Abstract
Study Objective
To determine if the concomitant use of nelfinavir (NFV) and proton pump inhibitors (PPIs) among HIV-positive individuals results in the loss of virologic control.
Design
Cohort study.
Setting
Kaiser Permanente Northern California (KPNC).
Patients
A total of 1,147 HIV-positive adults initiating NFV between November 1998 and June 2003 were included. The earlier date corresponds to the availability of the branched-DNA HIV viral load assay at KPNC and the later date corresponds to over-the-counter availability of PPIs.
Measurements and Main Results
The effect on two virologic outcomes, achieving undetectable HIV viral load and subsequent virologic rebound, were compared between subjects receiving NFV alone and NFV with PPIs. Cox proportional hazard models were utilized with adjustment for age, sex, race, HIV risk factors, hepatitis B or C co-infection, and other concurrent medications known to affect the metabolism of NFV. The use of PPIs had little effect on the ability to achieve an undetectable HIV viral load (adjusted hazard ratio (HR) = 0.82; 95% confidence interval (95% CI) = 0.58, 1.19; p = 0.29), but there was an approximate 50% increased risk of virologic rebound with the use of PPIs (adjusted HR = 1.53; 95% CI = 1.06, 2.19; p = 0.02). Short-term use of PPIs (defined as within 30 days of initial PPI dispensation) was not associated with increased risk of virologic rebound (HR = 1.07; 95% CI = 0.26, 4.41; p = 0.93) in comparison to no use.
Conclusion
Use of PPIs should be minimized or avoided in individuals who have attained an undetectable HIV viral load on a NFV-based antiretroviral regimen. However, concomitant use of these medications may be acceptable for indications where PPIs are required for < 30 days.
Keywords: nelfinavir, proton pump inhibitors, omeprazole, interaction, HIV, virologic rebound
Introduction
Gastrointestinal (GI) symptoms due to progressive deterioration of the general and local immune system of the mucosa of the GI tract1-4 and disturbances in gastric acid secretion due to morphological changes in parietal cells5,6 have been documented with a high frequency in human immunodeficiency virus (HIV)-positive individuals. Additionally, antiretroviral (ARV) medications, particularly the protease inhibitor (PI) class, have been recognized as frequent causes of GI complaints leading to ARV discontinuation.7-9
Proton pump inhibitors (PPIs), available over-the-counter since 2003, are commonly used to alleviate GI symptoms. Survey studies have indicated that approximately 30-40% of patients receiving ARV therapy10,11, and as high as 65% among patients on a PI-based ARV regimen12 have reported taking proton pump inhibitors (PPIs). Such high frequency of PPI use, however, is concerning since there are known drug-drug interactions between PPIs and PIs13-17 such as Nelfinavir mesylate (NFV, Viracept®; Agouron Pharmaceuticals, Inc., La Jolla, CA), a PI used in combination with other ARV medications for the treatment of HIV18.
Presently, options for treatment of GI disorders of individuals on a stable NFV-based regimen are: 1) utilization of non-PPI acid-reducing agents, which may not be as effective as PPIs; 2) modification of the patient’s ARV regimen, which may be difficult for patients tolerating and adequately responding to NFV; or 3) concomitant use of the two agents, with the hope that virologic control will not be lost. However, clinical data assuring virologic control are lacking, which is particularly concerning given virologic failure can result in disease progression and drug resistance and consequently may minimize future ARV treatment options.19-21 It is therefore crucial to determine the clinical significance of this interaction in order to inform clinicians and counsel patients regarding the risks associated with the concomitant use of these medications. Thus, the objective of our study was to establish the clinical significance, determined by time to undetectable HIV viral load and time to virologic rebound, of the concomitant use of PPIs and NFV-based ARV regimens in HIV-infected individuals.
Methods
Using a cohort design of NFV initiators, we determined the time to undetectability (< 75 copies/mL) and time to virologic rebound (HIV viral load ≥ 75 copies/mL after having attained undetectability) among those with and without concomitant PPIs in HIV-infected Kaiser Permanente Northern California (KPNC) members.
Setting, subjects, and follow-up
KPNC is a large integrated health care system that provides medical services to more than three million members and that maintains an HIV registry of all known HIV-positive members, as well as pharmacy, laboratory, and administrative databases. Approximately 97% of members obtain their prescription medications at the KPNC pharmacies, including patients whose medications are covered through the Ryan White AIDS Drug Assistance Program.
We searched the KPNC pharmacy databases for all individuals who had initiated a NFV-based ARV regimen from November 1, 1998 to June 20, 2003. The earlier date was chosen a priori because the branched-DNA HIV viral load assay (lower limit of detection = 75 copies/mL) was introduced to KPNC in October 1998. The study end date was also selected a priori to coincide with the Food and Drug Administration (FDA) approval date for the over-the-counter availability of omeprazole (OMP) resulting in a complete capture of PPI use based on pharmacy dispensation records. We utilized the KPNC HIV registry to gather data on individual’s sex, date of birth, race, and HIV risk factors. Laboratory values, such as CD4+ cell counts and HIV viral loads were obtained from the KPNC laboratory databases and health plan membership dates were obtained from the KPNC administrative databases.
The study population included HIV-positive KPNC members who were 18 years or older and who had received at least two dispensations of an FDA-approved dose of NFV (i.e., 1250mg orally twice daily or 750mg orally thrice daily). Individuals who did not have a recorded baseline HIV viral load prior to starting a NFV-based ARV regimen were excluded. In order to ensure continuity in NFV use, subjects with evidence of NFV refill delays of greater than 30 days were censored (refill delays were determined based on the difference between the actual refill date and the expected refill date of NFV). Subjects were followed until the earliest of the following: 1) discontinuation of NFV; 2) a coverage gap in KPNC membership of three or more months; 3) a refill delay exceeding 30 days; 4) attainment of study end point (undetectability or virologic rebound depending on the outcome); or 5) end of the study (i.e., June 20, 2003, corresponding to the date when PPIs became available over the counter).
Key predictors, outcomes, and covariates
Two approaches were used to classify PPI use, the primary predictor of interest. In both approaches, an episode was defined as the period of time when an individual was considered exposed or unexposed to PPIs. In the first approach, referred to as the “ever/never” analysis, patients were assigned as always exposed to PPIs once prescribed (i.e., analogous to an intention-to-treat analysis). Specifically, each patient contributed follow-up data to the NFV-only group (unexposed episode) until a PPI was initiated, at which point they were assigned to the NFV/PPI group (exposed episode) and remained in this group for the remainder of the follow-up, regardless of PPI discontinuation (Figure 1). In the second approach, referred to throughout the text as the “current use” analysis and analogous to an as-treated analysis, subjects on NFV and a PPI contributed follow-up data to the NFV/PPI group (exposed episode) only until 30 days after the last PPI refill in the episode was expected to run out based on days supply. Therefore, in this analysis, a single individual could contribute multiple exposure episodes to both groups depending on the presence or absence of PPIs. The 30-day buffer period was added to the expected discontinuation date of the PPI in an attempt to account for the PPI wash-out period, the possibility of as-needed PPI use during and after the prescribed timeframe, and to allocate enough time for observation of possible virologic rebound. Of note, in the ever/never and current use approaches, it was possible that the initiation of NFV superseded the PPI use, in which case the individual would automatically be included in the exposed group.
Figure 1.
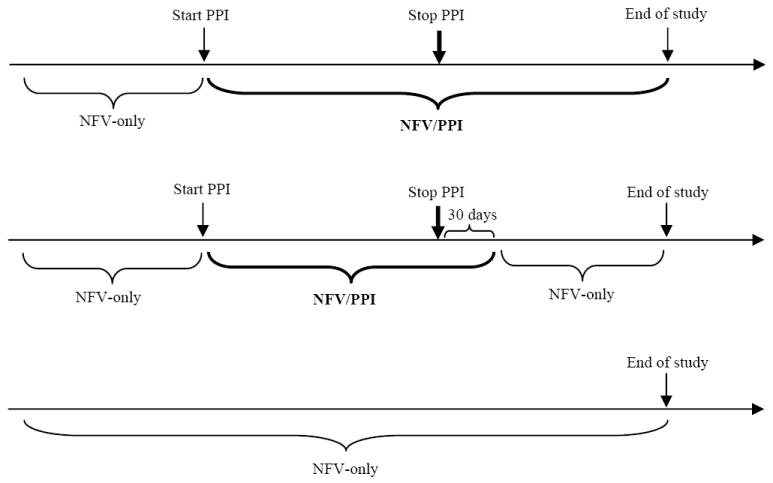
Schematic of the Ever/Never (top) and Current-use (middle) approaches to classify PPI exposure in comparison no PPI exposure (bottom)
Two key outcomes were considered: time to virologic undetectability and time to virologic rebound. For the virologic undetectability analysis, the start date was assigned as the date of NFV initiation and undetectability within six months of starting NFV was assessed. Subjects whose most recent HIV viral load test prior to NFV initiation was undetectable were excluded. For the virologic rebound analysis, the start date was assigned as the date when the individual’s HIV viral load first became undetectable (HIV viral load < 75 copies/mL). For subjects whose most recent HIV viral load test prior to NFV initiation was undetectable, the start date was assigned as the date of NFV initiation. Subjects were then followed until one detectable viral load or until they met one of the other data censoring criteria (defined above). We determined these two outcomes within each analytic approach (i.e., ever/never and current-use). Additionally, in the ever/never analysis, we determined risk of virologic rebound for short-term (< 30 days) and long-term (≥ 30 days) PPI use in comparison to no exposure in order to further investigate significant results.
Covariates in this study were age, race (non-White), HIV risk factor (intravenous drug use [yes/no]), hepatitis B co-infection (diagnosis of chronic hepatitis B and/or a positive surface antigen), hepatitis C co-infection (diagnosis of hepatitis C and/or a positive antibody or RNA test), and the presence of other medications such as liver cytochrome P450 (CYP) 3A inducers (bartiburates, carbamazepine, efavirenz, nevirapine, phenobarbital, phenytoin, pioglitazone, rifabutin, rifampin, tipranavir), CYP 2C19 inhibitors (chloramphenicol, cimetidine, felbamate, oxcarbazepine, fluoxtine, fluvoxamine, indomethacin, ketoconzole, modafinil, probenicid, ticlopidine, topiramate), or other medications (H2 receptor antagonists and anti-diarrheal medications).
Statistical analysis
Descriptive statistics were used to determine the baseline characteristics of the cohort for both the viral undetectability and rebound analyses. Next, Cox proportional hazard models22 were utilized to estimate unadjusted and adjusted hazard ratios (HR) and 95% confidence intervals (95% CI) for virologic outcomes using both approaches for classifying PPI use. Adjusted models included covariates for PPI use, age, race, HIV risk factor, use of H2 receptor antagonists or anti-diarrheal medications, use of CYP 3A inhibitors, use of 2C19 inducers, and co-infection with hepatitis B or C. A two-sided p-value < 0.05 was considered statistically significant. All analyses were performed using SAS software (v.9; SAS Institute, Inc., Cary, North Carolina). We received approval for this study from the KPNC Institutional Review Board with waiver of patient informed consent.
Results
We identified 1,465 eligible HIV-positive individuals who were listed in the KPNC HIV registry, had received at least two dispensations of an FDA-approved dose of NFV, and were ≥ 18 years of age. Study exclusions (which were not mutually exclusive) included 318 people without a baseline HIV viral load, with a lapse in KPNC membership of three months or more after starting NFV, or with a > 30-day NFV refill delay between the first actual refill date and the expected refill date, resulting in a final study population of 1,147 subjects. Within this cohort, 141 (12.3%) individuals were also prescribed PPIs at some point during NFV use. HIV viral loads were assessed at a mean of 2.5 per person per year and approximately 70% of the population received twice-daily dosing of NFV. Of the 1,147 individuals, 804 subjects were included in the time to undetectability analyses (343 individuals already had an undetectable viral load at baseline and therefore were not included in this analysis), and 784 subjects were included in the rebound analyses (363 individuals did not achieved an undetectable viral load and therefore were excluded from this analysis). Baseline characteristics of all subjects based on the analytic approach are shown in Table 1.
Table 1.
Baseline Characteristics
| Characteristic | Time to Undetectable Analysis (n = 804) | Time to Rebound Analysis (n = 784) |
|---|---|---|
| Age, n (%) | ||
| <40 years | 341 (42.4) | 310 (39.5) |
| 40-49 years | 277 (34.5) | 281 (35.8) |
| ≥50 years | 186 (23.1) | 193 (24.6) |
| Male gender, n (%) | 707 (87.9) | 682 (87.0) |
| Race, n (%) | ||
| White | 471 (58.6) | 484 (61.7) |
| Black | 156 (19.4) | 127 (16.2) |
| Hispanic | 117 (14.6) | 102 (13.0) |
| Other/unknown | 60 (7.5) | 71 (9.1) |
| HIV Risk Factors, n (%) | ||
| MSM | 422 (52.5) | 427 (54.5) |
| Heterosexual | 141 (17.5) | 133 (17.0) |
| IDU | 61 (7.6) | 49 (6.3) |
| Other/unknown | 180 (22.4) | 175 (22.3) |
| Hepatitis B, n (%) | 58 (7.2) | 55 (7.0) |
| Hepatitis C, n (%) | 82 (10.2) | 79 (10.1) |
| Mean baseline CD4+ cell count, n (%)a | ||
| < 200 cells/mm3 | 323 (41.4) | 224 (29.6) |
| 200-499 cells/mm3 | 342 (43.8) | 333 (44.1) |
| ≥ 500 cells/mm3 | 116 (14.9) | 199 (26.3) |
| Mean baseline HIV RNA, n (%)a | ||
| <75 copies/mL | - | 343 (43.8) |
| 75-999 copies/mL | 158 (19.7) | 105 (13.4) |
| 1,000-9,999 copies/mL | 169 (21.0) | 70 (8.9) |
| ≥ 10,000 copies/mL | 477 (59.3) | 266 (33.9) |
| Twice daily dosing of Nelfinavir, n (%) | 566 (70.4) | 570 (72.7) |
| Proton Pump Inhibitor use, n (%) | ||
| PPI use | 89 (11.1) | 103 (13.1) |
| PPI initiated before NFV | 41 (46.1)b | 36 (35.0)b |
| PPI initiated after NFV | 48 (53.9)b | 67 (65.0)b |
| Ever use of CYP 3A4 inducers, n (%) | 301 (37.4) | 224 (28.6) |
| Ever use of CYP 2C19 inhibitors, n (%) | 76 (9.5) | 85 (10.8) |
| Ever pregnant, n (%) | 15 (15.5) | 14 (13.7) |
Baseline laboratory values are from prior to the initiation of nelfinavir
Calculated based on percentage of total PPI use
Time to undetectability
In the ever/never analysis, 804 subjects contributed 29 undetectability events (i.e., HIV viral load < 75 copies/mL) in 34.1 person-years of PPI exposure and 414 undetectability events in 432.2 person-years of no PPI exposure. The use of a PPI was associated with approximately 18% reduced hazard of achieving an undetectable HIV viral load which did not reach statistical significance even after adjusting for covariates (HR = 0.82; 95% CI = 0.58, 1.19; p = 0.29) (Table 2).
Table 2.
Hazard Ratios (HR) and 95% Confidence Intervals (95% CI) for the Ever/Never and the Current-use Analyses
| Analysis | Outcome | Unadjusted HR (95% CI) | p-value | Adjusted HR (95% CI)a | p-value |
|---|---|---|---|---|---|
| Ever/Never | Undetectable | 0.83 (0.58, 1.23) | 0.37 | 0.82 (0.58, 1.19) | 0.29 |
| Rebound | 1.51 (1.06, 2.17) | 0.02 | 1.53 (1.06, 2.19) | 0.02 | |
| Current-use | Undetectable | 0.67 (0.42, 1.07) | 0.10 | 0.65 (0.40, 1.04) | 0.07 |
| Rebound | 1.16 (0.69, 1.96) | 0.57 | 1.22 (0.72, 2.06) | 0.46 |
Adjusted for age, race, HIV risk factor, use of H2 receptor antagonists or anti-diarrheal medications, use of inhibitors CYP 3A, use of inducers of 2C19, and co-infection with hepatitis B or C
Similarly for the current use analysis, we observed 19 undetectability events in 24.9 person-years of PPI exposure and 424 undetectability events in 441.3 person-years of no PPI exposure. Here, we observed an adjusted HR of 0.65, which trended toward statistical significance (95% CI = 0.40, 1.04; p = 0.07).
Time to rebound
For the ever/never analysis, we observed 34 rebound events in 91.9 person-years of PPI exposure and 248 rebound events in 973.0 person-years of no PPI exposure. Exposure to PPIs resulted in an increased likelihood of virologic rebound (HR (95% CI) = 1.51 (1.06, 2.17); p = 0.02), which remained statistically significant after adjusting for covariates (Table 2). To further investigate this significant result, we stratified follow-up into short-term (i.e., < 30 days) and long-term exposure to PPIs (i.e., ≥ 30 days). The risk of virologic rebound due to short-term use of PPIs was 1.07 (95% CI = 0.23, 4.41; p = 0.93) in comparison to no PPI exposure. Conversely, there was a 56% increased hazard of virologic rebound (HR = 1.56; 95% CI = 1.08, 2.26; p = 0.02) with long-term exposure to PPIs versus the unexposed group.
In the current-use analysis, 784 individuals contributed 15 events in 49.0 person-years of PPI exposure and 267 events in 1015.6 person-years of no PPI exposure. In contrast to the ever/never results, a statistically significant association was not seen with the use of PPIs and virologic rebound (HR (95% CI) = 1.16 (0.69, 1.96); p = 0.57). This association was also not statistically significant in the multivariable model (Table 2).
Additionally, rebound analyses revealed an association between the use of CYP 3A inducers with virologic rebound (HR (95% CI) = 1.31 (1.02, 1.67), p = 0.04), which was not demonstrated with the use of non-PPI CYP 2C19 inhibitors (HR (95% CI) = 0.75 (0.51, 1.10); p = 0.14). Lastly, the relationship between race and all virologic outcomes did not reach statistical significance (all p-values > 0.14).
Discussion
Here we demonstrate that the use of PPIs has little effect on the ability to achieve an undetectable HIV viral load. However, initiation of PPIs in individuals who are virologically suppressed on an NFV-based ARV regimen is associated with a 51% higher risk of virologic rebound. Additionally, our study indicated that this increased risk for rebound was limited to use longer than 30 days and that short-term use (i.e., < 30 days) of PPIs in patients on a stable NFV-based ARV regimen may be associated with minimal risk of virologic rebound.
Little information is available on the clinical implications of drug-drug interactions between most ARVs and acid-reducing agents;23 therefore, guidance for patient management relies solely on pharmacokinetic interaction studies in HIV-negative volunteers. Based on these studies17 and in spite of the lack of clinical research in HIV-positive volunteers, the co-administration of NFV and PPIs is considered a major drug-drug interaction which can result in the loss of virologic response and the development of drug resistance.18 Therefore, the strength of our study is that we evaluated the clinical consequences of the co-administration of these two medications and complemented the pharmacokinetic study17 that initially revealed this drug-drug interaction.
We utilized two analytic approaches to determine the consequences of the use of PPIs in subjects on a NFV-based ARV regimen. In the ever/never analysis, we took into account that PPIs may be used on an as-needed basis and therefore the calculated duration of the prescription may not be an accurate estimate of the timeframe that PPIs are actually taken. In the current-use approach, we attempted to determine the effect of PPIs on virologic outcomes assuming that patients actually took all doses of PPIs as prescribed. In both analyses, the ability to achieve undetectability was modestly compromised; however, in the ever/never analysis, patients who had filled a PPI prescription had an increased risk of virologic rebound in comparison to those who had not. Interestingly and despite the results in the ever/never analysis, the risk of virologic rebound in the current-use analytic approach was not as prominent. We believe that there are two potential explanations: 1) individuals were likely to have been using PPIs on an as-needed basis, therefore the date when the PPI prescription was expected to run out was not an accurate reflection of the timeframe that PPIs were actually taken; 2) the effect of PPIs on virologic rebound is likely to be cumulative, hence the analysis of each exposure episode as a separate and unrelated event may not have effectively revealed this association.
Distinct from other ARVs, NFV is metabolized by CYP 3A4 and CYP 2C19.24 The catalysis of NFV by CYP 2C19 leads to the formation of a major pharmacologically active metabolite, hydroxyl-t-butylamidenelfinavir (M8), which is as pharmacologically active as NFV, in vitro.25-27 Similarly, PPIs, such as OMP, have a high affinity for CYP 2C19 and competitively inhibit the activity of this isozyme.28 In one study, the increasing concentrations of OMP in NFV-treated patients led to a 75-98% reduction in rates of M8 formation.29 An open-label two-period, single-fixed-sequence study conducted on 20 HIV-negative subjects by Fang, et al revealed that the concomitant use of OMP (40 mg orally every 24 hours) with NFV (1,250 mg every 12 hours) decreased the pharmacokinetic exposure of NFV and M8 by 36-39% and 75-92%, respectively.17 The authors speculated that the two main mechanisms of this interaction were: 1) suppression of gastric acid secretion resulting in reduced NFV solubility (NFV demonstrates a pH-dependent solubility and becomes insoluble at a pH of greater than four18 and OMP can result in a median increase in gastric pH to approximately 4.530), and 2) competitive inhibition of CYP 2C19 by OMP resulting in a reduction in M8. The study concluded that the magnitude of this interaction increases the likelihood of virologic rebound and therefore, NFV and OMP should not be co-administered.
The co-administration of NFV and PPIs presents a unique interaction in that there are numerous factors that can contribute to reductions in the NFV and M8 plasma concentrations. Some of these factors include the subject’s race (activity of CYP 2C19 is genetically controlled), CYP 3A4 inducers, CYP 2C19 inhibitors, and the presence of diarrhea (reported in 14-20% of subjects taking NFV31). In a descriptive study, the authors reported that Black and Asian subjects had lower median M8 concentrations as compared to the Caucasian population.32 Conversely, in a subsequent study, little difference in antiviral response was seen among two groups of poor and extensive CYP 2C19 metabolizers.26,33 Similarly, we did not observe any racial differences in the antiviral response in our study. In the aforementioned study32, the concomitant use of CYP 3A4 inducers or CYP 2C19 inhibitors was associated with reductions in NFV and M8 concentrations. In our study, there was a statistically significant association between the presence of CYP 3A4 inducers and virologic rebound, which was not observed in the individuals who were prescribed non-PPI CYP 2C19 inhibitors.
There are some inherent limitations to our study. We utilized a cohort design, which does not allow for treatment assignment and therefore subject to potential selection biases or residual confounding. However, due to the report of the pharmacokinetic drug-drug interaction between PPIs and NFV17, their concomitant use is uncommon and therefore a cohort design was the most appropriate approach for our objectives. While the strength of KPNC pharmacy database is the complete capture of pharmacy refill data, these data do not represent the patient’s actual medication-taking behavior, especially for conditions where PPIs were used on an as-needed basis. Nevertheless, by limiting the study period to the date when PPIs became available over the counter and by using the ever/never versus the current-use analysis we attempted to capture the collective and the exposure-specific consequences of prescription PPIs on our primary outcomes. Similarly, we did not capture adherence to specific components of the patients’ ARV regimen. In addition, while our estimated hazard ratio for rebound associated with short term use was very close to the null, we note that the confidence interval is relatively wide given sample size limitations. Finally, we were unable to assess the presence of diarrhea and over-the-counter use of H2 receptor antagonists, which may have resulted in lower NFV and M8 plasma concentrations. However, we attempted to partially account for this issue by controlling for the presence of anti-diarrheal prescriptions and prescribed H2 receptor antagonists.
With the availability of newer and better-tolerated PIs, the advent of novel classes of ARV medications, and the change in the preferred regimens in the HIV treatment guidelines34, the use of NFV in clinical practice in the United States has decreased. However, NFV continues to be one of the most commonly used second-line PIs worldwide.35 Therefore, the identification of clinically significant drug-drug interactions between this PI and other medications commonly used by HIV-positive individuals remains a critical issue.
Conclusion
In summary, it has been established that HIV-positive individuals experience an elevated frequency of GI symptoms attributable to multiple conditions, such as infections, malignancies, medications, and disturbances in gastric acid secretion,1-9,35-41 and have a high utilization rate of acid-reducing agents10-12. Heart burn, gastro-esophageal reflux, and peptic ulcer have been reported as the major underlying causes for the use of PPIs and H2 receptor antagonists in HIV-positive patients.10 Currently, with the over-the-counter availability of PPIs, access to these medications have become simple and tracking of their use challenging. Due to these problems, as well as reports of numerous drug-drug interactions with the concomitant use of PPIs and ARV medications15-17, future research should investigate the expanding use of over-the-counter medications and the clinical significance of these interactions in order to instruct clinicians and advise patients on the risks and consequences of virologic failure. Additionally, health care providers should regularly inquire about and document their patients’ over-the-counter medication usage in order to safeguard against potential drug-drug interactions that may jeopardize HIV treatment goals.
Despite the lack of difference in the ability to attain an undetectable HIV viral load, the increased risk of virologic rebound once stabilized on a NFV-based regimen and following PPI initiation is clinically significant. Thus usage in virologically suppressed individuals should be minimized or avoided. For indication where PPIs are prescribed for a total duration of less than 30 days, it seems reasonable to continue NFV and closely monitor HIV viral load. However, for longer timeframes, the utilization of a non-PPI acid-reducing agent or the modification of the patient’s ARV regimen may be better options.
Acknowledgments
Francesca Aweeka, Pharm.D. for her mentoring and guidance on antiretroviral pharmacokinetics.
Sources of support: 2009 Kaiser Permanente Community Benefits Research Fund grant, the National Institute of Mental Health (award number F32MH086323), and National Institute of Allergy and Infectious Diseases (award number K01AI071725). The content is solely the responsibility of the authors and does not necessarily represent the official views of Kaiser Permanente or the National Institutes of Health.
Footnotes
Meetings at which data were presented: none
None of the authors have a commercial or other association that might pose a conflict of interest.
Contributor Information
Parya Saberi, Kaiser Permanente, Infectious Diseases and Pharmacy, Vallejo, CA; Department of Medicine, University of California San Francisco, San Francisco, CA.
Dilrini K. Ranatunga, Kaiser Permanente, Division of Research, Oakland, CA.
Charles P. Quesenberry, Jr, Kaiser Permanente, Division of Research, Oakland, CA.
Michael J. Silverberg, Kaiser Permanente, Division of Research, Oakland, CA.
References
- 1.Wilcox CM. Gastrointestinal consequences of infection with human immunodeficiency virus. In: Feldman M, Friedman LS, Sleisenger MH, editors. Sleisenger and Fordtran’s Gastrointestinal and liver diseases. Philadelphia: Saunders; 2002. pp. 487–497. [Google Scholar]
- 2.Smith PD, Quinn TC, Strober W, Janoff EN, Masur H. NIH conference. Gastrointestinal infection in AIDS. Ann Intern Med. 1992;116:63–77. doi: 10.7326/0003-4819-116-1-63. [DOI] [PubMed] [Google Scholar]
- 3.Ullrich R, Zeitz M, Riecken EO. Enteric immunologic abnormalities in human immunodeficiency virus infection. Semin Liver Dis. 1992;12:167–74. doi: 10.1055/s-2007-1007388. [DOI] [PubMed] [Google Scholar]
- 4.Bashir RM, Wilcox CM. Symptom-specific use of upper gastrointestinal endoscopy in human immunodeficiency virus infected patients yields high dividends. J Clin Gastroenterol. 1996;23:292–8. doi: 10.1097/00004836-199612000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Lake-Bakaar G, Elsakr M, Hagag N, et al. Changes in parietal cell structure and function in HIV disease. Dig Dis Sci. 1996;41:1398–408. doi: 10.1007/BF02088565. [DOI] [PubMed] [Google Scholar]
- 6.Welage LS, Carver PL, Revankar S, Pierson C, Kauffman CA. Alterations in gastric acidity in patients infected with human immunodeficiency virus. Clin Infect Dis. 1995;21:1431–8. doi: 10.1093/clinids/21.6.1431. [DOI] [PubMed] [Google Scholar]
- 7.Le Moing V, Chêne G, Leport C, et al. Impact of discontinuation of initial protease inhibitor therapy on further virological response in a cohort of human immunodeficiency virus-infected patients. Clin Infect Dis. 2002;34:239–47. doi: 10.1086/324354. [DOI] [PubMed] [Google Scholar]
- 8.d’Arminio Monforte A, Cozzi Lepri A, Rezza G, et al. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral-naïve patients. I.C.O.N.A. Study Group. Italian cohort of antiretroviral-naive patients. AIDS. 2000;14:499–507. doi: 10.1097/00002030-200003310-00005. [DOI] [PubMed] [Google Scholar]
- 9.Hänsel A, Bucher HC, Nöesch R, Battegay M. Reasons for discontinuation of first highly active antiretroviral therapy in a cohort of proteinase inhibitor-naïve HIV-infected patients. J Acquir Immune Defic Syndr. 2001;26:191–3. doi: 10.1097/00042560-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 10.Luber A, Garg V, Gharakhanian S. Vertex HIV Program Team. Survey of medication used by HIV-infected patients that affect gastrointestinal (GI) acidity and potential for negative drug interactions with HAART. Presented at the 7th International Congress on Drug Therapy in HIV Infection; Glasgow, UK. November 14-18, 2004. [Google Scholar]
- 11.Guessant S, Le Camus C, Lang JM. Survey on the gastric acid-modifying medications use in HIV-infectd (HIV+) patients on antiretroviral (ARV) treatment in France. Presented at the 10th European AIDS Conference; Dublin, Ireland. November 17-20, 2005. [Google Scholar]
- 12.van Lunzen J, Liess H, Arastéh K, Walli R, Daut B, Schürmann D. Concomitant use of gastric acid-reducing agents is frequent among HIV-1-infected patients receiving protease inhibitor-based highly active antiretroviral therapy. HIV Med. 2007;8:220–5. doi: 10.1111/j.1468-1293.2007.00456.x. [DOI] [PubMed] [Google Scholar]
- 13.Khanlou H, Farthing C. Co-administration of atazanavir with proton-pump inhibitors and H2 blockers. J Acquir Immune Defic Syndr. 2005;39:503. doi: 10.1097/01.qai.0000167477.20428.ce. [DOI] [PubMed] [Google Scholar]
- 14.Luber AD. Use of acid-reducing agents in protease inhibitor based HAART and the potential for negative treatment outcomes. AIDS Read. 2005;15:698–700. [PubMed] [Google Scholar]
- 15.Burger DM, Hugen PW, Kroon FP, et al. A pharmacokinetic interaction between the proton pump inhibitor omeprazole and the HIV protease inhibitor indinavir. AIDS. 1998;22:2080–2. doi: 10.1097/00002030-199815000-00025. [DOI] [PubMed] [Google Scholar]
- 16.Tomilo DL, Smith PF, Ogundele AB, et al. Inhibition of atazanavir oral absorption by lansoprazole gastric acid suppression in healthy volunteers. Pharmacotherapy. 2006;26:341–6. doi: 10.1592/phco.26.3.341. [DOI] [PubMed] [Google Scholar]
- 17.Fang AF, Damle BD, LaBadie RR, Crownover PH, Hewlett D, Jr, Glue PW. Significant decrease in nelfinavir systemic exposure after omeprazole coadministration in healthy subjects. Pharmacotherapy. 2008;28:42–50. doi: 10.1592/phco.28.1.42. [DOI] [PubMed] [Google Scholar]
- 18.Agouron Pharmaceuticals, Inc. Viracept (nelfinavir) package insert. La Jolla, CA: 2009. [Google Scholar]
- 19.Little SJ, Holte S, Routy JP, et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med. 2002;347:385–94. doi: 10.1056/NEJMoa013552. [DOI] [PubMed] [Google Scholar]
- 20.Grant RM, Hecht FM, Warmerdam M, et al. Time trends in primary HIV-1 drug resistance among recently infected persons. JAMA. 2002;288:181–8. doi: 10.1001/jama.288.2.181. [DOI] [PubMed] [Google Scholar]
- 21.Simon V, Vanderhoeven J, Hurley A, et al. Evolving patterns of HIV-1 resistance to antiretroviral agents in newly infected individuals. AIDS. 2002;16:1511–9. doi: 10.1097/00002030-200207260-00008. [DOI] [PubMed] [Google Scholar]
- 22.Cox DR, Oates D. Analysis of Survival Data. New York: Chapman & Hall; 1984. [Google Scholar]
- 23.Béïque L, Giguère P, la Porte C, Angel J. Interactions between protease inhibitors and acid-reducing agents: a systematic review. HIV Med. 2007;8:335–45. doi: 10.1111/j.1468-1293.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- 24.Sandoval TM, Grettenberger HM, Zhang KE, et al. Metabolism of nelfinavir mesylate, an HIV-1 protease inhibitor by human liver microsomes and recombinant human isoforms. Presented at the 12th Annual Meeting and Exposition of the American Association of Pharmaceutical Scientists; San Francisco, CA. November 15-19, 1998. [Google Scholar]
- 25.Zhang KE, Wu E, Patick AK, et al. Circulating metabolites of the human immunodeficiency virus protease inhibitor nelfinavir in humans: structural identification, levels in plasma, and antiviral activities. Antimicrob Agents Chemother. 2001;45:1086–93. doi: 10.1128/AAC.45.4.1086-1093.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lillibridge JH, Lee CA, Pithavala YK, et al. The role of polymorphic CYP2C19 in the metabolism of nelfinavir mesylate. Presented at the 12th Annual Meeting and Exposition of the American Association of Pharmaceutical Scientists; San Francisco, CA. November 15-19, 1998. [Google Scholar]
- 27.Zhang K, Wu E, Patick A, et al. Plasma metabolism of nelfinavir, a potent HIV protease inhibitor, in HIV positive patients: Quantitation by LC-MS/MS and antiviral activities. Presented at the 6th International Society for Study of Xenobiotics; Gothenburg, Sweden. June 30–July3, 1997. [Google Scholar]
- 28.Karam WG, Goldstein JA, Lasker JM, Ghanayem BI. Human CYP2C19 is a major omeprazole 5-hydroxylase, as demonstrated with recombinant cytochrome P450 enzymes. Drug Metab Dispos. 1996;24:1081–7. [PubMed] [Google Scholar]
- 29.Hirani VN, Raucy JL, Lasker JM. Conversion of the HIV protease inhibitor nelfinavir to the bioactive metabolite by human liver CYP2C19. Drug Metab Dispos. 2004;32:1462–7. doi: 10.1124/dmd.104.001743. [DOI] [PubMed] [Google Scholar]
- 30.Röhss K, Hasselgren G, Hedenström H. Effect of esomeprazole 40 mg vs omeprazole 40 mg on 24-hour intragastric pH in patients with symptoms of gastroesophageal reflux disease. Dig Dis Sci. 2002;47:954–8. doi: 10.1023/a:1015009300955. [DOI] [PubMed] [Google Scholar]
- 31.Saag MS, Tebas P, Sension M, et al. Randomized, double-blind comparison of two nelfinavir doses plus nucleosides in HIV-infected patients (Agouron study 511) AIDS. 2001;15:1971–8. doi: 10.1097/00002030-200110190-00009. [DOI] [PubMed] [Google Scholar]
- 32.Baede-van Dijk PA, Hugen PW, Verweij-van Wissen CP, Koopmans PP, Burger DM, Hekster YA. Analysis of variation in plasma concentrations of nelfinavir and its active metabolite M8 in HIV-positive patients. AIDS. 2001;15:991–8. doi: 10.1097/00002030-200105250-00007. [DOI] [PubMed] [Google Scholar]
- 33.Lillibridge JH, Lee CA, Pithavala YK, et al. The role of CYP2C19 in the formation of nelfinavir hydroxyl-t-butylamide (M8): in vitro/in vivo correlation. Presented at the 5th International Society for Study of Xenobiotics; Cairns, Australia. October 25–29, 1998. [Google Scholar]
- 34.Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [August 21, 2010];2009 December; Available from www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 35.WHO/UNAIDS. [August 21, 2010];Report on WHO/UNAIDS Meeting on Forecasting ARV needs up to 2010. 2005 November; Available from www.who.int/hiv/amds/ReportForecastingMeeting.pdf.
- 36.Chiu HM, Wu MS, Hung CC, Shun CT, Lin JT. Low prevalence of Helicobacter pylori but high prevalence of cytomegalovirus-associated peptic ulcer disease in AIDS patients: Comparative study of symptomatic subjects evaluated by endoscopy and CD4 counts. J Gastroenterol Hepatol. 2004;19:423–8. doi: 10.1111/j.1440-1746.2003.03278.x. [DOI] [PubMed] [Google Scholar]
- 37.Caciarelli AG, Marano BJ, Jr, Gualtieri NM, et al. Lower Helicobacter pylori infection and peptic ulcer disease prevalence in patients with AIDS and suppressed CD4 counts. Am J Gastroenterol. 1996;91:1783–4. [PubMed] [Google Scholar]
- 38.Varsky CG, Correa MC, Sarmiento N, et al. Prevalence and etiology or gastroduodenal ulcer in HIV positive patients: a comparative study of 497 symptomatic subjects evaluated by endoscopy. Am J Gastroenterol. 1998;93:935–40. doi: 10.1111/j.1572-0241.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- 39.Mach T, Skwara P, Biesiada G, Cieśla A, Macura A. Morphological changes of the upper gastrointestinal tract mucosa and Helicobacter pylori infection in HIV-positive patients with severe immunodeficiency and symptoms of dyspepsia. Med Sci Monit. 2007;13:CR14–9. [PubMed] [Google Scholar]
- 40.Marano BJ, Smith F, Bonanno CA. Helicobacter pylori prevalence in acquired immunodeficiency syndrome. Am J Gastroenterol. 1993;88:687–90. [PubMed] [Google Scholar]
- 41.Lichterfeld M, Lorenz C, Nischalke HD, Scheurlen C, Sauerbruch T, Rockstroh JK. Decreased prevalence of Helicobacter pylori infection in HIV patients with AIDS defining diseases. Z Gastroenterol. 2002;40:11–4. doi: 10.1055/s-2002-19637. [DOI] [PubMed] [Google Scholar]


