Abstract
Background:
Prolonged mechanical ventilation is increasingly common. It is expensive and associated with significant morbidity and mortality. Our objective is to comprehensively characterize patients admitted to a Ventilator Rehabilitation Unit (VRU) for weaning and identify characteristics associated with survival.
Methods:
182 consecutive patients over 3.5 years admitted to Temple University Hospital (TUH) VRU were characterized. Data were derived from comprehensive chart review and a prospectively collected computerized database. Survival was determined by hospital records and social security death index and mailed questionnaires.
Results:
Upon admission to the VRU, patients were hypoalbuminemic (albumin 2.3 ± 0.6 g/dL), anemic (hemoglobin 9.6 ± 1.4 g/dL), with moderate severity of illness (APACHE II score 10.7 + 4.1), and multiple comorbidities (Charlson index 4.3 + 2.3). In-hospital mortality (19%) was related to a higher Charlson Index score (P = 0.006; OR 1.08–1.6), and APACHE II score (P = 0.016; OR 1.03–1.29). In-hospital mortality was inversely related to admission albumin levels (P = 0.023; OR 0.17–0.9). The presence of COPD as a comorbid illness or primary determinant of respiratory failure and higher VRU admission APACHE II score predicted higher long-term mortality. Conversely, higher VRU admission hemoglobin was associated with better long term survival (OR 0.57–0.90; P = 0.0006).
Conclusion:
Patients receiving prolonged ventilation are hypoalbuminemic, anemic, have moderate severity of illness, and multiple comorbidities. Survival relates to these factors and the underlying illness precipitating respiratory failure, especially COPD.
Keywords: mechanical ventilation, mortality, weaning, ventilator rehabilitation, anemia, COPD
Introduction
Prolonged mechanical ventilation (PMV), generally defined as >14–21 days of continuous ventilation, is provided to an increasing number of patients leading to greater intensive care unit (ICU) patient-days, resource consumption and costs. About 2%–5% of the ICU population fit into this category which now accounts for 37% of all ICU costs.12 The financial burden of caring for an increased numbers of patients receiving prolonged ventilation has prompted acute care hospitals to transfer patients from the ICU to alternative sites of care for further weaning attempts and treatment.2 These alternative sites of care are usually geographically separate and have different medical, nursing, and ancillary staff patterns from the originating acute care hospital.3 Post-ICU ventilator facilities vary in purpose, organization and financial structure. A commercial model, called a long-term acute care facility (LTAC) contrasts to a not-for profit model such as a VRU or other dedicated weaning unit where the primary goal and direction of resources is toward progression of the patient toward ventilator independence and discharge toward home.
Survival to discharge data from non-ICU, non-acute care hospital weaning centers are varied, and range between 50%–94%. One year post discharge survival is reported between 23% and 53% with 3 year reported data between 25% and 56%.4–8 Morbidity among survivors is high.9 The variability in survival data reflects differences in single center patient selection, patient care practice, patient characteristics and outcomes reporting. With these limitations in mind we describe 182 patients treated over 3.5 years.
Herein, we present a comprehensive clinical and demographic characterization of patients receiving prolonged ventilation and describe their short-term (eg, at hospital discharge), and long-term survival (eg, years after discharge). Additionally, we describe the factors that are associated with an improved survival.
Methods
Setting
The VRU is an 18 bed unit within TUH. The unit is staffed by a board-certified pulmonary/critical care attending who leads the multidisciplinary team (Fig. 1). In addition to individualized and optimized medical care, all patients receive daily aggressive whole body rehabilitation and respiratory muscle training and daily spontaneous breathing trials.
Figure 1.
Outline of daily and long term planning in ventilator rehabilitation unit.
Adapted from reference 28, with permission from the authors.
Abbreviation: DME, durable medical equipment.
VRU admission and discharge criteria
Patients were referred from ICUs within the hospital (approximately 70%), and from outside ICUs, LTACs and nursing homes. Patients admitted to the VRU must breathe spontaneously ≥0.5 hours, have a tracheostomy, have hemodynamic stability and a willingness to participate in a physical rehabilitation program. Complete admission criteria are listed in Table 1. VRU discharge occurred upon resolution or control of medical illness, liberation from mechanical ventilation, confirmed need for home mechanical ventilation, or ICU transfer for new or worsening medical condition. Timing of discharge was influenced by patients’ level of social and psychological support, need for partial or total home mechanical ventilation or placement in skilled nursing facility for ongoing subacute care or nursing home placement.
Table 1.
Criteria for transfer from the intensive care unit to the ventilator rehabilitation unit.
| Respiratory stability | Non-respiratory medical stability |
|---|---|
| Airway: tracheostomy for invasive ventilation | Sepsis controlled |
| Secretions: manageable with infrequent suctioning | No uncontrolled hemorrhage |
| Oxygenation: FIO2 < 50%, PEEP < 5 cm H2O, SaO2 > 92% | No uncontrolled arrhythmias, heart failure, or unstable angina |
| Ventilator settings: stable, volume control | No coma |
| Patient assessment: comfortable, no increased WOB or dyspnea | Secure parenteral line |
| Weaning technique: tracheal collar | Secure alimentation route |
Abbreviations: PEEP, positive end-expiratory pressure; SaO2, oxygen saturation; WOB, work of breathing.
Study patient selection
Data were collected from consecutive patients receiving prolonged mechanical ventilation admitted to the VRU from 1/2000 to 7/2004. Clinical and demographic data were collected prospectively into an institutional review board (IRB) approved database. The IRB approved all aspects of the study.
Classification of respiratory failure
Patients were classified by cause of respiratory failure. Seven diagnostic groups were acute respiratory distress syndrome (ARDS)/Pneumonia, chronic obstructive pulmonary disease (COPD), congestive heart failure/coronary artery disease (CHF/CAD), neurological (Neuro), obesity, trauma and post-thoracic transplantation (heart, lung or heart-lung). Prior studies grouped patients similarly 4–6,10 with the exception of the newly added, obesity and thoracic transplant groups.
Assessed variables
Primary outcomes were survival to hospital discharge and post-discharge survival to one and 3 years. Survival was assessed for each of seven patient categories of respiratory failure and according to the presence or absence of COPD as a primary or comorbid diagnosis. Secondary outcomes were weaning from mechanical ventilatory support, and post-VRU-discharge disposition. Survival outcomes were determined from hospital and clinic records, the social security death index, and a questionnaire mailed to each patient’s last known address. Survival data collection terminated 3 years after the last patient was discharged.
Clinical data included: facility of origin, total hospital length of stay, length of ICU stay (defined as time spent in the ICU prior to VRU admission), length of VRU stay age, gender, co-morbidities using the Charlson index,11 APACHE II score, dialysis needs, body mass index (BMI), and presence or absence of the following CHF, COPD, diabetes mellitus (DM). Laboratory data included: serum albumin at hospital admission, at VRU admission and upon discharge, hemoglobin at VRU admission and transfusion requirements pre and post-VRU admission. Ventilator data included: duration of mechanical ventilation, peak and plateau pressures at VRU admission and on the last day of mechanical ventilation, PaO2 and PaCO2 arterial blood gas results and PaO2/FiO2 on ventilator support at VRU admission, maximum negative inspiratory pressure, and spontaneous respiratory rate and tidal volume.
Weaning techniques
The post-ICU weaning strategy exclusively employed augmenting daily tracheal collar (venti-trach mask) or T-piece spontaneous breathing trials with return to assist-control (AC) mode ventilation. The treating physicians and respiratory therapists assessed each patient early every day. In addition to clinical assessment and observation of spontaneous breathing respiratory mechanics {maximum inspiratory pressure (MIP), minute ventilation (VE) and its components of tidal volume (VT) and respiratory rate (f) and its ratio of the rapid shallow breathing index (f/VT)} were measured and recorded. Based on patient tolerance, stability of vital signs and adequate gas exchange patients were progressively and totally weaned from assisted ventilation with increasing daily efforts over days to weeks. Weaning success was defined as complete withdrawal of mechanical ventilation that persisted to hospital discharge.
Statistical analysis
Kaplan-Meier analysis was used to construct survival curves for all patients by diagnostic category. Log rank (Mantel-Cox) test was applied for pair-wise comparisons between categories of respiratory failure and patients grouped COPD vs. non-COPD.
Maximum likelihood estimates analysis was performed for univariate associations between each patient characteristic and outcome. A multivariate analysis using maximum likelihood estimates was performed for each significant univariate association (P ≤ 0.05).
Results
Patient characteristics
During the study period 182 patients were admitted to the VRU for PMV. Table 2 shows the mean, total hospital and ICU length of stays and duration of PMV. Table 3 shows patient characteristics at VRU admission. The study patients were generally older, with multiple comorbid illness, were severely hypoalbuminemic and anemic. Mean left ventricular ejection fraction determined by transthoracic echocardiogram (interpreted by cardiologist) was low normal (normal >45%). Table 4 shows respiratory mechanics at VRU admission and at discontinuation of mechanical ventilation. Overall MIP increased 6.3 cm H2O from 32.2 ± 11.9 to 38.5 ± 15.1) (P < 0.001). The most common cause of respiratory failure was pneumonia/ARDS (71 [39%]), followed by CHF/CAD (42 [23%]), COPD (26 [14%]), post thoracic organ transplant (14 [8%]), obesity (11 [6%]), neurological diseases (9 [5%]) and trauma (7 [4%]). Two patients were classified as other (tracheal stenosis and asthma) 20.3% (37) of the 182 experienced respiratory failure following surgery and were classified as pneumonia/ARDS, COPD, CHF/CAD or post thoracic organ transplant. The majority of these (25/37) were post-cardiac surgery who experience pneumonia or CHF as a cause of respiratory failure (Fig. 2).
Table 2.
Hours weaning and lengths of stay.
| Variable | Mean | Std Dev |
|---|---|---|
| # hours weaning prior to transfer to VRU | 9.7 | 7.3 |
| Total duration of mechanical ventilation (days) | 55.0 | 42.7 |
| ICU LOS (days) | 39.7 | 36.5 |
| VRU LOS (days) | 38.8 | 48.1 |
| Total hospital LOS (days) | 74.7 | 57.6 |
Note: ICU LOS and length of mechanical ventilation includes time at outside institutions; Total Hospital LOS includes the time only at Temple University Hospital.
Abbreviations: ICU, intensive care unit; LOS, length of stay; VRU, ventilator rehabilitation unit.
Table 3.
Patient characteristics (at VRU admission unless otherwise stated).
| Variable | Mean | Std Dev |
|---|---|---|
| Age (years) | 64.1 | 15.6 |
| Charlson Index (a.u.) | 4.3 | 2.3 |
| Left ventricular ejection fraction (%) | 45.2 | 15.3 |
| Albumin on hospital admission (g/dL) | 2.7 | 1.8 |
| Albumin on VRU admission (g/dL) | 2.3 | 0.6 |
| Albumin on discharge (g/dL) | 2.6 | 0.7 |
| APACHE II (a.u) | 10.7 | 4.1 |
| BMI (kg/m2) | 28.7 | 11.3 |
| Hemoglobin (mg/dL) | 9.7 | 1.4 |
Abbreviations: a.u., arbitrary units; APACHE II, acute physiology and chronic health evaluation II; BMI, body mass index.
Table 4.
Lung mechanics.
| Variable | Mean | Std Dev |
|---|---|---|
| VRU admission | ||
| MIP (cm H2O)* | 32.2 | 11.9 |
| VE (L/min) | 7.5 | 5.0 |
| PIP (cm H2O) | 29.5 | 7.5 |
| PP (cm H2O) | 22.7 | 6.7 |
| Last day of ventilation | ||
| MIP (cm H2O)* | 38.5 | 15.1 |
| VE (L/min) | 7.0 | 3.2 |
| PIP (cm H2O) | 27.6 | 6.5 |
| PP (cm H2O) | 20.5 | 5.6 |
Note:
P value ≤ 0.001 in comparison to VRU admission, all other variable were not significantly different.
Abbreviations: VRU, ventilator rehabilitation unit; MIP, maximal inspiratory pressure; VE, minute ventilation, PIP, peak inspiratory pressure; PP, plateau pressure.
Figure 2.
The majority of patients had pneumonia, acute lung injury, heart failure, and COPD as the primary etiology for respiratory failure.
81% of (147/182) patients admitted to the VRU survived to hospital discharge. Of these, 85% (125/147) were completely weaned from mechanical ventilation (Fig. 3). Of the 22 not weaned, 13 patients were weaned to nocturnal ventilation and 9 were deemed unweanable. Of the 9 patients requiring chronic mechanical ventilation 6 were discharged to hospice. Of the remaining 3, 1 had West Nile Virus with acute and chronic quadriparetic myopathy, 1 very severe COPD and the other overlap syndrome (COPD and obesity hypoventilation).
Figure 3.
Disposition of patients. Of all patients admitted to the ventilator rehabilitation unit, 81% survived and were discharged. 85% of the survivors were completely weaned from mechanical ventilation and 66% returned to home, either directly or after a period in a rehabilitation facility.
Factors predictive of weaning failure
Patient characteristics associated with successful weaning from mechanical ventilation (univariate analysis) are shown in Table 5. Presence of COPD, higher body mass index (BMI) and longer total hospital length of say were associated with weaning failure. This negative association was retained in multivariate analysis for higher BMI (OR 0.951; CI 0.918–0.985; P < 0.005). The following characteristics were unrelated to weaning outcome: duration of mechanical ventilation, pre-VRU hospital length of stay, peak, plateau and maximal inspiratory pressure, or the presence of hypercapnia at VRU admission.
Table 5.
Predictors of weaning success.
| P value | Odds ratio | |
|---|---|---|
| Variable-univariate analysis | ||
| Longer total hospital LOS | 0.027 | 0.988 (0.978–0.999) |
| COPD present | 0.016 | 0.009 (0.001–0.417) |
| Higher BMI | 0.007 | 0.877 (0.796–0.965) |
| Variable-multivariate analysis | ||
| Higher BMI | 0.005 | 0.951 (0.918–0.985) |
Abbreviations: LOS, length of stay; BMI, body mass index; COPD, chronic obstructive pulmonary disease.
In-hospital mortality
81% of patients admitted to the VRU survived to hospital discharge. In-hospital mortality after VRU admission was 19% (147/182) Patient characteristics associated with in-hospital death by univariate analysis (Table 6) include acute renal failure requiring hemo-dialysis, any hemo-dialysis, comorbid congestive heart failure, diabetes mellitus, higher Charlson co-morbidity index (more co-morbidities), APACHE II score (more severely ill), lower albumin at VRU admission, greater number of transfusions after VRU admission and higher total packed red cell transfusions received during hospitalization. The number of transfusions received prior to VRU admission was not associated with in-hospital mortality, even when corrected for transfusions received during surgery. The association with increased mortality was retained in multivariate analysis for higher Charlson co-morbidity index, higher APACHE II score and lower albumin on VRU admission.
Table 6.
Predictors of hospital mortality.
| P value | Odds ratio | |
|---|---|---|
| Variable-univariate analysis | ||
| Become dialysis dependent during hospitalization | 0.038 | 11.00 (1.14–106.43) |
| Dialysis dependent before respiratory failure | 0.01 | 2.96 (1.29–6.81) |
| Diabetes mellitus | 0.043 | 2.21 (1.03–4.75) |
| Congestive heart failure | 0.042 | 2.20 (1.03–4.69) |
| # Transfusions in VRU | <0.0001 | 1.48 (1.23–1.79) |
| Charlson index at VRU admission | <0.0001 | 1.43 (1.20–1.69) |
| APACHE II score at VRU admission | <0.0001 | 1.24 (1.12–1.37) |
| # Transfusions—total hospitalization | 0.026 | 1.049 (1.004–1.074) |
| Higher albumin at VRU admission | 0.01 | 0.39 (0.19–0.80) |
| Variable-multivariate analysis | ||
| Higher charlson index | 0.006 | 1.08–1.60 |
| Higher APACHE II score at VRU admission | 0.016 | 1.03–1.29 |
| Higher albumin at VRU admission | 0.027 | 0.17–0.90 |
Abbreviations: VRU, ventilator rehabilitation unit; APACHE II, acute physiology and chronic health evaluation II; BMI, body mass index.
Disposition
Ultimately 65% (96/147) of patients went home. 44.9% (66) were discharged directly to home, 20.4% (30) were discharged to a rehabilitation facility then to home. 17.7% (26) were discharged to a nursing home, 9.5% (14) were discharged to a skilled nursing facility, 3% (2) were transferred to hospitals closer to home after complete weaning. 24% of the 9 (6%) unweanable, 6 were discharged to hospice and 3 to an LTAC for PMV. Figure 3 illustrates post-VRU disposition for all patients.
One and three year mortality
Post-VRU discharge survival at 1 and 3 years was 75% and 59%. Patient characteristics associated with out-of-hospital death by univariate analysis (Table 7) include older age, lower hemoglobin, presence of COPD, higher APACHE II score at VRU admission and higher number of packed red cell transfusions after VRU admission. Charlson Index, APACHE II score and packed red cell transfusions were also associated with in-hospital death. The associations of higher APACHE II score on VRU admission, presence of COPD and lower hemoglobin with 1 and 3 year mortality were retained in multivariate analysis. Any diagnosis of COPD (primary or secondary) worsened post discharge survival (HR 1.91; 95% CI 1.075–3.42; P = 0.027) (Fig. 5).
Table 7.
Predictors of long-term mortality.
| P value | Hazard ratio | |
|---|---|---|
| Variable-univariate analysis | ||
| COPD present | 0.003 | 1.31–3.7 |
| Transfusions in VRU | <0.001 | 1.13–1.44 |
| APACHE II score (at VRU admission) | <0.001 | 1.08–1.29 |
| Age | 0.054 | 1.00–1.04 |
| Lower hemoglobin (at VRU admission) | 0.002 | 0.55–0.87 |
| Variable-multivariate analysis | ||
| COPD present | 0.027 | 1.08–3.42 |
| APACHE II score (VRU admission) | <0.0001 | 1.07–1.21 |
| Hemoglobin (VRU admission) | 0.006 | 0.56–0.90 |
Abbreviations: VRU, ventilator rehabilitation unit; APACHE II, acute physiology and chronic health evaluation II.
Figure 5.
Kaplan-Meier Survival Curve in COPD vs. non COPD patients. Patients with COPD (either as a primary cause of respiratory failure or as a comorbidity) had a significantly greater mortality than non-COPD patients.
Discussion
Generally our patients were in their 60’s, in the ICU about 30 days before transfer for weaning, with multiple co-morbidities, had a left ventricular ejection fraction of 45%, were hypoalbuminemic, anemic and had multiple comorbidities and an elevated APACHE II score. Multiviariate analysis demonstrated an association with higher BMI and failure to wean. In-hospital mortality was similarly association with higher Charlson co-morbidity index, higher APACHE II score and lower albumin on VRU admission. Post-VRU discharge mortality was associated by multivariate analysis with higher APACHE II score and lower hemoglobin on VRU admission and the presence of COPD. Older age was did not retain significant association with short or long-term mortality by multivariate analysis.
The literature contains several reports of the outcomes of individual weaning centers, each with center-specific admission and discharge criteria, weaning protocols, diagnostic and therapeutic capabilities and resource allocation.4–8,10,12–17 Each center treated a patient group unique in demographics, principal diagnoses, comorbidities, and severities of illness. Furthermore the published descriptions of patients differ in detail and scope. Direct comparison of the experiences of different centers must be limited and cautious. For example Gracey and colleagues reported the outcomes of >60% post-surgical patients as 90.2% hospital survival and 72% 2-year survival.15 Whereas Stoller reported 30% post-surgical patients as 84% hospital and only 32% 2-year survival.5 Such differences in patient populations make it difficult to compare results between studies and limit the application of the findings in a broader context.
The focus of post-PMV outcomes literature has evolved in small increments since its origins in the 1980’s. An early paper by Gillespie and colleagues examined weaning and hospital survival outcomes of patients requiring invasive ventilation >24 hours.14 In this setting, prior to a dedicated weaning unit model, they looked at the aggregate experience of the surgical and medical ICUs in a single hospital. 327 patients age 62.2 ± 17.7 years ventilated 11.8 ± 20.1 days had a 33.9% hospital mortality. The patients were subdivided into six groups of respiratory failure including post-surgical, medical, acute lung injury, respiratory failure with and without multisystem organ failure and previous lung disease (mostly COPD). Hospital mortality was highest in respiratory failure with multisystem organ failure. One-year mortality was highest in non-surgical COPD patients. This scheme was emulated by Gracy in 1992 who reported excellent hospital, one and two year survivals in a predominantly post-surgical population without multisystem organ failure and minimal chronic lung disease.18 Scheinhorn and colleagues recognized the need for diagnosis related weaning success/failure analysis. He classified 206 patients into one of five diagnostic groups: acute pneumonia with underlying COPD, aspiration pneumonia with underlying neuromuscular disease, cardiac/thoracic surgery, CABG with severe CHF and Sepsis with ARDS. Relatively small numbers in each group limited the results. The authors concluded that “Larger numbers of patients in each combined-diagnosis subgroup would allow us to translate the sub-group weaning outcomes into reasonable expectation of weaning success”.8 No diagnostic group related survival analysis was presented. Others applied unique respiratory failure diagnostic classification schemes to weaning success/failure analysis with varied results.4,7,10,17 Pilcher and colleagues describe in-hospital survival according to three categories of respiratory failure: neuromuscular/chest wall, COPD and post-surgical.10 Long-term survival was not reported. Quinnel and colleagues described the long term survival in 76 patients with respiratory failure secondary to COPD who were weaned from prolonged mechanical ventilation.16 However, in the subsequent literature there has been no clarification or standardization of a categorization scheme for causes of prolonged respiratory failure. As a result, survival outcomes analysis has been limited to less incisive analysis of the negative impact of patient attributes such as age, serum albumin and severity of illness.
The survival analysis presented in this report offers a comprehensively characterized group of patients receiving prolonged mechanical ventilation. Of particular importance, we extracted diagnostic etiologies for prolonged respiratory failure as documented by the treating physicians. The seven categories chosen were thought to reflect a primary physiologic mechanism that determined need for invasive mechanical ventilation. For example “CAD/CHF” indicates left ventricular dysfunction, volume overload, possible ischemia and hypoxemia; “neuro” indicates respiratory pump weakness, whole body weakness and risk for pneumonia; “COPD” indicates static and dynamic hyperinflation, bronchospasm and inspiratory muscle weakness and so forth. We chose these categories over the more general “surgical vs. non-surgical” because of the mechanistic information contained in each. It is important to recognize that weakness, impaired nutrition, swallowing dysfunction, and a need for total body rehabilitation is nearly universal in prolonged mechanical ventilation regardless of the etiology.
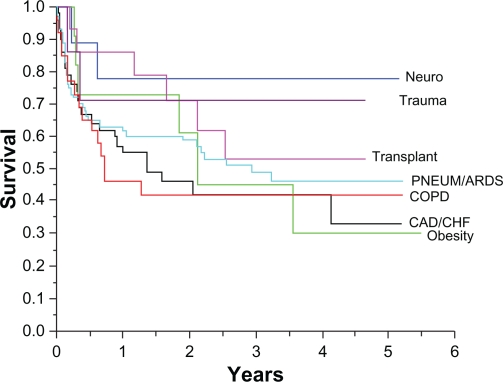
We demonstrated a statistically significant out-of-hospital survival difference between patients with neurological respiratory failure and obesity related respiratory failure (neuro < obesity, P = 0.05; Fig. 4). The neuro group included patients with acute catastrophic illness, such as stroke or Guillain-Barre syndrome, with a subsequent recovery of some or all functions, whereas the morbidly obese (with an average BMI of 56.4 kg/m2) with hypoventilation syndrome and right sided heart failure had morbid chronic complications secondary to their weight such as diabetes mellitus, supraventricular arrhythmia, venous thrombosis, pneumonia and difficulty using non-invasive positive pressure ventilation Non-significant visual trends toward survival differences between neuro and. COPD and neuro and CHF/CAD invoke the limitation of small patient numbers.
Figure 4.
Kaplan-Meier survival curve by primary cause for respiratory failure. Although not statistically significant, patients with trauma and neurological cause for respiratory failure showed a trend towards better survival than patients with chronic conditions (eg, COPD, morbid obesity).
Of all the patients who lived to be discharged (147), 85% were completely weaned of mechanical ventilation and only 6% were deemed unweanable. Interestingly, failure to wean was not related to lung mechanics, hypercapnia, or time spent on mechanical ventilation. The presence of COPD and severe obesity was associated with failure to wean. This is similar to Dasgupta’s results in which a cause of respiratory failure other than COPD was associated with improved weaning success.4 A significant increase was observed in pre-weaning MIP vs. the last day of mechanical ventilation, reinforcing the role of respiratory muscle weakness in prolonged respiratory failure and the importance of rehabilitation and restoration of skeletal muscle strength in successful weaning. Our rehabilitation protocol included daily physical therapy, inspiratory muscle strength training. Skeletal muscle weakness is undoubtedly more complex than simple deconditioning and likely results from systemic oxidative and nitrosydative stress and inflammation related to acute and chronic illness. This has been demonstrated in animal studies and in COPD. Prospective study of inflammatory biomarkers, optimal rehabilitation duration and frequency, skeletal and diaphragm muscle morphology and function during the ventilator rehabilitation and weaning process will give greater meaning to the observed improvement in MIP.
Long-term survival one year after VRU discharge was 75%, 67% at 2 years and 59% after 3 years similar to Gracey and colleagues. who reported 1, 2 and 3 year survival of 69%, 60% and 56% in a largely post-surgical group (60%).15 In contrast, only 26% of the present studies patients experienced respiratory failure after surgery vs. 60%. Our survival data is substantially higher than that reported by others in which the 1, 2 and 3 year survivals were 43%, 32% and 27%.5 It is difficult to compare results between centers. It is easy to speculate that inter-center differences are due to non-patient related factors such as experience, resources, protocols, and superior homecare after discharge. Our patient data correspond to the years 2000–2004. During the interval 1993–1996 to 2000–2004, new treatments may have contributed to better survival, such as new weaning techniques,19–21 protocol driven care,22–24 less use of sedation and paralytic agents,25 and better home care delivery.
Degree of anemia, higher APACHE II scores at VRU admission and the presence of COPD as a comorbidity correlate with poorer long term survival. Hemoglobin level is likely an epimarker for severity of illness. However it must be recognized that treatment of anemia with red cell transfusion or erythropoietin analogue may negatively influence survival.26,27
Summary
We report 81% survival to hospital discharge in a heterogeneous group of patients with respiratory failure requiring PMV and rehabilitation in a multidisciplinary VRU. Of the hospital survivors, 85% were completely weaned from invasive mechanical ventilation. Combined post-VRU discharge survival was 75% at 1 year and 59% at 3 years. Severity of illness at VRU admission characterized by APACHE II score and degree of anemia negatively influenced survival. Of the 7 diagnostic etiologies of prolonged respiratory failure “COPD” had the poorest post-VRU survival.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Cohen IL, Booth FVM. Cost Containment and Mechanical Ventilation in the United States New Horizon. 1994:183–290. [PubMed] [Google Scholar]
- 2.Scheinhorn DJ, Chao DC, Stearn-Hassenpflug M, Wallace WA. Outcomes in post-ICU mechanical ventilation: a therapist-implemented weaning protocol. Chest. 2001;119(1):236–42. doi: 10.1378/chest.119.1.236. [DOI] [PubMed] [Google Scholar]
- 3.Donat WE. Long Term Mechanical Ventilation. Marcel Dekker; 2001. Sites of care for long-term mechanical ventilation. [Google Scholar]
- 4.Dasgupta A, Rice R, Mascha E, Litaker D, Stoller JK. Four-year experience with a unit for long-term ventilation (respiratory special care unit) at the Cleveland Clinic Foundation. Chest. 1999;116(2):447–55. doi: 10.1378/chest.116.2.447. [DOI] [PubMed] [Google Scholar]
- 5.Stoller JK, Xu M, Mascha E, Rice R. Long-term outcomes for patients discharged from a long-term hospital-based weaning unit. Chest. 2003;124(5):1892–99. doi: 10.1378/chest.124.5.1892. [DOI] [PubMed] [Google Scholar]
- 6.Gracey DR, Hardy DC, Naessens JM, Silverstein MD, Hubmayr RD. The mayo ventilator-dependent rehabilitation unit: a 5-year experience. Mayo Clin Proc. 1997;72(1):13–9. doi: 10.4065/72.1.13. [DOI] [PubMed] [Google Scholar]
- 7.Bagley PH, Cooney E. A community-based regional ventilator weaning unit: development and outcomes. Chest. 1997;111(4):1024–9. doi: 10.1378/chest.111.4.1024. [DOI] [PubMed] [Google Scholar]
- 8.Scheinhorn DJ, Chao DC, Stearn-Hassenpflug M, LaBree LD, Heltsley DJ. Post-ICU mechanical ventilation: treatment of 1,123 patients at a regional weaning center. Chest. 1997;111(6):1654–9. doi: 10.1378/chest.111.6.1654. [DOI] [PubMed] [Google Scholar]
- 9.Unroe M ea. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: A Cohort Study. Annals of Internal Medicine. 2010;153:167. doi: 10.1059/0003-4819-153-3-201008030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilcher DV, Bailey MJ, Treacher DF, Hamid S, Williams AJ, Davidson AC. Outcomes, cost and long term survival of patients referred to a regional weaning centre. Thorax. 2005;60(3):187–92. doi: 10.1136/thx.2004.026500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Ai-Ping C, Lee KH, Lim TK. In-hospital and 5-year mortality of patients treated in the ICU for acute exacerbation of COPD: a retrospective study. Chest. 2005;128(2):518–24. doi: 10.1378/chest.128.2.518. [DOI] [PubMed] [Google Scholar]
- 13.Carson SS, Bach PB, Brzozowski L, Leff A. Outcomes after long-term acute care. An analysis of 133 mechanically ventilated patients. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1568–73. doi: 10.1164/ajrccm.159.5.9809002. [DOI] [PubMed] [Google Scholar]
- 14.Gillespie DJ, Marsh HM, Divertie MB, Meadows JA., 3rd Clinical outcome of respiratory failure in patients requiring prolonged (greater than 24 hours) mechanical ventilation. Chest. 1986;90(3):364–9. doi: 10.1378/chest.90.3.364. [DOI] [PubMed] [Google Scholar]
- 15.Gracey DR, Naessens JM, Viggiano RW, Koenig GE, Silverstein MD, Hubmayr RD. Outcome of patients cared for in a ventilator-dependent unit in a general hospital. Chest. 1995;107(2):494–9. doi: 10.1378/chest.107.2.494. [DOI] [PubMed] [Google Scholar]
- 16.Quinnell TG, Pilsworth S, Shneerson JM, Smith IE. Prolonged invasive ventilation following acute ventilatory failure in COPD: weaning results, survival, and the role of noninvasive ventilation. Chest. 2006;129(1):133–9. doi: 10.1378/chest.129.1.133. [DOI] [PubMed] [Google Scholar]
- 17.Schonhofer B, Euteneuer S, Nava S, Suchi S, Kohler D. Survival of mechanically ventilated patients admitted to a specialised weaning centre. Intensive Care Med. 2002;28(7):908–16. doi: 10.1007/s00134-002-1287-5. [DOI] [PubMed] [Google Scholar]
- 18.Gracey DR, Viggiano RW, Naessens JM, Hubmayr RD, Silverstein MD, Koenig GE. Outcomes of patients admitted to a chronic ventilator-dependent unit in an acute-care hospital. Mayo Clin Proc. 1992;67(2):131–6. doi: 10.1016/s0025-6196(12)61313-5. [DOI] [PubMed] [Google Scholar]
- 19.Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. 1991;324(21):1445–50. doi: 10.1056/NEJM199105233242101. [DOI] [PubMed] [Google Scholar]
- 20.Esteban A, Frutos F, Tobin MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation Spanish Lung Failure Collaborative Group. N Engl J Med. 1995;332(6):345–50. doi: 10.1056/NEJM199502093320601. [DOI] [PubMed] [Google Scholar]
- 21.Brochard L, Rauss A, Benito S, et al. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med. 1994;150(4):896–903. doi: 10.1164/ajrccm.150.4.7921460. [DOI] [PubMed] [Google Scholar]
- 22.The acute respiratory distress syndrome network. ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 23.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 24.Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 25.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342(20):1471–7. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 26.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 27.Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet. 2007;369(9559):381–8. doi: 10.1016/S0140-6736(07)60194-9. [DOI] [PubMed] [Google Scholar]
- 28.Martin UJ, Hinkapie L, Nimchuk M, et al. Impact of whole-body rehabilitation in patients receiving chronic mechanical ventilation. Crit Care Med. 33(10):2259–65. doi: 10.1097/01.ccm.0000181730.02238.9b. 2205. [DOI] [PubMed] [Google Scholar]