Summary
Armadillo family proteins known as plakophilins have been characterized as structural components of desmosomes that stabilize and strengthen adhesion by enhancing attachments with the intermediate filament cytoskeleton. However, plakophilins and their close relatives are emerging as versatile scaffolds for multiple signaling and metabolic processes that not only facilitate junction dynamics but also more globally regulate diverse cellular activities. While perturbation of plakophilin functions contribute to inherited diseases and cancer pathogenesis, the functional significance of the multiple PKP isoforms and the mechanisms by which their behaviors are regulated remain to be elucidated.
Introduction
Armadillo proteins in the p120ctn family are intercellular junction molecules in adherens junctions and desmosomes whose functions are rapidly expanding to include nuclear and signaling roles in development and tissue homeostasis. They can be divided into two subclasses: the plakophilins (PKPs) and the p120ctn-related proteins. The latter group includes p120, δ-catenin (also known as neural plakophilin related arm protein, NPRAP) and ARVCF (Armadillo Repeat gene deleted in Velo-Cardio-Facial syndrome), whereas the plakophilins include PKP1, 2 and 3. The PKPs localize to the cytoplasmic face of desmosomes where they participate in linking the intermediate filament cytoskeleton to the junctional plaque. PKP1 and 2 also localize to the nucleus, where their specific functions are poorly understood. A fourth protein, p0071 (sometimes referred to as PKP4), is also frequently associated with this group but its presence in desmosomes is a matter of debate [1,2]. The current state of knowledge of PKPs, their complex network of binding partners, and functions have been described in several comprehensive reviews (most recently reviewed in [3]). Here we elaborate on the concept that PKP armadillo-related proteins serve as multipurpose scaffolds that bring together a broad range of signaling and structural proteins to regulate cell adhesion, tissue homeostasis and disease.
PKP domain structure and distribution
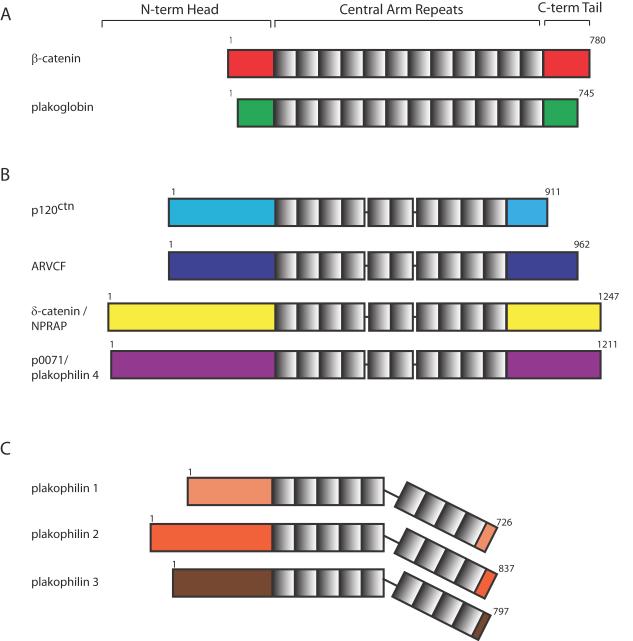
Like the founding members of the armadillo family, p120 proteins comprise a series of central armadillo repeats flanked by less conserved amino- and carboxyl terminal sequences (Fig. 1). In contrast to armadillo/β-catenin and plakoglobin, p120 and the PKPs have longer N-terminal domains, ranging from 246 to 348 amino acids. In PKPs, this N-terminus contains the sites for all known binding partners. The C-terminus of PKPs is typically quite small. High resolution structural data revealed 9 armadillo repeats, with a distinctive polypeptide “wedge” at residues 464-588 between repeats 5 and 6, resulting in a predicted bend in the structure that may provide a mechanism for regulating interactions with different partners (Fig. 1) [4].
Figure 1.
Comparison of armadillo-family protein structures. (A) Classical Armadillo family proteins β-catenin and plakoglobin (also known as γ-catenin). (B) p120ctn family armadillo proteins p120ctn, ARVCF, δ-catenin (NPRAP), and p0071 (plakophilin 4). (C) Plakophilin family armadillo proteins plakophilin 1, 2, and 3. Armadillo-family proteins consist of an amino-terminal head domain, a central armadillo repeat domain with variable number of repeats, and a carboxy-terminal tail. Numbers indicate amino acid residues.
The three PKPs exhibit differential but overlapping tissue distribution patterns. PKP1 is found in the desmosomes of differentiated layers of stratified epithelia [5-7]. PKP2 is expressed in all simple, complex and stratified epithelia as well as cardiac myocytes (where it is the only PKP expressed), germinal centers of lymph node follicles, a variety of tumors, and numerous cell lines [8,9]. PKP3 is more equally distributed among all the epidermal layers and is found in most simple and stratified epithelia except hepatocytes [10,11]. Unlike the classical armadillo proteins plakoglobin (PG) and β-catenin, p120-family proteins undergo variable degrees of RNA splicing. PKP1 and 2 each have two splice variants, 1a, 1b, 2a, 2b. The 1b isoform is localized only to the nucleus whereas 1a, 2a and 2b localize to both cytoplasm and nucleus [6,8]. These overlapping but distinct expression patterns allow for tissue-specific functions while ensuring some redundancy of critical core roles in tissue integrity or other functions.
PKPs are structural scaffolds that increase junction plaque density and mechanical strength
PKPs bind to a broad repertoire of desmosomal proteins including desmosomal cadherins, plakoglobin (PG), and desmoplakin (DP) as well as junction-associated intermediate filaments (IFs) and possibly actin [12,13]. Additional partnerships with adherens junction, nuclear and regulatory proteins, reviewed in detail in [3], support the idea that PKPs serve as scaffolds to coordinate adhesion and signaling (Fig. 2, 3). The ability to enhance the recruitment of DP to cell-cell contacts in keratinocytes is a core function shared by all PKPs [12,14-16]. In desmosome-containing epithelial cells, PKP2 is associated with DP in cytoplasmic precursor complexes that translocate to borders in the later stages of desmosome assembly [17]; its loss results in a loss of DP assembly competence and failure of DP to accumulate normally at borders [18]. Reconstitution studies in which PKP 1 or 2 are ectopically introduced into cells support the idea that PKPs collaborate with PG to cluster desmosome components into plaques with characteristic desmosome-like ultrastructure [19-21]. Thus, while PKPs provide vertical (cadherin-PKP-DP) links between the plasma membrane and IF cytoskeleton, they also facilitate lateral (PKP-PG-DP) protein-protein interactions to increase the density of the cortical structural network (Fig. 2).
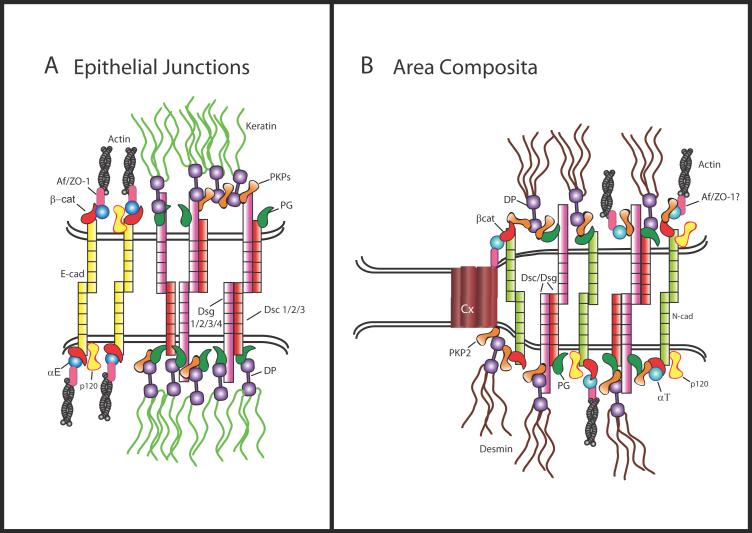
Figure 2.
Comparison of epithelial cell desmosome and adherens junctions (A) with cardiac myocyte mixed junction structures (B). In the desmosome, the cadherin family proteins (desmogleins [DSGs] and desmocollins [DSCs]) are single-pass transmembrane glycoproteins that bind through homo and heterodimeric interactions to cadherins on neighboring cells via their extracellular domains in a calcium-dependent manner. The cytoplasmic plaque consists of the armadillo-family proteins (plakoglobin [PG] and plakophilins [PKPs]), and the intermediate filament (IF)-binding plakins (desmoplakin [DP]). PG and the PKPs bind to the cytoplasmic tail of either DSG or DSC where they serve as linker proteins to DP and also participate in lateral expansion and clustering of the plaque. In the adherens junction E-cadherin forms intercellular attachments through hemophilic interactions with cadherins on neighboring cells and interacts with the armadillo protein β-catenin through its cytoplasmic domain. β-catenin, which is essential for adherens junction adhesion, binds α-catenin, which may anchor actin to the junctional plaque either directly or through other catenin binding proteins such as Z0-1 or afadin. In vertebrate cardiac myocytes hybrid junctions, or area compositae, occupy a majority of the intercalated disc membrane (B). These specialized junctions contain components common to adherens junctions and desmosomes, possibly facilitated in part by interactions between proteins such as PKP2 and αT-catenin. Connexin 43 associates with PKP2, raising the possibility that gap junctions may be intimately associated with the area composita or co-localize in intercalated disc precursors.
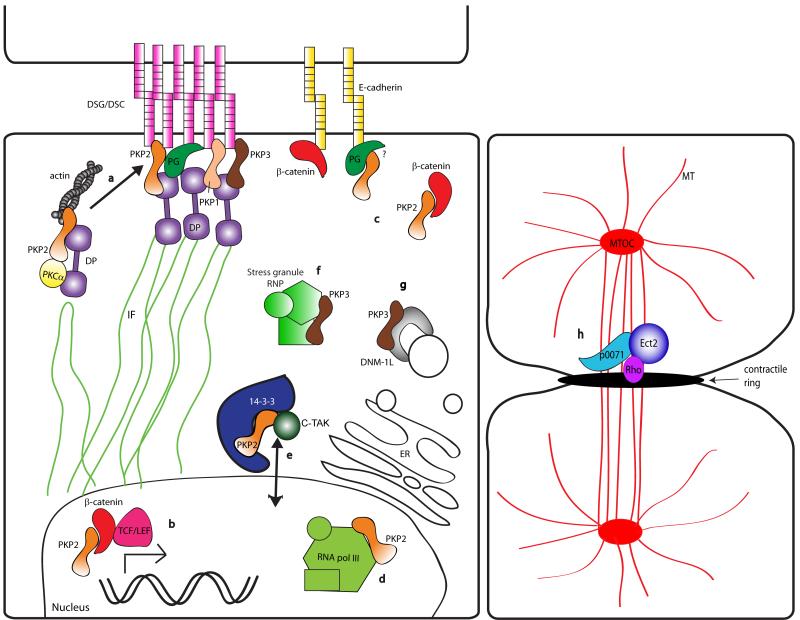
Figure 3.
Schematic of PKP family protein roles as multifunctional scaffolds. (a) PKP2 recruits PKCα to DP-containing desmosome precursors thereby modulating the DP-IF interaction, and potentially regulating actin reorganization. Direct or indirect interactions of PKP2 with actin may participate in precursor dynamics and promote their incorporation into junctions. PKP1, 2, and 3 all are found at the cytoplasmic face of the desmosome and link DP to cadherin tails. (b) PKP2 interacts with β-catenin and potentiates TCF/Lef-mediated transcriptional activity. (c) PKP2 interacts with extra-junctional β-catenin and is found in complexes with E-cadherin, possibly mediated via plakoglobin (PG). (d) Nuclear PKP2 interacts with the RNA polymerase III holoenzyme and may serve a role in rRNA and tRNA synthesis. (e) PKP2 is phosphorylated by c-TAK1, promoting 14-3-3 interaction with PKP2 and modulating PKP2-nuclear transport. (f) PKP3 colocalizes with large ribonucleoprotein complexes known as “stress granules”. (g) PKP3 interacts with DNM1L, a dynamin-related protein known to be found in endoplasmic reticulum (ER) tubules and secretory vesicles. (h) p0071 regulates recruitment and activity of Rho small GTPase via Ect2 at the midbody of a cell undergoing mitotic cell division. MT = microtubules; MTOC = microtubule organizing complex. Molecules depicted are not to scale.
PKPs are signaling scaffolds that regulate junction assembly and cytoskeletal dynamics
PKPs and their related family members serve as scaffolds not just for core junctional components but also for signaling proteins that orchestrate junction assembly. One such group of signaling molecules is the protein kinase C (PKC) family of serine/threonine kinases, which is composed of a number of related isoforms. Several members of both conventional and atypical PKC subtypes have been implicated in the regulation of cell adhesion, migration and cytoskeleton organization. In particular, PKCα is thought to be responsible for regulating a switch between a hyper-adhesive state present in mature tissues and a more dynamic state present in remodeling epithelia and healing wounds [22]. In addition, PKC activation has been reported to promote DP recruitment to cell-cell borders, bypassing signals normally required for desmosome assembly [23]. PKPs may provide one mechanism for harnessing PKC to perform these functions. We demonstrated that PKP2 interacts with PKCα and is required for PKCα association with DP (Fig. 3a). In PKP2 deficient cells, PKCα’s loss from DP complexes is associated with retention of DP on IF and failure of DP cytoplasmic precursors to translocate to nascent desmosomes, a behavior that is mimicked by a phospho-deficient mutant of DP [18]. PKC-dependent DP phosphorylation may result in a more dynamic association with IF required for desmosome precursor assembly. PKP2 can also bind IF in vitro [13] and is colocalized with IF in desmosome precursors and at cell-cell borders during junction assembly [17]. While it generally is not seen in widespread association with IF networks in cells, it is plausible that under certain conditions PKP2 may recruit PKC to IF to modulate local cytoskeletal dynamics important for junction assembly and maturation. PKP2 deficiency not only resulted in unscaffolding of PKCα from DP, but also hyperphosphorylation of the PKCα substrates MARCKS and adducin, both of which have been implicated in regulation of actin organization [18]. Based on the importance of actin organization for later stages of desmosome assembly [17], these data raise the possibility that PKP2-dependent regulation of DP-IF and remodeling of the cortical actin cytoskeleton may work in concert to recruit desmosome precursors to nascent junctions (Fig. 3a).
The p120ctn family of arm proteins has also been shown to regulate the actin dynamics via Rho GTPases (reviewed in [24]). Shape change indicators of p120-dependent inhibition of RhoA are also observed in cells expressing the PKP1 arm domain [12] or full length PKP2, the latter which is blocked by a single, highly conserved point mutation in the arm repeats (K. Green, unpublished observations). These data suggest that PKPs may coordinate actin remodeling through multiple signaling pathways. p0071/PKP4 is also a Rho signaling center, but in this case acts to spatially restrict RhoA and RhoGEF Ect2 to the region of the contractile ring during cytokinesis [25] (Fig. 3h).
Members of the armadillo family including p120ctn and β-catenin associate with microtubules (MT) and associated motor proteins including kinesins and dynein/dynactin. These interactions facilitate MT-dependent vesicular trafficking of junctional molecules and other proteins, as well as the capture and tethering of MT to the junctional plasma membrane ([26] and reviewed in [27]). p120ctn associates with kinesin indirectly via interactions with recently identified proteins Nezha and PLEKHA7 (described in [28]). Recently, studies linking PKPs to vesicle trafficking have begun to emerge. PKP3 binds to the dynamin-like protein DNM1L, a member of the dynamin family involved in vesicle trafficking within the secretory pathway in lung carcinoma cells [29] (Fig. 3g)., and in epithelial cells, p0071 translocates to the mitotic midbody via kinesin family member, KIF3b [30]. Collectively these data point to the PKP subfamily of catenins as multi-functional scaffolds that regulate the organization, function and protein interactions with all three non-muscle cytoskeletal networks (Fig. 3).
PKPs and Junctional Cross Talk
Desmosomes and adherens junctions (AJs) exist as distinct structural entities in epithelial tissues. However, their interdependence is evident during junction assembly and continues with their functional synergy at maturity, which contributes to the mechanical integrity of tissues [31,32]. In some cases AJ and desmosome components form specialized “mixed” junctions in endothelial and cardiac tissues. Their ability to interact with components of both junction types put armadillo proteins at center stage with respect to junction cross talk.
Even though normally restricted to desmosomes, PKPs exhibit the ability associate with AJ membrane domains when ectopically expressed or re-introduced into junction deficient cells [16,20]. In desmosome-deficient HT1080 cells, PKP2 recruits desmoplakin to endogenous classic cadherins, but when PG is also added, proteins sort out into separate AJ and desmosome domains [20]. PKP2 has also been shown to co-localize with and co-immunoprecipitate β-catenin when ectopically introduced into cultured keratinocytes (Fig. 3c) [16]. These findings may indicate a role for PKP2 sorting AJ and desmosome components during dynamic periods of junction assembly. The AJ protein p120 catenin has also been reported to associate with desmoglein under certain circumstances [33], suggesting a two way exchange of junctional partnerships.
Deletion or mutation of desmosome proteins can result in abnormal junction mixing, but this state is normal in the newly described mixed junctions of the cardiac myocyte [34]. It has been appreciated for a number of years that the adherens junction, desmosome and gap junction may act as a single functional unit [35,36]. During reassembly of intercellular junctions in cardiac myocytes, a “temporal intermingling” of adherens junctions and desmosomes was noted, with subsequent incorporation of gap junctions [37]. It has since been demonstrated that formation of mechanical junctions is a pre-requisite for gap junction formation. High resolution ultrastructural analysis has revealed that in higher vertebrates, components of the intercalated disc become intermixed perinatally in what is referred to as the “area composita” or “hybrid adhering junctions” [34,38] (Fig. 2). A possible contributor to this special organization is PKP2, the only PKP expressed in the heart. PKP2 uniquely interacts with the α-catenin form found in cardiac muscle, αT-catenin [16,39] but not epithelial or neuronal forms of this actin-binding adherens junction protein. PKP2 is essential for recruitment of DP to cardiac junctions and intercalated disc architecture and thus is required for normal electrical coupling and conduction in the heart [40-42].
p0071 (PKP4) associates with both classic and desmosomal cadherins [1,43] and may be involved in recruiting DP to classic cadherins. Differences between p0071 behavior and that of the PKPs are also highlighted by the observation that p0071 overexpression in keratinocytes leads to loss of desmosome components from the plasma membrane while membrane localization of AJ components is enhanced [44]. In contrast, overexpression of PKPs results in larger desmosomes and stronger adhesion [12,15,16,19,44]. Although we have much more to learn about the specific roles of these family members it seems reasonable to propose that they function in part to fine tune the interplay of junction structures and functions during dynamic tissue remodeling.
Non-junctional functions of PKPs: transcription, translation and cellular stress
While PKP2 is a constitutive component of desmosomes at sites of cell-cell contact within epithelial cells, it localization is limited to the nucleus in some epithelia and in fibroblasts, which do not typically form desmosomes [8]. Here, PKP2 interacts with the RNA polymerase III subunit (RPC155) of TFIIIB, an enzyme important for transcription of ribosomal and tRNA [45] (Fig. 3d). PKP2 may also play a role in gene transcription as it has been shown to interact with non-junctional β-catenin and potentiates β-catenin/TCF-mediated transcriptional activity [16] (Fig. 3b). Phosphorylation of PKP2 at Serine 82 by C-TAK1 regulates its interaction with a 14-3-3 protein and allows for PKP2 nuclear localization [46]. Specific signaling events regulate the translocation of cytoplasmic PKP2 to the nucleus, where it may act to anchor β-catenin to critical transcriptional machinery or possibly have transcriptional regulatory activity independent of β-catenin.
Less well understood but likely important protein-protein interactions have been identified between PKP3 and cytoplasmic RNA-binding proteins PABPC1, FXR1, and G3BP. PKP3 co-localizes with these ribonuclear-protein complexes in “stress granules” that serve as sites of stalled translocation initiation after heat or metabolic stress [47] (Fig. 3f).
p0071 has been revealed as a binding partner for PDZ domain proteins such as PAPIN (plakophilin-related armadillo repeat protein-interacting PSD-95/Dlg-A/ZO-1 (PDZ) protein). These proteins are thought to scaffold signaling complexes and target them different membrane domains [48-51]. p0071 has also been demonstrated to interact with ERBIN, a regulator of MAPK signaling pathways that is implicated in both positively and negatively affecting growth factor signaling in a context-dependent manner [51]. Collectively, these observations underscore the concept that PKPs participate widely in junctional, cytoplasmic and nuclear scaffolding.
Disorders of Junctions in Development and Inherited Disease: Role of PKP Scaffolding Disruption
The first human disease of the desmosome was reported in a family with a compound mutation in the PKP1, revealing a rare autosomal recessive disorder resulting in fragile skin with blistering upon mechanical trauma [52]. The trauma-induced blistering is thought to be caused by impaired recruitment of DP and associated IF, resulting in a reduction in desmosome number and size; however, alterations in ectodermal appendages such as nails and hair may reflect altered signaling during morphogenesis. While no human mutations in PKP3 have been identified to date, a mouse knock-out with hair follicle abnormalities also exhibited alterations in cutaneous inflammatory responses [53]. The latter finding is interesting in light of a reported association of p120ctn with NFκB-dependent inflammation through Rho GTPases [54].
Mutations in desmosomal proteins have also been associated with “Arrhythmogenic right ventricular cardiomyopathy/dysplasia” (ARVC), an inherited disorder of the cardiac muscle. The majority of familial ARVC cases are linked to mutations in PKP2 [55,56]. The disease is characterized by replacement of cardiac ventricular mass with fibroid and adipose tissue (“fibrofatty infiltration”), ventricular arrhythmias, and a high incidence of sudden death [55]. Loss of electrical synchrony can occur in the absence of major structural damage. Several possible mechanisms have been proposed for pathophysiology of ARVC. Several studies suggest that a disruption of the integrity of the gap junction plaque may constitute part of the arrhythmogenic substrate [57,58]. This hypothesis is supported by experimental evidence showing that loss of PKP2 leads to redistribution of connexins [41,42], and expression of ARVC-relevant PKP2 mutants seems to affect total Cx43 content [59]. A recent study has shown that, independent of the causative mutation, loss of immunodetectable plakoglobin at the intercalated disc is a consistent feature of the disease, and can serve as an important diagnostic tool for ARVC in afflicted individuals [57]. The contribution of mechanical stress responses versus other pathways to the fibrofatty infiltration is unknown. However, studies of a DP mouse model of ARVC implicated that alterations in β-catenin dependent signaling contribute to a transcriptionally driven conversion to an adipocyte cell fate [60]. PKP2’s role in regulating PKC signaling in epithelial cells suggests yet another potential pathway that could be affected in PKP2-deficient cardiac myocytes. It is clear from these studies that simple mechanical disruption of adhesion complexes is not the sole pathogenetic mechanism underlying these diseases.
PKPs’ role in cancer goes both ways
While loss of p120ctn has gained much attention recently as a participant in cancer progression due to its ability to stabilize classic cadherins [61], less is known about PKPs and cancer. Reduced PKP1 and 3 expression is correlated with desmosome instability, increased cell migration, and poor prognosis for patients with metastatic tumors [62,63], and PKP3 is transcriptionally repressed by the E-cadherin repressor ZEB1 in tumor cells [64]. Increased PKP cytoplasmic localization or loss of membrane staining was found in a number of oropharyngeal tumors [65,66]. In contrast, upregulation of PKP3 expression was observed in a panel of non-small cell lung cancer tumors and RNAi-mediated knock down suppressed cell growth whereas overexpression enhanced cell growth and motility in vitro [29]. Likewise, PKP2 expression correlated with poorer prognosis and was suggested to play an oncogenic role in oropharyngeal tumors [65]. The underlying reason for these apparently contradictory correlations between PKPs and cancer, and to what extent PKPs are causally related to tumor progression or repression, remains to be determined.
Conclusion and future challenges
Plakophilins are the next frontier in the armadillo family. While we have an appreciation of their potential as multi-functional protein scaffolds that direct structural or signaling molecules to specific locations, we know very little about how these interactions are tailored in different cellular contexts for specific functions. Association with kinases, and possibly with regulatory components of the Rho pathway, may help orchestrate the assembly dynamics of adhesive complexes with cytoskeletal remodeling and other functions. It seems likely that the PKP central armadillo repeats hold the key for regulating such functions, but future efforts to identify novel binding partners for this region as candidate regulators of these pathways will be required. How is PKP2 itself regulated? The finding that C-TAK mediated phosphorylation regulates nuclear localization is but one example of what is likely to be important pathways that regulate upstream events responsible for organizing these scaffolds. Finally, in light of p120’s potent role as an inhibitor of cadherin endocytic internalization [67], studies towards assessing PKPs role in trafficking are warranted, as are additional efforts to define yet elusive nuclear functions.
Acknowledgements
The authors would like to thank Andrew Kowalczyk for critical reading of this manuscript. Work in the authors’ labs is supported by National Institutes of Health grants HL087226 (to M. Delmar) and R01AR43380, R01AR41836 and R01CA122151 (to K.J. Green) A.E. Bass-Zubek was supported in part by grants from National Institutes of Health (T32CA009560) and the American Heart Association (0615631Z).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Articles of particular interest have been highlighted as:
• of special interest
•• of exceptional interest
- 1.Hatzfeld M, Green KJ, Sauter H. Targeting of p0071 to desmosomes and adherens junctions is mediated by different protein domains. J. Cell Sci. 2002;116:1219–1233. doi: 10.1242/jcs.00275. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann I, Schlechter T, Kuhn C, Hergt M, Franke WW. Protein p0071 - an armadillo plaque protein that characterizes a specific subtype of adherens junctions. J. Cell Sci. 2009;122:21–24. doi: 10.1242/jcs.043927. [DOI] [PubMed] [Google Scholar]
- 3••.Hatzfeld M. Plakophilins: Multifunctional proteins or just regulators of desmosomal adhesions? Biochim. Biophys. Acta. 2007;1773:69–77. doi: 10.1016/j.bbamcr.2006.04.009. A comprehensive review of the three main plakophilin isoforms outlines their genetic, structural, and functional similarities and differences in the context of cell adhesion, signaling and disease.
- 4.Choi H-J, Weis WI. Structure of the armadillo repeat domain of plakophilin 1. J. Mol. Biol. 2005;346:367–376. doi: 10.1016/j.jmb.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 5.Heid HW, Schmidt A, Zimbelmann R, Schafer S, Winter-Simanowski S, Stumpp S, Keith M, Figge U, Schnolzer M, Franke WW. Cell type-specific desmosomal plaque proteins of the plakoglobin family: plakophilin 1 (band 6 protein) Differentiation. 1994;58:113–131. doi: 10.1046/j.1432-0436.1995.5820113.x. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt A, Langbein L, Rode M, Pratzel S, Zimbelmann R, Franke WW. Plakophilins 1a and 1b: widespread nuclear proteins recruited in specific epithelial cells as desmosomal plaque components. Cell Tissue Res. 1997;290:481–499. doi: 10.1007/s004410050956. [DOI] [PubMed] [Google Scholar]
- 7.Moll I, Kurzen H, Langbein L, Franke WW. The distribution of the desmosomal protein, plakophilin 1, in human skin and skin tumors. J. Invest. Dermatol. 1997;108:139–146. doi: 10.1111/1523-1747.ep12332388. [DOI] [PubMed] [Google Scholar]
- 8.Mertens C, Kuhn C, Franke WW. Plakophilins 2a and 2b: constitutive proteins of dual location in the karyoplasm and the desmosomal plaque. J. Cell Biol. 1996;135:1009–1025. doi: 10.1083/jcb.135.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mertens C, Kuhn C, Moll R, Schwetlick I, Franke WW. Desmosomal plakophilin 2 as a differentiation marker in normal and malignant tissues. Differentiation. 1999;64:277–290. doi: 10.1046/j.1432-0436.1999.6450277.x. [DOI] [PubMed] [Google Scholar]
- 10.Bonne S, van Hengel J, Nollet F, Kools P, van Roy F. Plakophilin-3, a novel armadillo-like protein present in nuclei and desmosomes of epithelial cells. J. Cell Sci. 1999;112:2265–2276. doi: 10.1242/jcs.112.14.2265. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt A, Langbein L, Pratzel S, Rode M, Rackwitz HR, Franke WW. Plakophilin 3--a novel cell-type-specific desmosomal plaque protein. Differentiation. 1999;64:291–306. doi: 10.1046/j.1432-0436.1999.6450291.x. [DOI] [PubMed] [Google Scholar]
- 12.Hatzfeld M, Haffner C, Schulze K, Vinzens U. The function of plakophilin 1 in desmosome assembly and actin filament organization. J.Cell Biol. 2000;149:209–222. doi: 10.1083/jcb.149.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmann I, Mertens C, Brettel M, Nimmrich V, Schnolzer M, Herrmann H. Interaction of plakophilins with desmoplakin and intermediate filament proteins: an in vitro analysis. J. Cell Sci. 2000;113:2471–2483. doi: 10.1242/jcs.113.13.2471. [DOI] [PubMed] [Google Scholar]
- 14.Kowalczyk AP, Hatzfeld M, Bornslaeger EA, Kopp DS, Borgwardt JE, Corcoran CM, Settler A, Green KJ. The head domain of plakophilin-1 binds to desmoplakin and enhances its recruitment to desmosomes. Implications for cutaneous disease. J. Biol. Chem. 1999;274:18145–18148. doi: 10.1074/jbc.274.26.18145. [DOI] [PubMed] [Google Scholar]
- 15.Bonne S, Gilbert B, Hatzfeld M, Chen X, Green KJ, van Roy F. Defining desmosomal plakophilin-3 interactions. J. Cell Biol. 2003;161:403–416. doi: 10.1083/jcb.200303036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Bonne S, Hatzfeld M, van Roy F, Green KJ. Protein binding and functional characterization of plakophilin 2. Evidence for its diverse roles in desmosomes and beta-catenin signaling. J. Biol. Chem. 2002;277:10512–10522. doi: 10.1074/jbc.M108765200. [DOI] [PubMed] [Google Scholar]
- 17.Godsel LM, Hsieh SN, Amargo EV, Bass AE, Pascoe-McGillicuddy LT, Huen AC, Thorne ME, Gaudry CA, Park JK, Myung K, et al. Desmoplakin assembly dynamics in 4D: multiple phases differentially regulated by intermediate filaments and actin. J. Cell Biol. 2005;171:1045–1059. doi: 10.1083/jcb.200510038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Bass-Zubek AE, Hobbs RP, Amargo EV, Garcia NJ, Hsieh SN, Chen X, Wahl JKI, Denning MF, Green KJ. Plakophilin 2: a critical scaffold for PKCalpha that regulates intercellular junction assembly. J. Cell Biol. 2008;181:605–613. doi: 10.1083/jcb.200712133. This paper demonstrates a novel function for a PKP in the recruitment of a ubiquitous signaling protein, PKCα, to desmosomal precursors. PKP2 regulates cytoskeletal association of these precursors and is required for their proper assembly into junctions.
- 19.Bornslaeger EA, Godsel LM, Corcoran CM, Park JK, Hatzfeld M, Kowalczyk AP, Green KJ. Plakophilin 1 interferes with plakoglobin binding to desmoplakin, yet together with plakoglobin promotes clustering of desmosomal plaque complexes at cell-cell borders. J. Cell Sci. 2001;114:727–738. doi: 10.1242/jcs.114.4.727. [DOI] [PubMed] [Google Scholar]
- 20.Koeser J, Troyanovsky SM, Grund C, Franke WW. De novo formation of desmosomes in cultured cells upon transfection of genes encoding specific desmosomal components. Exp. Cell Res. 2003;285:114–130. doi: 10.1016/s0014-4827(03)00016-8. [DOI] [PubMed] [Google Scholar]
- 21.Sobolik-Delmaire T, Katafiasz D, Wahl JK., 3rd The carboxyl terminus of plakophilin-1 recruits it to the plasma membrane, whereas amino terminus recruits desmoplakin and promotes desmosome assembly. J. Biol. Chem. 2006;281:16962–16970. doi: 10.1074/jbc.M600570200. [DOI] [PubMed] [Google Scholar]
- 22.Wallis S, Lloyd S, Wise I, Ireland G, Fleming TP, Garrod D. The alpha isoform of protein kinase C is involved in signaling the response of desmosomes to wounding in cultured epithelial cells. Mol. Biol. Cell. 2000;11:1077–1092. doi: 10.1091/mbc.11.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Hengel J, Gohon L, Bruyneel E, Vermeulen S, Cornelissen M, Mareel M, von Roy F. Protein kinase C activation upregulates intercellular adhesion of alpha-catenin-negative human colon cancer cell variants via induction of desmosomes. J. Cell Biol. 1997;137:1103–1116. doi: 10.1083/jcb.137.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anastasiadis PZ. p120-ctn: A nexus for contextual signaling via Rho GTPases. Biochim. Biophys. Acta. 2007;1773:34–46. doi: 10.1016/j.bbamcr.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 25.Wolf A, Keil R, Gotzl O, Mun A, Schwarze K, Lederer M, Huttelmaier S, Hatzfeld M. The armadillo protein p0071 regulates Rho signalling during cytokinesis. Nat. Cell Biol. 2006;8:1432–1440. doi: 10.1038/ncb1504. [DOI] [PubMed] [Google Scholar]
- 26.Franz CM, Ridley AJ. p120 catenin associates with microtubules: inverse relationship between microtubule binding and Rho GTPase regulation. J. Biol. Chem. 2004;279:6588–6594. doi: 10.1074/jbc.M312812200. [DOI] [PubMed] [Google Scholar]
- 27.Stehbens SJ, Akhmanova A, Yap AS. Microtubules and cadherins: a neglected partnership. Front. Biosci. 2009;14:3159–3167. doi: 10.2741/3442. [DOI] [PubMed] [Google Scholar]
- 28.Meng W, Mushika Y, Ichii T, Takeichi M. Anchorage of Microtubule Minus Ends to Adherens Junctions Regulates Epithelial Cell-Cell Contacts. Cell. 2008;135:948–959. doi: 10.1016/j.cell.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 29.Furukawa C, Daigo Y, Ishikawa N, Kato T, Ito T, Tsuchiya E, Sone S, Nakamura Y. Plakophilin 3 oncogene as prognostic marker and therapeutic target for lung cancer. Cancer Res. 2005;65:7102–7110. doi: 10.1158/0008-5472.CAN-04-1877. [DOI] [PubMed] [Google Scholar]
- 30.Keil R, Kießling C, Hatzfeld M. Targeting of p0071 to the midbody depends on KIF3 J. Cell Sci. 2009;122:1174–1183. doi: 10.1242/jcs.045377. [DOI] [PubMed] [Google Scholar]
- 31.Huen AC, Park JK, Godsel LM, Chen X, Bannon LJ, Amargo EV, Hudson TY, Mongiu AK, Leigh IM, Kelsell DP, et al. Intermediate filament-membrane attachments function synergistically with actin-dependent contacts to regulate intercellular adhesive strength. J. Cell Biol. 2002;159:1005–1017. doi: 10.1083/jcb.200206098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasioukhin V, Bowers E, Bauer C, Degenstein L, Fuchs E. Desmoplakin is essential in epidermal sheet formation. Nat. Cell Biol. 2001;3:1076–1085. doi: 10.1038/ncb1201-1076. [DOI] [PubMed] [Google Scholar]
- 33.Kanno M, Aoyama Y, Isa Y, Yamamoto Y, Kitajima Y. p120 catenin is associated with desmogleins when desmosomes are assembled in high-Ca(2+) medium but not when disassembled in low-Ca(2+) medium in DJM-1 cells. J. Dermatol. 2008;35:317–324. doi: 10.1111/j.1346-8138.2008.00480.x. [DOI] [PubMed] [Google Scholar]
- 34.Franke WW, Borrmann CM, Grund C, Pieperhoff S. The area composita of adhering junctions connecting heart muscle cells of vertebrates. I. Molecular definition in intercalated disks of cardiomyocytes by immunoelectron microsocopy of desmosomal proteins. Eur. J. Cell Biol. 2006;85:69–82. doi: 10.1016/j.ejcb.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Delmar M. The intercalated disk as a single functional unit. Heart Rhythm. 2004;1:12–13. doi: 10.1016/j.hrthm.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Saffitz JE. Dependence of electrical coupling on mechanical coupling in cardiac myocytes: insights gained from cardiomyopathies caused by defects in cell-cell connections. Ann. N.Y. Acad. Sci. 2005;1047:336–344. doi: 10.1196/annals.1341.030. [DOI] [PubMed] [Google Scholar]
- 37.Kostin S, Hein S, Bauer EP, Schaper J. Spatiotemporal development and distribution of intercellular junctions in adult rat cardiomyocytes in culture. Circ. Res. 1999;85:154–167. doi: 10.1161/01.res.85.2.154. [DOI] [PubMed] [Google Scholar]
- 38•.Pieperhoff S, Franke WW. The area composita of adhering junctions connecting heart muscle cells of vertebrates. IV. Coalescence and amalgamation of desmosomal and adhaerens junction components. - Late processes in mammalian heart development Eur. J. Cell Biol. 2007;86:377–391. doi: 10.1016/j.ejcb.2007.04.001. This article demonstrates that the mixed cardiac adhesion junctions, the area composita, coalesce from once distant complexes, desmosomes and adherens junctions, as cardiac tissue matures.
- 39•.Goossens S, Janssens B, Bonne S, De Ryke R, Braet F, van Hengel J, van Roy F. A unique and specific interaction between {alpha}T-catenin and plakophilin-2 in the area composita, the mixed-type junctional structure of cardiac intercalated discs. J. Cell Sci. 2007;120:2126–2136. doi: 10.1242/jcs.004713. This article demonstrates a cardiac specific colocalization of, and interaction between PKP2 and α-catenin, suggesting a potential mechanism by which mixed cardiac junctions occur.
- 40.Grossmann KS, Grund C, Huelsken J, Behrend M, Erdmann B, Franke WW, Birchmeier W. Requirement of plakophilin 2 for heart morphogenesis and cardiac junction formation. J. Cell Biol. 2004;167:149–160. doi: 10.1083/jcb.200402096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Pieperhoff S, Schumacher H, Franke WW. The area composita of adhering junctions connecting heart muscle cells of vertebrates. V. The importance of plakophilin-2 demonstrated by small interference RNA-mediated knockdown in cultured rat cardiomyocytes. Eur. J. Cell Biol. 2008;87:399–411. doi: 10.1016/j.ejcb.2007.12.002. This article demonstrates that RNAi-mediated loss of PKP2 expression led to the disintegration of area compositae in the intercalated junctions of neonatal rat cardiomyocytes.
- 42••.Oxford EM, Musa H, Maass K, Coombs W, Taffet SM, Delmar M. Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circ. Res. 2007;101:703–711. doi: 10.1161/CIRCRESAHA.107.154252. This article is the first to demonstrate a direct interaction between PKP2 and gap junction protein connexin43, and provides supporting evidence for the idea that the pathogenetic mechanism of PKP2-related ARVC may be due to disruption of cardiac myocyte coupling.
- 43.Calkins CC, Hoepner BL, Law CM, Novak MR, Setzer SV, Hatzfeld M, Kowalczyk AP. The armadillo family protein p0071 is a VE-cadherin- and desmoplakin-binding protein. J. Biol. Chem. 2003;278:1774–1783. doi: 10.1074/jbc.M205693200. [DOI] [PubMed] [Google Scholar]
- 44.Setzer SV, Calkins CC, Garner J, Summers S, Green KJ, Kowalczyk AP. Comparative analysis of armadillo family proteins in the regulation of A431 epithelial cell junction assembly, adhesion and migration. J. Invest. Dermatol. 2004; 123:426–433. doi: 10.1111/j.0022-202X.2004.23319.x. [DOI] [PubMed] [Google Scholar]
- 45.Mertens C, Hofmann I, Wang Z, Teichmann M, Chong SS, Schnolzer M, Franke WW. Nuclear particles containing RNA polymerase III complexes associated with the junctional plaque protein plakophilin 2. Proc. Nat. Acad. Sci. 2001;98:7795–7800. doi: 10.1073/pnas.141219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muller J, Ritt DA, Copeland TD, Morrison DK. Functional analysis of C-TAK1 substrate binding and identification of PKP2 as a new C-TAK1 substrate. EMBO Journal. 2003;22:4431–4442. doi: 10.1093/emboj/cdg426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hofmann I, Casella M, Schnolzer M, Schlechter T, Spring H, Franke WW. Identification of the junctional plaque protein plakophilin 3 in cytoplasmic particles containing RNA-binding proteins and the recruitment of plakophilins 1 and 3 to stress granules. Mol. Biol. Cell. 2006;17:1388–1398. doi: 10.1091/mbc.E05-08-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deguchi M, Iizuka T, Hata Y, Nishimura W, Hirao K, Yao I, Kawabe H, Takai Y. PAPIN. A novel multiple PSD-95/Dlg-A/ZO-1 protein interacting with neural plakophilin-related armadillo repeat protein/delta-catenin and p0071. J. Biol. Chem. 2000;275:29875–29880. doi: 10.1074/jbc.M005384200. [DOI] [PubMed] [Google Scholar]
- 49.Ohno H, Hirabayashi S, Iizuka T, Ohnishi H, Fujita T, Hata Y. Localization of p0071-interacting proteins, plakophilin-related armadillo-repeat protein-interacting protein (PAPIN) and ERBIN, in epithelial cells. Oncogene. 2002;21:7042–7049. doi: 10.1038/sj.onc.1205852. [DOI] [PubMed] [Google Scholar]
- 50.Laura RP, Witt AS, Held HA, Gerstner R, Deshayes K, Koehler MF, Kosik KS, Sidhu SS, Lasky LA. The Erbin PDZ domain binds with high affinity and specificity to the carboxyl termini of delta-catenin and ARVCF. J. Biol. Chem. 2002;277:12906–12914. doi: 10.1074/jbc.M200818200. [DOI] [PubMed] [Google Scholar]
- 51.Izawa I, Nishizawa M, Tomono Y, Ohtakara K, Takahashi T, M. I ERBIN associates with p0071, an armadillo protein, at cell-cell junctions of epithelial cells. Genes Cells. 2002;7:475–485. doi: 10.1046/j.1365-2443.2002.00533.x. [DOI] [PubMed] [Google Scholar]
- 52.McGrath JA, McMillan JR, Shemanko CS, Runswick SK, Leigh IM, Lane EB, Garrod DR, Eady RA. Mutations in the plakophilin 1 gene result in ectodermal dysplasia/skin fragility syndrome. Nat. Genet. 1997;17:240–244. doi: 10.1038/ng1097-240. [DOI] [PubMed] [Google Scholar]
- 53.Sklyarova T, Bonné S, D’Hooge P, Denecker G, Goossens S, De Rycke R, Borgonie G, Bösl M, van Roy F, van Hengel J. Plakophilin-3-deficient mice develop hair coat abnormalities and are prone to cutaneous inflammation. J. Invest. Dermatol. 2008 doi: 10.1038/sj.jid.5701189. [DOI] [PubMed] [Google Scholar]
- 54.Perez-Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, Fuchs E. p120-catenin mediates inflammatory responses in the skin. Cell. 2006;124:631–644. doi: 10.1016/j.cell.2005.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerull B, Heuser A, Wichter T, Paul M, Basson CT, McDermott DA, Lerman BB, Markowitz SM, Ellinor PT, MacRae CA, et al. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat. Genet. 2004;36:1162–1164. doi: 10.1038/ng1461. [DOI] [PubMed] [Google Scholar]
- 56.van Tintelen JP, Entius MM, Bhuiyan ZA, Jongbloed R, Wiesfeld AC, Wilde AA, van der Smagt J, Boven LG, Mannens MM, I.M. vL, et al. Plakophilin-2 mutations are the major determinant of familial arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation. 2006;113:1650–1658. doi: 10.1161/CIRCULATIONAHA.105.609719. [DOI] [PubMed] [Google Scholar]
- 57.Asimaki A, Tandri H, Huang H, Halushka MK, Gautam S, Basso C, Thiene G, Tsatsopoulou A, Protonotarios N, McKenna WJ, et al. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N. Engl. J. Med. 2009;360:1075–1084. doi: 10.1056/NEJMoa0808138. [DOI] [PubMed] [Google Scholar]
- 58.Kaplan SR, Gard JJ, Protonotarios N, Tsatsopoulou A, Spiliopoulou C, Anastasakis A, Squarcioni CP, McKenna WJ, Thiene G, Basso C, et al. Remodeling of myocyte gap junctions in arrhythmogenic right ventricular cardiomyopathy due to a deletion in plakoglobin (Naxos disease) Heart Rhythm. 2004;1:3–11. doi: 10.1016/j.hrthm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 59•.Joshi-Mukherjee R, Coombs W, Musa H, Oxford E, Taffet S, Delmar M. Characterization of the molecular phenotype of two arrhythmogenic right ventricular cardiomyopathy (ARVC)-related plakophilin-2 (PKP2) mutations. Heart Rhythm. 2008;5:1715–1723. doi: 10.1016/j.hrthm.2008.09.009. This article investigates the molecular mechanism underlying ARVC via ectopic expression of ARVC-mutant PKP2. Disruption of interactions between mutant PKP2 and junctional proteins leads to reduced connexin43 and HSP90 expression.
- 60.Garcia-Gras E, Lombardi R, Giocondo MJ, Willerson JT, Schneider MD, Khoury DS, Marian AJ. Suppression of canonical Wnt/ß-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J. Clin. Invest. 2006;116:2012–2021. doi: 10.1172/JCI27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reynolds AB, Carnahan RH. Regulation of cadherin stability and turnover by p120ctn: implications in disease and cancer. Seminars in Cell and Developmental Biology. 2004;15:657–663. doi: 10.1016/j.semcdb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 62.South AP, Wan H, Stone MG, Dopping-Hepenstal PJ, Purkis PE, Marshall JF, Leigh IM, Eady RA, Hart IR, McGrath JA. Lack of plakophilin 1 increases keratinocyte migration and reduces desmosome stability. J. Cell Sci. 2003;116:3303–3314. doi: 10.1242/jcs.00636. [DOI] [PubMed] [Google Scholar]
- 63.Kundu ST, Gosavi P, Khapare N, Patel R, Hosing AS, Maru GB, Ingle A, Decaprio JA, Dalal SN. Plakophilin 3 downregulation leads to a decrease in cell adhesion and promotes metastasis. Int. J. Cancer. 2008;123:2303–2314. doi: 10.1002/ijc.23797. [DOI] [PubMed] [Google Scholar]
- 64.Aigner K, Descovich L, Mikula M, Sultan A, Dampier B, Bonné S, van Roy F, Mikulits W, Schreiber M, Brabletz T, et al. The transcription factor ZEB1 (deltaEF1) represses plakophilin 3 during human cancer progression. FEBS Lett. 2007;581:1617–1624. doi: 10.1016/j.febslet.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Papagerakis S, Shabana AH, Depondt J, Gehanno P, Forest N. Immunohistochemical localization of plakophilins (PKP1, PKP2, PKP3, and p0071) in primary oropharyngeal tumors: correlation with clinical parameters. Hum. Pathol. 2003;34:565–572. doi: 10.1016/s0046-8177(03)00174-6. [DOI] [PubMed] [Google Scholar]
- 66.Sobolik-Delmaire T, Katafiasz D, Keim SA, Mahoney M, Wahl JKI. Decreased plakophilin-1 expression promotes increased motility in HNSCC cells. Cell Commun. Adhes. 2007;14:99–109. doi: 10.1080/15419060701463082. [DOI] [PubMed] [Google Scholar]
- 67.Delva E, Kowalczyk AP. Regulation of cadherin trafficking. Traffic. 2009;10:259–267. doi: 10.1111/j.1600-0854.2008.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]