean number of positive cells per 100 high power fields (hpf).
Liver transplantation
Orthotopic liver transplantation (OLTx) was performed in a mouse strain combination (B10 to C3H; MHC class I, II and mHC disparities) with a high acceptance rate, using techniques described previously in detail (22), with minor modifications. No immunosuppressive therapy was used. The animals were killed 14 days after OLTx (three animals per group).
RESULTS
Growth and immunophenotype of liver DC progenitors
Approximately 2.5×106 low-density nonadherent cells with typical dendritic shape and other morphological features of DC (Fig. 1) could be harvested per liver after 7–10 days of culture in GM-CSF. Flow cytometric analysis (summarized in Table 1) confirmed that these cells expressed surface antigens associated with mouse DC, including CD45 (leukocyte-common antigen), heat-stable antigen, CD54 (ICAM-1), CD11b (MAC-1), and CD44 (nonpolymorphic determinant of Pgp.1 glycoprotein). In addition, staining of weak-to-moderate intensity was observed for the DC-restricted markers NLDC-145 (interdigitating cells), 33D1 and N418 and for F4/80 and CD32 (FcyRII). The intensity of expression of these markers on GM-CSF-stimulated spleen cells was similar, except that CD32 and CD11b were reduced and 33D1 and NLDC 145 were slightly more and less intense, respectively, compared with the liver-derived cells. In contrast to the spleen cell progeny, which were MHC class IIbright and positive for CTLA-4CR, the liver-derived, GM-CSF-stimulated cells were MHC class II−/dim and did not express detectable CTLA-4CR.
Figure 1.

Cytocentrifuge preparation showing cells released from GM-CSF-stimulated liver cell aggregates (day 10), which exhibit irregularly shaped, eccentric nuclei, absence of prominent granules, and distinct cytoplasmic processes (giemsa; ×600).
Table 1.
Immunophenotypic characteristics of nonadherent, GM-CSF stimulated mouse liver-derived DC progenitors harvested from liquid cultures: comparison with GM-CSF stimulated spleen-derived DC
| Antigen (mAb) | Liver DC progenitors |
Spleen- derived DC |
|---|---|---|
| Leukocyte-common Ag | ||
| CD45 | ++ | ++ |
| CD45RA;B220 | − | − |
| MHC Class II; I-Ek,d,p,r | −/dim | +++ |
| DC-restricted | ||
| Lymphoid DC (33D1) | + | + |
| Interdigitating cell (NLDC 145) | + | + |
| Myeloid (primarily) macrophage (F4/80) |
+ | + |
| Lymphoid (primarily) | ||
| CD3∈ | − | − |
| Heat-stable antigen (J11D) | ++ | ++ |
| NK cells: NK1.1 | − | − |
| Receptors/adhesins | ||
| CD32, FcγRII | + | + |
| CD11b, MAC-1α unit; C3biR | ++ | + |
| CD11c, p150/90 (N418) | + | NDa |
| CD44, Pgp-1 | ++ | ++ |
| CD54, ICAM-1 | ++ | ++ |
| CTLA-4 counter-receptors | − | ++ |
| CD25, p55; IL-2R | − | − |
Not determined.
Allostimulatory activity of GM-CSF-propagated liver-derived cells
Compared with freshly isolated B10.BR liver NPC or spleen cells, the GM-CSF-stimulated liver DC progenitors failed to induce proliferation in naive, allogeneic (B10; I-E−) splenic T cell populations (Fig. 2). Similar observations were made whether nondepleted or Ia-depleted cell populations were tested. GM-CSF-stimulated spleen-derived DC, however, which expressed much higher levels of surface MHC class II antigen and also CTLA-4CR, were efficient inducers of primary allogeneic T cell responses (data not shown).
Figure 2.
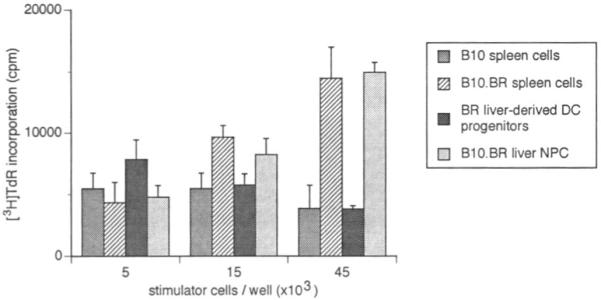
Absence of allostimulatory activity of γ-irradiated, GM-CSF-stimulated, B10.BR mouse liver–derived DC progenitors [ ] using naive, B10 (I-E−) splenic T cells as responders. The nonadherent, low-buoyant-density cells were harvested from 10-day GM-CSF-stimulated cultures and set up at various concentrations, with 4×105 responder T cells. Cultures were maintained for 72 hr; [3H]TdR was added 18 hr before harvesting. The MLR-stimulatory activity of freshly-isolated allogeneic (B10.BR) liver NPC [
] using naive, B10 (I-E−) splenic T cells as responders. The nonadherent, low-buoyant-density cells were harvested from 10-day GM-CSF-stimulated cultures and set up at various concentrations, with 4×105 responder T cells. Cultures were maintained for 72 hr; [3H]TdR was added 18 hr before harvesting. The MLR-stimulatory activity of freshly-isolated allogeneic (B10.BR) liver NPC [ ] and spleen cells [
] and spleen cells [ ] and of syngeneic (B10; [
] and of syngeneic (B10; [ ]) spleen cells is also shown. The results are expressed as mean counts per minute (cpm) ± 1 SD and are representative of 3 separate experiments.
]) spleen cells is also shown. The results are expressed as mean counts per minute (cpm) ± 1 SD and are representative of 3 separate experiments.
In vivo migration of liver-derived developing DC
We wished to determine the migratory ability of the liver-derived DC progenitors after their local (s.c.) or i.v. injection (in the latter instance to mimic possible consequences of liver transplantation). As shown in Figure 3, at least some of the liver-derived cells homed after footpad or i.v. injection almost exclusively to the T cell areas of recipients’ spleens, where they were identified by expression of donor MHC class II (I-Ek) in close proximity to arterioles. Similar observations were made in the draining popliteal lymph nodes of footpad-injected mice; occasional I-E+ cells were also observed in the thymic medulla. Many of these chimeric cells exhibited distinct dendritic morphology. Donor MHC class II+ (I-Ek+ ) cells were first observed in lymph node or spleen 24 hr after injection. Interestingly, structures resembling weakly donor MHC class II+ cells were also observed in lymph nodes at 24 hr (Fig. 3). Whether these were maturing donor-derived cells or recipient cells that had taken up shed donor MHC class II peptides was not determined. In the spleen, maximal numbers of I-Ek+ cells were found on day 5 (Fig. 4). Thereafter, numbers declined, but I-Ek+ cells were still detected in T cell areas 2 months after the injection of liver-derived DC progenitors. In addition to isolated cells, aggregates (Fig. 3e) and also rare dividing cells (Fig. 3h) were found within the first 5 days after injection. To address the question of whether up-regulation of MHC class II expression or selective migration of the small proportion of class II+ cells had occurred in vivo, Ia+ cells were removed before injection of the cultured cells. Similar observations were made whether nondepleted or Ia-depleted, liver-derived cells were injected; after s.c. injection, the mean number of positive cells per 100 high-power fields in the spleen at 5 days was 15.8±8.9 and 15.3±9.2 per 100 hfp, respectively.
Figure 3.
(a–h). Homing ability of GM-CSF-stimulated B10.BR liver–derived cells DC progenitors released in culture from proliferating cell aggregates (harvested on day 10). The cells (2.5×105) were injected s.c. (day 0) into one hind footpad of B10 (I-E−) recipients and detected by immunohistochemistry (I-E+) in cryostat sections of draining lymph nodes, spleen, or thymus 1 day to 2 months later. The sections were stained, using the ABC peroxidase procedure, with donor-specific mouse anti-I-Ek mAb, together with appropriate controls, (a) day 1, spleen; a strongly donor MHC class II–positive cell in the T-dependent area of recipient spleen, close to an arteriole (arrow); (b), day 1, lymph node; a single positive cell in the subcapsular space and some additional structures (arrows) that may represent lightly positive cells; (c) day 1, thymus; a positive cell in the medulla; (d) day 5, spleen; several strongly positive cells with dendritic morphology in T-dependent area near an arteriole; (e) day 5, spleen; isolated donor class II+ cells with dendritic morphology and an aggregate of similar cells, each of which expresses donor phenotype; (f) 2 weeks and (g) 2 months, spleen; showing positive cells and (h) day 4, spleen; a dividing donor class II+ cell in late metaphase following the injection of Ia-depleted, liver-derived DC progenitors (all × 1000).
Figure 4.
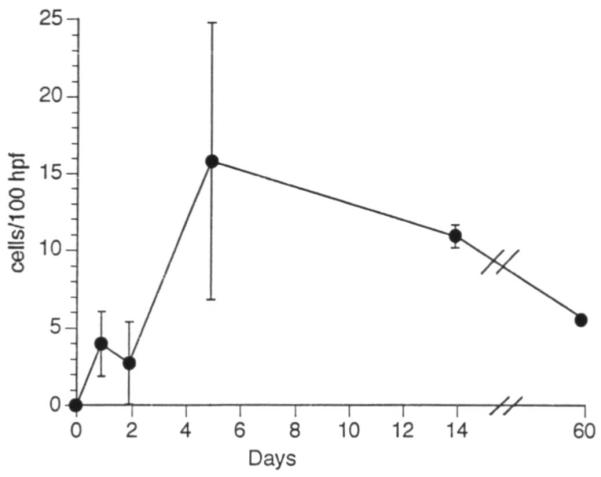
Mean number ± 1 SD of donor MHC class II+ cells with dendritic morphology observed in spleen per 100 high-power fields (hpf) 1–60 days after s.c. footpad injection of 2.5×l05 GM-CSF-stimulated, liver-derived DC progenitors.
Growth of cells expressing donor phenotype from bone marrow and spleen of liver-allografted mice
We next tested whether, using the same technique as was employed to propagate DC lineage cells from normal mouse liver, GM-CSF stimulated cells of donor phenotype could be propagated from the bone marrow or spleen of mice killed 14 days after allogeneic orthotopic liver transplantation (B10 → C3H). Flow cytometric analysis of donor and recipient MHC class I cell surface antigen expression in freshly isolated suspensions of bone marrow or spleen cells revealed a low incidence (<5%) of donor (H-2b+) cells, compared with 89% and 97% recipient cells (H-2k+), respectively. Ten days after the initiation of GM-CSF-stimulated cultures, in which selective enrichment for cells with DC characteristics was performed, a minor population of donor MHC class II+ cells could readily be identified both in the bone marrow and spleen cell populations (Fig. 5). Cells were also propagated for 10 days from the spleens of normal B10 (donor strain) and C3H (recipient strain) animals and stained both for I-Ab and I-Ek, with appropriate Ig isotype controls. No mAb crossreactivity was observed. These findings are therefore consistent with the ex vivo propagation of donor liver-derived DC from progenitors, or even stem cells, seeded in the lymphoid tissue of the organ allograft recipients.
Figure 5.
(a), donor MHC class II+ (I-Ab+) and (b), recipient MHC class II+ (I-Ek+) cells with dendritic morphology in 10-day GM-CSF-supplemented cultures of bone marrow-derived cells isolated from a C3H (H-2k) mouse recipient of a B10 (H-2b) liver allograft, 14 days after transplantation (×1000). (c), mouse IgG isotype control (×400). No staining of GM-CSF-stimulated C3H or B10 spleen cells (derived from normal mice) with anti-I-Ab or anti-I-Ek, respectively, was observed (data not shown).
DISCUSSION
Unlike GM-CSF-stimulated DC propagated from normal mouse blood (20), bone marrow (25), or spleen, the majority of GM-CSF-stimulated cells with DC characteristics derived from liver NPC express little or no cell surface MHC class II antigen. Furthermore, as we have shown here, the liver-derived DC progenitors fail to stimulate unprimed alloreactive T cells—in contrast to freshly isolated spleen cells or hepatic NPC, a finding in keeping with the very low intensity of MHC class II and CTLA-4CR detected on their surface. As shown previously (17), these cells also exhibit avid phagocytic activity—a property of immature DC. They can however, be induced to mature in vitro (confirming their DC lineage) by exposure for several days to the extracellular matrix protein type-1 collagen (17) that is spacially associated with MHC class II+ DC in the portal triads within normal liver. Recently, there has been increased speculation that such “immature” DC or DC progenitors present in non-lymphoid organs may constitute tolerogenic progenitors of chimeric cells in recipients of organ allografts (1, 17, 23).
A specialized property of bone marrow–derived DC is their capacity to migrate in vivo to T-dependent areas of peripheral lymphoid tissues (18, 19) wherein they present antigen to T cells. In this study, we assessed the homing ability of GM-CSF-stimulated, developing, liver-derived DC (low I-Ek expression or Ia− following complement-mediated lysis) that were injected either locally or systemically (to mimic possible sequelae of liver transplantation) into allogeneic B10 (I-E−) recipients. When the recipients were killed after 1 to 5 days, at least some of the injected cells were found to have migrated to T cell areas of the draining lymph node (after local injection) and spleen. In these sites, they were easily identified as donor by their strong cell surface staining for MHC class II (I-Ek). Significantly, the liver-derived cells or their progeny persisted in the spleen much longer (for at least 2 months) than those donor-derived DC described in the same tissue up to only 4 days after cardiac transplantation in unmodified mice that rejected their grafts within 8–12 days (18). This may, of course, reflect both qualitative and quantitative differences in the migratory DC populations derived from liver and heart. Either way, the persistence of injected chimeric cells of DC lineage within lymphoid tissue of unmodified allogeneic recipients is consistent with the failure of these animals to reject liver allografts (2). It is also in agreement with the survival of donor-derived leukocytes, including DC, within the central lymphoid tissue of unmodified murine liver allograft recipients for up to one year posttransplant (2). Donor chimeric leukocytes have similarly been observed in spleens of liver-allografted rats up to 300 days posttransplant (24).
The comparatively long survival and apparent maturation of liver DC progenitors homing to T cell–dependent areas of allogeneic lymphoid tissue is consistent with the contention (2, 24) that the destination of donor-derived leukocytes, migrating after whole organ transplantation, is lineage-specific, following routes similar to those taken by syngeneic cells of the same lineages. After transplantation of the liver or other organs, immature cells of DC lineage may undergo in vivo differentiation/maturation (as shown here in terms of the upregulation of cell surface MHC class II expression) under the regulatory influence of endogenous GM-CSF and other cytokines, such as tumor necrosis factor-a and IL-4, in an in vivo recapitulation of the results of in vitro maturation (25-27). The kinetics of this maturation process are likely to be influenced by the extent of host immunosuppression, although the general pathways of donor cell migration and differentiation have been shown to be much the same in liver-allografted rats treated with FK506 (24). The implications of the rapid egress from the liver (a potential hema-tolymphoid organ [28]) of progenitor DC with replicative capacity, exhibiting low cell surface MHC class II expression and low T cell–stimulating activity, are considerable. The implications of these findings are underscored both by the lengthy persistence of these cells in nonimmunosuppressed hosts and by our capacity to propagate cells of donor phenotype in vitro from the lymphoid tissue of liver allograft recipients. This latter observation is consistent with the detection of presumptive proliferating (10–15%) donor cells within the spleens of liver-allografted rats several days posttransplant (24). Taken together, these findings suggest a mechanistic basis for cytokine-driven perpetuation of donor leukocyte chimerism in recipients of liver and other organ grafts. They also provide support for the therapeutic strategy of augmentation of natural chimerism in human allograft recipients currently being evaluated at this medical center (29).
The possible functional role of chimeric cells of DC lineage and their progeny in organ allograft acceptance remains to be determined. The existence however, of subpopulations of murine DC with a veto function (inactivation of T helper cells or cytotoxic T cell precursors) has been proposed (30). In addition, MHC class IIdim allogeneic donor bone marrow cells, shown to exhibit veto cell activity, have been postulated to be immature DC (31). Although the precise basis of DC–T cell interactions that might lead to tolerance induction is uncertain, it is most likely to depend on the relative affinity or avidity (compared with “immunizing” antigen-presenting cells [APC]) of donor DC–T cell receptor interactions and on the expression on the former cells of critical adhesins and costimulatory molecules, such as members of the CD80 (B7–1/B7–2) family. These aspects of developing liver and bone marrow–derived DC both in vitro and in vivo are under further investigation in our laboratory.
Following local or systemic injection into nonimmunosup-pressed allogeneic recipients, liver-derived DC progenitors propagated in vitro homed to T-dependent areas of lymph nodes and spleen. They were detected by the expression of donor MHC class II antigen and they persisted for at least 2 months in the spleen, where rare dividing cells identified as donor were also detected. Using the same techniques employed to propagate DC progenitors from normal liver, both bone marrow and spleen cells obtained from liver allograft recipients 14 days after transplantation were cultured for 10 days in GM-CSF. This resulted in the propagation of recipient-derived cells and of cells expressing donor MHC class II antigens, suggesting the growth of donor cells from precursors seeded to host lymphoid tissue. These findings provide insight into the establishment and perpetuation of donor (APC) cell chimerism following transplantation of the liver and possibly other organs.
Acknowledgments
The authors are grateful to Dr. Ralph M. Steinman for providing monoclonal antibodies, Dr. Peter S. Linsley for the CTLA-4Ig fusion protein, Dr. Lan Gao for performing mouse liver transplants, Dr. A. Jake Demetris for expert advice and discussion, Mr. Michael Hsieh for skilled technical assistance, Dr. Jacky Woo for help with cell culture and flow cytometry, and Ms. Shelly L. Conklin for typing the manuscript.
Footnotes
Presented at the 13th Annual Meeting of the American Society of Transplant Physicians, May 16-18, 1994, Chicago, IL.
This work was supported in part by National Institutes of Health Grant DK 29961-14.
- APC
- antigen-presenting cell
- DC
- dendritic cells
- GM-CSF
- granulocyte-macrophage colony-stimulating factor
- Ia
- immune-associated antigen
- mAb
- monoclonal antibody
- MHC
- major histocompatibility complex
- NPC
- nonparenchymal cell
- RT
- room temperature
REFERENCES
- 1.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:96. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice: I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinman RM, Lustig DS, Cohn ZA. Identification of a novel cell in peripheral lymphoid organs of mice: III. Functional properties in vivo. J Exp Med. 1974;139:1431. doi: 10.1084/jem.139.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 6.Starzl TE, Marchioro TL, Porter KA, et al. Factors determining short- and long-term survival after orthotopic liver homotransplantation in the dog. Surgery. 1965;58:131. [PMC free article] [PubMed] [Google Scholar]
- 7.Calne RY, Sells RA, Pena JR, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;233:472. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman FA, Butcher GW, Davies HS, Brons G, Kamada N, Turel O. Techniques for orthotopic liver transplantation in the rat and some studies of the immunologic responses to fully allogeneic liver grafts. Transplant Proc. 1979;11:571. [PubMed] [Google Scholar]
- 9.Murase N, Demetris AJ, Kim DG, Todo S, Fung JJ, Starzl TE. Rejection of the multivisceral allograft in rats: a sequential analysis with comparison to isolated orthotopic small bowel and liver grafts. Surgery. 1990;108:880. [PMC free article] [PubMed] [Google Scholar]
- 10.Reyes J, Tzakis A, Ramos HC, et al. The frequent achievement of a drug free state after orthotopic liver transplantation. Transplant Proc. 1993;25:3315. [PMC free article] [PubMed] [Google Scholar]
- 11.Hart DNJ, Fabre JW. Demonstration and characterization of Ia-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues but not brain. J Exp Med. 1981;153:347. doi: 10.1084/jem.154.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klinkert WEF, Labadie JH, Bowers WE. Accessory and stimulating properties of dendritic cells and macrophages isolated from various rat tissues. J Exp Med. 1982;156:1. doi: 10.1084/jem.156.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steiniger B, Klempnauer J, Wonigeit K. Phenotype and histological distribution of interstitial dendritic cells in the rat pancreas, liver, heart, and kidney. Transplantation. 1984;38:169. doi: 10.1097/00007890-198408000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Witmer-Pack MD, Crowley MT, Inaba K, Steinman RM. Macrophages, but not dendritic cells, accumulate colloidal carbon following administration in situ. J Cell Sci. 1993;105:965. doi: 10.1242/jcs.105.4.965. [DOI] [PubMed] [Google Scholar]
- 15.Prickett TCR, Mckenzie JL, Hart DNJ. Characterization of interstitial dendritic cells in human liver. Transplantation. 1988;46:754. doi: 10.1097/00007890-198811000-00024. [DOI] [PubMed] [Google Scholar]
- 16.Woo J, Lu L, Rao AS, Li Y, Subbotin V, Starzl TE, Thomson AW. Isolation, phenotype and allostimulatory activity of mouse liver dendritic cells. Transplantation. 1994;58:484. doi: 10.1097/00007890-199408270-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu L, Woo J, Rao AS, et al. Propagation of dendritic cell progenitors from normal mouse liver using GM-CSF and their maturational development in the presence of type-1 collagen. J Exp Med. 1994;179:1823. doi: 10.1084/jem.179.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens, a novel pathway for initiation of rejection. J Exp Med. 1990;171:307. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsen CP, Steinman RM, Witmer-Pack W, Hankins DF, Morris PJ, Austyn JM. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990;172:1483. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inaba K, Steinman RM, Pack MW, et al. Identification of proliferating dendritic cell precursors in mouse blood. J Exp Med. 1992;175:1157. doi: 10.1084/jem.175.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linsley PS, Brady W, Urnes M, Grosmaire L, Damle N, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian S, Fung JJ, Demetris AJ, Ildstad ST, Starzl TE. Orthotopic liver transplantation in the mouse. Transplantation. 1991;52:562. doi: 10.1097/00007890-199109000-00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinman RM, Inaba K, Austyn JM. Donor cell chimerism in recipients of organ transplants. Hepatology. 1993;17:1153. [PubMed] [Google Scholar]
- 24.Demetris AJ, Murase N, Fujisaki S, Fung JJ, Rao AS, Starzl TE. Hematolymphoid cell trafficking, chimerism and tolerance after liver, bone marrow, and heart transplantation: rejection, GVH disease and the merging of the immune systems. Transplant Proc. 1993;25:3337. [PMC free article] [PubMed] [Google Scholar]
- 25.Romani N, Lenz A, Glassl H, et al. Cultured human Langerhans cells resemble lymphoid dendritic cells in phenotype and function. J Invest Dermatol. 1989;93:600. doi: 10.1111/1523-1747.ep12319727. [DOI] [PubMed] [Google Scholar]
- 26.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and down regulated by tumor necrosis factor a. J Exp Med. 1994;179:1190. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakamoto T, Saizawa T, Mabuchi A, Norose Y, Shoji T, Yokomuro K. The liver as a potential hematolymphoid organ examined from modifications occurring in the systemic and intrahepatic hematolymphoid system during liver regeneration after partial hepatectomy. Reg Immunol. 1992;4:1. [PubMed] [Google Scholar]
- 29.Fontes P, Rao AS, Demetris AJ, et al. Bone marrow augmentation of donor-cell chimerism in kidney, liver, heart, and pancreas islet transplantation. Lancet. 1994;344:151. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vremec D, Zorbas M, Scollay R, et al. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas JM, Carver FM, Kasten-Jolly J, et al. Further studies of veto activity in rhesus monkey bone marrow in relation to allograft tolerance and chimerism. Transplantation. 1994;57:101. doi: 10.1097/00007890-199401000-00018. [DOI] [PubMed] [Google Scholar]




