Abstract
A number of cell death pathways have been recognized. Though apoptosis and autophagy have been well characterized, programmed necrosis has recently received attention and may provide clinical alternatives to suppress resistant tumors. Necrosis is primarily characterized by large-scale permeabilization, swelling, and rupture of cell membranes and the release of pro-inflammatory cytokines. Traditionally, necrosis in cancer cells has been indicative of poor prognoses, as chronic inflammation was found to encourage tumor growth. Yet, many antitumor effects associated with necrosis have been discovered in certain settings, such as the formation of an effective antitumor immune response. In this way, finding ways to attenuate the pro-tumor effects of necrosis while engaging the antitumor pathways via drugs, radiation, and sensitization may prove valuable as a clinical focus for the future. We hypothesize that the use of Bcl-2 inhibitors may enhance necrotic death characterized by inflammation and antitumor immunity. In this article, we briefly review apoptosis and autophagy and reason how necrosis may be a suitable alternative therapeutic endpoint. We then highlight novel inhibitors of Bcl-2 that may provide clinical application of our hypothesis in the future.
Programmed Cell Death and Its Role in Cancer Therapy
In the past two decades, significant research has uncovered the importance of programmed cell death (PCD). This in an important process in the field of cancer biology because PCD is an essential process by which abundant or undesirable cells can be removed. When such PCD pathways become deregulated or mutated, various diseases such as cancer arise. The first of these cell death pathways to be discovered was apoptosis, found in Caenorhabditis elegans in the 1990s, leading to the discovery and characterization of a homologous pathway in mammalian eukaryotic cells. In fact, a hallmark of many cancers has been an observed resistance to the apoptotic mode of death within tumor cells (1).
However, a number of nonapoptotic cell death pathways have since been identified, including autophagy, necrosis, senescence, and mitotic catastrophe. Among these, autophagy and necrosis have garnered much attention in the past decade. For this article, we will review apoptosis and autophagy and then examine the benefits of necrosis, a pathway that may yield novel therapeutic approaches when apoptosis and autophagy fail. Furthermore, we will explore the effect of the pervasive anti-apoptotic Bcl-2/xl proteins in the context of nonapoptotic cell death. In particular, our work has involved the investigation of drug-induced radiosensitization to certain death pathways, a therapeutic approach that may be extended to necrosis. By understanding these cell death mechanisms, oncologists will be able to tailor various therapies including radiotherapy and chemotherapy to stimulate different modes of cell death in tumor cells while ensuring minimal death to normal cells.
The First Evidence of Programmed Cell Death: Apoptosis
The observation of similar morphology across dying cells of different tissues led to the realization of a regulated cell death pathway termed apoptosis in the 1970s. These morphological findings usually entail chromatin condensation, nuclear fragmentation, DNA fragmentation, membrane blebbing, and the maintained integrity of organelle structures along with the formation of apoptosomes. In this way, these signs are all characteristic of type 1, or apoptotic cell death. As the most understood of all cell death forms, apoptosis can be defined generally as a pathway involving the sequential proteolytic activation of Cys proteins called caspases, which in turn signal apoptotic machinery, most notably endonucleases.
The specific mechanisms behind apoptosis such as the caspase cascade prove difficult to examine in vivo because many pathways become interrelated or codependent. Multiple signals can regulate one protein or one signal can regulate many proteins. Unfortunately, such redundancy and complexity creates difficulty in examining the function of a Bcl-2 like proteins in vivo, though this complexity probably serves to achieve fine-tuned cellular homeostasis. It has been argued that the complexity of the apoptotic pathway allows for easier interference and thus apoptotic malfunction and drug resistance (2). This idea would advocate the use of other nonapoptotic death pathways in cancer therapy such as necrosis.
Nonapoptotic Cell Death: Autophagy
In addition to apoptosis, nonapoptotic death pathways had been observed for many years at the morphological level but with no mechanistic understanding. One such pathway was called autophagy, whereby double-membrane bound autophagosomes sequester cytoplasmic materials and organelles to be delivered to a lysosome for subsequent degradation. Interestingly, studies showed that when apoptosis was inhibited, cells could turn to the nonapoptotic pathways such as autophagy to facilitate death. Cells undergoing this pathway exhibit type II morphology, characterized by partial chromatin condensation, cell membrane blebbing, and the appearance of autophagosomes (3). Interestingly, the autophagic pathway can promote either cell survival or PCD, depending on the stimuli and environment (4). Autophagy has been observed during periods of stress such as nutrient deprivation, hypoxia, endoplasmic reticulum (ER) stress, inhibition of proteosomal degradation, accumulation of intracellular calcium, hyperthermia, and even pathogenic bacterial invasion as a defense mechanism (5, 6). Indeed, both yeast and mammalian cells require autophagy to maintain cell viability under starvation conditions, such as amino acid deficiency in order to maintain bioenergetics. Studies on tumor cell lines have similarly shown that upregulation of autophagy can increase cell viability in response to certain stress conditions. Therefore, investigating methods to inhibit autophagy in cancer cells could have significant clinical impact by suppressing a cellular survival attempt in response to genotoxic stress.
Necrosis: Breakdown on Cellular Breakdown
The catastrophic nature of necrotic morphology would give an impression of a pathway largely passive and unregulated caused by a massive amount of stress, in stark contrast to the intricately programmed apoptotic pathway. The primary molecular mechanism behind necrosis is believed to be the rapid depolarization of membranes leading to swelling and rupture of the plasma membrane (7). The morphological features of necrosis involve organelle swelling, rapid mitochondrial dysfunction, plasma membrane permeabilization, oxidative stress, and mitochondrial dysfunction (8). Unlike apoptosis there are no signs of nuclear fragmentation. A number of agents, scenarios, and pathways are linked with necrosis, including certain extracellular mediators, DNA damage, ATP depletion, calcium ion concentrations, reactive oxygen species (ROS), certain protein kinases, Poly (ADP-ribose) polymerase (PARP), the mitochondrion, Bcl-2 proteins, heat shock proteins, proteases, nucleases, and phospholipases (9).
Programmed necrosis is a form of necrosis induced by Death Receptor (DR) signaling rather than by nonspecific cellular damage (10). Much focus has been given specifically to programmed necrosis in recent years because of its cross-talks with both apoptosis and autophagy. Programmed necrosis shares the upstream tumor necrosis factor receptor (TNFR) pathway with apoptosis, specifically at the level of death induced signaling complex (DISC) formation. DISC is formed when the Fas/TNF DR, upon ligand binding, trimerizes and associates with its adaptor molecules and pro-apoptotic caspase-8. Adaptor molecules include Fas-associated death domain protein (FADD), TNFR-associated factor 2 (TRAF2), and TNFR-associated death domain protein (TRADD).
Reversion from apoptosis to programmed necrosis has been shown to occur when RIP, a serine/threonine protein kinase receptor-interacting protein, binds to FADD more efficiently than does caspase-8 (11). RIP kinase domain alone in its full-length dimerized state sufficiently induces kinase-dependent programmed necrotic cell death (12). The uncleaved RIP protein can only turn on programmed necrosis and not apoptosis, as evidenced when the cleavage-resistant RIP mutants conferred protection against TNF-induced apoptosis (13). Interestingly, caspase-8 can cleave RIP into two fragments, RIPc and RIPn, and turn off the programmed necrotic pathway. Overexpression of one of the cleavage products, RIPc, increases the TNF-induced apoptosis by promoting the TRADD and FADD interaction; however, its presence is not absolutely required in Fas or TNF-mediated apoptosis (14, 15). The second cleavage product, RIPn, contains the entire kinase domain of RIP and has been shown to play no role in TNF-induced nuclear factor-κB (NF-kB) activation or TNF-induced apoptosis (13). In short, the delicate balance between RIP binding to FADD and caspase 8-driven RIP cleavage seems to function as the diverging point between apoptosis and programmed necrosis.
Though the mechanism remains unclear, it is thought that RIP also induces mitochondrial dysfunction by dissociating cyclophilin D from ADP-ATP translocase (ANT) at the permeability transition pore complex (PTPC) thereby causing a membrane permeability transition (MPT), which depletes ATP while increasing ROS concentration (16). The mitochondrion becomes the central executor of cell death in this model, although necrosis activated MPT differs from the mitochondrial dysfunction mediated by Bax/Bak in apoptosis (17). Traditionally, chronic necrosis has been found to usually encourage tumor growth, leading to a poor prognosis. However, recent research indicates possible clinical benefits from the induction of acute necrosis and the subsequent antitumor immune response in certain cancers.
Clinical Implications of Necrosis
One of the main obstacles in treating cancer today is the induction of drug resistance in multiple types of tumors. These multidrug resistant tumors, however, remain susceptible to necrotic death. In this way, necrosis should become a focus of study in determining novel approaches to cancer prevention and treatment.
Necrosis and Immunogenicity
In contrast to necrosis, apoptosis has been regarded as an immune-suppressive cell death mechanism, though apoptotic immune stimulation may occur. Apoptotic silencing of the immune system occurs via signal pathways involving TGF-β and prostaglandin E2, as well as membrane-bound phosphotidylinositol presentation (18). Pro-apoptotic caspases silence inflammatory programmed necrotic pathways by cleavage of PARP among other immune-stimulating molecules. Conversely, necrosis is characterized by the release of cytokinines, which stimulate fibroblasts, macrophages, and dendritic cells (DCs) of the immune system (19).
Chronic necrotic inflammation has been attributed to tumor growth, which has led to much research on the inhibition of necrosis. Inflammation in other settings usually carries negative prognostic effects. Studies have found that the inhibition of necrosis by agents such as necrostatin-1 has been beneficial for certain diseases and injuries, such as brain ischemia (10). Usually, innate immune cells may stimulate inflammation and drive tumor formation in response to cell injury through the chronic release of pro-inflammatory mediators such as TNF-α and interleukin (IL)-6 (20). However, inflammation in established tumors may trigger a specific antitumor immune response (21), with the immune system serving a protective role against cancer. Unlike apoptotic cells, necrotic cells release maturation signals to DCs after membrane rupture, which can then become antigen-presenting cells (APCs) that may cross present antigens to cytotoxic T-cells (CTLs) and facilitate antitumor memory (22). Besides its roles as an architectural protein and transcription factor in the nucleus (23), HMGB1 is an important inflammatory adjuvant released by necrotic cells into the extracellular environment that may bind receptor for advanced glycation end product (RAGE) or Toll-like Receptor 4 (TLR4) on macrophages (24, 25). Conversely, apoptotic cells sequester HMGB1 in the nucleus, inhibiting an immune response. Whereas HMGB1-RAGE binding has been linked to tumor growth and migration (26), HMGB1-TLR4 binding may facilitate DC maturation, antitumor activity, and memory, as discovered in vivo (27). In fact, TLR4 binding was found to be critical in establishing the efficacy of radiotherapy and chemotherapy in stimulating CTL maturation (28). HMGB1 may provide an ideal clinical application in antitumor vaccines for both apoptotic and necrotic death. Interestingly, breast cancer patients with a deviant TLR4 allele exhibited a poor immunogenic anticancer response after therapy, owing to defective HMGB1-TLR4 binding. Though DCs still phagocytized dying tumor cells, the TLR4 defect allowed engulfed cell components to be digested quickly by lysosomes instead of being presented as antigens. In this way, patients with the TLR4 polymorphism could be treated by inhibiting the lysosomal activity with drugs such as chloroquine to allow effective antigen presentation (28). The TLR4-HMGB1 interaction should be further researched in order to improve clinical therapies to enhance antitumor immunogenicity.
Because RAGE interaction with advanced glycation end products (AGE) usually promotes immunogenic tumor growth, the search for RAGE inhibitors in addition to HMGB1 vaccines may offer clinical rewards. Cytotoxic effects attributed to RAGE inhibition is improbable due to low basal RAGE levels in healthy humans. The pathogenesis of RAGE-AGE binding is believed to be NF-κB activation, which encourages tumor growth, angiogenesis, and proliferation (29). A novel truncated form of RAGE called endogenous secreted RAGE (sRAGE) has been recently discovered that may actively bind AGE without initiating pathogenesis (30). In this way, sRAGE may be used as a drug antagonist that competes with RAGE for ligand binding, a phenomenon that may have viable clinical application in tumor suppression.
Two other molecules important in relaying “eat me” signals for dying cancer cells are phosphotidylserine and calreticulin (CRT) when presented on the cell surface. Whereas phosphotidylserine recognition by phagocytes suppresses immune response, as observed in apoptosis (31), CRT recognition spurs the maturation of DCs and macrophages (32). In fact, CRT may also increase immunogenicity in apoptotic tumor cells in response to radiation and anthracyclines (33). In this way, CRT regulation should be another target for advancing immunogenicity in dying necrotic or apoptotic cells.
The PARP Pathway and NF-κB
The antagonistic machinery between apoptosis and necrosis is evidenced by the mediator, PARP. During apoptosis, initiator and executioner caspases inhibit PARP by proteolytic cleavage. Conversely, in response to DNA damage, PARP will bio-energetically inhibit apoptosis by depleting ATP while inducing necrosis (34). Because PARP is responsible for genomic stability and repair, PARP inhibitors have been researched for their chemosensitizing potential for increasing DNA damage in cancer cells (35), thus enhancing apoptotic death in certain tumors. Specifically, we are studying the radiosensitive effects of a novel PARP inhibitor ABT-888, a drug that may provide significant apoptotic tumor suppression with ionizing radiation.
On the other hand, overexpression of PARP may also provide beneficial effects as a method of inducing necrosis rather than apoptosis in resistant cells. Much of the protumor effects of PARP activation arise from its activation of NF-κB as well as from RAGE binding. NF-κB promotes tumor proliferation, invasion, inflammation, angiogenesis, and metastasis, and may be activated by a number of other signals. However, NF-κB inhibition along with PARP overexpression may induce necrosis while reducing protumor activity. Examples of NF-κB inhibitors include the Ikb class of protein kinases, curcumin, BAY11, soy isoflavone genistein, parthnolide, and dehydroxymethylepoxyquinomycin (DHMEQ) (ref. 36). In addition, PARP releases pro-inflammatory molecules, such as HMGB1, which may stimulate an antitumor immune response in some settings.
Secondary Autophagy Follows Programmed Necrosis
Frequently the induction of programmed necrosis activates secondary autophagy as a common downstream effect, having only a minor role in cell death but perhaps major role in debris cleanup (10). However, the mechanism of this downstream signaling has not been defined. One could postulate that activation of RIP1K signaling increases concentrations of ROS, which activates the autophagic pathway in necrotic cells, as evidence of H202 was found to directly regulate Atg4 (37). However, further investigation is needed to test this hypothesis, as this downstream effect may be pleiotropically regulated.
Hypotheses and Clinical Implications of Bcl-2 Inhibition
Ever since its discovery, Bcl-2 has been shown to have far reaching effects across many pathways, aside from its anti-apoptotic binding of other members in the Bcl-2 family. Bcl-2 expression has been implicated in other tumor survival pathways via its prometastatic activity (38), as well as its pro-angiogenic activity as seen in its modulation of vascular endothelial growth factor (VEGF) expression (39). Bcl-2 has also been implicated in immunosilencing, and it has been shown that Bcl-2 inhibition provokes antitumor response to activated T cells (40). Furthermore, it has been shown that Bcl-2 could inhibit necrosis as it could apoptosis and autophagy, albeit via different pathways (see Fig. 1). Many drug-resistant or apoptotic-resistant cancers such as B-cell lymphoma (11) and small cell lung cancer (41) indicate Bcl-2 overexpression. The attenuation of Bcl-2 may prove favorable in certain clinical settings to enhance alternative modes of cell death, antitumor immune response, and drug efficacy.
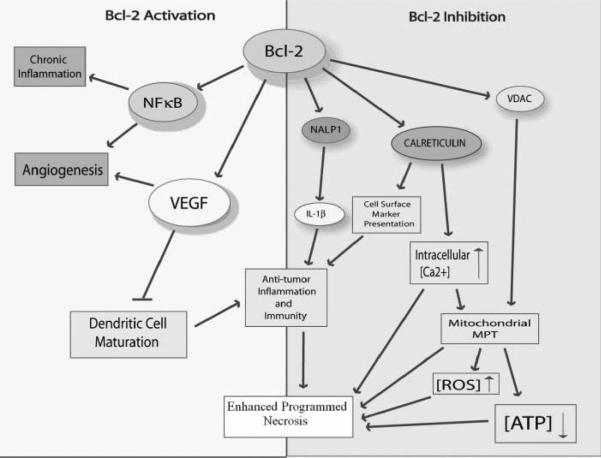
Figure 1.
Various pathways associated with the inactivation or activation of Bcl-2 and their generalized effects toward programmed necrosis initiation. On the left side, the activation of Bcl-2 leads to various protumor effects, such as angiogenesis and chronic inflammation. The activation of VEGF inhibits the maturation of DCs and antitumor inflammation, thus inhibiting programmed necrosis. The inhibition of Bcl-2 on the right side highlights many programmed necrotic effects, such as the activation of antitumor immunity by increased CRT and NALP1. Other programmed necrosis activators include the opening of VDAC to increase [ROS] and decrease [ATP].
Bcl-2, a Common Player in Apoptosis and Autophagy
Bcl-2 in Apoptosis
The mitochondrion serves not only as the cell's powerhouse, but also as its executioner. Capable of releasing a plethora of pro-apoptotic proteins, the mitochondrion is a necessary site of extensive regulation in all types of programmed cell death. The Bcl-2 family is an important group of proteins that was first noted to regulate the release of apoptotic proteins from the mitochondria, specifically by altering the permeability of the outer mitochondrial membrane. In this way, the Bcl-2 protein family consists of upstream activators of apoptotic signaling in relation to caspases. The Bcl-2 like family of proteins can be divided into three groups, an anti-apoptotic Bcl-2 group, a pro-apoptotic BH3 domain-only group, and the Bax and Bak adapter proteins. The pro-apoptotic BH3-only proteins activate Bax and Bak, which are essential downstream initiators of apoptosis located in the mitochondrial outer membrane. Examples of BH-3 only proteins include Bim, Puma, and Bid. In particular, Bid has been shown to be activated by caspase-8 and associate with Bax to induce channel formation, cytochrome c translocation, or membrane instability. The anti-apoptotic group is composed of proteins such as Bcl-2, Bcl-XL, and Mcl-1, which mostly inhibit apoptosis by sequestering BH3-only proteins, thus disallowing their activation of the Bax/Bak proteins. In recent years studies have revealed these proteins, specifically Bcl-2, to be the mediator of not only apoptosis, but also autophagy and programmed necrosis (Fig. 1).
Bcl-2 in Autophagy
Bcl-2 is an attractive target for modulating different modes of cell death in cancer therapy because it affects mediators of apoptosis, programmed necrosis, and autophagy (Fig. 2). The Bcl-2 family of proteins functions to inhibit autophagy, a pathway well known to confer genomic stability and tumor suppression. Overexpression of Bcl-2 can thereby serve dual purposes of inhibiting apoptosis and autophagy (Fig. 2). The pro-autophagic protein Beclin 1 is well known for its constitutive interaction with Bcl-2, which binds to the BH3 domain of Beclin 1 and prevents its activation. This is evidenced when the addition of ABT-737, a BH3-mimetic compound able to bind Bcl-2, sufficiently induces autophagy. Various autophagic inducers including nutrient starvation also triggers the dissociation of Beclin 1 from Bcl-2 (42–44).
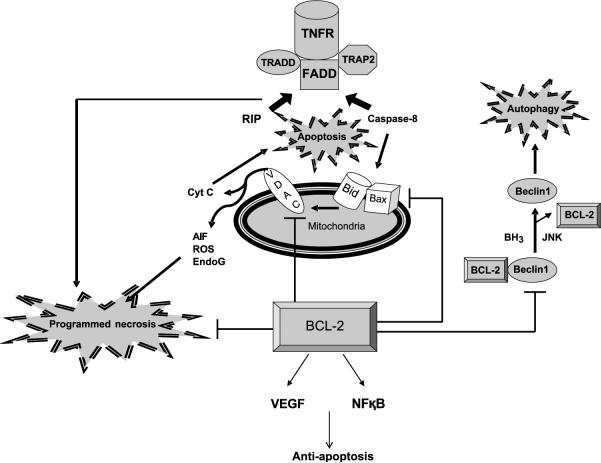
Figure 2.
Bcl-2 as an attractive target for modulating different modes of cell death in cancer therapy due to close communications with mediators of apoptosis, programmed necrosis, and autophagy. Sharing the common upstream TNF receptor, apoptosis and programmed necrosis seem to diverge when programmed necrosis protein RIP and pro-apoptotic caspase-8 compete to bind the adaptor molecule FADD for their activation. Bcl-2 is also shown here to block the VDAC and consequent disruption of mitochondrial permeability necessary for the release of programmed necrosis factors. On the other hand, Bcl-2 induces VEGF and NF-κB production that can suppress programmed necrosis. Downstream of caspase-8 lies the apoptotic pathway involving Bid and Bax oligomerization and consequent permeabilization of the mitochondrial membrane for cytochrome c release. Bcl-2 here acts to suppress the effects of Bid and Bax. Lastly, autophagy is activated when the inhibitory binding of Bcl-2 to Beclin 1 is relieved either by BH3-domain proteins or JNK phosphorylation of Bcl-2.
Interestingly, Beclin 1 and pro-apoptotic proteins Bak, Bad, and Bim share the same BH3 domain needed for their structural fit into the BH3-binding groove of Bcl-2. This suggests that the extent to which either becomes competitively displaced from Bcl-2 determines the delicate balance between apoptosis and autophagy. In fact, enforced overexpression of the BH3 protein Bad alone initiated autophagic signaling with or without caspase inhibition (43). However, Beclin 1 dissociation can occur independently of the competition with BH3-domain proteins. The phosphorylation of Bcl-2 by the stress-activated c-Jun N-terminal protein kinase 1 (JNK1) also relieves Beclin 1 from its functional inhibition (45). Alternatively, the competitive displacement of Beclin 1 by pro-apoptotic proteins may not be of significance at all in turning on autophagy, for only the ER-targeted but not the mitochondrion-targeted Bcl-2 has been shown to suppress autophagy (4, 42). In such case, Beclin 1: Bcl-2 complex located in the ER would not be exposed to a concentrated pool of mitochondrial pro-apoptotic proteins.
Of note, emerging evidences reveal that the point of crosstalk between apoptosis and autophagy may not be limited to Bcl-2. A recent study found that the pro-autophagic protein Atg5 interacts with FADD and possibly caspase-8 so as to link IFN-γ activated death to both autophagy and apoptosis (46). Further research will undoubtedly reveal multiple communicators between the two cell death pathways, all of which can serve as potential targets in cancer therapy.
Bcl-2 in Antitumor Immunity and Inflammation
Inflammation may provide beneficial clinical effects in promoting an immunogenic antitumor response in certain settings. As VEGF is known to inhibit the maturation of DCs (47), Bcl-2 may act to inhibit the innate antitumor immune response by promoting VEGF production (48). In this way, downregulation of VEGF by Bcl-2 inhibition may increase antitumor immunity during necrosis.
Another pathway whereby Bcl-2 modulates inflammatory activity involves a novel protein related to the NOD family, NALP1 (also called DEFCAP, NAC), which helps form the inflammasome complex by binding ASC, caspase-1, and caspase-5 (49). In turn caspase-1 cleaves intracellular pro-IL-1β into Il-1β, an important “alarm cytokine,” which is then secreted in the extracellular space triggering inflammation (50). Thus, regulation of NALP1 may be a potential target for inflammatory cancer therapy. Interestingly, a recent study linked Bcl-2 and Bcl-xL to dysregulation of the inflammatory response by the binding of Bcl-2 to NALP1, preventing caspase-1 activation (51). Thus, Bcl-2/xL inhibition may provide benefits, as inflammation may be clinically useful during programmed necrotic activation in order to generate antitumor immunity, especially in response to chemotherapy or radiotherapy (28). NALP1 is expressed across many tissues in the body (52), which may present many opportunities for clinical intervention. In particular, myeloid leukemia and lymphoma may have the greatest clinical potential because myeloid and lymphoid cells were found to have the most elevated NALP1 expression (53). Furthermore Bcl-2/xL are known to be overexpressed in leukemia cells (54), which may cause Bcl-2/xL inhibition to have a dramatic response in leukemic cells during necrosis. This hypothesis linking Bcl-2 inhibition and NALP1 mediated inflammation to improve programmed necrotic antitumor response will have to be investigated in future studies.
Another inflammatory mediator previously mentioned was NF-κB, which has been linked to protumor activity in chronic inflammation as well as pro-angiogenic activity (55). It has been found that the downregulation of Bcl-2 attenuates NF-κB activation because Bcl-2-mediated degradation cytoplasmic inhibitor IκB-α is essential for NF-κB activation (56). Indeed, the selective dysreglation of NF-κB would ensure the specific antitumor effects of inflammation while limiting its protumor effects.
Bcl-2 and Calreticulin
Bcl-2 overexpression has been shown to downregulate CRT and decrease intracellular [Ca2+], an apoptotic signal (57). CRT-mediated ER Ca2+ release and high intracellular [Ca2] have been implicated in the programmed necrotic pathway in C. Elegans (58). Thus, in apoptosis-resistant cells, it would be beneficial to upregulate CRT in order to maintain high [Ca2] to induce necrotic death. Apart from its role in regulating Ca2+ levels, CRT is a well-characterized surface marker signaling for immunogenic phagocytosis by DCs and macrophages. This dual role of CRT makes it a potent target for clinical therapy for enhancing immunogenicity and programmed necrosis. In this manner, the inhibition of Bcl-2 could favor the upregulation of CRT and help induce necrosis.
Bcl-2 and the Mitochondrial Permeability Transition
The MPT is the primary mechanism by which programmed necrotic death occurs by increasing ROS concentration and rupturing the mitochondrial membrane. Bcl-2 and Bcl-xL may directly inhibit the MPT in a manner different from the apoptotic pathway (3), because these proteins are capable of blocking the voltage-dependent anion channel (VDAC). Indeed, Bcl-2/xL is known to affect the PTPC in a cytochrome-C independent fashion (59). If this is the case, Bcl-2 inhibition would favor the induction of necrosis by ensuring the rupture of the mitochondrial membrane, leading to the depletion of ATP and the release of ROS into the cell.
Shikonin
A natural drug discovered in the roots of Lithospermum erythrorhizon has been shown to directly induce necrosis regardless of Bcl-2 and Bcl-xL levels. Furthermore, necrosis occurred independently of Fas/TNFR or P-glycoproteins, both of which contribute to drug resistance in tumor cells (60). In this way, shikonin may circumvent resistance to drugs such as Bcl-2 inhibitors. Interestingly, shikonin has also been found to downregulate Bcl-2 and Bcl-xL levels (61). This finding could have true clinical impact, because shikonin may be able to inhibit Bcl-2/xL levels while inducing necrosis during Bcl-2/xL overexpression. Thus, shikonin stands out as a strong candidate for the suppression of resistant cancer cells.
Approaches for Bcl-2 Inhibition
The connections between the downregulation of Bcl-2 and the tumor suppression warrant research into novel methods of inhibiting Bcl-2. A number of Bcl-2 inhibitors as well as other methods have been discovered in recent years, including small-molecule inhibitors, post-translational modification, and post-transcriptional modification, in which the latter includes siRNA gene silencing, Bcl-2 ribozymes, and antisense (AS) oligonucleotides.
Small-Molecule Bcl-2 Inhibitors
Research into Bcl-2 small-molecule inhibitors began soon after the realization of its anti-apoptotic activity. The first small-molecule inhibitors were discovered via structure-based computer screening for binding site similarities to pro-apoptotic elements such as Bak. A BH3 mimetic ABT-737 is a novel antagonist Bcl-2/xL inhibitor that has shown high antitumor efficacy as well as synergistic effects in conjunction with radiation and chemotherapy with reduced cytotoxicity (62). Other small-molecule inhibitors discussed in literature include WL-276, which inhibited drug-resistant prostate cancer as a BH3 mimetic (63), and TW-37, which attenuated expression of NF-κB and its downstream metastatic effectors such as VEGF, COX-2, and survivin, effectively inhibiting pancreatic tumor growth in vitro (64). Some small-molecule inhibitors of Bcl-2, such as ABT-737, have been successfully shown to sensitize cancer cells to antitumor immune response in apoptotic cells (40), and this effect should be thoroughly investigated in necrotic cells, as the immune response may be synergistically higher.
Yet another novel small-molecule Bcl-2 inhibitor is (−)-Gossypol (AT-101) (ref. 65). (−)- Gossypol was found to effectively improve antitumor activity in radioresistant prostate cancer (66), breast cancer (67), and many other cancers. Recently, a derivative of gossypol termed apogossypol (NSC736630) was found to exhibit much less human hepatotoxicity and gastrointestinal toxicity than its parent compound, while displaying higher in vivo antitumor activity and better pharmacokinetics, probably because of the lack of a reactive aldehyde group (68). In this way, apogossypol may provide significant clinical impact in bcl-2 inhibition along with necrosis.
Post-transcriptional Bcl-2 Inhibitors
A post-transcriptional method that may be used to abrogate Bcl-2 expression involves ribozymes, catalytic RNA molecules that can cleave cellular RNA. Hypothesized as early pre-evolutionary enzymes, ribozymes may be synthesized artificially to target specific mRNA and inhibit protein synthesis (69). In this way, ribozymes have been used to cleave Bcl-2 and significantly increase apoptotic death in prostate cancer and lymphoma among others (70, 71). Ribozyme activation during programmed necrosis should be investigated as well.
Another post-transcriptional method, AS oligonucleotides bind complimentary mRNA transcripts, which inhibits translation (72). AS Bcl-2 (G3139) binds the first six codons of Bcl-2 mRNA, which upregulates apoptosis and has been found to be an effective chemotherapeutic enhancer in various cancers (48, 73). Interestingly, G3139 has also been shown to carry immunogenic effects not attributed to its AS properties but to its two CpG-motifs on the oligodeoxynucleotide (ODN) that bind Toll-Like Receptor 9 (TLR9) on DCs and B cells, stimulating the release of inflammatory cytokines, chemokines, and interferons (74). Research should be conducted in applying AS Bcl-2 inhibition to resistant cells in conjunction with ATP-depleted conditions or other programmed necrotic stimuli, and whether CpG-TLR9 mediated immunogenicity may be synergistically upregulated during programmed necrosis to provoke an efficacious antitumor response.
In addition, siRNA is a powerful post-transcriptional tool that may be used to knock down Bcl-2 expression by RNA interference (RNAi) (ref. 75). Research into novel drug delivery vectors such as water-soluble lipopolymers (76), adeno-viruses (74), and, even more recently, nanoparticles such as semiconducting quantum dots and proton sponges (77), could yield dramatic clinical benefits for siRNA-based cancer therapy in the future, including the inhibition of Bcl-2.
Post-translational Methods of Inhibition
The ubiquitin-dependent proteosomal degradation of Bcl-2 may occur after dephosphorylation of Bcl-2 residues, such as Ser87 and Thr74 (78). Several proteins found responsible for phosphorylating Bcl-2 and maintaining its stability include PKA, PKC, and MAP kinases such as ERK2, all of which could be targets of clinical inhibition by dephosphorylation (79), which would subsequently lead to Bcl-2 dephosphorylation and inactivation. However, conflicting evidence has shown Bcl-2 dephosphorylation to increase resistance to apoptotic cell death, a variable that seems to depend on the cell type (80). In any case, investigation into post-translational Bcl-2 modifications should continue and could apply to the stimulation of programmed necrotic death in tumor cells.
Conclusions
In examining the various cell death pathways, we identified the importance of necrosis as an alternative focus for cancer suppression. Whereas necrosis and inflammation was traditionally believed to be negative, further research has erased this simplistic view. In certain settings, programmed necrosis or programmed necrosis may occur and be more effective in cells, such as those resistant to apoptosis. Furthermore, acute inflammation responses may trigger antitumor immunity via antigen presentation. In analyzing these ideas, the inhibition of Bcl-2 by various methods and drugs, such as gossypol, shikonin, AS oligonucleotides, and others, may enhance the efficacy of necrotic death via upregulating the immune response. Although Bcl-2 inhibitors had been researched first for upregulating apoptosis and autophagy in cancer cells, they may hold great promise in clinical programmed necrotic cancer therapy.
Acknowledgments
Grant support: The U.S. National Cancer Institute (NCI) Grant 1R01 CA125842-01A1.
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
References
- 1.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu X, Xuan Y. Bypassing cancer drug resistance by activating multiple death pathways-a proposal from the study of circumventing cancer drug resistance by induction of necroptosis. Cancer Lett. 2008;259:127–37. doi: 10.1016/j.canlet.2007.11.007. PubMed doi:10.1016/j.canlet.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu S, Kanaseki T, Mizushima N, et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–8. doi: 10.1038/ncb1192. PubMed doi:10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Polo RA, Boya P, Pauleau AL, et al. The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J Cell Sci. 2005;118:3091–102. doi: 10.1242/jcs.02447. PubMed doi:10.1242/jcs.02447. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–66. doi: 10.1016/j.cell.2004.11.038. PubMed doi:10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 6.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–77. doi: 10.1016/s1534-5807(04)00099-1. PubMed doi:10.1016/S1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 7.Lemasters JJ, Nieminen AL, Qian T, et al. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366:177–96. doi: 10.1016/s0005-2728(98)00112-1. PubMed doi: 10.1016/S0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 8.Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–30. doi: 10.1126/science.1059108. PubMed doi:10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Proskuryakov SY, Konoplyannikov AG, Gabai VL. Necrosis: a specific form of programmed cell death? Exp Cell Res. 2003;283:1–16. doi: 10.1016/s0014-4827(02)00027-7. PubMed doi:10.1016/S0014-4827(02)00027-7. [DOI] [PubMed] [Google Scholar]
- 10.Degterev A, Huang Z, Boyce M, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–9. doi: 10.1038/nchembio711. PubMed doi:10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 11.Vanden Berghe T, Kalai M, van Loo G, Declercq W, Vandenabeele P. Disruption of HSP90 function reverts tumor necrosis factor-induced necrosis to apoptosis. J Biol Chem. 2003;278:5622–9. doi: 10.1074/jbc.M208925200. PubMed doi:10.1074/ jbc.M208925200. [DOI] [PubMed] [Google Scholar]
- 12.Holler N, Zaru R, Micheau O, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–95. doi: 10.1038/82732. PubMed doi:10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 13.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–26. doi: 10.1101/gad.13.19.2514. PubMed doi:10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanger BZ, Leder P, Lee TH, Kim E, Seed B. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell. 1995;81:513–23. doi: 10.1016/0092-8674(95)90072-1. PubMed doi:10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 15.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. PubMed doi:10.1016/S1074-7613(00)80535-X. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y, Temkin V, Liu H, Pope RM. NF-κB protects macrophages from lipopolysaccharide-induced cell death: the role of caspase 8 and receptor-interacting protein. J Biol Chem. 2005;280:41827–34. doi: 10.1074/jbc.M510849200. PubMed doi: 10.1074/jbc.M510849200. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa T, Shimizu S, Watanabe T, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–8. doi: 10.1038/nature03317. PubMed doi:10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 18.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890–8. doi: 10.1172/JCI1112. PubMed doi:10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandenabeele P, Vanden Berghe T, Festjens N. Caspase inhibitors promote alternative cell death pathways. Sci STKE. 2006;2006:pe44. doi: 10.1126/stke.3582006pe44. [DOI] [PubMed] [Google Scholar]
- 20.Hagemann T, Balkwill F, Lawrence T. Inflammation and cancer: a double-edged sword. Cancer Cell. 2007;12:300–1. doi: 10.1016/j.ccr.2007.10.005. PubMed doi: 10.1016/j.ccr.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Degenhardt K, Mathew R, Beaudoin B, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. PubMed doi:10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–34. doi: 10.1084/jem.191.3.423. PubMed doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller S, Scaffidi P, Degryse B, et al. New EMBO members' review: the double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J. 2001;20:4337–40. doi: 10.1093/emboj/20.16.4337. PubMed doi:10.1093/emboj/20.16.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu M, Wang H, Ding A, et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–9. doi: 10.1097/01.shk.0000225404.51320.82. PubMed doi:10.1097/01. shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 25.Kakizaki T, Hamada N, Wada S, et al. Distinct modes of cell death by ionizing radiation observed in two lines of feline T-lymphocytes. J Radiat Res (Tokyo) 2006;47:237–43. doi: 10.1269/jrr.0618. PubMed doi:10.1269/jrr.0618. [DOI] [PubMed] [Google Scholar]
- 26.Taguchi A, Blood DC, del Toro G, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–60. doi: 10.1038/35012626. PubMed doi:10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 27.Rovere-Querini P, Capobianco A, Scaffidi P, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–30. doi: 10.1038/sj.embor.7400205. PubMed doi:10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. PubMed doi:10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 29.Gilmore T, Gapuzan ME, Kalaitzidis D, Starczynowski D. Rel/NF-κ B/I κ B signal transduction in the generation and treatment of human cancer. Cancer Lett. 2002;181:1–9. doi: 10.1016/s0304-3835(01)00795-9. PubMed doi:10.1016/S0304-3835(01)00795-9. [DOI] [PubMed] [Google Scholar]
- 30.Koyama H, Yamamoto H, Nishizawa Y. RAGE and soluble RAGE: potential therapeutic targets for cardiovascular diseases. Mol Med. 2007;13:625–35. doi: 10.2119/2007-00087.Koyama. PubMed doi:10.2119/2007-00087.Koyama. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferraro-Peyret C, Quemeneur L, Flacher M, Revillard JP, Genestier L. Caspase-independent phosphatidylserine exposure during apoptosis of primary T lymphocytes. J Immunol. 2002;169:4805–10. doi: 10.4049/jimmunol.169.9.4805. PubMed. [DOI] [PubMed] [Google Scholar]
- 32.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. PubMed doi:10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 33.Obeid M, Panaretakis T, Joza N, et al. Calreticulin exposure is required for the immunogenicity of γ-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14:1848–50. doi: 10.1038/sj.cdd.4402201. PubMed doi:10.1038/sj. cdd.4402201. [DOI] [PubMed] [Google Scholar]
- 34.Zong WX, Ditsworth D, Bauer DE, Wang ZQ, Thompson CB. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004;18:1272–82. doi: 10.1101/gad.1199904. PubMed doi:10.1101/gad.1199904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albert JM, Cao C, Kim KW, et al. Inhibition of poly(ADP-ribose) polymerase enhances cell death and improves tumor growth delay in irradiated lung cancer models. Clin Cancer Res. 2007;13:3033–42. doi: 10.1158/1078-0432.CCR-06-2872. PubMed doi: 10.1158/1078-0432.CCR-06-2872. [DOI] [PubMed] [Google Scholar]
- 36.Maeda S, Omata M. Inflammation and cancer: role of nuclear factor-κB activation. Cancer Sci. 2008;99:836–42. doi: 10.1111/j.1349-7006.2008.00763.x. PubMed doi:10.1111/j.1349-7006.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–60. doi: 10.1038/sj.emboj.7601623. PubMed doi:10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi J, Choi K, Benveniste EN, et al. Bcl-2 promotes invasion and lung metastasis by inducing matrix metalloproteinase-2. Cancer Res. 2005;65:5554–60. doi: 10.1158/0008-5472.CAN-04-4570. PubMed doi:10.1158/0008-5472.CAN-04-4570. [DOI] [PubMed] [Google Scholar]
- 39.Biroccio A, Candiloro A, Mottolese M, et al. Bcl-2 overexpression and hypoxia synergistically act to modulate vascular endothelial growth factor expression and in vivo angiogenesis in a breast carcinoma line. FASEB J. 2000;14:652–60. doi: 10.1096/fasebj.14.5.652. PubMed. [DOI] [PubMed] [Google Scholar]
- 40.Lickliter JD, Cox J, McCarron J, et al. Small-molecule Bcl-2 inhibitors sensitise tumour cells to immune-mediated destruction. Br J Cancer. 2007;96:600–8. doi: 10.1038/sj.bjc.6603599. PubMed doi:10.1038/sj.bjc.6603599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang XH, Kleeman LK, Jiang HH, et al. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–96. doi: 10.1128/jvi.72.11.8586-8596.1998. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–39. doi: 10.1016/j.cell.2005.07.002. PubMed doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Maiuri MC, Le Toumelin G, Criollo A, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–39. doi: 10.1038/sj.emboj.7601689. PubMed doi:10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Criollo A, Maiuri MC, Tasdemir E, et al. Regulation of autophagy by the inositol trisphosphate receptor. Cell Death Differ. 2007;14:1029–39. doi: 10.1038/sj.cdd.4402099. PubMed. [DOI] [PubMed] [Google Scholar]
- 45.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–88. doi: 10.1016/j.molcel.2008.06.001. PubMed doi:10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pyo JO, Jang MH, Kwon YK, et al. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. J Biol Chem. 2005;280:20722–9. doi: 10.1074/jbc.M413934200. PubMed doi: 10.1074/jbc.M413934200. [DOI] [PubMed] [Google Scholar]
- 47.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–103. doi: 10.1038/nm1096-1096. PubMed doi:10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 48.Kim R, Emi M, Matsuura K, Tanabe K. Antisense and nonantisense effects of antisense Bcl-2 on multiple roles of Bcl-2 as a chemosensitizer in cancer therapy. Cancer Gene Ther. 2007;14:1–11. doi: 10.1038/sj.cgt.7700986. PubMed doi: 10.1038/sj.cgt.7700986. [DOI] [PubMed] [Google Scholar]
- 49.Kataoka T, Budd RC, Holler N, et al. The caspase-8 inhibitor FLIP promotes activation of NF-κB and Erk signaling pathways. Curr Biol. 2000;10:640–8. doi: 10.1016/s0960-9822(00)00512-1. PubMed doi:10.1016/S0960-9822(00)00512-1. [DOI] [PubMed] [Google Scholar]
- 50.Jaattela M, Tschopp J. Caspase-independent cell death in T lymphocytes. Nat Immunol. 2003;4:416–23. doi: 10.1038/ni0503-416. PubMed doi:10.1038/ni0503-416. [DOI] [PubMed] [Google Scholar]
- 51.Bruey JM, Bruey-Sedano N, Luciano F, et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007;129:45–56. doi: 10.1016/j.cell.2007.01.045. PubMed doi:10.1016/j.cell.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 52.Hlaing T, Guo RF, Dilley KA, et al. Molecular cloning and characterization of DEFCAP-L and -S, two isoforms of a novel member of the mammalian Ced-4 family of apoptosis proteins. J Biol Chem. 2001;276:9230–8. doi: 10.1074/jbc.M009853200. PubMed doi:10.1074/jbc.M009853200. [DOI] [PubMed] [Google Scholar]
- 53.Kummer JA, Broekhuizen R, Everett H, et al. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55:443–52. doi: 10.1369/jhc.6A7101.2006. PubMed doi:10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- 54.Nuessler V, Stotzer O, Gullis E, et al. Bcl-2, bax and bcl-xL expression in human sensitive and resistant leukemia cell lines. Leukemia. 1999;13:1864–72. doi: 10.1038/sj.leu.2401571. PubMed doi:10.1038/sj/leu/2401571. [DOI] [PubMed] [Google Scholar]
- 55.Seo KH, Ko HM, Choi JH, et al. Essential role for platelet-activating factor-induced NF-κB activation in macrophage-derived angiogenesis. Eur J Immunol. 2004;34:2129–37. doi: 10.1002/eji.200424957. PubMed doi:10.1002/eji.200424957. [DOI] [PubMed] [Google Scholar]
- 56.Regula KM, Ens K, Kirshenbaum LA. IKK β is required for Bcl-2-mediated NF-κ B activation in ventricular myocytes. J Biol Chem. 2002;277:38676–82. doi: 10.1074/jbc.M206175200. PubMed doi:10.1074/jbc.M206175200. [DOI] [PubMed] [Google Scholar]
- 57.Vanden Abeele F, Skryma R, Shuba Y, et al. Bcl-2-dependent modulation of Ca(2+) homeostasis and store-operated channels in prostate cancer cells. Cancer Cell. 2002;1:169–79. doi: 10.1016/s1535-6108(02)00034-x. PubMed doi:10.1016/S1535-6108(02)00034-X. [DOI] [PubMed] [Google Scholar]
- 58.Xu K, Tavernarakis N, Driscoll M. Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca(2+) release from the endoplasmic reticulum. Neuron. 2001;31:957–71. doi: 10.1016/s0896-6273(01)00432-9. PubMed doi: 10.1016/S0896-6273(01)00432-9. [DOI] [PubMed] [Google Scholar]
- 59.Zamzami N, Brenner C, Marzo I, Susin SA, Kroemer G. Subcellular and submitochondrial mode of action of Bcl-2-like oncoproteins. Oncogene. 1998;16:2265–82. doi: 10.1038/sj.onc.1201989. PubMed doi:10.1038/sj.onc.1201989. [DOI] [PubMed] [Google Scholar]
- 60.Han W, Li L, Qiu S, et al. Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol Cancer Ther. 2007;6:1641–9. doi: 10.1158/1535-7163.MCT-06-0511. PubMed doi:10.1158/1535-7163.MCT-06-0511. [DOI] [PubMed] [Google Scholar]
- 61.Kuma A, Hatano M, Matsui M, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–6. doi: 10.1038/nature03029. PubMed doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 62.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. PubMed doi:10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 63.Wang L, Sloper DT, Addo SN, Tian D, Slaton JW, Xing C. WL-276, an antagonist against Bcl-2 proteins, overcomes drug resistance and suppresses prostate tumor growth. Cancer Res. 2008;68:4377–83. doi: 10.1158/0008-5472.CAN-07-6590. PubMed doi:10.1158/0008-5472.CAN-07-6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Z, Song W, Aboukameel A, et al. TW-37, a small-molecule inhibitor of Bcl-2, inhibits cell growth and invasion in pancreatic cancer. Int J Cancer. 2008;123:958–66. doi: 10.1002/ijc.23610. PubMed doi:10.1002/ijc.23610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Kitada S, Leone M, Sareth S, Zhai D, Reed JC, Pellecchia M. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem. 2003;46:4259–64. doi: 10.1021/jm030190z. PubMed doi:10.1021/jm030190z. [DOI] [PubMed] [Google Scholar]
- 66.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–64. doi: 10.1172/JCI26373. PubMed doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ye W, Chang HL, Wang LS, et al. Modulation of multidrug resistance gene expression in human breast cancer cells by (-)-gossypol-enriched cottonseed oil. Anticancer Res. 2007;27:107–16. PubMed. [PubMed] [Google Scholar]
- 68.Kitada S, Kress CL, Krajewska M, Jia L, Pellecchia M, Reed JC. Bcl-2 antagonist apogossypol (NSC736630) displays single-agent activity in Bcl-2-transgenic mice and has superior efficacy with less toxicity compared with gossypol (NSC19048) Blood. 2008;111:3211–9. doi: 10.1182/blood-2007-09-113647. PubMed doi:10.1182/blood-2007-09-113647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khan AU. Ribozyme: a clinical tool. Clin Chim Acta. 2006;367:20–7. doi: 10.1016/j.cca.2005.11.023. PubMed doi:10.1016/j.cca.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 70.Dorai T, Goluboff ET, Olsson CA, Buttyan R. Development of a hammerhead ribozyme against BCL-2. II. Ribozyme treatment sensitizes hormone-resistant prostate cancer cells to apoptotic agents. Anticancer Res. 1997;17:3307–12. PubMed. [PubMed] [Google Scholar]
- 71.Luzi E, Papucci L, Schiavone N, et al. Downregulation of bcl-2 expression in lymphoma cells by bcl-2 ARE-targeted modified, synthetic ribozyme. Cancer Gene Ther. 2003;10:201–8. doi: 10.1038/sj.cgt.7700556. PubMed doi:10.1038/sj. cgt.7700556. [DOI] [PubMed] [Google Scholar]
- 72.Weiss B, Davidkova G, Zhou LW. Antisense RNA gene therapy for studying and modulating biological processes. Cell Mol Life Sci. 1999;55:334–58. doi: 10.1007/s000180050296. PubMed doi:10.1007/s000180050296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pepper C, Hooper K, Thomas A, Hoy T, Bentley P. Bcl-2 antisense oligonucleotides enhance the cytotoxicity of chlorambucil in B-cell chronic lymphocytic leukaemia cells. Leuk Lymphoma. 2001;42:491–8. doi: 10.3109/10428190109064606. PubMed doi:10.3109/10428190109064606. [DOI] [PubMed] [Google Scholar]
- 74.Lin Y, Choksi S, Shen HM, et al. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem. 2004;279:10822–8. doi: 10.1074/jbc.M313141200. PubMed doi:10.1074/jbc.M313141200. [DOI] [PubMed] [Google Scholar]
- 75.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8. doi: 10.1038/35078107. PubMed doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 76.Kim WJ, Chang CW, Lee M, Kim SW. Efficient siRNA delivery using water soluble lipopolymer for anti-angiogenic gene therapy. J Control Release. 2007;118:357–63. doi: 10.1016/j.jconrel.2006.12.026. PubMed doi:10.1016/j.jconrel.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 77.Yezhelyev MV, Qi L, O'Regan RM, Nie S, Gao X. Proton-sponge coated quantum dots for siRNA delivery and intracellular imaging. J Am Chem Soc. 2008;130:9006–12. doi: 10.1021/ja800086u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dimmeler S, Breitschopf K, Haendeler J, Zeiher AM. Dephosphorylation targets Bcl-2 for ubiquitin-dependent degradation: a link between the apoptosome and the proteasome pathway. J Exp Med. 1999;189:1815–22. doi: 10.1084/jem.189.11.1815. PubMed doi:10.1084/jem.189.11.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Breitschopf K, Haendeler J, Malchow P, Zeiher AM, Dimmeler S. Posttranslational modification of Bcl-2 facilitates its proteasome-dependent degradation: molecular characterization of the involved signaling pathway. Mol Cell Biol. 2000;20:1886–96. doi: 10.1128/mcb.20.5.1886-1896.2000. PubMed doi:10.1128/MCB.20.5.1886-1896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simizu S, Tamura Y, Osada H. Dephosphorylation of Bcl-2 by protein phosphatase 2A results in apoptosis resistance. Cancer Sci. 2004;95:266–70. doi: 10.1111/j.1349-7006.2004.tb02214.x. PubMed doi:10.1111/j.1349-7006.2004.tb02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]