Summary
Crosstalk exists in mammalian cells between cholesterol trafficking and innate immunity signaling. Apolipoprotein A-I (apoA-I), a serum apolipoprotein that induces anti-atherogenic efflux of macrophage cholesterol, is widely described as anti-inflammatory because it neutralizes bacterial lipopolysaccharide. Conversely, lipopolysaccharide-induced inflammation is pro-atherogenic. However, whether innate immunity plays an endogenous, physiological role in host cholesterol homeostasis in the absence of infection is undetermined. We report that apoA-I signals in the macrophage through Toll like Receptor (TLR)2, TLR4, and CD14, utilizing Myeloid Differentiation Primary Response Protein 88 (MyD88)-dependent and –independent pathways, to activate nuclear factor-κB and induce cytokines. MyD88 plays a critical role in reverse cholesterol transport in vitro and in vivo, in part through promoting ATP-Binding Cassette A1 transporter upregulation. Taken together, this work identifies apoA-I as an endogenous stimulus of innate immunity that couples cholesterol trafficking to inflammation through MyD88, and identifies innate immunity as a physiologic signal in cholesterol homeostasis.
Introduction
In the mammalian plasma membrane, two-way crosstalk occurs between endogenous cholesterol trafficking pathways and innate immune responses to pathogens by Toll like Receptors (TLRs). Bacterial lipopolysaccharide (LPS) activates signals in macrophages by recruiting TLR4 and its intracellular adaptors, such as Myeloid Differentiation Primary Response Protein 88 (MyD88), to cholesterol-rich lipid raft membrane microdomains (Triantafilou et al., 2002). The cholesterol content of membrane rafts can itself regulate cellular signaling by determining the selective localization of signaling proteins to these microdomains (Foster et al., 2003). Indeed, pharmacologic or genetic manipulation of membrane cholesterol may suffice to activate TLRs (Sun et al., 2009). In turn, raft cholesterol content is normally regulated by homeostatic traffic of cholesterol from the plasma membrane to extracellular acceptors, such as apolipoprotein A-I (apoA-I), in particular (Murphy et al., 2008). Thus, apoA-I, a lipid-binding protein that induces cholesterol export from the cell, is positioned as a pivotal regulator of innate immunity.
ApoA-I, a 243-amino acid serum apolipoprotein, is proposed to contribute to atheroprotection in part by promoting reverse cholesterol transport (RCT), the process by which cholesterol in macrophages and other cells is exported to apoA-I for transport via the blood to bile and then feces. ApoA-I is moreover widely considered anti-inflammatory in the context of LPS, an activity likely due both to its binding/neutralization of LPS and to its effect on raft lipid (Berbee et al., 2005). Conversely, LPS inhibits RCT (McGillicuddy et al., 2009), and innate immunity is conventionally considered pro-atherogenic (Bjorkbacka et al., 2004b; Khovidhunkit et al., 2004; Michelsen et al., 2004). Recent reports in the lung, however, indicate that innate immunity is not restricted to exogenous microbial challenges, but may also play non-pathogen-related homeostatic roles by responding to endogenous ligands (Zhang et al., 2006). As innate immunity is generally considered a response to external insult, there has been no investigation to date of whether it plays endogenous, physiological roles in cholesterol trafficking in the absence of infection.
Chemical extraction of membrane cholesterol into the extracellular space by cyclodextrins (CDs) impairs subsequent cell signaling responses to LPS by displacing raft lipid and protein (Fessler et al., 2004). It is perhaps less well-recognized that CDs themselves nonetheless acutely induce responses in leukocytes through TLR4 and MyD88 (Fessler et al., 2004; Flemming et al., 2004). This suggests that, though raft cholesterol is necessary for TLR4 activation by LPS as a ligand, acute, primary fluxes in membrane lipid may nevertheless be sufficient events to trigger the protein-protein interactions of innate immunity. ApoA-I exerts effects on plasma membrane lipid grossly similar to those of CDs (Murphy et al., 2008), and, moreover, structurally responds to membrane lipid in a manner strikingly similar to the type I viral fusion protein family (Oda et al., 2003), members of which stimulate TLR4 (Kurt-Jones et al., 2000). We thus reasoned that apoA-I might also trigger TLR4-/MyD88-dependent signals in the macrophage. Secondly, as apoA-I-elicited intracellular signals regulate efflux of cellular cholesterol (Nofer et al., 2003), we further hypothesized that MyD88 might then regulate mobilization of cellular cholesterol to apoA-I. Herein, we report that apoA-I indeed stimulates a pro-inflammatory cascade to nuclear factor (NF)-κB-dependent cytokine induction through TLR4 and TLR2 and their adaptors in the macrophage. In addition, we demonstrate that MyD88 plays a crucial role in apoA-I-dependent RCT in vitro and in vivo. Taken together, we propose that innate immune signals mediated by MyD88 serve as physiologic ‘second messengers’ in the homeostatic, anti-atherogenic RCT pathway.
Results
ApoA-I activates MyD88-dependent signals in macrophages
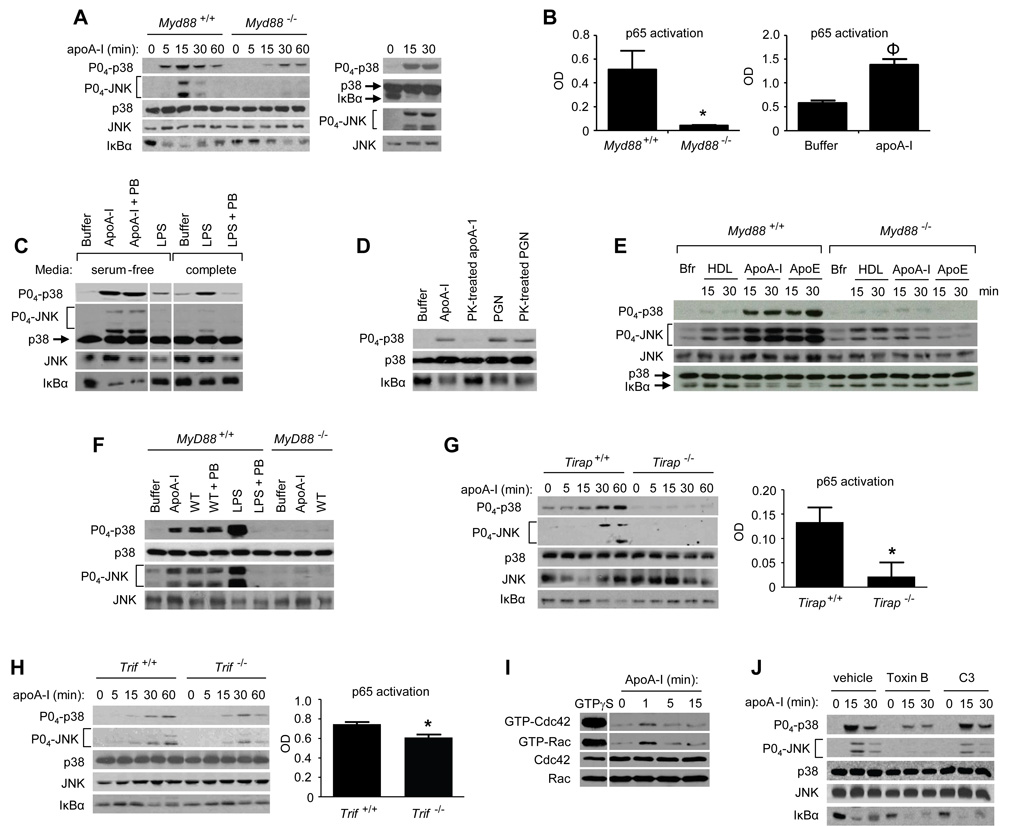
Chemical extraction of cholesterol from the plasma membrane of leukocytes with CDs induces an acute pro-inflammatory signaling cascade that approximates TLR4 responses, likely due to secondary translocation of proteins to/from membrane microdomains (Fessler et al., 2004; Flemming et al., 2004). MyD88 is an intracellular TLR adaptor that transduces an early phase of mitogen-activated protein kinase (MAPK) and NF-κB activation by LPS (O'Neill and Bowie, 2007). As previously reported for B cells (Flemming et al., 2004), we confirmed in macrophages that methyl-β-CD activates mitogen-activated protein kinases (MAPKs) and triggers degradation of IκBα, an indicator of NF-κB activation (Schuster and Nelson, 2000), through an MyD88-dependent mechanism (Fig. S1). ApoA-I, an apolipoprotein cholesterol acceptor, similarly activated p38, c-Jun N-terminal kinase (JNK), and NF-κB through MyD88 (Fig. 1A, left). In Myd88−/− macrophages, as compared to Myd88+/+, apoA-I-induced p38 activation and IκBα degradation were delayed, and JNK activation was markedly attenuated, similar to LPS (Fig. S2A). Human monocyte-derived macrophages were similarly responsive to apoA-I (Fig.1A, right). ApoA-I activated DNA binding of p65 NF-κB in nuclear isolates of murine and human macrophages, and did so through an MyD88-dependent mechanism in the former case (Fig. 1B).
Figure 1. ApoA-I activates macrophages through MyD88-dependent and – independent pathways.
(A) Peritoneal exudate macrophages (PEMs) from Myd88+/+ and Myd88−/− mice were treated with apoA-I (10 µg/ml)(Left [L]), and human monocyte-derived macrophages were treated with apoA-I (50 µg/ml)(Right [R]) for times indicated. Lysates were then immunoblotted for indicated targets. (B) Nuclear isolates from Myd88+/+ or Myd88−/− PEMs (L) or human monocyte-derived macrophages (R) were assayed for p65 activation after apoA-I exposure (20 min, 10 µg/ml)(*, P=0.04; ϕ, P=0.004). (C–F) Cells were exposed as described and then immunoblotted for the indicated targets. (C) PEMs were cholesterol-loaded and cultured in serum-free/0.2% BSA media, or left unloaded and cultured in complete (10% FBS) media, and then treated with apoA-I (30 µg/ml) or LPS (100 ng/ml, 15 min) in the presence and absence of polymyxin B (PB). Lysates were then probed for the indicated targets. Lane 4 (LPS in serum-free media) was translocated from original location on gel for juxtaposition. (D) PEMs were exposed to apoA-I (10 µg/ml) or PGN (10 µg/ml) that had been left untreated or pretreated with proteinase K (PK). (E) PEMs were treated with buffer (Bfr), HDL (100 ug/ml), apoA-I (30 µg/ml), or apoE (30 µg/ml), and lysates probed for the indicated targets. (F) Myd88+/+ or Myd88−/− PEMs were exposed to buffer, serum-isolated apoA-I (30 µg/ml), WT recombinant apoA-I (20 µg/ml), or LPS in presence or absence of PB as shown, and then probed for the indicated targets. (G) Tirap+/+ and Tirap−/− PEMs were treated with apoA-I (10 µg/ml), lysates were immunoblotted for indicated targets, and nuclear isolates assayed for p65 activity (20 min exposure; *, P=0.03). (H) Trif+/+ and Trif−/− PEMs were treated with apoA-I, lysates immunoblotted for indicated targets, and nuclear isolates assayed for p65 activity (75 min exposure; *, P=0.02). (I) C57BL/6 PEMs were stimulated as shown and then assayed for activated (GTP-bound) Cdc42 and Rac. GTPγS-treated lysates (juxtaposed) are shown as positive control, and total Cdc42 and Rac expression in whole cell lysate are also shown. Data shown are representative of 2 independent experiments. (J) C57BL/6 PEMs were pretreated (4h) with C. difficile toxin B (500 ng/ml) or C3 transferase (5 µg/ml), treated with PB, stimulated with apoA-I (10 µg/ml), and then immunoblotted as shown. Data are results from 3 independent experiments. All values are means ± s.e.m. See also Fig. S1.
Commercial serum-derived lipid-free apoA-I, which was initially found to have ~27 pg LPS contamination per microgram of protein by Limulus test, was column-repurified and found thereafter to have undetectable levels of LPS (i.e., <0.06 EU [<6 pg] per 10 µg apoA-I). The experiments in this report used this repurified apoA-I under serum-free conditions, which are devoid of LPS-binding protein. Though under these conditions 100 ng/ml LPS had minimal activity upon p38, JNK, and NF-κB (Fig. 1C), polymyxin B (PB, 25 µg/ml), an LPS-neutralizing agent, was nevertheless used in all experiments in order to address the possibility that apoA-I-bound LPS could escape detection and elicit signals. Whereas LPS at 100 ng/ml (Fig. 1C) and indeed at 2 µg/ml (data not shown), namely >3×105-fold higher than the Limulus detection threshold, was neutralized by PB, apoA-I signaling was unaffected (Fig. 1C). Unlike peptidoglycan (PGN)(Fig. 1D) and LPS (Fig. S2B), which, as expected, were protease-resistant, pretreatment of apoA-I with protease ablated its signaling activity (Fig. 1D). Like apoA-I, apoE, another amphipathic α-helical apolipoprotein, also activated MAPKs and NF-κB in an MyD88-dependent fashion (Fig.1E). By contrast, although apoA-I comprises ~80% of high density lipoprotein (HDL) protein (Lee et al., 2004), HDL did not activate NF-κB, even at higher concentrations than used for apoA-I, had minimal activity upon p38, and activated JNK in an MyD88-independent fashion (Fig. 1E). PB abolished p38 activation by macrophages co-exposed to 10 ng/ml LPS and 10 µg/ml HDL (data not shown), indicating that apolipoproteins do not shelter LPS from PB neutralization in our system.
ApoA-I has complex, lipid- and oxidation-sensitive secondary structure that impacts its function, and these modifications occur in vivo (Lagerstedt et al., 2007). To corroborate our results with serum-isolated, delipidated apoA-I, we tested bacterially expressed, column-repurified wild type (wt) apoA-I (Lagerstedt et al., 2007; Oda et al., 2003).
Recombinant wt apoA-I activated p38 and JNK in murine macrophages through an MyD88-dependent mechanism (Fig. 1F). Unlike LPS, this activity was unaffected by PB. Serum-isolated, native apoA-I was used in all experiments that follow except where specified.
In addition to MyD88, other adaptor proteins play important roles in mediating signals from TLRs to the cell interior. Toll-interleukin 1 receptor (TIR) domain-containing adaptor protein (TIRAP) is a co-adaptor with MyD88 for TLR2 and TLR4 signals, bridging MyD88 to the intracellular domains of these two receptors (O'Neill and Bowie, 2007). We thus questioned whether TIRAP mediates the apoA-I signal. Tirap−/− macrophages indeed displayed similar attenuation of apoA-I-induced p38 and IκBα signals as observed in Myd88−/− macrophages (Fig. 1G, Fig. S2C). Given the delayed signaling in Myd88−/− cells (Fig. 1A), we also queried whether an additional adaptor was involved. TIR-domain-containing adapter-inducing interferon-β (TRIF), an adaptor commonly referred to as the MyD88-independent pathway, transduces a late phase of LPS-induced MAPK and NF-κB activation (O'Neill and Bowie, 2007). Trif−/− macrophages indeed had attenuated activation of p38, JNK, and NF-κB at late time points of apoA-I exposure (60 min for p38/JNK; 75 min for p65 NF-κB)(Fig. 1H), suggesting that TRIF mediates a late phase of apoA-I signaling.
ApoA-I activates the Rho GTPase Cdc42 upstream of MAPKs in fibroblasts (Argmann et al., 2005; Nofer et al., 2003). Rho GTPases also regulate TLR2- and TLR4-dependent signaling (Fessler et al., 2004). Testing the macrophage, we found that Cdc42 and Rac are activated by apoA-I (Fig. 1I). Pretreatment of macrophages with C. difficile toxin B, which inhibits RhoA, Cdc42, and Rac, attenuated apoA-I-induced activation of p38 and JNK but not IκBα degradation, whereas C3 transferase, a selective RhoA inhibitor, had no effect (Fig. 1J). Taken together, these results suggest that Cdc42 or Rac lies upstream of MAPK but not NF-κB activation in the apoA-I cascade, and that the pathways to MAPK and NF-κB are distinct.
ApoA-I signals through TLR2 and TLR4 in macrophages
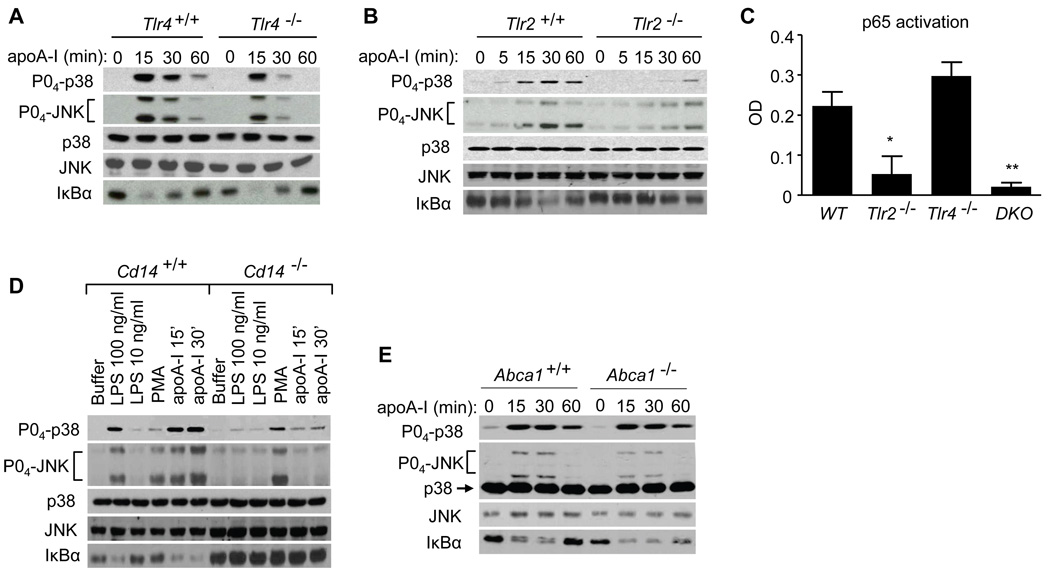
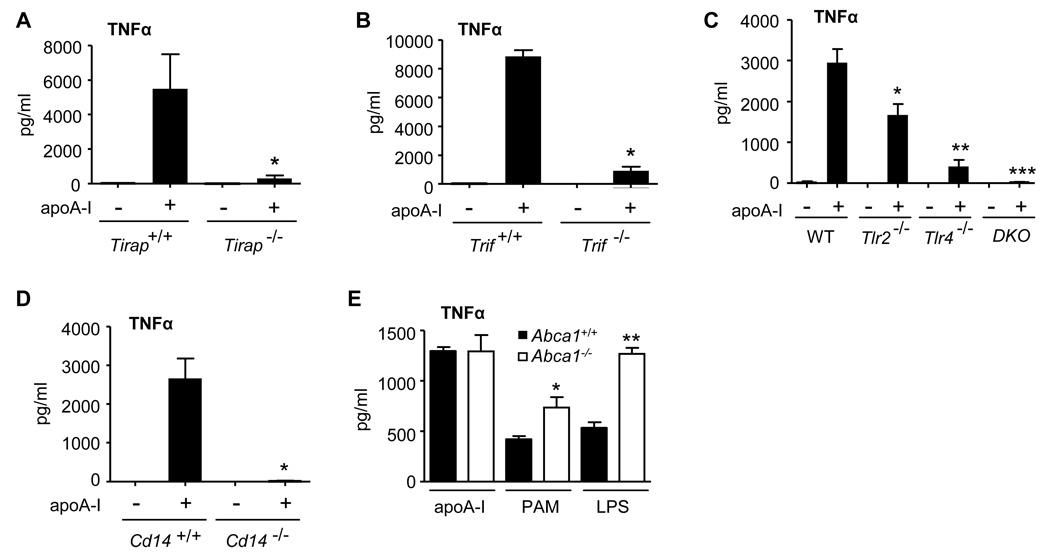
Given our findings that the TLR4 adaptors MyD88, TIRAP, and TRIF regulate apoA-I signaling in the macrophage, we next determined whether TLR4 is itself involved. Unlike LPS signaling, which, as expected, was abolished in Tlr4−/− macrophages (Fig. S2D), apoA-I activated MAPKs in these cells (Fig. 2A). Compared to Tlr4+/+, Tlr4−/− macrophages had intact IκBα degradation, but attenuated p38 and JNK activation at 30 and 60 minutes of exposure (Fig. 2A). The marked attenuation of all signals in Myd88−/− and Tirap−/− macrophages (Fig. 1), compared with the modest attenuation of MAPKs and intact degradation of IκBα in Tlr4−/− macrophages, suggested that apoA-I might utilize an additional MyD88- and TIRAP-dependent TLR, namely TLR2 (O'Neill and Bowie, 2007). Indeed, Tlr2−/− macrophages, which, as expected, responded to LPS and poly(I:C) but not to the TLR2 stimulus Pam3CSK4 (Fig. S2E), had attenuated and delayed activation of p38 and JNK in response to apoA-I similar to Myd88−/− macrophages (Fig. 2B), confirming that apoA-I signaling in the macrophage is also TLR2-dependent. Consistent with the IκBα findings for Tlr2−/− and Tlr4−/− cells, apoA-I-induced activation of p65 was attenuated in Tlr2−/− and in Tlr2−/−Tlr4−/− cells, but was intact in Tlr4−/− cells (Fig. 2C). CD14 is a co-receptor for TLR2 and TLR4 stimuli (Kurt-Jones et al., 2000); hence, we questioned whether it regulates apoA-I signaling. ApoA-I-induced MAPK activation and IκBα degradation were indeed attenuated in Cd14−/− macrophages (Fig. 2D). ATP-Binding Cassette Transporter A1 (ABCA1), a putative receptor for apoA-I, is reported to lie upstream of apoA-I-induced MAPK activation in fibroblasts (Nofer et al., 2003; Nofer et al., 2006), and to negatively regulate LPS signaling in macrophages (Zhu et al., 2008). However, we found no difference in apoA-I signaling between Abca1+/+ and Abca1−/− macrophages (Fig. 2E), indicating that the pathway we have identified in macrophages is distinct from that reported in fibroblasts.
Figure 2. ApoA-I signals through TLR2 and TLR4 in the macrophage.
(A, B) Wild type, Tlr2−/−, and Tlr4−/− PEMs were treated with apoA-I (10 µg/ml) and lysates immunobloted. (C) Wild type, Tlr2−/−Tlr4−/− or Tlr2−/−Tlr4−/− double knockout (DKO) PEM nuclear isolates were assayed for p65 activation after apoA-I exposure (20 min; 10 µg/ml; *, P=0.03, and **, P=0.009 compared to WT). (D) Cd14+/+ and Cd14−/− PEMs were treated and immunoblotted as shown (PMA = phorbol myristate acetate, 32 nM, 15 min; apoA-I, 30 µg/ml). (E) Abca1−/− and Abca1+/+ PEMs were exposed to apoA-I (10 µg/ml) and lysates probed as shown. Data are representative of 3 independent experiments. See also Fig. S2.
ApoA-I induces MyD88-dependent cytokines and inflammation
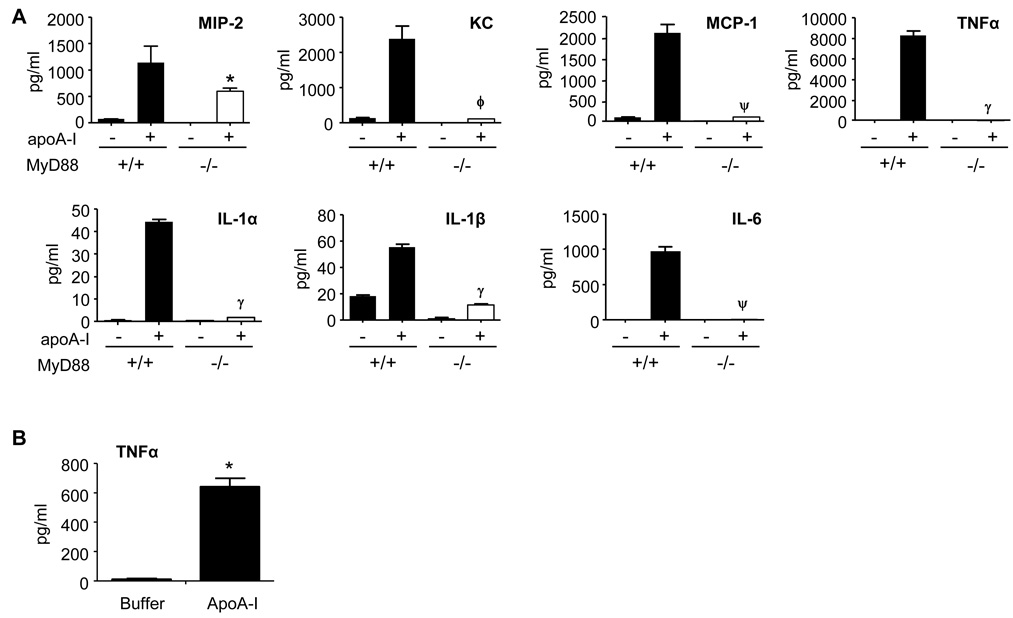
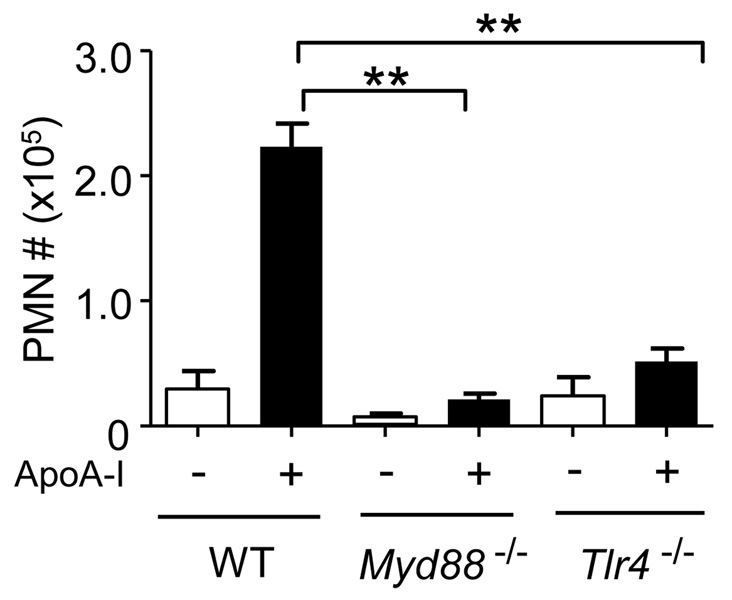
Our finding that apoA-I activates NF-κB was somewhat unexpected given that apoA-I has conventionally been considered anti-inflammatory (Berbee et al., 2005). To confirm and characterize this pro-inflammatory response, we analyzed conditioned media and found that apoA-I induces several NF-κB-dependent cytokines in murine (Fig. 3A) and human (Fig. 3B) macrophages. Like NF-κB activation, cytokine induction was dramatically dependent upon MyD88, albeit to degrees that varied among the cytokines (Fig. 3A). Microarray analysis confirmed that a suite of pro-inflammatory genes is induced by serum-isolated apoA-I with varying dependence upon MyD88, and that MyD88 regulates >6% of the apoA-I-induced transcriptome (Table S1). PB abolished LPS-induced TNFα, but did not affect TNFα induction by apoA-I; moreover, protease treatment of apoA-I abolished its ability to induce TNFα, but did not affect LPS or Pam3CSK4 (Fig. S2F). Bacterially expressed wt apoA-I protein also induced TNFα in a PB-resistant, MyD88-dependent fashion (Fig. S2G). Given the suite of pro-inflammatory cytokines induced by apoA-I in vitro, we investigated whether apoA-I could induce inflammation in vivo by injecting mice i.p. with serum-isolated apoA-I. Unlike C57BL/6 mice injected with BSA or saline, neutrophils were recruited to the peritoneum of apoA-I-injected mice (Fig. S3), indicating acute inflammation. ApoA-I-elicited neutrophil recruitment to the peritoneum was nearly abolished in Myd88−/− and Tlr4−/− mice (Fig. 4), indicating the dependence of apoA-I-induced peritonitis upon these two proteins.
Figure 3. ApoA-I induces MyD88-dependent cytokine induction.
(A) Myd88+/+ and Myd88−/− PEMs were exposed to buffer or apoA-I (10 µg/ml, 24h), and cytokines quantified in media supernatants by Bioplex assay. *, P=0.02; ϕ, P=0.003; ψ, P=.0006; γ, P<0.0001 compared to respective wt. Data is representative of two experiments with triplicate samples. (B) Human monocyte-derived macrophages were exposed to apoA-I (25 µg/ml, 2h), and TNFα quantified in media supernatants (ELISA). Data are results from 3 independent experiments. *, P=0.0004. See also Table S1.
Figure 4. ApoA-I elicits TLR4/MyD88-dependent inflammation in vivo.
C57BL/6 (‘WT’), Myd88−/−, and Tlr4−/− mice were injected i.p. with 50 µg of delipidated BSA (“−“) or endotoxin-free apoA-I (“+”). Neutrophils (PMN) were counted in peritoneal lavage fluid. Data are from 2 experiments, n=8–10 mice per bar in graph. All values are means ± s.e.m. **, P<0.0001. See also Fig. S3.
TNFα production was also significantly attenuated in Tirap−/−, Trif−/−, Tlr2−/−, Tlr4 −/−, and Cd14−/− macrophages stimulated with serum-isolated apoA-I (Fig. 5A–D) with additive contributions of TLR2 and TLR4 revealed by Tlr2−/−Tlr4−/− macrophages (Fig. 5C). By contrast, whereas Abca1−/− macrophages were markedly hyper-responsive to both LPS and Pam3CSK4, inducing elevated levels of TNFα protein consistent with prior reports (Zhu et al., 2008), they produced wild type levels of TNFα in response to apoA-I (Fig. 5E).
Figure 5. ApoA-I induction of TNFα is TIRAP-, TRIF-, TLR2-, TLR4-, and CD14-dependent, but ABCA1-independent.
(A–D) PEMs from strains shown were stimulated with apoA-I (30 µg/ml, 20h), and TNFα quantified in media supernatants (ELISA). Data are results from 3 independent experiments. A, *P=0.04; B, * P=0.001; C *, P=0.04 compared to WT, **, P=0.015 and ***, P=0.003 both compared to Tlr2−/−; D, *, P=0.006. (E) Abca1+/+ and Abca1−/− cholesterol-loaded PEMs were exposed (6h) to apoA-I (30 µg/ml), Pam3CSK4 (PAM, 100 ng/ml), or LPS (100 ng/ml) (*, P<0.01; **, P<0.001).
Reverse cholesterol transport requires MyD88
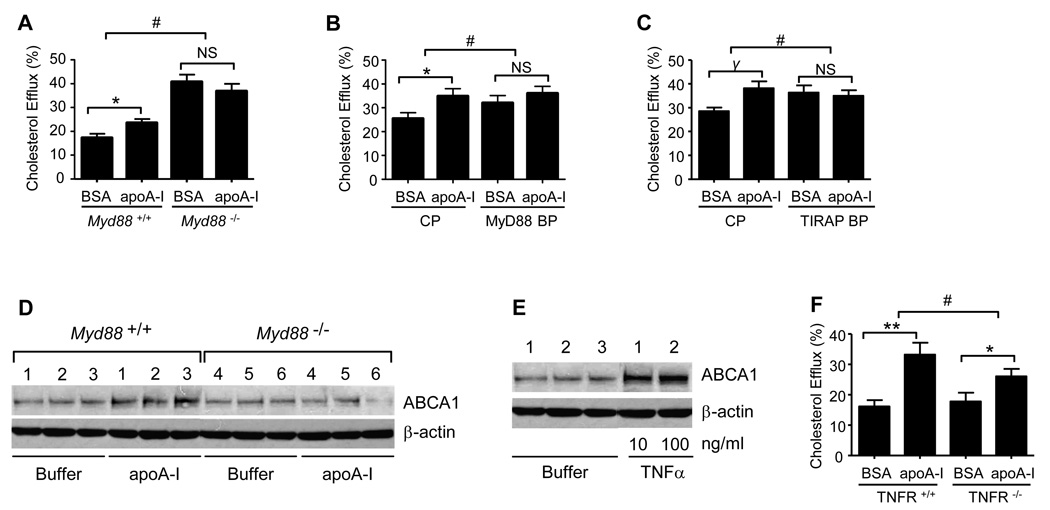
Given the marked MyD88-dependence of apoA-I signaling, and previous reports that acute intracellular signals elicited by apoA-I regulate cholesterol efflux in the fibroblast (Nofer et al., 2003; Nofer et al., 2006), we next investigated whether MyD88 regulates apoA-I-induced cholesterol efflux from the macrophage. ApoA-I plays a central role in induction of ABCA1-dependent cholesterol efflux from cells as the first step of the RCT pathway (Gaus et al., 2004). Compared to wt, Myd88−/− macrophages had elevated cholesterol efflux into BSA-containing medium; however, unlike wt macrophages, Myd88−/− cells displayed no apoA-I-induced cholesterol efflux above BSA background (Fig. 6A). These results, derived from scintillation counting of 3H-cholesterol, were confirmed by cholesterol mass analysis (Fig. S4A). ApoA-I-induced cholesterol efflux was also significantly decreased in human macrophages treated with cell-permeant inhibitors of MyD88 and TIRAP (Fig. 6B–C). ApoA-I is reported to promote ABCA1-dependent cholesterol efflux in part through inducing post-translational upregulation of ABCA1 (Wang et al., 2003). Consistent with a role for MyD88 in this phenomenon, we found that apoA-I-induced upregulation of ABCA1 was attenuated in Myd88−/− macrophages (Fig. 6D). TNFα is reported to enhance cholesterol efflux by upregulating ABCA1 (Gerbod-Giannone et al., 2006). Consistent with this, TNFα treatment upregulated ABCA1 protein in peritoneal macrophages (Fig. 6E). Our finding that apoA-I induces MyD88-dependent TNFα production (Fig. 3A) thus prompted us to determine whether autocrine signaling from this cytokine might promote apoA-I-induced cholesterol efflux from the macrophage. Consistent with this, apoA-I-induced cholesterol efflux was indeed attenuated in TNF receptor-deficient macrophages (Fig. 6F). Rho GTPases and JNK reportedly regulate apoA-I-induced cholesterol efflux in fibroblasts (Nofer et al., 2003). Given our finding that JNK is activated downstream of MyD88 in a Rho-dependent fashion (Figs. 1I–J), we next questioned whether Rho GTPases regulate macrophage cholesterol efflux. Like the case for MyD88 deletion (Fig. 6, Fig. S4A), toxin B increased cholesterol efflux to BSA, but blocked apoA-I-induced cholesterol efflux above that to BSA carrier (Fig. S4B).
Figure 6. Macrophage cholesterol efflux requires MyD88.
(A) 3H-Cholesterol efflux to BSA and apoA-I was quantified for Myd88+/+ and Myd88−/− PEMs. Data is representative of three experiments. *P=0.02; #, P<0.001. (B, C) Human monocyte-derived macrophages were pretreated with 22R–OH-cholesterol and 9 cis-retinoic acid (24h), and 100 µM MyD88 blocking peptide (BP), TIRAP BP, or control peptide (CP) (4h) prior to measurement of 3H-cholesterol efflux to BSA or apoA-I (100 µg/ml). *, P=0.02; γ, P=0.006; #, P<0.01 for comparisons of both MyD88 and TIRAP BP to CP. Data are results from 3 independent experiments. (D) PEMs from 3 Myd88+/+ and 3 MyD88−/− mice were exposed to buffer or apoA-I (10 µg/ml, 24h), after which equal protein aliquots of whole cell lysate were immunoblotted for ABCA1 and β-actin. Data are representative of 2 independent experiments. (E) C57BL/6 PEMs were exposed to buffer (n=3 mice) or to 10 or 100 ng/ml TNFα (24h). Equal protein aliquots of whole cell lysate were then immunoblotted for the targets as shown. Data are representative of five independent experiments. (F) TNFR+/+ and TNFR−/− PEMs were analyzed for 3H-cholesterol efflux to BSA or apoA-I. Data are results from 6 different experiments. **, P=0.0004; *, P=0.002; #, P<0.01. All values are means ± s.e.m. See also Fig. S4.
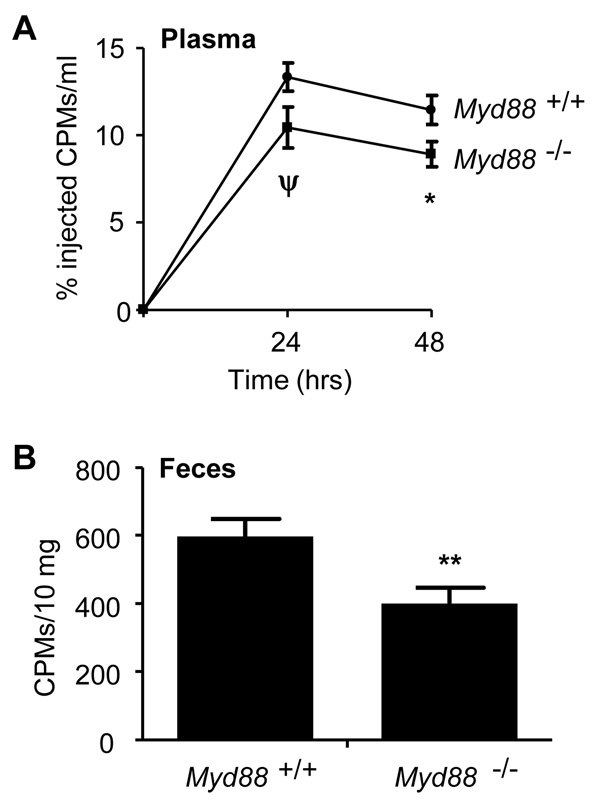
Finally, in order to confirm physiological relevance, we adopted a well-established method of measurement of RCT in vivo (Naik et al., 2006; Navab et al., 2004; Rotllan et al., 2005; Wang et al., 2007; Zhang et al., 2003). In order to determine whether macrophage MyD88 regulates RCT in vivo, Myd88+/+ and Myd88−/− macrophages were loaded ex vivo with 3H-cholesterol/acetylated LDL and then injected i.p. into separate Myd88+/+ recipients. 3H-cholesterol was then monitored in plasma and feces to quantify bodily disposal of cholesterol from the transplanted macrophages. Transport of 3H-cholesterol into the plasma and feces was significantly reduced from Myd88−/− as compared to Myd88+/+ donor macrophages (Fig. 7), a reduction from wt comparable to that previously reported for Abca1−/− macrophages (Wang et al., 2007). These data confirm an important role for macrophage MyD88 in RCT in vivo. Taken together, our study has identified a role for innate immunity as a physiologic signal in RCT, and indicates that innate immunity and RCT are coupled processes.
Figure 7. In vivo reverse cholesterol transport requires MyD88.
Reverse cholesterol transport of 3H-cholesterol into plasma (A) and feces (B) was assayed at different time points following i.p. injection of 3H-cholesterol-loaded (~1.25 E6 counts per minute [CPMs]) Myd88+/+ or Myd88−/− PEMs into Myd88+/+ recipient mice. Feces were assayed at 48h. ψ, P=0.056; *, P=0.038; **, P=0.01. Data are from 3 separate experiments, n=8 per experiment. All values are means ± s.e.m.
Discussion
This report establishes a critical link between a heretofore unrecognized activity of apoA-I upon innate immunity and its well-established anti-atherogenic function. We report that apoA-I utilizes TLR2, TLR4, CD14, and the TLR adaptors MyD88, TIRAP, and TRIF, to activate a cascade involving Rho GTPases, MAPKs, and NF-κB, and culminating in induction of cytokines. This response is shared by lipid-free apoE, suggesting a possible class effect of the exchangeable amphipathic α-helical apolipoproteins. In support of this, apoC-III was recently reported to activate NF-κB in THP-1 cells through interactions with TLR2 (Kawakami et al., 2008). The response is not triggered, however, by HDL. As HDL, like lipid-free apoA-I, extracts cellular cholesterol, albeit from different membrane microdomains (Drobnik et al., 2002), membrane cholesterol extraction per se does not appear sufficient for pathway activation. Corroboration of our results with recombinant apoA-I suggests that neither in vivo (e.g., oxidation) nor ex vivo (i.e., preparative) modifications of the native protein are likely to explain our results.
Multiple approaches were undertaken to exclude the confounding effects of contaminating pathogen-associated molecular patterns (PAMPs). ApoA-I-induced NF-κB activation was TLR4-independent and unaffected by PB, and LPS did not induce TNFα under culture conditions in which apoA-I did. Unlike LPS, PGN, and Pam3CSK4, apoA-I was inactivated by proteolysis. Unlike apoA-I, both LTA and flagellin are TLR4- and TRIF-independent, and flagellin and other non-TLR2/TLR4 stimuli are TIRAP-independent (O'Neill and Bowie, 2007). Significant independent contributions from TLRs other than TLR2 and TLR4 were excluded by the DKO data. Abca1−/− macrophages were hyper-responsive to LPS and Pam3CSK4 but had normal responses to apoA-I (Fig. 5E)(Zhu et al., 2008). Serum-purified apoA-I from two sources showed similar results as did lipid-free apoE and bacterially-expressed apoA-I, whereas HDL did not activate the pathway. Most importantly, in vivo RCT, induced by endogenous apolipoproteins, was also MyD88-dependent. We cannot exclude low-level endotoxemia in the mouse. Indeed, endotoxemia occurs in ostensibly healthy humans (Wiedermann et al., 1999). Hence, apoA-I in vivo may not be naïve to PAMPs and may possibly signal under less purified conditions than we established in vitro.
It has been proposed that intracellular signals triggered by apoA-I in fibroblasts, such as JNK activation, regulate ABCA1-dependent efflux of cellular cholesterol (Gaus et al., 2004; Nofer et al., 2003). Regulation of ABCA1 expression is an important mechanism of control of cholesterol efflux to apoA-I. Whereas apoA-I upregulates ABCA1 in macrophages through intracellular signals (Wang et al., 2003), LPS impairs cholesterol efflux through downregulation of ABCA1 (Baranova et al., 2002; Khovidhunkit et al., 2003; McGillicuddy et al., 2009). We propose that MyD88 promotes apoA-I-induced cholesterol efflux at least in part through promoting ABCA1 upregulation (Fig. 6D). By contrast, Myd88−/− macrophages display enhanced cholesterol efflux to BSA-containing media (Fig. 6A); thus, whether MyD88 also suppresses ‘diffusional’ cholesterol efflux through a distinct mechanism is a question worthy of future study. Notably, we identify for the first time that macrophages exposed to apoA-I in vitro, a model system used by numerous groups to study cholesterol efflux, express and release multiple cytokines through an MyD88-dependent mechanism. We propose that MyD88 may promote apoA-I-induced cholesterol efflux at least in part through inducing TNFα, thus allowing for its autocrine feedback upon the macrophage (Fig. 6F). Whether the Liver X Receptors regulate apoA-I-induced cholesterol efflux, in part, through effects on TNFα induction is an interesting question worthy of future study.
While acute cellular exposure to lipid-free apoA-I, as performed by us and many others (Gaus et al., 2004; Nofer et al., 2003; Nofer et al., 2006), permits identification of the cell response to apoA-I, the situation is different in vivo, where macrophages are tonically exposed to apoA-I, most of it in the form of HDL. Continuous exposure may possibly induce a form of apoA-I tolerance, with suppression of the pro-inflammatory response to apoA-I, as well as to other raft-dependent stimuli. By analogy, CDs impair cellular responsiveness to raft-dependent stimuli including LPS, after acutely inducing pro-inflammatory signals (Fessler et al., 2004). This said, our RCT data (Fig. 7) do suggest that MyD88 exerts regulatory tone upon apolipoprotein-dependent cholesterol trafficking in vivo. Perhaps more importantly, although it is accepted that ~5–10% (~50–100 µg/ml) of apoA-I is in lipid-free form in vivo, it has been shown that in vitro incubation of lipid-free apoA-I with plasma and injection of lipid-free apoA-I into rodents both lead to very rapid (<5 min) incorporation of the apolipoprotein into HDL (Lee et al., 2004). Hence, because of the nature of our approach, our findings are not inconsistent with those of others that apoA-I is anti-inflammatory in vivo and in the context of LPS. The lipid-free population of apoA-I in vivo is determined as a balance among incorporation into HDL, release from HDL, and lipidation by ABCA1 to form nascent HDL; these events are proposed to occur in the subendothelial space (Curtiss et al., 2006). Thus, it is likely that the pro-inflammatory potential of apoA-I is veiled in vivo particularly in the general circulation, whereas low HDL or conditions modifying HDL composition may partition apoA-I more towards pro-inflammatory activity at diseased tissue sites. Indeed, inflammatory states are associated with release of lipid-poor apoA-I from HDL (Jahangiri et al., 2009), raising the possibility that apoA-I may then feed forward to promote further inflammation. ApoA-I is found within atherosclerotic lesions (Mucchiano et al., 2001a; Mucchiano et al., 2001b), indeed correlating and colocalizing with inflammatory markers in unstable carotid plaques from symptomatic patients (Patel et al., 2009).
In summary, we report that apoA-I utilizes MyD88 as a signal in RCT. These findings add to a growing literature that has identified overlapping roles for several host proteins in lipid trafficking and innate immunity, suggesting common origins for and coupling between metabolism and host defense. This may not be surprising insofar as microbial and dietary lipids are coupled in their absorption from the gut and in their systemic circulation (Cani et al., 2007), and TLR2 and TLR4 stimuli are generally modified lipids of the bacterial membrane (e.g., LTA, LPS) that perturb host lipid metabolism (Khovidhunkit et al., 2004) through interactions with host lipid-binding proteins and membranes. Macrophages and inflammation are critical contributors to atherogenesis, but both also have anti-atherogenic activity (Levin et al., 2005; Shi et al., 2000). Thus, while MyD88-derived inflammation contributes to atherosclerosis by recruiting monocytes to atheromas (Bjorkbacka et al., 2004b; Michelsen et al., 2004), macrophage and endothelial MyD88 may have distinct roles. Future investigations will be necessary to discriminate whether macrophage MyD88 counteracts atherosclerosis through promoting RCT under permissive conditions.
Experimental Procedures
Reagents
The ProteoSpin Endotoxin Removal kit was from Norgen Biotek. The Limulus amebocyte lysate pyrogent test kit (sensitivity 0.06 EU/ml) was from Cambrex. PB, PGN, poly(I:C), mβCD (Sigma), human apoE (Calbiochem), human HDL (Intracel), Pam3CSK4 (InvivoGen), C3 transferase (Cytoskeleton), E. coli 0111:B4 LPS (List Biological), and proteinase K (Invitrogen) were purchased. C. difficile toxin B was kindly provided by Dr. Ingo Just. TIRAP and MyD88 inhibitory peptides and control peptide were from Imgenex. Lipid-free human apoA-I was purchased from two vendors (Calbiochem [≥ 95% purity], Sigma [>85% purity]) with similar results. Protein purity was confirmed by Sypro Ruby staining of 10 µg protein resolved by SDS-PAGE.
Mice
Myd88-, Tlr2-, Tlr4-, Trif-, and Tirap–deficient mice have been described (Horng et al., 2002; Kawai et al., 1999; Takeuchi et al., 1999; Yamamoto et al., 2003), and were all crossed >8 generations onto C57BL/6 background before use. Tlr2-/Tlr4-deficient mice were generated by crossing Tlr2- and Tlr4-deficient strains. Cd14-deficient mice on a C57BL/6 background, age- and gender-matched C57BL/6 controls, and Tnfrsf1a−/− Tnfrsf1b−/− (p55−/−p75−/−) mice and B6129SF2/J controls were purchased from Jackson Laboratories. Macrophage-specific Abca1-deficient mice (Zhu et al., 2008) were on a mixed B6;129 background and were compared to littermate controls. All experiments were performed in accordance with the Animal Welfare Act and the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals after review of the protocol by the NIEHS Animal Care and Use Committee.
Macrophage Harvests
Endotoxin-free reagents and plastics were used in all experiments. For murine peritoneal macrophage harvests, mice were injected i.p. with Brewer’s thioglycollate (2 ml, 4%) and euthanized 96 hours later. The peritoneum was washed with 10 ml ice cold PBS three times. Cells were spun down (1,000x RPM, 6 min, 4°C) and washed twice with sterile PBS. Cells were then resuspended in appropriate media, counted, and plated for experiments. Human peripheral monocytes were isolated from whole blood by discontinuous plasma Percoll centrifugation and differentiated to macrophages as described (Haslett et al., 1985).
Cell Exposures
For apoA-I exposures, peritoneal exudate macrophages were plated at 1–2 ×106/well in a 12-well plate with DMEM/0.1% FBS and allowed to settle for 2 h before adding cholesterol loading media (DMEM / 0.1% FBS / 2 mg/ml delipidated BSA /10 µg/ml cholesterol). Human macrophages were plated at 5×106/well in a 12-well plate with X-vivo media/10% human serum and were given cholesterol loading media on Day 7. The following day, cells were washed with PBS and media was changed to DMEM/2mg/ml BSA. Polymyxin B (25µg/ml) was added 30 minutes prior to apoA-I in all experiments except where indicated. LPS and Pam3CSK4 exposures were conducted upon non-cholesterol-loaded macrophages in complete media (DMEM/10% FBS) unless otherwise specified. In some experiments as indicated, apoA-I, LPS, or PGN were pretreated with proteinase K (0.4 µl enzyme/rxn, 37°C overnight, followed by 80°C for 25 min for heat inactivation).
Western Blotting
Cells were lysed and equivalent protein loads resolved by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were probed with antibodies against β-actin (#4967; Cell Signaling); phospho-p38 (Thr180/Tyr182; Cell Signaling), phospho-JNK (Thr183/Tyr185; Cell Signaling), p38 (C-20: sc-535; Santa Cruz), JNK1 (C17:sc-474; Santa Cruz), IκBα (C-21; sc-371; Santa Cruz), Rac (clone 23A8, Upstate), Cdc42 (P1; sc-98; Santa Cruz), and ABCA1 (Zhu et al., 2008).
p65 Activation Assay
Nuclear extracts were isolated using the NE-PER kit (Pierce) and equal nuclear protein aliquots (Bradford assay) analyzed with the TransAM NF-κB p65 kit (Active Motif) per manufacturer’s conditions. Data is depicted as buffer-corrected apoA-I-induced p65 binding (i.e., apoA-I-induced p65 activity minus buffer-induced p65 activity), except where noted.
Rho GTPase Activity Assays
Cdc42 and Rac activity in macrophage lysates (22×106) were quantified with the Rac Activation Assay Kit (Upstate, Lack Placid, NY) per manufacturer’s instructions, followed by detection with mouse anti-Rac (Upstate) and rabbit anti-Cdc42 (Santa Cruz) antibodies.
Cytokine Measurement
Media supernatants were analyzed by either the Bioplex multiplex bead assay (BioRad) or ELISA to mouse or human TNFα (eBioscience), per manufacturer’s specifications.
Intraperitoneal Injection of ApoA-I
Mice were injected i.p. with column-repurified apoA-I (50 µg), delipidated low-endotoxin BSA (50 µg), 1x PBS (pH, 7.4), or left untreated. Six hrs later, mice were euthanized, and peritoneal cells were lavaged and counted. Cytospins were prepared, stained by the Hema 3 Manual Staining System (Fisher), and leukocyte subpopulations counted.
Cholesterol Efflux Assay
PEMs were plated at 50% confluence in a 24-well plate (~2.5×105 cells/well) in RPMI 1640 with 10% FBS, and human macrophages at ~2×106 cells/well in X-vivo media/10% human serum for 7 days to allow for differentiation. The following day, medium was changed to RPMI plus 10% lipoprotein deficient FBS (Intracel). Twenty four hours later, cells were loaded with 1 µCi/mL [3H] cholesterol (NEN Life Science Products) in the presence of 50 µg acetylated LDL (Kalen)/mL in a total volume of 250 µl. The next day, cells were washed with 1x PBS and were allowed to equilibrate for 4h in RPMI/0.2% lipid-free BSA. Cells were treated in RPMI/0.2% BSA with or without apoA-I in the presence or absence of 10 µM 22(R)-hydroxycholesterol (LXR agonist; Sigma) and 1 µM 9 cis-retinoic acid (RXR agonist; Sigma) for 24h. The medium was then collected. Cells were lysed with 200 µl of 0.2N NaOH (RT, 1h) and the lysate was collected; each well was then washed with 50 µl of 0.2N NaOH and the two fractions combined. Radioactivity was measured (counts per minute [CPM]) from the medium and lysates. Cholesterol efflux to BSA or apoA-I was calculated as the radioactivity (CPMs) recovered in the medium divided by the sum of radioactivity in medium plus lysate.
Reverse Cholesterol Transport Assay
The following protocol was adapted (Zhang et al., 2003). Myd88+/+ or Myd88−/− PEMs (~4×107) were plated at 2×106/well in a 6-well plate in RPMI/10% FBS. The following day, wells were washed and loaded with 25 µg/mL acLDL and 5 µCi/well [3H]-cholesterol (60h). The cells were then washed 2x with RPMI/0.2% BSA and allowed to equilibrate (4h). Cells were then washed again in RPMI/0.2% BSA, gently scraped with a cell lifter, spun (1,000x RPM, 6 min, 4°) and resuspended in RPMI/0.2% BSA. Radioactivity was measured from each cell prep and an equal number (~1.25×106) CPM was injected into Myd88+/+ recipient mice. Plasma was collected at 24h and 48h. A 2 µl sample was measured in a scintillation counter. CPMs/ml plasma are reported as % of injected CPMs. Feces were collected at 48h, weighed and soaked in distilled water (1mL water/100 mg) overnight at 4°C. The next day, an equal volume of ethanol was added and the samples were homogenized. A 200µl aliquot of each homogenized sample was measured on a scintillation counter (Naik et al., 2006).
Cholesterol Mass Analysis
Cholesterol was quantified by gas-liquid chromatography.
ApoA-I expression
Protein was expressed in E. coli and purified using standard procedures.
Microarray analysis
Agilent Whole Mouse Genome (014868) 4×44 multiplex format oligo arrays (Agilent Technologies) were used.
Statistics
All data are presented as means ±SEM. P<0.05 was considered statistically significant for all assays. Cholesterol efflux was analyzed by two-tailed t-test; the difference between apoA-I and control was further subjected to a two-way Analysis of Variance (ANOVA) with genotype and experiment as the two factors (JMP software). Plasma samples were analyzed by two-way ANOVA using Prism software (GraphPad). Cytokine, p65 assays, and PMN counts were analyzed by two-tailed t-test. In the microarray analyses, differentially expressed genes (p<0.01) were determined by error-weighted ANOVA with multiple test correction (Bonferroni) using Rosetta Resolver®.
Further details on cholesterol mass analysis, apoA-I expression, and microarray analysis are available in the Supplement.
Supplementary Material
Acknowledgments
We thank Zhuowei Li and John Hollingsworth for assistance with PEM harvest, Grace Kissling for statistical consultation, Marcia Moss and Robert Petrovich for protein expression, Donald Cook and Farhad Imani for manuscript review, Ingo Just for providing C. difficile toxin B, Shizuo Akira for providing Tlr2−/−, Tlr4−/−, Trif−/−, and Myd88−/− mice, and Ruslan Medzhitov for providing Tirap−/− mice. This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences, and by NIH grants P01-HL49373 and R01 HL94525 (JSP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argmann CA, Edwards JY, Sawyez CG, O'Neil CH, Hegele RA, Pickering JG, Huff MW. Regulation of macrophage cholesterol efflux through hydroxymethylglutaryl-CoA reductase inhibition: a role for RhoA in ABCA1-mediated cholesterol efflux. The Journal of biological chemistry. 2005;280:22212–22221. doi: 10.1074/jbc.M502761200. [DOI] [PubMed] [Google Scholar]
- Baranova I, Vishnyakova T, Bocharov A, Chen Z, Remaley AT, Stonik J, Eggerman TL, Patterson AP. Lipopolysaccharide down regulates both scavenger receptor B1 and ATP binding cassette transporter A1 in RAW cells. Infect Immun. 2002;70:2995–3003. doi: 10.1128/IAI.70.6.2995-3003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbee JF, Havekes LM, Rensen PC. Apolipoproteins modulate the inflammatory response to lipopolysaccharide. J Endotoxin Res. 2005;11:97–103. doi: 10.1179/096805105X35215. [DOI] [PubMed] [Google Scholar]
- Bjorkbacka H, Fitzgerald KA, Huet F, Li X, Gregory JA, Lee MA, Ordija CM, Dowley NE, Golenbock DT, Freeman MW. The induction of macrophage gene expression by LPS predominantly utilizes Myd88-independent signaling cascades. Physiological genomics. 2004a;19:319–330. doi: 10.1152/physiolgenomics.00128.2004. [DOI] [PubMed] [Google Scholar]
- Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nature medicine. 2004b;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Curtiss LK, Valenta DT, Hime NJ, Rye KA. What is so special about apolipoprotein AI in reverse cholesterol transport? Arteriosclerosis, thrombosis, and vascular biology. 2006;26:12–19. doi: 10.1161/01.ATV.0000194291.94269.5a. [DOI] [PubMed] [Google Scholar]
- Drobnik W, Borsukova H, Bottcher A, Pfeiffer A, Liebisch G, Schutz GJ, Schindler H, Schmitz G. Apo AI/ABCA1-dependent and HDL3-mediated lipid efflux from compositionally distinct cholesterol-based microdomains. Traffic. 2002;3:268–278. doi: 10.1034/j.1600-0854.2002.030404.x. [DOI] [PubMed] [Google Scholar]
- Fessler MB, Arndt PG, Frasch SC, Lieber JG, Johnson CA, Murphy RC, Nick JA, Bratton DL, Malcolm KC, Worthen GS. Lipid rafts regulate lipopolysaccharide-induced activation of Cdc42 and inflammatory functions of the human neutrophil. The Journal of biological chemistry. 2004;279:39989–39998. doi: 10.1074/jbc.M401080200. [DOI] [PubMed] [Google Scholar]
- Flemming JA, Perkins KH, Luus L, Ferguson AR, Corley RB. Disruption of membrane cholesterol stimulates MyD88-dependent NF-kappaB activation in immature B cells. Cell Immunol. 2004;229:68–77. doi: 10.1016/j.cellimm.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Foster LJ, De Hoog CL, Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci U S A. 2003;100:5813–5818. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaus K, Kritharides L, Schmitz G, Boettcher A, Drobnik W, Langmann T, Quinn CM, Death A, Dean RT, Jessup W. Apolipoprotein A-1 interaction with plasma membrane lipid rafts controls cholesterol export from macrophages. Faseb J. 2004;18:574–576. doi: 10.1096/fj.03-0486fje. [DOI] [PubMed] [Google Scholar]
- Gerbod-Giannone MC, Li Y, Holleboom A, Han S, Hsu LC, Tabas I, Tall AR. TNFalpha induces ABCA1 through NF-kappaB in macrophages and in phagocytes ingesting apoptotic cells. Proc Natl Acad Sci U S A. 2006;103:3112–3117. doi: 10.1073/pnas.0510345103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett C, Guthrie LA, Kopaniak MM, Johnston RB, Jr, Henson PM. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985;119:101–110. [PMC free article] [PubMed] [Google Scholar]
- Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420:329–333. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- Jahangiri A, de Beer MC, Noffsinger V, Tannock LR, Ramaiah C, Webb NR, van der Westhuyzen DR, de Beer FC. HDL remodeling during the acute phase response. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:261–267. doi: 10.1161/ATVBAHA.108.178681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- Kawakami A, Osaka M, Aikawa M, Uematsu S, Akira S, Libby P, Shimokado K, Sacks FM, Yoshida M. Toll-like receptor 2 mediates apolipoprotein CIII-induced monocyte activation. Circulation research. 2008;103:1402–1409. doi: 10.1161/CIRCRESAHA.108.178426. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. Journal of lipid research. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- Khovidhunkit W, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Endotoxin down-regulates ABCG5 and ABCG8 in mouse liver and ABCA1 and ABCG1 in J774 murine macrophages: differential role of LXR. Journal of lipid research. 2003;44:1728–1736. doi: 10.1194/jlr.M300100-JLR200. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- Lagerstedt JO, Budamagunta MS, Oda MN, Voss JC. Electron paramagnetic resonance spectroscopy of site-directed spin labels reveals the structural heterogeneity in the N-terminal domain of apoA-I in solution. The Journal of biological chemistry. 2007;282:9143–9149. doi: 10.1074/jbc.M608717200. [DOI] [PubMed] [Google Scholar]
- Lee JY, Lanningham-Foster L, Boudyguina EY, Smith TL, Young ER, Colvin PL, Thomas MJ, Parks JS. Prebeta high density lipoprotein has two metabolic fates in human apolipoprotein A-I transgenic mice. Journal of lipid research. 2004;45:716–728. doi: 10.1194/jlr.M300422-JLR200. [DOI] [PubMed] [Google Scholar]
- Levin N, Bischoff ED, Daige CL, Thomas D, Vu CT, Heyman RA, Tangirala RK, Schulman IG. Macrophage liver X receptor is required for antiatherogenic activity of LXR agonists. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:135–142. doi: 10.1161/01.ATV.0000150044.84012.68. [DOI] [PubMed] [Google Scholar]
- McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucchiano GI, Haggqvist B, Sletten K, Westermark P. Apolipoprotein A-1-derived amyloid in atherosclerotic plaques of the human aorta. The Journal of pathology. 2001a;193:270–275. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH753>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Mucchiano GI, Jonasson L, Haggqvist B, Einarsson E, Westermark P. Apolipoprotein A-I-derived amyloid in atherosclerosis. Its association with plasma levels of apolipoprotein A-I and cholesterol. American journal of clinical pathology. 2001b;115:298–303. doi: 10.1309/PJE6-X9E5-LX6K-NELY. [DOI] [PubMed] [Google Scholar]
- Murphy AJ, Woollard KJ, Hoang A, Mukhamedova N, Stirzaker RA, McCormick SP, Remaley AT, Sviridov D, Chin-Dusting J. High-Density Lipoprotein Reduces the Human Monocyte Inflammatory Response. Arteriosclerosis, thrombosis, and vascular biology. 2008 doi: 10.1161/ATVBAHA.108.168690. [DOI] [PubMed] [Google Scholar]
- Naik SU, Wang X, Da Silva JS, Jaye M, Macphee CH, Reilly MP, Billheimer JT, Rothblat GH, Rader DJ. Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation. 2006;113:90–97. doi: 10.1161/CIRCULATIONAHA.105.560177. [DOI] [PubMed] [Google Scholar]
- Navab M, Anantharamaiah GM, Reddy ST, Hama S, Hough G, Grijalva VR, Wagner AC, Frank JS, Datta G, Garber D, Fogelman AM. Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation. 2004;109:3215–3220. doi: 10.1161/01.CIR.0000134275.90823.87. [DOI] [PubMed] [Google Scholar]
- Nofer JR, Feuerborn R, Levkau B, Sokoll A, Seedorf U, Assmann G. Involvement of Cdc42 signaling in apoA-I-induced cholesterol efflux. The Journal of biological chemistry. 2003;278:53055–53062. doi: 10.1074/jbc.M305673200. [DOI] [PubMed] [Google Scholar]
- Nofer JR, Remaley AT, Feuerborn R, Wolinnska I, Engel T, von Eckardstein A, Assmann G. Apolipoprotein A-I activates Cdc42 signaling through the ABCA1 transporter. Journal of lipid research. 2006;47:794–803. doi: 10.1194/jlr.M500502-JLR200. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- Oda MN, Forte TM, Ryan RO, Voss JC. The C-terminal domain of apolipoprotein A-I contains a lipid-sensitive conformational trigger. Nat Struct Biol. 2003;10:455–460. doi: 10.1038/nsb931. [DOI] [PubMed] [Google Scholar]
- Patel S, Chung SH, White G, Bao S, Celermajer DS. The "atheroprotective" mediators apolipoproteinA-I and Foxp3 are over-abundant in unstable carotid plaques. International journal of cardiology. 2009 doi: 10.1016/j.ijcard.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Rotllan N, Ribas V, Calpe-Berdiel L, Martin-Campos JM, Blanco-Vaca F, Escola-Gil JC. Overexpression of human apolipoprotein A-II in transgenic mice does not impair macrophage-specific reverse cholesterol transport in vivo. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:e128–e132. doi: 10.1161/01.ATV.0000175760.28378.80. [DOI] [PubMed] [Google Scholar]
- Schuster JM, Nelson PS. Toll receptors: an expanding role in our understanding of human disease. J Leukoc Biol. 2000;67:767–773. [PubMed] [Google Scholar]
- Shi W, Wang X, Wang NJ, McBride WH, Lusis AJ. Effect of macrophage-derived apolipoprotein E on established atherosclerosis in apolipoprotein E-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:2261–2266. doi: 10.1161/01.atv.20.10.2261. [DOI] [PubMed] [Google Scholar]
- Sun Y, Ishibashi M, Seimon T, Lee M, Sharma SM, Fitzgerald KA, Samokhin AO, Wang Y, Sayers S, Aikawa M, Jerome WG, Ostrowski MC, Bromme D, Libby P, Tabas IA, Welch CL, Tall AR. Free cholesterol accumulation in macrophage membranes activates Toll-like receptors and p38 mitogen-activated protein kinase and induces cathepsin K. Circulation research. 2009;104:455–465. doi: 10.1161/CIRCRESAHA.108.182568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002;115:2603–2611. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- Wang N, Chen W, Linsel-Nitschke P, Martinez LO, Agerholm-Larsen B, Silver DL, Tall AR. A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoA-I. The Journal of clinical investigation. 2003;111:99–107. doi: 10.1172/JCI16808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. The Journal of clinical investigation. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, Willeit J. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck Study. J Am Coll Cardiol. 1999;34:1975–1981. doi: 10.1016/s0735-1097(99)00448-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Science. Vol. 301. New York, N.Y.: 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway; pp. 640–643. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shan P, Jiang G, Cohn L, Lee PJ. Toll-like receptor 4 deficiency causes pulmonary emphysema. The Journal of clinical investigation. 2006;116:3050–3059. doi: 10.1172/JCI28139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zanotti I, Reilly MP, Glick JM, Rothblat GH, Rader DJ. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108:661–663. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
- Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS. Increased Cellular Free Cholesterol in Macrophage-specific Abca1 Knock-out Mice Enhances Pro-inflammatory Response of Macrophages. The Journal of biological chemistry. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.