Abstract
Background:
The aim of this work was to study the ultrasonographic (USG) features of knee joints in relation to clinical and laboratory measures in patients with juvenile rheumatoid arthritis (JRA), and also to evaluate the accuracy of ultrasound in the diagnosis of local joint activity.
Methods:
This study included 20 with JRA and 20 matched and apparently healthy controls. All patients were subjected to full history taking, careful clinical examination and laboratory investigation. The knee joints of all patients and control were examined with plain radiography and ultrasonography on the same day of clinical examination using ultrasound to detect synovial thickness and effusion at the knee.
Results:
Mean USG knee synovial thickness was significantly greater in JRA patients versus controls (4.2 ± 2.4 mm versus 1.7 ± 0.3 mm, P < 0.001). Although knee effusion was not detected in any of the controls, it was demonstrated in 90% of JRA patients, with a mean effusion volume of 3.8 ± 3.1 mL. There was a statistically significant difference (P < 0.001) between clinically active and inactive knees with regard to knee synovial thickness. Mean knee effusion volume was significantly (P < 0.05) higher in the clinically active than in the clinically inactive knees. Patients with high disease activity had a significantly (P < 0.05) higher knee synovial thickness and knee effusion volume than patients with low and moderate disease activity. Significantly (P < 0.05) positive correlations were found between knee synovial thickness and articular index (AI) scores, disease activity score, clinical knee scores, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels. Significant positive correlations (P < 0.05) were found between knee effusion volume and AI scores, visual analog scores, disease activity scores, clinical knee scores, ESR, and CRP levels. Significant negative correlations (P < 0.05) were found between knee effusion volumes and hemoglobin levels.
Conclusion:
UGS-detected parameters represent a reliable index of JRA disease activity with a higher sensitivity for knee synovial thickness and higher specificity for knee effusion.
Keywords: juvenile rheumatoid arthritis, synovial thickness, knee effusion, ultrasonography
Introduction
Juvenile rheumatoid arthritis (JRA) is a chronic multisystem inflammatory disease with prominent joint manifestation.1 It is the most common rheumatic disease of childhood.2 The knee is the most commonly affected joint in JRA, and overall accounts for the most disability.3 Clinical parameters in the early stages of the disease have been shown to be very useful for predicting the articular outcome of JRA. Therefore, they could constitute a good instrument to help clinicians tailor the best therapy for their patients.4 Clinical evaluation of symptomatic joints is frequently supplemented with plain radiographs. However, radiographic changes mostly represent late and indirect signs of rheumatic disease.5
In addition, early detection of joint involvement would allow the treating doctor to intervene at the appropriate time and prescribe suitable medication.6 Based on these facts and on the limitation of plain radiographs for detecting radiological changes, there is a real need for other radiological variables to assess the joints in patients with arthritis.7
Ultrasonography (USG) has considerable advantages over other imaging methods, including noninvasiveness, speed of performance, relatively low cost, ability to scan multiple joints, repeatability, and high patient acceptability.8 The aim of this work was to study the USG features of the knee joints in patients with JRA, to correlate these features with clinical and laboratory parameters of JRA, and to evaluate the accuracy of USG in the diagnosis of local joint activity in JRA.
Methods
This study included 20 patients fulfilling the criteria of Cassidy et al9 for the diagnosis of JRA (Group 1) as well as 20 apparently healthy age- and gender-matched children as controls (Group 2). Patients in Group 1 comprised nine males (45%) and 11 females (55%), whose mean age was 9.2 ± 3.9 years, with a mean disease duration of 39.1 ± 32.4 months. Eight patients (40%) had polyarticular onset JRA, seven patients (35%) had pauciarticular onset, and five patients (25%) had systemic onset JRA. All patients were subjected to full history taking, clinical examination with stress of the locomotor system, and the following measurements were recorded: Ritchie articular index score for assessing joint tenderness,10 clinical assessment of the knee according to Sureda et al,11 including presence of pain (score 1 or absence score 0), degree of swelling (score 0 absence, score 1 mild, score 2 moderate, score 3 severe), degree of limitation of extension (no limitation score 0, <5° limitation of extension score 1, <10° limitation of extension score 2, <15° limitation of extension score 3, >15° limitation of extension score 4). Patients with a mean total score ≥1 were classified as having active knee involvement, while patients with normal physical findings were considered to be in clinical remission. Functional capacity of the patients was assessed using the Steinbrocker grading system12 and the juvenile arthritis functional assessment report (JAFAR) according to Lovell et al.13 Clinical assessment of disease activity was performed using the modified disease activity score of 28 joint count (DAS 28). DAS is a statistically derived index consisting of number of tender joints, number of swollen joints, erythrocyte sedimentation rate, and global disease activity.14 Laboratory investigations were performed for all patients, including determination of hemoglobin concentration (g/dL), erythrocyte sedimentation rate by Westergrens method, C-reactive protein detection by the latex agglutination slide test, and rheumatoid factor slit lamp for antinuclear antibodies. Conventional radiological examination of the knee joint in the anteroposterior and lateral projections were obtained on the same day as the ultrasound examination. USG was done for both knees of all patients and controls on the same day as the clinical and laboratory investigation using the Megas Esaote SpA with a 7.5 mHz linear probe and color Doppler. Knee ultrasound was obtained by sets of sagittal images of the suprapatellar bursa with the patient in the recumbent position and the knee in 30% flexion. A standardized procedure similar to that used previously by other investigators11 was followed. The ultrasound transducer was positioned longitudinally above the patella, and the synovial thickness was measured when the probe touched the middle portion of the basis patella, measurement of total synovial thickness (with electronic calipers and corresponding to the largest anteroposterior diameter of the suprapatellar pouch) was performed by applying firm compression with the transducer to express the suprapatellar fluid into the joint recesses. Assessment of intra-articular fluid was performed by measuring the length of the suprapatellar bursa. Longitudinal images were obtained with manual compression of the lateral synovial recesses to express all intra-articular fluid into the suprapatellar bursa. Transverse diameters were also obtained for the longitudinal images, followed by depth measurements in the transverse images. The volume of fluid was then calculated.
Results
Table 1 shows the clinical, laboratory, and USG characteristics of the 20 JRA patients. In our study, clinical knee joint involvement was present in 15 patients (75%) of 20 patients examined (Table 1). Six patients (40%) had polyarticular onset disease, five patients (33.3%) had pauciarticular onset, and four patients (26.7%) had systemic onset disease. Bilateral knee joint involvement was found in 11 patients (73.3%), while unilateral involvement was found in four patients (26.7%). Clinical knee joint involvement was observed in 26 of the 40 knees examined.
Table 1.
Clinical, laboratory, and ultrasonographic characteristics of 20 patients with juvenile rheumatoid arthritis.
| Age, years, mean ± SD (range) | 9.2 ± 3.9 (4–16) |
| Gender, male/female (%) | 9/11 (45%–55%) |
| Disease onset (%) | Pauci/poly/syst 7/8/5 (35%/40%/25%) |
| Disease duration/months, mean ± SD (range) | 39.1 ± 32.4 (7−120) |
| Articular index, mean ± SD | 16.4 ± 10.6 |
| Visual analog scale (cm), mean ± SD | 5.1 ± 1.9 |
| Disease activity score, mean ± SD | 4.0 ± 1.1 |
| Functional capacity grading (%) | 1/2/3 8/8/4 (40%/40%/20%) |
| JAFAR score, mean ± SD | 8.4 ± 4.4 |
| Clinical knee score, mean ± SD | 2.7 ± 2.5 |
| Hemoglobin (g/dL), mean ± SD | 10.0 ± 1.4 |
| ESR level (mm/hour), mean ± SD | 47.2 ± 17.5 |
| CRP level (mg/L), mean ± SD | 29.2 ± 20.9 |
| Rheumatoid factor (%) | 5 positive/15 negative (25%−75%) |
| USG knee synovial thickness (mm), mean ± SD | 4.2 ± 2.4 |
| USG knee effusion volume (mL), mean ± SD | 3.8 ± 3.1 |
Abbreviations: JAFAR, juvenile arthritis functional assessment report; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; USG, ultrasonographic; SD, standard deviation; syst, systemic; poly, polyarticular; pauci, pauciarticular.
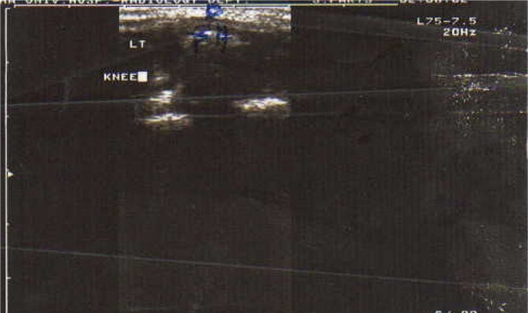
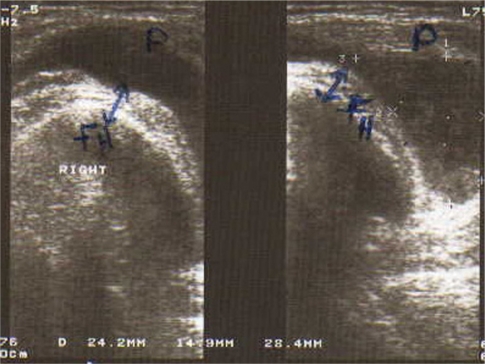
Mean ± standard deviation (SD) USG knee synovial thickness was 4.2 ± 2.4 mm in the JRA patients and 1.7 ± 0.3 mm in the control group. This difference was statistically significant (P < 0.001). Although knee effusion was not detected in any of the control subjects, it was demonstrated in 90% of the JRA patients, with a mean effusion volume of 3.8 ± 3.1 mL (Figure 1 and 2).
Figure 1.
Ultrasound of the right knee joint (ventral longitudinal scan of suprapatellar pouch) showing normal knee synovial thickness. Note that no effusion is detected.
Abbreviations: P, patella; FH, femoral head.
Figure 2.
Ultrasound of right knee joint (ventral longitudinal scan of the suprapatellar pouch) showing pathological knee synovial thickness (arrow) and knee effusion in a patient with juvenile rheumatoid arthritis.
In the JRA patients, mean knee synovial thickness was 5.2 ± 2.3 mm in the clinical active knees and 2.3 ± 1.0 mm in the clinically inactive knees. This difference was statistically significant (P < 0.001). Mean USG knee effusion volume was significantly higher in clinically active knees than in clinically inactive knees (4.8 ± 3.6 mL and 2.0 ± 0.9 mL, respectively, P < 0.05).
There was a highly statistically significant (P < 0.001) difference in levels of C-reactive protein and erythrocyte sedimentation rate between patients with low, moderate, and high disease activity, being highest in the first hour in those with high disease activity (60.9 ± 15.6 mm and 50.3 ± 24.6 mg/L, respectively). There was a statistically significant (P < 0.05) difference between patients for articular index score and JAFAR scores, being highest in patients with high disease activity (25.9 ± 11.0 and 11.1 ± 4.9, respectively). Patients with high disease activity had significantly (P < 0.05) lower hemoglobin levels (9.4 ± 1.0 g/dL).
There was no significant difference for disease duration, visual analog score, or clinical knee score. Patients with high disease activity had a significantly (P < 0.05) greater USG knee synovial thickness (6.7 ± 1.7 mm) and USG knee effusion volume (7.9 ± 5.2 mL) than patients with low or moderate disease activity.
Patients with functional capacity Grade 3 had had a significantly (P < 0.001) longer mean disease duration (60.3 ± 25.4 months) and significantly (P < 0.005) higher mean JAFAR score (12.4 ± 3.7) than patients with Grade 1 and Grade 2 functional capacity. There was statistically significant difference between the patients with Grade 1, 2, and 3 functional capacity with regard to articular index score, visual analog score, DAS score, clinical knee score, hemoglobin level, erythrocyte sedimentation rate, C-reactive protein, USG knee synovial thickness, or USG knee effusion volume.
From our calculations, the upper limit of normal value (cutoff point) for USG knee synovial thickness was 2.3 mm, recorded as the mean for controls + 2 SD. In our study, 26 of the 40 knees examined were clinically active. Twenty-five of the 26 clinically active knees showed pathological USG synovial thickness >2.3 mm. Seven clinically inactive knees showed USG pathological synovial thickness >2.3 mm. Two control knees showed USG pathological thickness >2.3 mm. These results yielded a sensitivity of 82.5% and a specificity of 95%, a positive predictive value of 94.3%, and a negative predictive value of 84.4%. None of our control subjects showed intra-articular knee effusion, whereas USG knee effusion was detected in 90% of our JRA patients, indicating 100% specificity of ultrasound in detecting effusion in clinically active joints.
Discussion
JRA is one of the most common inflammatory diseases of childhood and is a major cause of disability.15 Several clinical studies done in patients with JRA have shown that the knee is the joint most frequently affected.16 History-taking and physical examination supplemented with radiological investigation are the usual tools to make the diagnosis of JRA.17 Radiographic changes mostly represent late and indirect signs of synovial disease.18
Over the past few years, rheumatologists have become increasingly interested in detection of synovitis and bony erosions in both small and large joints.19 It has several advantages over magnetic resonance imaging, including cost, immediate availability in the clinic, and the ability to scan multiple joints simultaneously.20,21 The demographic characteristics of our patients were similar to those reported by Fedrizzi et al,22 whose study comprised 35 patients with JRA of mean age nine (2–16) years. Their disease duration ranged between six months and 11 years, with a mean duration of three years. Systemic onset was documented in 17% of patients, polyarticular onset in 34%, and pauciarticular onset in 49%. Our clinical data are also consistent with those of Argyropoulou et al.23 Their study included 28 patients with JRA, comprising 13 patients (46.5%) with polyarticular onset, eight (28.5%) with pauciarticular onset, and seven (25%) with systemic onset. In our study, clinical knee joint activity was present in 15 (75%) of 20 patients examined (26 of 40 knees). Bilateral knee joint involvement was found in 11 patients (73.3%), while unilateral involvement was found in four patients (26.7%). Of 15 patients, six (40%) had polyarticular onset, five (33.3%) had pauciarticular onset, and four (26.7%) had systemic onset disease. El Miedany et al3 stated that the knee is the joint most frequently affected. In our study, we examined the knees by USG for detection of both synovial thickness and effusion volume, and found a mean USG synovial thickness of the knee joints in JRA patients of 4.2 ± 2.4 mm which was significantly (P < 0.001) higher than the mean value of 1.7 ± 0.3 recorded in our control subjects. These results are consistent with those reported by Sureda et al,11 for 36 children with JRA and 30 healthy controls. They found that mean synovial thickness of the knee joint was significantly (P < 0.001) greater in patients with JRA than in the controls (5.2 ± 3 mm versus 2.7 ± 0.8 mm, respectively).
Our results differ slightly from those of Barbuti et al,24 who investigated the role of USG in follow-up of the knee joint, and undertook 594 examinations in 240 children with JRA, reporting a mean synovial thickness of 6 mm (range 2.5–11) mm in JRA patients with affected knees compared with a mean synovial thickness of 2.7 (range 1.0–4.5) mm in normal subjects. This variability of synovial thickness results could be attributed to the large number of patients examined in their study. Our results also relatively close to those of EL-Miedany and his colleagues,3 who studied the ultrasound versus magnetic resonance imaging in evaluating the knee joint in patients with JRA. They reported that the synovial thickness of the knee joint was significantly increased in patients with JRA, with a mean of 5.2 ± 2.5 mm on both ultrasound and magnetic resonance imaging, compared with a mean thickness of 2.7 mm ± 0.8 mm in the control group. the authors also stated that, in healthy children, visualization of the synovial membrane may be difficult because it is extremely thin and has echogenicity similar to that of the surrounding tissue. In our study, there was a highly statistically significant difference (P < 0.001) between the mean USG synovial thickness of clinically active (5.2 ± 2.3 mm) and clinically inactive knees (2.3 ± 1 mm). There was also a statistically significant (P < 0.05) difference in the mean USG knee synovial thickness between patients with JRA according to their disease activity score, being greatest in those with a high activity score (Grade 3) who had a mean thickness of 6.0 ± 1.7 mm. This agrees with work done by Cellerinui et al,25 who found a statistically significant (P < 0.001) increase in the mean USG synovial thickness of clinically active knee joints versus clinically silent joints. Our finding contrast with those reported by Frosch et al,26 who did not find a significant difference in either the number of patients with synovial thickness or the mean synovial thickness between clinically active and inactive arthritis at follow-up examinations. In their study, the volume of fluid in the suprapatellar bursa in JRA knees ranged between 2.4 ± 15 mL, with a mean of 3.8 ± 3.1 mL. Sureda et al11 reported similar findings whereby ultrasound could not detect knee effusion in healthy children and demonstrated effusion in 60% of documented clinically active knees. In their study, the length of the suprapatellar bursa was measured rather than the volume of the effusion. Also El-Miedany et al3 detected mild to moderate joint effusion in 18 out of 38 cases of JRA by assessment of the length of the suprapatellar bursa. Frosch et al26 found a marked effusion within the suprapatellar pouch by measuring the largest anterioposterior diameter of the suprapatellar pouch in mm. In our study, we measured the length, width, and depth of the bursa to determine the actual volume of the effusion in the suprapatellar bursa. We assumed that this would be a more accurate assessment method, especially after noticing the variability in the depth and width of the bursa. Comparing the mean USG knee effusion volume in our JRA patients according to their local disease activity, we found a significant increase (P < 0.05) in mean USG effusion in active knees compared with inactive knees. This finding is in agreement with that of Cellerini et al25 who reported a statistically significant increase in articular effusion from clinically active knee joints versus clinically silent joints. This also coincides with a study done with Forsch et al26 who found a highly significant (P < 0.001) difference in the number of patients with joint effusion as well as in the mean USG joint effusion volume between patients with clinically active and inactive knee arthritis at follow-up examinations. In our study, there was a significant positive correlation (P < 0.05), between mean USG synovial thickness of the knee and the clinical knee scores (r = 0.71), articular indices (r = 0.74), DAS (r = 0.73), erythrocyte sedimentation rate (r = 0.61), and C-reactive protein levels (r = 0.5). There was also a significant positive correlation (P < 0.05) between mean USG knee effusion volumes and articular indices (r = 0.64), VAS (r = 0.41), DAS (r = 0.83), clinical knee scores (r = 0.85), erythrocyte sedimentation rate (r = 0.44) and C-reactive protein levels (r = 0.45). This coincides with the findings of Frosch et al26 who found a strong positive significant correlation (P < 0.001) between sonographic parameters, especially joint effusion, and the clinical findings of knee joint examination. With regard to USG knee synovial thickness, we found a sensitivity of 82.5%, a specificity of 95%, a positive predictive value of 94.3%, and a negative predictive value of 84.4%. For knee joint effusion, none was detected in the control group, but it was detected in 90% of the patients, indicating 100% specificity of ultrasound for detecting effusion in clinically active joints. This coincides with a report by Cellerini et al,25 who commented that ultrasound is particularly sensitive in the detection of synovial effusion and its differentiation from synovial thickness. In their study, ultrasound demonstrated effusion and synovial thickness in 70% of clinically silent joints. This is also in agreement with the findings of El Miedany et al,3 who found mild to moderate effusion on ultrasound in 18 of 38 cases (47%), and confirms a report by Kaye et al27 who stated that ultrasound is a sensitive method for detecting the presence of small suprapatellar effusion. A prospective study is recommended to assess whether USGs finding could be predictive of early relapse in order to treat a silent joint, as long as diagnosis and therapy is routinely established on a clinical basis.
Table 2.
Correlation coefficients between clinical and laboratory variables of patients with juvenile rheumatoid arthritis in relation to their ultrasonographic findings.
| USG knee synovial thickness | USG knee effusion volume | |
|---|---|---|
| Disease duration (month) | 0.18 | 0.23 |
| AI score | 0.74* | 0.64* |
| VAS (cm) | 0.21 | 0.41* |
| DAS score | 0.73* | 0.83* |
| JAFAR score | 0.13 | 0.37 |
| Clinical knee score | 0.71* | 0.85* |
| Clinical hip score | 0.23 | 0.15 |
| Hb level (gm/dL) | −0.31 | −0.81* |
| ESR level (mm first hour) | 0.61* | 0.44* |
| CRP level (mg/L) | 0.51* | 0.45* |
Notes: Critical value(r) = 0.9; P > 0.05 insignificant; P < 0.05* = significant.
Abbreviations: AI, articular index; DAS, disease activity score; VAS, visual analog scale; JAFAR, juvenile arthritis functional assessment record; Hb, hemoglobin level; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; USG, ultrasonography.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Kakati P, Sodhi KS, Sandhu MS, Singh S, Katariya S, Khandelwal N. Clinical and ultrasound assessment of the knee in children with juvenile rheumatoid arthritis. Ind J Pediatr. 2007;74:831–6. doi: 10.1007/s12098-007-0148-1. [DOI] [PubMed] [Google Scholar]
- 2.Lang BA, Schneider R, Reilly BJ, Silverman ED, Laxer RM. Radiologic features of systemic onset juvenile rheumatoid arthritis. J Rheumatol. 1995;22:168–73. [PubMed] [Google Scholar]
- 3.El-Miedany YM, Housny IH, Mansour HM, Mourad HG, Mehanna AM, Megeed MA. Ultrasound versus MRI in the evaluation of juvenile idiopathic arthritis of the knee. Joint Bone Spine. 2001;68:222–30. doi: 10.1016/s1297-319x(01)00269-x. [DOI] [PubMed] [Google Scholar]
- 4.Modesto C, Woo P, Garcia G, Merino R, Anal C. Systemic onset juvenile chronic arthritis, polyarticular pattern and hip involvement as markers for a bad prognosis. Clin Exp Rheumatol. 2001;19:211–7. [PubMed] [Google Scholar]
- 5.Naredo E, Bonilla G, Gamero F, Uson J, Carmona L, Laffon A. Assessment of inflammatory activity in rheumatoid arthritis: a comparative study of clinical evaluation with grey scale and power Doppler ultrasonography. Ann Rheum Dis. 2005;64:375–81. doi: 10.1136/ard.2004.023929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J, Lee SK, Suh JS, Yoon M, Song JH, Lee CH. Magnetic resonance imaging of the wrist in defining remission of rheumatoid arthritis. J Rheumatol. 1997;24:1303–8. [PubMed] [Google Scholar]
- 7.Pinals RS, Masi AT, Larsen RA. Preliminary criteria for clinical remission in rheumatoid arthritis. Arthritis Rheum. 1981;24:1308–15. doi: 10.1002/art.1780241012. [DOI] [PubMed] [Google Scholar]
- 8.Naredo E, Möller I, Moragues C. Interobserver reliability in musculoskeletal ultrasonography: Results from a “Teach the Teachers” rheumatologist course. Ann Rheum Dis. 2006;65:14–9. doi: 10.1136/ard.2005.037382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassidy JT, Levinson JE, Bass JC, et al. A study of classification criteria for a diagnosis of juvenile rheumatoid arthritis. Arthritis Rheum. 1986;29:274–81. doi: 10.1002/art.1780290216. [DOI] [PubMed] [Google Scholar]
- 10.Ritchie DM, Boyle JE, McInnes JM, Jasni MK. Clinical studies with an articular index for assessment of joint tenderness in patients with rheumatoid arthritis. Q J Med. 1968;147:393–406. [PubMed] [Google Scholar]
- 11.Sureda D, Quiroga S, Arnal C, Boronat M, Andreu J, Casas L. Juvenile rheumatoid arthritis of the knee: Evaluation with US. Radiology. 1994;190:403–6. doi: 10.1148/radiology.190.2.8284388. [DOI] [PubMed] [Google Scholar]
- 12.Steinbrocker O, Trager GH, Butterman RC. Theraputic criteria in juvenile rheumatoid arthritis. JAMA. 1949;140:659–62. doi: 10.1001/jama.1949.02900430001001. [DOI] [PubMed] [Google Scholar]
- 13.Lovell DJ, Howe S, Shear A, et al. Development of a disability measurement tool for juvenile rheumatoid arthritis: The arthritis functional assessment scale. Arthritis Rheum. 1989;32:1390–5. doi: 10.1002/anr.1780321107. [DOI] [PubMed] [Google Scholar]
- 14.Prevo MLL, Vanthof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 15.Horneff G, Schmeling H, Biedermann T, et al. The German etanercept registry for treatment of juvenile idiopathic arthritis. Ann Rheum Dis. 2004;63:1638–44. doi: 10.1136/ard.2003.014886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karim Z, Wakefield RJ, Quinn M, et al. Validation and reproducibility of ultrasonography in the detection of synovitis in the knee. Arthritis Rheum. 2004;50:387–94. doi: 10.1002/art.20054. [DOI] [PubMed] [Google Scholar]
- 17.Bierma SMA, Bohnen AM, Verhaar JAN, Prins A, Ginaikaramata AZ, Lameris JS. Sonography for hip joint effusion in adults with hip pain. Ann Rheum Dis. 2000;59:178–82. doi: 10.1136/ard.59.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kane D, Balint PV, Sturrock RD. Ultrasonography is superior to clinical examination in detection and localization of the knee joint effusion in rheumatoid arthritis. J Rheumatol. 2003;30:966–71. [PubMed] [Google Scholar]
- 19.Alarcon GS, Lopez-Ben R, Moreland W. High resolution ultrasound for the study of target joints in rheumatoid arthritis. Arthritis Rheum. 2002;46:1969–81. doi: 10.1002/art.10310. [DOI] [PubMed] [Google Scholar]
- 20.Wakefield RJ, Green MJ, Marzo-Orteg OH, et al. Should oligoarthritis be reclassified? Ultrasound reveals high prevalence of subclinical disease. Ann Rheum Dis. 2004;63:382–5. doi: 10.1136/ard.2003.007062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson K. Imaging of juvenile idiopathic arthritis. Pediatr Radiol. 2006;36:743–58. doi: 10.1007/s00247-006-0199-x. [DOI] [PubMed] [Google Scholar]
- 22.Fedrizzi MS, Ronchezel MV, Hilario MO, et al. Ultrasonography in the early diagnosis of hip joint involvement in juvenile rheumatoid arthritis. J Rheumatol. 1997;24:1820–5. [PubMed] [Google Scholar]
- 23.Argyropoulou MI, Fanis SL, Tenakis T, Fremidis SC, Simopoulou A. The role of MRI in the evaluation of hip joint disease in clinical subtype of juvenile idiopathic arthritis. Br J Radiol. 2002;75:229–33. doi: 10.1259/bjr.75.891.750229. [DOI] [PubMed] [Google Scholar]
- 24.Barbuti D, Bergami G, Vecchioli Scaldazza AA. Role of ultrasonography of the knee on the follow-up of juvenile rheumatoid arthritis. Radiol Med. 1997;93:27–32. Italian. [PubMed] [Google Scholar]
- 25.Cellerini M, Salti S, Trapani S, D’Elia G, Falcini F, Villari N. Correlation between clinical and ultrasound assessment of the knee in children with mono-articular or pauci-articular juvenile rheumatoid arthritis. Pediatr Radiol. 1999;29:117–23. doi: 10.1007/s002470050554. [DOI] [PubMed] [Google Scholar]
- 26.Frosch M, Foell D, Ganser G, Roth J. Arthrosonography of hip and knee joints in the follow up of juvenile rheumatoid arthritis. Ann Rheum Dis. 2003;62:242–4. doi: 10.1136/ard.62.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaye JJ. Arthritis: Roles of radiography and other imaging techniques in evaluation. Radiology. 1990;177:601–8. doi: 10.1148/radiology.177.3.2243957. [DOI] [PubMed] [Google Scholar]