Abstract
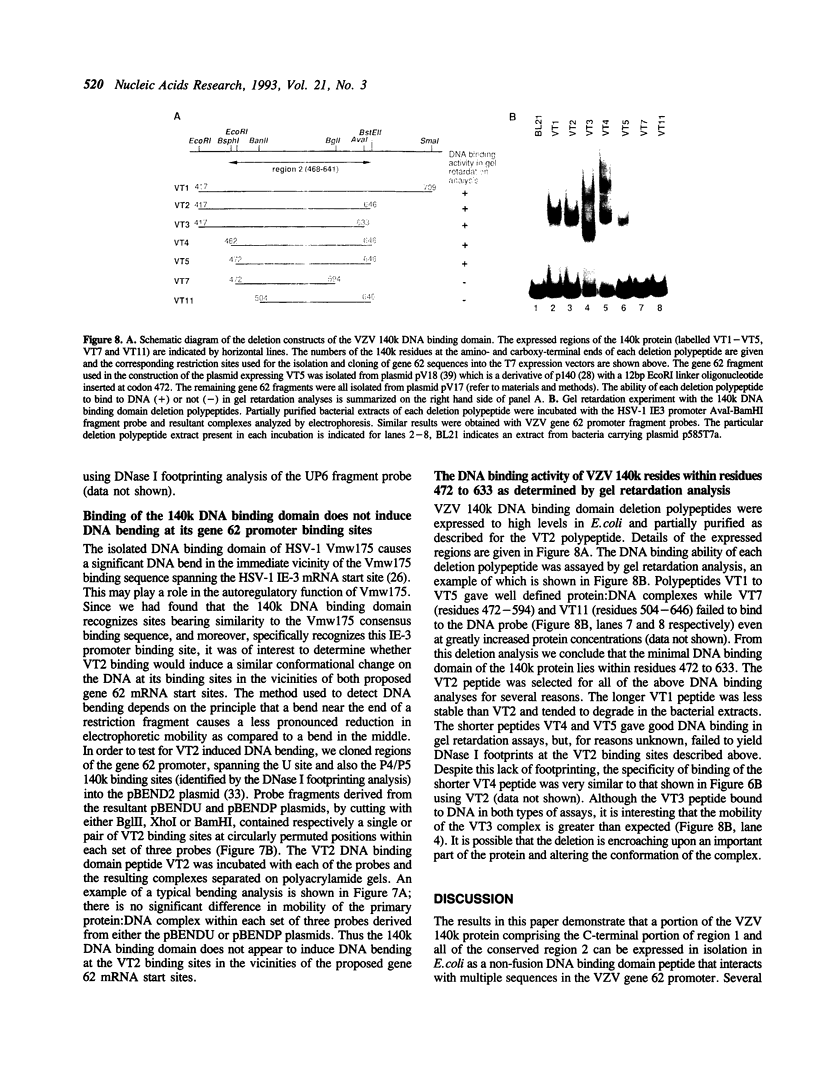
Varicella-zoster virus gene 62 encodes a protein with predicted Mr of 140,000D (VZV 140k) that shares extensive predicted amino acid sequence homology with the major immediate early (IE) transcriptional regulator protein of herpes simplex virus type 1 (HSV-1) Vmw175. The integrity of highly conserved region 2 is essential for the DNA binding and transcriptional regulatory functions of Vmw175. Similarly, an insertion mutation in region 2 (codons 468-641) of 140k eliminates the transcriptional repression and activation functions of this protein. We have expressed a fragment of 140k which encompasses region 2 as a non-fusion polypeptide in bacteria. This 140k DNA binding domain peptide (codons 417-646) binds to numerous DNA sequences throughout the VZV gene 62 promoter region. It induces multiple regions of protection from DNase I digestion, flanked by sites of DNase I hypersensitivity. Several of the sites recognized can be considered to be divergent forms of the consensus sequence which is recognized by Vmw175. However, by use of a panel of mutagenized probe fragments, we found that the 140k DNA binding domain was less sequence-specific than Vmw175 in its interactions with DNA. Consistent with this, the homologous Vmw175 DNA binding domain, and also intact Vmw175, recognize the gene 62 binding sites much less efficiently than the 140k DNA binding domain. Also in contrast to the situation with Vmw175, the 140k DNA binding domain failed to induce DNA bending when occupying the binding sites in its own promoter. Deletion analysis has mapped the minimal DNA binding domain of the VZV 140k protein, as measured in gel retardation analysis, to lie within residues 472 to 633. The differences in binding characteristics of the DNA binding domains of the homologous VZV 140k and HSV-1 Vmw175 IE proteins may account for the subtle differences in their regulatory activities in transfection assays and during virus growth in tissue culture.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggin M., Farrell P. J., Barrell B. G. Transcription and DNA sequence of the BamHI L fragment of B95-8 Epstein-Barr virus. EMBO J. 1984 May;3(5):1083–1090. doi: 10.1002/j.1460-2075.1984.tb01933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. B., Watson R. J., Wilkie N. M. Temporal regulation of herpes simplex virus type 1 transcription: location of transcripts on the viral genome. Cell. 1977 Sep;12(1):275–285. doi: 10.1016/0092-8674(77)90205-7. [DOI] [PubMed] [Google Scholar]
- Cromlish W. A., Abmayr S. M., Workman J. L., Horikoshi M., Roeder R. G. Transcriptionally active immediate-early protein of pseudorabies virus binds to specific sites on class II gene promoters. J Virol. 1989 May;63(5):1869–1876. doi: 10.1128/jvi.63.5.1869-1876.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- DeLuca N. A., McCarthy A. M., Schaffer P. A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985 Nov;56(2):558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca N. A., Schaffer P. A. Physical and functional domains of the herpes simplex virus transcriptional regulatory protein ICP4. J Virol. 1988 Mar;62(3):732–743. doi: 10.1128/jvi.62.3.732-743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDonato J. A., Muller M. T. DNA binding and gene regulation by the herpes simplex virus type 1 protein ICP4 and involvement of the TATA element. J Virol. 1989 Sep;63(9):3737–3747. doi: 10.1128/jvi.63.9.3737-3747.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDonato J. A., Spitzner J. R., Muller M. T. A predictive model for DNA recognition by the herpes simplex virus protein ICP4. J Mol Biol. 1991 Jun 5;219(3):451–470. doi: 10.1016/0022-2836(91)90186-a. [DOI] [PubMed] [Google Scholar]
- Disney G. H., Everett R. D. A herpes simplex virus type 1 recombinant with both copies of the Vmw175 coding sequences replaced by the homologous varicella-zoster virus open reading frame. J Gen Virol. 1990 Nov;71(Pt 11):2681–2689. doi: 10.1099/0022-1317-71-11-2681. [DOI] [PubMed] [Google Scholar]
- Disney G. H., McKee T. A., Preston C. M., Everett R. D. The product of varicella-zoster virus gene 62 autoregulates its own promoter. J Gen Virol. 1990 Dec;71(Pt 12):2999–3003. doi: 10.1099/0022-1317-71-12-2999. [DOI] [PubMed] [Google Scholar]
- Everett R. D., DiDonato J., Elliott M., Muller M. Herpes simplex virus type 1 polypeptide ICP4 bends DNA. Nucleic Acids Res. 1992 Mar 25;20(6):1229–1233. doi: 10.1093/nar/20.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D., Dunlop M. Trans activation of plasmid-borne promoters by adenovirus and several herpes group viruses. Nucleic Acids Res. 1984 Aug 10;12(15):5969–5978. doi: 10.1093/nar/12.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D., Elliott M., Hope G., Orr A. Purification of the DNA binding domain of herpes simplex virus type 1 immediate-early protein Vmw175 as a homodimer and extensive mutagenesis of its DNA recognition site. Nucleic Acids Res. 1991 Sep 25;19(18):4901–4908. doi: 10.1093/nar/19.18.4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D., Paterson T., Elliott M. The major transcriptional regulatory protein of herpes simplex virus type 1 includes a protease resistant DNA binding domain. Nucleic Acids Res. 1990 Aug 11;18(15):4579–4585. doi: 10.1093/nar/18.15.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D. The regulation of transcription of viral and cellular genes by herpesvirus immediate-early gene products (review). Anticancer Res. 1987 Jul-Aug;7(4A):589–604. [PubMed] [Google Scholar]
- Everett R. D. Trans activation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 1984 Dec 20;3(13):3135–3141. doi: 10.1002/j.1460-2075.1984.tb02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felser J. M., Kinchington P. R., Inchauspe G., Straus S. E., Ostrove J. M. Cell lines containing varicella-zoster virus open reading frame 62 and expressing the "IE" 175 protein complement ICP4 mutants of herpes simplex virus type 1. J Virol. 1988 Jun;62(6):2076–2082. doi: 10.1128/jvi.62.6.2076-2082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felser J. M., Straus S. E., Ostrove J. M. Varicella-zoster virus complements herpes simplex virus type 1 temperature-sensitive mutants. J Virol. 1987 Jan;61(1):225–228. doi: 10.1128/jvi.61.1.225-228.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inchauspe G., Ostrove J. M. Differential regulation by varicella-zoster virus (VZV) and herpes simplex virus type-1 trans-activating genes. Virology. 1989 Dec;173(2):710–714. doi: 10.1016/0042-6822(89)90584-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Brauer D. H. Complete DNA sequence of the short repeat region in the genome of herpes simplex virus type 1. Nucleic Acids Res. 1986 Feb 25;14(4):1727–1745. doi: 10.1093/nar/14.4.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee T. A., Disney G. H., Everett R. D., Preston C. M. Control of expression of the varicella-zoster virus major immediate early gene. J Gen Virol. 1990 Apr;71(Pt 4):897–906. doi: 10.1099/0022-1317-71-4-897. [DOI] [PubMed] [Google Scholar]
- Muller M. T. Binding of the herpes simplex virus immediate-early gene product ICP4 to its own transcription start site. J Virol. 1987 Mar;61(3):858–865. doi: 10.1128/jvi.61.3.858-865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson T., Everett R. D. Mutational dissection of the HSV-1 immediate-early protein Vmw175 involved in transcriptional transactivation and repression. Virology. 1988 Sep;166(1):186–196. doi: 10.1016/0042-6822(88)90160-2. [DOI] [PubMed] [Google Scholar]
- Paterson T., Everett R. D. The regions of the herpes simplex virus type 1 immediate early protein Vmw175 required for site specific DNA binding closely correspond to those involved in transcriptional regulation. Nucleic Acids Res. 1988 Dec 9;16(23):11005–11025. doi: 10.1093/nar/16.23.11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer L. I., Everett R. D., Tedder D. G., Elliott M., Litman B. Nucleotides within both proximal and distal parts of the consensus sequence are important for specific DNA recognition by the herpes simplex virus regulatory protein ICP4. Nucleic Acids Res. 1991 Feb 11;19(3):477–483. doi: 10.1093/nar/19.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J Virol. 1979 Jan;29(1):275–284. doi: 10.1128/jvi.29.1.275-284.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. S., Boundy A., O'Hare P., Pizzorno M. C., Ciufo D. M., Hayward G. S. Direct correlation between a negative autoregulatory response element at the cap site of the herpes simplex virus type 1 IE175 (alpha 4) promoter and a specific binding site for the IE175 (ICP4) protein. J Virol. 1988 Nov;62(11):4307–4320. doi: 10.1128/jvi.62.11.4307-4320.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki K., Hyman R. W. The immediate early proteins of varicella-zoster virus. Virology. 1987 Feb;156(2):423–426. doi: 10.1016/0042-6822(87)90423-5. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Thali M., Rusconi S., Schaffner W. Immediate early protein of pseudorabies virus is a general transactivator but stimulates only suboptimally utilized promoters. A clue to specificity? J Mol Biol. 1990 Sep 20;215(2):301–311. doi: 10.1016/S0022-2836(05)80348-1. [DOI] [PubMed] [Google Scholar]
- Wu C. L., Wilcox K. W. Codons 262 to 490 from the herpes simplex virus ICP4 gene are sufficient to encode a sequence-specific DNA binding protein. Nucleic Acids Res. 1990 Feb 11;18(3):531–538. doi: 10.1093/nar/18.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. L., Wilcox K. W. The conserved DNA-binding domains encoded by the herpes simplex virus type 1 ICP4, pseudorabies virus IE180, and varicella-zoster virus ORF62 genes recognize similar sites in the corresponding promoters. J Virol. 1991 Mar;65(3):1149–1159. doi: 10.1128/jvi.65.3.1149-1159.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieb C., Kim J., Adhya S. DNA bending by negative regulatory proteins: Gal and Lac repressors. Genes Dev. 1989 May;3(5):606–611. doi: 10.1101/gad.3.5.606. [DOI] [PubMed] [Google Scholar]








