Abstract
Autism spectrum disorders (ASD) are characterized by significant social impairments, including deficits in orienting attention following social cues. Behavioral studies investigating social orienting in ASD, however, have yielded mixed results, as the use of naturalistic paradigms typically reveals clear deficits whereas computerized laboratory experiments often report normative behavior. The present study is the first to examine the neural mechanisms underlying social orienting in ASD in order to provide new insight into the social attention impairments that characterize this disorder. Using fMRI, we examined the neural correlates of social orienting in children and adolescents with ASD and in a matched sample of typically developing (TD) controls while they performed a spatial cueing paradigm with social (eye gaze) and nonsocial (arrow) cues. Cues were either directional (indicating left or right) or neutral (indicating no direction), and directional cues were uninformative of the upcoming target location in order to engage automatic processes by minimizing expectations. Behavioral results demonstrated intact orienting effects for social and nonsocial cues, with no differences between groups. The imaging results, however, revealed clear group differences in brain activity. When attention was directed by social cues compared to nonsocial cues, the TD group showed increased activity in frontoparietal attention networks, visual processing regions, and the striatum, whereas the ASD group only showed increased activity in the superior parietal lobule. Significant group × cue type interactions confirmed greater responsivity in task-relevant networks for social cues than nonsocial cues in TD as compared to ASD, despite similar behavioral performance. These results indicate that, in the autistic brain, social cues are not assigned the same privileged status as they are in the typically developing brain. These findings provide the first empirical evidence that the neural circuitry involved in social orienting is disrupted in ASD and highlight that normative behavioral performance in a laboratory setting may reflect compensatory mechanisms rather than intact social attention.
Keywords: autism, attention, functional magnetic resonance imaging, gaze, social cue
1. Introduction
Autism spectrum disorders (ASD) are characterized by profound deficits in social communication and interaction. One of the most notable aspects of these social impairments is reduced orienting in response to social cues (e.g., eye gaze, pointing gestures). Converging behavioral and neural evidence shows that this deficit is not simply the result of impaired sensory processing of social stimuli, but rather a more specific impairment in social attention. For example, neuroimaging studies have demonstrated decreased activity in brain regions involved in processing faces, emotions, and voices in ASD (Critchley et al., 2000; Dalton et al., 2005; Gervais et al., 2004). Yet, when ASD individuals are cued to attend to the social stimuli, activity in these regions normalizes (Hadjikhani et al., 2004; Wang et al., 2004; Wang et al., 2007). Pelphrey et al. (2005) also found that individuals with ASD showed normal activation of the superior temporal sulcus (STS) when viewing gaze shifts. However, STS activity varied depending on the intentions conveyed by the gaze shift in control participants, while no such difference was found in the ASD group. Thus, impaired orienting in response to social cues (social orienting) in ASD likely results from impaired attentional responses to social stimuli.
Naturalistic studies investigating social orienting provide compelling behavioral evidence for impaired utilization of social cues among children with ASD. For example, these children fail to orient their attention toward social stimuli significantly more than typically developing (TD) children and those with Down Syndrome (Dawson et al., 1998). Moreover, ASD children fail to shift their attention toward novel objects selectively when these objects are cued socially by a head turn and gaze shift (Leekam et al., 2000). Consistent with these findings, retrospective studies examining the home movies of infants prior to ASD diagnosis have shown that those infants who will later be diagnosed with ASD displayed less social orienting behavior, including reduced orienting to faces and following pointing gestures (Osterling and Dawson, 1994; Osterling et al., 2002; Werner et al., 2000). Thus, both orienting toward a social stimulus and orienting toward an object that is cued by a social stimulus are clearly impaired in children with ASD in naturalistic situations.
Surprisingly, computerized laboratory experiments do not show similar deficits. Social orienting can be tested in the laboratory using a variant of Posner’s spatial cueing paradigm (1980) in which a social spatial cue (e.g., eyes gazing to the side) precedes a target stimulus. Even when the direction of the cue (gaze) is not predictive of the location of the upcoming target, adults and children show faster responses to targets occurring in the cued location than in an uncued location (Driver et al., 1999; Friesen and Kingstone, 1998; Ristic et al., 2002). This facilitation effectis thought to reflect an automatic shift in attention toward the cued location (Friesen et al., 2005; Langton et al., 2000). Most gaze cueing studies in ASD adults and children report intact facilitation effects and no differences in those effects between ASD and TD controls using dynamic gaze cues (Chawarska et al., 2003; Swettenham et al., 2003), static gaze cues (Kylliainen and Hietanen, 2004; Vlamings et al., 2005), and even counterpredictive gaze cues (Senju et al., 2004). To our knowledge, only two studies using a computerized gaze cueing paradigm found impaired social orienting in ASD (Goldberg et al., 2008; Ristic et al., 2005). Thus, most of the evidence suggests that both children and adults with ASD automatically orient toward the location indicated by gaze cues presented on a computer screen.
It should be noted, however, that some differences have been observed between ASD and TD performance despite intact facilitation effects. For example, one study found that TD adults responded more slowly for social cues than for nonsocial cues, whereas ASD adults showed no difference (Vlamings et al., 2005), and another showed that TD children were slower in social cueing tasks than ASD children (Chawarska et al., 2003). Others found that TD children responded at similar speeds to social and nonsocial cues, while ASD children were faster for social cues (Senju et al., 2004). Further, using counterpredictive cues, Senju et al. demonstrated that gaze cues were more effective than arrow cues in automatically orienting attention in TD children, with no such difference in ASD children. Still, the failure to find differences in the facilitation effect of social cues is perplexing, especially in light of the clear impairments reported in naturalistic paradigms.
Considering the underlying neural mechanisms involved in social orienting may help reconcile these discrepant findings. Neuroimaging studies investigating gaze perception have found that the superior temporal sulcus (STS) plays a prominent role in processing gaze (Hoffman and Haxby, 2000; Hooker et al., 2003). In addition, when Hoffman and Haxby (2000) compared neural activity for viewing averted gaze to that for viewing directed gaze (toward the participant), they also found stronger activity in the intraparietal sulcus (IPs), a region of the posterior parietal cortex (PPC) that is part of a frontoparietal network consistently implicated in attentional orienting (Corbetta and Shulman, 2002; Mesulam, 1981). Heightened STS and PPC activity has also been found among adults and children when viewing gaze shifts (Mosconi et al., 2005; Pelphrey et al., 2003). Moreover, neurotypical adults displayed differential activation in the STS and PPC when comparing gaze shifts that met versus those that violated expectations (Pelphrey et al., 2003), whereas ASD adults did not (Pelphrey et al., 2005). Thus, activity in the STS and PPC has already provided some clues as to how individuals with ASD process social stimuli.
Only a handful of neuroimaging studies have investigated social orienting using a spatial cueing paradigm among neurotypical adults. While a few of these studies reported overlapping activation for social and nonsocial cues in frontoparietal regions (Greene et al., 2009; Sato et al., 2009; Tipper et al., 2008), most of these studies found differential activation during social cueing compared to nonsocial cueing, including heightened activity in the extrastriate cortex (Engell et al., 2010; Greene et al., 2009; Hietanen et al., 2006; Tipper et al., 2008), the inferior frontal gyrus (Engell et al., 2010), the medial frontal cortex (Tipper et al., 2008), and the STS (Kingstone et al., 2004), as well as reduced activity in frontoparietal regions (Hietanen et al., 2006). Thus, the typical adult brain treats social and nonsocial cues somewhat differently.
Understanding how the ASD brain processes social compared to nonsocial cues should help explain the discrepant behavioral results found in naturalistic and experimental studies. One possibility is that individuals with ASD process gaze cues as nonsocial cues, using nonsocial mechanisms that rely on lower-level directional properties of eye gaze rather than on its social significance for orienting attention (Nation and Penny, 2008). Thus, orienting behavior in simple laboratory experiments may appear intact even though there are differences in the brain that account for the impairments seen in real life situations. Thus, the goal of the present study was to use fMRI in order to reveal differences in processing even when they are not apparent in behavior. Children and adolescents with and without ASD underwent fMRI while they performed a spatial cueing task that included social (eye gaze) and nonsocial (arrow) cues. We predicted that the ASD and TD groups would show no differences at the behavioral level, but would show variation in brain activity, reflecting underlying group differences in the processing of social cues. Specifically, if individuals with ASD treat social cues as nonsocial, we would expect them to display fewer differences in brain activity between the cue types than the TD group.
2. Methods
2.1 Participants
Our sample included 22 high-functioning children and adolescents with ASD (20 male; 19 right-handed) and 21 TD children and adolescents (19 male; 18 right-handed) matched by age, IQ, and extent of head motion while in the MRI scanner (see Table 1). Two additional ASD children participated in the study, but were excluded from subsequent behavioral and imaging analyses, one for excessive eye movements and one for excessive head movement during scanning. Participants were recruited through the UCLA Center for Autism Treatment and Research, fliers in the local community, and from a pool of participants who previously participated in research studies at UCLA. Participants and parents provided written consent according to guidelines specified by the Institutional Review Board at the University of California, Los Angeles. Prior diagnosis of ASD was confirmed using the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000) and the Autism Diagnostic Interview-Revised (ADI-R; Lord et al., 1994), as well as by expert clinical judgment based on DSM-IV diagnostic criteria. One participant’s diagnosis was confirmed by the ADOS and by clinical judgment, but the ADI was not administered. All participants had a full scale IQ of 80 or higher, based on either the Wechsler Scale of Abbreviated Intelligence (Wechsler, 1999) or the full Wechsler Intelligence Scale for Children (Wechsler, 1991). All participants were screened to rule out head trauma, history of neurological or psychological disorders, substance abuse or other serious medical conditions.
Table 1.
Subject Demographics listed as “mean (standard deviation)”
| TD | ASD | |
|---|---|---|
| Chronological Age | 13.19 (2.44) | 12.95 (2.46) |
| Age range | 10 – 17 | 9 – 17 |
| Full Scale IQ | 110.48(14.10) | 103.25 (13.93) |
| Mean absolute motion (mm) | 0.27 (0.20) | 0.39 (0.25) |
| Mean relative motion (mm) | 0.09 (0.07) | 0.11 (.08) |
| ADOS (Social Subscale) | NA | 7.50 (1.77) |
| ADOS (Communication Subscale) | NA | 3.18 (1.84) |
| ADI (Social Subscale) | NA | 21.48 (4.34) |
| ADI (Communication Subscale) | NA | 16.80 (4.20) |
2.2 Behavioral Task
The software program MacStim 3.2.1 (WhiteAnt Occasional Publishing, West Melbourne, VIC, Australia) was used to present stimuli and record reaction time (RT) data. Visual stimuli were presented through magnet-compatible goggles and responses were collected unimanually from a magnet-compatible button box (Resonance Technology, Northridge, CA).
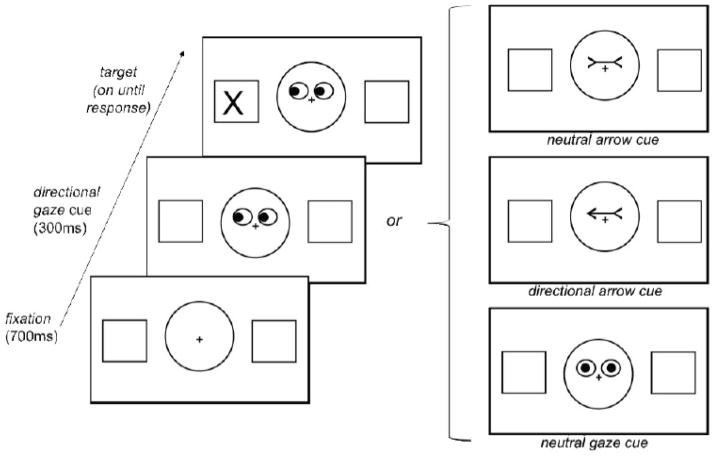
Figure 1 illustrates the experimental procedure. Each trial began with a fixation stimulus for 700ms (black fixation cross surrounded by a circle, with a box on the left and the right sides of the screen). This was followed by the appearance of a cue just above fixation for 300ms. There were four possible cue types: a) directional gaze: schematic eyes looking left or right, b) neutral gaze: schematic eyes looking straight ahead, c) directional arrow: line with an arrowhead on each end pointing left or right, or d) neutral arrow: line with arrowheads both pointing inward. With the cue still on the screen, a target ‘X’ then appeared in one of the two peripheral boxes and remained on the screen until the participant responded. This was followed by the fixation stimulus for a duration calculated to maintain the length of each trial to 4000ms. The directional cues were valid or invalid in identifying the location of the upcoming target. In order to engage automatic processes by minimizing expectations, these cues were uninformative, such that they were valid in only 50% of the trials.
Figure 1.
Experimental procedure of the behavioral task.
Participants first performed a block of 8 practice trials outside the scanner, and were allowed to repeat the practice block until they were comfortable with the task. It was emphasized in the instructions that the cues did not predict the location of the target. It was also emphasized to remain fixated on the central cross at all times in order to measure attentional shifts independent of eye movement. The experimenter ensured that they could complete an entire practice block without moving their eyes before continuing. In the scanner, participants completed two functional runs and were reminded of the instructions before each run. A run consisted of four blocks of experimental trials, each block showing one of the four cue types for 12 trials, the order of which was counterbalanced across participants within each group. Valid and invalid cues were randomized within each block. Experimental blocks were separated by baseline blocks (showing the fixation stimulus) for 12 seconds. At the beginning of the baseline block, the word “LOOK” was presented for 500ms, and at the end, the words “FIND THE X” were presented for 500ms. The initial baseline lasted 18 seconds, as the first 2 TRs of each run were deleted to allow for scanner warm up. Participants responded via a button box situated on their torso while in a supine position in the scanner. Their task was to press the button corresponding to the location of the target (left or right) with the index and middle fingers of the dominant hand as quickly and accurately as possible.
2.3 Behavioral Analysis
Behavioral data from 2 of the 22 ASD participants and from 1 of the 21 TD participants were lost due to software malfunction. Therefore, behavioral analyses were conducted on 20 participants in each group. Trials with reaction times (RT) faster than 150ms and slower than 1000ms were considered attentional errors and removed from analysis. There was no significant difference between groups in the number of trials that were excluded (p > .1). A repeated-measures ANOVA was conducted on RT data for correct trials2 with Cue type (gaze, arrow) and Validity (valid, invalid) as within-subjects factors, and with Group (ASD, TD) as a between-subjects factor. Paired-samples t-tests were then conducted to investigate the specific facilitation effects. Neutral cues were not included in the behavioral analysis, as their purpose was to account for visual differences in the stimuli for the imaging analysis. Yet, RT data for neutral cue conditions can be viewed in Table 2 for the interested reader.
Table 2.
Mean reaction time for each condition in each group.
| Cue | TD | ASD | ||
|---|---|---|---|---|
| Gaze | Arrow | Gaze | Arrow | |
| Valid | 405.6(77.7) | 421.2(81.1) | 437.0(108.8) | 447.0(99.3) |
| Invalid | 436.5(73.4) | 470.6(89.3) | 464.8(99.3) | 510.6(110.9) |
| Neutral | 412.8(74.2) | 423.5(75.1) | 436.9(94.3) | 440.8(81.2) |
Note. Values are listed as “mean (standard deviation)” in msec.
2.4 Imaging Acquisition
Images were acquired using a Siemens Trio 3.0 Tesla MRI scanner. Two sets of high-resolution anatomical images were acquired for registration purposes: 1) an MP-RAGE structural volume (TR = 2300ms, TE = 2.84ms, flip angle = 9°) with 160 sagittal slices, 1.2mm thick, and 1mm × 1mm in-plane resolution, and 2) a T2-weighted co-planar volume (TR = 5000ms, TE = 34ms, flip angle = 90°) with 34transverse slices covering the whole brain, 4mm, a 128 × 128 matrix, and an in-plane resolution of 1.5 mm × 1.5 mm. Each functional run involved the acquisition of 80 EPI volumes (gradient-echo, TR = 3000ms, TE = 28ms, flip angle = 90°), each with 34transverse slices, 4mm thick, and a 64 × 64 matrix yielding an in -plane resolution of 3 mm × 3 mm. Each functional run lasted 4 minutes and 6 seconds.
2.5 Imaging Analysis
Analyses were performed using FSL Version 4.1.4 (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). Preprocessing included motion correction to the mean image, spatial smoothing (Gaussian kernel FWHM = 6mm), mean-based intensity normalization of all volumes by the same factor, and high-pass temporal filtering (0.01 Hz). Functional data were linearly registered to a common stereotaxic space using a three-step process. Functional images were first registered to the in-plane T2 image (6 degrees of freedom), then to the high-resolution T1 MP-RAGE (6 degrees of freedom), and finally to the MNI152 brain (12 degrees of freedom).
Statistical analysis was carried out using FEAT (FMRI Expert Analysis Tool) Version 5.98, part of FSL. We modeled the BOLD response using a separate explanatory variable (EV) for each of the four cue conditions of the task. The design was convolved with a double-gamma hemodynamic response function (HRF) to produce an expected BOLD response, and the temporal derivative of this timecourse was included in the model for each EV. Functional data were then fitted to the model using FSL’s implementation of the general linear model. The two runs of functional data for each participant were first combined using a higher-level fixed-effects analysis. Then, data from all participants were passed into a higher-level mixed-effects analysis for within-group and between-group comparisons. Higher-level group analyses were carried out using FLAME (FMRIB’s Local Analysis of Mixed Effects) stage 1 and stage 2 (Beckmann et al., 2003; Woolrich, 2008; Woolrich et al., 2004). Z (Gaussianised T/F) statistic images were thresholded at Z > 2.3 with a (corrected) cluster significant threshold of p = .05 (Worsley, 2001). We included absolute motion as a covariate in our between-group analyses, since it appeared to be larger in the ASD group than in the TD group, though not statistically significant (p = .104), and we wanted to ensure that our results were not driven by differences in motion in the scanner3.
To qualify the interactions in our analysis, as well as to explore the relationship between brain activity and symptom severity in the ASD group, we extracted the parameter estimates from regions that showed more activity for social orienting vs. nonsocial orienting. Specifically, regions of interest (ROIs) were generated from the functional activity for the directional gaze > directional arrow contrast and the 2 (gaze vs. arrow) × 2 (directional vs. neutral) interactions, and then constrained with an anatomical ROI as defined by the Harvard-Oxford Cortical and Subcortical Structural Atlases.
2.6 Eye Tracking
To ensure central fixation during the functional scans, participants’ eye movements were monitored using an MRI-compatible infrared camera attached to the right side of the stimulus presentation goggles, connected to ViewPoint EyeTracker® software (Arrington Research Inc, Scottsdale, Az). Quantifiable eye tracking data were obtained, for at least one functional run, from 28 participants (13 ASD, 15TD). Unusable data ensued due to difficulties with calibration, goggle shift, and eye blinks throughout the duration of the scan. For all participants, however, the experimenters verified fixation by watching the participants’ eye and fixation trajectories online via the eye tracker computer. As mentioned previously, one participant was excluded due to excessive eye movements.
Regions of interest (ROIs) were drawn on the behavioral stimuli screen, with the central fixation ROI defined as the space within the central circle of the fixation stimulus. Fixation was quantified by summing the total time of saccades away from the central ROI over the total time of stimulus presentation. Both groups maintained fixation over 99% of the time (ASD 99.1%, TD 99.6%) with no significant difference between groups, t(26) = 2.06, p = .28.
3. Results
3.1 Behavioral Results
Table 2 lists the mean reaction time for each condition in each group. The 2 (Cue type) × 2 (Validity) × 2 (Group) ANOVA revealed a significant main effect of Cue type, with faster RT for gaze cues (M= 436, SD= 62.2) than for arrow cues (M= 462.4, SD = 65.8), F(1, 38) = 18.19, p < .001, and a main effect of Validity, with faster RT for the valid condition (M = 427.7, SD= 63.4) than for the invalid condition (M = 470.6, SD= 64.1), F(1, 38) = 58.62, p < .001. There was also a significant interaction of Cue type × Validity, F(1, 38) = 8.88, p = .005, driven by a larger facilitation effect for arrow cues (56.5ms) than for gaze cues (29.3ms), t(39) = 2.98, p = .005. There was no main effect and no interactions with Group, demonstrating similar behavior across groups.
In the ASD group, Paired-samples t-tests revealed a significant facilitation effect for the arrow cue, with faster RT for the valid condition (M = 447, SD = 81.1) than for the invalid condition (M = 510.6, SD = 110.9), t(19) = 5.71, p < .001. There was also a significant facilitation effect for the gaze cue, with faster RT for the valid condition (M = 437, SD = 108.8) than for the invalid condition (M = 464.8, SD = 99.3), t(19) = 3.64, p = .002. Similarly, in the TD group, Paired-samples t-tests revealed a significant facilitation effect for the arrow cue, with faster RT for the valid condition (M= 421.2, SD = 81.1) than for the invalid condition (M= 470.6, SD= 89.3), t(19) = 5.52, p < .001, and a significant facilitation effect for the gaze cue, with faster RT for the valid condition (M = 405.6, SD= 77.7) than for the invalid condition (M= 436.5, SD= 73.4), t(19) = 2.48, p = .023.
3.2 Imaging Results
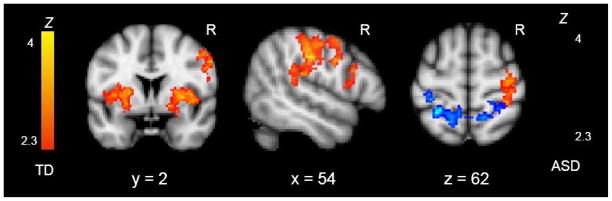
To identify brain regions that differed in activity during social vs. nonsocial orienting, we first examined regions with more activity for the directional gaze cue than for the directional arrow cue (directional gaze > directional arrow; the reverse contrast did not yield any significant activity in either group). Peak activation coordinates are reported in Table 3a for the TD group, and in Table 4a for the ASD group. As can be seen in Figure 2, in the TD group there was significant activity in frontoparietal regions, including the inferior frontal gyrus (IFG), premotor cortex, precentral gyrus, and supramarginal gyrus (SMG), largely lateralized to the right hemisphere, along with activity in lower level visual regions. There were also large and significant clusters of activity in bilateral putamen, extending into the insula. In the ASD group, the same contrast demonstrated significant activation only in the superior parietal lobule (SPL).
Table 3.
Peak activation coordinates (MNI) for the within-group analyses in the TD group.
| Anatomical region | Side | x | y | z | Max Z-score |
|---|---|---|---|---|---|
| Directional Gaze > Directional Arrow | |||||
| Cuneus | R | 2 | −96 | 8 | 4.63 |
| L | −12 | −96 | 12 | 4.20 | |
| IFG | R | 58 | 16 | 26 | 3.71 |
| Insula | R | 36 | 4 | 6 | 3.94 |
| L | −38 | −6 | −4 | 3.46 | |
| Postcentral gyrus | R | 52 | −20 | 34 | 3.93 |
| Premotor cortex | R | 24 | −18 | 74 | 4.13 |
| R | 60 | 2 | 38 | 3.82 | |
| Putamen | R | 26 | 0 | 6 | 3.57 |
| L | −22 | 2 | 6 | 3.44 | |
| SMG | R | 62 | −24 | 18 | 3.94 |
| 2 (Gaze, Arrow) × 2 (Directional, Neutral) Interaction | |||||
| Cingulate gyrus | R | 4 | 10 | 34 | 3.75 |
| L | −10 | 2 | 36 | 3.56 | |
| Cuneus | R | 10 | −64 | 12 | 3.80 |
| L | −8 | −72 | 8 | 4.42 | |
| Fusiform gyrus | R | 38 | −48 | −16 | 4.31 |
| IFG | R | 52 | 14 | 16 | 3.78 |
| Insula | L | −38 | −4 | −2 | 4.00 |
| Lingual gyrus | R | 8 | −86 | −4 | 3.93 |
| L | −8 | −94 | 8 | 3.90 | |
| LOC | R | 30 | −86 | 0 | 3.56 |
| L | −42 | −74 | 14 | 3.74 | |
| Postcentral gyrus | R | 52 | −22 | 36 | 4.50 |
| L | −48 | −24 | 38 | 4.53 | |
| Putamen | R | 22 | 4 | −4 | 4.40 |
| L | −26 | 0 | 2 | 3.93 | |
| SPL | R | 26 | −46 | 70 | 4.03 |
| L | −38 | −42 | 68 | 3.79 | |
MNI = Montreal Neurological Institute; TD = typical development; IFG = inferior frontal gyrus; LOC = lateral occipital cortex; SMG = supramarginal gyrus; SPL = superior parietal lobule; Thresholded at Z > 2.3 (p < .01), corrected for multiple comparisons at the cluster level (p < .05).
Table 4.
Peak activation coordinates (MNI) for the within-group analyses in the ASD group.
| Anatomical region | Side | x | y | z | Max Z-score |
|---|---|---|---|---|---|
| Directional Gaze > Directional Arrow | |||||
| SPL | R | 12 | −48 | 64 | 3.57 |
| L | −26 | −46 | 64 | 4.45 | |
| 2 (Gaze, Arrow) × 2 (Directional, Neutral) Interaction | |||||
| Cuneus | R | 16 | −96 | 18 | 4.72 |
| L | −10 | −80 | 24 | 3.18 | |
MNI = Montreal Neurological Institute; ASD = autism spectrum disorders; SPL = superior parietal lobule; Thresholded at Z > 2.3 (p < .01), corrected for multiple comparisons at the cluster level (p < .05).
Figure 2.
Z statistic activation maps of the Directional Gaze > Directional Arrow contrast for each group (corrected for multiple comparisons at the cluster level, p < .05). Color barsindicate Z statistic. Coronal slice shows activity in bilateral putamen and right premotor cortex; sagittal slice shows frontoparietal activity; transverse slice shows activity in SPL and postcentral gyrus.
Direct comparison between groups confirmed greater activity in the TD group than in the ASD group for the directional gaze > directional arrow contrast. Figure 3 displays the results for this group comparison and peak activation coordinates are reported in Table 5a. The TD > ASD analysis demonstrated significant activity in bilateral IFG, and in the right superior temporal gyrus (STG), middle temporal gyrus (MTG), and SMG. The activity along the ventral portion of the STG and the dorsal portion of the MTG correspond to the superior temporal sulcus (STS), a region we expected to differentiate between social and nonsocial cues. There was also significant bilateral putamen activity, extending into the insula. As shown in Figure 3, the interaction was driven by greater activity for the directional gaze than for the directional arrow in the TD group, with the opposite pattern in the ASD group. We do note that we did not report greater activity in the STS for the gaze cue than for the arrow cue in the TD group alone. While this activation did not survive statistical threshold in the within-group analysis, the opposing patterns in the TD and ASD groups allowed it to emerge as significant in the between-group analysis. These interaction effects were qualified by signal increases (vs. baseline) for directional gaze cues and signal decreases for directional arrow cues in the TD group, along with signal decreases for directional gaze cues in the ASD group, in all regions except the STS where the TD group did not show a signal increase for directional gaze cues. The ASD > TD analysis did not yield any significant activity.
Figure 3.
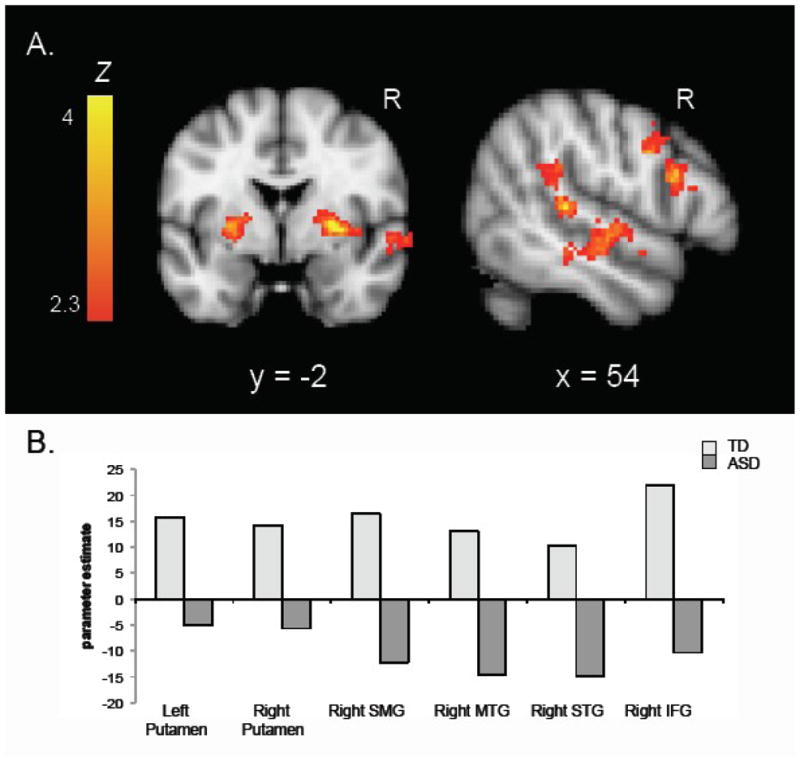
A. Z statistic activation maps of the 2 (TD vs. ASD) × 2 (Directional Gaze vs. Directional Arrow) interaction (corrected for multiple comparisons at the cluster level, p < .05). Color barsindicate Z statistic. Coronal slice shows activity in bilateral putamen and right STS; sagittal slice shows activity in right STS and IFG. B. Parameter estimates for the directional gaze > directional arrow contrast for each group in regions shown in A.
Table 5.
Peak activation coordinates (MNI) for the Group (TD > ASD) interactions
| Anatomical region | Side | x | y | z | Max Z-score |
|---|---|---|---|---|---|
| Gaze directional > Arrow directional | |||||
| IFG | R | 56 | 14 | 22 | 3.76 |
| L | −46 | 32 | 10 | 4.04 | |
| Insula | R | 42 | 2 | 0 | 3.64 |
| MTG | R | 50 | −18 | −12 | 4.50 |
| Putamen | R | 28 | 0 | 2 | 4.36 |
| L | −24 | 2 | 0 | 4.14 | |
| SMG | R | 52 | −36 | 26 | 3.09 |
| STG | R | 54 | −32 | 8 | 3.94 |
| R | 54 | −14 | −6 | 3.26 | |
| 2 (Gaze, Arrow) × 2 (Directional, Neutral) Interaction | |||||
| IFG | L | −52 | 18 | 6 | 3.71 |
| L | −40 | 16 | 22 | 3.95 | |
| MFG | L | −26 | 46 | −4 | 4.59 |
| MTG | L | −46 | −60 | 0 | 3.33 |
| OcG | L | −24 | −88 | −14 | 4.21 |
| Putamen | R | 24 | 4 | −4 | 4.51 |
| L | −26 | 2 | 0 | 4.22 | |
MNI = Montreal Neurological Institute; IFG = inferior frontal gyrus; MFG = middle frontal gyrus; MTG = middle temporal gyrus; OcG = occipital gyrus; SMG = supramarginal gyrus; STG = superior temporal gyrus. Thresholded at Z > 2.3 (p < .01), corrected for multiple comparisons at the cluster level (p < .05), covarying out absolute motion.
In order to account for the visual characteristics of the cues, we then examined the 2 (Gaze vs. Arrow) × 2 (Directional vs. Neutral) interaction, specifically entered as [directional gaze > neutral gaze] > [directional arrow > neutral arrow] for each group. Figure 4 displays the results from this interaction for each group. Peak activation coordinates are reported in Table 3b for the TD group and in Table 4b for the ASD group. The TD group showed significant cortical activity in the right IFG and fusiform gyrus, and in bilateral superior parietal lobule (SPL), postcentral gyrus, cingulate cortex, lateral occipital cortex, cuneus, and lingual gyrus. Again, there was significant activity in bilateral putamen that extended into the insula. This interaction was qualified by a general pattern of signal increases (vs. baseline) for directional gaze cues and signal at or below baseline for all other conditions (neutral gaze, directional arrow, neutral arrow); this was the case for all regions except the cuneus where signal was below baseline for all conditions, albeit not significantly so for directional gaze cues. In the ASD group, a significant interaction effect was only observed in the cuneus; as per the TD group, this interaction effect was qualified by a reduced signal decrease for the directional gaze cue than for the other conditions (as compared to baseline).
Figure 4.
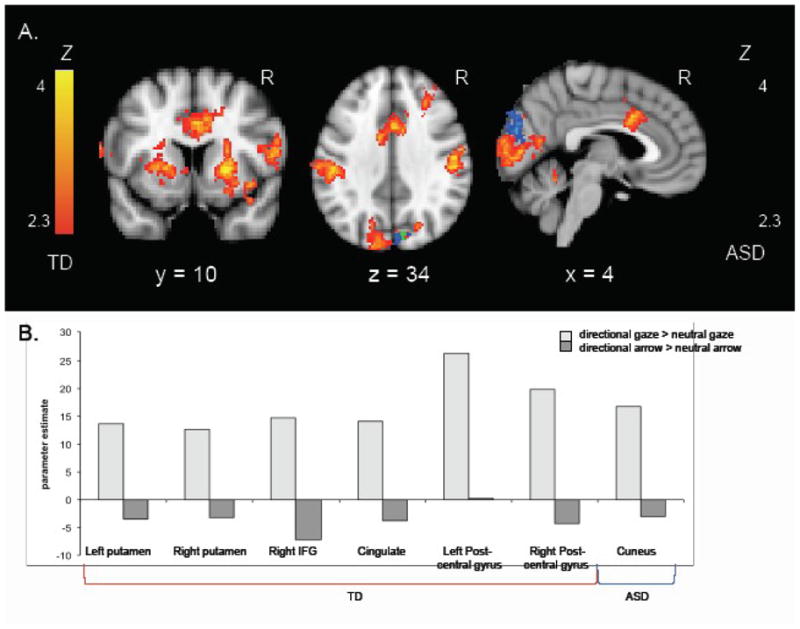
A. Z statistic activation maps of the 2 (Gaze vs. Arrow) × 2 (Directional vs. Neutral) interaction for each group (corrected for multiple comparisons at the cluster level, p < .05). Color barsindicate Z statistic; TD group shown in red-yellow, ASD group shown in blue-light blue, overlap shown in green. Coronal slice shows activity in bilateral putamen, cingulate, and right IFG; transverse slice shows activity in bilateral postcentral gyrus, cuneus, cingulate, and right middle frontal gyrus; sagittal slice shows activity in cingulate and visual cortices. B. Parameter estimates for the directional gaze > neutral gaze and directional arrow > neutral arrow contrasts for regions shown in A.
Direct group comparisons for this interaction effect revealed significant cortical activity in the left IFG, middle frontal gyrus (MFG), MTG, and occipital gyrus, and subcortical activity in bilateral putamen (Table 5b). This reflected greater activity for the directional gaze cue than all other conditions in the TD group and either less activity for the directional gaze cue than the other conditions (IFG, MFG, putamen) or no difference in activity between the directional gaze cue and the other conditions (MTG) in the ASD group, confirming that the three-way interaction was driven by increased activity for social orienting in the TD group that was not present in the ASD group. Since the TD group showed bilateral cortical activity in the within-group analysis, we examined the between-group analysis thresholded at p < .05, corrected for multiple comparisons, and confirmed group differences in bilateral frontoparietal regions as well as in the STS.
When we further explored whether symptom severity in the ASD group modulated activity within regions of the frontoparietal attention network where the TD group showed greater activity for directional gaze cues, we did not find any significant correlations between brain activity and symptom severity as indexed by the ADI social subscale and the ADOS subscales.
4. Discussion
This is the first study to investigate the neural correlates of social orienting in autism using a spatial cueing paradigm. One of the most striking results was that TD and ASD children and adolescents demonstrated similar social orienting behavior in the laboratory task, yet the brain activity underlying that behavior showed clear group differences. The TD group exhibited greater activity for social cues than for nonsocial cues in many regions, while the ASD group showed less distinction that also differed anatomically. These results are consistent with the hypothesis that social cues are not assigned the same privileged status in the autistic brain as in the typically developing brain.
Behaviorally, both TD and ASD children and adolescents demonstrated facilitation effects for gaze and arrow cues with no differences between groups. This is consistent with previous studies conducted in laboratory settings (Chawarska et al., 2003; Kylliainen and Hietanen, 2004; Senju et al., 2004; Swettenham et al., 2003), displaying “normal” orienting responses by social and nonsocial cues in ASD. Additionally, both groups were faster for gaze-cued trials than for arrow-cued trials, suggesting a comparable distinction between social and nonsocial cues between groups. However, the similar orienting behavior did not translate into similar brain activity in the two groups. Rather, the TD group showed extensive differences in neural activity for social and nonsocial cues, while the ASD group only showed differences in one parietal region and lower-level visual regions.
The TD group demonstrated greater brain activity in several regions for social cues than for nonsocial cues, supporting the idea that social cues have a special status in the neurotypical brain. Given the behavioral result that participants were faster for gaze-cued trials than for arrow-cued trials, one might argue that differences in brain activity simply reflect differences in reaction time. However, brain activity typically increases for conditions with slower reaction times, whereas our participants showed increased activity when reaction times were faster (gaze cue condition). Specifically, gaze cues elicited more activity in frontoparietal regions, including the IFG, premotor cortex, SPL, and SMG, which have been identified as part of a network for orienting attention. The posterior parietal cortex (PPC), which encompasses the SPL and SMG, and ventral prefrontal regions are consistently implicated in disorders of spatial attention (Bisiach et al., 1981; Heilman et al., 2003; Mesulam, 1999) and are reliably activated in neuroimaging studies of spatial orienting (for a review, see Corbetta and Shulman, 2002). Additional frontoparietal activity was found in the cingulate cortex and postcentral gyrus. The anterior cingulate cortex is reliably activated in studies of executive attention (Bush et al., 2000), and both precentral and postcentral gyri have been shown to activate during target response in spatial orienting tasks like the one used here (Hopfinger et al., 2000). Thus, our data show that TD children and adolescents engage several frontoparietal attention regions to a greater extent when their attention is cued by a social stimulus compared to a nonsocial stimulus.
While previous fMRI studies in adults also found greater activity for social cues than for nonsocial cues, such activity was not reported in the PPC, and only Engell et al. (2010) reported a cluster in the IFG. Further, Hietanen et al. (2006), whose paradigm was most similar to ours, reported greater frontoparietal activity for arrow cues than for gaze cues. They concluded that gaze cues are more automatic than arrow cues based on previous neuroimaging data showing larger extents of activation for controlled (endogenous, top-down) than for automatic (exogenous, bottom-up) orienting (Kim et al., 1999; Rosen et al., 1999). Not only did we find greater frontoparietal activity for gaze cues than for arrow cues, but we also did not find any regions that were more active for arrow cues than for gaze cues. Thus, the different patterns of results in our study and in previous studies of adults may suggest that children are still developing automaticity for orienting attention in response to social stimuli. Post-hoc regression analyses with age in the TD group lend some support to this notion, as we found that activity for gaze cueing within the right SMG decreased as age increased (r = −.46, p = .036).
The TD group also showed greater activity in visual processing areas for gaze cues, notably in the right fusiform gyrus and in the lateral occipital cortex (LOC). It is not surprising that regions involved in visual processing of social stimuli showed more activity for gaze cues than for arrows cues. However, our results did not show stronger activation in these regions for all gaze cues compared to all arrow cues. Rather, directional gaze cues elicited more activity than all other conditions, including neutral gaze cues. Previous neuroimaging data have shown that the fusiform gyrus is not only reliably activated by face perception, but is also modulated by selective attention (Hoffman and Haxby, 2000; Hooker et al., 2003). Thus, it is possible that even though our participants knew that the cues did not predict the location of the target and were instructed to ignore the direction of the cues, they paid more attention to the directional gaze cues, resulting in increased fusiform activity. Likewise, the LOC showed increased activity for directional gaze cues compared to all other condition. This is consistent with previous neuroimaging studies of social orienting in adults that reported greater LOC activity for social cues than for nonsocial cues (Engell et al., 2010; Greene et al., 2009; Tipper et al., 2008). Our data extend these findings to developing populations, and confirm that LOC activity was not simply driven by visual processing of social stimuli.
The putamen also showed robust bilateral activation for directional gaze cues compared to the other cue conditions. This finding was unexpected, but suggests a role for the striatum in social orienting during typical development. It is well known that the putamen lies at the center of motor circuitry, with anatomical connections to cortical motor and somatosensory regions (for a review, see Alexander et al., 1986). Interestingly, it has been shown that cells in the putamen may respond when a stimulus is important to behavior, but not when the behavioral significance is removed (Evarts et al., 1984). Thus, it is possible that directional gaze cues were interpreted as more behaviorally significant than the other cue conditions. The clusters of activity in the putamen also extended into the insula, consistent with previous studies that found activation in the putamen/insula region for spatial cueing tasks (Gitelman et al., 1999; Hopfinger et al., 2000). Further research is necessary to understand the link between the striatum/insula regions and social orienting and whether this relationship is only observed during development.
The ASD group showed much less difference in brain activity between gaze cues and arrow cues than the TD group, with differential responses for social and nonsocial cues only observed in the SPL and visual association cortices. One explanation for this result is that the ASD group paid less attention to the task than the TD group or did not look at the cues as much. However, we found no differences in the behavioral effects between groups, and we were able to verify that both groups looked at the center of the screen (where the cue appeared) throughout the task. Another possibility is that individuals with ASD relied upon visual analysis of gaze direction rather than automatically making use of eye gaze due to its inherent social significance. Further, the observed SPL activity may reflect recruitment of top-down attentional resources, as this region is considered part of the dorsal frontoparietal attention network that supports controlled, endogenous shifts in attention (Corbetta and Shulman, 2002).
Direct comparison of brain activity between groups verified differences in activity in several regions when attention was directed by gaze cues vs. arrow cues in TD as compared to ASD. Interestingly, these interactions revealed an opposite pattern of responses in the STS between groups, such that TD children showed decreased activity for arrow cues whereas children with ASD showed decreased activity for gaze cues. The STS is reliably involved in perception of biological stimuli, particularly eye gaze (Puce and Perrett, 2003). Moreover, Pelphrey and Carter (2008) have suggested that the STS may also be involved in utilizing eye gaze to understand the intentions of others. Here we demonstrate that the STS may also play a role in utilizing social cues to orient attention during typical development. In ASD, however, the STS may not be sensitive to the social meaning conveyed by eye-gaze (e.g., Pelphrey et al., 2005), consistent with our results showing that the ASD group treated social cues similarly to the way in which the TD group treated nonsocial cues.
Taken together, our findings indicate that the ASD brain does not distinguish between social and nonsocial cues in the same way as the TD brain, relying upon different strategies to arrive at similar behavior. While this study did not examine the developmental trajectory of gaze processing, it is possible that high-functioning children and adolescents with ASD may have learned that gaze direction conveys meaning about the surrounding environment. Hence, typical behavior may be achieved through more ‘effortful’ orienting responses based on this acquired knowledge. To this end, our results could help explain why most prior laboratory experiments reported intact social orienting behavior in ASD. While individuals with ASD may be able to utilize lower-level physical properties of eye gaze and thus direct their attention in a controlled setting, they do not assign special social significance to such stimuli. As suggested by Nation and Penny (2008), individuals with ASD engage nonsocial mechanisms to process social cues. In more complex naturalistic paradigms as well as in daily life, these nonsocial mechanisms may not be otherwise engaged or may not function as efficiently, resulting in altered social orienting. Face and gaze processing impairments in ASD could originate from dysfunction in the basic neural system for face processing, or from abnormal development of that system due to lack of experience with faces (for a review, see Dawson et al., 2002). Either way, early social orienting deficits can directly impact the ability to establish joint attention, which is notably impaired in autism, leading to a cascade of negative consequences for subsequent development (Dawson et al., 2002; Mundy et al., 1990; Mundy et al., 1986; Sheinkopf, 2005).
5. Conclusions
In sum, the present findings have a number of important implications. First, they help reconcile some of the discrepant findings in the literature, highlighting the need to develop more ecologically valid paradigms to study social orienting in the laboratory. Second, they add to a growing body of work (e.g., Wang et al., 2007) showing significant abnormalities in the autistic brain, even in the presence of intact behavioral performance. These observations indicate that inferences on the integrity of any brain system based solely on behavioral data can be misguided. Lastly, our findings may also have some applied implications as they could ultimately inform the development of new interventions designed to foster attention to social cues in noisier contexts that more closely approximate real life circumstances.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development [P50 HD055784] and Autism Speaks [4854 to D.J.G.]. For generous support the authors also wish to thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, Ahmanson Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Northstar Fund, and the FPR-UCLA Center for Culture, Brain, and Development. This project was in part also supported by grants (RR12169, RR13642 and RR00865) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCR or NIH.
Footnotes
Error rates did not differ between groups: ASD = 2.2% ASD, TD = 2.4%.
IQ was lower in the ASD group than the TD group despite no significant statistical group difference as well (p = .114). However, when we included absolute motion as a covariate, the difference in IQ became even less significant (p = .213), voiding the need to add IQ as a covariate in the fMRI analyses as well.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Capitani E, Luzzatti C, Perani D. Brain and conscious representation of outside reality. Neuropsychologia. 1981;19:543–551. doi: 10.1016/0028-3932(81)90020-8. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Volkmar F. Automatic attention cueing through eye movement in 2-year-old children with autism. Child Dev. 2003;74:1108–1122. doi: 10.1111/1467-8624.00595. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, Williams SC, Van Amelsvoort T, Robertson DM, Rowe A, Phillips M, McAlonan G, Howlin P, Murphy DG. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123 (Pt 11):2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, Davidson RJ. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. J Autism Dev Disord. 1998;28:479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb S, Schellenberg GD, Dager S, Friedman S, Aylward E, Richards T. Defining the broader phenotype of autism: genetic, brain, and behavioral perspectives. Dev Psychopathol. 2002;14:581–611. doi: 10.1017/s0954579402003103. [DOI] [PubMed] [Google Scholar]
- Driver J, Davis G, Ricciardelli P, Kidd P, Maxwell E, Baron-Cohen S. Gaze perception triggers reflexive visuospatial orienting. Visual Cognition. 1999;6:509–540. [Google Scholar]
- Engell AD, Nummenmaa L, Oosterhof NN, Henson RN, Haxby JV, Calder AJ. Differential activation of frontoparietal attention networks by social and symbolic spatial cues. Soc Cogn Affect Neurosci. 2010 doi: 10.1093/scan/nsq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts EV, Kimura M, Wurtz RH, Hikosaka O. Behavioral correlates of activity in basal ganglia neurons. Trends in Neurosciences. 1984;7:447–453. [Google Scholar]
- Friesen CK, Kingstone A. The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychonomic Bulletin & Review. 1998;5:490–495. [Google Scholar]
- Friesen CK, Moore C, Kingstone A. Does gaze direction really trigger a reflexive shift of spatial attention? Brain and Cognition. 2005;57:66–69. doi: 10.1016/j.bandc.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Gervais H, Belin P, Boddaert N, Leboyer M, Coez A, Sfaello I, Barthelemy C, Brunelle F, Samson Y, Zilbovicius M. Abnormal cortical voice processing in autism. Nat Neurosci. 2004;7:801–802. doi: 10.1038/nn1291. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, Mesulam M. A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999;122 ( Pt 6):1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Goldberg MC, Mostow AJ, Vecera SP, Larson JC, Mostofsky SH, Mahone EM, Denckla MB. Evidence for impairments in using static line drawings of eye gaze cues to orient visual-spatial attention in children with high functioning autism. J Autism Dev Disord. 2008;38:1405–1413. doi: 10.1007/s10803-007-0506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DJ, Mooshagian E, Kaplan JT, Zaidel E, Iacoboni M. The neural correlates of social attention: automatic orienting to social and nonsocial cues. Psychol Res. 2009;73:499–511. doi: 10.1007/s00426-009-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Chabris CF, Clark J, Steele S, McGrath L, Vangel M, Aharon I, Feczko E, Harris GJ, Tager-Flusberg H. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage. 2004;22:1141–1150. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Hamilton AF, Brindley RM, Frith U. Imitation and action understanding in autistic spectrum disorders: how valid is the hypothesis of a deficit in the mirror neuron system? Neuropsychologia. 2007;45:1859–1868. doi: 10.1016/j.neuropsychologia.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Watson RT, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. Oxford University Press; New York, NY: 2003. pp. 296–346. [Google Scholar]
- Hietanen JK, Nummenmaa L, Nyman MJ, Parkkola R, Hamalainen H. Automatic attention orienting by social and symbolic cues activates different neural networks: an fMRI study. Neuroimage. 2006;33:406–413. doi: 10.1016/j.neuroimage.2006.06.048. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat Neurosci. 2000;3:80–84. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Paller KA, Gitelman DR, Parrish TB, Mesulam MM, Reber PJ. Brain networks for analyzing eye gaze. Brain Res Cogn Brain Res. 2003;17:406–418. doi: 10.1016/s0926-6410(03)00143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Kim YH, Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Mesulam MM. The large-scale neural network for spatial attention displays multifunctional overlap but differential asymmetry. Neuroimage. 1999;9:269–277. doi: 10.1006/nimg.1999.0408. [DOI] [PubMed] [Google Scholar]
- Kingstone A, Tipper C, Ristic J, Ngan E. The eyes have it!: an fMRI investigation. Brain Cogn. 2004;55:269–271. doi: 10.1016/j.bandc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- Kylliainen A, Hietanen JK. Attention orienting by another’s gaze direction in children with autism. J Child Psychol Psychiatry. 2004;45:435–444. doi: 10.1111/j.1469-7610.2004.00235.x. [DOI] [PubMed] [Google Scholar]
- Langton SR, Watt RJ, Bruce II. Do the eyes have it? Cues to the direction of social attention. Trends Cogn Sci. 2000;4:50–59. doi: 10.1016/s1364-6613(99)01436-9. [DOI] [PubMed] [Google Scholar]
- Leekam SR, Lopez B, Moore C. Attention and joint attention in preschool children with autism. Dev Psychol. 2000;36:261–273. doi: 10.1037//0012-1649.36.2.261. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EHJ, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule--Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview--Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi MW, Mack PB, McCarthy G, Pelphrey KA. Taking an “intentional stance” on eye-gaze shifts: a functional neuroimaging study of social perception in children. Neuroimage. 2005;27:247–252. doi: 10.1016/j.neuroimage.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Kasari C. A longitudinal study of joint attention and language development in autistic children. J Autism Dev Disord. 1990;20:115–128. doi: 10.1007/BF02206861. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Ungerer J, Sherman T. Defining the social deficits of autism: the contribution of non-verbal communication measures. J Child Psychol Psychiatry. 1986;27:657–669. doi: 10.1111/j.1469-7610.1986.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Nation K, Penny S. Sensitivity to eye gaze in autism: is it normal? Is it automatic? Is it social? Dev Psychopathol. 2008;20:79–97. doi: 10.1017/S0954579408000047. [DOI] [PubMed] [Google Scholar]
- Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. J Autism Dev Disord. 1994;24:247–257. doi: 10.1007/BF02172225. [DOI] [PubMed] [Google Scholar]
- Osterling JA, Dawson G, Munson JA. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Dev Psychopathol. 2002;14:239–251. doi: 10.1017/s0954579402002031. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Carter EJ. Brain mechanisms for social perception: lessons from autism and typical development. Ann N Y Acad Sci. 2008;1145:283–299. doi: 10.1196/annals.1416.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Singerman JD, Allison T, McCarthy G. Brain activation evoked by perception of gaze shifts: the influence of context. Neuropsychologia. 2003;41:156–170. doi: 10.1016/s0028-3932(02)00146-x. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Puce A, Perrett D. Electrophysiology and brain imaging of biological motion. Philos Trans R Soc Lond B Biol Sci. 2003;358:435–445. doi: 10.1098/rstb.2002.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic J, Friesen CK, Kingstone A. Are eyes special? It depends on how you look at it. Psychonomic Bulletin & Review. 2002;9:507–513. doi: 10.3758/bf03196306. [DOI] [PubMed] [Google Scholar]
- Ristic J, Mottron L, Friesen CK, Iarocci G, Burack JA, Kingstone A. Eyes are special but not for everyone: the case of autism. Brain Res Cogn Brain Res. 2005;24:715–718. doi: 10.1016/j.cogbrainres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Rao SM, Caffarra P, Scaglioni A, Bobholz JA, Woodley SJ, Hammeke TA, Cunningham JM, Prieto TE, Binder JR. Neural basis of endogenous and exogenous spatial orienting. A functional MRI study. J Cogn Neurosci. 1999;11:135–152. doi: 10.1162/089892999563283. [DOI] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Uono S, Yoshikawa S. Commonalities in the neural mechanisms underlying automatic attentional shifts by gaze, gestures, and symbols. Neuroimage. 2009;45:984–992. doi: 10.1016/j.neuroimage.2008.12.052. [DOI] [PubMed] [Google Scholar]
- Senju A, Tojo Y, Dairoku H, Hasegawa T. Reflexive orienting in response to eye gaze and an arrow in children with and without autism. J Child Psychol Psychiatry. 2004;45:445–458. doi: 10.1111/j.1469-7610.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- Sheinkopf SJ. Hot topics in autism: cognitive deficits, cognitive style, and joint attention dysfunction. Med Health R I. 2005;88:152–153. 157–158. [PubMed] [Google Scholar]
- Swettenham J, Condie S, Campbell R, Milne E, Coleman M. Does the perception of moving eyes trigger reflexive visual orienting in autism? Philos Trans R Soc Lond B Biol Sci. 2003;358:325–334. doi: 10.1098/rstb.2002.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper CM, Handy TC, Giesbrecht B, Kingstone AF. Brain Responses to Biological Relevance. J Cogn Neurosci. 2008;20:879–891. doi: 10.1162/jocn.2008.20510. [DOI] [PubMed] [Google Scholar]
- Vlamings PH, Stauder JE, van Son IA, Mottron L. Atypical visual orienting to gaze- and arrow-cues in adults with high functioning autism. J Autism Dev Disord. 2005;35:267–277. doi: 10.1007/s10803-005-3289-y. [DOI] [PubMed] [Google Scholar]
- Wang AT, Dapretto M, Hariri AR, Sigman M, Bookheimer SY. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Wang AT, Lee SS, Sigman M, Dapretto M. Reading affect in the face and voice: neural correlates of interpreting communicative intent in children and adolescents with autism spectrum disorders. Arch Gen Psychiatry. 2007;64:698–708. doi: 10.1001/archpsyc.64.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children. 3. The Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Werner E, Dawson G, Osterling J, Dinno N. Brief report: Recognition of autism spectrum disorder before one year of age: a retrospective study based on home videotapes. J Autism Dev Disord. 2000;30:157–162. doi: 10.1023/a:1005463707029. [DOI] [PubMed] [Google Scholar]
- Woolrich M. Robust group analysis using outlier inference. Neuroimage. 2008;41:286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. OUP; 2001. [Google Scholar]