Abstract
The calcium ion (Ca2+) plays fundamental roles in orchestrating dynamic changes in the function and structure of nerve cell circuits in the brain. The endoplasmic reticulum (ER) is a particularly intriguing organelle that actively removes Ca2+ from the cytoplasm, and can release stored Ca2+ through ER membrane receptor channels responsive to either the lipid messenger IP3 or to cytosolic Ca2+. Emerging findings suggest that perturbed ER Ca2+ homeostasis contributes to the dysfunction and degeneration of neurons that occurs in Alzheimer’s disease (AD). Interestingly, mutations in presenilin-1 (PS1) (an integral membrane protein in the ER of neurons) that cause early-onset inherited AD increase the pool of ER Ca2+ available for release, and enhance Ca2+ release through ER IP3- and ryanodine-sensitive channels. By enhancing Ca2+ flux across the ER membrane, PS1 mutations may exaggerate Ca2+ signaling in synaptic terminals and thereby render them vulnerable to dysfunction and degeneration in the settings of aging and amyloid accumulation in AD.
Approximately 5 million Americans and 30 million individuals world-wide are currently afflicted with Alzheimer’s disease (AD), and is estimated that these numbers will more than double by the year 2050 because of the rapid increase in individuals who live to an age (65+ years) when AD most commonly strikes (1). Nerve cell circuits involved in short-term memory, including those in the entorhinal cortex, hippocampus, prefrontal cortex and basal forebrain are affected early in the AD process and exhibit the most neuronal loss as the disease progresses. Advancing age is the major risk factor for AD, and quantitative structural magnetic resonance imaging data suggest that many of the same brain regions that degenerate in AD exhibit progressive, albeit slower, atrophy during aging in cognitively normal subjects (2). Additional risk factors for AD include a sedentary lifestyle, diabetes, traumatic brain injury and clinical depression. It is believed that each of these risk factors may render neurons vulnerable to AD by increasing levels of oxidative stress and promoting cellular energy deficits in neurons. Why some individuals with one or more of these risk factors escape AD is unknown, but may involve a “cognitive reserve” and/or increased levels of “neuroprotective factors” (3, 4).
Studies of the brains of living and deceased AD patients, of genetic causes of rare inherited forms of AD, and of experimental models of AD have elucidated the sequence of events that occur in nerve cells that result in their dysfunction and death (5, 6). The accumulation of amyloid β-peptide (Aβ), a 40–42 amino acid self-aggregating peptide that forms compact fibrillar deposits (plaques) and diffuse non-fibrillar aggregates, occurs in affected brain regions early in the disease process. Aβ is produced when the β-amyloid precursor protein (APP; an integral membrane protein) is cleaved first at the N-terminus of Aβ by β-secretase and then at the C-terminus of Aβ by γ-secretase (5). Mutations in APP located within or immediately adjacent to Aβ are responsible for a few rare cases of dominantly inherited familial AD; in each case the mutation increases the production Aβ1-42 (7). Experiments using cultured neurons and mice that produce excessive amounts of human Aβ have shown that Aβ can cause synaptic dysfunction and neuronal degeneration, and that these adverse effects of Aβ occur when the peptide is in the early stages of self-aggregation. Aβ can damage neurons by inducing membrane-associated oxidative stress resulting in disruption of Ca2+ regulation in a manner that renders neurons vulnerable to Ca2+ overload, particularly under conditions of impaired mitochondrial energy production (8).
While the vast majority of cases of AD occur late in life and are sporadic, approximately 5–10% of cases have an early onset (4th and 5th decades of life) and are inherited in autosomal dominant manner. In 1995 Sherrington et al. reported the discovery of five different missense mutations in a gene on chromosome 14 that co-segregated with early-onset familial AD (FAD); the gene, which was initially named S182, is now known as presenilin-1 (PS1) (9). Later that year a mutation in a highly homologous gene located on chromosome 1 (presenilin-2; PS2) was shown to cause FAD in a Volga German kindred (10). Now more than 100 different FAD mutations in PS1 have been reported and most of these mutations increase the production of Aβ1-42 when expressed in cultured cells and transgenic mice. PS1 is itself the aspartyl protease responsible for γ-secretase cleavage of APP and functions in a complex with 3 other protein components (nicastrin, Pen-2 and Aph-1) of the γ-secretase complex. The mechanism by which PS1 mutations cause AD therefore involves increased production of Aβ1-42 which then aggregates and damages neurons (11). However, an additional mechanism of mutant PS1 action involving intense sparks of Ca2+ emanating from within neurons was then discovered (Figure 1).
Figure 1.
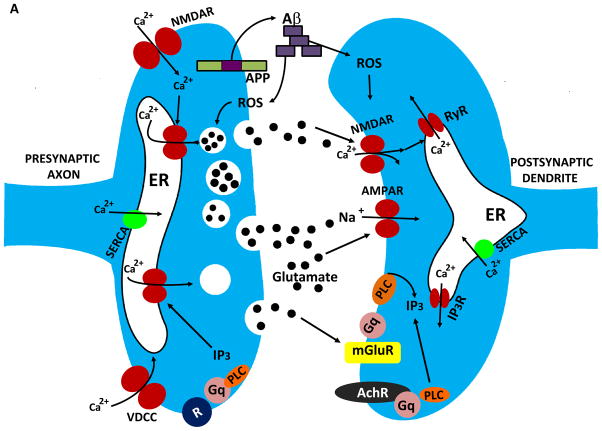
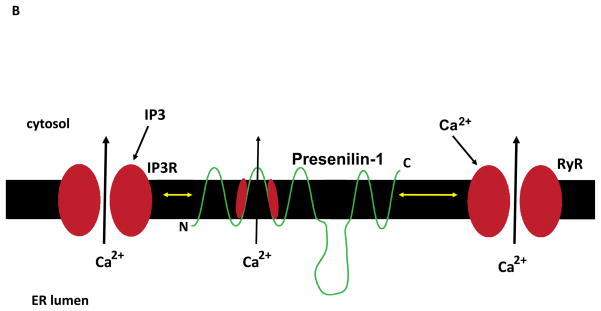
Mechanisms that regulate synaptic calcium dynamics, and their possible roles in the pathogenesis of Alzheimer’s disease (AD). A. The endoplasmic reticulum (ER) is present in both presynaptic axon terminals (left) and postsynaptic dendrites (right) where it regulates Ca2+-mediated processes involved in synaptic transmission and structural plasticity. In response to membrane depolarization, Ca2+ enters presynaptic terminals through voltage-dependent Ca2+ channels (VDCC) and N-methyl-D-aspartate (NMDA) glutamate receptors. The elevated cytosolic Ca2+ then triggers Ca2+ release through ryanodine receptors (RyR), and Ca2+ may also be released through IP3 receptors (IP3R) in response to activation of metabotropic receptors (R). The elevated Ca2+ concentration in the cytosol triggers fusion of glutamate-containing synaptic vesicles with the synaptic plasma membrane resulting in release of glutamate into the synaptic cleft. The β-amyloid precursor protein (APP) is present in the presynaptic membrane, where it can be proteolytically processed to release the amyloid β-peptide (Aβ). Aβ then self-aggregates on pre- and post-synaptic membranes where it causes oxidative stress and membrane lipid peroxidation which can disrupt cellular Ca2+ homeostasis. Glutamate binds postsynaptic AMPA receptors resulting in sodium influx, membrane depolarization and calcium influx through NMDA receptors and VDCC. Glutamate also binds metabotropic receptors (mGluR) coupled to the GTP-binding protein Gq which then activates phospholipase C (PLC) resulting in generation of IP3. SERCA, sarco- (smooth-) endoplasmic reticulum Ca2+-ATPase. AMPAR (alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor). B. Presenilin-1 (PS1) is an integral membrane protein that may modulate ER Ca2+ dynamics, thereby playing roles in synaptic plasticity and the pathogenesis of AD. PS1 can interact with IP3R and RyR, and mutations in PS1 that cause early-onset inherited AD enhance the amount of Ca2+ released in response to IP3 or Ca2+ influx. PS1 may itself function as a Ca2+ leak channel and PS1 mutations may compromise this function of PS1 resulting in an increased intraluminal Ca2+ concentration.
It was reported that mutant PS1 (L286V mutation) perturbs Ca2+ homeostasis in neuronal cells in a manner characterized by increased release of Ca2+ from the ER in response to agonists that activate inositol trisphosphate (IP3) receptors (12). Similar results were obtained in Ca2+ imaging studies of neurons from PS1 mutant (M146V) knockin mice which exhibited enhanced Ca2+ responses to glutamate receptor stimulation as a result of increased Ca2+ release from ER IP3 and ryanodine receptors (13, 14). Additional studies showed that levels of ryanodine receptors are elevated and caffeine-induced Ca2+ release is enhanced in primary hippocampal neurons from PS1 mutant knockin mice (15). Fibroblasts isolated from mutant PS1 knockin mice also exhibited a marked potentiation in the amplitude of Ca2+ transients evoked by IP3 and ER store-operated Ca2+ entry through plasma membrane Ca2+ channels was impaired (16). The notion that the size of the ER Ca2+ pool is abnormally large in neurons expressing mutant PS1 is supported by evidence suggesting that PS1 normally functions as a Ca2+ leak channel in the ER membrane and that FAD PS1 mutations disrupt this leak channel function (17).
In a recent article in Science Signaling, Cheung et al. (18) directly recorded ER IP3 receptor currents in lymphocytes from FAD (PS1 mutant) patients and in neurons from PS1 mutant mice. Mutations in PS1 increased the probability that the IP3 receptor channels were in an open state resulting in enhanced Ca2+ release when cells were stimulated. IP3 receptor channels in cells expressing mutant PS1 exhibited increased open times and, in many cases, dramatic bursts of repetitive openings for extended time periods suggesting an effect of mutant PS1 on modal gating of the channels. Modal gating has been previously described for ryanodine receptors in the ER of skeletal muscle cells and hippocampal neurons (19), and was recently reported to also occur in IP3 receptor channels where three modes were detected, a low activity mode, a fast kinetic mode and a burst mode (20). The new findings reveal that similar modal gating of IP3 receptor channels occurs in neurons and that several different FAD PS1 proteins shift the gating such that fast kinetic and burst modes are increased (18). It remains to be established how this shift in modal gating of IP3 receptors caused by FAD PS1 impacts on specific physiological processes mediated by Ca2+ in neurons. One possibility is that increased release of ER Ca2+ in presynaptic terminals enhances glutamate release. Wild type PS may play a role in regulating neurotransmitter release because long-term potentiation (LTP) and short-term plasticity at CA1 synapses are decreased after presynaptic but not postsynaptic deletion of PSs (21). This presynaptic function of PSs was apparently due to an effect of PSs on ER Ca2+ release because depletion of ER Ca2+ stores with thapsigargin or blockade of ryanodine receptors mimicks and occludes the effects of presynaptic PS depletion. However, excessive release of Ca2+ in presynaptic terminals caused by FAD mutant PS1 may ultimately impair synaptic plasticity by causing depletion of the neurotransmitter or by enhancing long-term depression of the postsynaptic response (22).
In a study that combined whole-cell patch-clamp recordings with flash photolysis and Ca2+ imaging in brain slices from wild type and PS1 mutant mice, Stutzmann et al. (23) showed that IP3-evoked Ca2+ responses were 3 times greater in cortical neurons from PS1 mutant mice compared to wild type mice. Interestingly, electrical excitability of PS1 mutant neurons was decreased as the result of Ca2+-mediated activation of K+ channels. Because Ca2+ release from ER plays important roles in multiple postsynaptic processes including LTP and LTD (24), a second possibility is that FAD PS1 mutations enhance postsynaptic responses by increasing the release of Ca2+ from IP3- and/or ryanodine-sensitive ER in dendrites, Interestingly, whereas IP3 receptor activation enhances LTP at hippocampal CA1 synapses (25), NMDA receptor-independent LTP is facilitated in mice lacking the type 3 ryanodine receptor (26). Assuming that FAD PS1 mutations enhance Ca2+ release from both IP3- and ryanodine-sensitive stores in dendrites, Ca2+-mediated processes involved in postsynaptic plasticity could be altered in ways that impair learning and memory.
The best known biological activity of PS1 is that it is an aspartyl protease that cleaves APP and the Notch receptor resulting in the intracellular release of APP and Notch C-terminal domains (AICD and NICD, respectively) (5). The NICD translocates to the nucleus where it functions as a transcriptional regulator, and this may also be the case with AICD. Notch signaling plays a major role in regulating the fate of neural progenitor cells, and may also be involved in synaptic plasticity and neuron survival (27, 28). There is evidence that γ-secretase-mediated Notch and APP cleavage by PS1 may modulate cellular Ca2+ signaling (29), although the details of the underlying molecular mechanism(s) is unknown.
How might the perturbed ER Ca2+ release caused by PS1 mutations contribute to the cognitive deficits and neurodegeneration in AD? Electrophysiological recordings of synaptic transmission at CA1 synapses in hippocampal slices from PS1 mutant knockin and control mice revealed age-related changes in two different forms of LTP, the widely-studied early phase of LTP (E-LTP), and late-LTP (L-LTP) which requires gene transcription and protein synthesis (30). Young mice exhibit enhanced E-LTP, but impaired maintenance of L-LTP, while older mice are impaired in both E-LTP and L-LTP. In another study it was shown that cholinergic modulation of hippocampal synaptic plasticity is impaired in PS1 mutant knockin mice (31). Activation of muscarinic acetylcholine receptors induces Ca2+ release from IP3-sensitive stores and enhances LTP in normal mice, but inhibits LTP in PS1 mutant mice. The NMDA current is decreased in hippocampal CA1 neurons of PS1 mutant mice and is restored by intracellular Ca2+chelation. Muscarinic receptor- and NMDA receptor-mediated components of synaptic plasticity were also impaired in 3xTgAD mice that express PS1, APP and tau mutations and exhibit Aβ and tau pathologies (31). In 3xTgAD mice there is an aberrantly large Ca2+ release through ER ryanodine receptors which may be due to increased levels of the type 2 ryanodine receptor (32). In other studies it was shown that FAD PS1 mutations increase the vulnerability of neurons to reduced energy availability and increased oxidative stress, conditions that occur in normal aging and AD (33, 34). The enhanced ER Ca2+ release has been shown to occur in synaptic terminals of PS1 mutant mice in studies that further demonstrated an adverse effect of this perturbation of synaptic Ca2+ homeostasis on mitochondrial function (35). Collectively, the findings described above suggest that perturbed ER Ca2+ regulation contributes to synaptic dysfunction and neuronal degeneration in experimental models relevant to AD.
What are the relationships between perturbed ER Ca2+ regulation and the Aβ and tau pathologies in AD. Aβ has been shown to elevate intracellular Ca2+ levels in cultured neurons by impairing Ca2+ extrusion and by enhancing Ca2+ influx through plasma membrane voltage-dependent and glutamate-activated Ca2+ channels (5). Recent in vivo calcium imaging studies in a mouse model of AD have demonstrated aberrant elevations of intracellular Ca2+ levels in neurites associated with Aβ deposits (36). Release of ER Ca2+ may also contribute to neuronal degeneration in AD because dantrolene, which blocks ryanodine receptors, can protect neurons from being damaged by Aβ (37 Guo et al., 1997). Aβ also directly induces oxidative stress in neurons which may further disrupt Ca2+ homeostasis and exacerbate amyloidogenic processing of APP (38 Jo et al., 2008) thereby fostering a viscous neurodegenerative cycle. There is also evidence that elevated intracellular Ca2+ levels contribute to tau hyperphosphorylation and self-aggregation to form neurofibrillary tangles. For example, sustained elevations of intracellular Ca2+ levels caused by overactivation of glutamate receptors can increase tau phosphorylation in cultured hippocampal neurons (39), and can induce phosphorylation of tau and APP, followed by accumulation of Aβ inside of cultured neurons (40). Postmortem analysis of brain tissue sections from AD patients have provided evidence for elevated Ca2+ levels in tangle-bearing neurons compared to healthier neurons (41).
Future research should clarify the specific contributions of perturbed ER Ca2+ handling to the cellular events that underlie synaptic dysfunction and neuronal degeneration in AD. While elevated pools of ER Ca2+ resulting from FAD PS1 mutations have been widely documented in a range of cell culture and animal models, the molecular basis of this alteration remains unknown. In addition, it is unclear if and how altered ER Ca2+ regulation is involved in the more common late-onset forms of AD. One possibility which merits further investigation is that both age- and disease-related processes impose adverse (oxidative and proteotoxic) stress on the ER (42). The emphasis of translational research on AD has, thus far, focused mainly on reducing Aβ production, enhancing Aβ removal, or modulating cholinergic and glutamatergic neurotransmission (5). The findings described above suggest that stabilization of ER Ca2+ levels is an additional therapeutic approach that merits testing. This might be achieved using dietary and exercise interventions that increase ER stress resistance (43) or drugs that suppress activation of IP3 or ryanodine receptors (although side effects of such drugs are likely given the important functions of ER Ca2+ release in neuronal plasticity).
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging, NIH. I thank KC Alexander for helping with the preparation of Figure 1.
References
- 1.Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2009;5:234–270. doi: 10.1016/j.jalz.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM. One-year brain atrophy evident in healthy aging. J Neurosci. 2009;29:15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arumugam TV, Gleichmann M, Tang SC, Mattson MP. Hormesis/preconditioning mechanisms, the nervous system and aging. Ageing Res Rev. 2006;5:165–178. doi: 10.1016/j.arr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Cohen AD, Price J, Weissfeld L, James J, Rosario B, Bi W, Nebes R, Saxton J, Snitz BE, Aizenstein HA, Wolk DA, Dekosky ST, Mathis CA, Klunk WE. Basal cerebral metabolism may modulate the cognitive effects of Abeta in mild cognitive impairment: an example of brain reserve. J Neurosci. 2009;29:14770–14778. doi: 10.1523/JNEUROSCI.3669-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, Laviolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, Johnson KA. Functional Alterations in Memory Networks in Early Alzheimer’s Disease. Neuromolecular Med. 2010 doi: 10.1007/s12017-009-8109-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 8.Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin J-F, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, Da Silva HAR, Haines JL, Pericak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, St George-Hyslop PH. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 10.Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, Crowley AC, Fu YH, Guenette SY, Galas D, Nemens E, Wijsman EM, Bird TD, Schellenberg GD, Tanzi RE. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 11.Kimberly WT, Xia W, Rahmati T, Wolfe MS, Selkoe DJ. The transmembrane aspartates in presenilin 1 and 2 are obligatory for gamma-secretase activity and amyloid beta-protein generation. J Biol Chem. 2000;275:3173–3178. doi: 10.1074/jbc.275.5.3173. [DOI] [PubMed] [Google Scholar]
- 12.Guo Q, Furukawa K, Sopher BL, Pham DG, Xie J, Robinson N, Martin GM, Mattson MP. Alzheimer’s PS-1 mutation perturbs calcium homeostasis and sensitizes PC12 cells to death induced by amyloid beta-peptide. Neuroreport. 1996;8:379–383. doi: 10.1097/00001756-199612200-00074. [DOI] [PubMed] [Google Scholar]
- 13.Guo Q, Fu W, Sopher BL, Miller MW, Ware CB, Martin GM, Mattson MP. Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nat Med. 1999;5:101–106. doi: 10.1038/4789. [DOI] [PubMed] [Google Scholar]
- 14.Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol Chem. 2000;275:18195–18200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- 15.Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol Chem. 2000;275:18195–18200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- 16.Leissring MA, Akbari Y, Fanger CM, Cahalan MD, Mattson MP, LaFerla FM. Capacitative calcium entry deficits and elevated luminal calcium content in mutant presenilin-1 knockin mice. J Cell Biol. 2000;149:793–798. doi: 10.1083/jcb.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer’s disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung KH, Mei L, Mak DD, Hayashi I, Iwatsubo T, Kang DE, Foskett JK. Gain of function Alzheimer’s disease presenilin regulation of InsP3 receptor modal gating in patient cells and AD mouse neurons. Science Signaling. 2010 doi: 10.1126/scisignal.2000818. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armisén R, Sierralta J, Vélez P, Naranjo D, Suárez-Isla BA. Modal gating in neuronal and skeletal muscle ryanodine-sensitive Ca2+ release channels. Am J Physiol. 1996;271:C144–153. doi: 10.1152/ajpcell.1996.271.1.C144. [DOI] [PubMed] [Google Scholar]
- 20.Ionescu L, White C, Cheung KH, Shuai J, Parker I, Pearson JE, Foskett JK, Mak DO. Mode switching is the major mechanism of ligand regulation of InsP3 receptor calcium release channels. J Gen Physiol. 2007;130:631–645. doi: 10.1085/jgp.200709859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang C, Wu B, Beglopoulos V, Wines-Samuelson M, Zhang D, Dragatsis I, Südhof TC, Shen J. Presenilins are essential for regulating neurotransmitter release. Nature. 2009;460:632–636. doi: 10.1038/nature08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unni VK, Zakharenko SS, Zablow L, DeCostanzo AJ, Siegelbaum SA. Calcium release from presynaptic ryanodine-sensitive stores is required for long-term depression at hippocampal CA3-CA3 pyramidal neuron synapses. J Neurosci. 2004;24:9612–9622. doi: 10.1523/JNEUROSCI.5583-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stutzmann GE, Caccamo A, LaFerla F, Parker I. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer’s-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J Neurosci. 2004;24:508–513. doi: 10.1523/JNEUROSCI.4386-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park MK, Choi YM, Kang YK, Petersen OH. The endoplasmic reticulum as an integrator of multiple dendritic events. Neuroscientist. 2008;14:68–77. doi: 10.1177/1073858407305691. [DOI] [PubMed] [Google Scholar]
- 25.Fernández de Sevilla D, Núñunez A, Borde M, Malinow R, Buño W. Cholinergic-mediated IP3-receptor activation induces long-lasting synaptic enhancement in CA1 pyramidal neurons. J Neurosci. 28:1469–1478. doi: 10.1523/JNEUROSCI.2723-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Futatsugi A, Kato K, Ogura H, Li ST, Nagata E, Kuwajima G, Tanaka K, Itohara S, Mikoshiba K. Facilitation of NMDAR-independent LTP and spatial learning in mutant mice lacking ryanodine receptor type 3. Neuron. 1999;24:701–713. doi: 10.1016/s0896-6273(00)81123-x. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Chan SL, Miele L, Yao PJ, Mackes J, Ingram DK, Mattson MP, Furukawa K. Involvement of Notch signaling in hippocampal synaptic plasticity. Proc Natl Acad Sci U S A. 2004;101:9458–9462. doi: 10.1073/pnas.0308126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arumugam TV, Chan SL, Jo DG, Yilmaz G, Tang SC, Cheng A, Gleichmann M, Okun E, Dixit VD, Chigurupati S, Mughal MR, Ouyang X, Miele L, Magnus T, Poosala S, Granger DN, Mattson MP. Gamma secretase-mediated Notch signaling worsens brain damage and functional outcome in ischemic stroke. Nat Med. 2006;12:621–623. doi: 10.1038/nm1403. [DOI] [PubMed] [Google Scholar]
- 29.Leissring MA, Murphy MP, Mead TR, Akbari Y, Sugarman MC, Jannatipour M, Anliker B, Müller U, Saftig P, De Strooper B, Wolfe MS, Golde TE, LaFerla FM. A physiologic signaling role for the gamma -secretase-derived intracellular fragment of APP. Proc Natl Acad Sci USA. 2002;99:4697–4702. doi: 10.1073/pnas.072033799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Auffret A, Gautheron V, Mattson MP, Mariani J, Rovira C. Progressive age-related Impairment of the late long-term potentiation in Alzheimer’s disease presenilin-1 mutant knock-in mice. J Alzheimers Dis. 2009 doi: 10.3233/JAD-2010-1302. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Greig NH, Yu QS, Mattson MP. Presenilin-1 mutation impairs cholinergic modulation of synaptic plasticity and suppresses NMDA currents in hippocampus slices. Neurobiol Aging. 2009;30:1061–1068. doi: 10.1016/j.neurobiolaging.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakroborty S, Goussakov I, Miller MB, Stutzmann GE. Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3xTg-AD mice. J Neurosci. 2009;29:9458–9470. doi: 10.1523/JNEUROSCI.2047-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller JN, Guo Q, Holtsberg FW, Bruce-Keller AJ, Mattson MP. Increased sensitivity to mitochondrial toxin-induced apoptosis in neural cells expressing mutant presenilin-1 is linked to perturbed calcium homeostasis and enhanced oxyradical production. J Neurosci. 1998;18:4439–4450. doi: 10.1523/JNEUROSCI.18-12-04439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattson MP, Zhu H, Yu J, Kindy MS. Presenilin-1 mutation increases neuronal vulnerability to focal ischemia in vivo and to hypoxia and glucose deprivation in cell culture: involvement of perturbed calcium homeostasis. J Neurosci. 2000;20:1358–1364. doi: 10.1523/JNEUROSCI.20-04-01358.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Begley JG, Duan W, Chan S, Duff K, Mattson MP. Altered calcium homeostasis and mitochondrial dysfunction in cortical synaptic compartments of presenilin-1 mutant mice. J Neurochem. 1999;72:1030–1039. doi: 10.1046/j.1471-4159.1999.0721030.x. [DOI] [PubMed] [Google Scholar]
- 36.Kuchibhotla KV, Goldman S, Lattarulo C, Wu HY, Hyman BT, Bacskai BJ. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo W, Sopher BL, Furukawa K, Pham DG, Robinson N, Martin GM, Mattson MP. Alzheimer’s presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: involvement of calcium and oxyradicals. J Neurosci. 1997;17:4212–4222. doi: 10.1523/JNEUROSCI.17-11-04212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jo DG, Arumugam TV, Woo HN, Park JS, Tang SC, Mughal M, Hyun DH, Park JH, Choi YH, Gwon AR, Camandola S, Cheng A, Cai H, Song W, Markesbery WR, Mattson MP. Evidence that gamma-secretase mediates oxidative stress-induced beta-secretase expression in Alzheimer’s disease. Neurobiol Aging. 2008 Aug 5; doi: 10.1016/j.neurobiolaging.2008.07.003. [Epub ahead of print] (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattson MP. Antigenic changes similar to those seen in neurofibrillary tangles are elicited by glutamate and Ca2+ influx in cultured hippocampal neurons. Neuron. 1990;4:105–117. doi: 10.1016/0896-6273(90)90447-n. [DOI] [PubMed] [Google Scholar]
- 40.Pierrot N, Santos SF, Feyt C, Morel M, Brion JP, Octave JN. Calcium-mediated transient phosphorylation of tau and amyloid precursor protein followed by intraneuronal amyloid-beta accumulation. J Biol Chem. 2006;281:39907–39914. doi: 10.1074/jbc.M606015200. [DOI] [PubMed] [Google Scholar]
- 41.Nixon RA, Saito KI, Grynspan F, Griffin WR, Katayama S, Honda T, Mohan PS, Shea TB, Beermann M. Calcium-activated neutral proteinase (calpain) system in aging and Alzheimer’s disease. Ann N Y Acad Sci. 1994;747:77–91. doi: 10.1111/j.1749-6632.1994.tb44402.x. [DOI] [PubMed] [Google Scholar]
- 42.Salminen A, Kauppinen A, Suuronen T, Kaarniranta K, Ojala J. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. J Neuroinflammation. 2009;6:41. doi: 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol. 2009 doi: 10.1002/ana.21798. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]