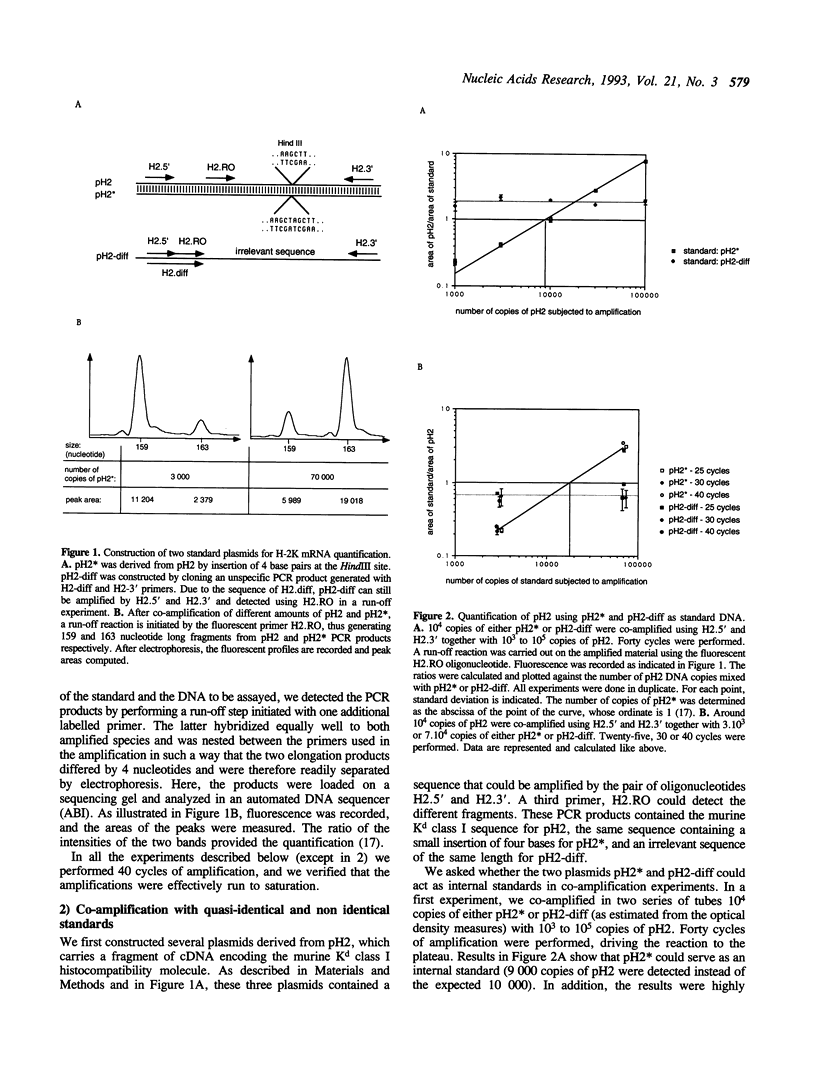
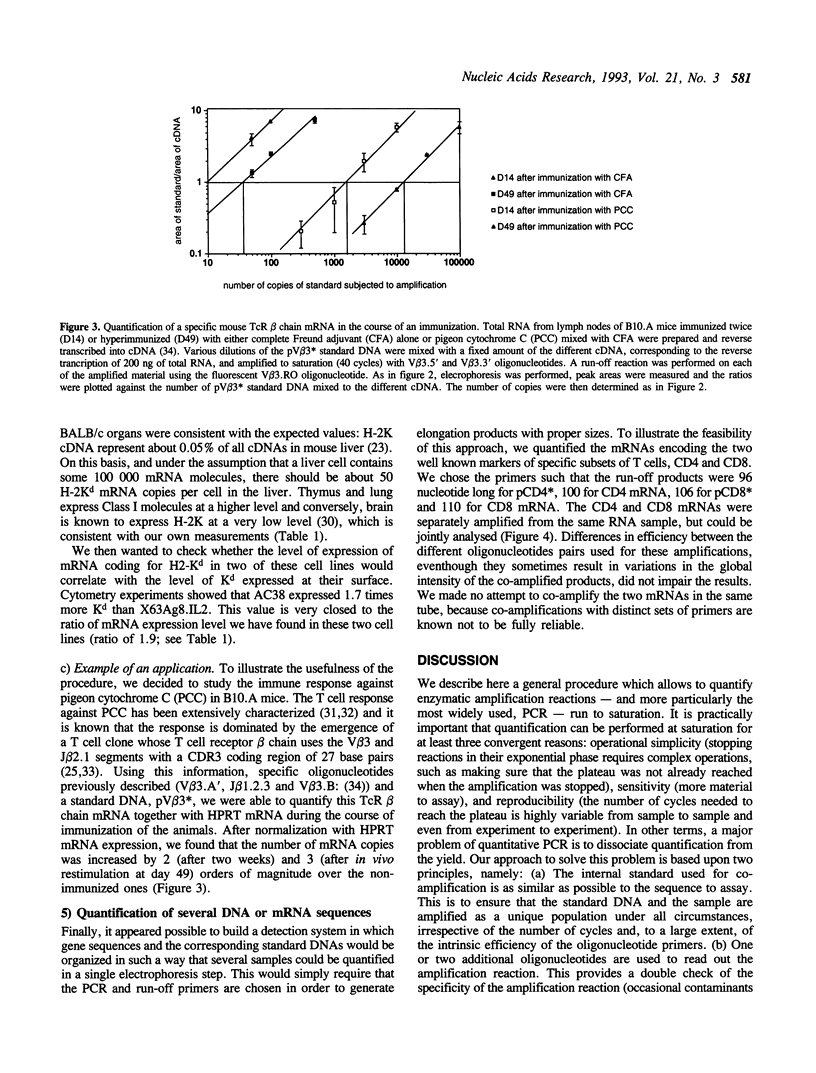
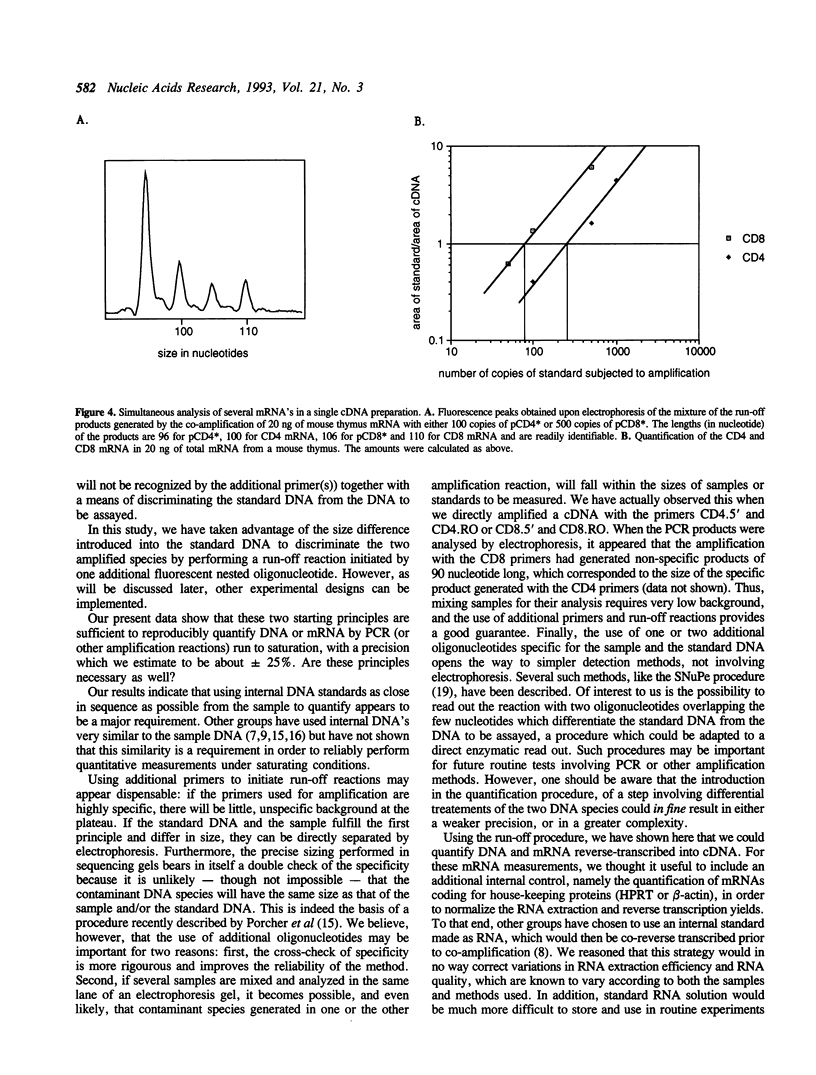
Abstract
In vitro enzymatic amplification of nucleic acids by PCR or other techniques is a very sensitive method to detect rare DNA segments. We present here a protocol that allows the rapid, sensitive and precise quantification of DNA molecules using PCR amplification run to saturation. The DNA (or cDNA) to be assayed is co-amplified with known amounts of an internal standard DNA. We show that the latter must be almost identical to the assayed DNA, otherwise quantification at the plateau is unreliable. The read-out of the amplification involves one or two additional oligonucleotides. Using fluorescent oligonucleotides as primers in run-off reactions together with an automated DNA sequencer, we could measure the level of expression of several genes, like the murine MHC class I H-2Kd or a specific T cell receptor beta chain transcript in the course of an immunization. mRNA levels were normalized by measuring in a similar manner the number of transcripts encoding the housekeeping gene HPRT. Finally, our procedure might allow the rapid analysis of a large number of samples at the same time, as illustrated by the simultaneous analysis of the mRNAs encoding the CD4 and CD8 murine T cell markers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballagi-Pordány A., Ballagi-Pordány A., Funa K. Quantitative determination of mRNA phenotypes by the polymerase chain reaction. Anal Biochem. 1991 Jul;196(1):89–94. doi: 10.1016/0003-2697(91)90122-a. [DOI] [PubMed] [Google Scholar]
- Becker-André M., Hahlbrock K. Absolute mRNA quantification using the polymerase chain reaction (PCR). A novel approach by a PCR aided transcript titration assay (PATTY). Nucleic Acids Res. 1989 Nov 25;17(22):9437–9446. doi: 10.1093/nar/17.22.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelly J., Montarras D., Pinset C., Berwald-Netter Y., Kaplan J. C., Kahn A. Quantitative estimation of minor mRNAs by cDNA-polymerase chain reaction. Application to dystrophin mRNA in cultured myogenic and brain cells. Eur J Biochem. 1990 Feb 14;187(3):691–698. doi: 10.1111/j.1432-1033.1990.tb15355.x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cochet M., Pannetier C., Regnault A., Darche S., Leclerc C., Kourilsky P. Molecular detection and in vivo analysis of the specific T cell response to a protein antigen. Eur J Immunol. 1992 Oct;22(10):2639–2647. doi: 10.1002/eji.1830221025. [DOI] [PubMed] [Google Scholar]
- Compton J. Nucleic acid sequence-based amplification. Nature. 1991 Mar 7;350(6313):91–92. doi: 10.1038/350091a0. [DOI] [PubMed] [Google Scholar]
- David-Watine B., Israël A., Kourilsky P. The regulation and expression of MHC class I genes. Immunol Today. 1990 Aug;11(8):286–292. doi: 10.1016/0167-5699(90)90114-o. [DOI] [PubMed] [Google Scholar]
- Erlich H. A., Gelfand D., Sninsky J. J. Recent advances in the polymerase chain reaction. Science. 1991 Jun 21;252(5013):1643–1651. doi: 10.1126/science.2047872. [DOI] [PubMed] [Google Scholar]
- Fink P. J., Blair M. J., Matis L. A., Hedrick S. M. Molecular analysis of the influences of positive selection, tolerance induction, and antigen presentation on the T cell receptor repertoire. J Exp Med. 1990 Jul 1;172(1):139–150. doi: 10.1084/jem.172.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink P. J., Matis L. A., McElligott D. L., Bookman M., Hedrick S. M. Correlations between T-cell specificity and the structure of the antigen receptor. Nature. 1986 May 15;321(6067):219–226. doi: 10.1038/321219a0. [DOI] [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guatelli J. C., Whitfield K. M., Kwoh D. Y., Barringer K. J., Richman D. D., Gingeras T. R. Isothermal, in vitro amplification of nucleic acids by a multienzyme reaction modeled after retroviral replication. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1874–1878. doi: 10.1073/pnas.87.5.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. M. Specificity of the T cell receptor for antigen. Adv Immunol. 1988;43:193–234. doi: 10.1016/s0065-2776(08)60366-1. [DOI] [PubMed] [Google Scholar]
- Hoof T., Riordan J. R., Tümmler B. Quantitation of mRNA by the kinetic polymerase chain reaction assay: a tool for monitoring P-glycoprotein gene expression. Anal Biochem. 1991 Jul;196(1):161–169. doi: 10.1016/0003-2697(91)90133-e. [DOI] [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Kuppuswamy M. N., Hoffmann J. W., Kasper C. K., Spitzer S. G., Groce S. L., Bajaj S. P. Single nucleotide primer extension to detect genetic diseases: experimental application to hemophilia B (factor IX) and cystic fibrosis genes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1143–1147. doi: 10.1073/pnas.88.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwoh D. Y., Davis G. R., Whitfield K. M., Chappelle H. L., DiMichele L. J., Gingeras T. R. Transcription-based amplification system and detection of amplified human immunodeficiency virus type 1 with a bead-based sandwich hybridization format. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1173–1177. doi: 10.1073/pnas.86.4.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalanne J. L., Delarbre C., Gachelin G., Kourilsky P. A cDNA clone containing the entire coding sequence of a mouse H-2Kd histocompatibility antigen. Nucleic Acids Res. 1983 Mar 11;11(5):1567–1577. doi: 10.1093/nar/11.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. W., Konecki D. S., Brennand J., Caskey C. T. Structure, expression, and mutation of the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2147–2151. doi: 10.1073/pnas.81.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Meyerhans A., Vartanian J. P., Wain-Hobson S. DNA recombination during PCR. Nucleic Acids Res. 1990 Apr 11;18(7):1687–1691. doi: 10.1093/nar/18.7.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannetier C., Cochet M., Darche S., Kourilsky P. Une méthode de dosage quantitatif d'acides nucléiques par amplification enzymatique (méthode PCR) à saturation. C R Acad Sci III. 1992;315(7):271–277. [PubMed] [Google Scholar]
- Platzer C., Richter G., Uberla K., Müller W., Blöcker H., Diamantstein T., Blankenstein T. Analysis of cytokine mRNA levels in interleukin-4-transgenic mice by quantitative polymerase chain reaction. Eur J Immunol. 1992 May;22(5):1179–1184. doi: 10.1002/eji.1830220511. [DOI] [PubMed] [Google Scholar]
- Porcher C., Malinge M. C., Picat C., Grandchamp B. A simplified method for determination of specific DNA or RNA copy number using quantitative PCR and an automatic DNA sequencer. Biotechniques. 1992 Jul;13(1):106–114. [PubMed] [Google Scholar]
- Rebai N., Mercier P., Kristensen T., Devaux C., Malissen B., Mawas C., Pierres M. Murine H-2Dd-reactive monoclonal antibodies recognize shared antigenic determinant(s) on human HLA-B7 or HLA-B27 molecules or both. Immunogenetics. 1983;17(4):357–370. doi: 10.1007/BF00372455. [DOI] [PubMed] [Google Scholar]
- Robinson M. O., Simon M. I. Determining transcript number using the polymerase chain reaction: Pgk-2, mP2, and PGK-2 transgene mRNA levels during spermatogenesis. Nucleic Acids Res. 1991 Apr 11;19(7):1557–1562. doi: 10.1093/nar/19.7.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer-Sam J., LeBon J. M., Dai A., Riggs A. D. A sensitive, quantitative assay for measurement of allele-specific transcripts differing by a single nucleotide. PCR Methods Appl. 1992 Feb;1(3):160–163. doi: 10.1101/gr.1.3.160. [DOI] [PubMed] [Google Scholar]
- Sorger S. B., Hedrick S. M., Fink P. J., Bookman M. A., Matis L. A. Generation of diversity in T cell receptor repertoire specific for pigeon cytochrome c. J Exp Med. 1987 Feb 1;165(2):279–301. doi: 10.1084/jem.165.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvänen A. C., Aalto-Setälä K., Harju L., Kontula K., Söderlund H. A primer-guided nucleotide incorporation assay in the genotyping of apolipoprotein E. Genomics. 1990 Dec;8(4):684–692. doi: 10.1016/0888-7543(90)90255-s. [DOI] [PubMed] [Google Scholar]
- Walker G. T., Fraiser M. S., Schram J. L., Little M. C., Nadeau J. G., Malinowski D. P. Strand displacement amplification--an isothermal, in vitro DNA amplification technique. Nucleic Acids Res. 1992 Apr 11;20(7):1691–1696. doi: 10.1093/nar/20.7.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]



