Abstract
We have attributed organ engraftment to clonal exhaustion-deletion of host-versus-graft and graft-versus-host reactions that are reciprocally induced and governed by migratory donor and recipient leukocytes. The so-called donor passenger leukocytes that migrate from the allograft into the recipients have been thoroughly studied (chimerism), but not the donor leukocytes that remain in, or return to, the transplanted organ. Therefore, using flow cytometry we determined the percentage and lineages of donor leukocytes in cell suspensions prepared from Lewis (LEW) cardiac allografts to 100 days posttransplantation. The LEW hearts were transplanted to naïve untreated Brown Norway (BN) recipients (group 2), to naïve BN recipients treated with a 28-day or continuous course of tacrolimus (TAC) (groups 3 and 4), and to drug-free BN recipients pretolerized by earlier bone marrow cell (BMC) or orthotopic LEW liver transplantation (groups 5 and 6). The findings in the heart cell suspensions were correlated with the results from parallel histopathologic-immunocytochemical studies and other studies of the grafts and of host tissues. Although the LEW heart allografts were rejected in 9.6 days by the unmodified recipients of group 2, all beat for 100 days in the recipients of groups 3 through 6. Nevertheless, all of the long-surviving cardiac allografts (but not the isografts in group 1) were the targets of an immune reaction at 5 days, reflected by dramatic increases in the ratio of leukocytes to nonleukocyte nucleated cells from normal values of 1:5–1:6 to 1:1–5:1 and by manifold other evidence of a major inflammatory event. The acute changes returned to baseline by 100 days in the chronic rejection (CR) free hearts of groups 4 and 6, but not in the CR-afflicted hearts of short-course TAC group 3 or the less-severely damaged hearts of the BMC-prime group 5. The freedom from CR in groups 4 and 6 was associated with a large donor contribution to the intracardiac leukocyte population at 5 days (28.6% and 22% in the respective groups) and at 100 days (30.5% in group 4 and 8.4% in group 6) compared with 2% and 1.2% at 100 days in the CR-blighted allografts of the partially tolerant animals of groups 3 and 5. Whether large or small, the donor leukocyte fraction always included a subset of class II leukocytes that had histopathologic features of dendritic cells. These class II+ cells were of mixed myeloid (CD11-b/c+) and lymphoid lineages; their migration was markedly inhibited by TAC and accelerated by donor-specific priming and TAC discontinuance. Although a large donor leukocyte population and a normal leukocyte/nonleukocyte cell ratio were associated with freedom from CR, these findings and the lineage profile of the intracardiac leukocytes were not associated with tolerance in the animals of groups 3 and 4 under active TAC treatment. The findings in this study, singly and in their entirety, are compatible with our previously proposed leukocyte migration-localization paradigm of organ allograft acceptance and tolerance.
The adaptive host-versus-graft (HVG) immune response to an allograft is analogous to the immune response to intracellular noncytopathic microorganisms1–4 with more complex consequences because the mobile immunocompetent passenger leukocytes of an organ mount a contemporaneous graft-versus-host (GVH) reaction.4–7 We have proposed that the HVG and GVH reactions, each driven by the immunogenic cells of the opposing leukocyte population, are regulated by the migration and localization of the respective leukocytes.4 In this view, transplantation immunology is incomplete without accurate knowledge of the routes of migration, destination, and lineages of both populations of mobile leukocytes.
The traffic of different lineages of donor cells from the graft to the lymphoid organs of the host and the heterogenous persistence of these cells (i.e., microchimerism) in nonlymphoid as well as lymphoid areas have been extensively documented in experimental studies of organ allograft acceptance and tolerance.8–12 Conversely, investigations of leukocytes in the transplanted organ have been concerned almost exclusively with the host-infiltrating lymphocytes and other host-effector leukocytes.
The present study provides information about the proportions of donor and recipient leukocytes and their lineages in rat heart allografts transplanted to untreated or immunosuppressed naïve recipients and nonimmunosuppressed recipients that had been rendered partially or completely tolerant by the previous transplantation of donor-strain bone marrow cells (BMCs) or an orthotopic liver. Particular attention was focused on the leukocytes of myeloid precursor origin (i.e., nonlymphoid cells) that classically have been associated with rejection13,14 and, more recently, with tolerance.5–11, 15 The results allow a better understanding of the interactions of the existing donor and recipient immunocyte populations, how transplantation tolerance is acquired, and especially the pathogenesis of chronic rejection (CR).
Materials and Methods
Animals and Surgical Procedures
Male Brown Norway (BN, RT1n) and Lewis (LEW, RT1l) rats, which differ at class I and II major histocompatibility complex (MHC) and minor histocompatibility complex (mHC) loci, were purchased from Harlan Sprague Dawley Inc (Indianapolis, IN) and kept in a specific pathogen-free animal facility. Maintenance of the animals and performance of all experiments conformed to the guidelines of the Council of Animal Care at the University of Pittsburgh (Pittsburgh, PA). Hearts from 200- to 250-g naïve LEW donors were transplanted to the abdominal site of 250- to 300-g BN recipients or isograft controls (group 1) to LEW recipients (Table 1). The methods of LEW→BN orthotopic liver transplantation and LEW BMC preparation and infusion into BN recipients were as previously reported.11,16
Table 1.
Experimental Groups and Controls
| Group | Recipient Priming | Post-Heart TX Immunosuppression |
Previously Reported Effects9,11,16 |
|---|---|---|---|
| 0. Normal LEW heart | None | None | Not applicable |
| 1. Isograft control (LEW → LEW) | None | None | None |
| 2. No treatment | None | None | Acute irreversible rejection at 9 days |
| 3. Short TAC | None | Short-course TAC days 0–28* | CR associated with decline of microchimerism |
| 4. Long TAC | None | Continuous TAC days 0–100† | Freedom from acute rejection and CR associated with stable microchimerism |
| 5. BMC prime | 2.5 × 108 LEW BMCs day −100; TAC days −100 to −87, and days −80 and −73‡ | None | Partial drug-independent tolerance but subtle CR less severe than in group 3, associated with decline of chimerism |
| 6. Liver TX prime | LEW orthotopic liver on day −100; same TAC therapy as group 5‡ | None | Complete drug-free tolerance associated with robust microchimerism |
NOTE. Heart transplantations were on day 0.
Abbreviation: TX, transplantation.
1.0 mg/kg/d of TAC days 0–13, 20, and 27.
1.0 mg/kg/d of TAC days 0–13 and once weekly thereafter until animals were killed at 100 days.
1.0 mg/kg/d of TAC days −100 to −87 and additional doses on days −80 and −73. Animals were drug free for 73 days before test heart transplantation.
Experimental Design
Normal LEW hearts, isografts (group 1), and allografts were studied under the conditions listed in Table 1. Naïve BN allograft recipients either were not treated after transplantation (group 2) or were immunosuppressed with tacrolimus (TAC; Fujisawa Pharmaceutical Co Ltd, Osaka, Japan) for 28 days only (group 3) or for the 100 days’ duration of the experiments (group 4).
Other BN recipients were preconditioned 100 days in advance of the cardiac transplantations with an infusion of 2.5 × 108 LEW BMCs (group 5) or with the orthotopic transplantation of a LEW liver (group 6). Beginning on the day of the priming BMCs or liver transplantations, the animals were treated with the same 28-day course of TAC used for the naïve heart recipients of group 3. At the time of the challenge heart transplantations, these animals had been TAC-free for 73 days and had no trace of TAC in their blood.
Heart-cell suspensions
The percentage of leukocytes (CD45+ cells) relative to the number of cardiac myocytes and other nonleukocyte (CD45−) cells was determined by flow cytometry of cell suspensions prepared from normal and transplanted LEW hearts. The control CD45+/CD45− ratio (1:4 to 1:5 in normal LEW hearts and isografts [group 1]) was compared with the ratios in cell suspensions of allografts (groups 2 through 6) removed when the animals were killed 5 and 100 days after transplantation under the experimental conditions listed in Table 1. The donor and recipient proportions of the total CD45+ population and of the myeloid subpopulations were directly determined. The CD45+ leukocytes not stained with the myeloid lineage–specific monoclonal antibody (mAb; Table 2) were assumed to be mostly lymphoid cells.
Table 2.
Panel of Monoclonal Antibodies Used in This Study
| Clone | Specificity | Isotype | Concentration | Supplier |
|---|---|---|---|---|
| Lineages | ||||
| OX1 | CD45, leukocyte common antigen | Mouse IgG1, κ | 1:200 | PharMingen* |
| OX42 | CD11b/c, myeloid cells | Mouse IgG2a, κ | 1:100 | PharMingen |
| ED1 | CD68, monocytes/infiltrative macrophages | Mouse IgG1 | 1:100 | Serotec† |
| ED2 | Tissue macrophages | Mouse IgG1 | 1:100 | Serotec |
| Costimulatory molecules | ||||
| 3H5 | CD80, B7-1 | Mouse IgG1, κ | 1:100 | PharMingen |
| 24F | CD86, B7-2 | Mouse IgG1, κ | 1:100 | PharMingen |
| MHC antigens | ||||
| 163 | Class I MHC on LEW (RT1.Al) | Rat IgG2b | 1:400 | Dr H.W. Kunz‡ |
| 42 | Class I MHC on BN (RT1.An) | Rat IgG2a | 1:500 | Dr H.W. Kunz |
| L21-6 | Class II MHC on LEW | Mouse IgG1 | 1:1,200 | Dr Y. Iwaki‡ |
Abbreviation: IgG, immunoglobulin G.
San Diego, CA.
Kidlington, Oxford, UK.
University of Pittsburgh, Pittsburgh, PA.
Intact tissues
In separate experiments, tissue sections of the transplanted hearts and host spleens were studied with immunohistochemistry (IHC). For these studies, 3 to 6 animals in all 6 experimental groups were killed 1, 3, 5, and 9 to 10 days after heart transplantation. In groups 1 and 3 through 6, in which all hearts continued to beat for the variable periods of observation before the animals were killed, recipients also were killed at 15, 30, and 100 days. Portions of the heart grafts and recipient spleens (and in group 6, of the priming liver allografts) were fixed in formalin for routine histopathologic examination, placed in optimal cold temperature (OCT) compound, and snap-frozen for messenger RNA (mRNA) extraction.
Cell Isolation From Heart Grafts
At the time the animals were killed, the hearts were perfused in situ through the aorta with 20 mL of Hank’s balanced salt solution followed by 20 mL of RPMI-1640 containing 0.05% collagenase (Type B; Boehringer Mannheim, Mannheim, Germany), 0.003% DNase (type IV; Sigma, St Louis, MO), 10 mmol/L of N-2-hydroxyethylpiperazine-propanesulfonic acid (HEPES), 1 mmol/L of l-glutamine, 50 µg/mL of gentamicin, and 5% fetal bovine serum (all from Life Technologies, Grand Island, NY).
The bloodless hearts were cut into small pieces, digested in a water bath for 60 minutes at 37°C, and then passed through nylon mesh. Isolated cells were washed twice with RPMI-1640 and further purified by centrifugation over Ficoll-Paque (specific gravity, 1.077; Biotech AB, Uppsala, Sweden).
Flow Cytometry
After the Ficoll-Paque centrifugation, the heart-cell suspensions were analyzed with 3-color flow cytometry, using the mAbs listed in Table 2. Normal splenocytes were used in each experiment to confirm the accuracy of staining of the mAbs.
Total leukocytes
Phycoerythrin-conjugated OX1 (CD45) was used to distinguish hematolymphopoietic cells from parenchymal cells.
Donor and recipient phenotypes
This distinction was made with affinity-purified biotinylated mAb 163 and mAb 42, which are specific for the RT1.A1 antigen (LEW MHC class I) and the RT1.An antigen (BN MHC class I), respectively.17 Cy-chrome–conjugated streptavidin (PharMingen, San Diego, CA) was used as a secondary antibody. In addition, donor MHC class II+ cells were identified by mAb L21-6, which is specific for MHC class II antigens on LEW, but not BN, cells.18
Nonlymphoid leukocytes
The subset of CD45+ leukocytes expressing CD11b/c (myeloid precursor–derived cells) was delineated by staining with OX42 antibody. OX42 is a marker for leukocyte integrin Mac-1 (CD11b, CD18) and p150.95 (CD11c, CD18) on macrophages, monocytes, dendritic cells (DCs), and granulocytes.
Macrophages
ED1 mAb was used to identify cells of the mononuclear/phagocyte system.19,20 Tissue macrophages were identified with mAb ED2,19,21 the specificity of which is similar to mAb BMAC-5.22
IHC
Frozen samples in OCT compound were sectioned at 4 µm and stained with a routine indirect avidin-biotin complex method, as previously described.23 Sequential changes in the numbers and location of class II+ donor cells were determined with the L21-6 mAb.18 The number of positively stained cells was determined by blindly counting the number of labeled cells in 20 high-power fields (HPFs)/section (original magnification ×400). When the section contained more than 5 L21-6+ cells/HPF, double labeling was performed with other mAbs that included 3H5 (B7-1, CD80), 24F (B7-2, CD86), ED1, and ED2 (Table 2). Isotype-matched nonimmune antibody was used for negative control for each assay.
Programmed Cell Death
Apoptosis
After formalin-fixed sections were deparaffinized, the slides were treated with proteinase K (40 mg/mL; Sigma) for 15 minutes at 37°C. Apoptosis was quantitated with the terminal deoxynucleotidyl transferase–mediated biotin deoxyuridine triphosphase nick end labeling (TUNEL) method using Apop Tag kit (Intergen, Purchase, NY). Positively stained cells were counted according to the procedure used in IHC (previously discussed).
Caspase-3–like activity
A frozen portion of the heart allograft was used to determine caspase-3–like activity. A portion of the heart allograft was homogenized in 10 mmol/L of HEPES (pH 7.4) containing the protease inhibitors (0.5 mmol/L of phenylmethylsufonyl fluoride, 5 µg/mL of aprotinin, 5 µg/mL of pepstatin, and 10 µg/mL of leupeptin). Crude cytosol was obtained after centrifugation at 12,000g for 20 minutes at 4°C. The enzyme reaction mixture contained 200 µg of cytosolic protein and 200 µmol/L of AC-DEVD-pNA in 150 µL of reaction buffer (100 mmol/L of HEPES, 20% glycerol, 5 mmol/L of dithiothreitol [DTT] and protease inhibitors). The enzyme reaction was initiated by adding the substrate to a 96-well plate containing the enzyme solution at 37°C. The caspase-3–like activity was calculated from the initial velocity by measuring the increased absorbency at 405 nm every 15 minutes. The reaction mixture without enzyme or substrate was used as a control.
Ribonuclease Protection Assay of Cytokines
Total RNA was extracted from frozen samples by using TRIZOL Reagent (Life Technologies) according to the manufacturer’s instructions. The concentration of RNA was determined by UV spectrophotometer at 260 nm. The ribonuclease (RNase) protection assay (RPA) was performed by using commercially available kits and following the manufacturer’s instructions (all from PharMingen).
Radiolabeled antisense RNA multiple probes were synthesized using an in vitro transcription kit and rat cytokine multiprobe template set (rCK-1), which included probes for cytokines (interleukin-1α [IL-1α], IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, tumor necrosis factor-α [TNF-α], TNF-β, and interferon-γ) and housekeeping genes (L32 and GAPDH). 32P-labeled probes (8.0 × 105 cpm) and sample RNA (5 µg) were hybridized at 56°C for 12 to 16 hours, and single-stranded RNAs including antisense RNA probes were digested. The protected RNA duplexes were isolated by RNase inactivation/precipitation solution (Ambion Inc, Austin, TX) and electrophoresed in a standard sequencing gel. Dried gels were exposed to Storage Phosphor Screen (Molecular Dynamics, Sunnyvale, CA) for 12 hours at room temperature, and the radioactivity of each band was measured by β-scan (PhosphorImager; Molecular Dynamics) and NIH Image (download from http://rsb.info.nih.gov/nih-image/). The results were normalized to GAPDH and expressed as ratios of cytokine-GAPDH.
Mixed Lymphocyte Reactions
In vitro antidonor reactivity of the primed BN recipients was assayed with 1-way mixed leukocyte reactions. Responder cervical lymph node lymphocytes were obtained just before challenge heart transplantation from the BN rats primed 100 days previously with LEW BMCs (group 5) or LEW liver grafts (group 6) and from naïve BN rats (control). Lymph node lymphocytes from naïve BN (recipient strain), LEW (donor strain), and ACI (third-party) rats were used as stimulator cells. Technical details have been reported.11 After determining thymidine uptake with a liquid scintillation counter (Wallace Inc, Gaithersberg, MD), the stimulation index (SI) was calculated as follows:
Statistical Analysis
Results of flow cytometry, IHC, and RPA are presented as mean ± SD. Data were analyzed by 1-way analysis of variance (ANOVA), Fisher’s PLSD test, and Student’s t-test. Statistical significance is established at P less than .05.
Results
Graft Outcome
Naïve nontreated recipients
Although the LEW isografts (group 1) had indefinite survival, the LEW cardiac allografts (group 2) were irreversibly rejected with cessation of heartbeat after 9.6 ± 1.3 days (n = 7).
Immunosuppressed or primed recipients
None of the allografts in groups 3 through 6 was lost to rejection. At the time the animals were killed, there were no discernible differences between groups in the gross appearance or estimated strength of heartbeat. CR was undetectable by histopathologic examination of the transplanted hearts of continuous-TAC-treatment group 4 and liver-primed group 6 (Table 1).
With microscopic examination of tissue sections, the presence of CR in the hearts of short-course-TAC group 3 and BMC-primed group 5 was obvious with evaluations recorded9,11,16 without knowledge of the experimental groups. The findings of CR included obliterative arteriopathy and multiple perivascular and subendocardial lymphocytic infiltrates similar to the “Quilty” lesions seen in human cardiac allografts.16 They were the most advanced in the cardiac allografts of short-course-TAC group 3.
Flow Cytometry of Heart-Cell Suspensions
CD45+ cells
Between one fourth and one fifth of the nucleated cells in suspensions prepared from normal LEW hearts or 5-day LEW isografts stained with the pan leukocyte OX1 mAb (Table 3). The leukocyte fraction increased 4-fold by 5 days (to >80%) in allografts transplanted to untreated (group 2) or BMC-primed BN recipients (group 5), and it doubled when the transplantations were to TAC-treated (groups 3 and 4) or liver-primed recipients (group 6; Table 3).
Table 3.
Leukocyte (CD45+ Cell) Percentage of Contribution to Total Nucleated Cells in Suspensions Prepared From LEW Hearts 5 and 100 Days After Isotransplantation or Allotransplantation
| Group | No. | Day 5: %CD45+ Cells |
No. | Day 100: %CD45+ Cells |
|---|---|---|---|---|
| 0. Normal heart | 8 | 19.9 ± 2.5 | 8 | 19.9 ± 2.5 |
| 1. Isograft control | 6 | 25.0 ± 5.8 | 3 | 13.0 ± 0.7 |
| 2. No treatment | 5 | 81.8 ± 8.7* | NA | NA |
| 3. Short TAC | 5 | 45.4 ± 9.0* | 3 | 81.9 ± 5.4* |
| 4. Long TAC | 5 | 45.4 ± 9.0* | 3 | 14.7 ± 4.0 |
| 5. BMC prime | 6 | 86.4 ± 10.7* | 3 | 47.3 ± 4.7* |
| 6. Liver TX prime | 5 | 52.6 ± 7.1* | 3 | 20.8 ± 1.4 |
NOTE. Normal heart data from naïve animals that did not undergo transplantation.
Abbreviation: NA, not applicable.
P < .0001 compared with the normal heart and isografts.
After 100 days, 47% of the cells in the heart allografts of BMC-primed group 5 still were CD45+, and in the short-course-TAC group 3, the leukocytes accounted for 82% of cells. By this time, the leukocytenonleukocyte (CD45+-CD45−) ratio in the CR-free allografts of the continuous-TAC group 4 and the drug-free but liver-primed group 6 had returned to the range of normal hearts.
Donor-recipient phenotypes
Five days after transplantation, only 2.5% to 3% of the CD45+ cells remained LEW (i.e., costained with mAb 163) in the heart allografts of the untreated naïve recipients (group 2) and the heart allografts of recipients primed with LEW BMCs (group 5; Table 4). In contrast, 28.6% of the CD45+ cells remained LEW in the 5-day hearts transplanted to TAC-treated naïve recipients (groups 3 and 4), and 22% were still LEW in the cardiac allografts of liver-primed recipients (group 6).
Table 4.
Percentage Contribution of LEW and BN Cells to the Leukocyte (CD45+) Population in the Cell Suspensions Shown in Table 3 5 and 100 Days After Isotransplantation or Allotransplantation
| Day 5% of CD45+ Cells |
Day 100 % of CD45+ Cells |
|||||
|---|---|---|---|---|---|---|
| Group | No. | LEW Class I (LEW Class II) |
BN Class I | No. | LEW Class I (LEW Class II) |
BN Class I |
| 0. Normal heart | 8 | 98.7 ± 1.17 (24.9 ± 4.7) | NT | 8 | 98.7 ± 1.17 (24.9 ± 4.7) | NT |
| 1. Isograft control | 6 | 98.6 ± 0.7 (28.7 ± 4.4) | 0.6 ± 0.3 | 3 | 99.2 ± 0.4 (29.2 ± 0.2) | 0.8 ± 0.2 |
| 2. No treatment | 5 | 3.0 ± 0.9 (1.8 ± 0.5) | 97.4 ±1.0 | NA | NA | NA |
| 3. Short TAC | 5 | 28.6 ± 6.1 (3.3 ± 0.2) | 69.4 ± 2.6 | 3 | 2.0 ± 0.8 (<1.0) | 98.2 ± 0.9 |
| 4. Long TAC | 5 | 28.6 ± 6.1 (3.3 ± 0.2) | 69.4 ± 2.6 | 3 | 30.5 ± 7.4 (4.3 ± 1.6) | 69.0 ± 9.0 |
| 5. BMC prime | 6 | 2.5 ±1.1 (1.7 ± 0.4) | 97.8 ± 0.9 | 3 | 1.2 ± 0.7 (<1.0) | 98.2 ± 1.0 |
| 6. Liver TX prime | 5 | 22.0 ± 7.6 (9.2 ± 3.9) | 79.3 ± 8.7 | 3 | 8.4 ± 2.4 (2.0 ± 0.5) | 92.0 ± 2.8 |
NOTE. DC-rich subset of L21-6+ LEW cells is in parentheses. Normal heart data from naïve animals that did not undergo transplantation.
Abbreviations: TX, transplantation; NA, not applicable; NT, not tested.
At 100 days, LEW (donor) cells accounted for 1% to 2% of the CD45+ population in the heart allografts of groups 3 (short-course TAC) and 5 (BMC primed) compared with 30.5% in the continuous-TAC group 4 and 8.4% in the liver-primed group 6 (Table 4).
CD11b/c+ cells
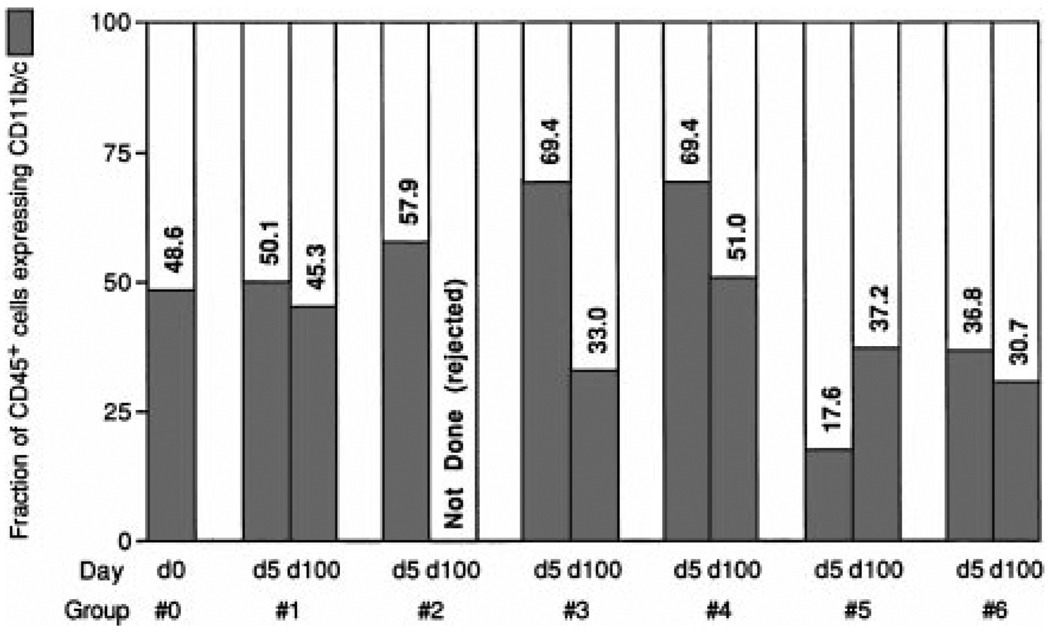
Leukocytes coexpressing CD11b/c made up approximately half the CD45+ leukocytes of normal LEW hearts and LEW isografts throughout the 100 days of the experiment (Table 5). In allografts, the CD11b/c+ fraction was sustained only in naïve recipients treated continuously with TAC (group 4; Fig. 1). In the BMC- and liver-primed groups 5 and 6, this fraction was already reduced by 5 days, and in the short-course-TAC group 3, the depletion occurred after discontinuance of treatment (Fig. 1; Table 5). By inference, the 67% to 69% of leukocytes that were CD11b/c− in the 100-day allografts of drug-free recipients groups 3, 5, and 6 consisted of lymphoid cells compared with approximately 50% in 100-day isografts (group 1) and the TAC-protected allografts of group 4 (Fig. 1).
Table 5.
Percentage Contribution of CDllb/c+ Cells to the Leukocyte (CD45+) Population in the Cell Suspensions Shown in Table 3
| Day 5 |
Day 100 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Group | No | Total CDllb/c+ |
LEW+ CDllb/c+ (LEW Class II+ CDllb/c+) |
BN+ CDllb/c+ |
No. | Total CDllb/c+ |
LEW+CDllb/c+ (LEW Class II+ CDllb/c+) |
BN+ CDllb/c+ |
| 0. Normal heart | 8 | 48.6 ± 7.0 | 48.6 ± 7.0 (14.6 ± 4.6) | NT | 8 | 48.6 ± 7.0 | 48.6 ± 7.0 (14.6 ± 4.6) | NT |
| 1. Isografr control | 6 | 50.1 ± 11.2 | 48.9 ± 12.4 (21.6 ± 4.5) | 0.5 ± 0.3 | 3 | 45.3 ± 0.3 | 45.1 ± 0.1 (23.4 ± 1.9) | 0.2 ± 0.1 |
| 2. No treatment | 5 | 57.9 ± 12.2 | 1.7 ±0.5 (1.3 ±0.1) | 56.2 ± 11.7 | NA | NA | NA | NA |
| 3. Short TAC | 5 | 69.4 ± 2.6 | 17.8 ±0.1 (2.3 ±0.1) | 51.6 ± 2.7 | 3 | 33.0 ± 7.2 | 1.5 ± 0.4 (<1.0) | 31.5 ± 6.9 |
| 4. Long TAC | 5 | 69.4 ± 2.6 | 17.8 ±0.1 (2.3 ±0.1) | 51.6 ± 2.7 | 3 | 51.0 ± 11.3 | 25.2 ± 8.0 (1.4 ± 0.1) | 25.8 ± 4.3 |
| 5. BMC prime | 6 | 17.6 ± 6.1 | 3.3 ± 1.8 (1.0 ± 0.2) | 14.3 ± 4.4 | 3 | 37.2 ± 7.4 | 1.6 ± 0.2 (<1.0) | 31.8 ± 4.8 |
| 6. Liver TX prime | 5 | 36.8 ± 10.0 | 13.8 ± 5.8 (5.8 ± 3.8) | 23.0 ± 5.2 | 3 | 30.7 ± 7.9 | 5.9 ± 1.3 (1.7 ± 0.2) | 24.8 ± 9.2 |
NOTE. LEW cells that contribute to the total CDllb/c+ population in all allograft groups are shown separately; DC-rich subset of L21-6+ LEW cells that also are CDllb/c+ is in parentheses. All numbers are shown as percentage of CD45+ cells. Normal heart data from naïve animals that did not undergo transplantation.
Abbreviations: TX, transplantation; NA, not applicable; NT, not tested.
Figure 1.
Fraction of CD45+ cells (leukocytes) that were CD11b/c+ (myeloid precursor–derived) in the cell suspensions of control (group 0) and transplanted LEW isografts (group 1) and allografts (groups 2 through 6) after 5 and 100 days’ residence in the host. Note the low CD11b/c representation at day 5 in the BMC-primed group 5 and the liver-primed group 6. At day 100, the CD11b/c fraction remained low in these groups, and it had been reduced in group 3 after discontinuance of TAC.
The CD11b/c+ cells included donor (LEW) as well as recipient (BN) leukocytes in all groups of allografts at both time points (Table 5). However, the hearts in the continuous-TAC group 4 differed from all other 100-day allografts in that fully half of their CD11b/c+ cells were donor, a representation of donor cells many times greater than in any other experimental group (Table 5).
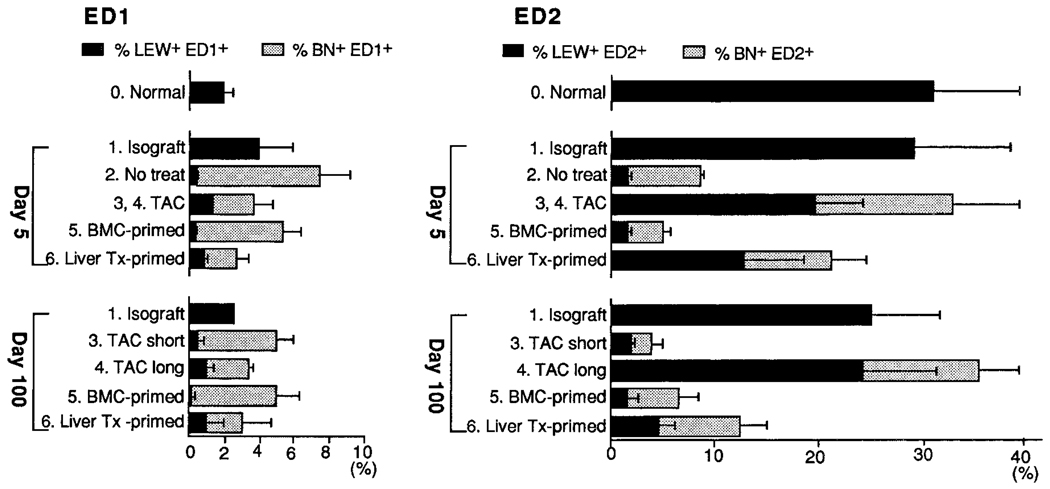
ED1+/ED2+ cells
Approximately 30% of the CD45+ cells in the cell suspensions of normal LEW hearts and isografts stained with the ED2 mAb, which has been reported to identify tissue macrophages19,21 (Fig. 2). Except in animals under continuous treatment with TAC (group 4), a remarkable reduction in ED2+ cells was seen over the course of 100 days in all groups of allografts. By day 100, the majority of the reduced numbers of ED2+ cells in the hearts of groups 3, 5, and 6 were recipient phenotype. Conversely, the fraction of ED2+ cells in the continuous-TAC heart allografts (group 4) was fully maintained; the majority was made up by LEW (donor) cells (Fig. 2).
Figure 2.
Flow cytometric analysis of heart-cell suspensions obtained from different experimental groups 5 and 100 days after transplantation (Tx). Subsets of ED1+ and ED2+ macrophages are shown with a breakdown of LEW (donor) and BN (recipient) constituencies. All data are expressed as percentages of CD45+ cells.
In normal LEW hearts, the monocytes/phagocytes identified with ED1 mAb were only 1/15 as numerous as the ED2+ cells. Contrary to the reduced ED2+ cell population in most of the allograft groups, the ED1 macrophage fraction was increased in all groups from the very small percentage (≤2%) found in normal hearts. The increases at 5 days were most dramatic (to 7.6%) in the untreated group 2 hearts. The vast majority of these cells were BN (Fig. 2).
At 100 days, when the histopathologic determinations of CR were made, the ED1 fraction was greatest in the CR-afflicted allografts of the short-course-TAC group 3 and the heart allografts of the BMC-primed group 5 (both 5%). Smaller increases (to 3.5%) were seen in the CR-free allografts of the continuous-TAC group 4 and the liver-primed group 6 (Fig. 2). Similar to the findings at 5 days, most of the ED1 cells were BN (recipient).
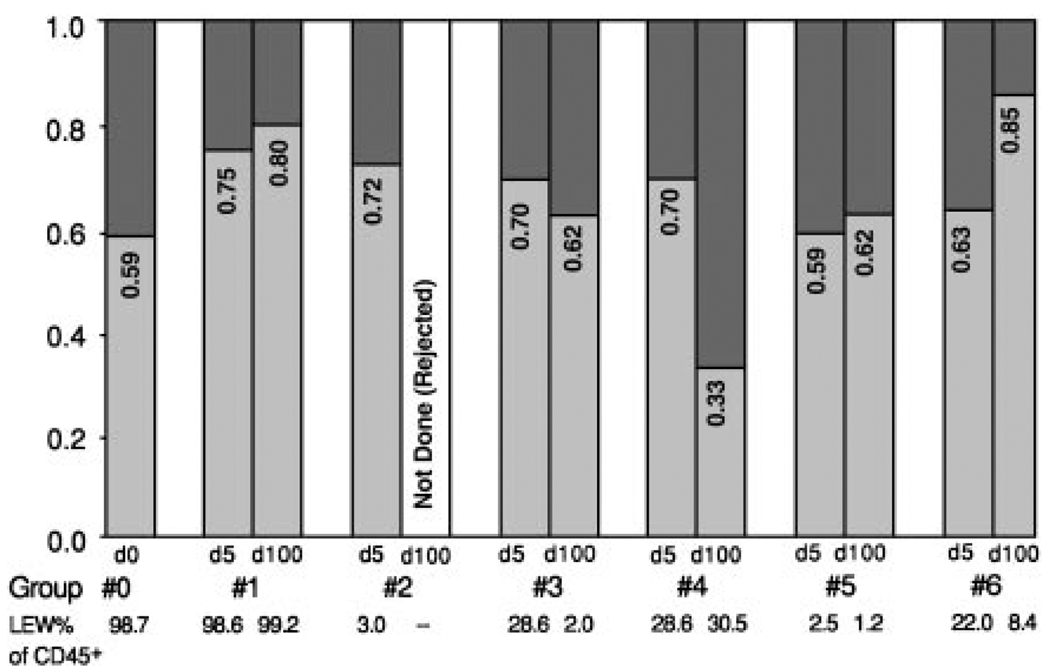
The L21-6+ population
These LEW-specific MHC class II+ cells, which have many characteristics of DCs,8–11 made up 24.9% of the total CD45+ leukocytes of normal hearts (Table 4). Less than two thirds of the L21-6+ cells (a fraction of 0.59) coexpressed CD11b/c (light shade, Fig. 3). By inference, the remaining fraction of L21-6+ leukocytes in normal hearts (~41%) consisted of lymphoid cells (dark shade, Fig. 3).
Figure 3.
The myeloid (i.e., CD11b/c+) fraction (light shading) of LEW-specific MHC class II+ (L21-6+) leukocytes in cell suspensions prepared from normal LEW hearts (group 0) and LEW isografts (group 1) and allografts (groups 2 through 6) 5 and 100 days after transplantation. Note that 59% to 85% of the L21-6+ cells are myeloid derived except in the 100-day hearts of recipients under active TAC therapy, in which the L21-6+ CD11b/c+ cells are a distinct minority (33%).
The CD11b/c+ fraction of the L21-6+ population in the 5-day and 100-day allografts was not different from that in the isografts, except in the 100-day hearts of recipients treated with continuous TAC (group 4). In the exceptional group 4, the myeloid fraction of the L21-6+ cells in the 100-day allografts was only 0.33 (Fig. 3), leaving 0.67 of presumed L21-6+ lymphoid cells.
IHC
LEW MHC class II+ (L21-6+) leukocytes
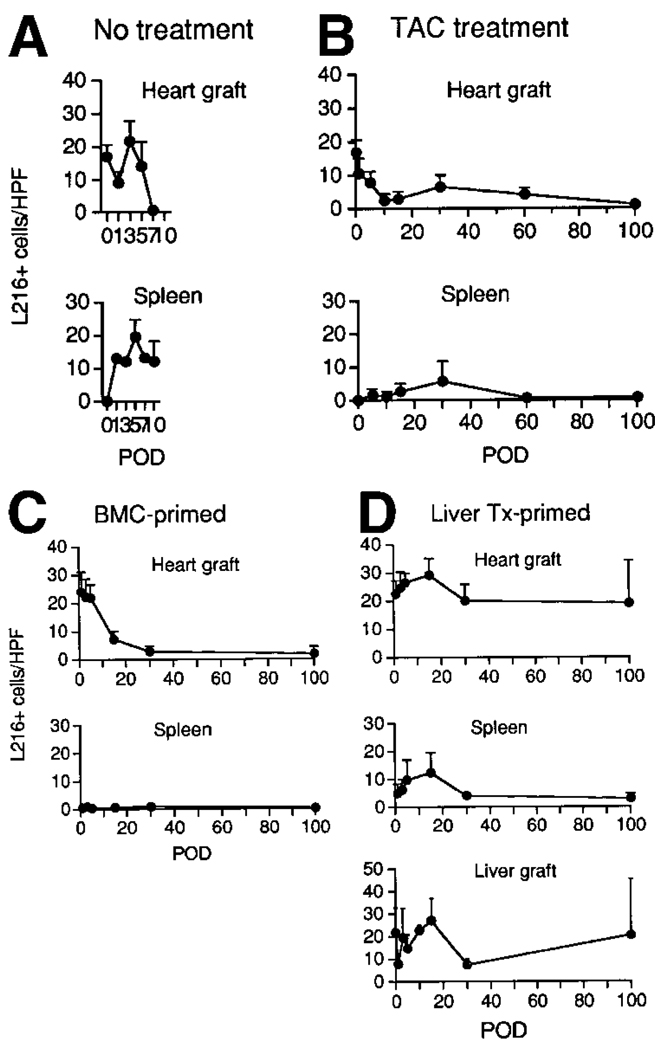
Throughout the normal LEW heart, strong staining was seen on homogeneously distributed spindled interstitial cells that had the morphological characteristics of DCs. These cells rapidly disappeared from the rejecting LEW cardiac allografts transplanted to untreated BN rats (group 2) while appearing in the recipient spleens (Fig. 4A).
Figure 4.
Sequential changes of L21-6+ cells in tissue sections of LEW heart allografts and BN recipient spleens after transplantation into (A) naïve animals without immunosuppression (group 2), (B) under continuous-TAC (group 4), (C) BMC-primed (group 5), and (D) liver transplant (Tx)-primed (group 6) recipients. For group 6, data are included from the LEW liver allografts that had been used for priming and been in place for 100 days by the time of challenge heart transplantation. After immunohistochemical staining, the number of L21-6+ cells was determined in 20 HPFs at original magnification × 400. Data from 3 to 6 animals at each time point are expressed as mean ± SD. (POD, postoperative day.)
Similarly, L21-6+ cells were promptly reduced in the LEW heart allografts of naïve BN recipients treated continuously with TAC (group 4), but small numbers were detectable at 30 days and after 100 days (Fig. 4B). L21-6+ cells were not seen in the spleens of these TAC-treated recipients until 30 days posttransplantation, and then only in small numbers (Fig. 4B). It could not be determined whether the paucity of L21-6+ cells in the spleen at both 30 and 100 days in group 4 was caused by the absence of these cells or by downregulation of MHC class II antigens, as described with deoxyspergualin.24
L21-6+ cells also declined rapidly in the heart allografts of the drug-free BMC-primed recipients of group 5, but they were rarely seen in the spleen (Fig. 4C). Between 30 and 100 days, they remained sparse in both locations.
Conversely, the L21-6+ cells in the heart allografts of the liver-primed recipients (group 6) were at least as numerous as in normal LEW hearts throughout the entire 100 days after cardiac transplantation (Fig. 4D). They also transiently increased in the host spleen, peaked there at 15 days, and returned to baseline levels by 30 days. The donor specificity of the L21-6 mAb was shown by the total absence of staining in naïve BN spleens. These findings in the liver-primed group 6 and also those in group 5 confirmed previous reported observations.16
In addition to the heart studies, the L21-6+ cell population of the priming LEW livers was determined before hepatic transplantation, during the 100-day residence of the priming liver before challenge heart transplantation (data not shown), and for 100 days after the heart transplantations. After transplantation of the challenge LEW hearts, the number of L21-6+ cells in the priming liver allografts was changed very little by the challenge heart transplantation at the time or for the next 100 days’ residence of the cardiac grafts (Fig. 4D).
The normal nontransplanted LEW liver expressed only 6.5 ± 3.6 L21-6+ cells/HPF, most with the morphological features of DCs. Thus, the expression of 20 to 30 of these class II+ cells/HPF in the liver allograft at the time of challenge heart transplantation and for the subsequent 100 days was 3 to 5 times greater than normal. The cells had the typical appearance and location of DCs (portal triad) and Kupffer cells (sinusoids).
Costimulatory molecule expression
CD80 (B7-1) was not detected with single-color IHC staining in transplanted cardiac isografts or allografts (or in the host spleens) of any of the animals of groups 1 through 6 (data not shown). The validity of this observation was supported by strong CD80 expression in the spleens of lipopolysaccharide-injected naïve BN rats (positive control). The staining was of large and irregularly shaped cells located in the splenic periarteriolar lymphoid sheaths and small round cells in the splenic germinal centers (presumably DCs and macrophages).
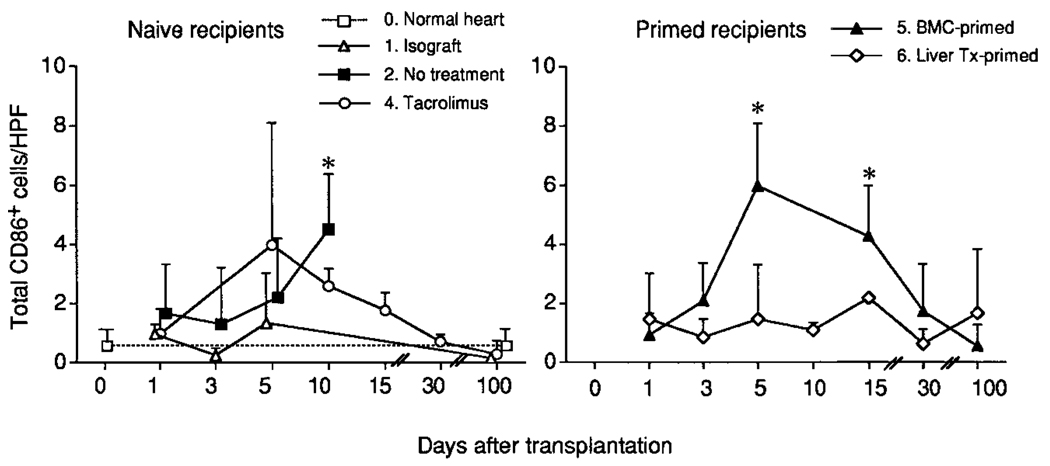
In contrast to the negative CD80 observations, interstitial dendritic-shaped cells in normal LEW hearts were CD86+ (B7-2) at a frequency of 0.50 ± 0.67/ HPF, a rate similar to that in LEW cardiac isografts from 1 to 100 days after their transplantation (group 1; Fig. 5, left). In contrast, CD86+ cells increased 5- to 10-fold by days 5 through 10 in the LEW heart allografts of naïve, untreated BN recipients (group 2) and naïve recipients immunosuppressed with TAC (group 4). The CD86+ cells were found in aggregates located mainly in the interstitium, perivascular area, and epicardium, suggesting that they were infiltrating recipient leukocytes. Of interest, no difference in the number, location, and appearance of these cells could be distinguished to 10 days in the transplanted hearts of untreated group 2 and the continuous-TAC group 4; however, the CD86+ cells subsequently declined in group 4. These analyses were not performed in the short-course-TAC group 3.
Figure 5.
Sequential determination in tissue sections of CD86 (B7-2) expression in different types of heart allografts. After immunohistochemical staining, the number of CD86+ cells was determined in 20 HPFs at original magnification × 400. Data from 3 to 6 animals at each time point are expressed as mean ± SD. *P < .05 versus normal heart (1-way ANOVA).
CD86 expression was most frequently observed in the LEW heart allografts transplanted to the BMC-primed BN recipients of group 5 (Fig. 5, right). The CD86+ cells appeared 3 days after challenge heart transplantation, reached a zenith of 6 ± 2.1/HPF after 5 days (a 10- to 20-fold increase), and returned to normal levels by 100 days. With blind reading of the slides, the findings in the BMC-primed group 5 at day 5 could not be distinguished from those in the allografts of group 2 (no treatment) or group 4 (TAC treatment).
The heart allografts transplanted to liver-primed BN recipients were unique in that CD86+ cells were found only slightly more frequently throughout the 100 days posttransplantation than in LEW isografts and normal LEW hearts (Fig. 5, right).
Identification of donor CD86+ cells
Double immuno-staining with anti-CD86 mAb 24F and donor-specific L21-6 was performed on heart allografts obtained on posttransplantation day 5, the time of highest CD86+ cell yield. In groups 2 through 5, the focally aggregated CD86+ cells at day 5 were L21-6−, confirming the impression from single CD86 staining that these were infiltrating recipient cells rather than donor leukocytes.
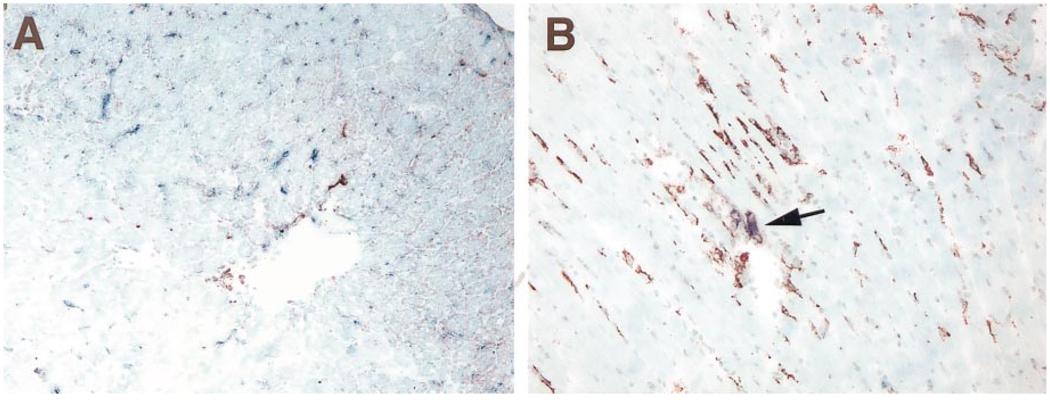
The findings at day 5 in the heart allografts transplanted to liver-primed recipients were of special interest and also in agreement with the single-staining results with L21-6 or mAb 24F (CD86). Almost no L21-6− (recipient) cells were found to express CD86. The majority of the abundant L21-6+ (donor) cells also were CD86−, but some coexpressed CD86 (Fig. 6). The observation that donor rather than recipient class II+ DC-like cells in the transplanted hearts of these pretolerized animals coexpressed CD86 (B7-2) was consistent with our previous demonstration that naïve passenger leukocytes from an allograft transplanted to a pretolerized (i.e., now defenseless) recipient could mount an unopposed GVH reaction similar to that in parent-to-offspring F1 hybrid models.9
Figure 6.
Double immunohistochemical stain of LEW MHC class II and CD86 with L21-6 mAb (3-amino-9-ethylcarbazole; red) and 24F mAb (blue alkaline phosphatase substrate kit IV; blue) in heart allografts 5 days after transplantation. (A) Untreated naïve BN recipient of group 2. Note many CD86+ cells associated with disappearance of L21-6+ cells and the upregulation of MHC class II on the microvasculature. (B) Heart allograft of group 6 in liver-primed recipient. Note double-positive purple donor cells (L21-6+, CD86+; arrow) among many single-stained L21-6+ cells but almost no single-stained CD86+ cells in the interstitium of the heart graft, i.e., a few MHC class II+ donor cells with upregulated B7-2 but essentially no recipient cells because the recipient was pretolerized to the donor. (Original magnification ×200.)
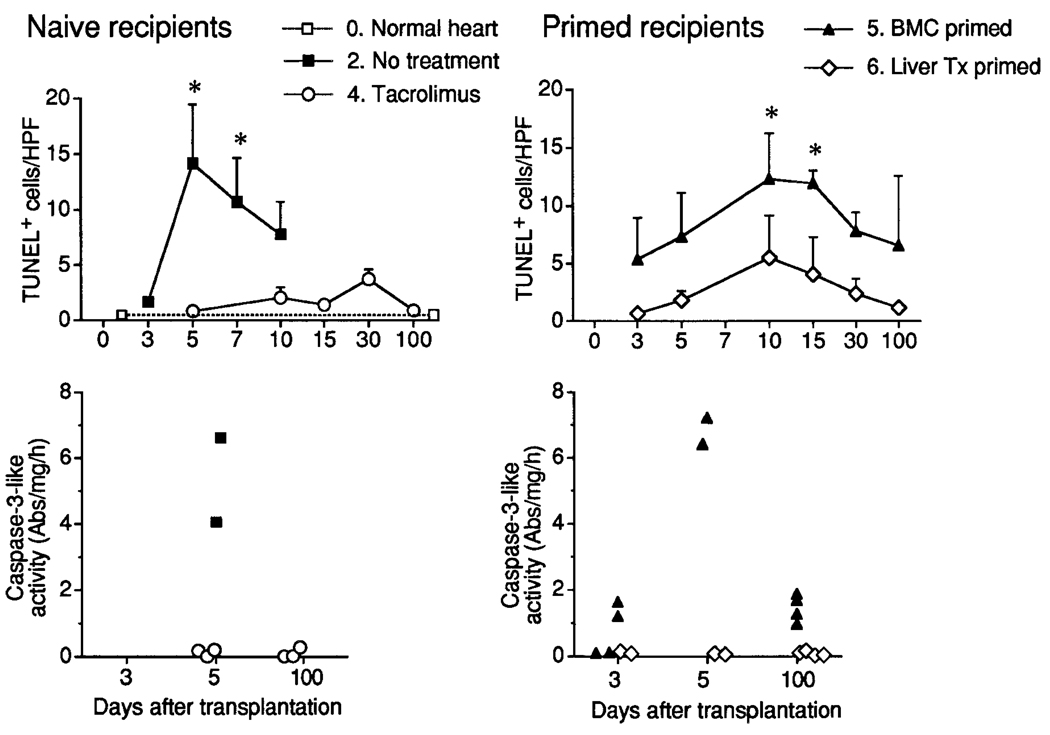
Apoptosis
Normal LEW hearts had 0.4 ± 0.1 TUNEL-positive cells/HPF. This count increased dramatically to a peak at 5 days in the cardiac allografts of untreated naïve BN recipients (group 2) in association with increases in caspase-3–like activity and then receded as the hearts rejected (Fig. 7, left). An increase in TUNEL-positive cells was low grade by comparison and delayed in the TAC-treated naïve recipient group 4, and there was little or no parallel change in caspase-3–like activity (Fig. 7, left).
Figure 7.
Apoptosis determined by TUNEL assay and caspase-3–like activity in different types of heart allografts. The number of TUNEL-positive cells in heart allograft sections was determined in 20 HPFs at original magnification ×400. Data from 3 to 5 animals at each time point are expressed as mean ± SD. In caspase-3–like activity, each dot represents the result from an individual animal.*P < .05 versus normal heart (1-way ANOVA).
The TUNEL+ cell count in LEW hearts of the BMC- and liver-primed groups 5 and 6 increased several fold during the first 2 weeks after transplantation and remained elevated throughout the 100 days of observation (Fig. 7, right). Compared with the values in normal hearts, the increases were significant at 10 and 15 days in group 5, but not in group 6. Elevations in caspase-3–like activity greater than that of normal heart controls were recorded at posttransplantation days 3, 5, and 100 in 8 of the 10 hearts removed from the BMC-primed group, but not 8 hearts retrieved from liver-primed recipients (Fig. 7, right).
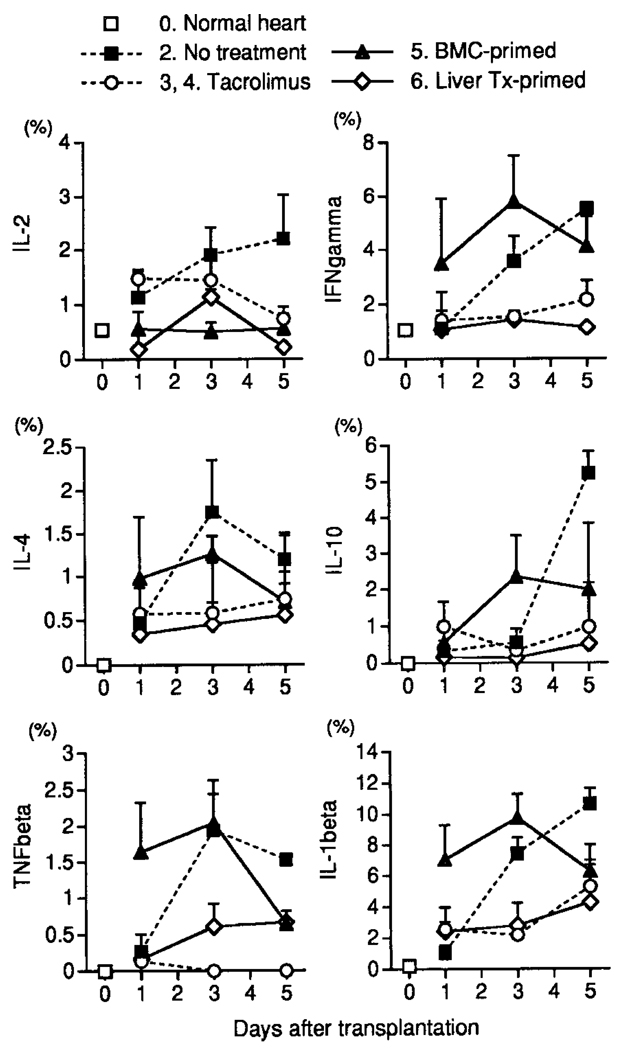
Cytokine Profiles
The elevations in mRNA of 6 representative cytokines in the LEW allograft of groups 2 through 6 are shown for the first 5 posttransplantation days in Figure 8. The seemingly large increases in mRNA of IL-1β were illusory because of the exquisite sensitivity of the assay. The increases in the untreated group 2 were blunted but not prevented by treatment with TAC (groups 3 and 4).
Figure 8.
Cytokine mRNA levels in heart grafts determined by RPA. (IFN, interferon.)
Interestingly, the increases in mRNA of cytokines in allografts transplanted to BMC-primed recipients (group 5) were just as prompt and as great or greater than those in the hearts transplanted to the untreated naïve recipients of group 2, with the exception of IL-2. However, the increases in group 5 were quickly reversible. In comparison, the cytokine profile was relatively little affected in the liver-primed recipients by challenge heart transplantation.
Mixed Leukocyte Reaction
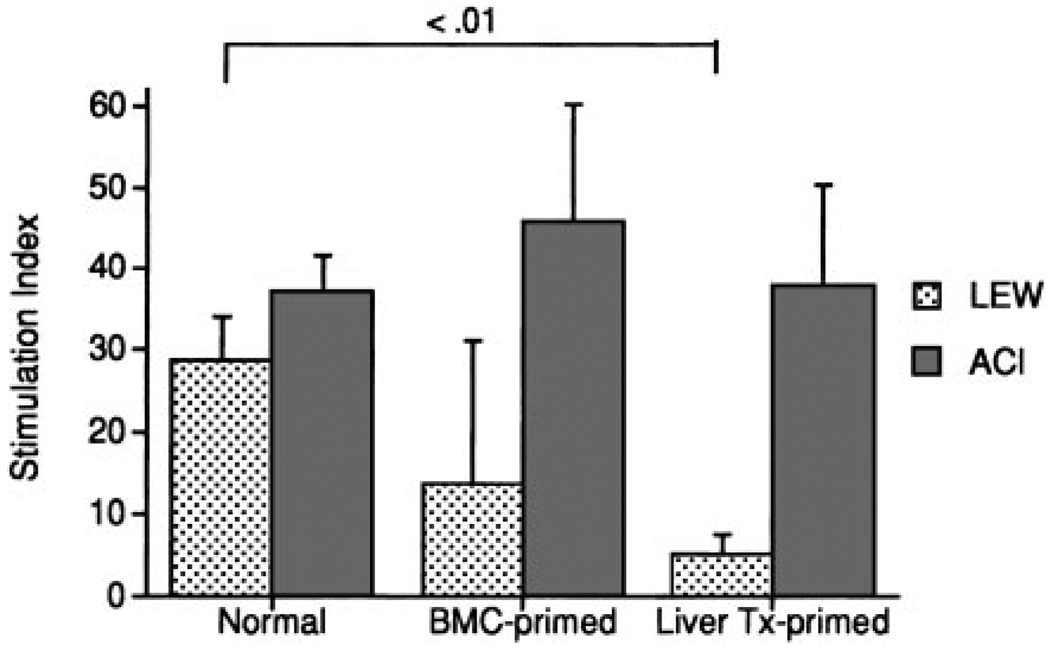
Donor-specific immune reactivity just before challenge transplantation with LEW hearts (Fig. 9) was most depressed in the liver-primed BN recipients (group 5; P < .01), and less so in BMC-primed recipients (group 4; P = not significant). The response to ACI (third-party) stimulatory cells was normal.
Figure 9.
Mixed lymphocyte reactions of cervical lymph node lymphocytes from normal BN rats and BN rats primed 100 days previously with BMCs or a liver allograft under a short course of TAC. Mean ± SD of 4 to 6 animals.
Discussion
Evidence has accumulated consistent with the paradigm that organ engraftment results from an acute double immune reaction in which donor and recipient leukocytes induce reciprocal clonal expansion followed by peripheral clonal deletion,4–7,25 the maintenance of which requires the persistence of donor leukocytes.11,26 We have further proposed that the migration and localization of the leukocytes regulates the HVG and GVH responses,4 exemplifying an immune governance principle that applies to all antigens.4,27–29 In essence, HVG and GVH cytotoxic T-cell responses either are not induced (immune indifference) or cannot be maintained4 unless the respective donor and recipient alloantigens reach organized lymphoid collections that are epitomized by, but not limited to, the lymphoid organs.
It could be postulated that recipient DCs or other antigen-presenting leukocytes can endocytose apoptotic and/or necrotic donor cells and transport the peptide residue to lymphoid organs,30–33 theoretically orchestrating T-cell immunity, including the preservation of self.34 We have argued instead that the only mobile donor antigen capable of reaching host lymphoid organs and inducing enough of an HVG response to cause clonal exhaustion-deletion is the donor passenger leukocyte population. The donor leukocytes migrate preferentially to the recipient lymphoid organs,9,35,36 where they may induce and exhaust antigraft T cells. Reciprocally, activated antihost donor T cells among the passenger leukocytes either cause GVH disease or are deleted.4
This concept mandates evaluation of numerous previously unchallenged dogmas of transplantation immunology. For example, it has long been assumed that passenger leukocytes of an allograft are highly immunogenic, whereas parenchymal cells are not. The leukocyte immunogenicity has been widely attributed to their expression of MHC class II and/or costimulatory (e.g., B7) molecules. Contrary to this assumption, the passenger leukocytes in the antigen migration-localization paradigm only appear to be immunogenic because of their ability to migrate to lymphoid organs, whereas the so-called nonimmunogenicity of organ parenchymal cells is caused by their immobilization within the organ architecture. This would readily explain the otherwise enigmatic observation by Bumgardner et al37–39 that when hepatocytes are isolated and infused, they are as immunogenic, if not more so, than hepatic passenger leukocytes.
It also is apparent that experiments in which donor leukocytes are subtracted from an organ (epitomized by the use of “parking” models) are inappropriate for studies of tolerance mechanisms because the essential tolerogenic step of immune activation is eliminated at the outset. Moreover, the studies reported here show that the donor-strain leukocytes in allografts parked in ostensibly tolerant recipients are not uniformly replaced, as commonly believed. Finally, the absence of CR in organ allografts during the parking or after re-transplantation cannot be assumed without examining the allograft histopathologically and without analysis of its leukocyte composition.
Although the host lymphoid organs undoubtedly have the crucial role in the double-immune reaction of transplant rejection, the observations reported here are consistent with the possibility that immune responses also may be induced locally in the organ allografts, which quickly develop the immunogenic-tolerogenic characteristics of an ectopic organized lymphoid collection. Evidence of an intragraft GVH reaction mounted by the graft’s passenger leukocytes has been reported in animals40 and humans.41 Consistent with such reports, B7 was expressed by donor but almost no recipient cells in the LEW heart allografts of the present study that had been transplanted to BN recipients who were immunologically defenseless because they had been pretolerized with an orthotopic LEW liver (group 6). The other way around, we23 and others42 have described antigraft host T cells assembled in rosettes around donor DCs in rejecting rat organ allografts, suggesting the local induction of an HVG response.
By 5 days, the cardiac allografts in all our experimental groups had become major repositories of donor and recipient leukocytes, replete with cytokines and growth factors and with cell-to-cell proximity of multiple leukocyte lineages, even in the highly pretolerized recipients (liver-primed group 6) and the less efficiently pretolerized recipients of group 5 that had been primed with donor BMCs. Sakamoto et al43 have shown that the allograft, with parenchymal cells that are syngeneic to the donor passenger leukocytes, provides the optimal microenvironment for donor leukocytes, including the precursor and stem cells that presumably are responsible for the multilineage nature of microchimerism, as well as macrochimerism.9,10 Thus, the organ allograft may be a critical site from which donor leukocytes are renewed and exported to destinations in the host.43
By 100 days, the ratio of leukocytes to nonleukocyte cells had returned to normal (1:4 to 1:5) in the CR-free heart grafts of the continuous-TAC and the liver-primed recipients (groups 4 and 6). However, the ratio remained elevated to 1:1 in the hearts of the BMC-primed group 5 that had moderate CR and to 5:1 in the severely CR-afflicted hearts of the short-course-TAC group 3. Despite the finding in the hearts of group 3 that leukocytes were 5 times more frequent than all other nucleated cells combined, these 100-day hearts had a strong heartbeat and appeared normal.
At both 5 and 100 days, the allograft leukocytes in all experimental groups included donor as well as recipient phenotypes. The greatest proportions of donor leukocytes at both times were in the CR-free heart allografts that had the smallest (most normal) total leukocyte fraction, i.e., those of the continuous-TAC and liver-primed groups 4 and 6. In the group 6 recipients, the flow cytometry analyses of the total donor leukocyte fraction and class II+ donor subset identified with the L21-6 mAb were generally consistent with immunohistochemical studies of tissue sections. In both the heart allografts and priming liver allografts of group 6 recipients, robust levels of donor class II+ leukocytes within organ allografts paralleled but tended to exceed the donor leukocyte chimerism in recipient tissues, as we reported previously.9 In analogous clinical observations, O’Connell et al44 reported that a large number of donor leukocytes in bronchoalveolar lavage specimens from human lung recipients was associated with the absence in the allograft of bronchiolitis obliterans, the hallmark lesion of CR in pulmonary allografts.45
Although a high proportion of donor leukocytes in the heart grafts correlated with the CR-free state, it did not predict the extent to which donor-specific nonreactivity had developed. The presence of a large percentage of donor leukocytes in the cell suspensions prepared from CR-free heart allografts in group 4 was clearly dependent on a once-weekly maintenance dose of TAC. When the weekly treatment was stopped at posttransplantation day 27 (group 3), the incompleteness of the tolerance was quantifiable by the extent of CR; nevertheless, it was enough to allow such hearts to consistently survive longer than 200 days.11
The poor correlation of donor cells in the allografts (and also in recipient tissues) with donor-specific non-reactivity also was obvious at the low end of the donor leukocyte scale. The sparse donor leukocytes at 5 days in the highly durable hearts of the drug-free BMC-primed recipients (group 5) were not distinguishable by the methods used in our study from those in the irreversibly rejecting allografts of the untreated group 2, underscoring the futility of equating arbitrary levels of chimerism with tolerance.46–49
Differences in lineage profiles of the graft leukocytes did not correlate with outcome. However, several findings were noteworthy. First, both donor and recipient leukocytes always were multilineage. Second, the depletion of donor ED2+ cells (tissue macrophages) from the heart allografts was accelerated by priming (similar to analogous findings of Armstrong et al50), but it was markedly inhibited by immunosuppression. Third, donor ED1+ macrophages (monocytes/phagocytes) also were depleted in the 100-day heart allografts of all experimental groups, but they were replaced by disproportionate numbers of infiltrating ED1+ host cells, consistent with previous claims that these macrophages are important effector cells in both acute rejection and CR. 51–53
Finally, the subpopulation of donor leukocytes in the allograft cell suspensions stained by the LEW MHC class II–specific L21-6 mAb and previously identified as DCs9,11,54,55 made up only a small fraction of the numerous donor leukocytes contained in the CR-free cardiac allografts of groups 4 and 6. However, when the donor cells were less than 3% of the total leukocytes, the L21-6+ cells made up the majority, raising the possibility that this tiny residual population of migratory cells had lost the ability to mount a GVH reaction, whereas selectively retaining the capacity of antigen presentation. This interpretation is consistent with the hypothesis that periodic leakage of donor leukocytes from the nonlymphoid to the lymphoid compartment maintains stable-state clonal exhaustion-deletion4,29 similar to that in tolerant mouse-liver recipients56 and a model of autoimmune diabetes mellitus.57,58
Whether they were present in large or small numbers, between half and two thirds of the L21-6+ cells in the 100-day cell suspension expressed CD11b/c and were believed to be the classic myeloid DCs originally described by Steinman and Cohn.13,14 The remaining nonmyeloid L21-6+ cells may have been DCs of lymphoid origin59,60 or class II–expressing lymphocytes. Contrary to a current hypothesis,61 there was no correlation between the percentage of the ostensible lymphoid DCs in the L21-6+ fraction and donor-specific nonreactivity.
Collectively, the studies reported here indicate that the extent of CR defines the incompleteness of tolerance. Once initiated, the HVG and GVH immune responses can be terminated in 2 ways, both involving apoptosis. If 1 leukocyte population eliminates the other, its antigen-specific clonal expansion ceases, shutting down the secretion of IL-2 and other molecules. The resulting passive apoptosis of the cytokine-deficient clone requires new protein synthesis, is strongly inhibited by Bc1–2 and related antiapoptotic molecules (consistent with experiments by Hancock et al62), and is believed to involve mitochondrial apoptosis mechanisms rather than such death cytokines as Fas ligand (FasL) and TNF (summarized by Lenardo et al63).
When the alloantigen cannot be eliminated, the continuing response may be terminated by the so-called activation-associated clonal exhaustion-deletion, the seminal mechanism of acquired tolerance4,6,7 that has been shown in several nontransplant and transplant tolerance models.64–72 Although this kind of apoptosis involves FasL and TNF and occurs by different molecular pathways than those of antigen withdrawal,63 there is evidence that both kinds of apoptosis are involved.73,74 Organ allografts develop CR if the MHC-restricted HVG reaction becomes unrelenting when it is not terminated either by rejection of the allograft and its peripheralized leukocytes or by the induction of donor-specific tolerance to a level less than the threshold for destructive immunity.4 Although nonimmunologic factors may contribute to the immunopathological characteristics of CR,75–79 evidence supporting a straightforword donor-specific HVG response as the core cause is overwhelming.80–84
This pathogenesis explains very well why it has been so difficult in human organ recipients to achieve the closely related objectives of drug-free tolerance and freedom from CR. The prevention with immunosuppression of destructive immunity (i.e., rejection) for long enough to allow the variable induction of tolerance has been the sine qua non of clinical organ transplantation. Organ transplantation has flourished with minimal reliance on HLA matching more because of the advent of increasingly potent immunosuppressants than any other factor. However, because tolerance induction depends on the acute clonal activation that is interdicted, the penalty may be the inability to ever stop drug therapy.68,85 If maintenance immunosuppression is reduced to less than the threshold necessary to complement the variably incomplete tolerance induced largely at the outset, CR ensues coincident with disappearance of the donor leukocytes from the host tissues and allograft.4,11 This chain of events was evident in our naïve heart recipients treated with TAC. TAC significantly reduced the immune reaction, judged by studies of costimulatory molecule expression, cytokine profiles of heart tissue extracts, in vivo test of host immune reactivity, and apoptosis. The subsequent dependence on immunosuppression to maintain a stable state was analogous to that in the vast majority of partially tolerant human recipients of long-surviving organ allografts.
Clinical efforts to facilitate tolerance in organ recipients with adjunct donor BMCs have been hampered by the need to administer the same potentially antitolerogenic immunosuppression as that used for conventional organ transplantation. The low-level chimerism normally found in organ recipients has been increased many fold by the additional load of donor leukocytes and has been reported in some studies to result in a greater incidence of donor-specific nonreactivity.86–88 However, discontinuance of immunosuppression has not been achieved.
Acknowledgments
Supported in part by grants no. DK29961, R01AI/DK38899, and R01 DK 54232 from the National Institutes of Health.
References
- 1.Zinkernagel RM, Doherty PC. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semi-allogeneic system. Nature. 1974;248:701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]
- 2.Doherty PC, Zinkernagel RM. A biological role for the major histocompatibility antigens. Lancet. 1975;1:1406–1409. doi: 10.1016/s0140-6736(75)92610-0. [DOI] [PubMed] [Google Scholar]
- 3.Zinkernagel RM, Doherty PC. The discovery of MHC restriction. Immunol Today. 1997;18:14–17. doi: 10.1016/s0167-5699(97)80008-4. [DOI] [PubMed] [Google Scholar]
- 4.Starzl TE, Zinkernagel ZR. Antigen localization and migration in immunity and tolerance. N Engl J Med. 1998;339:1905–1913. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Demetris AJ, Trucco M, Ramos H, Zeevi A, Rudert WA, et al. Systemic chimerism in human female recipients of male livers. Lancet. 1992;340:876–877. doi: 10.1016/0140-6736(92)93286-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Demetris AJ, Trucco M, Murase N, Ricordi C, Ildstad S, et al. Cell migration and chimerism after whole-organ transplantation: The basis of graft acceptance. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 8.Murase N, Demetris AJ, Woo J, Tanabe M, Furuya T, Todo S, Starzl TE. Graft-versus-host disease (GVHD) after BN to LEW compared to LEW to BN rat intestinal transplantation under FK 506. Transplantation. 1993;55:1–7. doi: 10.1097/00007890-199301000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demetris AJ, Murase N, Fujisaki S, Fung JJ, Rao AS, Starzl TE. Hematolymphoid cell trafficking, microchimerism, and GVH reactions after liver, bone marrow, and heart transplantation. Transplant Proc. 1993;25:3337–3344. [PMC free article] [PubMed] [Google Scholar]
- 10.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: Tolerance and donor cell chimerism. Hepatology. 1994;19:916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murase N, Starzl TE, Tanabe M, Fujisaki S, Miyazawa H, Ye Q, et al. Variable chimerism, graft-versus-host disease, and tolerance after different kinds of cell and whole organ transplantation from Lewis to brown Norway rats. Transplantation. 1995;60:158–171. doi: 10.1097/00007890-199507000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terakura M, Murase N, Demetris AJ, Ye Q, Thomson A, Starzl TE. Lymphoid/non-lymphoid compartmentalization of donor leukocyte chimerism in rat recipients of heart allografts, with or without adjunct bone marrow. Transplantation. 1998;66:350–357. doi: 10.1097/00007890-199808150-00012. [DOI] [PubMed] [Google Scholar]
- 13.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinman RM. The dendritic cell system and its role in immunogenicity. Ann Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 15.Thomson AW, Lu L, Murase N, Demetris AJ, Rao AS, Starzl TE. Microchimerism, dendritic cell progenitors and transplantation tolerance. Stem Cells. 1995;13:622–639. doi: 10.1002/stem.5530130607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demetris AJ, Murase N, Ye Q, Galvao FH, Richert C, Saad R, et al. Analysis of chronic rejection and obliterative arteriopathy. Possible contributions of donor antigen-presenting cells and lymphatic disruption. Am J Pathol. 1997;150:563–578. [PMC free article] [PubMed] [Google Scholar]
- 17.Gill TJD, Kunz HW, Misra DN, Hassett AL. The major histocompatibility complex of the rat. Transplantation. 1987;43:773–785. [PubMed] [Google Scholar]
- 18.Yagihashi A, Takahashi S, Murase N, Starzl TE, Iwaki Y. A monoclonal antibody (L21-6) recognizing an invariant chain expressed on the cell surface in rats with the exception of the BN (RT1n): A study of tissue and strain distributions. Transplant Proc. 1995;27:1519–1521. [PMC free article] [PubMed] [Google Scholar]
- 19.Dijkstra CD, Dopp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: Distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2, and ED3. Immunology. 1985;54:589–599. [PMC free article] [PubMed] [Google Scholar]
- 20.Damoiseaux JG, Dopp EA, Calame W, Chao D, MacPherson GG, Dijkstra CD. Rat macrophage lysosomal membrane antigen recognized by monoclonal antibody ED1. Immunology. 1994;83:140–147. [PMC free article] [PubMed] [Google Scholar]
- 21.Barbe E, Damoiseaux JG, Dopp EA, Dijkstra CD. Characterization and expression of the antigen present on resident rat macrophages recognized by monoclonal antibody ED2. Immunobiology. 1990;182:88–99. doi: 10.1016/S0171-2985(11)80586-3. [DOI] [PubMed] [Google Scholar]
- 22.Spencer SC, Fabre JW. Characterization of the tissue macrophage and the interstitial dendritic cell as distinct leukocytes normally resident in the connective tissue of rat heart. J Exp Med. 1990;171:1841–1851. doi: 10.1084/jem.171.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demetris A, Qian S, Sun H, Fung JJ, Yagihashi A, Murase N, et al. Early events in liver allograft rejection: Delineation of sites of simultaneous intragraft and recipient lymphoid tissue sensitization. Am J Pathol. 1991;138:609–618. [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas JM, Contreras JL, Jiang XL, Eckhoff DE, Wang PX, Hubbard WJ, et al. Peritransplant tolerance induction in macaques: Early events reflecting the unique synergy between immunotoxin and deoxyspergualin. Transplantation. 1999;68:1660–1673. doi: 10.1097/00007890-199912150-00009. [DOI] [PubMed] [Google Scholar]
- 25.Starzl TE, Demetris AJ, Murase N, Trucco M, Thomson AW, Rao AS. The lost chord: Microchimerism. Immunol Today. 1996;17:577–584. doi: 10.1016/s0167-5699(96)10070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehl S, Aichele P, Ramseier H, Barchet W, Hombach J, Pircher H, et al. Antigen persistence and time of T-cell tolerization determine the efficacy of tolerization protocols for prevention of skin graft rejection. Nat Med. 1998;4:1015–1019. doi: 10.1038/2001. [DOI] [PubMed] [Google Scholar]
- 27.Zinkernagel RM. Immunology taught by viruses. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]
- 28.Zinkernagel RM, Ehl S, Aichele P, Oehen S, Kundig T, Hengartner H. Antigen localization regulates immune responses in a dose- and time-dependent fashion: A geographical view of immune reactivity. Immunol Rev. 1997;156:199–209. doi: 10.1111/j.1600-065x.1997.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 29.Zinkernagel RM, Hengartner H. Antiviral immunity. Immunol Today. 1997;18:258–260. doi: 10.1016/s0167-5699(97)80017-5. [DOI] [PubMed] [Google Scholar]
- 30.Zocchi MR, Poggi A, Rubartelli A. The RGD-containing domain of exogenous HIV-1 Tat inhibits the engulfment of apoptotic bodies by dendritic cells. AIDS. 1997;11:1227–1235. doi: 10.1097/00002030-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 32.Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–443. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: Exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunohistostimulatory dendritic cells. J Exp Med. 2000;191:423–433. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nemlander A, Soots A, von Willebrand E, Husberg B, Hayry P. Redistribution of renal allograft-responding leukocytes during rejection. II. Kinetics and specificity. J Exp Med. 1982;156:1087–1100. doi: 10.1084/jem.156.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel route for initiation of rejection. J Exp Med. 1990;171:307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bumgardner GL, Li J, Heininger M, Orosz CG. Costimulation pathways in host immune responses to allogeneic hepatocytes. Transplantation. 1998;66:1841–1845. doi: 10.1097/00007890-199812270-00047. [DOI] [PubMed] [Google Scholar]
- 38.Bumgardner GL, Heininger M, Li J, Xia D, Parker-Thornburg J, Ferguson RM, Orosz CG. A functional model of hepatocyte transplantation for in vivo immunologic studies. Transplantation. 1998;65:53–61. doi: 10.1097/00007890-199801150-00011. [DOI] [PubMed] [Google Scholar]
- 39.Bumgardner GL, Li J, Heininger M, Ferguson RM, Orosz CG. In vivo immunogenicity of purified allogeneic hepatocytes in a murine hepatocyte transplant model. Transplantation. 1998;65:47–52. doi: 10.1097/00007890-199801150-00010. [DOI] [PubMed] [Google Scholar]
- 40.Elkins WL, Guttmann RD. Pathogenesis of a local graft versus host reaction: Immunogenicity of circulating host leukocytes. Science. 1968;159:1250–1251. doi: 10.1126/science.159.3820.1250. [DOI] [PubMed] [Google Scholar]
- 41.Hayry P, von Willebrand E. Transplant aspiration cytology in the evaluation of a renal allograft. In: Touraine JL, Traeger J, Bétuel H, Brochier J, Dubernard JM, Revillard JP, Triau R, editors. Transplantation and clinical immunology. vol 15. Amsterdam: Excerpta Medica; 1983. pp. 124–137. [Google Scholar]
- 42.Forbes RD, Parfrey NA, Gomersall M, Darden AG, Guttmann RD. Dendritic cell-lymphoid cell aggregation and major histocompatibility antigen expression during rat cardiac allograft rejection. J Exp Med. 1986;164:1239–1258. doi: 10.1084/jem.164.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakamoto T, Ye Q, Lu L, Demetris AJ, Starzl TE, Murase N. Donor hematopoietic progenitor cells in nonmyeloablated rat recipients of allogeneic bone marrow and liver grafts. Transplantation. 1999;67:833–840. doi: 10.1097/00007890-199903270-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Connell PJ, Mba-Jonas A, Leverson GE, Heisey DM, Meyer KC, Love RB, Burlingham WJ. Stable lung allograft outcome correlates with the presence of intragraft donor-derived leukocytes. Transplantation. 1998;66:1167–1174. doi: 10.1097/00007890-199811150-00010. [DOI] [PubMed] [Google Scholar]
- 45.Burke CM, Theodore J, Dawkins KD, Yousem SA, Blank N, Billingham ME, et al. Post-transplant obliterative bronchiolitis and other late lung sequelae in human heart-lung transplantation. Chest. 1984;86:824–829. doi: 10.1378/chest.86.6.824. [DOI] [PubMed] [Google Scholar]
- 46.Wekerle T, Sykes M. Mixed chimerism as an approach for the induction of transplantation tolerance. Transplantation. 1999;68:459–467. doi: 10.1097/00007890-199908270-00001. [DOI] [PubMed] [Google Scholar]
- 47.Wren SM, Hronakes ML, Ildstad ST. The requirement for allogeneic chimerism for second transfer of tolerance from mixed allogeneic chimeras (A+B→A) to secondary recipients. Transplantation. 1992;54:1031–1040. doi: 10.1097/00007890-199212000-00017. [DOI] [PubMed] [Google Scholar]
- 48.Bushell A, Pearson TC, Morris PJ, Wood KJ. Donor-recipient microchimerism and tolerance induction. Transplantation. 1996;61:170–172. [Google Scholar]
- 49.Wood K, Sachs DH. Chimerism and transplantation tolerance: Cause and effect. Immunol Today. 1996;17:584–588. doi: 10.1016/s0167-5699(96)10069-4. [DOI] [PubMed] [Google Scholar]
- 50.Armstrong HE, Bolton EM, McMillan I, Spencer SC, Bradley JA. Prolonged survival of actively enhanced rat renal allografts despite accelerated cellular infiltration and rapid induction of both class I and class II MHC antigens. J Exp Med. 1987;165:891–907. doi: 10.1084/jem.165.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paul LC, Grothman GT, Benediktsson H, Davidoff AL, Rozing J. Macrophage subpopulations in normal and transplanted heart and kidney tissues in the rat. Transplantation. 1992;53:157–162. doi: 10.1097/00007890-199201000-00032. [DOI] [PubMed] [Google Scholar]
- 52.Cramer DV, Wu GD, Chapman FA, Gajulis E, Wang HK, Makowka L. Lymphocytic subsets and histopathologic changes associated with the development of heart transplant arteriosclerosis. J Heart Lung Transplant. 1992;11:458–466. [PubMed] [Google Scholar]
- 53.Adams DH, Wyner LR, Karnovsky MJ. Experimental graft arteriosclerosis. II. Immunocytochemical analysis of lesion development. Transplantation. 1993;56:794–799. doi: 10.1097/00007890-199310000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Murase N, Demetris AJ, Woo J, Furuya T, Nalesnik M, Tanabe M, et al. Lymphocyte traffic and graft-versus-host disease after fully allogeneic small bowel transplantation. Transplant Proc. 1991;23:3246–3247. [PMC free article] [PubMed] [Google Scholar]
- 55.Demetris AJ, Murase N, Starzl TE. Donor dendritic cells after liver and heart allotransplantation under short-term immunosuppression. Lancet. 1992;339:1610. doi: 10.1016/0140-6736(92)91875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dahmen U, Qian S, Rao AS, Demetris AJ, Fu F, Sun H, et al. Split tolerance induced by orthotopic liver transplantation in mice. Transplantation. 1994;58:1–8. doi: 10.1097/00007890-199407000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohashi PS, Oehen S, Buerki K, Pircher HP, Ohashi CT, Odermatt B, et al. Ablation of “tolerance” and induction of diabetes by viruses infection in viral antigen transgenic mice. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 58.Ohashi PS, Oehen S, Aichele P, Pircher H, Odermatt B, Herrera P, et al. Induction of diabetes is influenced by the infectious virus and local expression of MHC class I and tumor necrosis factor-α. J Immunol. 1993;150:5185–5194. [PubMed] [Google Scholar]
- 59.Vremec D, Zorbas M, Scollay R, Saunders DJ, Ardavin CF, Wu L, Shortman K. The surface phenotype of dendritic cells purified from mouse thymus and spleen: Investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shortman K, Vremec D, Corcoran LM, Georgopoulos K, Lucas K, Wu L. The linkage between T-cell and dendritic cell development in the mouse thymus. Immunol Rev. 165:39–46. doi: 10.1111/j.1600-065x.1998.tb01228.x. 199. [DOI] [PubMed] [Google Scholar]
- 61.Fazekas de St Groth B. The evolution of self-tolerance: A new cell arises to meet the challenge of self-reactivity. Immunol Today. 1998;19:448–454. doi: 10.1016/s0167-5699(98)01328-0. [DOI] [PubMed] [Google Scholar]
- 62.Hancock WW, Buelow R, Sayegh MH, Turka L. Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nat Med. 1998;4:1392–1396. doi: 10.1038/3982. [DOI] [PubMed] [Google Scholar]
- 63.Lenardo M, Chan FK, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature lymphocyte apoptosis-immune regulation in a dynamic and unpredictable antigen environment. Ann Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 64.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: Clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 65.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 66.Critchfield J, Racke M, Zuniga-Pflucker J, Cannella B, Raine CS, Goverman J, Lenardo MJ. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science. 1994;263:1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 67.Aichele P, Brduscha-Riem K, Zinkernagel RM, Hengartner H, Pircher H. T cell priming versus T cell tolerance induced by synthetic peptides. J Exp Med. 1995;182:261–266. doi: 10.1084/jem.182.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bishop GA, Sun J, DeCruz DJ, Rokahr KL, Sedgwick JD, Sheil AG, et al. Tolerance to rat liver allografts. III. Donor cell migration and tolerance-associated cytokine production in peripheral lymphoid tissues. J Immunol. 1996;156:4925–4931. [PubMed] [Google Scholar]
- 69.Qian S, Lu L, Fu F, Li Y, Starzl TE, Fung JJ, Thomson AW. Apoptosis within spontaneously accepted mouse liver allografts: Evidence for deletion of cytotoxic T cells and implications for tolerance induction. J Immunol. 1997;158:4654–4661. [PMC free article] [PubMed] [Google Scholar]
- 70.Meyer D, Baumgardt S, Loeffeler S, Czub S, Otto C, Gassel HJ, et al. Apoptosis of T lymphocytes in liver and/or small bowel allografts during tolerance induction. Transplantation. 1998;66:1530–1536. doi: 10.1097/00007890-199812150-00018. [DOI] [PubMed] [Google Scholar]
- 71.Wekerle T, Sayegh MH, Hill J, Zhao Y, Chandraker A, Swenson KG, et al. Extrathymic T cell deletion and allogeneic stem cell engraftment induced with costimulatory blockade is followed by central T cell tolerance. J Exp Med. 1998;187:2037–2044. doi: 10.1084/jem.187.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu L, Li W, Zhong C, Qian S, Fung JJ, Thomson AW, Starzl TE. Increased apoptosis of immunoreactive host cells and augmented donor leukocyte chimerism, not sustained inhibition of B7 molecule expression are associated with prolonged cardiac allograft survival: In mice preconditioned with immature donor dendritic cells plus anti-CD40L mAb. Transplantation. 1999;68:747–757. doi: 10.1097/00007890-199909270-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 74.Wells AD, Li XC, Li Y, Walsh MC, Zheng XX, Wu Z, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 75.Tilney NL. Chronic rejection. In: Ginns LC, Cosimi AB, Morris PJ, editors. Transplantation. Malden: Blackwell Scientific; 1999. pp. 43–53. 56–58. [Google Scholar]
- 76.Hayry P. Commentary on Tilney NL: Chronic rejection. In: Ginns LC, Cosimi AB, Morris PJ, editors. Transplantation. Malden: Blackwell Scientific; 1999. pp. 53–59. [Google Scholar]
- 77.Mackenzie HS, Tullius SG, Heemann UW, Azuma H, Rennke HG, Brenner BM, Tilney NL. Nephron supply is a major determinant of long-term renal allograft outcome. J Clin Invest. 1994;94:2148–2152. doi: 10.1172/JCI117571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yilmaz S, Paavonen T, Hayry P. Chronic rejection of rat renal allografts. II. The impact of prolonged ischemia time on transplant histology. Transplantation. 1992;53:823–827. doi: 10.1097/00007890-199204000-00023. [DOI] [PubMed] [Google Scholar]
- 79.Chertow GM, Milford EL, Mackenzie HS, Brenner BM. Antigen-independent determinants of cadaveric kidney transplant failure. JAMA. 1996;276:1732–1736. [PubMed] [Google Scholar]
- 80.Cramer DV, Qian SQ, Harnaha J, Chapman FA, Estes LW, Starzl TE, Makowka L. Cardiac transplantation in the rat. I. The effect of histocompatibility differences on graft arteriosclerosis. Transplantation. 1989;47:414–419. doi: 10.1097/00007890-198903000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hancock WH, Whitley WD, Tullius SG, Heemann UW, Wasowska B, Baldwin WM, III, Tilney NL. Cytokines, adhesion molecules, and the pathogenesis of chronic rejection of rat renal allografts. Transplantation. 1993;56:643–650. doi: 10.1097/00007890-199309000-00028. [DOI] [PubMed] [Google Scholar]
- 82.Russell ME, Hancock WW, Akalin E, Wallace AF, Glysing-Jensen T, Willett TA, Sayegh MH. Chronic cardiac rejection in the LEW to F344 rat model: Blockade of CD28-B7 costimulation by CTLA4Ig modulates T cell and macrophage activation and attenuates arteriosclerosis. J Clin Invest. 1996;97:833–838. doi: 10.1172/JCI118483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Azuma H, Chandraker A, Nadeau K, Hancock WW, Carpenter CB, Tilney NL, Sayegh MH. Blockade of T cell costimulation prevents development of experimental chronic allograft rejection. Proc Natl Acad Sci U S A. 1996;93:12439–12444. doi: 10.1073/pnas.93.22.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murphy B, Kim KS, Buelow R, Sayegh MH, Hancock WW. Synthetic MHC class I peptide prolongs cardiac survival and attenuates transplant arteriosclerosis in the Lewis→Fischer 344 model of chronic allograft rejection. Transplantation. 1997;64:14–19. doi: 10.1097/00007890-199707150-00004. [DOI] [PubMed] [Google Scholar]
- 85.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 86.Fontes P, Rao A, Demetris AJ, Zeevi A, Trucco M, Carroll P, et al. Augmentation with bone marrow of donor leukocyte migration for kidney, liver, heart, and pancreas islet transplantation. Lancet. 1994;344:151–155. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salgar S, Shapiro R, Dodson F, Corry R, McCurry K, Zeevi A, et al. Infusion of donor leukocytes to induce tolerance in organ allograft recipients. J Leukoc Biol. 1999;66:310–314. doi: 10.1002/jlb.66.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garcia Morales R, Esquenazi V, Zucker K, Gomez CI, Fuller L, Carreno M, et al. Assessment of the effects of cadaver donor bone marrow on kidney allograft recipient blood cell chimerism by a novel technique combining PCR and flow cytometry (PCR-FLOW) Transplantation. 1996;62:1149–1160. doi: 10.1097/00007890-199610270-00021. [DOI] [PubMed] [Google Scholar]