Abstract
The ability to distinguish ‘self’ from ‘nonself’ is the most fundamental aspect of any immune system. The evolutionary solution in plants to the problems of perceiving and responding to pathogens involves surveillance of nonself, damaged-self and altered-self as danger signals. This is reflected in basal resistance or non-host resistance, which is the innate immune response that protects plants against the majority of pathogens. In the case of surveillance of nonself, plants utilize receptor-like proteins or -kinases (RLP/Ks) as pattern recognition receptors (PRRs), which can detect conserved pathogen/microbe-associated molecular pattern (P/MAMP) molecules. P/MAMP detection serves as an early warning system for the presence of a wide range of potential pathogens and the timely activation of plant defense mechanisms. However, adapted microbes express a suite of effector proteins that often interfere or act as suppressors of these defenses. In response, plants have evolved a second line of defense that includes intracellular nucleotide binding leucine-rich repeat (NB-LRR)-containing resistance proteins, which recognize isolate-specific pathogen effectors once the cell wall has been compromised. This host-immunity acts within the species level and is controlled by polymorphic host genes, where resistance protein-mediated activation of defense is based on an ‘altered-self’ recognition mechanism.
Key words: effector, innate immunity, nonself, pathogen-associated molecular pattern, pattern recognition receptors, plant defense, resistance
Introduction: The Age Old Question of “What is Self?”
The ability to distinguish self from nonself is the most fundamental aspect of an immune system. Although expressing an apparent passivity associated with their sedentary lifestyle and, being simultaneously exposed to evolving pathogens as well as environmental stresses, plants have evolved a unique metabolic plasticity that allows them to perceive pathogens and unleash effective defense strategies.1 Since a plant's entire immune response is not based on an adaptive/acquired system as seen in mammals, it would appear to be an evolutionary ancient defense mechanism able to genetically distinguish ‘self’ from ‘nonself’ and result in downstream cascades to counter pathogen attack or eliminate the pathogen. The question therefore arises how such a system could perceive so many diverse pathogen-derived signals when the mechanism originated before the evolving variables in potential invaders and when plants are unable to acquire or adapt specifically, whilst simultaneously being exposed to environmental stresses? In this context it is important to define ‘self’ and ‘nonself’ during a defense response and to focus on how plants distinguish between self and nonself/damaged-self/altered-self using the perception and signal transduction mechanisms available.
Self.
The philosophical debate regarding self appears to be mute when considering plant cells that are surrounded by a cell wall. In this biological context everything originating from within the wall is self and all molecules of foreign origin outside the cell wall are nonself. This definition applies to the self recognition (i.e., the identification of the same genetic homology) that is observed in the specific example of pollen recognition in the same species, from the same plant during self incompatibility (SI).
The established system of self/nonself recognition in SI systems utilizes receptor-ligand type interactions to perceive, recognize and reject incompatible pollen when the same S-haplotype is expressed by both pollen and pistil. Thus, SI prevents self-pollination. Although SI responses are generally comprised of a self/nonself recognition process, SI systems have evolved independently (as exemplified in the Solanaceae, Papaveraceae and Brassicaceae) and do not utilize one molecular mechanism exclusively. Rather, SI encompasses a collection of divergent cellular responses leading to pollen rejection.2–4 In Brassicaceae, the recognition of self and self-incompatibility are components of a receptor-ligand based mechanism that utilizes an S receptor kinase (SRK) to perceive and reject self-pollen. SRK is an S-domain RLK, which in turn is part of the RLK family, some members of which represent PRRs involved in the detection of P/MAMPs (discussed below). S-domain RLKs also occur in species that do not exhibit self-incompatibility and exhibit upregulated gene expression profiles in response to wounding and pathogen recognition, suggesting that they may fulfill a role in perception and/or defense. Although evolution may have driven expansion of certain RLK families to serve roles in particular physiological processes, this may not exclude these receptor types from functioning in different programs.1 The evolutionary origins of plant SI, centered on the hypothesis that SI evolved from a defense pathway, was discussed by Nasrallah.5 Parallels exist where plant SI and plant immunity have similar outcomes, such as the elimination of undesirable cells or organisms. Interestingly, the process of pollen rejection is closely associated with rapid and effective proteolytic events, including the ubiquitin-proteasome pathway and the vacuolar sorting pathways; processes that are also of great importance in plant defense. SI is not further discussed and the reader is referred to Zhang et al.4 for a recent review on the subject and to Sanabria et al.1 for the conceptual and mechanistic links between microbial recognition and self-incompatibility.
Nonself.
The definition for self, as described above, is not entirely sufficient. However, with regards to immune responses, it appears to be more constructive to define what plants perceive as nonself. In the same biological context, nonself should be redefined as a biological molecule/organism that the plant perceives to be (I) of different origin, e.g., pathogenic species recognized during a defense response (discussed in detail below) or (II) of different state, e.g., altered or damaged cellular components recognized during routine ‘house-keeping’ maintenance and regulatory metabolism (discussed in detail below).
The Constant Battle between Self and Nonself: Principles of Immunity
During co-evolution with pathogens, plants have evolved systems to distinguish self and nonself based on the detection of P/MAMPs. PAMPs may be described as invariant epitopes within molecules that are fundamental to the pathogens' fitness. They are widely distributed among different microbes, absent from the host and recognized by a wide array of potential hosts (Table 1). PAMP-triggered immunity (PTI), constitutes the first line of inducible defense against infectious disease.10,11 In response, many Gram-negative bacteria inject effector proteins, previously termed avirulence (Avr) proteins, into the host cells, through type III secretion systems, which suppress the P/MAMP-mediated immune responses.12–15 As a counter move, plants have co-evolved specific resistance (R) proteins to recognize the effector proteins.10,13,14 This then leads to effector-triggered immunity (ETI) and/or the hypersensitive response (HR) representing a form of programmed cell death.14,16 Moreover, host inhibition of bacterial virulence effectors can trigger immunity to infection.17 And so the cycle continues, (Figure 1), thus perpetuating the constant battle between pathogens and plants, described as an “arms race between pattern recognition receptors in plants and effectors in microbial pathogens”.18
Table 1.
Summary of selected PAMPs* recognized by plants (adapted from Nürnberger and Lipka, Schwessinger and Zipfel)6,7
| PAMP | Plant | Pathogen(s) | Active epitope |
| β-glucans | Rice Legume | Fungi (Pyricularia oryzae), Oomycetes (Phytophthora spp.,), Brown algae | Tetraglucosyl glucitol, branced hepta-β-glucoside, linear oligo-β-glucosides |
| Cerebrosides A, C | Rice | Fungi (Magnaporthe spp.,) | Sphingoid base |
| Chitin/Chitosan | Arabidopsis, rice, tomato and wheat | All fungi | Chitin oligosaccharides (degree of polymerization >3) |
| Cold shock protein | Solanaceae | Gram-negative bacteria, Gram-positive bacteria | RNP-1 motif (amino terminal fragment of cold shock protein) |
| Elongation factor (EF-Tu) | Brassicaceae | Gram-negative bacteria | elf18 (N-acetylated amino terminal fragment of EF-Tu) |
| Ergosterol | Tomato | All fungi | |
| Flagellin | Most plants (except rice) | Gram-negative bacteria | flg22 (amino terminal fragment of flagellin) |
| Harpin (HrpZ) | Various plants | Gram-negative bacteria (Pseudomonads, Erwinia) | Undefined |
| Invertase | Tomato | Yeast | N-mannosylated peptide (fragment of the peptide) |
| Lipid-transfer proteins (elicitins) | Tobacco | Oomycetes (Phytophthora spp., Pythium spp.,) | Undefined |
| LPS | Arabidopsis, pepper and tobacco | Gram-negative bacteria (Xanthomonads, Pseudomonads, Burkholderia spp.) | Lipid A/inner core/Glucosamine backbone/Combinations of motifs? |
| Necrosis-inducing protein | Many dicot plants | Bacteria (Bacillus spp.,), Fungi (Fusarium spp., Verticillium spp.,) Oomycetes (Phytophthora spp., Pythium spp.) | Undefined |
| Peptidoglycan | Arabidopsis and tobacco | Gram-positive bacteria | Muramyl dipeptide |
| Rhamnolipids** | Grapevine | Pseudomonas species | Mono-/dirhamnolipids |
| Siderophores*** | Tobacco | Undefined | Pseudomonas fluorenscens |
| Sulphated fucans | Tobacco | Brown algae | Fucan oligosaccharide |
| Transglutaminase | Parsley and potato | Oomycetes (Phytophthora spp.) | Pep-13 motif (surface-exposed epitope of the transglutaminase) |
| Xylanase | Tobacco and tomato | Fungi (Trichoderma spp.) | TKLGE pentapeptide (suface-exposed epitope of xylanase) |
Figure 1.
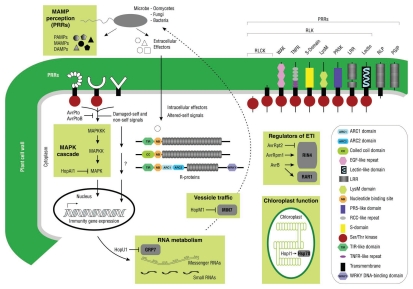
Perception systems for damaged-self, altered-self and nonself signals in plants. Pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), and pathogen-derived effectors are perceived as nonself danger signals. Extracellular P/MAMPs originating from prototypical microbes and DAMPs generated by their enzymes, are recognized via pattern recognition receptors (PRRs). Pathogen effectors injected into the cell are detected, directly or indirectly, by intracellular resistance (R) proteins and associated proteins. The domain organization of typical extracellular and intracellular receptors in plants is shown.21,141 Successful pathogens have acquired the ability to interfere or suppress generated signals and to circumvent plant defenses (e.g., AvrPto, AvrProB, AvrRpt2, HopAl1, HopU1, HopM1, Hopi1).146 Six possible targets for interference or suppression include: (1) MAMP perception through PRRs, (2) the MAPK cascade, (3) RNA metabolism, (4) vesicle trafficking, (5) regulation of ETI/PTI and (6) chloroplast function (adapted from Boller and He,18 Boller and Felix,19 as well as Tör et al.147).
Innate immunity.
Plants possess an innate immune system consisting of PTI and ETI that detects and defends against potentially dangerous microbes.14,16,18,19 It draws its origins from a phylogenetically ancient form of immunity that is common to all Metazoa and Viridiplantae,20 which precedes SI.1,21 The innate immune system in plants is unable to acquire or specifically adapt like the animal adaptive immune system.22 Rather, it relies on a spectrum of predetermined receptors expressed in non-mobile cells. These receptors may be proteins with similar morphologies or proteins that are able to multi-task between different functions in order to compensate for the inability to acquire antibodies.
Basal resistance, non-host and host immunity.
Non-host immunity refers to an evolutionary ancient, multilayered resistance mechanism consisting of constitutive and inducible components.23 Non-host immunity remains operative even in susceptible plants to limit pathogen growth and is associated with the release of molecules (ligands or elicitors) derived from the pathogen.24 In addition, it is also associated with peptides or oligouronides originating from hydrolytic events during the interaction between plants and pathogens and acting as endogenous elicitors. These are analogous to the ‘danger signals’ of the vertebrate immune system, such as heat-shock proteins, nucleotides, reactive oxygen intermediates, extracellular-matrix breakdown products, neuromediators and cytokines.25 Basal resistance is the innate immune response that protects plants against the majority of pathogens. In addition, another, more recently evolved form of immunity is operative in plants. Host-immunity acts within the species level and is controlled by polymorphic host genes, such as the R genes, the products of which interact, directly or indirectly, with secreted avr proteins or effectors of the pathogen.20,26
Current models of plant immunity; PAMP- vs. Effector-triggered immunity.
Two branches to the plant immune system are now recognized: PTI and ETI, associated with different perception mechanisms in the host.14,16
PTI. PTI refers to the inducible responses activated upon recognition of conserved P/MAMPs such as lipopolysaccharides (LPS), peptidoglycan and flagellin of bacteria, or chitin and glucan of fungi etc. It has been reported that P/MAMPs can interact, either directly or indirectly, with each other as well as the cell wall matrix of the host. This interaction would influence the speed, magnitude, versatility as well as the organization of the defense response.27 Recent evidence indicates that some identified PRRs are members of the RLK family, e.g., the flagellin receptor (FLS2) and elongation factor Tu (EF-Tu)-receptor (EFR).28,29 The work regarding flagellin and EF-Tu, indicates that there must be a requirement for numerous such signal perception and transduction systems in plants able to recognize all potential invaders.29,30 Indeed, sequencing of the Arabidopsis thaliana genome has revealed the presence of >400 RLK sequences with various receptor configurations, of which those containing a leucine-rich repeat (LRR) in the extra-cellular domain constitute the largest group with 216 members.30,32 The diversity and large number of plant RLKs suggest that they may be involved in the perception of a wide range of stimuli. Other PRRs are also found amongst non-RLK proteins such as Glycine max β-glucan elicitor binding protein (GmGBP), Lycopersicon esculentum ethylene-inducing xylanase (LeEIX2) and chitin elicitor-binding protein (CeBIP) for perception of β-glucans (soybean), xylanase (tomato) and chitin fragments (rice) respectively.33–35
ETI. The second branch of the plant immune system, ETI, in contrast acts mostly inside the cell, using polymorphic resistance proteins encoded by R genes, reviewed by Liu et al.36 as well as Tameling and Takken.37 R gene-mediated resistance is a form of host-immunity activated upon recognition of an avirulence factor, a pathogen effector protein that elicits resistance (via direct recognition of the effector by the plant, or via their action on targeted host molecules, i.e., indirectly). Since R genes act in a race-specific manner, there are few that confer broad-spectrum resistance.
‘Zigzag’ model and ETS. A ‘zigzag’ model to illustrate the quantitative output of the plant immune system as well as to illustrate the evolutionary relationship between PTI and ETI was recently proposed.16 In phase 1, P/MAMPs are recognized by PRRs, resulting in PTI that can stop further colonization. In phase 2, successful pathogens deploy effectors that contribute towards pathogen virulence. When effectors suppress or interfere with PTI, it results in effector-triggered susceptibility (ETS).15,38 In phase 3, an effector is specifically recognized by an R protein, which results in ETI. ETI is regarded as an accelerated and amplified PTI response, which results in disease resistance and may lead to an HR at the infection site. In phase 4, natural selection drives pathogens to avoid ETI. This is achieved by either shedding or diversifying the recognized effector gene, or by acquiring effectors that suppress ETI. Thereafter, natural selection results in the evolution of new R specificities leading to ETI being triggered again.
Downstream signaling. Similar to PTI, R-protein triggered immunity is also linked to reactive oxygen intermediate accumulation and activation of defense genes, but the two responses differ quantitatively and kinetically. The outcome of ETI can lead to programmed cell death of the host cell in the form of the HR in order to limit the spread of the infection. It results in local induced/acquired resistance (LAR), acting at the site of infection to contain the invader, and systemic acquired resistance (SAR), which induces defenses in distal, non-infected parts of plants after activation of local resistance. Due to the fact that PTI and ETI have similar output responses, it is possible that the downstream signaling pathways converge.39 It should be noted that SAR has also been demonstrated to be induced by recognition of PAMPs like LPS40 and that certain PAMPs like flagellin and flg22 can cause an HR.41 Both salicylic acid (SA) and jasmonic acid (JA) are required for P/MAMP-induced defense responses.42 P/MAMP, as well as effector-triggered processes are linked to SA pathways,43 therefore, SA-mediated responses may be an important part of R gene-mediated defense.
Biochemistry of Perception and Recognition: Nonself Detection
Perception of pathogens by plants.
The ability to monitor microbial presence at the cell surface is essential for plant defense mechanisms. Plant innate immunity is activated either upon perception of P/MAMPs by PRRs or upon recognition of pathogen race-specific effector molecules by R processes (Fig. 1). Recognition of potential pathogen-derived molecules or pathogen activity in planta, results in signal initiation and signal transduction, culminating in the activation or de-repression of defense-associated genes. The recognition specificities in the different kingdoms probably arose independently in order to recognize highly conserved molecules.14,16
The plant cell wall as a sensor of integrity.
The structure of the plant cell wall distinguishes it from all other eukaryotic cells. It represents the first barrier to an invading pathogen. If the invasion is halted, cellular damage is minimized and no other defensive actions are required. In this context the plant cell wall is not only a rigid or static structure used for mechanical support; it exists as a highly dynamic and responsive structure in a relationship with the plasma membrane and cytoskeleton, where the external and internal environments are joined, and where information from external stimuli is relayed.44 The plant cell is able to perceive changes to the cell wall, be responsive and adapt with regards to growth and development, as well as stresses, e.g., wounding and pathogen attack, which was reviewed by Humphrey et al.44
Pathogen attack may lead to cell damage and influence the cell wall integrity. When the cell wall responds to stress or change, it may be due to the recognition of its ‘damaged-self,’ through damage-associated molecular pattern molecules (DAMPs). The stress or change is perceived by a sensor or sentinels and the plant responds to the change in a defensive manner. An example of recognition of the plant’s ‘damaged-self’ is when pathogen-secreted or endogenous plant polygalacturonases or pectate lyases cause enzymatic degradation of pectin in the plant cell wall, i.e., an altered/damaged state of self. Here, the polygalacturonase-inhibiting protein, an extracellular LRR R protein, interacts to generate oligogalacturonides, which are perceived by a sentinel in order to generate a signal, triggering defense related responses.45–47 Other potential DAMPs include cellodextrins and cutin monomers, originating as degradation products from the plant cell wall cellulose and cutin layers. Plants can perceive modifications of the cuticle and activate a multi-factorial defense response.48 However, sentinels that alert the plant to activate defense responses in response to DAMPs have only recently been explored.
In addition to signals generated due to cell wall degradation, conditions that lead to a decrease in cellulose content (e.g., due to loss of function mutations in cellulose synthase (CESA) or chemical inhibition of cellulose synthase/cell wall synthesis) are associated with a corresponding increase in defense-associated cell wall strengthening through lignin and callose synthesis.49–52 This implies a feedback mechanism involving sensors of wall integrity. In addition, these conditions cause constitutive expression of genes associated with JA or ethylene signaling.53 The synthesis of these hormones is usually associated with responses to pathogens, wounding and drought.54–56
The protein components in the cell wall probably play a determining role in perception and these include a variety of potential sensors. Arabinogalactan proteins (AGPs) are regarded as potential sensors of wall integrity.44 AGPs are glycosylphos-phatidylinositol (GPI)-anchored proteins (GAPs). GAPs may play a role in cell surface signaling, adhesion, matrix remodeling and pathogen response.57 In addition, leucine-rich extensin (LRX) proteins that bind to the cellulose microfibrils, have also been identified as potential cell wall sensors. The wall-associated kinases (WAKs) are the best characterized of the potential cell wall receptors and are ideally situated for sensing and signaling from the cell wall.58 WAK expression can be induced in response to pathogen attack and, being able to bind pectin fragments and oligogalacturonides,59 may serve as potential sensors of damaged-self. Other RLKs found associated with the cell wall include lectin receptor kinases, a subset of which are found in plasma membrane-cell wall adhesions and proline-rich extensin-like receptor kinases (PERKs) involved in sensing of cell wall damage due to wounding by pathogens.60 THESEUS1, a receptor kinase, is a new candidate for sensing cell wall integrity, but has not been proven to play a role in defense.44 Furthermore, various plasma membrane proteins with extracellular domains, such as RLPs and RLKs important in relaying information from external stimuli (discussed below), interact with and within the wall matrix.
Sentinels of nonself: pattern recognition receptors.
Plants have evolved a large range of potential immune receptors (refer to Tables 2–531 y) that recognize P/MAMPs as determinants of nonself, or mediate effector perception in the form of ‘sentinels.’ Sentinels may contain pattern recognition domains combined with accessory domains that participate in signal relay. The diversity of P/MAMPs and the identification of the corresponding PRRs, a term describing a functional category, were recently reviewed.19 Receptors, a term describing a molecular category, which detect microbial patterns can either be surface based or intracellular receptors (Fig. 1). Surface receptors are known to detect primarily microbe-derived elicitors (including P/MAMPs, if the molecule contains a conserved ‘pattern’), as well, in certain cases, avirulence effectors such as Xa21, in which case they are regarded as R gene products. The surface receptors include RLKs, RLPs and extracellular binding proteins that may form part of multi-component recognition complexes.61 The few P/MAMP receptors identified thus far in plants are all surface receptors that physically interact with their cognate ligands.61 In contrast, interaction between effectors and the intracellular R proteins (which can contain a LRR domain) probably occurs indirectly through a multi-member complex.62
Table 2.
Plant LRR-RLKs associated with plant-microbe interactions, innate immunity and defense
| Gene | Plant | Class | Type | Function | Perception | Putative ligands | References |
| CURL3 | Solanum esculentum | LRR | RD-kinase | Putatively systemin perception during wounding; possibly brassinosteroid perception *Orthologue of Sr160 | Nonself recognition: altered self | Systemin BL | 107 |
| DIPM1 to 4 | Malus x domestica | LRR | Non-RD-kinase | Disease resistance and plant-pathogen interaction signaling | Nonself recognition: different origins | DspA/E | 108 |
| EFR | Arabidopsis thaliana | LRR | Non-RD-kinase | Disease resistance and plant-pathogen interaction signaling | Nonself recognition: different origins | EF-Tu (elf18) | 29 |
| ERECTA | Arabidopsis thaliana | LRR | RD-kinase | Resistance to Ralstonia solanacearum | Nonself recognition: different origins | 109 | |
| FLS2 | Arabidopsis thaliana | LRR | Non-RD-kinase | Flagellin perception | Nonself recognition: different origins | flg22 | 28 |
| HAR1 | Lotus japonicas | LRR | RD-kinase | Nodule development during nitrogen fixation symbiosis *Orthologue of NARK | Symbiotic relationship, recognition of different origins | 110 | |
| LRPKm1 | Malus x domestica | LRR | RD-kinase | Disease resistance and plant-pathogen interaction signaling. Highly induced expression during incompatible reactions and lower induction during compatible interactions with Venturia inaequalis | Nonself recognition: different origins | 111 | |
| Nork | Medicago sativa | LRR | Non-RD-kinase | Bacterial symbiosis. root nodule and mycorrhiza formation *Orthologue of SYMRK | Symbiotic relationship, recognition of different origins | 112 | |
| PEPR1 | Arabidopsis thaliana | LRR | RD-kinase | Activation of defense and possible positive feedback loop mechanism to amplify PAMP-induced responses. | Nonself recognition: altered self | AtPep1 | 113 |
| SIRK | Arabidopsis thaliana | LRR | RD-kinase | Upregulated during senescence and pathogen challenge | Nonself recognition: different origins | 114 | |
| SR160 | Lycopersicon esculentum | LRR | RD-kinase | Putatively systemin perception during wounding; possibly brassinosteroid perception *Orthologue of CURL3 | Nonself recognition: altered self | Systemin BL | 115 |
| SYMRK | Lotus japonicus | LRR | RD-kinase | Bacterial symbiosis. root nodule and mycorrhiza formation *Orthologue of Nork | Symbiotic relationship, recognition of different origins | 116 | |
| Xa21 and Xa26 | Oryza sativa | LRR | Non-RD-kinase | Specific disease resistance to Xanthomonas oryzae pv oryzae | Nonself recognition: different origins | AvrXa21 elicitor | 117, 118 |
Table 5.
Dual functioning in plant signaling
| Component | Dual Function | Reference |
| BAK1 | Is associated with developmental regulation through the plant hormone receptor BRI 1, but also has a functional role in PRR-dependant signaling which initiates innate immunity | 105, 106 |
| ERECTA | Affects development of aerial organs by controlling organ size and shape, and is also involved in disease resistance | 142, 143 |
| HAESE/RLK5 | Functions in both developmental processes (abscission) and defense (hypersensitive cell death) | 100 |
| LTP1 | In wheat, it binds putative receptors for elicitins. | 144 |
| In tobacco, it binds jasmonic acid providing protection against Phytophthora parasitica similar to that invoked by elicitin | ||
| LysM type receptors | *CEBiP is a chitin oligosaccharide elicitor binding protein with two LysM motifs with a proposed role in chitin signaling and transcriptional regulation | 35 |
| *NRF1 and NRF5 are two LysM receptor kinases found in Lotus japonicus, which are putative receptors for lipochitooligosaccharide Nod-factors | 127 | |
| *LYK3, found in Medicago truncatula, is involved in specific recognition during later stages of bacterial infection | 145 | |
| Mi gene product | Confers resistance to nematodes as well as aphids | 85 |
| PERK | Upregulated by wounding and infection in Brassica, also plays a role in regulation of growth in Arabidopsis | 146, 147 |
| Plant gp91PHOX NADPH oxidase | Although involved in the oxidative burst, it also functions in a variety of developmental and physiological processes | 103 |
| RIN4 | Guardee—interacts with two different R genes, RPM1 and RPS2 | 92 |
| RPM1 | Able to recognize two different Avr effectors | 85 |
| RPP8/HRT | Recognizes both viral and oomycete pathogens | 85 |
| Rx/Gpa gene | Confer both viral and nematode resistance | 85 |
| WAK1 | Involved with an epidermal growth factor EGF-like motif linked to plant growth, also up regulated in response to pathogen infection and exogenous salicylic acid | 139 |
Extracellular sentinels—receptor-like proteins/kinases (RLP/Ks). According to the zig-zag model, plants will first be exposed to pathogen-derived elicitors or PAMPs, which upon perception will trigger PTI. Plants are therefore dependent on the initial local recognition of the invader to activate defenses, and this is where perception by RLP/Ks can play a determining role.
In Arabidopsis, 610 RLK and 56 RLP have been identified, but only a limited number have been functionally characterized and even fewer are reported to act as immune receptors.63,64 Many Arabidopsis genes encoding RLK and RLP were found to be induced upon the amino-terminal fragment of flagellin (flg22) or EF-Tu treatment, suggesting that they may function as immune receptors.65 Indeed, 27 out of a total 216 LRR-RLK in Arabidopsis were found to be transcriptionally induced upon treatment with flg22 or EF-Tu.61,65 In addition to FLS2, the EFR was identified as a novel PRR and is a LRR-RLK.28,29,66 Among the upregulated genes, there also were three encoding RLK containing lysine motifs (LysM) in their extracellular domains, which potentially could recognize carbohydrate microbial structures containing N-acetyl glucosamine (GlcNAc). It appears that P/MAMPs also trigger enhanced expression of their own cognate receptors, as reported for flg22, EF-Tu and chitin.29,35,65
The receptor protein kinases (RPKs) can be classified according to different substrate specificities (tyrosine, serine/threonine or histidine) in the kinase domains.67,68 RLKs form part of the receptor serine/threonine kinase (RSTK) family, also known as the interleukin-1 receptor-associated kinase (IRAK/Pelle) family. The RLKs can be subdivided into transmembrane receptor kinases (TMRKs) and receptor-like cytoplasmic kinases (RLCKs) if an extracellular domain is absent.63 RLKs are transmembrane proteins with versatile N-terminal extracellular domains and a C-terminal intracellular kinase domain related to the Drosophila Pelle kinase.21,69 They are classified according to their extracellular domains, except for those who do not have a signal peptide and/or a transmembrane region, referred to as RLCKs. TMRKs can be further grouped into arginine and aspartate (RD)-kinases, non-RD kinases and RD-minus kinases.68 Although only a few RLKs have been shown to play a role in either development, plant defense or even symbiotic interactions, their large number and diversity suggest that they may be able to recognize and respond to a variety of stimuli. Despite the small number of non-RD kinases (10% of the Arabidopsis kinome), kinases known or predicted to function in PRR signaling fall into the non-RD class. The reader is referred to Dardick and Ronald,70 for a review on receptor signaling through non-RD kinases, predicted to function in PRR signaling and thought to be involved in pathogen recognition and innate immunity. RLPs differ from RLKs in that they contain an extracellular domain and a membrane-spanning domain, but they lack an intracellular activation domain. Therefore, they require interaction with adaptor molecule(s) or RLCKs for signal transduction.
The proposed evolutionary relationships between receptor kinase family members arose from an ancient duplication event leading to the divergence of RLKs/Pelle from receptor tyrosine kinases (RTKs)/Raf.21,64 In the case of PTI, the evolutionary history of the plant RLKs indicate that the kinase domains were recruited numerous times by fusion with different extracellular domains to form the subfamilies found in Arabidopsis. Subfamilies are assigned based on kinase phylogeny and are shown according to the domain organization of the majority of members in a given subfamily.21,69
Diverse sequence motifs are present in the extracellular domains of RLKs and these motifs are potentially responsible for interactions with other proteins, carbohydrates or lipids.69 The data indicates that RLKs involved in resistance or defense responses may have been duplicated or retained at higher rates in a lineage-specific fashion.31 The preferential expansion of defense/resistance-related RLKs could be the consequence of strong selection pressure for recognizing pathogens.31 The large family of plant RLK proteins, therefore, contain distinct protein kinases where each might play a unique role in cellular signaling.71 These probably comprise receptors for further P/MAMP recognition.29 In addition, in certain cases, plant defense mechanisms seem to exhibit ‘multi-tasking,’ i.e., the use and application of pre-existing biochemical modules or systems to compensate for evolving variables in potential invaders (discussed below).
The members of the RLK family are divided into classes. The S-class/domain RLKs share homology with the self-incompatibility-locus glycoproteins (SLG) from Brassica.72 The extracellular domain has 12 characteristic conserved cysteines, CX5 CX5 CX7 CXCXN CX7CXN CX3 CX3 CXCXN C. Usually, 10 cysteines are absolutely conserved.73 A conserved PTDT box was observed in seven different S-domain RLKs.71 The LRR class contains conserved repeats of leucines, LX2 LX2 LX2 LXLX2 XN XLXGXIPX2 and the regions are also surrounded by paired cysteines.74,75 The lectin-like class has an extracellular domain that shares homology with lectin proteins.63 Some RLKs bind to plant cell-wall components. The extracellular domains of cell wall-associated kinase (WAK)-type RLKs are associated with pectin, a structural component in the middle lamella and primary cell wall.21,76 WAKs contain an extracellular domain with similarity to epidermal growth factor (EGF)-like domains.44 The tumor necrosis factor receptor (TNFR) class has a repeat motif that resembles the extracellular domain of the mammalian tumor necrosis factor receptor.77 The pathogenesis-related (PR) class contains all 16 cysteines in the extracellular domain that are conserved in PR5 antimicrobial proteins.78 The chitinase-like class has an extracellular domain homologous to both class V tobacco and Bacillus WL-12 A chitinases.79 The cysteine-rich repeat (CRR) class has one or more repeats of the C-X8-C-X2-C motif.80 RLKs containing lysine motifs in their extracellular domains are characterized as the LysM class. The ‘miscellaneous’ or ‘other’ types of RLKs include those with extracellular domains that do not share homology with other known proteins, contain unique motifs, and therefore cannot be grouped into the above mentioned classes.
Receptor proteins have also been identified that lack the characteristic RLK kinase domain (i.e., RLPs), or proteins that are functional kinases that lack extracellular ligand binding domains (RLCKs). In some cases the proteins have an intracellular kinase domain, as well as a transmembrane region, but only have a short extracellular domain. Tables 2 and 3 serve to summarize which RLKs are involved with disease resistance and/or associated with plant-microbe interactions. The role and regulation of RLKs that have been identified in elicitor-initiated defense responses and as incompatibility-locus glycoproteins (SLG) from Brassica.72 The extracellular domain has 12 characteristic conserved cysteines, CX5 CX5 CX7 CXCXN CX7CXN CX3 CX3 CXCXN C. Usually, 10 cysteines are absolutely conserved.73 A conserved PTDT box was observed in seven different S-domain RLKs.71 The LRR class contains conserved repeats of leucines, LX2 LX2 LX2 LXLX2 XN XLXGXIPX2 and the regions are also surrounded by paired cysteines.74,75 The lectin-like class has an extracellular domain that shares homology with lectin proteins.63 Some RLKs bind to plant cell-wall components. The extracellular domains of cell wall-associated kinase (WAK)-type RLKs are associated with pectin, a structural component in the middle lamella and primary cell wall.21,76 WAKs contain an extracellular domain with similarity to epidermal growth factor (EGF)-like domains.44 The tumor necrosis factor receptor (TNFR) class has a repeat motif that resembles the extracellular domain of the mammalian tumor necrosis factor receptor.77 The pathogenesis-related (PR) class contains all 16 cysteines in the extracellular domain that are conserved in PR5 antimicrobial proteins.78 The chitinase-like class has an extracellular domain homologous to both class V tobacco and Bacillus WL-12 A chitinases.79 The cysteine-rich repeat (CRR) class has one or more repeats of the C-X8-C-X2-C motif.80 RLKs containing lysine motifs in their extracellular domains are characterized as the LysM class. The ‘miscellaneous’ or ‘other’ types of RLKs include those with extracellular domains that do not share homology with other known proteins, contain unique motifs, and therefore cannot be grouped into the above mentioned classes. Receptor proteins have also been identified that lack the characteristic RLK kinase domain (i.e., RLPs), or proteins that are functional kinases that lack extracellular ligand binding domains (RLCKs). In some cases the proteins have an intracellular kinase domain, as well as a transmembrane region, but only have a short extracellular domain. Tables 2 and 3 serve to summarize which RLKs are involved with disease resistance and/or associated with plant-microbe interactions. The role and regulation of RLKs that have been identified in elicitor-initiated defense responses and as dominant R genes in race-specific pathogen defense, was recently reviewed.81
Table 3.
Non-LRR RLKs and RLPs associated with plant-microbe interactions, innate immunity and defense
| Gene | Plant | Class | Type | Function | Perception | Putative ligands | References |
| At-RLK3 | Arabidopsis thaliana | DUF-26 | RD-kinase | Expressed under oxidative stress and pathogen attack | Nonself recognition: altered self | 119 | |
| CERK1 | Arabidopsis thaliana | LysM | RD-kinase | Chitin elicitor signaling *Homologue of LysM RLK1 | Nonself recognition: different origins | chitin | 120 |
| CHRK1 | Nicotiana tabacum | Chitinase | RD-kinase | Induced by tobacco mosaic virus, Phytophthora parastitica | Nonself recognition: different origins | 79 | |
| LecRK-a1 | Arabidopsis thaliana | Lectin | RLP | Expressed during wounding | Nonself recognition: altered self | 121 | |
| LRK10 | Triticum aestivum | ‘Other’/S-domain | Non-RD-kinase | Specific resistance to wheat rust fungi | Nonself recognition: different origins | 122, 123 | |
| LYK3 | Medicago truncatula | LysM | RD-kinase | Involved in early events during nitrogen fixation symbiosis (symbiotic interactions) *Orthologue of NFR1 | Symbiotic relationship, recognition of different origins | Nod factors | 124 |
| LYK4 | Medicago truncatula | LysM | RD-kinase | Involved in early events during nitrogen fixation symbiosis (symbiotic interactions) | Symbiotic relationship, recognition of different origins | Nod factors | 124 |
| LysM RLK1 | Arabidopsis thaliana | LysM | RD-kinase | Chitin elicitor signalling *Homologue of CerK1 | Nonself recognition: different origins | chitin | 125 |
| NARK | Glycine max | RD-kinase | Nodule development during nitrogen fixation symbiosis *Orthologue of HAR1 | Symbiotic relationship, recognition of different origins | 126 | ||
| NFR1 | Lotus japonicas | LysM | RD-kinase | Involved in early events during nitrogen fixation symbiosis (symbiotic interactions) *Orthologue of LYK3 | Symbiotic relationship, recognition of different origins | Nod factors | 127 |
| NFR5 | Lotus japonicas | LysM | RD-kinase | Involved in early events during nitrogen fixation symbiosis (symbiotic interactions) *Orthologue of SYM10 | Symbiotic relationship, recognition of different origins | Nod factors | 128 |
| PBS1 | Arabidopsis thaliana | RLCK | RD-kinase | Specific resistance to Pseudomonas syringae pv phaseolicola | Nonself recognition: different origins | 129 | |
| PERK1 | Brassica napus | RLK | RD-kinase | Sensing of cell wall damage due to wounding by pathogens | Nonself recognition: altered self | 60 | |
| Pi-d2 | Oryza sativa | LecrK/S-domain | Non-RD-kinase | R gene that confers resistance to blast disease | Nonself recognition: different origins | 130 | |
| PnLPK | Populus nigra var. Italic (Lombardy poplar) | Lectin-like | RD-kinase | Expressed during wounding | Nonself recognition: altered self | 131 | |
| PR5K | Arabidopsis thaliana | PR5 | Non-RD-kinase | Disease/stress response | Nonself recognition: altered self | 78 | |
| PvRK20-1 | Phaseolus vulgaris | DUF-26 | RD-kinase | Expressed during wounding and plant-microbe interactions | Nonself recognition: altered self | 132 | |
| RFO1 | Arabidopsis thaliana | WAK/EGF | RD-kinase | Involved in resistance response to Fusarium oxysporum | Nonself recognition: different origins | 133 | |
| RKC1 | Arabidopsis thaliana | DUF-26 | RD-kinase | Salicylic acid inducible | Nonself recognition: altered self | 100 | |
| RKS1, 2 | Arabidopsis thaliana | S-domain | RD-kinase | Salicylic acid inducible | Nonself recognition: altered self | 100 | |
| RLK1 | Arabidopsis thaliana | S-domain | RLP | Salicylic acid inducible | Nonself recognition: altered self | 134 | |
| RLK4 | Arabidopsis thaliana | DUF26 | RD-kinase | Salicylic acid inducible | Nonself recognition: altered self | 135 | |
| SFR1, 2 | Brassica oleracea | S-domain | RD-kinase | Defense response signaling, wounding, pathogenic (Xanthomonas campestris, Ralstonia solanacearum) and non-pathogenic (Escherichia coli; E. coli) bacterial infection | Nonself recognition: different origins & altered self | 136 | |
| SYM10 | Pisum sativum | LysM | RD-kinase | Involved in early events during nitrogen fixation symbiosis (symbiotic interactions) *Orthologue of NFR5 | Symbiotic relationship, recognition of different origins | Nod factors | 128 |
| WAK1 to 4 | Arabidopsis thaliana | WAK/EGF | RD-kinase | Cell expansion and disease response, Pseudomonas syringae (compatible), repressed by wounding | Nonself recognition: altered self | 137–140 |
Intracellular sentinels—R proteins. A second class of immune receptors encoded by R genes, which occur mainly intracellularly, has the capacity to perceive isolate-specific pathogen effectors encoded by AVR genes of the pathogen. This perception can occur either directly or indirectly by sensing the host proteins upon which effectors have acted.37,61,82,83 Some R proteins structurally resemble RLK and RLP receptors and probably evolved from PAMP receptors through the loss of a kinase domain.70 However, as exceptions, some intracellular R proteins can consist of one (Pto and Fen) or even two kinase domains (RPG1). Similar to RLKs, R genes subfamilies have evolutionary expansion patterns that show lineage-specific expansions marked by tandem duplicates.64,70 Some R genes are more rapidly evolving as components of the plant immune system, compared to the evolution of P/MAMP receptors.84
It has been recognized that plants might not possess enough R genes to intercept all potential avirulence determinants, due to the diversity of pathogens and their associated effectors. According to Dangl and Jones,85 the likelihood of the known R genes linked to defense being able to recognize all the possible effector signals, where ‘a surprisingly small number of genes mediate recognition of all possible pathogen-encoded effectors’ is questionable. For this reason, bacterial effector recognition has likely evolved as an indirect mechanism (within a complex),62 with a limited repertoire of plant resistance receptors.14
The mechanisms of R gene-mediated immunity may be explained by the ‘gene-for-gene’ genetic model or the ‘guard hypothesis’ molecular model. A ‘guard’ can refer to a typical R protein, whereas the ‘guardee’ represents a target of pathogen effectors.85 Many plant R proteins might be activated indirectly by pathogen-encoded effectors, and not by direct recognition.85 This form of ‘guard hypothesis’ implies that R proteins are able to indirectly recognize pathogen effectors by monitoring the structural integrity of the host cell targets following effector action. The R proteins in question are, thus, activated as sensors or sentinels of ‘pathogen-induced altered-self’ molecular patterns and can thereby potentially perceive the presence of more than one effector protein. Plants are able to sense an ‘infectious-self,’ where the host molecules that are normally not available for recognition (but rather are released following microbe detection, wounding or during infection), are recognized as an altered-self.86 This ‘altered/nonself’ concept can explain how plants can recognize a diverse set of pathogens and pathogen-specific molecules, using a relatively limited number of pathogen receptors. A recent modification to the model describes ‘decoys’ that mimic effector targets in the plant in order to trap the pathogen in a recognition event.87
Much information has been gained from molecular studies of R genes about their organization within genomes and their functional domains. Although polymorphic, and divided into several classes, common structural modules found in intracellular plant R proteins are a C-terminal LRR domain, which is believed to sense microbe-derived signals, and a central nucleotide-binding (NB) domain (Table 4). The NB domain is part of a larger NB-ARC domain (due to its occurrence in plant R proteins, the apoptotic protease-activating factor, APAF-1, and its Caenorhabditis elegans homolog CED-4).88 These NB-ARC domain proteins belong to the family of STAND (signal transduction ATPases with numerous domains) NTPases. STAND ATPases are modular proteins and display a wide range of fusions to domains involved in protein-protein or protein-DNA interactions, small molecule-binding domains, as well as catalytic domains involved in signal transduction.
Table 4.
Some plant R proteins associated with plant-microbe interactions and innate immunity (adapted from Dickinson)141
| Class | Protein Structure | Gene | Plant | Function | Perception | Putative ligands |
| 1 | EILP | Nicotiana tabacum | Non-host disease resistance, induced by hyphal wall component elicitor, Pseudomonas syringae pv. glycinae, Pseudomonas syringae pv. tabaci | Nonself recognition: different origins and altered self | ||
| L | Resistance against Melampsora lini | Nonself recognition: altered self | AvrL567 | |||
| M | Linum usitatissimum | Resistance against Melampsora lini | Nonself recognition: altered self | AM | ||
| TIR-NBS-LRR | P | Resistance against Melampsora lini | Nonself recognition: altered self | AvrP4, AvrP123 | ||
| N | Nicotiana tabacum | Resistance against Tobacco mosaic virus | Nonself recognition: altered self | TMV replicase | ||
| RPP1 | Resistance against Peronospora parasitica | Nonself recognition: altered self | ATR1 | |||
| RPP5 | Arabidopsis thaliana | Resistance against Peronospora parasitica | Nonself recognition: altered self | AvrRPP5 | ||
| RPS4 | Resistance against Pseudomonas syringae | Nonself recognition: altered self | AvrRps4 | |||
| Prf | Solanum esculentum | Resistance against Pseudomonas syringae | Nonself recognition: altered self | AvrPto | ||
| MI | Resistance against Melodogyne incognita | Nonself recognition: altered self | ||||
| Gpa2/Rx1 | Solanum tuberosum | Resistance against Globodera pallida and Potato virus X | Nonself recognition: altered self | |||
| CC-NBS-LRR | RPS2 | Arabidopsis thaliana | Resistance against Pseudomonas syringae | Nonself recognition: altered self | AvrRpt2 | |
| RPS5 | Resistance against Pseudomonas syringae | Nonself recognition: altered self | AvrPphB | |||
| RPM1 | Resistance against Pseudomonas syringae | Nonself recognition: altered self | AvrRpm1, AvrB | |||
| RPP8/HRT | Resistance against Peronospora and Turnip crinkle virus | Nonself recognition: altered self | AvrRPP8 | |||
| NBS-LRR | Bs2 | Capsicum chacoense | Resistance against Xanthomonas campestris | Nonself recognition: altered self | AvrBs2 | |
| Dm3 | Lactuca serriola | Resistance against Bremia lactuca | Nonself recognition: altered self | Avr3 | ||
| I2 | Solanum esculentum | Resistance against Fusarium oxysporum | Nonself recognition: altered self | |||
| Cre3 | Triticum aestivum | Resistance against Heterodera avenae | Nonself recognition: altered self | |||
| Xa1 | Resistance against Xanthomonas oryzae | Nonself recognition: altered self | AvrXa1 | |||
| Pib | Oryza sativa | Resistance against Magnaporthe grisea | Nonself recognition: altered self | |||
| Pi-ta | Resistance against Magnaporthe grisea | Nonself recognition: altered self | AvrPita | |||
| Rp1 | Zea mays | Resistance against Puccinia sorghi | Nonself recognition: altered self | |||
| Mla | Hordeum vulgare | Resistance against Blumeris graminis | Nonself recognition: altered self | AvrMla | ||
| TIR-NBS-LRR-NLS-WRKY | RRS1-R | Arabidopsis thaliana | Resistance against Ralstonia solanacearum | Nonself recognition: altered self | PopP2 | |
| 2 | LRR-TM (RLP) | Cf-2, Cf-4, Cf-5, Cf-9 | Solanum esculentum | Resistance against Cladosporium fulvum | Nonself recognition: different origins | Avr2, Avr4, Avr5, Avr9 |
| Ve1, Ve2 | Resistance against Verticillium albo-atrum and V. dahliae | Nonself recognition: different origins | ||||
| 3 | Kinase | Pto | Specific resistance to Pseudomonas syringae pv tomato | Nonself recognition: altered self | AvrPto | |
| PtI1 | Specific resistance to Pseudomonas syringae pv tomato | Nonself recognition: altered self | ||||
| PBS1 | Arabidopsis thaliana | Resistance against Pseudomonas syringae | Nonself recognition: altered self | AvrPphB | ||
| Kinase-Kinase | Rpg1 | Hordeum vulgare | Resistance against Puccinia graminis | Nonself recognition: altered self | AvrB | |
| 4 | LRR-TM-Kinase (RLK) | Xa21 | Oryza sativa | Resistance against Xanthamonas oryzae | Nonself recognition: different origins | |
| FLS2 | Arabidopsis thaliana | Resistance against | Nonself recognition: different origins | |||
| 5 | Unique | HS1 pro-1 | Beta vulgaris | Resistance against Heterodera schachtii | Nonself recognition: altered self | |
| 6 | Membrane protein | RPW8 | Arabidopsis thaliana | Resistance against Erysiphe | Nonself recognition: altered self | |
| mlo | Hordeum vulgare | Resistance against Blumeria graminis | Nonself recognition: altered self | |||
| 7 | Toxin reductase | Hm1 | Zea mays | Resistance against Cochliobolus carbonum | Nonself recognition: altered self |
These immune sensor proteins are considered to act as regulatory signal transduction switches where the regulatory switch, scaffolding and occasionally, sensory as well as signal-generating moieties are integrated into a single multidomain protein.89,90 In addition, a structurally diverse range of domains was co-opted during evolution and is found on the N-terminal side of the NB domain. These include the coiled coil (CC, formerly referred to as leucine zipper) or TOLL/interleukin-1 receptor (TIR) domains (Fig. 1). In the case of RRS1, a WRKY DNA-binding domain is located at the C-terminus.
Current data points to the existence of the R proteins in auto-repressed conformations in the absence of a cognate pathogen effector. Direct or indirect recognition of effectors by the polymorphic LRR regions initiates conformational changes and ADP/ATP exchange that renders the respective N-terminal effector domains accessible for interactions with downstream targets.91
Various studies have shown that the C-terminal part of the LRR domain provides pathogen recognition specificity. Hence, the LRR domain has a dual function; it provides auto-inhibition and it translates pathogen recognition into activation. How exactly the LRR domains recognize a pathogen or pathogen action is unclear. Whereas some R proteins bind effectors directly, others require an intermediary host factor(s). This factor often interacts with the N-terminal domain of the R protein and could represent either the virulence target (thereby acting as a guardee) or a target mimic (thereby acting as a decoy).87,92 In this situation, the LRR domain is likely involved in sensing the effector-induced perturbations/altered-self of the target.
Many R genes contain nuclear localization signals.93,94 Recent data indicate that members of the TIR- and CC-type of R protein families function inside the nucleus with nucleocytoplasmic partitioning occurring upon activation. Inside the nucleus, the N-terminal domains of the activated receptor can act as signal relays to transcription factors of the WRKY class.94 The subgroup of R proteins that have co-opted a WRKY-domain, may exhibit direct DNA-binding capacity. Members of the WRKY transcription factors bind to cis-acting regulatory elements called W-boxes and can act as repressors of PAMP-triggered immune responses whilst others act as positive regulators.95,96
De-repression of defense genes could thus amplify PAMP-triggered responses and integrate signals generated by defenseassociated RLKs and R proteins.38 It is considered likely that, in addition to interference with WRKY repressors, other potential convergence points between P/MAMP- and R protein-triggered signaling pathways exist.94 P/MAMP-triggered and mitogen-activated protein kinase (MAPK)-dependent phosphorylation of R proteins can modulate effector-triggered receptor-activity and/or nucleo-cytoplasmic receptor partitioning.38 This offers an explanation of how perception of nonself structures by RLP/Ks and R proteins can lead to transcriptional activation of defense-responsive genes, thereby linking receptor function to transcriptional reprogramming of the host cells for pathogen defense.38
Upregulation of Surveillance and a Primed State
Perception of general elicitors such as LPS and flagellin from bacteria by plants, resembles recognition based on PAMPs in animals.97,98 As all types of plant immunity may be considered innate, the response to PAMPs should be considered as an expression of basal resistance. Genes expressed in Arabidopsis in response to elicitation by flg22,99 indicate that a considerable number of the upregulated genes can be classified as being involved in signal perception (RLK and R genes) and signal transduction. This indicates a positive feedback regulation operating in innate immunity with transcriptional activation of the components involved in the perception and signaling.65 Similar results were found in a transcriptional microarray analysis of genes expressed in Arabidopsis in response to elicitation by LPS (TAIR accession expression set 100808727, Nürnberger T 2006).
Many receptors are transcriptionally activated upon perception of their ligands as well as SA, an effector of SAR.100 Wang et al.101 provided evidence for a model where the RPW8 resistance gene from Arabidopsis could be induced by invasion of powdery mildew isolates and amplified by a SA-feedback circuit, leading to activation of defense responses, via a conserved basal resistance pathway in a non-race-specific manner. In the case of LRR-RLK genes, 49 were found to be upregulated upon either PAMP elicitation and/or pathogen infection.83
Recent data indicate the existence of similar and complementary, but independent perception systems, for different PAMPs (e.g., flg22 and elf18), where perception of one PAMP at a binding site induces higher amounts of binding sites for a second PAMP, and vice versa. Interestingly, the genes for the RLKs FLS2 and EFR, are also induced by LPS and other PAMPs.29 Signaling cascades generated by these independent receptors converge to lead to the activation of plant innate immunity systems.29 If that is generally applicable, plants seem to induce the gene products recognizing the attacking pathogen, thus activating the plant's surveillance system and thereby sensitize or ‘prime’ the rest of the plant to control the spread of the pathogen. It would also imply sensitization of the innate immune system to perceive and respond to the attacking pathogen, analogous to what constitutes local and systemic induced resistance.102 The upregulated expression of RLK and R genes presumably leads to an enhanced sensitivity of the plant to further stimuli, sensing the presence of invading microorganisms with other PAMPs or effector signals, i.e., a primed or sensitized state.1
Dual Functioning in Plant Signaling
There is currently no conclusive evidence for evolutionary conservation of an ancient P/MAMP detection system,32,85,103 and independent recruitment of components during evolution is equally plausible. Moreover, there are also various examples where a specific type of biochemical module or protein appears to be used to fulfill a requirement in more than one process, i.e., dual functioning or ‘multi-tasking.’ Since the pre-existing mechanisms of innate immunity must be specifically utilized to distinguish plant from pathogen, the question arises if it is possible that there might be a sharing of receptors between similar signal molecules, such as a general receptor and/or co-receptor complex for PAMPs with common molecular architectures.
The re-use of highly evolved processes for diverse functions was recently pointed out.103 It was concluded that a form of the ‘guard hypothesis’ best explains how plants can potentially recognize a diverse set of pathogens and pathogen-specific molecules, using a relatively limited number of pathogen receptors, but emphasizes that (in addition to PTI) the evolutionary solution in plants to identify pathogens involves surveillance of ‘self vs. altered-self,’ whereas the evolutionary solution in the adaptive immune response in vertebrates involves detection of foreign antigens.
The work regarding innate immunity, with specific reference to flagellin, has lead to the perception of a one-to-one specific recognition of pathogen (ligand) by the host (receptor) in plants, as also observed in animal and insect adaptive (and innate) immune responses.29,30,99 However, it is not necessarily a specific one-to-one recognition system (such as the Avr-R model). Rather, plant defense mechanisms may follow an adaptation of the guard hypothesis, such as ‘one post, multiple guards’.92 In addition, many R proteins (guards) may perceive the presence of more than one effector protein, whether that protein comes from pathogens with similar or different lifestyles.85
An example of independent recruitment of biochemical components for different functions is the LRR motif. LRR domains are found in transmembrane proteins, -kinases and intracellular R proteins. Collectively, LRRs appear to be involved in a range of processes from development to intercellular communication and to disease resistance.14,104 A number of LRR transmembrane and intracellular proteins act as integral components of ligand perception complexes during ETI.85 In addition, the LRR motif also plays an important role in PRRs in the evolutionary older PTI.83
Is it possible that the same type of receptor could perceive different signals, for example both PAMP signals for defense and MAMP rhizobial signals for symbiosis; although the downstream signaling and the outcome of the plant-microbe interactions are different?10 An important recent discovery is the role that co-receptors might play in receptor-ligand interactions, and it has been suggested that co-receptors might modulate receptor specificity.105 The brassinosteroid receptor BRI1-associated kinase (BAK1), may be upregulated and seems to be a crucial component of plant disease resistance and a positive regulator/general signaling adaptor/signal amplifier in signaling, and exerts this activity independent of brassinosteroids.86,106 In addition to BAK1 interacting with FLS2 in a stimulus-dependent manner, it may also have a common role as an adaptor or co-receptor for the regulation of various other receptors. BAK1 is thus not only associated with developmental regulation through the hormone receptor BRI1, but also has a functional role in PRR-dependent signaling which initiates innate immunity.11,105 Other examples of multifunctional proteins, in addition to BAK1, are compiled in Table 5, and include multiple applications of specific motifs and the re-use of highly evolved processes for diverse functions.
Conclusion
The concept of innate immunity centers on the recognition of ‘nonself‗ components, which is accomplished by sentinels. Plants have evolved a unique metabolic plasticity that allows them to perceive pathogens and unleash effective defense strategies, but how the plant can distinguish between itself and pathogens during a defense response has only recently been explored. This highly evolved surveillance system in plants is able to detect a broad range of signals originating from microbes or damaged plant tissues, initiating sophisticated molecular mechanisms that result in defense. Microbe/pathogen-associated molecular pattern molecules, damage-associated molecular pattern molecules, virulence factors, secreted proteins and processed peptides can be recognized directly or indirectly by this surveillance system. Together, receptor-like kinases or receptor like proteins, as membrane bound signaling molecules with an extracellular receptor domain and intracellular nucleotide binding-leucine-rich repeat proteins as receptors of pathogen-secreted effector proteins, provide an early warning system for the presence of potential pathogens and activate protective immune signaling in plants. Much remains to be discovered, e.g., how different perception mechanisms in plants, based on self, damaged-self, altered-self and nonself, are employed for different threats, and how those signals are transduced within the inter-connected relay system observed during defense responses.
Abbreviations
- Avr
avirulence
- AGP
arabinogalactan protein
- BAK
BRI1-associated kinase
- BRI
brassinosteroid insensitive (receptor)
- CC
coiled coil
- CRR
cysteine-rich repeat
- DAMP
damage-associated molecular pattern
- EFR
elongation factor Tu receptor
- EGF
epidermal growth factor
- ETI
effector-triggered immunity
- ETS
effector-triggered susceptibility
- FLS
flagellin (sensing) binding receptor
- GAP
GPI-associated protein
- GlcNac
N-acetyl glucosamine
- GPI
glycosylphospatidylinositol
- HR
hypersensitive response
- IRAK
interleukin-1 receptor-associated kinase
- JA
jasmonic acid
- LAR
local induced/acquired resistance
- LRR
leucine-rich repeat
- LRX
leucine-rich extensin
- LPS
lipopolysaccharides
- LysM
lysine motif
- MAPK
mitogenactivated protein kinase
- NB
nucleotide-binding
- P/MAMP
pathogen/microbe-associated molecular pattern
- PR
pathogenesisrelated
- PRR
pattern recognition receptor
- PTI
pathogen-triggered immunity
- R
resistance
- RD
arginine-aspartate
- TIR
toll/interleukin-1 receptor
- RPK
receptor protein kinase
- RLCK
receptor-like cytoplasmic kinase
- RLK
receptor-like kinase
- RLP
receptor-like protein
- RSTK
receptor serine/threonine kinase
- RTK
receptor tyrosine kinase
- SA
salicylic acid
- SAR
systemic induced resistance
- SI
self incompatibility
- SLG
self incompatibility locus glycoprotein
- SRK
S receptor kinase
- TMRK
trans membrane receptor kinase
- TNFR
tumor necrosis factor receptor
- WAK
wall-associated kinase
- WRKY
trp-arg-lys-tyr
- PERK
proline-rich extensin-like receptor kinase
Footnotes
Previously published online: www.landesbioscience.com/journals/selfnonself/article/10442
References
- 1.Sanabria NM, Goring D, Nürnberger T, Dubery IA. Self/nonself perception and recognition mechanisms in plants: a comparison of self-incompatibility and innate immunity. New Phytol. 2008;178:503–514. doi: 10.1111/j.1469-8137.2008.02403.x. [DOI] [PubMed] [Google Scholar]
- 2.Takayama S, Isogai A. Self-incompatibility in plants. Annu Rev Plant Biol. 2005;56:467–489. doi: 10.1146/annurev.arplant.56.032604.144249. [DOI] [PubMed] [Google Scholar]
- 3.Wheeler MJ, Franklin-Tong VE. Specifying self-recognition: peptides lead the way. New Phytol. 2007;175:597–599. doi: 10.1111/j.1469-8137.2007.02200.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Zhao Z, Xue Y. Roles of proteolysis in plant self-incompatibility. Annu Rev Plant Biol. 2009;60:21–42. doi: 10.1146/annurev.arplant.043008.092108. [DOI] [PubMed] [Google Scholar]
- 5.Nasrallah JB. Recognition and rejection of self in plant self-incompatibility: comparisons to animal histocompatibility. Trends Immunol. 2005;26:412–418. doi: 10.1016/j.it.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Nürnberger T, Lipka V. Non-host resistance in plants: new insights into an old phenomenon. Molec Plant Pathol. 2005;6:335–345. doi: 10.1111/j.1364-3703.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- 7.Schwessinger B, Zipfel C. News from the frontline: recent insights into PAMP-triggered immunity in plants. Curr Opin Plant Biol. 2008;11:389–395. doi: 10.1016/j.pbi.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Varnier AL, Sanchez L, Vatsa P, Boudesocque L, Garcia-Brugger A, Rabenoelina F, et al. Bacterial rhamnolipids are novel MAMPs conferring resistance to Botrytis cinerea in grapevine. Plant Cell Environ. 2009;32:178–193. doi: 10.1111/j.1365-3040.2008.01911.x. [DOI] [PubMed] [Google Scholar]
- 9.Van Loon LC, Bakker PA, van der Heijdt WH, Wendehenne D, Pugin A. Early responses of tobacco suspension cells to rhizobacterial elicitors of induced systemic resistance. Molec Plant Microbe Interact. 2008;21:1609–1621. doi: 10.1094/MPMI-21-12-1609. [DOI] [PubMed] [Google Scholar]
- 10.Shan L, He P, Sheen J. Endless hide-and-seek: Dynamic co-evolution in plant-bacterium warfare. J Integr Plant Biol. 2007;49:105–111. [Google Scholar]
- 11.Nicaise V, Roux M, Zipfel C. Recent advances in PAMP-triggered immunity against bacteria: Pattern recognition receptors watch over and raise alarm. Plant Physiol. 2009;150:1638–1647. doi: 10.1104/pp.109.139709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim MG, da Cunha L, McFall AJ, Belkhadir Y, DebRoy S, Dangl JL, Mackey D. Two Pseudomonas syringae type III effectors inhibit RIN4-regulated basal defence in Arabidopsis. Cell. 2005;121:749–759. doi: 10.1016/j.cell.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Abramovitch RB, Anderson JC, Martin GB. Bacterial elicitation and evasion of plant innate immunity. Nat Rev Mol Cell Biol. 2006;7:601–611. doi: 10.1038/nrm1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: shaping the evolution of the plant immune response. Cell. 2006;124:803–114. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 15.He P, Shan L, Sheen J. Elicitation and suppression of microbe-associated molecular pattern-triggered immunity in plant-microbe interactions. Cell Microbiol. 2007;9:1385–1396. doi: 10.1111/j.1462-5822.2007.00944.x. [DOI] [PubMed] [Google Scholar]
- 16.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 17.Ntoukakis V, Mucyn TS, Gimenez-Ibanez S, Chapman HC, Gutierrez JR, Balmuth AL, et al. Host inhibition of a bacterial virulence effector triggers immunity to infection. Science. 2009;324:784–787. doi: 10.1126/science.1169430. [DOI] [PubMed] [Google Scholar]
- 18.Boller T, He SH. Innate immunity in plants: An arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science. 2009;324:742–744. doi: 10.1126/science.1171647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boller T, Felix G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- 20.Fluhr R. Sentinels of disease: Plant resistance genes. Plant Physiol. 2001;127:1367–1374. [PMC free article] [PubMed] [Google Scholar]
- 21.Shiu S, Bleecker AB. Plant receptor-like kinase gene family: diversity, function and signaling. Science STKE. 2001;113:22. doi: 10.1126/stke.2001.113.re22. [DOI] [PubMed] [Google Scholar]
- 22.Iriti M, Faoro F. Review of innate and specific immunity in plants and animals. Mycopathologia. 2007;164:57–64. doi: 10.1007/s11046-007-9026-7. [DOI] [PubMed] [Google Scholar]
- 23.Thordal-Christensen H. Fresh insights into processes of nonhost resistance. Curr Opin Plant Biol. 2003;6:351–357. doi: 10.1016/s1369-5266(03)00063-3. [DOI] [PubMed] [Google Scholar]
- 24.Gust AA, Biswas R, Lenz HD, Rauhut T, Ranf S, Kemmerling B, et al. Bacteria-derived peptidoglycans constitute pathogen-associated molecular patterns triggering innate immunity in Arabidopsis. J Biol Chem. 2007;282:32338–32348. doi: 10.1074/jbc.M704886200. [DOI] [PubMed] [Google Scholar]
- 25.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 26.Jones DA, Takemoto D. Plant innate immunity—direct and indirect recognition of general and specific pathogen-associated molecules. Curr Opin Immunol. 2004;16:48–62. doi: 10.1016/j.coi.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Aslam SN, Erbs G, Morrissey KL, Newman M, Chinchilla D, Boller T, et al. Microbe-associated molecular pattern (MAMP) signatures, synergy, size and charge: influences onperception or mobility and the host defence responses. Mol Plant Pathol. 2009;10:375–387. doi: 10.1111/j.1364-3703.2009.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gómez-Gómez L, Boller T. FLS2: an LRR receptorlike kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell. 2000;5:1003–1011. doi: 10.1016/s1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- 29.Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JDG, Boller T, et al. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125:749–760. doi: 10.1016/j.cell.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 30.Gómez-Gómez L, Boller T. Flagellin perception: a paradigm for innate immunity. Trends Plant Sci. 2002;7:251–256. doi: 10.1016/s1360-1385(02)02261-6. [DOI] [PubMed] [Google Scholar]
- 31.Shiu S-H, Karlowski WM, Pan R, Tzeng Y-H, Mayer KFX, Li W-H. Comparative analysis of the receptor-like kinase family in Arabidopsis and Rice. Plant Cell. 2004;16:1220–1234. doi: 10.1105/tpc.020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ingle RA, Carstens M, Denby KJ. PAMP recognition and the plant-pathogen arms race. BioEssays. 2006;28:880–889. doi: 10.1002/bies.20457. [DOI] [PubMed] [Google Scholar]
- 33.Umemoto N, Kakitani M, Iwamatsu A, Yoshikawa M, Yamaoka N, Ishida I. The structure and function of a soybean beta-glucan-elicitor-binding protein. Proc Natl Acad Sci USA. 1997;94:1029–1034. doi: 10.1073/pnas.94.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ron M, Avni A. The receptor for the fungal elicitor ethylene-inducing xylanase is a member of a resistance-like gene family in tomato. Plant Cell. 2004;16:1604–1615. doi: 10.1105/tpc.022475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaku H, Nishizawa Y, Ishii-Minami N, Akimoto-Tomiyama C, Dohmae N, Takio N, et al. Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc Natl Acad Sci USA. 2006;103:11086–11091. doi: 10.1073/pnas.0508882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Liu X, Dai L, Wang G. Recent progress in elucidating the structure, function and evolution of disease resistance genes in plants. J Genet Genomics. 2007;34:765–776. doi: 10.1016/S1673-8527(07)60087-3. [DOI] [PubMed] [Google Scholar]
- 37.Tameling WIL, Takken FLW. Resistance proteins: scouts of the plant innate immune system. Eur J Plant Pathol. 2008;121:243–255. [Google Scholar]
- 38.Shan L, He P, Li J, Heese A, Peck SC, Nürnberger T, et al. Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe. 2008;4:17–27. doi: 10.1016/j.chom.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen Q-H, Schulze-Lefert P. Rumble in the nuclear jungle: compartmentalization, trafficking and nuclear action of plant immune receptors. EMBO J. 2007;26:4293–4301. doi: 10.1038/sj.emboj.7601854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mishina TE, Zeier J. Pathogen-associated molecular pattern recognition rather than development of tissue necrosis contributes to bacterial induction of systemic acquired resistance in Arabidopsis. Plant J. 2007;50:500–513. doi: 10.1111/j.1365-313X.2007.03067.x. [DOI] [PubMed] [Google Scholar]
- 41.Naito K, Taguchi F, Suzuki T, Inagaki Y, Toyoda K, Shiraishi T, et al. Amino acid sequence of bacterial microbe-associated molecular pattern flg22 is required for virulence. Mol Plant Microbe Interact. 2008;21:1165–1174. doi: 10.1094/MPMI-21-9-1165. [DOI] [PubMed] [Google Scholar]
- 42.Halim VA, Altmann S, Ellinger D, Eschen-Lippold L, Miersch O, Schell D, et al. PAMP-induced defense responses in potato require both salicylic acid and jasmonic acid. Plant J. 2009;57:230–242. doi: 10.1111/j.1365-313X.2008.03688.x. [DOI] [PubMed] [Google Scholar]
- 43.Tsuda K, Sato M, Glazebrook J, Cohen JD, Katagiri F. Interplay between MAMP-triggered and SA-mediated defense responses. Plant J. 2008;53:763–775. doi: 10.1111/j.1365-313X.2007.03369.x. [DOI] [PubMed] [Google Scholar]
- 44.Humphrey TV, Bonetta DT, Goring DR. Sentinels at the wall: cell wall receptors and sensors. New Phytologist. 2007;176:7–21. doi: 10.1111/j.1469-8137.2007.02192.x. [DOI] [PubMed] [Google Scholar]
- 45.De Lorenzo G, D'Ovidio R, Cervone F. The role of polygalacturonase-inhibiting proteins (PGIPS) in defense against pathogenic fungi. Annu Rev Phytopathol. 2001;39:313–335. doi: 10.1146/annurev.phyto.39.1.313. [DOI] [PubMed] [Google Scholar]
- 46.Cote F, Hahn MG. Oligosaccharins—structures and signal-transduction. Plant Mol Biol. 1994;26:1379–1411. doi: 10.1007/BF00016481. [DOI] [PubMed] [Google Scholar]
- 47.Mattei B, Galletti R, Manfredini C, Pontiggia D, Salvi G, Spadoni S, et al. Recognition and signaling in the cell wall: The case of endopolygalacturonase, PGIP and oligogalacturonides. Plant Biosystems. 2005;139:24–27. [Google Scholar]
- 48.Chassot C, Nawrath C, Métraux J-P. Cuticular defects lead to full immunity to a major plant pathogen. Plant J. 2007;49:972–980. doi: 10.1111/j.1365-313X.2006.03017.x. [DOI] [PubMed] [Google Scholar]
- 49.Caño-Delgado A, Penfield S, Smith C, Catley M, Bevan M. Reduced cellulose synthesis invokes lignification and defense responses in Arabidopsis thaliana. Plant J. 2003;34:351–362. doi: 10.1046/j.1365-313x.2003.01729.x. [DOI] [PubMed] [Google Scholar]
- 50.Chen Z, Hong X, Zhang H, Wang Y, Li X, Zhu JK, et al. Disruption of the cellulose synthase gene, AtCesA8/IRX1 enhances drought and osmotic stress tolerance in Arabidopsis. Plant J. 2005;43:273–283. doi: 10.1111/j.1365-313X.2005.02452.x. [DOI] [PubMed] [Google Scholar]
- 51.Hernandez-Blanco C, Feng DX, Hu J, Sanchez-Vallet A, Deslandes L, Llorente F, et al. Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell. 2007;19:890–903. doi: 10.1105/tpc.106.048058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nickle TC, Meinke DW. A cytokinesis-defective mutant of Arabidopsis (cyt1) characterized by embryonic lethality, incomplete cell walls and excessive callose accumulation. Plant J. 1998;15:321–332. doi: 10.1046/j.1365-313x.1998.00212.x. [DOI] [PubMed] [Google Scholar]
- 53.Ellis C, Karafyllidis I, Wasternack C, Turner JG. The Arabidopsis mutant cev 1 links cell wall signaling to jasmonate and ethylene responses. Plant Cell. 2002;14:1557–1566. doi: 10.1105/tpc.002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Creelman RA, Tierney ML, Mullet JE. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci USA. 1992;89:4938–4941. doi: 10.1073/pnas.89.11.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Creelman RA, Mullet JE. Jasmonic acid distribution and action in plants: Regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci USA. 1995;92:4114–4119. doi: 10.1073/pnas.92.10.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Penninckx IA, Thomma BP, Buchala A, Metraux JP, Broekaert WF. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell. 1998;10:2103–2113. doi: 10.1105/tpc.10.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borner GHH, Sherrier DJ, Stevens TJ, Arkin IT, Dupree P. Prediction of glycosylphosphatidylinositol-anchored proteins in Arabidopsis. A genomic analysis. Plant Physiol. 2002;129:486–499. doi: 10.1104/pp.010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anderson CM, Wagner TA, Perret M, He ZH, He D, Kohorn BD. WAKs: cell wall-associated kinases linking the cytoplasm to the extracellular matrix. Plant Molec Biol. 2001;47:197–206. [PubMed] [Google Scholar]
- 59.Decreux A, Messiaen Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation. Plant Cell Physiol. 2005;46:268–278. doi: 10.1093/pcp/pci026. [DOI] [PubMed] [Google Scholar]
- 60.Silva NF, Goring DR. The proline-rich, extensin-like receptor kinase-1 (PERK1) gene is rapidly induced by wounding. Plant Mol Biol. 2002;50:667–685. doi: 10.1023/a:1019951120788. [DOI] [PubMed] [Google Scholar]
- 61.Altenbach D, Robatzek S. Pattern recognition receptors: from the cell surface to intracellular dynamics. Mol Plant Micr Interact. 2007;20:1031–1039. doi: 10.1094/MPMI-20-9-1031. [DOI] [PubMed] [Google Scholar]
- 62.Shirasu K. The HSP90-SGT1 chaperone complex for NLR immune sensors. Annu Rev Plant Biol. 2009;60:139–164. doi: 10.1146/annurev.arplant.59.032607.092906. [DOI] [PubMed] [Google Scholar]
- 63.Shiu S, Bleecker AB. Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol. 2003;132:530–543. doi: 10.1104/pp.103.021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fritz-Laylin LK, Krishnamurthy N, Tör M, Sjölander KV, Jones DG. Phylogenomic analysis of the receptor-like proteins of rice and Arabidopsis. Plant Physiol. 2005;138:611–623. doi: 10.1104/pp.104.054452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JDG, Felix G, et al. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;428:764–767. doi: 10.1038/nature02485. [DOI] [PubMed] [Google Scholar]
- 66.Chinchilla D, Bauer Z, Regenass M, Boller T, Felix G. The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell. 2006;18:465–476. doi: 10.1105/tpc.105.036574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Becraft PW. Receptor kinase signaling in plant development. Annu Rev Cell Dev Biol. 2002;18:163–192. doi: 10.1146/annurev.cellbio.18.012502.083431. [DOI] [PubMed] [Google Scholar]
- 68.Afzal AJ, Wood AJ, Lightfoot DA. Plant receptor-like serine threonine kinases: roles in signaling and plant defense. Mol Plant Micr Interact. 2008;21:507–517. doi: 10.1094/MPMI-21-5-0507. [DOI] [PubMed] [Google Scholar]
- 69.Shiu S, Bleecker AB. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc Natl Acad Sci USA. 2001;98:10763–10768. doi: 10.1073/pnas.181141598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dardick C, Ronald P. Plant and animal pathogen recognition receptors signal through non-RD kinases. PLoS Pathogens. 2006;2:14–28. doi: 10.1371/journal.ppat.0020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walker JC. Structure and function of the receptor-like kinases of higher plants. Plant Mol Biol. 1994;26:1599–1609. doi: 10.1007/BF00016492. [DOI] [PubMed] [Google Scholar]
- 72.Nasrallah JB, Yu S, Nasrallah ME. Self-incompatibility genes of Brassica oleracea: Expression, isolation and structure. Proc Natl Acad Sci USA. 1988;85:5551–5555. doi: 10.1073/pnas.85.15.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Torii KU, Clark SE. Receptor-like kinases in plant development. Adv Bot Res. 2000;32:225–267. [Google Scholar]
- 74.Takemoto D, Hayashi M, Doke N, Nishimura M, Kawakita K. Isolation for the gene for EILP, an elicitor-inducible LRR receptor-like protein from tobacco by differential display. Plant Cell Physiol. 2000;41:458–464. doi: 10.1093/pcp/41.4.458. [DOI] [PubMed] [Google Scholar]
- 75.Kobe B, Deisenhofer J. A structural basis of the interactions between leucine-rich repeats and protein ligands. Nature. 1995;374:183–186. doi: 10.1038/374183a0. [DOI] [PubMed] [Google Scholar]
- 76.Wagner TA, Kohorn BD. Wall-associated kinases are expressed throughout plant development and are required for cell expansion. Plant Cell. 2001;13:303–318. doi: 10.1105/tpc.13.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Loetscher H, Pan YE, Lahm H, Gentz R, Brockhaus M, Tabuchi H, et al. Molecular cloning and expression of the human 55 kD tumor necrosis factor receptor. Cell. 1990;61:351–359. doi: 10.1016/0092-8674(90)90815-v. [DOI] [PubMed] [Google Scholar]
- 78.Wang X, Zafian P, Choudhary M, Lawton M. The PR5K receptor protein kinase from Arabidopsis thaliana is structurally related to a family of plant defence proteins. Proc Natl Acad Sci USA. 1996;93:2598–2602. doi: 10.1073/pnas.93.6.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim YS, Lee JH, Yoon GM, Cho HS, Park SW, Suh MC, et al. CHRK1, a chitinase-related receptor-like kinase in tobacco. Plant Physiol. 2000;123:905–915. doi: 10.1104/pp.123.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen Z. A superfamily of proteins with novel cysteine-rich repeats. Plant Physiol. 2001;126:473–476. doi: 10.1104/pp.126.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goff KE, Ramonell KM. The role and regulation of receptor-like kinases in plant defense. Gene Regul Syst Bio. 2007;1:167–175. [PMC free article] [PubMed] [Google Scholar]
- 82.Martin GB, Bogdanove AJ, Sessa G. Understanding the functions of plant disease resistance proteins. Annu Rev Plant Biol. 2003;54:23–61. doi: 10.1146/annurev.arplant.54.031902.135035. [DOI] [PubMed] [Google Scholar]
- 83.Nürnberger T, Kemmerling B. Receptor protein kinases—pattern recognition receptors in plant immunity. Trends Plant Sci. 2006;11:519–522. doi: 10.1016/j.tplants.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 84.Bent AF, Mackey D. Elicitors, effectors and R genes: the new paradigm and a lifetime supply of questions. Annu Rev Phytopathol. 2007;45:399–436. doi: 10.1146/annurev.phyto.45.062806.094427. [DOI] [PubMed] [Google Scholar]
- 85.Dangl JL, Jones JD. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- 86.Zipfel C. Pattern-recognition receptors in plant innate immunity. Curr Opin Immunol. 2008;20:10–16. doi: 10.1016/j.coi.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 87.Van der Hoorn RAL, Kamoun S. From guard to decoy: a new model for perception of plant pathogen effectors. Plant Cell. 2008;20:2009–2017. doi: 10.1105/tpc.108.060194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van der Biezen EA, Jones JDG. The NB-ARC domain; a novel signaling motif shared by plant resistance gene products and regulators of cell death in animals. Curr Biol. 1998;8:226–228. doi: 10.1016/s0960-9822(98)70145-9. [DOI] [PubMed] [Google Scholar]
- 89.Leipe DD, Koonin EV, Aravind I. STAND, a class of P-loop NTPases including animal and plant regulators of programmed cell death: multiple, complex domain archotechtures, unusual phyletic patterns and evolution by horizontal gene transfer. J Mol Biol. 2004;343:1–28. doi: 10.1016/j.jmb.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 90.Takken FLW, Albrecht M, Tameling WIL. Resistance proteins: molecular switches of plant defense. Curr Opin Plant Biol. 2006;9:383–390. doi: 10.1016/j.pbi.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 91.Takken FLW, Tameling WIL. To nibble at plant resistance proteins. Science. 2009;324:744–746. doi: 10.1126/science.1171666. [DOI] [PubMed] [Google Scholar]
- 92.Marathe R, Dinesh-Kumar S. Plant defense: One post, multiple guards? Mol Cell. 2003;11:284–286. doi: 10.1016/s1097-2765(03)00072-8. [DOI] [PubMed] [Google Scholar]
- 93.Meyers BC, Kozik A, Griego A, Kuang HH, Michelmore RW. Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell. 2003;15:809–834. doi: 10.1105/tpc.009308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shen Q-H, Saijo Y, Mauch S, Biskup C, Bieri S, Keller B, et al. Nuclear activity of MLA immune receptors links isolate-specific and basal disease-resistance responses. Science. 2007;315:1098–1103. doi: 10.1126/science.1136372. [DOI] [PubMed] [Google Scholar]
- 95.Du L, Chen Z. Identification of genes encoding receptor-like protein kinases as possible targets of pathogen- and salicyclic acid induced WRKY DNA-binding proteins in Arabidopsis. Plant J. 2000;24:837–847. doi: 10.1046/j.1365-313x.2000.00923.x. [DOI] [PubMed] [Google Scholar]
- 96.Ulker B, Somssich IE. WRKY trancription factors: from DNA binding towards biological function. Curr Opin Plant Biol. 2004;7:491–498. doi: 10.1016/j.pbi.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 97.Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 98.Zipfel C, Felix G. Plants and animals: a different taste for microbes? Curr Opin Plant Biol. 2005;8:353–360. doi: 10.1016/j.pbi.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 99.Navarro L, Zipfel C, Rowland O, Keller I, Robatzek S, Boller T, et al. The transcriptional innate immune response to flg22; interplay and overlap with Avr gene-dependent defense responses and bacterial pathogenesis. Plant Physiol. 2004;135:113–128. doi: 10.1104/pp.103.036749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ohtake Y, Takahashi T, Komeda Y. Salicylic acid induces the expression of a number of receptor-like kinase genes in Arabidopsis thaliana. Plant Cell Physiol. 2000;41:1038–1044. doi: 10.1093/pcp/pcd028. [DOI] [PubMed] [Google Scholar]
- 101.Wang H, Chevalier D, Larue C, Ki Cho S, Walker JC. The protein phosphatases and protein kinases of Arabidopsis thaliana. In: Somerville CR, Meyerowitz EM, editors. The Arabidopsis Book. Rockville MD: American Society of Plant Biologists; 2007. ( www.aspb.org/publications/arabidopsis) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sanabria NM, Dubery IA. Differential display profiling of the Nicotiana response to LPS reveals elements of plant basal resistance. Biochem Biophys Res Commun. 2006;344:1001–1007. doi: 10.1016/j.bbrc.2006.03.216. [DOI] [PubMed] [Google Scholar]
- 103.Ausubel F. Are innate immune signaling pathways in plants and animals conserved? Nat Immunol. 2005;6:973–979. doi: 10.1038/ni1253. [DOI] [PubMed] [Google Scholar]
- 104.Zhang X. Leucine-rich repeat receptor-like kinases in plants. Plant Mol Biol Rep. 1998;16:301–311. [Google Scholar]
- 105.Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JDG, et al. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defense. Nature. 2007;448:497–501. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- 106.Kemmerling B, Schwedt A, Rodriguez P, Mazzotta S, Frank M, Abu Qamar S, et al. A brassinolide-independent role for the BRI1 associated receptor kinase 1 (BAK1) in plant cell death control. Curr Biol. 2007;17:1116–1122. doi: 10.1016/j.cub.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 107.Scheer JM, Ryan CA. The systemin receptor SR160 from Lycopersicon peruvianum is a member of the LRR receptor kinase family. Proc Natl Acad Sci USA. 2002;99:9585–9590. doi: 10.1073/pnas.132266499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meng X, Bonasera JM, Kim JF, Nissinen RM, Beer SV. Apple proteins that interact with DspA/E, a pathogenicity effector of Erwinia amylovora, the fire blight pathogen. Molec Plant Micr Interact. 2006;19:53–61. doi: 10.1094/MPMI-19-0053. [DOI] [PubMed] [Google Scholar]
- 109.Torii KU, Mitsukawa N, Oosumi T, Matsuura Y, Yokoyama R, Whittier RF, et al. The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular Leucine-rich repeats. Plant Cell. 1996;8:735–746. doi: 10.1105/tpc.8.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nishimura R, Hayashi M, Wu GJ, Kouchi H, Imaizumi-Anraku H, Murakami Y, et al. HAR1 mediates systemic regulation of symbiotic organ development. Nature. 2002;420:426–429. doi: 10.1038/nature01231. [DOI] [PubMed] [Google Scholar]
- 111.Komjanc M, Festi S, Rizzotti L, Cattivelli L, Cervone F, De Lorenzo G. A leucine-rich repeat receptor-like protein kinase (LRPKm1) gene is induced in Malus x domestica by Venturia inaequalis infection and salicylic acid treatment. Plant Mol Biol. 1999;40:945–957. doi: 10.1023/a:1006275924882. [DOI] [PubMed] [Google Scholar]
- 112.Endre G, Kereszt A, Kevei Z, Mihacea S, Kaló P, Kiss GB. A receptor kinase gene regulating symbiotic nodule development. Nature. 2002;417:962–966. doi: 10.1038/nature00842. [DOI] [PubMed] [Google Scholar]
- 113.Ryan CA, Huffaker A, Yamaguchi Y. New insights into innate immunity in Arabidopsis. Cell Microbiol. 2007;9:1902–1908. doi: 10.1111/j.1462-5822.2007.00991.x. [DOI] [PubMed] [Google Scholar]
- 114.Robatzek S, Somssich IE. Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev. 2002;16:1139–1149. doi: 10.1101/gad.222702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Montoya T, Nomura T, Farrar K, Kaneta T, Yokota T, Bishop GJ. Cloning the tomato Curl3 gene highlights the putative dual role of the leucine-rich repeat receptor kinase tBRI1/SR160 in plant steroid hormone and peptide hormone signaling. Plant Cell. 2002;14:3163–3176. doi: 10.1105/tpc.006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stracke S, Kistner C, Yoshida S, Mulder L, Sato S, Kaneko T, et al. A plant receptor-like kinase required for both bacterial and fungal symbiosis. Nature. 2002;417:959–962. doi: 10.1038/nature00841. [DOI] [PubMed] [Google Scholar]
- 117.Song WY, Wang GL, Chen LL, Kim HS, Pi LY, Holsten T, et al. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science. 1995;270:1804–1806. doi: 10.1126/science.270.5243.1804. [DOI] [PubMed] [Google Scholar]
- 118.Sun X, Cao Y, Yang Z, Xu C, Li X, Wang S. Xa26, a gene conferring resistance to Xanthomonas oryzae pv. oryzae in rice, encodes an LRR receptor kinase-like protein. Plant J. 2004;37:517–527. doi: 10.1046/j.1365-313x.2003.01976.x. [DOI] [PubMed] [Google Scholar]
- 119.Czernic P, Visser B, Sun W, Savoure A, Deslandes L, Marco Y, et al. Characterisation of an Arabidopsis thaliana receptor-like protein kinase gene activated by oxidative stress and pathogen attack. Plant J. 1999;18:321–327. doi: 10.1046/j.1365-313x.1999.00447.x. [DOI] [PubMed] [Google Scholar]
- 120.Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, et al. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA. 2007;104:19613–19618. doi: 10.1073/pnas.0705147104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Riou C, Hervé C, Pacquit V, Dabos P, Bernard Lescure B. Expression of an Arabidopsis lectin kinase receptor gene, lecRK-a1, is induced during senescence, wounding and in response to oligogalacturonic acids. Plant Physiol Biochem. 2002;40:431–438. [Google Scholar]
- 122.Feuillet C, Schachermayr G, Keller B. Molecular cloning of a new receptor-like kinase gene encoded at the Lr10 disease resistance locus of wheat. Plant J. 1997;11:45–52. doi: 10.1046/j.1365-313x.1997.11010045.x. [DOI] [PubMed] [Google Scholar]
- 123.Feuillet C, Reuzeau C, Kjellbom P, Keller B. Molecular characterization of a new type of receptor-like kinase (wlrk) gene family in wheat. Plant Mol Biol. 1998;37:943–953. doi: 10.1023/a:1006062016593. [DOI] [PubMed] [Google Scholar]
- 124.Limpens E, Franken C, Smit P, Willemse J, Bisseling T, Geurts R. LysM domain receptor kinases regulating rhizobial Nod factor-induced infection. Science. 2003;302:630–633. doi: 10.1126/science.1090074. [DOI] [PubMed] [Google Scholar]
- 125.Wan JW, Zhang X, Neecer D, Ramonell KM, Clough S, Kim S, et al. A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell. 2008;20:471–481. doi: 10.1105/tpc.107.056754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Searle IR, Men AE, Laniya TS, Buzas DM, Iturbe-Ormaetxe I, Carroll BJ. Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase. Science. 2003;299:109–112. doi: 10.1126/science.1077937. [DOI] [PubMed] [Google Scholar]
- 127.Radutoiu S, Madsen LH, Madsen EB, Felle HH, Umehara Y, Gronlund M, et al. Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature. 2003;425:585–592. doi: 10.1038/nature02039. [DOI] [PubMed] [Google Scholar]
- 128.Madsen EB, Madsen LH, Radutoiu S, Olbryt M, Rakwalska M, Szczyglowski K, et al. A receptor kinase gene of the LysM type is involved in legume perception of rhizobial signals. Nature. 2003;425:637–640. doi: 10.1038/nature02045. [DOI] [PubMed] [Google Scholar]
- 129.Swiderski MR, Innes RW. The Arabidopsis PBS1 resistance gene encodes a member of a novel protein kinase subfamily. Plant J. 2001;26:101–112. doi: 10.1046/j.1365-313x.2001.01014.x. [DOI] [PubMed] [Google Scholar]
- 130.Chen X, Shang J, Chen D, Lei C, Zou Y, Zhai W, et al. A B-lectin receptor kinase gene conferring rice blast resistance. Plant J. 2006;46:794–804. doi: 10.1111/j.1365-313X.2006.02739.x. [DOI] [PubMed] [Google Scholar]
- 131.Nishiguchi M, Yoshida Y, Sumizono T, Tazaki K. A receptor-like protein kinase with a lectin-like domain from Lombardy poplar: gene expression in response to wounding and characterization of phosphorylation activity. Mol Genet Genom. 2002;267:506–514. doi: 10.1007/s00438-002-0683-4. [DOI] [PubMed] [Google Scholar]
- 132.Lange J, Xie ZP, Broughton WJ, Vögeli-Lange R, Boller T. A gene encoding a receptor-like protein kinase in the roots of common bean is differentially regulated in response to pathogens, symbionts and nodulation factors. Plant Sci. 1999;142:133–145. [Google Scholar]
- 133.Diener AC, Ausubel FM. RESISTANCE TO FUSARIUM OXYSPORUM 1, a dominant Arabidopsis disease-resistance gene, is not race specific. Genetics. 2005;171:305–321. doi: 10.1534/genetics.105.042218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Walker JC. Receptor-like protein kinase genes of Arabidopsis thaliana. Plant J. 1993;3:451–456. doi: 10.1111/j.1365-313x.1993.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 135.Coello P, Sassen A, Haywood V, Davis KR, Walker JC. Biochemical characterisation and expression of RLK4, a receptor-like kinase from Arabidopsis thaliana. Plant Sci. 1999;142:83–91. [Google Scholar]
- 136.Pastuglia M, Roby D, Dumas C, Cock JM. Rapid induction by wounding and bacterial infection of an S gene family receptor-like kinase gene in Brassica oleracea. Plant Cell. 1997;9:49–60. doi: 10.1105/tpc.9.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kohorn BD, Lane S, Smith TA. An Arabidopsis serine/threonine kinase homologue with an epidermal growth factor repeat selected in yeast for its specificity for a thylakoid membrane protein. Proc Natl Acad Sci USA. 1992;89:10989–10992. doi: 10.1073/pnas.89.22.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.He Z-H, Fujiki M, Kohorn BD. A cell-wall associated receptor-like protein kinase. J Biol Chem. 1996;271:19789–19793. doi: 10.1074/jbc.271.33.19789. [DOI] [PubMed] [Google Scholar]
- 139.He Z-H, He D, Kohorn BD. Requirements for the induced expression of a cell wall associated receptor kinase for survival during the pathogen response. Plant J. 1998;14:55–63. doi: 10.1046/j.1365-313x.1998.00092.x. [DOI] [PubMed] [Google Scholar]
- 140.He Z-H, Cheeseman I, He D, Kohorn BD. A cluster of five cell wall-associated receptor kinase genes, Wak1-5, are expressed in specific organs of Arabidopsis. Plant Mol Biol. 1999;39:1189–1196. doi: 10.1023/a:1006197318246. [DOI] [PubMed] [Google Scholar]
- 141.Dickinson M. Molecular plant pathology. New York: BIOS Scientific Publishers; 2003. Resistance genes; pp. 160–173. [Google Scholar]
- 142.Shpak ED, Lakeman MB, Torii KU. Dominantnegative receptor uncovers redundancy in the Arabidopsis ERECTA leucine-rich repeat receptor-like kinase signaling pathway that regulates organ shape. Plant Cell. 2003;15:1095–1110. doi: 10.1105/tpc.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Godiard L, Sauviac L, Torii KU, Grenon O, Mangin B, Grimsley NH. ERECTA, an LRR receptor-like kinase protein controlling development pleiotropically affects resistance to bacterial wilt. Plant J. 2003;36:353–365. doi: 10.1046/j.1365-313x.2003.01877.x. [DOI] [PubMed] [Google Scholar]
- 144.Buhot N, Gomes E, Milat ML, Ponchet M, Maroin D, Lequeu J, et al. Modulation of the biological activity of a tobacco LTP1 by lipid complexation. Mol Biol Cell. 2004;15:5047–5052. doi: 10.1091/mbc.E04-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Knogge W, Scheel D. LysM receptor recognize friend and foe. Proc Natl Acad Sci USA. 2006;103:10829–10830. doi: 10.1073/pnas.0604601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nakhamchik A, Zhao Z, Provart NJ, Shiu S-H, Keatley SK, Cameron RK, et al. A comprehensive expression analysis of the Arabidopsis proline-rich extensin-like receptor kinase family using bioinformatic and experimental approaches. Plant Cell Physiol. 2004;45:1875–1881. doi: 10.1093/pcp/pch206. [DOI] [PubMed] [Google Scholar]
- 147.Haffani YZ, Silva NF, Sewter SK, Aldea MG, Zhao Z, Nakhamchik A, et al. Altered expression of PERK receptor kinases in Arabidopsis leads to changes in growth and floral organ formation. Plant Signal Behav. 2006;1:251–260. doi: 10.4161/psb.1.5.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Block A, Li G, Fu ZQ, Alfano JR. Phytopathogen type III effector weaponry and their plant targets. Curr Opin Plant Biol. 2008;11:396–403. doi: 10.1016/j.jbi.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tör M, Lotze MT, Holton N. Receptor-mediated signalling in plants: molecular patterns and programmes. J Exp Bot. 2009;60:3645–3654. doi: 10.1093/jxb/erp233. [DOI] [PMC free article] [PubMed] [Google Scholar]