Abstract
Ongoing debates in the pharmaceutical sector about intellectual property, pricing and reimbursement, and public research investments have a common denominator: the pursuit of innovation. However, there is little clarity about what constitutes a true pharmaceutical innovation, and as a result there is confusion about what kind of new products should be pursued, protected and encouraged through health policy and clinical practice. If the concept of pharmaceutical innovation can be clarified, then it may become easier for health policy-makers and practitioners to evaluate, adopt and procure products in ways that appropriately recognize, encourage and give priority to truly valuable pharmaceutical innovations.
Ongoing debates in the pharmaceutical sector about intellectual property,1, 2 pricing and reimbursement,3, 4 and public research investments5 have a common denominator: the pursuit of innovation. However, there is little clarity about what constitutes a true pharmaceutical innovation, and as a result there is confusion about what kind of new products should be pursued, protected and encouraged through health policy and clinical practice.6 If the concept of pharmaceutical innovation can be clarified, then it may become easier for health policy-makers and practitioners to evaluate, adopt and procure products in ways that appropriately recognize, encourage and give priority to truly valuable pharmaceutical innovations.
To describe a product as innovative implies that it has properties that are worthy of recognition and reward. The term suggests that the product has unique value. However, notions of value are a matter of perspective. Commercial value, for example, is generally assessed from the perspective of a firm’s profitability. The perceived societal value of ordinary goods is often defined by consumer preferences as reflected by their willingness to pay for products that they perceive to be “good value for money.” However, pharmaceuticals are not ordinary goods.
Pharmaceutical products have no intrinsic value to patients or to society; rather, their value lies in the health outcomes they generate. Pharmaceuticals are licensed for sale on the basis of whether they safely and efficaciously address a health care need, not because patients might have preferences concerning their shape, colour, taste or brand. Although characteristics like shape, colour, taste or brand may play a role in improving health outcomes — perhaps by increasing treatment adherence — it is the measurable improvements in health outcomes that generate value for society. Product characteristics are analogous to surrogate endpoints in clinical trials insofar as they are of societal value only to the extent that they predict clinical or “hard” endpoints.7, 8
Although the concepts of novelty and innovation are often associated with one another,9 defining the societal value of pharmaceuticals exclusively in terms of the production of health outcomes implies that product novelty alone does not constitute pharmaceutical innovation. New chemical structures or mechanisms of action do not necessarily generate improved health outcomes:10, 11 a new pharmaceutical product must also have some degree of effectiveness (net of treatment risks).6 It should be noted that effectiveness alone is not enough to qualify a new product as an innovation. A generic drug, for example, may safely and efficaciously address a health care need — and may provide value to patients and society — but it would hardly be considered an innovation. Thus, neither novelty nor effectiveness alone is enough to qualify as pharmaceutical innovation. Even the combination of novelty and effectiveness is not enough.
Pharmaceutical innovation requires novelty of effectiveness. Pharmaceutical innovations create value to society by making it possible to generate improvements in patient health (net of treatment risks) that were previously unattainable. It is the uniqueness of such health improvements that defines pharmaceutical innovations. A drug can be considered a pharmaceutical innovation only if it meets otherwise unmet or inadequately met health care needs. This will depend on its efficacy, safety and convenience of use relative to the technologies available when it is introduced. For example, cimetidine, the prototypicalhistamine-2 receptor antagonist, was considered a pharmaceutical innovation when it was introduced in 1977 because it safely and effectively addressed a previously inadequately met need.12 However, the notion of pharmaceutical innovation is time-dependent. Competition and technological change mean that the standard by which the unique value of a pharmaceutical innovation is measured — the ability to address health care needs that are otherwise not addressable — will change over time. Neither cimetidine nor other histamine-2 receptor antagonists would be considered innovations today because the outcomes they generate have been matched and even surpassed by other technologies.
Replicating outcomes obtainable with existing treatments is important for market competition but it does not represent innovation. However, surpassing existing levels of performance in terms of established efficacy, safety or both would be considered pharmaceutical innovation. Again, consider advances in gastroenterology: the first proton pump inhibitor, omeprazole, was an innovation when it was introduced in 1989 because it met a given need more effectively than histamine-2 receptor antagonists. Proton pump inhibitors have since become the mainstay of treatment for acid-related gastrointestinal disease in adults. Although they continue to generate valuable outcomes, they would no longer be considered innovations.
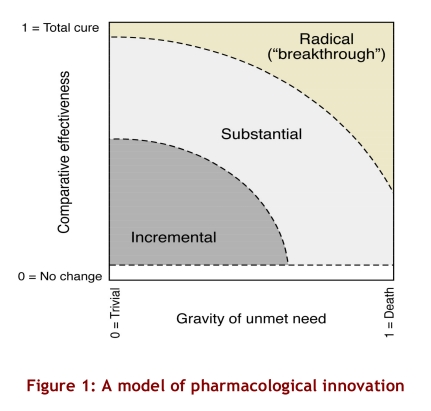
A pharmaceutical innovation may be thought of as incremental, substantial or radical according to the significance of the unmet health care need it addresses (gravity of unmet need) and the extent to which it improves net health outcomes related to that need (comparative effectiveness)(Figure 1). The gravity of an unmet need can be thought of as the gap between the health status that patients with a particular medical condition could attain with existing technologies and the health status they could expect if they did not have that medical condition. The lowest gravity of unmet need (zero) represents cases in which patients experience no deprivation in health status if they receive existing treatments or cases in which the nature of the condition is trivial in terms of health status (e.g., male pattern baldness). The highest gravity of unmet need (1) represents cases in which the condition has a prognosis of immediate death given existing treatment options.
Figure 1.
A model of pharmacological innovation
Gravity of unmet need establishes the potential for pharmaceutical innovation (i.e., the maximum improvement in health status that a new drug might offer, over and above existing technologies). For a condition with a low gravity of unmet need, such as colour blindness, there is a limited potential for pharmaceutical innovation. To determine the level of pharmaceutical innovation that a drug actually achieves, one must also examine its comparative effectiveness in terms of net improvements in health outcomes, taking into account the negative effects of the drug (e.g., side effects and adverse events). Drugs with zero comparative effectiveness offer no improvement in health outcomes compared with existing treatments. The highest value of comparative effectiveness (a value of 1) indicates the ideal (and seldom, if ever, realized) situation in which a drug is perfectly safe and entirely closes the gap between the health status attainable with prevailing treatments and the ideal health status for the treated population. The categories of innovation in Figure 1 are drawn with a lower border because a medicine must offer some level of comparative health benefit to be considered an innovation, no matter how grave the condition it aims to remedy.
The greater the gravity of the unmet need addressed by a new treatment, or the greater its comparative effectiveness in addressing that need, the greater the degree of pharmaceutical innovation. Radical innovations, or “breakthroughs,” are moderately to highly effective treatments for conditions that would otherwise significantly reduce the quality or length of life or both, or treatments that provide a near-total cure in cases in which the prevailing unmet needs are more moderate. Substantial innovations offer fair to modest improvements in health outcomes for patients with grave unmet needs, or substantial improvements over existing treatments for patients whose unmet health care needs are less serious. Finally, incremental innovations offer minor to moderate improvements over existing treatments for patients whose unmet needs are moderate to trivial.
Breakthrough drugs (or radical pharmaceutical innovations) generate the most significant societal value through their unique ability to generate improvements in health outcomes not otherwise possible. To qualify as a breakthrough, a new drug must offer significant improvements over existing treatments, even when the prevailing unmet needs are dire. For example, a drug that briefly extends the life expectancy of terminally ill patients might be considered an innovation, but to be considered a breakthrough it would have to provide these patients with a quantity and quality of life close to what they could have expected in the absence of the underlying illness. For this reason, the breakthrough category does not intersect with the lower horizontal line of Figure 1.
There is limited scope for pharmaceutical innovation for conditions for which existing therapies offer relatively good outcomes. New treatments within a drug class may offer modest therapeutic gains in efficacy or safety, but being different does not in itself constitute innovation. For example, early and late entrants into a drug class might each be more effective or better tolerated among certain population subgroups. However, late entrants will not represent significant pharmaceutical innovation unless they are systematically superior to early ones.
The value of new drugs that produce outcomes similar to those achievable with other treatment options lies not in innovation but in the potential competition that these products may bring to the marketplace. Such competition may have value for consumers and for society more generally through reduced costs per outcome achieved. However, the cost of developing such medicines includes the significant investment and the risks to participants in various stages of clinical trials, as well as the funds spent on marketing efforts to differentiate the new drug from existing treatments, and all of these costs divert resources from the pursuit of treatments to meet more substantial unmet health needs.1, 13, 14
Ultimately, it is commercial value that drives investments and activities in the private sector. Firms may strive for commercial performance by developing drugs that effectively address grave, unmet health care needs. Firms may also be commercially innovative without generating pharmaceutical innovations, such as when they develop a marketing campaign that builds brand loyalty for a product that is comparable to existing alternatives. Indeed, when Figure 1 is viewed from a societal perspective on a global or national basis, it appears that most of the commercial activity in the pharmaceutical market is focused on product development and related marketing activities in therapeutic areas in which new products would at best provide incremental advances in population health. That is, much of the innovation in this sector is commercial, not pharmaceutical.
The fact that commercial incentives are not always aligned with the production of major pharmaceutical innovations is evident not only in the global divide between burden of illness and drug research and development,15, 16 but also in the share of product development, marketing and sales in wealthy countries that is accounted for by medicines that offer little or no advantage over established treatment alternatives.17 Between 1993 and 2004, only one-third of US applications for the licensing of new molecular entities were promising enough to qualify for priority review by the US Food and Drugs Administration).18 The proportion of new drugs that represent true breakthroughs is likely lower. Between 1981 and 2000, Prescrire International rated only 74 (3%) of nearly 2300 new drugs or new indications for existing drugs as major or important therapeutic gains.19 Fewer than 10% of recently developed biotechnology drugs and cancer treatments have been deemed to offer substantial improvements with respect to hard clinical endpoints.20, 21
Ultimately, the pharmaceutical industry is not to blame. The industry’s focus on research and marketing activities for products that do not dramatically advance attainable health outcomes results from the way drugs are appraised, selected and purchased by health practitioners, patients, policy-makers and insurers. If these actors placed a premium on true pharmaceutical innovation—demonstrably safe and effective treatments for previously unmet needs—and encouraged competition among technologies that produce equivalent health outcomes, private investments in research and development would be stimulated in the areas of greatest value to society.4
Disclaimer
The views presented here are solely those of the authors and not necessarily those of the CIHR, the MSFHR, or of the Commonwealth Fund, its directors, officers, or staff.
Acknowledgments
Steve Morgan is supported by a New Investigator award from the Canadian Institutes of Health Research (CIHR) and a Scholar Award from the Michael Smith Foundation for Health Research (MSFHR). Ruth Lopert gratefully acknowledges the support of the Commonwealth Fund.
Biographies
Steven Morgan is an assistant professor in the University of British Columbia’s Department of Health Care and Epidemiology and leads the research program in pharmaceutical policy at the UBC Centre for Health Services and Policy Research, Vancouver, BC.
Ruth Lopert is a visiting associate professor with the Department of Health Policy at George Washington University, Washington, DC.
Devon Greyson is an information specialist at the UBC Centre for Health Services and Policy Research.
Footnotes
Competing interests: None declared.
Funding source: There was no direct funding for this study.
References
- 1.Barton John H, Emanuel Ezekiel J. The patents-based pharmaceutical development process: rationale, problems, and potential reforms. JAMA. 2005;294(16):2075–82. doi: 10.1001/jama.294.16.2075. [DOI] [PubMed] [Google Scholar]
- 2.Hollis A. Drugs for rare diseases: paying for innovation. In: Beach C, Chaykowski R, Shortt S, et al, editors. Health services restructuring in Canada: new evidence and new directions. 2005. [Google Scholar]
- 3.International Trade Administration. Pharmaceutical price controls in OECD countries: implications for U.S. consumers, pricing, research and development, and innovation. Washington: US Department of Commerce; 2004. [Google Scholar]
- 4.UK Office of Fair Trading. The Pharmaceutical Price Regulation Scheme report to Parliament. London: The Office; 2007. [Google Scholar]
- 5.Cooksey D. A review of UK health research funding. London: Her Majesty’s Stationery Office; 2006. [Google Scholar]
- 6.International Society of Drug Bulletins. ISDB declaration on therapeutic advance in the use of medicines. Paris: International Society of Drug Bulletins; 2001. [Google Scholar]
- 7.Barton John H, Emanuel Ezekiel J. The patents-based pharmaceutical development process: rationale, problems, and potential reforms. JAMA. 2005;294(16):2075–82. doi: 10.1001/jama.294.16.2075. [DOI] [PubMed] [Google Scholar]
- 8.Bucher HC, Guyatt GH, Cook DJ, Holbrook A, McAlister FA Evidence-Based Medicine Working Group. Users’ guides to the medical literature: XIX. Applying clinical trial results. A. How to use an article measuring the effect of an intervention on surrogate end points. JAMA. 1999;282(8):771–778. doi: 10.1001/jama.282.8.771. http://jama.ama-assn.org/cgi/pmidlookup?view=long&pmid=10463714. [DOI] [PubMed] [Google Scholar]
- 9.Oxford English dictionary online. Oxford: Oxford University Press; 2007. [Google Scholar]
- 10.Wardell W M, DiRaddo J. The measurement of pharmaceutical innovation. J Clin Pharmacol. 1980;20(1):1–9. doi: 10.1002/j.1552-4604.1980.tb01660.x. [DOI] [PubMed] [Google Scholar]
- 11.Wardell W M, DiRaddo J, Weintraub M. The measurement of therapeutic value. J Clin Pharmacol. 1980;20(2-3):77–90. doi: 10.1002/j.1552-4604.1980.tb02529.x. [DOI] [PubMed] [Google Scholar]
- 12.Hoogerwerf WA, Pasricha PJ. Pharmacotherapy of gastric acidity, peptic ulcers, and gastroesophageal reflux disease. In: Brunton Laurence L, Lazo John, Parker Keith., editors. Goodman & Gilman’s the pharmacological basis of therapeutics. 11th ed 2006. [Google Scholar]
- 13.Garattini S. Are me-too drugs justified? J Nephrol. 1997;10(6):283–294. [PubMed] [Google Scholar]
- 14.Montaner J S, O'Shaughnessy M V, Schechter M T. Industry-sponsored clinical research: a double-edged sword. Lancet. 2001;358(9296):1893–1895. doi: 10.1016/S0140-6736(01)06891-X. [DOI] [PubMed] [Google Scholar]
- 15.Trouiller Patrice, Olliaro Piero, Torreele Els, Orbinski James, Laing Richard, Ford Nathan. Drug development for neglected diseases: a deficient market and a public-health policy failure. Lancet. 2002;359(9324):2188–2194. doi: 10.1016/S0140-6736(02)09096-7. [DOI] [PubMed] [Google Scholar]
- 16.Winters David J. Expanding global research and development for neglected diseases. Bull World Health Organ. 2006 May 17;84(5):414–416. doi: 10.1590/S0042-96862006000500025. http://www.scielosp.org/scielo.php?script=sci_arttext&pid=S0042-96862006000500025&lng=en&nrm=iso&tlng=en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan Steven G, Bassett Kenneth L, Wright James M, Evans Robert G, Barer Morris L, Caetano Patricia A, Black Charlyn D. "Breakthrough" drugs and growth in expenditure on prescription drugs in Canada. BMJ. 2005 Sep 02;331(7520):815–6. doi: 10.1136/bmj.38582.703866.AE. http://bmj.com/cgi/pmidlookup?view=long&pmid=16141448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Government Accountability Office. New drug development: science, business, regulatory, and intellectual property issues cited as hampering drug development efforts [report to Congressional requesters] Washington: Government Accountability Office; 2006. http://www.gao.gov/products/GAO-07-49. [Google Scholar]
- 19.A look back at 2000: overabundance and deregulation. Prescrire Int. 2001 Apr;10(52):52–54. [PubMed] [Google Scholar]
- 20.Garattini S, Bertele V. Efficacy, safety, and cost of new anticancer drugs. BMJ. 2002;325(7358):269–271. doi: 10.1136/bmj.331.7521.895. http://www.bmj.com/cgi/content/full/325/7358/269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joppi Roberta, Bertele' Vittorio, Garattini Silvio. Disappointing biotech. BMJ. 2005;331(7521):895–897. doi: 10.1136/bmj.331.7521.895. http://bmj.com/cgi/pmidlookup?view=long&pmid=16223825. [DOI] [PMC free article] [PubMed] [Google Scholar]