Abstract
Receptor-mediated transmembrane signaling plays an important role in health and disease. Recent significant advances in our understanding of the molecular mechanisms linking ligand binding to receptor activation revealed previously unrecognized striking similarities in the basic structural principles of function of numerous cell surface receptors. In this work, I demonstrate that the Signaling Chain Homooligomerization (SCHOOL)-based mechanism represents a general biological mechanism of transmembrane signal transduction mediated by a variety of functionally unrelated single- and multichain activating receptors. within the SCHOOL platform, ligand binding-induced receptor clustering is translated across the membrane into protein oligomerization in cytoplasmic milieu. This platform resolves a long-standing puzzle in transmembrane signal transduction and reveals the major driving forces coupling recognition and activation functions at the level of protein-protein interactions—biochemical processes that can be influenced and controlled. The basic principles of transmembrane signaling learned from the SCHOOL model can be used in different fields of immunology, virology, molecular and cell biology and others to describe, explain and predict various phenomena and processes mediated by a variety of functionally diverse and unrelated receptors. Beyond providing novel perspectives for fundamental research, the platform opens new avenues for drug discovery and development.
Key words: immune signaling, T cell activation, B cell activation, T cell receptor, TCR, B cell receptor, BCR, Fc receptors, natural killer cell receptors, NK receptors, triggering receptors expressed on myeloid cells, TREM, platelet collagen receptor, glycoprotein VI, GPVI, intrinsically disordered proteins, multichain immune recognition receptors, MIRR, immunoreceptor tyrosine-based activation motif, ITAM, signaling chain homooligomerization model, SCHOOL model, cell activation, mechanistic model, protein-protein interactions, transmembrane interactions, cytoplasmic homointeractions, receptor clustering, receptor orientation, ligand-receptor complex lifetime, signal propagation, receptor tyrosine kinases
Cell surface receptors are integral membrane proteins and, as such, consist of three basic domains: extracellular (EC) ligand-binding domains, transmembrane (TM) domains and cytoplasmic (CYTO) signaling (or effector) domains. Upon recognition and binding of a specific ligand, cell surface receptors transmit this information into the interior of the cell, activating intracellular signaling pathways and resulting in a cellular response such as proliferation, differentiation, apoptosis, degranulation, the secretion of preformed and newly formed mediators, phagocytosis of particles, endocytosis, cytotoxicity against target cells, etc. Receptor-mediated TM signaling plays an important role in health and disease.1,2 Thus, modulation of signal transduction across the plasma membrane that can be used to modulate the cell response is not only of fundamental importance but has important clinical applications as well. However, until recently, there was no clear molecular understanding of the mechanisms underlying TM signaling. This significantly impeded progress in fundamental studies in biology and life sciences as well as in the development of new therapies.
Here I describe a general platform for receptor-mediated signaling, the Signaling Chain Homooligomerization (SCHOOL) platform. Within this platform, receptor oligomerization (clustering) is induced or tuned upon multivalent ligand binding outside the cell. Then, it is translated across the membrane into protein oligomerization inside the cell with formation of competent signaling oligomers in CYTO milieu being necessary and sufficient to trigger receptor activation. This uncovers for the first time the major driving forces behind coupling recognition and activation functions at the level of protein-protein interactions—biochemical processes that can be influenced and controlled. The platform also reveals previously unrecognized striking similarities in the basic mechanistic principles of function of numerous functionally diverse and unrelated cell surface receptors.
Structural Classification of Cell Surface Receptors
Based on location of binding and signaling (effector) domains, functionally diverse and unrelated cell surface receptors can be structurally classified into two main families: those in which binding and signaling domains are located on the same protein chain, the so-called single-chain receptors (SRs, Fig. 1), and those in which binding and signaling domains are intriguingly located on separate subunits, the so-called multichain receptors (Fig. 2). Because many multichain activating receptors are immune receptors, they are all commonly referred to as multichain immune recognition receptors (MIRRs).2–4
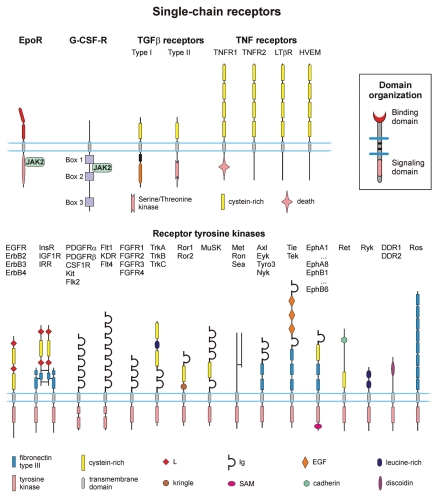
Figure 1.
The extracellular portion of the receptors is on top and the cytoplasmic portion is on bottom. The lengths of the receptors as shown are only approximately to scale. The inset shows SR domain organization. Abbreviations: EpoR, erythropoietin receptor; G-CSF-R, granulocyte colony-stimulating factor receptor; TGFβ, transforming growth factor-beta; TNF, tumor necrosis factor; JAK, Janus kinase; EGFR, epidermal growth factor receptor; InsR, insulin receptor; IGF1R, insulin-like growth factor I receptor; IRR, insulin receptor-related receptor; PDGFR, platelet-derived growth factor receptor; CSF1R, colony-stimulating-factor 1 receptor; FGFR, fibroblast growth factor receptor; MuSK, muscle-specific receptor tyrosine kinase; Eph, ephrin; DDR, discoidin domain receptor; Flt1, KDR and Flt4, vascular endothelial growth factor (VEGF) receptors.
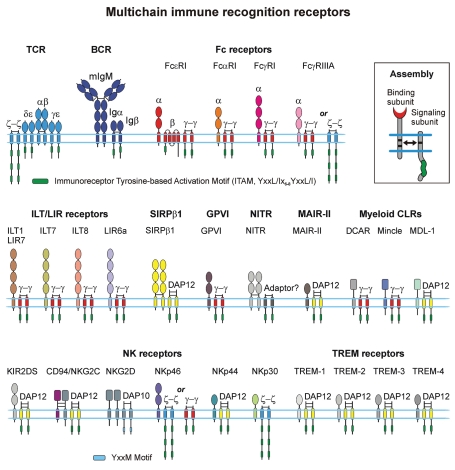
Figure 2.
Schematic presentation of the MIRRs expressed on many different immune cells including T and B cells, natural killer cells, mast cells, macrophages, basophils, neutrophils, eosinophils, dendritic cells and platelets. The inset shows MIRR assembly. The extracellular recognition domains and intracellular ITAM-containing signaling domains are located on separate subunits bound together by noncovalent transmembrane interactions (solid arrow). ITAMs/YxxM are shown by green. Curved lines depict intrinsic disorder of the cytoplasmic domains of MIRR signaling subunits. Abbreviations: BCR, B cell receptor; CLR, C-type lectin receptor; DAP-10 and DAP-12, DNAX adapter proteins of 10 and 12 kD, respectively; DCAR, dendritic cell immunoactivating receptor; GPVI, glycoprotein VI; ILT, Ig-like transcript; KIR, killer cell Ig-like receptor; LIR, leukocyte Ig-like receptor; MAIR-II, myeloid-associated Ig-like receptor; MDL-1, myeloid DAP12-associating lectin 1; NITR, novel immune-type receptor; NK, natural killer cells; SIRP, signal regulatory protein; TCR, T cell receptor; TREM receptors, triggering receptors expressed on myeloid cells.
Examples of SRs include receptor tyrosine kinases (RTKs) that are TM glycoproteins consisting of a variable EC N-terminal domain, a single membrane spanning domain, and a large CYTO portion composed of a juxtamembrane domain, the highly conserved tyrosine kinase domain and a C-terminal regulatory region (Fig. 1).5 RTKs activate numerous intracellular signaling pathways, leading to a variety of cell responses. These receptors are triggered by the binding of their cognate ligands and transduce the recognition signal to the cytoplasm by phosphorylating CYTO tyrosine residues on the receptors themselves (autophosphorylation) and on downstream signaling proteins. The proteins of the tumor necrosis factor (TNF) receptor superfamily6 are a group of SRs critically involved in the maintenance of homeostasis of the immune system (Fig. 1). Triggered by their corresponding ligands, these receptors either induce cell death or promote cell survival of immune cells. Transforming growth factor-β (TGFβ) is a potent regulatory cytokine which inhibits the development of immunopathology to self or non-harmful antigens without compromising immune responses to pathogens.7 The TGFβ superfamily functions via binding to type I and II TM serine/threonine kinase receptors that belong to the SR family (Fig. 1).
Functionally diverse members of the MIRR family are expressed on many different immune cells, including T and B cells, natural killer (NK) cells, mast cells, macrophages, basophils, neutrophils, eosinophils, dendritic cells (DCs) and platelets.2–4,8 Figure 2 shows typical examples of MIRRs including the T cell receptor (TCR) complex, the B cell receptor (BCR) complex, Fc receptors (e.g., FcεRI, FcαRI, FcγRI and FcγRIII), NK receptors (e.g., NKG2D, CD94/NKG2C, KIR2DS, NKp30, NKp44 and NKp46), immunoglobulin (Ig)-like transcripts and leukocyte Ig-like receptors (ILTs and LIRs, respectively), signal regulatory proteins (SIRPs), dendritic cell immunoactivating receptor (DCAR), myeloid DNAX adapter protein of 12 kD (DAP12)-associating lectin 1 (MDL-1), blood DC antigen 2 protein (BDCA2), novel immune-type receptor (NITR), myeloid-associated Ig-like receptor (MAIR-II), triggering receptors expressed on myeloid cells (TREMs) and the platelet collagen receptor, glycoprotein VI (GPVI). For more information on the structure and function of these and other MIRRs, I refer the reader to recent reviews.2,9–28 The MIRR ligand-binding subunits are integral membrane proteins with small intracellular domains that are themselves inert with regard to signaling. Signaling is achieved through the association of the ligand-binding chains with signal-transducing subunits that contain in their CYTO domains one or more copies of the immunoreceptor tyrosine-based activation motifs (ITAMs) with two appropriately spaced tyrosines (YxxL/Ix6–8YxxL/I; where x denotes non-conserved residues)29 or the YxxM motif,30,31 found in the DAP10 CYTO domain31 (Fig. 2). The association of the MIRR subunits in resting cells is driven mostly by the non-covalent TM interactions between recognition and signaling components (Fig. 2) and plays a key role in receptor assembly, integrity and surface expression.4,15–17,19,24,27,32–43
SCHOOL Platform of Receptor-Mediated Signaling
Basic concept.
Within the single- and multichain receptor families, the similar architecture of the receptors dictates similar mechanisms of receptor triggering which in turn provide the similarity of the therapeutic targets revealed at the level of protein-protein interactions involved in receptor-mediated signaling.2,44 This builds the structural basis for the development of novel pharmacological approaches as well as the transfer of our current and future clinical knowledge, experience and therapeutic strategies between seemingly unrelated diseases mediated by receptors within SR and MIRR families. As shown and described in detail below, the SCHOOL platform reveals striking similarities between members of these two families in mechanistic principles of receptor-mediated TM signal transduction.
Ligand-induced oligomerization of cell surface receptors is frequently employed in receptor-mediated TM signaling,5,45–47 with dimerization of receptors being the most frequent. Thus, the receptor dimer can be considered as an “elementary stimulatory unit” leading to a cell response. The fact that binding of multivalent but not monovalent ligand and subsequent receptor clustering are required for induction of the signaling cascade2,5,45,47–77 raises the following question: What is the molecular mechanism by which clustering of the EC binding domains leads to the generation of the activation signal by intracellular signaling domains?
According to the SCHOOL platform, signaling chain homooligomerization and formation of competent signaling oligomers in CYTO milieu provides the necessary and sufficient event to trigger receptors of both structural families (SRs and MIRRs) and induce cell activation (Fig. 3). Within the platform, receptor oligomerization induced or tuned upon ligand binding outside the cell is translated across the membrane into protein oligomerization in CYTO milieu, thus providing a general platform for receptor-mediated signaling. Intriguingly, in contrast to well-structured CYTO signaling domains of SRs, CYTO domains of MIRR signaling subunits belong to a novel class of intrinsically disordered proteins (IDPs; i.e., proteins that lack a well-defined ordered structure under physiological conditions in vitro).78–80 The recently discovered ability of these IDPs to homooligomerize,78,81 represents a missing and key piece of the MIRR triggering puzzle to accomplish our molecular understanding of receptor-mediated signaling (Fig. 3).
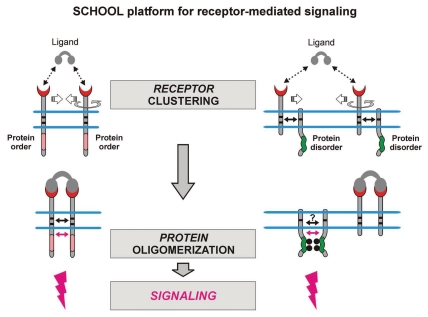
Figure 3.
Receptor oligomerization (clustering) induced upon ligand binding outside the cell is translated across the membrane into protein oligomerization inside the cell with cytoplasmic homointeractions representing the major driving force of receptor triggering. Small solid black and magenta arrows indicate specific interunit hetero- and homointeractions between transmembrane and cytoplasmic domains, respectively. Circular arrows indicate ligand-induced receptor reorientation. Phosphate groups are shown as gray circles. Abbreviation: SCHOOL, signaling chain homooligomerization.
In my opinion, we encounter here one of the most intriguing questions: Why for MIRRs, the receptors with EC recognition and intracellular signaling domains located on separate protein chains, did nature selected to use a functional link between protein disorder and oligomericity? Since its discovery in 2004,78 the unusual and unique biophysical phenomenon of IDP homooligomerization has become of more and more interest to biophysicists and biochemists,82,83 and one can expect that further multidisciplinary studies will clarify this question of great interest and practical utility.
Major driving forces.
The SCHOOL model was initially developed in 2004 for MIRR-mediated TM signaling.49 Later, the SCHOOL-based mechanism has been suggested as a general mechanism for members of both receptor families—SRs and MIRRs.2,44
Introducing the homotypic interactions between the CYTO domains of receptor protein chains (SR) or of receptor signaling subunits (MIRR) as one of the key interactions involved in receptor triggering and TM signaling, the plausible and easily testable SCHOOL model thus defines this process as an outcome of the interplay between three major driving forces: ligand-receptor EC interactions, interreceptor (SR) and intrareceptor (MIRR) TM interactions and interreceptor CYTO homointeractions (Table 1, Fig. 4).
Table 1.
Major driving forces in receptor triggering and transmembrane signaling as revealed by the SCHOOL model
| Protein-protein interactions* | Interaction milieu | Role in receptor triggering/signaling |
| SR and MIRR: Between antigen/ligand and receptor recognition domain(s) | EC | SR and MIRR: Cluster receptors in sufficient interreceptor proximity and correct (permissive) orientation relative to each other to promote the interreceptor CYTO homointeractions between receptor signaling domains (subunits), resulting in formation of competent signaling oligomers and thus initiating the downstream signaling cascade |
| SR: Between ligand-engaged receptors in a receptor cluster | TM | SR: Define the overall rigid geometry and topology of a receptor cluster. Promote interreceptor CYTO homointeractions between signaling domains |
| MIRR: Between MIRR recognition and signaling subunits in resting receptors** | MIRR: Define the overall rigid geometry and topology of the MIRR. Maintain the integrity of a functional receptor in resting cells. Balance opposing interactions, the CYTO homointeractions, thus helping to discriminate ligands/antigens in their functional ability to cluster MIRRs in sufficient interreceptor proximity and correct (permissive) orientation relative to each other to promote formation of competent signaling subunit oligomers | |
| SR: Homointeractions between signaling domains | CYTO | SR and MIRR: Lead to formation of competent signaling subunit oligomers, thus initiating the downstream signaling cascade |
| MIRR: Homointeractions between MIRR signaling subunit(s)** |
For MIRRs, all three protein-protein interactions, namely ligand-receptor EC interactions as well as intrareceptor TM heterointeractions and interreceptor CYTO homointeractions fall within the similar micromolar affinity range and are characterized by relatively rapid kinetics;
Within the SCHOOL model, these TM and CYTO interactions represent the opposing forces that balance resting and differently triggered patterns of MIRR receptor triggering and signaling; Abbreviations: CYTO, cytoplasmic; EC, extracellular; MIRR, multichain immune recognition receptor; SCHOOL model, signaling chain homooligomerization model; SR, single-chain receptor; TM, transmembrane.
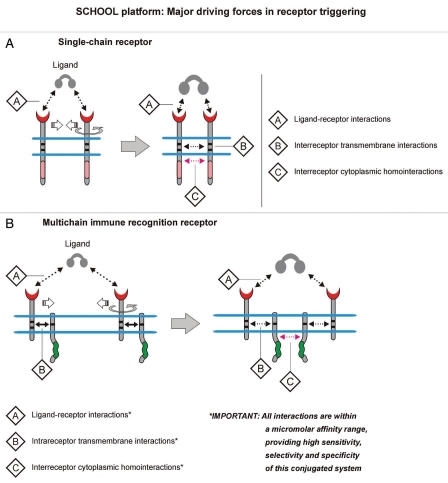
Figure 4.
Major driving forces in receptor triggering. Within the SCHOOL model, receptor triggering and signaling is an outcome of the ligand-induced interplay between three key protein-protein interactions: (1) ligand-receptor interactions, (2) interreceptor [single-chain receptors, (A)] and intrareceptor [multichain immune recognition receptors, (B)] transmembrane interactions, and (3) interreceptor cytoplasmic homointeractions. Circular arrows indicate ligand-induced receptor reorientation. Abbreviation: SCHOOL, signaling chain homooligomerization.
Interestingly, in RTK-mediated signaling, a weak dimerization propensity for all RTK TM domains allows for a tight control of the ratio between receptor monomers and dimers.84–88
In MIRR-mediated signaling, all three protein-protein interactions, namely antigen/ligand-MIRR EC interactions as well as intrareceptor TM heterointeractions and interreceptor CYTO homointeractions (Fig. 4B, Table 1), intriguingly fall within the similar micromolar affinity range and are characterized by relatively rapid kinetics.78,81,89–95 Interestingly, the homooligomerization of the intrinsically disordered CYTO domains of MIRR signaling subunits is not accompanied by a disorder-to-order transition and is best described by a two-step monomer-dimertetramer fast dynamic equilibrium with monomer-dimer dissociation constants in the micromolar affinity range.78,81 Together, these findings are in line with the known dependence of the overall binding affinity between proteins on the function of the protein complex. For example, obligate homodimers are strongly associated with nano- or picomolar binding affinity while, in contrast, proteins that associate and dissociate in response to changes in their environment, such as the majority of signal transduction mediators, tend to bind more weakly. Thus, this conjugated and well-balanced system of interprotein interactions (Table 1, Fig. 4B) provides the ideal basis to explain the molecular mechanisms of the ability of MIRRs to transduce the EC information about recognition of different ligands/antigens across the cell membrane in highly specific and sensitive manner and translate it into different activation signals, thus triggering different intracellular pathways and resulting in different cell responses.
Major restraints.
Within the SCHOOL platform of receptor-mediated signaling, the necessity and sufficiency of formation of competent signaling oligomers mediated by homointeractions between well-structured (SRs) or intrinsically disordered (MIRRs) CYTO signaling domains to trigger receptor function dictates several important restraints on receptor-mediated signaling (Table 2):
sufficient interreceptor proximity in receptor dimers/oligomers.
correct (permissive for signaling) relative orientation of the receptors in receptor dimers/oligomers.
long enough duration of the receptor-ligand interaction that generally correlates with the strength (affinity/avidity) of the ligand.
sufficient lifetime of an individual receptor in receptor dimers/oligomers.
Table 2.
Major restraints for receptor-mediated signaling imposed within the SCHOOL platform by the overall structural architecture and topology of receptors in combination with the major driving forces in receptor triggering and transmembrane signaling
| Restraints | Functional significance |
| Sufficient interreceptor proximity in receptor dimers/oligomers | Two or more antigen/ligand-clustered receptors should be in sufficient proximity to each other to initiate interreceptor TM (SRs) and CYTO homointeractions between SRs or signaling subunits of MIRRs with subsequent formation of competent signaling oligomers |
| Correct (permissive for signaling) relative orientation of the receptors in receptor dimers/oligomers | Within two or more antigen/ligand-clustered receptors, receptor chains of SRs or particular signaling subunit(s) of MIRRs should be in correct orientation relative to each other to initiate CYTO homointeractions between SRs or between the signaling subunits of MIRRs with subsequent formation of competent signaling oligomers. |
| Long enough duration of the receptor-ligand interaction that generally correlates with the strength (affinity/avidity) of the ligand | Main protein-protein interactions involved in receptor triggering and TM signaling fall into a similar low/moderate (micromolar) affinity range. For this reason, the multivalent antigen/ligand-receptor contact should last long enough to bring two or more receptors in sufficient proximity and correct relative orientation toward each other and hold them together to promote the interreceptor CYTO homointeractions between SRs or between the signaling subunits of MIRRs, resulting in formation of competent signaling oligomers and thus initiating the downstream signaling cascade. |
| Sufficient lifetime of an individual receptor in receptor dimers/oligomers | Similarly to a restraint on duration of antigen/ligand-receptor contact, in order to initiate the downstream signaling cascade, a lifetime of an individual receptor in antigen/ligand-clustered receptors should be sufficient to promote the interreceptor CYTO homointeractions between SRs or between the signaling subunits of MIRRs. |
Abbreviations: CYTO, cytoplasmic; SR, single-chain receptor; MIRR, multichain immune recognition receptor; SCHOOL model, signaling chain homooligomerization model; TM, transmembrane.
As described in more detail below, these general mechanistic principles are common for SRs and MIRRs linking mechanistically a variety of structurally and functionally diverse receptors.
Trinity of description, explanation and prediction.
Based on well-defined biochemical processes such as specific protein-protein interactions, the SCHOOL model represents the first general mechanistic model of receptor-mediated signaling and can be also defined as a dynamic, continuous, spatially homogeneous, descriptive and explanatory model.96 This model describes and explains molecular mechanisms and the main driving forces of TM signal transduction for structurally different and functionally unrelated members of the SR and MIRR families. Thus, the basic principles of TM signaling learned from the platform can be used in different fields of immunology and cell biology to describe processes that are mediated by a variety of cell surface receptors.2,4,44,49,97,98 Besides the ability to describe general principles of receptor-mediated signal transduction, the SCHOOL model provides a mechanistic explanation for specific processes behind “outside-in” receptor signaling that remain unclear. Since it was first published in 2004 for MIRR signaling,49 the model has also predicted several experimental observations that have been later reported for different immune cells.
By definition, the utility of scientific models is evaluated by their abilities to explain past observations, predict future observations and control events as well as by their simplicity or even aesthetic appeal. The distinct features demonstrating the utility of the SCHOOL model are described in detail below for specific receptors.
SCHOOL Model of Single-Chain Receptor Signaling
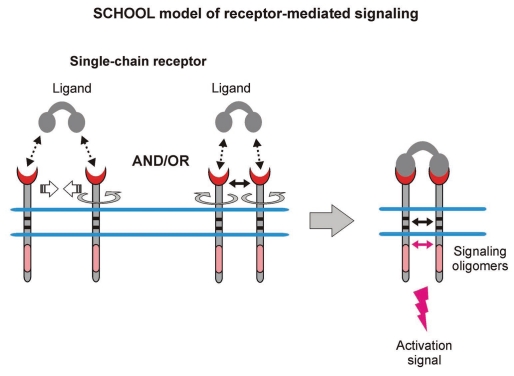
In contrast to MIRR-mediated TM signal transduction, the molecular mechanisms underlying SR (e.g., RTK) signaling have been fairly well delineated and suggest that intracellular formation of competent signaling oligomers plays a crucial role in receptor triggering (Fig. 5), thus proving the SCHOOL concept of SR signaling (Fig. 3). Within the SCHOOL model of SR signaling (Fig. 5), multivalent ligand binding results in receptor re-orientation and dimerization (oligomerization) mediated by interreceptor TM interactions84,85,87 and in subsequent formation of competent signaling oligomers in the cytoplasm.45,48,50,51,87,88,99–105 In RTKs, for example, this leads to trans-autophosphorylation at defined CYTO tyrosines through intrinsic kinase activity.88 RTKs and some other SRs such as, for example, members of the TNF receptor superfamily105,106 can exist as pre-assembled dimers/oligomers on the cell surface. In this scenario, within the SCHOOL model, binding to multivalent ligand results in reorientation of receptors in these oligomers to adopt an inter-unit geometry permissive for further receptor activation (Fig. 5).
Figure 5.
Ligand-induced SR clustering and reorientation (in pre-existing SR clusters, ligand binding induces receptor reorientation) results in SR oligomerization mediated by transmembrane interactions. In these oligomers, receptors are in sufficient proximity and adopt a correct (permissive) relative orientation and geometry to promote homointeractions between cytoplasmic domains. Within the model, formation of competent signaling oligomers in cytoplasmic milieu is necessary and sufficient to generate the activation signal (for receptor tyrosine kinases, this means trans-autophosphorylation of Tyr residues in cytoplasmic signaling sequences), thus triggering downstream signaling pathways. Protein-protein transmembrane and cytoplasmic interactions are shown by solid black and magenta arrows, respectively. Abbreviation: SCHOOL, signaling chain homooligomerization.
Proximity, orientation and oligomerization of signaling domains.
RTKs are believed to transduce biochemical signals via lateral dimerization in the plasma membrane, thus suggesting a crucial role of proximity in RTK-mediated signaling.77,85,88,107–109 On the other hand, all the RTKs shown (Fig. 1), with the exception of the insulin and the insulin-like growth factor receptors, exist in equilibrium between monomers and dimers in the membrane of resting cells. This raises a question: Why does dimerization of receptors in resting cells not result in receptor triggering and cell activation? The answer is simple enough. Inter-unit orientation in receptor dimers has to be correct (permissive for signaling) to promote oligomerization of CYTO signaling domains. In RTK-mediated signaling, a conformational change has been proposed to occur in the EC domain upon ligand binding, leading to the rotation of the whole receptor.85,102,110,111 In line with this, the activation of the Neu RTK occurs only for a specific TM dimer interface, the rotation of which leads to periodic oscillations in kinase activity.112 Furthermore, the rotation of the kinase domain with respect to the TM domain by inserting residues into the C-terminal TM flanking region restores the kinase activity. For EGFR, the ligand-induced rotation of the EGFR EC domain has been reported to be transmitted to the receptor TM domain.110
Recent structural studies revealed a critical role of the orientation of receptor dimers in erythropoietin receptor (EpoR)-mediated transmembrane signaling.113–115 These studies show that the activating efficiency of EpoR, and by inference each of the cytokine receptor complexes, depends critically on the separation, orientation and relative disposition of bound receptors, suggesting a tight coupling of the EC domain orientation to the CYTO signaling events.
The type I TM glycoprotein gp130 is the commonly used signaling receptor chain of all interleukin (IL)-6-type cytokines (i.e., IL-6).116 Intriguingly, signal transduction via IL-6 requires not only gp130 homodimerization but also the correct relative orientation of the gp130 CYTO regions in ligand-specific receptor dimer, suggesting that subtle changes in the orientation of the receptor chains relative to each other might result in very different responses.117 Enforcement of gp130 dimerization is not sufficient for receptor activation but additional conformational requirements must be fulfilled.118 Therefore, like antibody-induced dimerization of the MIRRs (as described below), dimerization of the cytokine receptors by monoclonal antibodies is in most cases not enough to induce signal transduction.119 Together, these findings117–119 suggest that in cytokine receptor signaling, dimerization of not just EC but rather CYTO domains of the gp130 signaling subunit is critically required to trigger the receptor and initiate the signaling cascade. Interestingly, many members of the TNF receptor superfamily were once thought to signal through ligand-induced receptor trimerization. However, recently, these receptors have been shown to exist as pre-assembled oligomers on the cell surface.105,106 This suggests that upon the binding of the trimeric ligand, not only oligomerization (trimerization) of these single-chain receptors, but also the correct intermolecular relative orientation within trimers, plays a crucial role in signaling. Recently, ligand-induced formation of surface receptor oligomers has been reported for a member of the TNF receptor family, the Fas receptor.104 This SR has a CYTO death domain (DD) that upon receptor stimulation with a trivalent ligand, binds to the homologous DD of the adaptor protein Fas-associated death domain protein (FADD) and homotrimerizes, thus initiating the caspase signaling cascade. Interestingly, a mutation in Fas CYTO domain (T225K) linked to autoimmune lymphoproliferative syndrome impairs receptor oligomerization and inhibits Fas-mediated signaling but retains the ability to interact with FADD.104 This indicates that homointeractions between Fas CYTO tails have an important role in the receptor triggering. Similarly, CYTO domain-mediated dimerization of toll-like receptor 4 (TLR4) has been recently reported to play an important role in the TLR4 triggering and signal transduction.120,121
Thus, these and other studies reported to date strongly support the general SCHOOL platform concept and the SCHOOL model of SR-mediated signaling in particular, by showing that ligand-induced receptor dimerization is translated into protein dimerization in TM milieu and that the TM dimer interface contains the critical structural information that positions the receptor CYTO domains in a way permissive for oligomerization and signaling (Figs. 3–5).
SCHOOL Model of Multichain Receptor Signaling
In this work, I refer to multichain activating receptors (Fig. 2) that share a common organizing principle—their EC recognition module(s) and intracellular signaling module(s) are found on separate subunits that are noncovalently associated through their TM domains, as to multichain immune recognition receptors (MIRRs). It should be noted, however, that members of this family are not necessarily immune-related (an example is the major collagen receptor on platelets, GPVI).
The MIRR-mediated activation signal can be divided into four parts: (1) the EC recognition of a multivalent ligand/antigen resulting in the aggregation, or clustering, of the MIRRs, (2) MIRR triggering and TM signal transduction, (3) phosphorylation of the ITAM or YxxM tyrosine residues by protein tyrosine kinases (PTKs) and activation of specific intracellular pathways and (4) the activation of genes in the nucleus. The EC recognition of a ligand/antigen, the MIRR-triggered biochemical cascades and the mechanisms of gene activation are understood in significant detail. However, the mechanism by which the MIRR transduces the recognition information via receptor TM and juxtamembrane (JM) regions into intracellular biochemical events (part 2) has been a long-standing mystery. In other words, the key question remained unanswered: what is the molecular mechanism by which clustering of the EC recognition domains of MIRRs leads to receptor triggering and tyrosine phosphorylation of the intracellular ITAMs or YxxMs, thus initiating specific pathways and resulting in immune cell functional outcomes? It was also not known how this putative mechanism could explain the intriguing ability of immune cells to discern and differentially respond to slightly different ligands.
MIRR-mediated signal transduction plays an important role in health and disease, making these receptors attractive targets for rational intervention in a variety of immune disorders.1,13,16,19,122–125 Thus, future therapeutic strategies depend on our detailed understanding of the molecular mechanisms underlying MIRR triggering and subsequent TM signal transduction. In addition, understanding these mechanisms would give us a new handle in dissecting the basic structural and functional aspects of the immune response.
Despite numerous models of MIRR-mediated TM signal transduction suggested for particular MIRRs (e.g., TCR, BCR, Fc receptors, NK receptors, etc.), no current model fully explains how ligand-induced TM signal transduction commences at the molecular level. As a consequence, these models are mostly descriptive, do not explain mechanistically a vast majority of the specific processes behind “outside-in” MIRR signaling, and do not reveal clinically important points of therapeutic intervention. In addition, since the term “MIRR” was first introduced in 1992,3 and MIRR-triggered signaling pathways were hypothesized to be similar,3,70,126–128 no general mechanistic model of MIRR-mediated immune cell activation has been suggested to date with the exception of the SCHOOL model.4,49,97,129,130 This impeded our advanced understanding of the immune response, and more importantly, prevented the potential transfer of therapeutic strategies between seemingly disparate immune disorders.
Basic concept and major stages.
A novel unusual biophysical phenomenon, the homointeractions of intrinsically disordered CYTO domains of ITAM-containing MIRR signaling subunits, has recently been discovered.78 Demonstrating that intrinsically disordered proteins do not necessarily undergo a transition between disordered and ordered states upon interaction,44,79,81 this finding opposes the generally accepted view on the behavior of IDPs. Perhaps even more intriguing is the fact that no chemical shift changes and significant changes in peak intensities are observed in the 1H-15N heteronuclear single quantum coherence (HSQC) spectra of 15N-labeled ζcyt upon dimerization.78,81 Later, the natural propensity of the random-coil TCRζ CYTO domain to homodimerize has been independently confirmed by other investigators.131 Interestingly, the homooligomerization of CYTO domains of MIRR signaling subunits is best described by a two-step monomer-dimer-tetramer fast dynamic equilibrium with dissociation constants in the micromolar affinity range.78,81 As mentioned above, the overall binding affinity between proteins depends on the function of the protein complex and proteins that associate and dissociate in response to changes in their environment, such as the majority of signal transduction mediators, tend to bind more weakly. In this context, micromolar binding affinities in combination with a rapid association and dissociation kinetics78 make the homotypic CYTO interactions between MIRR signaling subunits a valid candidate for involvement in MIRR-mediated signal transduction.
Uncovering a crucial physiological role of these unique homointeractions, the SCHOOL model suggests that formation of competent MIRR signaling subunit oligomers is necessary and sufficient to trigger the receptors and induce TM signal transduction and the downstream signaling sequence (Figs. 6 and 7).4,44,49,97,130 Intracellularly, the need for MIRR dimerization is consistent with the suggested structural hypothesis of cross-phosphorylation70,132 that assumes that (1) the kinase(s) responsible for catalyzing ITAM Tyr residue phosphorylations exist associated with the receptors, and (2) for steric reasons, these kinases cannot phosphorylate tyrosine residues on chains of the same receptor complex. Upon dimerization/oligomerization, these kinases phosphorylate the tyrosines of a distinct receptor complex (cross-phosphorylation, or transphosphorylation), thus triggering the receptor.70
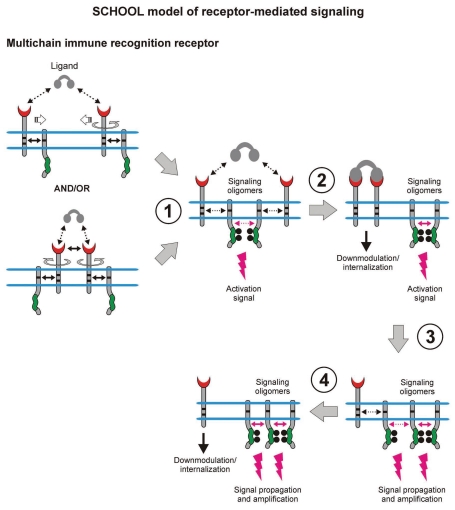
Figure 6.
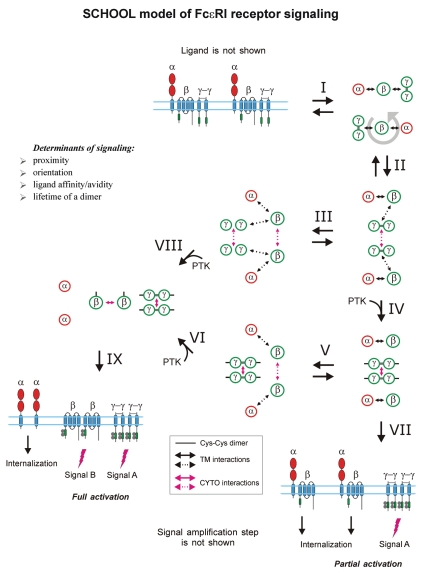
Within the model, formation of competent signaling oligomers in cytoplasmic milieu is necessary and sufficient to generate the activation signal, thus triggering downstream signaling pathways. Ligand-induced MIRR clustering and reorientation (in pre-existing MIRR clusters, ligand binding induces receptor reorientation) lead to formation of a dimeric/oligomeric intermediate (stage 1). In this intermediate, receptors are in sufficient proximity and adopt the correct (permissive) relative orientation and geometry to promote trans-homointeractions between cytoplasmic domains of signaling subunits resulting in formation of competent signaling oligomers. Upon formation of signaling oligomers, PTKs phosphorylate the tyrosine residues in the cytoplasmic signaling motifs, the immunoreceptor tyrosine-based activation or YxxM motifs (ITAMs/YxxM, shown by green), that leads to generation of the activation signal, dissociation of signaling oligomers and internalization of the engaged binding subunits (stage 1). Next, the signaling oligomers sequentially homointeract with the relevant signaling subunits of nonengaged receptors resulting in formation of higher-order signaling oligomers, thus propagating and amplifying the signals (stages 3 and 4). This also leads to the release and subsequent internalization of the nonengaged ligand-binding MIRR subunits (stage 4). Circular arrows indicate ligand-induced receptor reorientation. Black and magenta arrows indicate specific intersubunit hetero- and homointeractions between transmembrane and cytoplasmic domains, respectively. All interchain interactions in a dimeric intermediate are shown by dotted arrows reflecting their transition state. Curved lines depict intrinsic disorder of the cytoplasmic domains of MIRR signaling subunits. Phosphate groups are shown as dark circles. Abbreviation: PTK, protein tyrosine kinase; SCHOOL, signaling chain homooligomerization.
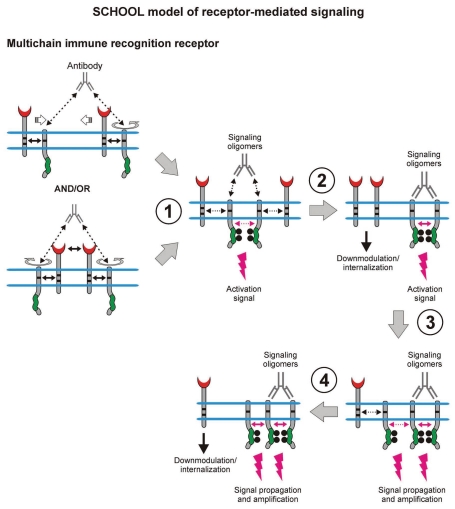
Figure 7.
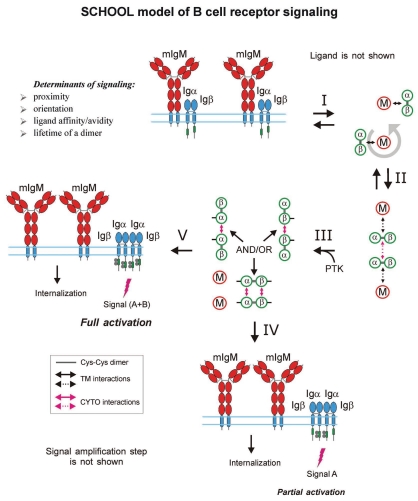
Within the model, formation of competent signaling oligomers in cytoplasmic milieu is necessary and sufficient to generate the activation signal, thus triggering downstream signaling pathways. Receptor clustering and reorientation (and/or receptor reorientation in pre-existing MIRR clusters) induced by antibodies to recognition (not shown) or signaling subunits (e.g., anti-TCRα, anti-TCRβ, anti-CD3ε, anti-Igβ, etc.,) lead to formation of a dimeric/oligomeric intermediate (stage 1). In this intermediate, receptors are in sufficient proximity and adopt the correct (permissive) relative orientation and geometry to promote trans-homointeractions between cytoplasmic domains of signaling subunits resulting in formation of competent signaling oligomers. Further stages and outcomes are similar to those described in the legend to Figure 6.
Within the model, MIRR engagement by multivalent antigen (Fig. 6) or anti-MIRR antibodies (e.g., anti-CD3ε and anti-TCRβ for TCR or anti-Igβ antibodies for BCR; Fig. 7) leads to receptor clustering coupled with a multi-step structural reorganization driven by the homooligomerization of MIRR signaling subunits (Figs. 6 and 7). Ligand-induced MIRR clustering leads to receptor reorientation and formation of a dimeric/oligomeric intermediate in which signaling chains from different receptor units start to trans-homointeract and form signaling oligomers (Figs. 6 and 7, stage 1). Upon formation of signaling oligomers, PTKs phosphorylate the tyrosine residues in the ITAMs located on the CYTO tails of MIRR signaling subunits, leading to the generation of intracellular activation signal(s), dissociation of signaling oligomers and internalization of the engaged MIRR ligand-binding subunits (Figs. 6 and 7, stage 2). Signaling oligomers then interact with the signaling subunits of nonengaged receptors resulting in formation of higher-order signaling oligomers, thus propagating and amplifying the activation signal and resulting in internalization of the non-engaged MIRR recognition subunits (Figs. 6 and 7, stages 3 and 4).
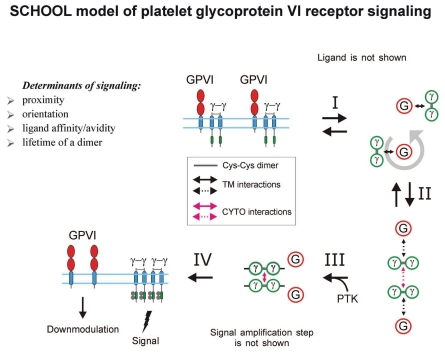
Similar to SRs, some MIRRs such as TCR and major platelet collagen receptor GPVI, can exist as pre-assembled oligomers on the cell surface.71,133,134 In these oligomers, multivalent ligand binding or antibody stimulation results in re-orientation of receptors to adopt an interunit geometry permissive for further receptor activation (Figs. 6 and 7).
The model also assumes that the diversity of the immune cell response is partly provided by the combinatorial nature of MIRR-mediated signaling. Signal diversification may be achieved through different patterns of MIRR signaling subunit oligomerization4,49,97 in combination with distinct activation signals provided by different MIRR signaling modules135–146 and/or different ITAMs located on the same signaling module (e.g., TCRζ chain).147 Thus, according to the model, the diversity of cell functional outcomes in response to different ligands is higher with the more different signaling subunits the MIRR complex has.
According to the SCHOOL model, MIRR triggering and TM signaling induced by binding to multivalent ligand/antigen or anti-MIRR antibodies can be divided into four major stages (Figs. 6 and 7):
Dynamic lateral clustering and rotation with subsequent formation of the intermediate complex. Ligand/antigen/antibody brings two or more MIRRs together in sufficient proximity and correct relative orientation toward each other to promote the interreceptor homointeractions between signaling subunits. Once initiated, these homointeractions weaken the intrareceptor TM interactions between recognition and signaling subunits. A signaling-competent oligomeric intermediate complex is formed, bringing together the CYTO domains of the signaling subunits, protein kinases and various adaptor/effector proteins, to create a competent, activated receptor complex. In the signaling subunit oligomers formed, the ITAM Tyr residues become phosphorylated, thus starting the signaling cascade.
Dissociation and internalization. Signaling oligomers dissociate from the engaged ligand-recognition subunits, which are then internalized.
Interactions with nonengaged receptors, lateral signal propagation and amplification. Signaling oligomers interact with the signaling subunits of nonengaged receptors resulting in formation of higher-order signaling oligomers, thus propagating and amplifying the activation signal.
Dissociation and internalization. Signaling oligomers dissociate from the nonengaged ligand-recognition subunits, which later are internalized.
Major driving forces.
As described above, within the SCHOOL model, there are three major driving forces of MIRR signaling: antigen/ligand-MIRR EC interactions, intrareceptor TM interactions and interreceptor CYTO interactions (see also Table 1 and Fig. 4B), and an outcome of the interplay between these forces defines MIRR triggering and activation. Antigen/ligand-MIRR interactions are generally of low affinity (micromolar range) and have rapid association and dissociation kinetics (reviewed in ref. 92). This low-affinity binding, in combination with fast kinetics, allows immune cells to recognize and discriminate a variety of antigens/ligands with high specificity, selectivity and sensitivity in order to respond with a variety of biological responses. Considering that EC and TM regions of MIRRs are well-ordered receptor segments while MIRR signaling CYTO domains are intrinsically disordered,2,78,79,81 an important and intriguing question is raised: how do MIRRs transduce highly ordered antigen recognition/discrimination EC information across the cell membrane into intracellular biochemical events, triggering specific pathways and resulting in a specific functional outcome?
Despite intensive studies of MIRR-mediated TM signal transduction, the only model that can answer this question and even more important, mechanistically explain how this signaling starts, is the SCHOOL model.4,49,97,130 As described above, all three major protein-protein interactions that define MIRR signaling are characterized by micromolar affinity and relatively rapid kinetics.78,89–95 Thus, this conjugated and well-balanced system of interprotein interactions can explain the molecular mechanisms of the ability of MIRRs to transduce the recognition/discrimination information across the cell membrane and translate it into different activation signals, thus triggering different intracellular pathways and resulting in different cell responses. Within the model, the MIRR-generated intracellular activation signals are combinatorial in nature and involve multiple components such as different ITAM Tyr phosphorylation patterns135–147 as well as formation of functionally different competent signaling oligomers formed by the CYTO homooligomerization of different MIRR signaling subunits.4,49,97,98,130 This system also explains mechanistically high specificity, selectivity and sensitivity of immune cells in recognition and discrimination of different antigens/ligands and how this recognition/discrimination results in different functional outcomes. This is particularly important for the TCR148 that has four different signaling subunits, namely ζ, CD3ε, CD3δ and CD3γ, known to play different roles in T cell biology.130,149 In addition, in contrast to other MIRR signaling subunits, ζ has three ITAMs that can provide differential Tyr phosphorylation patterns in response to different ligands, initiating different intracellular signaling pathways. Thus, within the model, TCR-mediated signaling and cell activation has the highest combinatorial potential as compared to other MIRRs, explaining a high variability of distinct TCR-triggered intracellular signaling pathways and therefore distinct T cell functional responses depending on the nature of the stimulus.4,49,97,98,130
Major restraints.
Interactions between TM helices of recognition and signaling MIRR subunits maintain receptor integrity in unstimulated cells (Fig. 2)4,15–17,19,24,25,27,32–43,49,97,98,130,150–155 and determine the relative positions of these subunits in the receptor complex (angles, distances, etc.,), thus dictating the overall geometry and topology of MIRRs. Within the SCHOOL model, this overall structural architecture of MIRRs, in combination with the requirement to initiate interreceptor CYTO homointeractions between receptor signaling subunits (Figs. 3, 6 and 7), impose several restraints for multivalent antigen/ligand-induced MIRR triggering (Table 2):4,49,97,98,
sufficient interreceptor proximity in MIRR dimers/oligomers,
correct (permissive) relative orientation of the receptors in MIRR dimers/oligomers,
long enough duration of the MIRR-ligand interaction that generally correlates with the strength (affinity/avidity) of the ligand, and
sufficient lifetime of an individual receptor in MIRR dimers/oligomers.
The importance of these factors for productive MIRR triggering is strongly supported by a growing body of evidence10,38,48,54,56,62,67,73,94,95,142,156–184 and is discussed in detail below.
The restraints imposed by the SCHOOL model play an especially important role during the first stage of MIRR triggering (Figs. 6 and 7), at which point these spatial, structural and temporal requirements (correct relative orientation, suf- ficient proximity, long enough duration of the MIRR-ligand interaction and lifetime of MIRR dimers/oligomers) should be fulfilled to favor initiation of trans-homointeractions between MIRR signaling subunits and formation of competent signaling subunit oligomers. If these requirements are not fulfilled at this “final decision-making” point, the formed MIRR dimers/oligomers may dissociate from the ligand and remain signaling incompetent and/or break apart to its initial monomeric receptor complexes. Also, at this stage, slightly different ligands may bring two or more MIRRs in different relative orientations that favor homointeractions between different signaling subunits and result in formation of different signaling oligomers or combinations, thus initiating distinct signaling pathways. This mechanism can explain the ability of MIRRs to differentially activate a variety of signaling pathways depending on the nature of the stimulus.
Within the proposed model, the signaling oligomers formed dissociate from ligand-binding chains, which later are internalized (Figs. 6 and 7, stage 2). This dissociation mechanism provides a structural and mechanistic basis for our improved understanding of many immunological phenomena, such as adaptive T cell tolerance or anergy,185–191 differential biological role of CD3 chains,192 ligand- or antibody-induced exposure of a cryptic polyproline sequence in the CYTO domain of CD3ε,165,193–195 BCR desensitization,196–199 human cytomegalovirus (CMV) escape from NK attack200 and others. The dissociation mechanism also allows the initially formed signaling oligomers to sequentially homointeract with the signaling subunits of nonengaged receptors (Figs. 6 and 7, stages 3 and 4) resulting in formation of higher-order signaling oligomers, thus propagating and amplifying the signal. This leads to dissociation and subsequent internalization of the nonengaged ligand-binding subunits. Thus, as with bacterial chemoreceptors,201–203 the SCHOOL model-based mechanism of MIRR-mediated cell activation suggests spreading (propagation) activation signal from engaged to nonengaged receptors within receptor clusters.
Finally, it should be noted that similar spatial, structural and temporal restraints are imposed within the proposed model for MIRR triggering by not only antigen (Fig. 6) but also the anti-MIRR (Fig. 7), antibodies such as anti-TCRα, anti-TCRβ, anti-CD3ε, anti-Igβ and others. This can explain differential immune cell functional outcomes mediated by MIRRs depending on the specificity of the antibodies.159,160,163–165,204–208
Advantages.
The plausible and easily testable SCHOOL model is fundamentally different from those numerous models that have been previously suggested for particular MIRRs and has several important advantages.4,49,97,98,130
This is the first general mechanistic model for all MIRRs known to date, including TCR, BCR, Fc receptors, NK receptors, ILTs, LIRs, SIRPs, DCAR, BDCA-2, MDL-1, NITR, TREMs, GPVI and others, and for those that will be discovered in the future. Thus, assuming that the general principles underlying MIRR triggering and TM signaling mechanisms are similar for all MIRRs, the SCHOOL model can be easily applied to any particular receptor of the MIRR family.
This is the first model that is based on specific protein-protein interactions (Fig. 4B)—biochemical processes that can be influenced and controlled,209–213 and specific inhibition and/or modulation of these interactions provides a promising novel approach for rational drug design, as revealed by recent progress in the design of inhibitory antibodies, peptides and small molecules.129,213–220
Introducing the CYTO homointeractions between MIRR signaling subunits as one of the key elements of MIRR triggering and signaling, the SCHOOL model imposes functionally important restraints (Table 2) and suggests molecular mechanisms for the vast majority of unexplained immunological observations accumulated to date.4,49,97,98,130
Unraveling the molecular mechanisms underlying MIRR triggering and subsequent TM signaling, the model suggests unique and powerful tools to study the immune response and a means to control and/or modulate it.4,49,97,98,130,221,222
Based on specific protein-protein interactions, the SCHOOL model reveals new therapeutic targets for the treatment of a variety of disorders mediated by immune cells.4,44,49,97,98,129,221–223
Finally, an important application of the SCHOOL model is that similar therapeutic strategies targeting key protein-protein interactions involved in MIRR triggering and TM signal transduction may be used to treat diverse immune-mediated diseases.44,98,129,222 This assumes that clinical knowledge, experience and therapeutic strategies can be transferred between seemingly disparate immune disorders or used to develop novel pharmacological approaches and that a general pharmaceutical approach may be used to treat diverse immune disorders.
Supportive Evidence for the SCHOOL Model of Multichain Receptor Signaling
The SCHOOL model was initially developed as a general model for the structurally related MIRR family members, i.e., for all receptors that have EC recognition and intracellular signaling modules located on separate receptor subunits. For this reason, in order to support the main concept and assumptions of the model, a rapidly growing body of evidence from studies of various MIRRs is used in this work.
Clustering and proximity.
Within the SCHOOL model, in order to trigger the MIRR, two or more receptors should be clustered/oligomerized in sufficient proximity to each other to initiate homointeractions between signaling subunits with subsequent formation of competent signaling subunit oligomers (Figs. 6 and 7).4,49,97,130 To date, these spatial restraints imposed by the model on MIRR triggering and initiation of the signaling cascade are consistent with the experimental data observed.
T cell receptor. There is a growing line of structural, biophysical and cellular evidence suggesting that ligand-specific TCR oligomerization is critical to generate a functional signal and that TCR dimerization constitutes a necessary and sufficient step for triggering T cell activation.48,52,57–60,65–67,72,74,157,224–232 These findings clearly demonstrate that dimeric/oligomeric antigens are able to stimulate T cells, whereas monomeric fail to do so. Interestingly, a correlation between antigenicity and repetitiveness of major histocompatibility complex (MHC)-bound peptides (pMHCs) has been also shown.157 For dimeric pMHC class I and II complexes, the ability to trigger T cells has been reported to decrease with increasing length of the connecting spacer.233,234 Recently, by testing well-defined dimeric, tetrameric and octameric pMHC complexes containing rigid polyproline spacers of different lengths, it has been also shown that their ability to activate cytotoxic T lymphocytes decreases as the distance between their subunit MHC complexes increases.156 Intriguingly, the pre-TCR complex has been shown to form oligomers spontaneously, in a ligand-independent manner.235,236 This oligomerization is mediated by specific charged residues in the EC domain of the pre-TCRα chain and is necessary and sufficient to induce autonomous signaling and stimulate pre-TCR function.235,236 Recently, TCR-coreceptor complexes from naive or activated CD4+ or CD8+ T cells have been found to exist as either dimers or tetramers, whereas no monomers or multimers were detected.230
B cell receptor. Similar to the TCR-induced signaling, the BCR activation signal is shown to be triggered by cross-linking of receptors through multivalent antigen,10,54,56,166–168,237 thus confirming the necessity of BCR clustering for competent signaling and cell activation.232 Interestingly, as shown for the pre-BCR in 2007, the ability of the purified recombinant receptor to dimerize indicates that accessory protein(s) are not required for dimerization, and by extension, pre-BCR signaling through multimerization can occur in a ligand-independent fashion.55 Showing strong similarities to the observations reported for the pre-TCR-mediated signaling,235,236 these findings are well consistent with the molecular mechanisms proposed by the SCHOOL model.
Fc receptors. Multichain Fc receptors, such as FcεRI, FcαRI, FcγRI and FcγRIII have been known to initiate cell signaling following interactions with multivalent ligands that induce their clustering.35,62,63,132,140,163,169–173,238–241 FcεRI aggregates as small as dimers have been reported to be capable of providing an effective activation signal to cause mediator secretion.163 Using a set of chemically well-defined ligands of valences 1–3, the magnitude of the cellular response has been demonstrated to dramatically increase as the valency of a ligand raises from two to three.62 Trivalent ligands with rigid double-stranded DNA spacers have been shown to effectively stimulate FcεRI-mediated degranulation responses in a length-dependent manner, providing direct evidence for receptor trans-phosphorylation as a key step in the mechanism of signaling by this receptor, whereas long bivalent ligands with flexible spacers have been demonstrated to be very potent inhibitors of mast cell degranulation stimulated by multivalent antigen.174 In other studies, the spacing of receptors in ligand-specific FcεRI aggregates has been also shown to be important for generating the activation signal.239
NK receptors. Multivalent ligand-induced receptor oligomerization is presumed to be a common mechanism for initiating NK receptor-mediated signaling.175–177 Also, structural and biochemical studies of the NKG2D receptor68,242,243 have demonstrated that the receptor exists as a dimer not only in the crystal but also at the surface of unstimulated NK cells. However, in contrast to pre-BCR and pre-TCR, this ligand-independent dimerization does not trigger the receptor and initiate downstream signaling, suggesting that dimerization is necessary but not sufficient to trigger the receptor.
Glycoprotein VI. Collagen, a natural ligand of GPVI, contains the GPVI-binding GPO (glycine-proline-hydroxyproline) motifs that form about 10% of the fibrillar collagen sequence and thus represent multiple GPVI-binding sites.244 Using a series of collagen-like model peptides containing GPO motifs of increasing length within (GPP)n sequences, Smethurst et al.245 have demonstrated that platelet aggregation and protein tyrosine phosphorylation can be induced only by cross-linked peptides that contain two or more GPO triplets. Multimeric snake venom proteins such as convulxin also strongly activate GPVI in a multimer size-dependent manner,246,247 suggesting that clustering of GPVI receptors through multiple binding events leads to activation. Structural studies have revealed a dimeric state of GPVI and 2 parallel grooves on the GPVI dimer surface as collagen-binding sites with an orientation and spacing of these grooves precisely matching the dimensions of an intact collagen fiber.64 These findings provide a structural basis for GPVI signaling mechanisms in which collagen-induced GPVI clustering triggers a signaling cascade via the FcRγ-chain. In 2007, GPVI-FcRγ-chain oligomerization on the surface of unstimulated platelets has been directly demonstrated,71 suggesting that, like dimerization of NKG2D, oligomerization of GPVI is necessary but not sufficient to trigger the receptor. In 2009, using Fab antibodies that bind to GPVI dimer but not to GPVI monomer, it has been shown that GPVI is present as a functionally relevant dimer on the platelet surface.134 Convulxin, a C-type lectin-like protein from the venom of the South American rattlesnake that functions as a potent agonist of GPVI, has been reported recently to form a dimer in solution and bind eight copies of GPVI.248
Other MIRRs. Human TREM-1 receptor has been shown to exist as a “head-to-tail” dimer in crystal, suggesting that the dimeric TREM-1 most likely contains two distinct ligand-binding sites.69 High-avidity ligands are thought to trigger TREM-1 and TREM-2, suggesting that formation of multivalent ligand-receptor complexes is a necessary step in TREM-1-mediated cell activation.24,177 Murine paired immunoglobulin-like receptor (PIR)-A and human leucocyte immunoglobulin-like receptor (LILR)-A2 (ILT/LIR7) complexed with the FcRγ signaling chain through their TM domains are also required to be clustered by a multivalent ligand in order to initiate TM signaling.25,249 Recently, it has been shown that integrin signaling in neutrophils and macrophages requires ITAM-containing adaptors, DAP-12 and FcRγ, suggesting that integrin signaling-mediated activation of cellular responses in these cells proceeds by an MIRR-like mechanism.250 Homotypic associations involving TM domains have been reported to represent a driving force for integrin activation, thus providing a structural basis for the coincidence of ligand-induced integrin clustering and activation.251,252
Orientation.
A rapidly growing body of experimental evidence strongly supports the importance of interreceptor orientation within ligand-specific MIRR dimers/oligomers for receptor triggering and generation of an activation signal. These findings strongly support the orientational restraints imposed by the SCHOOL model on the initiation of interreceptor homointeractions between signaling subunits in order to trigger MIRRs (Table 2, Figs. 6 and 7).4,49,97,130 Suggesting the importance of relative orientation,4,49,97 the model explains for the first time why random encounters of MIRRs by lateral diffusion or oligomeric forms of MIRRs existing in unstimulated cells68,126,133,253–255 and platelets71 do not result in MIRR triggering and cell activation.
T cell receptor. While direct biophysical measurements of the interreceptor relative orientation in ligand-specific TCR dimers/oligomers have not yet been performed, several lines of evidence indicate that relative orientation plays an important role in TCR-mediated cell activation. Using monoclonal antibodies (mAbs) specific to the TCR, it has been shown that T cell activation does not correlate with the affinity of the mAbs but rather with the recognized epitope.208 In other studies, triggering of different epitopes of the TCR-CD3-ζ2 receptor complex has been also reported to induce different modes of T cell activation,204–207 suggesting that TCR signaling is not a simple “on-off ” switch through cross-linking and/or clustering. In addition, high concentrations of anti-TCR, but not anti-CD3, induce a proliferative response without antibody cross-linking.206 Also, anti-TCR and anti-CD3 have been demonstrated to be different in their capacity to induce responsiveness to IL-4,207 and in their requirement for costimulatory signals.204 Yang and Parkhouse have reported that stimulation of T cells with a panel of anti-CD3 mAb recognizing different epitopes has differential functional consequences.159 This demonstrated, for the first time, that differences in activation mechanisms exist not only between TCR and CD3, but also between epitopes within CD3, and the authors postulated that occupancy of different CD3 epitopes may result in different degrees of conformational change in the receptor complex.159 These results were further confirmed in other studies.256 In thymocytes, only anti-TCRβ Ab but not anti-TCRα Ab cause long-term TCR downmodulation.160 Using three-dimensional fluorescence quantitation methods, signaling-induced reorientation of T cell receptors that cannot be mediated by simple passive diffusion has been shown to take place during immunological synapse formation.257 In 2007, a change in the orientation of the TCR with respect to the membrane induced by binding to pMHC was proposed to play an important role in TCR signaling.142 In line with the SCHOOL model, conclusions about the importance of interreceptor orientation in the ligand-specific TCR dimers/oligomers have been also made in 2007 by Minguet et al.258 who suggested the so-called permissive geometry model of TCR signaling.231 Recently, the SCHOOL mechanism-suggested role of the rotation of the TCRs in regulation of downstream signaling pathways has been confirmed in homology modeling and molecular dynamics simulation studies on the positioning of autoimmune TCR-Ob.2F3 and TCR-Ob.3D1 on the MBP85-99/HLA-DR2 complex.259 In contrast to these multiple independent studies, Cochran et al.233 have reported that intermolecular orientation is not critical for triggering T cell activation. However, to address this issue, the authors have used in their studies pMHC dimers coupled via flexible chemical cross-linkers that do not prevent rotation of pMHC molecules around their long axis. This assumption is further supported by the authors' findings that estimated distances for the used cross-linkers in fully extended conformations (50, 70 and 90 Å) did not correlate with the apparent hydrodynamic diameter values experimentally determined for the corresponding cross-linked pMHC dimers in the surprisingly narrow range of 70 to 75 Å.233 Thus, these dimers cannot be considered as conformationally constrained, thus suggesting a lack of control over the interreceptor orientation in these experiments.233
The three-dimensional structures of the three A6-TCR/peptide/HLA-A2 complexes that generate very different T cell signals have been found to be remarkably similar to each other and to the wild-type agonist complex, suggesting that different signals are not generated by different ligand-induced conformational changes in the αβTCR.260 This is in agreement with the SCHOOL model proposing that different signaling oligomers can be formed and therefore different T cell signals can be generated depending on the intermolecular relative orientation in the ligand-specific TCR dimers/oligomers rather than ligandinduced EC conformational changes.4,49,97
In summary, a vast majority of the experimental findings reported so far strongly support an importance of interreceptor relative orientation in ligand-specific TCR clusters for TCR triggering and cell activation.
B cell receptor. BCRs have been proposed and confirmed to organize into oligomeric clusters on the B cell surface.126,253–255 The observed basal BCR clustering does not result in receptor triggering and subsequent cell activation suggesting that the oligomerization of the BCR is necessary but not sufficient for receptor activation,255 and that interreceptor relative orientation in the BCR dimers/oligomers plays an important role in receptor triggering. The differential effects of the point mutations in various parts of the TM sequence of BCR membrane Ig (mIg) have been reported to differentially affect B cell activation induced by mono- or polyvalent anti-mIg antibodies, thus providing more evidence for importance of correct intermolecular orientation in BCR signaling.41
Fc receptors. As shown for FcεRI, it is not only the number of crosslinked receptors that determines the magnitude of mediator secretion-causing signal induced by different mAbs, but also the relative orientation of receptors within the produced dimers, thus suggesting the importance of the orientational restraint in ligand-specific FceRI dimers/oligomers for generating competent activation signal.163,164,171,178,179,261 Further, in the IgA receptor, FcαRI, a positively charged arginine residue within the TM domain of ligand recognition α chain promotes association with the signaling FcRγ chain.153 Studies of signaling through mutants of the FcαRI have shown that a vertical relocation of this TM positive charge does not have any significant effect on proximal and distal receptor functions, whereas a lateral transfer of the positive charge completely abrogates these functions.38 A possible explanation for these findings is that a vertical relocation of the noncovalent electrostatic bond does not change interreceptor relative orientation within the receptor dimers/oligomers formed upon multivalent ligand stimulation while lateral transfer does.
NK receptors. Existence of dimeric NKG2D receptor complexes in both NKG2D crystals and at the surface of unstimulated NK cells68,242,243 suggests that not only dimerization, but also relative orientation of receptors within ligand-specific NKG2D dimers/oligomers, plays an important role in receptor triggering.
Glycoprotein VI. Similar to NKG2D receptor complexes, GPVI has been found to form a back-to-back dimer in the GPVI crystal64 and to exist in an oligomeric state on the surface of unstimulated platelets,71 suggesting an important role of interreceptor relative orientation within these oligomers in GPVI signaling.
Oligomerization of signaling subunits.
According to the SCHOOL model, homooligomerization of the CYTO domains of MIRR signaling subunits drives formation of competent signaling oligomers, leading to triggering of the receptor and initiation of the signaling cascade (Figs. 6 and 7).4,49,97,130 Importantly, this homooligomerization also plays a crucial role in amplification and lateral propagation of the activation signal(s) (Figs. 6 and 7). The model also suggests that depending on the nature of stimuli, different signaling subunits can be oligomerized and become phosphorylated, thus triggering distinct signaling pathways and resulting in different functional outcomes.4,49,97,98 The experimental data obtained to date for different MIRRs strongly support this part of the main concept of the SCHOOL model.
The ability of the random-coil TCRζ CYTO domain to oligomerize was first reported in 2004,78 and later, independently confirmed in cell studies on the activity of membrane-anchored chimeric β2m/peptide molecules fused with the CYTO domain of ζ chain.131 Similarly, the propensity of the BCR Igα and Igβ signaling subunits to oligomerize78 has been recently independently confirmed and demonstrated to result in the ability of the BCR Igα/Igβ heterodimer to assemble into oligomers.262
Both in vitro and in vivo studies have shown that dimerization of CD3ε is critical and sufficient to substitute for a pre-TCR signal and drive double-positive transition, suggesting that the property of the pre-TCR responsible for β-selection is the autonomous formation of oligomers, which brings CD3 signaling subunits in close proximity to each other.235,236 These findings further confirm the ability of CD3ε to dimerize, first reported in 2004 for the CD3ε CYTO domain,78 and proves the physiological importance of this dimerization suggested by the SCHOOL model.4,49,97,98 Interestingly, the CD3ε EC domain has been recently shown to form a homodimeric structure in vitro.263
As reported,264 FceRIβ and γ signaling subunits independently dissociate from a ligand-binding α chain immediately after crosslinking with multivalent ligand. Moreover, these signaling subunits dissociate in the oligomerized form. Interestingly, only γ chains are oligomerized on surfaces of cells stimulated with a suboptimal concentration of antigen, while β chains remain dispersed.264 In contrast, stimulation of cells with an optimal concentration of antigen results in the distinct oligomerization of both signaling subunits.264
Dissociation.
Within the SCHOOL model, dissociation of competent signaling oligomers from both engaged and nonengaged ligand-recognition subunits upon multivalent ligand stimulation, plays an important role in MIRR triggering, initiation of the signaling cascade, and signal amplification and propagation (Figs. 6 and 7).4,49,97 Experimental data accumulated to date strongly support this dissociation mechanism.
In activated T cells, the CD3 and ζ signaling chains have been shown to independently dissociate from the remaining receptor subunits.265–268 In line with the SCHOOL model, TCRs lacking ζ are endocytosed more rapidly than completely assembled receptors.269 Further, degradation of ζ promoted by its interaction with the lysosomal protein LAPTM5 has been recently shown to result in TCR downmodulation and to represent a unique mechanism for the control of surface TCR expression and T cell activation.270 For BCR, it has been reported that, upon binding of moderate-to low-affinity antigen, the Igα/Igβ subunits physically dissociate from mIg resulting in BCR desensitization.196 Interestingly, although desensitized cells fail to respond to receptor ligation by a high dose of antigen or by anti-Igλ antibodies, the dissociated Igα/Igβ signaling complex retains signaling function if aggregated by anti-Igβ antibodies.196 In this context, similar mechanisms are proposed by the SCHOOL model to be involved in the BCR desensitization,196,197,199 T cell clonal anergy186,189,190,271,272 and in the inhibition of T cell activation by the so-called TCR core peptide (CP).273 The ligand-mediated physical dissociation of the activated BCR complex has been later confirmed in other studies.274 In 2005,275 using primary murine B cells, it has been found that while >95% of the mIg is internalized following anti- Ig-induced aggregation, 20–30% of Igβ remains on the surface, suggesting that mIg and Igβ may function independently following the initial stages of signal transduction. As mentioned, upon crosslinking of the FcεRI with multivalent ligand, oligomerized signaling β and γ chains immediately dissociate from a ligand-recognition α chain.264
Duration of the ligand-receptor contact.
The SCHOOL model of MIRR signaling suggests that the multivalent ligand-receptor contact should last long enough to bring two or more MIRRs in sufficient proximity and correct relative orientation toward each other and hold them together to promote the interreceptor CYTO homointeractions between signaling subunits, thus initiating the downstream signaling cascade (Figs. 6 and 7).4,49,97,130 It should be noted that duration of the MIRR-ligand interaction generally correlates with the strength (affinity/avidity) of the ligand. Clearly, the strength of the ligand determines not only duration of the ligand-MIRR contact but also lifetime of an individual receptor in the engaged MIRR dimer/oligomer. These important aspects of the model are also consistent with the experimental data accumulated so far.
In T cells, the results of multiple reports show a broad correlation between the duration of TCR-ligand interaction and ligand potency.91,276,277 The importance of prolonged binding to antigen-presenting cells for T cell fate decisions has also been recently reported.278,279 A similar interpretation is possible for the data on a revised model of kinetic proofreading in which the duration of TCR engagement regulates the efficiency with which signals trickle through the rapidly reversible early activation pathways to induce later responses.280,281 It is also known that the off-rate of ligand binding plays a role in determining the specificity of the TCR-generated signal in a population of T cells that can discriminate between self and nonself in the thymus.282 Also, the number of TCR ITAMs required for efficient positive or negative selection has been reported to vary depending upon the affinity of the TCR/ligand interaction.283 In studies on T cell activation by bacterial superantigens, a simple relationship between the affinity of the Staphylococcus enterotoxin C3 (SEC3)-TCR interaction and the functional responses has been proposed; stronger binding results in stronger T cell responses.92 As recently shown, short-lived pMHC ligands induce anergy in T cell clones in vitro and specific memory T cells in vivo.284 Total signal strength has been demonstrated to determine the capacity of primed T cells to respond to homeostatic cytokines, to survive cytokine withdrawal and to accumulate in vivo.285 The strength of antigen stimulation is also known to regulate T cell progression through thresholds of proliferation, differentiation and death.286 The diffusion trapping of interacting TCRs and pMHCs in the plasma membrane has also been recently suggested to play an important role in TCR triggering.287 Interestingly, the affinity that defines the threshold for negative selection has been determined in studies using three transgenic mouse strains expressing distinct class I MHC-restricted TCRs.288 The authors also concluded that the affinity threshold for self-tolerance appears to be a constant for cytotoxic T lymphocytes.288
Similar to T cells, the B cell response to antigen varies as a function of antigen/BCR interaction affinity.289 As demonstrated, above the threshold, concentration of antigen required to trigger a response decreases as the affinity increases.289 BCR signal strength has been shown to determine B cell fate.290 Importantly, continuous receptor signaling of a defined amplitude appears to be critical for development and survival of mature B cells.291 It is also known that, upon binding of moderate- to low- but not high-affinity antigen, the Igα/Igβ subunits physically dissociate from mIg resulting in BCR desensitization.196 A critical role of receptor affinity in antigen-driven selection of B cell clones in vivo has also been suggested based on studies of stable B cell transfectants.292 Recently, the strength of the initial BCR-triggered activation signal has been proposed to finally determine the eventual duration of BCR signaling and the rate of its transmission through downstream pathways.293
A great body of evidence shows that the capacity of downstream signaling by an individual FcεRI depends on its capacity to remain in a cluster and is therefore influenced by the ligand affinity/avidity.62,94,173,180–182,294 The ability of a similar signaling mechanism to trigger distinct FcεRI-mediated mast cell responses like mediator release and survival has been reported to be determined by the FcRγ signal strength or duration.181,295 Interestingly, recent findings redefine FcαRI as a bifunctional inhibitory/activating receptor of the immune system that mediates both antiand proinflammatory functions of IgA, depending on ligand multimericity and duration of multivalent ligand-induced receptor signaling.183 In platelets, affinity/avidity of interaction of GPVI with collagen or convulxin has been suggested to play an important role in receptor signaling and GPVI-mediated platelet activation.95,247
For more information on the important role of the ligand-MIRR complex lifetime in receptor triggering I refer the reader to recent reviews.277,281,294,296–298
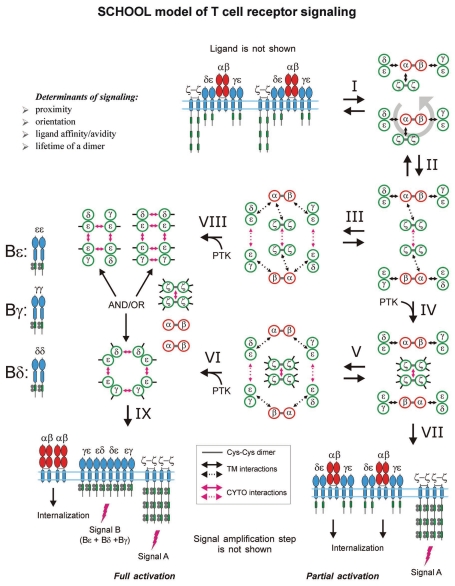
SCHOOL Model of T Cell Receptor Signaling
Description.
The TCR is a multisubunit complex composed of the ligand-binding clonotypic αβ heterodimer, as well as the heterodimeric CD3δε and CD3γε signaling components and the disulfide-linked ζ homodimer that contain one (CD3ε, γ and δ chains) or three (ζ) ITAMs, respectively (Figs. 2 and 8). This receptor complex provides an intriguing ability of T cells to discern and differentially respond to MHC-bound peptides that can differ by only a single amino acid. The mechanism by which the precise ligand-binding specificities of the TCR are converted into the distinct intracellular signaling processes and diverse functional outcomes has been one of the most controversial topics in T cell immunology. The SCHOOL model suggests not only the mechanism of TCR triggering and cell activation that can explain the majority of immunological phenomena observed experimentally but also proposes distinct ways to control and modulate the T cell-mediated immune response.
Figure 8.
SCHOOL model of T cell receptor (TCR) signaling. Interaction with multivalent ligand (not shown) clusters the receptors and pushes them to reorientate (I), to bring signaling subunits into a correct (permissive) relative orientation and in sufficient proximity in the formed receptor oligomer (for illustrative purposes, receptor dimer is shown), and thus to promote the trans-homointeractions between ζ molecules (II). Then, two alternative pathways can take a place depending on the nature of activating stimuli. First is going through a stage IV resulting in formation of ζ2 dimer (dimer of dimers) and phosphorylation of the ζ ITAM tyrosines, thus triggering the activation signal A. Then, the signaling ζ oligomers formed subsequently dissociate from the TCR-CD3 complex, resulting in internalization of the remaining engaged TCR-CD3 complexes (VII). This pathway leads to partial (or incomplete) T cell activation. Alternatively, the intermediate complex formed at the stage II can undergo further rearrangements, starting trans-homointeractions between CD3 proteins (III) and resulting in formation of an oligomeric intermediate. The stages I, II and III can be reversible or irreversible depending on interreceptor proximity and relative orientation of the receptors in TCR dimers/oligomers as well as on time duration of the TCR-ligand contact and lifetime of the receptor in TCR dimers/oligomers that generally correlate with the nature of the stimulus and its specificity and affinity/avidity. Next, in the signaling oligomers formed (III), the ITAM tyrosines undergo phosphorylation by PTKs that leads to generation of the activation signal, dissociation of signaling oligomers and internalization of the remaining engaged TCRαβ chains (VIII, XI). This pathway provides at least two different activation signals from the ζ and CD3 signaling oligomers (signals A and B), respectively, and results in full T cell activation. The distinct signaling through ζ and CD3 oligomers (or through various combinations of signaling chains in CD3 oligomeric structures) might be also responsible for distinct functions such as T cell proliferation, effector functions, T cell survival, pathogen clearance, TCR anergy, etc. In addition, the signaling oligomers formed can sequentially interact with the signaling subunits of nonengaged TCRs resulting in formation of higher-order signaling oligomers, thus amplifying and propagating the activation signal (not shown). Also, this leads to the release and subsequent internalization of the remaining nonengaged TCR complexes and/or TCRαβ chains (not shown). Immunoreceptor tyrosine-based activation motifs (ITAMs) are shown as green rectangles. TCR-CD3-ζ components are represented as whole polypeptides and as a simplified axial view. Circular arrows indicate ligand-induced receptor reorientation. Black and magenta arrows indicate specific intersubunit hetero- and homointeractions between transmembrane and cytoplasmic domains, respectively. All interchain interactions in intermediate complexes are shown by dotted arrows reflecting their transition state. Phosphate groups are shown as filled gray circles. In an axial view, one solid small black line depicts one phosphorylated ITAM domain. Abbreviations: PTK, protein tyrosine kinase; SCHOOL, signaling chain homooligomerization.
The overall rigid geometry and topology of the TCR is defined by electrostatic interactions between TCRαβ TM domains and TM domains of different signaling dimers: CD3γε, CD3δε and ζ2.32,33,41 Interestingly, the TCRζ subunit seems to have a unique and dynamic relationship with the TCR-CD3 complex since only this signaling homodimer appears to turn over independently from the rest of the TCR complex on the cell surface.299 Assuming that different TCR signaling modules provide distinct signaling and T cell functional outcomes,44,49,97,130,135,300,301 the SCHOOL model of T cell activation suggests that depending on the nature of activating stimuli, two or more TCRs can be clustered to dimer/oligomer in different relative orientations that promote homointeractions between different signaling subunits. This results in formation of distinct CD3 and/or ζ signaling oligomers and their activation through the phosphorylation of the corresponding ITAM tyrosines (Fig. 8), thus initiating distinct signaling cascades and leading to distinct functional outcomes.
Within the model (Fig. 8), two or more TCRs are clustered to dimer/oligomer with sufficient interreceptor proximity upon binding with multivalent ligand, and simultaneously rotate around the receptor axis perpendicular to the membrane to adopt a correct relative orientation toward each other, permissive of initiating the trans-homointeractions between ζ molecules. Until the ζ ITAM tyrosines are phosphorylated by PTK(s), this process is reversible and its reversibility can depend on duration of the TCR-ligand contact that generally correlates with the strength (affinity/avidity) of the ligand, and sufficient lifetime of a receptor in TCR dimers/oligomers. At this point of bifurcation, two alternative pathways (Fig. 8, stages IV and III) leading to partial or full T cell activation, respectively, can take place depending on the nature of activating stimuli. As a result, either ζ or both ζ and CD3 signaling oligomers are formed with subsequent phosphorylation of ITAM tyrosines by PTKs and dissociation from remaining TCR-CD3 complexes or TCRαβ chains. At this irreversible stage, downstream signaling events are triggered. Later, the remaining TCR-CD3 complexes or TCRαβ chains are internalized. According to the proposed model, at least two different activation signals (shown in the Fig. 8 as signals A and B) can be provided from the ζ and CD3 signaling oligomers, and both signals are required for full activation of T cells. Within the model, the different activation signals Bε, Bδ and Bγ are delivered through CD3ε, δ and γ signaling oligomers, respectively (Fig. 8). Thus, distinct signaling is achieved through ζ and CD3 signaling oligomers and/or through various combinations of signaling chains in oligomeric CD3 structures (Fig. 8). Then, the signaling oligomers formed from the initially engaged TCR dimer/oligomer can sequentially homointeract with the relevant signaling subunits of nonengaged TCRs resulting in formation of higher-order signaling oligomers with their subsequent phosphorylation and dissociation from ligand-binding subunits (as illustrated for all MIRRs in Figs. 6 and 7). This process leads to amplification and lateral propagation of the activation signal(s). Later, the remaining nonengaged TCR-CD3 complexes or TCRαβ chains are internalized.
Therefore, in the context of the model, TCR clustering by the MHCs bound to agonist, partial agonist or antagonist peptides results in formation of receptor dimers/oligomers with similar interreceptor proximity but different intermolecular orientation. This does or does not lead to the initiation of homointeractions between different signaling subunits with their subsequent oligomerization and ITAM Tyr phosphorylation, providing distinct signaling and T cell functional outcomes. This mechanism is also proposed for T cell activation mediated by other stimuli such as anti-TCRα, anti-TCRβ, anti-CD3ε, etc.
Comparison to other models.
There exist numerous models of TCR triggering and their modifications,2 including, but not limited to, a kinetic proofreading model,280,296,298,302–306 serial triggering model,162,307–310 serial encounter model,311 conformational models,126,165,193–195,258,312–319 permissive geometry model258 and clustering,52,59,60,75,224,258 segregation320–322 and mechanosensor256 models. However, despite the rapidly growing number of models and their modifications, no current model explains at the molecular level: (1) how ligand-induced TCR TM signaling commences, and (2) how this process occurs differentially for altered ligands or in altered cellular contexts.
Some of the models suggested so far were rejected in further studies. An example is a conformational model that suggests a lipid-dependent folding transition of the TCRζ CYTO domain to be a molecular switch linking ligand-induced TCR clustering and phosphorylation of the ζ ITAM tyrosines.315 Later, similar conformational model of TCR triggering was suggested based on a lipid-dependent folding transition of CD3ε CYTO domain.323,324 However, other studies have shown that the membrane binding mode of the ζ and CD3ε CYTO domains depends on lipid composition and revealed that lipid bilayers of the membrane models used315,323,324 are unstable and fuse and rupture upon protein binding, thus highlighting the importance of the choice of an appropriate membrane model for protein-lipid interactions studies. Binding of the ζ and CD3ε CYTO domains to stable lipid bilayers is not accompanied by a structural transition to a folded form, thus contradicting this conformational model.79,80 In addition, in contrast to the finding reported,315 phosphorylated ζ is still able to bind to stable lipid bilayers,79,80 further contradicting the suggested conformational model.315
In addition, most of the current models have been developed by investigators to describe their own experimental data. As a consequence, these models are mostly descriptive and often fail by trying to explain most of the immunological data accumulated to date. Many of the models suggested to date simply describe a phenomenon but not the mechanisms underlying the phenomenon. Examples include clustering models52,59,60,75,224,258 that describe a requirement for multivalent ligand to trigger TCR but do not explain the specific molecular mechanisms underlying those observations. Importantly, the lack of these mechanisms in a vast majority of the existing models prohibits the identification of clinically important points of therapeutic intervention. Table 3 illustrates comparative features of the currently existing models and demonstrates how these distinctive models for the first time can be readily combined into one model, the SCHOOL model of TCR triggering and TM signaling.
Table 3.
Comparison of different models for TCR triggering
| Model | Requirements/restraints imposed (+) or not (−) by a model | |||
| Ligand multivalency | Relative interreceptor orientation in TCR oligomers | Duration of ligand-MIRR contact/lifetime of TCR oligomers | Ligand affinity/avidity | |
| Kinetic proofreading | − | − | + | + |
| Serial triggering | − | − | + | + |
| Serial encounter | − | − | + | + |
| Conformational | − | − | + | + |
| Permissive geometry | + | + | − | − |
| Clustering | + | − | − | + |
| Segregation | − | − | + | + |
| Mechanosensor | − | − | + | + |
| SCHOOL | + | + | + | + |
Utility.
The powerful ability of the SCHOOL model to describe, explain and predict TCR-related immunological phenomena, providing a mechanistic explanation at the molecular level, is illustrated in Table 4. Selected examples are also described below in more detail.
Table 4.
Molecular mechanisms suggested or predicted by the SCHOOL model to underlie selected T cell-mediated immunological phenomena and observations
| Phenomenon | Observation | Mechanism |
| Inhibitory effect of TCR CP | TCR CP inhibits Ag- but not Ab-stimulated TM signal transduction and efficiently abrogates T cell-mediated immune responses in mice and man in vitro and in vivo.273,326,330,334,335 | TCR CP disrupts TCrα-CD3δε and TCrα-ζ TM interactions resulting in predissociation of these signaling subunits from the remaining complex, and thus preventing the formation of signaling oligomers upon Ag but not Ab stimulation and, consequently, inhibiting T cell activation.4,49,97,98,129 |
| Diversity of TCR-mediated cell response | Precise ligand-binding specificities of the TCR are converted into diverse functional outcomes.380–382 | Slightly different ligands bring two or more TCRs in different relative orientations that favor homointeractions between different signaling subunits and result in formation of different signaling oligomers or their combinations, thus initiating distinct signaling pathways and leading to diverse T cell functional outcomes.4,49,97 Thus, the signaling pathway and the direction of the response depends on the type of TCR signaling subunit(s) that is (are) oligomerized and ITAM-phosphorylated upon ligand stimulation. |
| Different TCR signaling subunits engage partially distinct signaling pathways.143–146 | ||
| CD3 signaling subunits play differential biological role as revealed by human immunodeficiencies.192 | ||
| T cell clonal anergy | Ag-unresponsive anergic T cells fail to produce IL-2 but produce comparable amounts of IFNγ and proliferate to similar extents in response to immobilized anti-CD3/CD28 mAbs.186 | Depending on the quality of Ag (affinity, avidity, specificity, etc.,) stimulation can induce dissociation of TCR CD3 and/or ζ signaling subunits from the remaining TCrαβ subunits and/or TCrαβ-CD3 complexes, respectively, thus preventing Ag186- or anti-TCR188- but not anti-CD3 mAbs186-mediated formation of signaling oligomers and generation of activation signal (Figs. 6–8). |
| Ag-induced tolerance in vivo is accompanied by altered early TCR-mediated signaling events.271 | ||
| T cell anergy is induced by activating but not by non-activating anti-CD3.272 | Depending on epitope location, anti-CD3 stimulation can induce formation of CD3 but not ζ signaling oligomers, thus leading to partial cell activation and preventing Ag-mediated T cell response. Depending on dissociated subunit(s), TCR-mediated signaling events in anergic cells and therefore the functional outcomes can be altered differently. | |
| T cell self-tolerance depends on the TCR's affinity for pMHC ligand, and the threshold for negative selection falls within the micromolar affinity range.288 | ||
| Comodulation of non-engaged TCRs | Activation of T cells with pMHC, bacterial superantigens, or anti-Vβ antibodies downmodulates not only directly stimulated (engaged) TCR complexes but also unstimulated (nonengaged) ones.268,307,383–386 | Upon ligand stimulation, signaling oligomers dissociate from the remaining engaged TCRs that undergo internalization. Then, the dissociated signaling oligomers sequentially interact with the signaling subunits of nonengaged TCRs resulting in the release and subsequent internalization of the remaining nonengaged TCRαβ-CD3 complexes or TCRαβ chains. internalization and intracellular fate may be different for TCR-CD3 complexes lacking ζ chain or for TCRαβ chains remaining on the cell surface after dissociation of either ζ or both ζ and CD3 signaling oligomers, respectively.49,97,130 |
| In the IS, only a small fraction of the TCR is bound to specific pMHCs.321 | ||
| TCR signaling initiation and following lateral signal propagation and amplification | TCR signaling is initiated and sustained in microclusters and is terminated in the TCR-rich central supramolecular activation cluster (cSMAC), a structure from which TCR are sorted for degradation.184 | The initially formed signaling oligomers initiate TCR signaling, dissociate from the remaining engaged TCRs and interact with the signaling subunits of nonengaged TCRs, thus propagating the activation signal to nonengaged receptors and resulting in signal amplification and lateral propagation. |
| Exposure of the CD3εcyt epitope | Ligand engagement of TCR- results in exposure of a cryptic proline-rich CD3εcyt epitope that is a binding site for the adaptor protein, Nck.165,193 | During full T cell activation, dissociation of CD3εγ and/or CD3εδ signaling oligomers from TCRαβ chains (Fig. 8) induces the release/unmasking of the CD3εcyt epitope. Thus, within the SCHOOL model, the ligand-induced exposure of the epitope is effect not cause of TCR triggering. During partial T cell activation, formation of only ζ signaling oligomers and their dissociation from the remaining TCR-CD3 complexes (Fig. 8) do not release/unmask the CD3εcyt epitope. |
| The CD3εcyt epitope is recognized by antibody APA1/1 and is only detected when the TCR is fully activated.194,195 in the IS, distribution of APA1/1 epitope is more restricted than ζ, CD3ε and Tyr-phosphorylated proteins.194 | ||
| Inhibitory action of HIV-1 gp41 FP | HIV-1 FP colocalizes with CD4 and TCR molecules, coprecipitates with the TCR, and inhibits Ag- but not Ab-specific T cell proliferation and proinflammatory cytokine secretion in vitro.387 | Similarly to the TCR-CP, the HIV-1 gp41 FP disrupts TCRα-CD3δε and TCRα-ζ2 TM interactions resulting in dissociation of these signaling subunits from the remaining complex, and thus preventing the formation of signaling oligomers upon Ag but not Ab stimulation and, consequently, inhibiting Ag- but not Ab-mediated T cell activation.4,221,339 |
| The peptide blocks the TCR/CD3 TM interactions needed for Ag-triggered T cell activation.388 | ||
| Pre-TCR signaling | Spontaneous pre-TCR oligomerization mediated by the pre-TCRα chain results in ligand-independent receptor triggering and TM signaling crucial for early T cell development.235,236 | Oligomerization of the pre-TCR through the pre-TCRα chain brings CD3 and ζ signaling subunits in close proximity and proper relative orientation, thus promoting formation of signaling oligomers and generating the activation signal. remarkably, as predicted by the SCHOOL model, formation of CD3ε dimers/oligomers is necessary and sufficient to induce the CD3ε ITAM Tyr phosphorylation and lead to cell response. |
| Forced dimerization of CD3ε is sufficient to simulate pre-TCR function and promote β-selection.235 | ||
| Differential TCR signaling | Point mutations in the TCRβ TM domain impair the development and function of CD8+ memory T cells without affecting primary effector T cell responses.351 | These mutations disrupt TCRβ-CD3εγ TM interactions and result in functional disconnection or predissociation of CD3εγ. This prevents the formation of CD3γ signaling oligomers and therefore the generation of CD3γ-related activation signal (signal Bγ, Fig. 8). |
| Epitope-dependent mAb stimulation | T cell activation induced by mAbs specific for the TCR does not correlate with the affinity of the mAbs but rather with the recognized epitope.208 | Clustering/oligomerization of TCRs by different antibodies results in different intermolecular relative orientations within receptor cluster/oligomer that promote (or do not) homointeractions between different signaling subunits, leading to the formation of different CD3 and/or ζ signaling subunit oligomers and therefore to different functional outcomes. If intermolecular relative orientation in the antibody-crosslinked TCR cluster/oligomer does not promote homointeractions between CD3 and/or ζ signaling subunits, this antibody will not stimulate T cell response. |
| Triggering of different epitopes of the TCR-CD3-ζ2 receptor complex depends on the mAb specificity and induces different modes of T cell activation.159,204–207 | ||
| In thymocytes, only anti-TCRβ but not anti-TCRα Ab reagents cause long-term TCR downmodulation.160 | ||
| Coexistence of mono- and multivalent (oligomeric) TCRs in resting cells | Monovalent TCRs coexist in intact resting cells with multivalent complexes with two or more ligand-binding TCRαβ subunits,133,389 raising a question: why does this basal TCR clustering not lead to receptor triggering whereas ligand-induced clustering does? | In resting cells, receptors within multivalent TCR complex have the relative orientation that does not promote homointeractions between CD3 and/or ζ signaling chains. Upon stimulation with multivalent ligand, these receptors adopt proper orientation relative to each other, starting homotypic interactions between signaling subunits and resulting in generation of the activation signal. A similar mechanistic explanation can also account for the existence of dimeric or tetrameric TCR-CD3-coreceptor complexes in naive CD4+ or CD8+ T cells.230 |
Abbreviations: Ab, antibody; Ag, antigen; CP, core peptide; FP, fusion peptide; IFN, interferon; IS, immunological synapse; HIV, human immunodeficiency virus; mAb, monoclonal antibody; pMHC, major histocompatibility complex (MHC)-bound peptide; TCR, T cell antigen receptor; TM, transmembrane; ζcyt, TCR ζ cytoplasmic domain.
Clinically relevant TCR CP, or TCR mimic peptide, represents a synthetic peptide corresponding to the sequence of the TM region of the ligand-binding TCRα chain critical for TCR assembly and function.325 This and similar TM peptides capable of inhibiting antigen-stimulated TCR-mediated T cell activation were first reported in 1997.326 Since that time, despite extensive basic and clinical studies of these peptides,273,327–336 the molecular mechanisms of action of these clinically relevant peptides have not been elucidated until 2004 when the SCHOOL model was first introduced.49 Within this model,4,49,97,98,129,221,222 the TCR CP competes with the TCRα chain for binding to CD3δε and ζ hetero- and homodimers, respectively, thus resulting in functional disconnection and predissociation of the signaling subunits from the remaining receptor complex. The proposed mechanism is the only mechanism consistent with all experimental and clinical data reported to date for TCR TM peptides and their lipid and/or sugar conjugates. The model also predicts that the same mechanisms of inhibitory action can be applied to MIRR TM peptides corresponding to the TM regions of not only the MIRR recognition subunits but the corresponding signaling subunits as well.4,49,97,98,129,222 This was recently confirmed experimentally334,337 by showing that the synthetic peptides corresponding to the sequences of the TM regions of the signaling CD3 (δ, ε or γ) and ζ subunits are able to inhibit the immune response in vivo. Importantly, the SCHOOL model is the first model that not only clearly explains the molecular mechanisms of action of TCR TM peptides4,49,97,98,129,222 but also extends the concept of their action through these mechanisms to other TM peptides of MIRRs and to the MIRR-mediated processes involved in viral pathogenesis.4,98,129,221,222,338–340
Interestingly, the model suggests a molecular explanation for the apparent discrepancies in in vitro and in vivo activities of cell-permeable chemical inducers of dimerization.341–343 In 1993, it was reported that in vitro chemically induced dimerization/oligomerization of the TCRζ CYTO domain results in T cell activation, as measured with a reporter gene assay.341 Later, activation of a chimeric receptor, containing binding domains for chemical inducers of dimerization fused to the CYTO tail of TCRζ chain, after stimulation with chemical dimerizers in Jurkat cells has been confirmed to show tyrosine phosphorylation of the TCRζ chain chimera, recruitment of phosphorylated Zap70, and generation of NFAT.342 However, in vivo studies demonstrated that signaling did not lead to increased expression of activation markers, T cell proliferation or apoptosis.342 The authors concluded that signaling through ζ alone is not sufficient to generate downstream events leading to full T cell activation or thymocyte selection; instead, additional CD3 components must be required to induce a functional response in primary thymocytes and peripheral T cells.342 Within the model, formation of both CD3γε/δε and ζ signaling oligomers is needed to provide competent activation signal(s) resulting in full cell activation (signals A and B, Fig. 8). Formation of only ζ signaling oligomers leads to partial T cell activation (signal A, Fig. 8).
The model also explains the apparent discrepancy in CD3 TM peptide activity between in vitro and in vivo T cell inhibition.334 It has been shown that the CD3δ and CD3γ TM peptides do not impact T cell function in vitro (the CD3ε TM peptide has not been used in the reported in vitro experiments because of solubility issues) but that all three CD3 (ε, δ and γ) TM peptides decrease signs of inflammation in an adjuvant-induced arthritis rat model in vivo and inhibit the immune response.334 Within the SCHOOL model,44,49,98,130,222 the CD3δ and CD3γ TM peptides disconnect the corresponding signaling subunits (CD3δ and CD3γ, respectively) from the remaining receptor complex. Thus, these subunits do not participate in further processes upon antigen stimulation. On the other hand, the previously reported in vitro activation studies with T cells lacking CD3γ and/or CD3δ CYTO domains indicate that antigen-stimulated induction of cytokine secretion and T cell proliferation are intact,344–346 thus explaining the absence of inhibitory effect of the CD3δ and CD3γ TM peptides in the in vitro activation assays used.334 However, in vivo deficiency either of CD3δ or CD3γ results in severe immunodeficiency disorders.192,347–349 This could explain the inhibitory effect observed in the in vivo studies for all three CD3 TM peptides.334 Thus, these experimental data confirm that our ability to selectively “disconnect” specific signaling subunits using the MIRR TM peptides in line with the SCHOOL model can provide a powerful tool to study MIRR functions and immune cell signaling.4,98
Interestingly, studies of T lymphocytes expressing a TCR with a mutant TCRβ TM domain have shown that upon antigen stimulation, these cells are similar to wild-type cells in terms of IL-2 secretion, IL-2 receptor expression and early activation and signaling events such as CD69 expression, Ca2+ flux, and CD3ε and ζ phosphorylation, but are specifically defective in undergoing activation-induced cell death.350 Point mutations in the TCRβ TM domain has been also reported to impair the development and function of CD8+ memory T cells without affecting primary effector T cell responses.351 Considering that in the TCR-CD3-ζ2 complex, the TCRβ TM domain is critical for interaction with the CD3γε signaling heterodimer,33 one can suggest that in a mutant TCR, the association of the CD3γε with the TCRβ chain is impaired. Upon antigen stimulation, this impaired (weakened) association prevents formation of CD3γε signaling oligomers and thus excludes CD3γ (but not CD3ε, because in the CD3εδ heterodimer of the TCR-CD3-ζ2 complex, there is another CD3ε chain capable of signaling independently of CD3ε in the CD3γε) from further participation in signaling and results in the lack of the activation signal Bγ (Fig. 8). Thus, within the model, only those signaling events that involve CD3γ (i.e., apoptotic response but not early activation and signaling events345,346,352,353) should be affected by a mutation of the TCRβ TM domain. This is in a good agreement with the data reported.350,351 Also, in this context, functional effect of this mutation should be and is very similar to the one observed by Collier et al. for CD3γ TM peptide,334 therefore providing more evidence for importance and utility of the proposed model and the MIRR TM peptides in studies on immune signaling.
The remarkable feature of the SCHOOL model is that it has a high predictive quality (Table 4) by generalizing molecular mechanisms of action and therefore potential therapeutic targets for all MIRRs.4,49,97,98,129,130,222
SCHOOL Model of FcεRI Receptor Signaling
Description and utility.
Structurally, all Fc receptors can be divided into two major categories: single- (FcγRIIA and FcγRIIA) and multichain (FcεRI, FcαRI, FcγRI and FcγRIIIA) receptors. Multichain Fc receptors, in turn, can be divided into two subcategories: receptors that contain one (FcαRI, FcγRI, FcγRIIIA) or two (FcεRI) signaling subunits (Fig. 2). To date, no general model has been suggested to explain at the molecular level how Fc receptor-mediated signaling commences.
As a general model of MIRR signaling, the SCHOOL model describes the molecular mechanisms underlying the receptor triggering for all multichain Fc receptors.4,49,97,130 The model also suggests that the FcεRI receptor that contains two different signaling subunits, β and γ (or FcRγ), has more capabilities to induce distinct signaling pathways and, therefore, lead to different functional outcomes as compared to the Fc receptors that contain only one signaling subunit (FcRγ) (Fig. 2).
The FcεRI receptor consists of a ligand-binding α subunit and two kinds of signaling subunits, a β chain and disulfide-linked homodimeric γ chains (Figs. 2 and 9). It plays a pivotal role in the initiation of allergic reactions when antigen crosslinks IgE antibodies bound to FcεRI on tissue mast cells or blood basophils.123,241,354–356
Figure 9.
SCHOOL model of FcεRI signaling. Interaction with multivalent ligand (not shown) clusters the receptors and pushes them to reorientate (I), to bring signaling subunits into a correct (permissive) relative orientation and in sufficient proximity in the formed receptor oligomer (for illustrative purposes, receptor dimer is shown), and thus to promote the trans-homointeractions between γ homodimers (II). Then, two alternative pathways can take a place depending on the nature of activating stimuli. First is going through a stage IV resulting in formation of γ2 dimer (dimer of dimers) and phosphorylation of the γ ITAM tyrosines, thus triggering the activation signal A. Then, the signaling γ oligomers formed subsequently dissociate from the α/β complex, resulting in internalization of the remaining engaged complexes (VII). Alternatively, the intermediate complex formed at the stage II can undergo further rearrangements, starting trans-homointeractions between β chains (III) and resulting in formation of an oligomeric intermediate. Stages I, II and III can be reversible or irreversible depending on interreceptor proximity and relative orientation of the receptors in FcεRI dimers/oligomers as well as on time duration of the receptor-ligand contact and lifetime of the receptor in FcεRI dimers/oligomers that generally correlate with the nature of the stimulus and its specificity and affinity/avidity. Next, in the signaling oligomers formed (III), the γ and β ITAM tyrosines undergo phosphorylation by PTKs that leads to generation of the activation signal, dissociation of signaling oligomers and internalization of the engaged α chains (VIII, XI). This pathway provides two different activation signals from the γ and β signaling oligomers (signals A and B), respectively, and results in full cell activation. In addition, the signaling oligomers formed can sequentially interact with the signaling subunits of nonengaged FcεRIs resulting in formation of higher-order signaling oligomers, thus amplifying and propagating the activation signal (not shown). Also, this leads to the release and subsequent internalization of the nonengaged α and/or αβ chains (not shown). Immunoreceptor tyrosine-based activation motifs (ITAMs) are shown as green rectangles. FceRIα, β and γ components are represented as whole polypeptides and as a simplified axial view. Black and magenta arrows indicate specific intersubunit hetero- and homointeractions between transmembrane and cytoplasmic domains, respectively. All interchain interactions in intermediate complexes are shown by dotted arrows reflecting their transition state. Circular arrow indicates ligand-induced receptor reorientation. Phosphate groups are shown as filled gray circles. In an axial view, one solid small black line depicts one phosphorylated ITAM domain. Abbreviations: PTK, protein tyrosine kinase; SCHOOL, signaling chain homooligomerization; FcεRI, high affinity IgE receptor.
In resting cells, like with other MIRRs, intrareceptor TM interactions between FcεRIα, β and γ chains define the overall rigid geometry and topology of the FcεRI.35–37,39,43,155 Within the proposed model, upon stimulation with multivalent ligand, two or more FcεRIs are brought into close proximity and adopt a correct relative orientation, initiating the interreceptor trans-homointeractions between signaling subunits and weakening the intrareceptor TM interactions (stages I and II, Fig. 9). Then, depending on the duration of the FcεRI-ligand interaction (affinity/avidity of the ligand), the receptors can either go back to a resting state or forward to an active state, in which β and/or γ signaling oligomers are formed (stages III and IV, Fig. 4), thus promoting ITAM Tyr phosphorylation and generation of activation signal. Assuming that two different FcεRI signaling subunits, γ and β, provide distinct signaling,139,140,264,357–363 the model suggests49,97,130 that depending on the nature of ligand, the FcεRIs can be clustered to dimer/oligomer in different relative orientations that, in turn, promote homotypic interactions between different signaling subunits (Fig. 9). This leads to formation of distinct, γ and/or β, signaling oligomers, phosphorylation of the corresponding ITAM tyrosines and generation of different activation signals (signals A and B, Fig. 9), resulting in diverse functional outcomes. The formed β and/or γ oligomers can sequentially interact with β and/or γ subunits of nonengaged FcεRIs (as illustrated for all MIRRs in Figs. 6 and 7), thus amplifying and propagating the activation signal.
Interestingly, several mathematical models have been recently developed for the early signaling events mediated by FcεRI.364–367 Through model simulations, it has been shown how changing the ligand concentration, and consequently the concentration of receptor aggregates, can change the nature of a cellular response as well as its amplitude. These models are largely based on the recently suggested sequence of early events in FcεRI signaling.173,368 Combining the basic organizing principles of the SCHOOL model with the existing mathematical models might significantly improve our understanding the spatiotemporal organization of FcεRI-mediated signal transduction as well as our ability to predict how this system will behave under a variety of experimental conditions.
Selected examples illustrating the ability of the SCHOOL model to provide a mechanistic explanation for FcεRI-related immunological phenomena are shown in Table 5. These and other findings mentioned above strongly support the validity and utility of the proposed activation model for the FcεRI.
Table 5.
Molecular mechanisms suggested or predicted by the SCHOOL model to underlie selected FCεRI-mediated immunological phenomena and observations
| Phenomenon | Observation | Mechanism |
| FcεRI-mediated signaling and cellular responses | Signaling capacity of FcεRIs depends on the driving forces leading to their clustering and also on fine tuning provided by both lifetime (or receptor capacity to remain in a cluster that is influenced by the ligand affinity) and interreceptor relative orientation in the FcεRI dimers/oligomers.179,294 | Triggering FcεRI requires close proximity of the receptors and a correct relative orientation in the FceRIs clustered (or altered in preexisting clusters) by multivalent ligand binding (Fig. 9).4,49,97,130 It also requires the ligand-receptor contact to last long enough to initiate the trans-homointeractions between signaling subunits and weaken the intrareceptor TM interactions, thus resulting in formation of signaling FcεRIβ and/or FcεRIγ oligomers and generation of activation signal (Fig. 9).4,49,97 |
| There is no simple correlation between multivalent ligand-promoted FcεRI clustering and FcεRI-mediated cellular responses, such as cell degranulation.178 | ||
| The ratio of late to early FcεRI-stimulated events correlates with the affinity of a ligand for the receptor-bound IgE.94 | Receptor clustering induced upon binding to multivalent ligand is necessary but not sufficient for the initiation of FcεRI signaling. To commence signal transduction, two or more clustered receptors should adopt a correct relative orientation toward each other permissive of initiating the trans-homointeractions between β and/or γ subunits, and remain in a cluster long enough to promote these interactions and therefore formation of signaling oligomers (Fig. 9).4,49,97 | |
| Orientational restraint in ligand-specific FceRI dimers/oligomers determines the magnitude of mediator secretion-causing signal induced by different mAbs.163,164,171,178,179,261 | ||
| Different roles of β and γ subunits in signaling | The FcεRI β and γ subunits play different roles in signaling.357–363 The γ chain aggregation alone can evoke cellular responses,360,363 while the β chain acts as an amplifier for signaling.139 Also, β chains can elicit a signal in a γ chain-independent manner.264 | Depending on the nature of ligand (i.e., its specificity, affinity and avidity), the FcεRIs are clustered to dimer/oligomer in different relative orientations that promote homotypic interactions between different signaling subunits (Fig. 9). As a result, different, β and/or γ, signaling oligomers are formed, generating distinct activation signals and therefore distinct signaling pathways.4,49,97 |
| Lateral propagation of activation signal | FcεRI-activated mast cells propagate signals from small signaling domains around dimerized/oligomerized receptors; formation of large FcεRI aggregates promotes both strong receptor triggering and rapid termination of the signaling responses.63 | The initially formed β and/or γ signaling oligomers initiate FcεRI signaling, dissociate from the remaining engaged receptors and interact with the signaling subunits of nonengaged FcεRIs, thus propagating the activation signal to nonengaged receptors and resulting in signal amplification and lateral propagation. |
Abbreviations: FcεRI, high affinity IgE receptor; mAb, monoclonal antibody; TM, transmembrane.
SCHOOL Model of B Cell Receptor Signaling
Description and utility.
The BCR is a multimeric complex composed of mIg noncovalently associated with a disulfide-linked Igα/Igβ heterodimer that is responsible for signal transduction. In the resting state, like with other MIRRs, intrareceptor TM interactions between mIg and Igα/Igβ subunits define the overall rigid geometry and topology of the BCR.34,40,41,369 In cells, this receptor transduces signals leading to a variety of biologic responses including antigen receptor editing, apoptotic death, developmental progression, cell activation, proliferation and survival. Despite several BCR triggering and cell activation models that have been suggested,126,168,237,370–372 no model fully explains the molecular mechanisms underlying spatiotemporal organization of BCR-triggered TM signal transduction.
Within the SCHOOL model, two or more BCRs are brought into close proximity and adopt a correct relative orientation upon receptor engagement with multivalent ligand (Fig. 10). At this point, the trans-homointeractions between Igα and Igβ molecules are initiated, weakening the TM interactions within the BCR (Fig. 10, stages I and II). Then, depending on the duration of ligand-BCR interaction and therefore on the affinity/avidity of ligand, the receptors can go either back to resting state or forward to active state, in which signaling Igα/Igβ oligomers are formed, thus promoting ITAM Tyr phosphorylation and generation of activation signal (Fig. 10, stage III). Considering that Igα and Igβ chains can play different physiological roles,136–138,373 the model suggests that depending on the nature of stimuli, different Igα/Igβ signaling oligomers can form, thus resulting in phosphorylation of Igα and/or Igβ ITAM tyrosines and induction of distinct signaling pathways. Further, once formed, these oligomers can sequentially interact with Igα/Igβ subunits of nonengaged BCRs (as illustrated for all MIRRs in Figs. 6 and 7), thus propagating and amplifying the activation signal and favoring the formation and stabilization of supramolecular complexes that can promote sustained signaling. In this context, it can also be suggested that the more BCRs are initially engaged and/or the higher is the affinity/avidity of antigen, the faster is signaling cluster formation.
Figure 10.
Interaction with multivalent ligand (not shown) clusters the receptors and pushes them to reorientate (I), to bring signaling subunits into a correct (permissive) relative orientation and in sufficient proximity in the formed receptor oligomer (for illustrative purposes, receptor dimers are shown), and thus to promote the trans-homointeractions between Igα/Igβ heterodimers (II). Formation of competent signaling oligomers results in phosphorylation of the ITAM tyrosines, thus triggering the activation signal (III). Then, the signaling oligomers formed subsequently dissociate from mIg, resulting in internalization of the remaining engaged receptor chains (IV). Stages I and II can be reversible or irreversible depending on interreceptor proximity and relative orientation of the receptors in ligand-specific dimers/oligomers as well as on time duration of the receptor-ligand contact and lifetime of the receptor in these dimers/oligomers that generally correlate with the nature of the stimulus and its specificity and affinity/avidity. The BCR signaling module contains two different signaling chains, Igα and Igβ. This provides a possibility of the signal and cell response diversity depending on the particular set of the Igα and/or Igβ ITAM tyrosines that become phosphorylated. Further, the signaling oligomers formed can sequentially interact with the signaling subunits of nonengaged receptors resulting in formation of higher-order signaling oligomers, thus amplifying and propagating the activation signal (not shown). Also, this leads to the release and subsequent internalization/downmodulation of the nonengaged mIg chains (not shown). Immunoreceptor tyrosine-based activation motifs (ITAMs) are shown as green rectangles. Receptor components are represented as whole polypeptides and as a simplified axial view. Black and magenta arrows indicate specific intersubunit hetero- and homointeractions between transmembrane and cytoplasmic domains, respectively. All interchain interactions in intermediate complexes are shown by dotted arrows reflecting their transition state. Circular arrow indicates ligand-induced receptor reorientation. Phosphate groups are shown as filled gray circles. In an axial view, one solid small black line depicts one phosphorylated ITAM domain. Abbreviations: PTK, protein tyrosine kinase; SCHOOL, signaling chain homooligomerization; BCR, B cell receptor.
In contrast to Igβ and other ITAM-containing proteins, the dynamic equilibrium between monomeric and oligomeric species of Igα is slow, and this protein forms stable homooligomers (mostly, dimers and tetramers) even at very low protein concentrations.78 Formation of the stable Igα/Igβ clusters/oligomers may be particularly important for sustained signaling during the synapse formation between B cell and antigen-displaying target cell and subsequent antigen acquisition.374 Also, as recently shown,375 plasma membrane association of Igα/Igβ complexes results in a generation of biologically relevant basal signaling while the ability of the BCR to interact with both conventional as well as nonconventional EC ligands is eliminated.
As illustrated in Table 6 by several selected examples, the proposed model is capable of providing a mechanistic explanation for BCR-related immunological phenomena. Thus, a vast majority of the experimental findings reported so far strongly support the validity and utility of this activation model for the BCR.
Table 6.
Molecular mechanisms suggested or predicted by the SCHOOL model to underlie selected BCR-mediated immunological phenomena and observations
| Phenomenon | Observation | Mechanism | |
| BCR-mediated signaling and cellular responses | B cell response is induced by multivalent but not monovalent ligand stimulation,1 and Ag valency influences B cell responses by modulating the stability of BCr-signaling microdomains and BCR trafficking.2 | Triggering BCR requires multivalent ligand-induced clustering of the BCRs in a close proximity and a correct relative orientation in the formed clusters (or reorientation of the receptors in preexisting oligomers/clusters) (Fig. 10).3–6 Also, the more BCRs are initially engaged, the faster is Igα/Igβ signaling oligomer formation and the stronger is the amplified activation signal. | |
| Formation of Igα/Igβ oligomers | Signaling Igα/Igβ heterodimer assembles into oligomers upon ligand stimulation.7 | Upon multivalent ligand stimulation, BCRs are clustered in close proximity and correct relative orientation, thus promoting homotypic interactions between Igα/Igβ signaling subunits. This leads to formation of signaling oligomers and phosphorylation of the ITAM tyrosines, thus initiating the signaling cascade.3–6 | |
| Comodulation of nonengaged BCRs | Unligated BCRs cluster with BCRs engaged by multivalent ligands.1 | Upon multivalent ligand stimulation, signaling Igα/Igβ oligomers dissociate from the remaining engaged migs that undergo internalization. Then, the dissociated oligomers sequentially interact with the signaling subunits of nonengaged receptors resulting in their activation and therefore the signal amplification and propagation. This also leads to the release and subsequent internalization of the remaining nonengaged mIg chains.3,4,6 | |
| The extent of BCR internalization is not correlated with Ag valency, suggesting that BCR signaling and internalization are distinct processes.1 | |||
| Upon anti-Ig-induced BCR clustering, >95% of the mIg is internalized, whereas 20–30% of Igβ remains on the surface.8 | |||
| B cell tolerance/BCR desensitization | Monomeric hen egg lysozyme (HEL) efficiently engages the specific BCR, however, presentation of HEL-derived epitopes is impaired compared to multivalent antigens.9 | Monovalent or moderate to low-affinity Ag stimulation induces dissociation of BCR Igα/Igβ signaling subunits from the remaining mIg, thus preventing Ag- or anti-Ig- but not anti-Igβ mAbs-mediated formation of signaling oligomers and generation of activation signal (Figs. 6, 7 and 10).3,4,6 The remaining mig chains are internalized. | |
| Soluble monovalent Ag, administered intravenously, induces B cell tolerance.10,11 | |||
| Upon binding of moderate- to low-affinity Ag, physical dissociation of the Igα/Igβ subunits from mIg results in BCR desensitization.12 However, these desensitized cells can be still activated by anti-Igβ antibodies.12 | Within the SCHOOL model, the ligand-induced dissociation of signaling subunits from ligand recognition subunits is suggested to be a general molecular mechanism underlying T and B cell tolerance, BCR desensitization, and TM peptide-modulated T and other immune cell response.3–5,13,14 | ||
Abbreviations: Ag, antigen; BCR, B cell antigen receptor; mAb, monoclonal antibody; mIg, membrane immunoglobulin; TM, transmembrane.
SCHOOL Model of Platelet Glycoprotein VI Receptor Signaling
Description and utility.
Studies of patients deficient in GPVI identified this platelet membrane protein as a physiological collagen receptor. This receptor is noncovalently associated with FcRγ, the ITAM-containing homodimeric signaling module. The GPVI-FcRγ receptor complex induces platelet activation when it binds to collagen or other agonists, and GPVI-deficient platelets lack specifically collagen-induced aggregation and the ability to form thrombi on a collagen surface under flow conditions.16,376 The selective inhibition of GPVI and/or its signaling is thought by most experts in the field to inhibit thrombosis without affecting hemostatic plug formation. Thus, future therapeutic strategies targeting platelet-mediated disease depend on our detailed understanding of the molecular mechanisms underlying GPVI triggering and subsequent TM signal transduction. In addition, knowing these mechanisms would give us a new handle in dissecting the basic structural and functional aspects of thrombus formation.
In 2006, GPVI has been reported to form a back-to-back dimer in the GPVI crystal.64 Based on these findings, a model for GPVI signaling has been suggested, in which GPVI clustering triggers a signaling cascade via the FcRγ chain coreceptor.64 Despite its apparent similarity to the SCHOOL model,49 it does not explain the existence of oligomeric GPVI-FcRγ complexes at the surface of unstimulated platelets71 and does not suggest specific protein-protein interactions involved in the molecular mechanisms underlying the GPVI-triggered signaling. These findings64,71 raise an important and intriguing question: why does the observed basal receptor dimerization not lead to receptor triggering and subsequent platelet activation whereas agonist-induced receptor crosslinking/clustering does?
Despite extensive studies of the GPVI-FcRγ receptor complex and its mechanism of action,16,244,377,378 the only model that can answer this question and even more important, mechanistically explain how GPVI-mediated TM signaling begins, is the SCHOOL model.4,49,97,98,130,223,338,379 Within this model, GPVI-mediated platelet activation is a result of the interplay between GPVI-FcRγ TM interactions, the association of two TM Asp residues in the FcRγ homodimer with the TM Arg residue of GPVI,36 that maintain receptor integrity in platelets under basal conditions, and homointeractions between FcRγ subunits that lead to formation of signaling oligomers and initiation of a signaling response (Figs. 4B and 11). Binding of the multivalent ligand (collagen) to two or more GPVI-FcRγ receptor complexes pushes the receptors to cluster, rotate and adopt an appropriate orientation relative to each other (Fig. 11, stages I and II), at which point the trans-homointeractions between FcRγ molecules are initiated. Upon formation of FcRγ signaling oligomers, the Srcfamily kinases Fyn or Lyn phosphorylate the tyrosine residues in the FcRγ ITAM that leads to generation of the activation signal (Fig. 11, stage III) and subsequent dissociation of FcRγ signaling oligomers and downmodulation of the remaining engaged GPVI subunits (Fig. 11, stage IV). Later, the dissociated oligomeric FcRγ chains can interact with FcRγ subunits of the nonengaged GPVI-FcRγ complexes, resulting in formation of higher-order signaling oligomers and their subsequent phosphorylation, thus providing lateral signal propagation and amplification (as illustrated for all MIRRs in Figs. 6 and 7).
Figure 11.
SCHOOL model of GPVI signaling. Interaction with multivalent ligand (not shown) clusters the receptors and pushes them to reorientate (I), to bring signaling subunits into a correct (permissive) relative orientation and in sufficient proximity in the formed receptor oligomer (for illustrative purposes, receptor dimer is shown), and thus to promote the trans-homointeractions between FcRγ homodimers (II). Formation of competent signaling oligomers results in phosphorylation of the ITAM tyrosines, thus triggering the activation signal. Then, the signaling oligomers formed subsequently dissociate from GPVI, resulting in downmodulation of the remaining engaged receptor chains (IV). Stages I and II can be reversible or irreversible depending on interreceptor proximity and relative orientation of the receptors in ligand-specific dimers/oligomers as well as on time duration of the receptor-ligand contact and lifetime of the receptor in these dimers/oligomers that generally correlate with the nature of the stimulus and its specificity and affinity/avidity. Further, the signaling oligomers formed can sequentially interact with the signaling subunits of nonengaged receptors resulting in formation of higher-order signaling oligomers, thus amplifying and propagating the activation signal (not shown). Also, this leads to the release and subsequent downmodulation of the nonengaged GPVI chains (not shown). Immunoreceptor tyrosine-based activation motifs (ITAMs) are shown as green rectangles. Receptor components are represented as whole polypeptides and as a simplified axial view. Black and magenta arrows indicate specific intersubunit hetero- and homointeractions between transmembrane and cytoplasmic domains, respectively. All interchain interactions in intermediate complexes are shown by dotted arrows reflecting their transition state. Circular arrow indicates ligand-induced receptor reorientation. Phosphate groups are shown as filled gray circles. In an axial view, one solid small black line depicts one phosphorylated ITAM domain. Abbreviations: PTK, protein tyrosine kinase; SCHOOL, signaling chain homooligomerization; GPVI, glycoprotein VI receptor.
Thus, for the preformed oligomeric receptor complexes existing in unstimulated platelets as found by Berlanga et al.71 the proposed model suggests that under basal conditions, the overall geometry of the receptor dimer keeps FcRγ chains apart, whereas stimulation by collagen results in breakage of GPVI-GPVI EC interactions and reorientation of signaling FcRγ homodimers, thus bringing them into close proximity and appropriate relative orientation permissive of initiating the FcRγ homointeractions and receptor triggering.
Intriguingly, suggesting how binding to collagen triggers the GPVI-mediated signal cascade at the molecular level, the SCHOOL model reveals GPVI-FcRγ TM interactions as a novel therapeutic target for the prevention and treatment of platelet-mediated thrombotic events.4,98,129,222,223,338,379 Preliminary experimental results provided support for this novel concept of platelet inhibition and resulted in the development of novel class of promising platelet inhibitors.223,338
Thus, the experimental evidence accumulated to date on the GPVI-mediated TM signal transduction and platelet activation strongly support the validity and utility of the proposed activation model for this receptor.
SCHOOL Model of Other Multichain Immune Recognition Receptor Signaling
As illustrated in Figure 2, a structural assembly of many MIRRs, such as FcαRI, FcγRI, FcγRIIIA, ILT/LIR receptors, DCAR, NK and TREM receptors, etc., is very similar to that of the GPVI receptor; all these receptors have a ligand-recognition subunit and one homodimeric signaling subunit. Another CLR receptor, BDCA-2 (not shown in Fig. 2), is known to signal through the associated FcRγ signaling chain and has a typical MIRR assembly similar to that of DCAR (Fig. 2).8 Thus, the basic principles of GPVI triggering and TM signaling suggested by the SCHOOL model can be easily applied to these and other, structurally related, MIRRs. Selected examples illustrating the capability of the SCHOOL model to provide a mechanistic explanation for immunological phenomena mediated by these receptors are shown in Table 7.
Table 7.
Molecular mechanisms suggested or predicted by the SCHOOL model to underlie selected MIRR-mediated immunological phenomena and observations
| Phenomenon | Observation | Mechanism |
| FcαRI-mediated signaling and cellular responses | Vertical relocation of the TM positive charge responsible for FcαRI-FcRγ association does not effect on calcium flux, MAPK phosphorylation, and IL-2 release, whereas its lateral transfer completely abrogates these functions.38 | Vertical relocation of the noncovalent electrostatic bond does not affect interreceptor relative orientation within the FcαRI dimers/oligomers formed upon multivalent ligand stimulation, whereas lateral transfer does, thus preventing formation of FcRγ signaling oligomers and initiation of signaling cascade. |
| NKR-mediated signaling and cellular response | Short CPs derived from the TM sequence of NKRs inhibit NK cell cytolytic activity.337 | NKR CPs disrupt the TM interactions between NKR ligand-binding subunits and associated homodimeric signaling subunits, such as ζ-ζ, γ-γ or DAP-12.4,98,129,222 |
| Immune escape in hCMV pathogenesis | hCMV tegument protein pp65 interacts directly with NKp30, leading to dissociation of the linked ζ signaling subunit and, consequently, to reduced killing.200 | Binding to pp65 protein affects the NKp30-ζ2 TM interactions resulting in dissociation of the ζ signaling subunit from the remaining complex, and thus preventing the formation of ζ signaling oligomers upon ligand stimulation and, consequently, inhibiting NK cell cytolytic activity.4,98,129,222 |
| TREM-mediated signaling | Structurally similar receptors, TREM-1 and TREM-2 (Fig. 2) that contain the same signaling subunit, DAP-12, show activating and inhibitory functions.24,177 | Depending on the affinity/avidity of the ligand, ligand stimulation can result in: (1) receptor clustering, formation of oligomeric signaling subunits and generation of the activation signal (TREM-1, activating function), or (2) dissociation of signaling subunit from the engaged receptor and unmasking a specific “inhibitory” epitope(s) in the cytoplasmic tail of ligand recognition subunit (TREM-2, inhibitory function). |
Abbreviations: Ag, antigen; CPs, core peptides; hCMV, human cytomegalovirus; DAP-12, DNAX activation protein 12; mAb, monoclonal antibody; MAPK, mitogen-activated protein kinase; NKRs, natural killer cell receptors; TM, transmembrane; TREM, triggering receptors expressed on myeloid cells.
Conclusions
The crucial role of receptor-mediated signaling in health and disease assumes that our understanding of the underlying molecular mechanisms and methods to modulate the cell response through control of transmembrane signal transduction can contribute significantly towards the improvement of existing therapies and the design of new therapeutic strategies for a diverse set of disorders. Recently discovered functional link between protein intrinsic disorder and oligomericity in structurally related multichain activating receptors represents a missing piece of the long-standing puzzle of signaling and reveals striking similarities in the basic mechanistic principles of function of most single- and multichain receptors. In this context, suggesting that formation of competent signaling oligomers mediated by homointeractions between well-structured (SRs) or intrinsically disordered (MIRRs) cytoplasmic signaling domains is a necessary and sufficient event to trigger receptor function, the SCHOOL-based mechanism represents a general biological mechanism of transmembrane signal transduction. Thus, within the SCHOOL platform, ligand binding-induced receptor clustering is translated across the membrane into protein oligomerization in cytoplasmic milieu. The basic principles of transmembrane signaling learned from the SCHOOL model can be used in different fields of immunology, virology, molecular and cell biology and others to describe, explain and predict various phenomena and processes mediated by a variety of functionally diverse and unrelated receptors. Beyond providing novel perspectives for fundamental research, the platform opens new avenues for drug discovery and development.
For MIRRs, the proposed SCHOOL model is the first general model that provides a set of basic principles of MIRR signaling and mechanistically explains how MIRR-mediated signaling commences and what the major driving forces and restraints of MIRR triggering/signaling are. Furthermore, this model is the first model that can describe, explain and predict numerous MIRR-mediated immunological phenomena. Thus, this model represents a powerful tool that can be used in dissecting the basic structural and functional aspects of the immune response and in further using this knowledge in both fundamental and clinical fields. In addition, revealing the major driving forces and fundamental stages of MIRR triggering and TM signal transduction, the model identifies effective ways of modulating the immune response.
Importantly, by generalizing mechanistic features of MIRR signaling, the SCHOOL model shows how the similar structural architecture of the MIRRs dictates similar mechanisms of MIRR triggering and subsequent TM signal transduction, and furthermore, reveals similar therapeutic targets in seemingly unrelated diseases. This permits the transfer of accumulated knowledge and pharmacological approaches between seemingly disparate immune disorders and builds the molecular basis for existing and future therapeutic strategies. Impressively, applications of this model have already illustrated how do the similar molecular mechanisms of MIRR signaling revealed by the model work in seemingly unrelated fields, such, for example, as T cell-mediated diseases, platelet-mediated thrombosis, and viral entry into target cells without triggering host-defense response.
In conclusion, I sincerely hope that the model and issues presented in this work will stimulate debate and new research to further test and apply the proposed model, thus opening new horizons in our multidisciplinary knowledge and generating new perspectives for the effective prevention and/or treatment of numerous disorders.
Footnotes
Previously published online: www.landesbioscience.com/journals/selfnonself/article/10832
References
- 1.Rudd CE. Disabled receptor signaling and new primary immunodeficiency disorders. N Engl J Med. 2006;354:1874–1877. doi: 10.1056/NEJMp068062. [DOI] [PubMed] [Google Scholar]
- 2.Sigalov AB, editor. Multichain Immune Recognition Receptor Signaling: From Spatiotemporal Organization to Human Disease. New York: Springer-Verlag; 2008. [PubMed] [Google Scholar]
- 3.Keegan AD, Paul WE. Multichain immune recognition receptors: similarities in structure and signaling pathways. Immunol Today. 1992;13:63–68. doi: 10.1016/0167-5699(92)90136-U. [DOI] [PubMed] [Google Scholar]
- 4.Sigalov AB. Immune cell signaling: a novel mechanistic model reveals new therapeutic targets. Trends Pharmacol Sci. 2006;27:518–524. doi: 10.1016/j.tips.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Hubbard SR, Till JH. Protein tyrosine kinase structure and function. Annu Rev Biochem. 2000;69:373–398. doi: 10.1146/annurev.biochem.69.1.373. [DOI] [PubMed] [Google Scholar]
- 6.Zhou T, Mountz JD, Kimberly RP. Immunobiology of tumor necrosis factor receptor superfamily. Immunol Res. 2002;26:323–336. doi: 10.1385/IR:26:1-3:323. [DOI] [PubMed] [Google Scholar]
- 7.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-β regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 8.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krogsgaard M, Davis MM. How T cells ‘see’ antigen. Nat Immunol. 2005;6:239–245. doi: 10.1038/ni1173. [DOI] [PubMed] [Google Scholar]
- 10.DeFranco AL. B-cell activation 2000. Immunol Rev. 2000;176:5–9. [PubMed] [Google Scholar]
- 11.Dal Porto JM, Gauld SB, Merrell KT, Mills D, Pugh-Bernard AE, Cambier J. B cell antigen receptor signaling 101. Mol Immunol. 2004;41:599–613. doi: 10.1016/j.molimm.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Takai T. Fc receptors and their role in immune regulation and autoimmunity. J Clin Immunol. 2005;25:1–18. doi: 10.1007/s10875-005-0353-8. [DOI] [PubMed] [Google Scholar]
- 13.Takai T. Fc receptors: their diverse functions in immunity and immune disorders. Springer Semin Immunopathol. 2006;28:303–304. doi: 10.1007/s00281-006-0055-y. [DOI] [PubMed] [Google Scholar]
- 14.Colonna M, Nakajima H, Navarro F, Lopez-Botet M. A novel family of Ig-like receptors for HLA class I molecules that modulate function of lymphoid and myeloid cells. J Leukoc Biol. 1999;66:375–381. doi: 10.1002/jlb.66.3.375. [DOI] [PubMed] [Google Scholar]
- 15.Borrego F, Kabat J, Kim DK, Lieto L, Maasho K, Pena J, et al. Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol Immunol. 2002;38:637–660. doi: 10.1016/s0161-5890(01)00107-9. [DOI] [PubMed] [Google Scholar]
- 16.Moroi M, Jung SM. Platelet glycoprotein VI: its structure and function. Thromb Res. 2004;114:221–233. doi: 10.1016/j.thromres.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 17.Barclay AN, Brown MH. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6:457–464. doi: 10.1038/nri1859. [DOI] [PubMed] [Google Scholar]
- 18.Kanazawa N, Tashiro K, Miyachi Y. Signaling and immune regulatory role of the dendritic cell immunoreceptor (DCIR) family lectins: DCIR, DCAR, dectin-2 and BDCA-2. Immunobiology. 2004;209:179–190. doi: 10.1016/j.imbio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Biassoni R, Cantoni C, Falco M, Pende D, Millo R, Moretta L, et al. Human natural killer cell activating receptors. Mol Immunol. 2000;37:1015–1024. doi: 10.1016/s0161-5890(01)00018-9. [DOI] [PubMed] [Google Scholar]
- 20.Biassoni R, Cantoni C, Marras D, Giron-Michel J, Falco M, Moretta L, et al. Human natural killer cell receptors: insights into their molecular function and structure. J Cell Mol Med. 2003;7:376–387. doi: 10.1111/j.1582-4934.2003.tb00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoki N, Kimura S, Xing Z. Role of DAP12 in innate and adaptive immune responses. Curr Pharm Des. 2003;9:7–10. doi: 10.2174/1381612033392503. [DOI] [PubMed] [Google Scholar]
- 22.Bakker AB, Baker E, Sutherland GR, Phillips JH, Lanier LL. Myeloid DAP12-associating lectin (MDL)-1 is a cell surface receptor involved in the activation of myeloid cells. Proc Natl Acad Sci USA. 1999;96:9792–9796. doi: 10.1073/pnas.96.17.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Berg TK, Yoder JA, Litman GW. On the origins of adaptive immunity: innate immune receptors join the tale. Trends Immunol. 2004;25:11–16. doi: 10.1016/j.it.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 25.Takai T. Paired immunoglobulin-like receptors and their MHC class I recognition. Immunology. 2005;115:433–440. doi: 10.1111/j.1365-2567.2005.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakahashi C, Tahara-Hanaoka S, Totsuka N, Okoshi Y, Takai T, Ohkohchi N, et al. Dual assemblies of an activating immune receptor, MAIR-II, with ITAM-bearing adapters DAP12 and FcRgamma chain on peritoneal macrophages. J Immunol. 2007;178:765–770. doi: 10.4049/jimmunol.178.2.765. [DOI] [PubMed] [Google Scholar]
- 27.Fujimoto M, Takatsu H, Ohno H. CMRF-35-like molecule-5 constitutes novel paired receptors, with CMRF-35-like molecule-1, to transduce activation signal upon association with FcRγ. Int Immunol. 2006;18:1499–1508. doi: 10.1093/intimm/dxl083. [DOI] [PubMed] [Google Scholar]
- 28.Stewart CA, Vivier E, Colonna M. Strategies of natural killer cell recognition and signaling. Curr Top Microbiol Immunol. 2006;298:1–21. doi: 10.1007/3-540-27743-9_1. [DOI] [PubMed] [Google Scholar]
- 29.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. [PubMed] [Google Scholar]
- 30.Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser WG, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 31.Wu J, Cherwinski H, Spies T, Phillips JH, Lanier LL. DAP10 and DAP12 form distinct, but functionally cooperative, receptor complexes in natural killer cells. J Exp Med. 2000;192:1059–1068. doi: 10.1084/jem.192.7.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manolios N, Bonifacino JS, Klausner RD. Transmembrane helical interactions and the assembly of the T cell receptor complex. Science. 1990;249:274–277. doi: 10.1126/science.2142801. [DOI] [PubMed] [Google Scholar]
- 33.Call ME, Pyrdol J, Wiedmann M, Wucherpfennig KW. The organizing principle in the formation of the T cell receptor-CD3 complex. Cell. 2002;111:967–979. doi: 10.1016/s0092-8674(02)01194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michnoff CH, Parikh VS, Lelsz DL, Tucker PW. Mutations within the NH2-terminal transmembrane domain of membrane immunoglobulin (Ig) M alters Ig-α and Ig-β association and signal transduction. J Biol Chem. 1994;269:24237–24244. [PubMed] [Google Scholar]
- 35.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 36.Feng J, Garrity D, Call ME, Moffett H, Wucherpfennig KW. Convergence on a distinctive assembly mechanism by unrelated families of activating immune receptors. Immunity. 2005;22:427–438. doi: 10.1016/j.immuni.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng J, Call ME, Wucherpfennig KW. The assembly of diverse immune receptors is focused on a polar membrane-embedded interaction site. PLoS Biol. 2006;4:142. doi: 10.1371/journal.pbio.0040142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakema JE, de Haij S, den Hartog-Jager CF, Bakker J, Vidarsson G, van Egmond M, et al. Signaling through mutants of the IgA receptor CD89 and consequences for Fc receptor γ-chain interaction. J Immunol. 2006;176:3603–3610. doi: 10.4049/jimmunol.176.6.3603. [DOI] [PubMed] [Google Scholar]
- 39.Varin-Blank N, Metzger H. Surface expression of mutated subunits of the high affinity mast cell receptor for IgE. J Biol Chem. 1990;265:15685–15694. [PubMed] [Google Scholar]
- 40.Stevens TL, Blum JH, Foy SP, Matsuuchi L, DeFranco AL. A mutation of the mu transmembrane that disrupts endoplasmic reticulum retention. Effects on association with accessory proteins and signal transduction. J Immunol. 1994;152:4397–4406. [PubMed] [Google Scholar]
- 41.Zidovetzki R, Rost B, Pecht I. Role of transmembrane domains in the functions of B- and T-cell receptors. Immunol Lett. 1998;64:97–107. doi: 10.1016/s0165-2478(98)00100-x. [DOI] [PubMed] [Google Scholar]
- 42.Blum JH, Stevens TL, DeFranco AL. Role of the mu immunoglobulin heavy chain transmembrane and cytoplasmic domains in B cell antigen receptor expression and signal transduction. J Biol Chem. 1993;268:27236–27245. [PubMed] [Google Scholar]
- 43.Ra C, Jouvin MH, Kinet JP. Complete structure of the mouse mast cell receptor for IgE (FcepsilonRI) and surface expression of chimeric receptors (ratmouse-human) on transfected cells. J Biol Chem. 1989;264:15323–15327. [PubMed] [Google Scholar]
- 44.Sigalov AB. Protein intrinsic disorder and oligomericity in cell signaling. Mol Biosyst. 2010 doi: 10.1039/B916030M. [DOI] [PubMed] [Google Scholar]
- 45.Heldin CH. Dimerization of cell surface receptors in signal transduction. Cell. 1995;80:213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- 46.Metzger H. Transmembrane signaling: the joy of aggregation. J Immunol. 1992;149:1477–1487. [PubMed] [Google Scholar]
- 47.Lemmon MA, Schlessinger J. Regulation of signal transduction and signal diversity by receptor oligomerization. Trends Biochem Sci. 1994;19:459–463. doi: 10.1016/0968-0004(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 48.Klemm JD, Schreiber SL, Crabtree GR. Dimerization as a regulatory mechanism in signal transduction. Annu Rev Immunol. 1998;16:569–592. doi: 10.1146/annurev.immunol.16.1.569. [DOI] [PubMed] [Google Scholar]
- 49.Sigalov AB. Multichain immune recognition receptor signaling: different players, same game? Trends Immunol. 2004;25:583–589. doi: 10.1016/j.it.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 50.Cooper JA, Qian H. A mechanism for SRC kinase-dependent signaling by noncatalytic receptors. Biochemistry. 2008;47:5681–5688. doi: 10.1021/bi8003044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiss A, Schlessinger J. Switching signals on or off by receptor dimerization. Cell. 1998;94:277–280. doi: 10.1016/s0092-8674(00)81469-5. [DOI] [PubMed] [Google Scholar]
- 52.Boniface JJ, Rabinowitz JD, Wulfing C, Hampl J, Reich Z, Altman JD, et al. Initiation of signal transduction through the T cell receptor requires the multivalent engagement of peptide/MHC ligands. Immunity. 1998;9:459–466. doi: 10.1016/s1074-7613(00)80629-9. [DOI] [PubMed] [Google Scholar]
- 53.Holowka D, Baird B. Antigen-mediated IGE receptor aggregation and signaling: a window on cell surface structure and dynamics. Annu Rev Biophys Biomol Struct. 1996;25:79–112. doi: 10.1146/annurev.bb.25.060196.000455. [DOI] [PubMed] [Google Scholar]
- 54.Thyagarajan R, Arunkumar N, Song W. Polyvalent antigens stabilize B cell antigen receptor surface signaling microdomains. J Immunol. 2003;170:6099–6106. doi: 10.4049/jimmunol.170.12.6099. [DOI] [PubMed] [Google Scholar]
- 55.Bankovich AJ, Raunser S, Juo ZS, Walz T, Davis MM, Garcia KC. Structural insight into pre-B cell receptor function. Science. 2007;316:291–294. doi: 10.1126/science.1139412. [DOI] [PubMed] [Google Scholar]
- 56.Puffer EB, Pontrello JK, Hollenbeck JJ, Kink JA, Kiessling LL. Activating B cell signaling with defined multivalent ligands. ACS Chem Biol. 2007;2:252–262. doi: 10.1021/cb600489g. [DOI] [PubMed] [Google Scholar]
- 57.Deng L, Langley RJ, Brown PH, Xu G, Teng L, Wang Q, et al. Structural basis for the recognition of mutant self by a tumor-specific, MHC class II-restricted T cell receptor. Nat Immunol. 2007;8:398–408. doi: 10.1038/ni1447. [DOI] [PubMed] [Google Scholar]
- 58.Adams EJ, Chien YH, Garcia KC. Structure of a γδ T cell receptor in complex with the nonclassical MHC T22. Science. 2005;308:227–231. doi: 10.1126/science.1106885. [DOI] [PubMed] [Google Scholar]
- 59.Bachmann MF, Ohashi PS. The role of T-cell receptor dimerization in T-cell activation. Immunol Today. 1999;20:568–576. doi: 10.1016/s0167-5699(99)01543-1. [DOI] [PubMed] [Google Scholar]
- 60.Bachmann MF, Salzmann M, Oxenius A, Ohashi PS. Formation of TCR dimers/trimers as a crucial step for T cell activation. Eur J Immunol. 1998;28:2571–2579. doi: 10.1002/(SICI)1521-4141(199808)28:08<2571::AID-IMMU2571>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 61.DeFranco AL, Gold MR, Jakway JP. B-lymphocyte signal transduction in response to anti-immunoglobulin and bacterial lipopolysaccharide. Immunol Rev. 1987;95:161–176. doi: 10.1111/j.1600-065x.1987.tb00504.x. [DOI] [PubMed] [Google Scholar]
- 62.Posner RG, Savage PB, Peters AS, Macias A, DelGado J, Zwartz G, et al. A quantitative approach for studying IgE-FcεRI aggregation. Mol Immunol. 2002;38:1221–1228. doi: 10.1016/s0161-5890(02)00067-6. [DOI] [PubMed] [Google Scholar]
- 63.Draberova L, Lebduska P, Halova I, Tolar P, Stokrova J, Tolarova H, et al. Signaling assemblies formed in mast cells activated via Fcε receptor I dimers. Eur J Immunol. 2004;34:2209–2219. doi: 10.1002/eji.200322663. [DOI] [PubMed] [Google Scholar]
- 64.Horii K, Kahn ML, Herr AB. Structural basis for platelet collagen responses by the immune-type receptor glycoprotein VI. Blood. 2006;108:936–942. doi: 10.1182/blood-2006-01-010215. [DOI] [PubMed] [Google Scholar]
- 65.Cochran JR, Cameron TO, Stern LJ. The relationship of MHC-peptide binding and T cell activation probed using chemically defined MHC class II oligomers. Immunity. 2000;12:241–250. doi: 10.1016/s1074-7613(00)80177-6. [DOI] [PubMed] [Google Scholar]
- 66.Fahmy TM, Bieler JG, Schneck JP. Probing T cell membrane organization using dimeric MHC-Ig complexes. J Immunol Methods. 2002;268:93–106. doi: 10.1016/s0022-1759(02)00203-x. [DOI] [PubMed] [Google Scholar]
- 67.Kiessling LL, Gestwicki JE, Strong LE. Synthetic multivalent ligands as probes of signal transduction. Angew Chem Int Ed Engl. 2006;45:2348–2368. doi: 10.1002/anie.200502794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garrity D, Call ME, Feng J, Wucherpfennig KW. The activating NKG2D receptor assembles in the membrane with two signaling dimers into a hexameric structure. Proc Natl Acad Sci USA. 2005;102:7641–7646. doi: 10.1073/pnas.0502439102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Radaev S, Kattah M, Rostro B, Colonna M, Sun PD. Crystal structure of the human myeloid cell activating receptor TREM-1. Structure. 2003;11:1527–1535. doi: 10.1016/j.str.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 70.Ortega E. How do multichain immune recognition receptors signal? A structural hypothesis. Mol Immunol. 1995;32:941–945. doi: 10.1016/0161-5890(95)00070-u. [DOI] [PubMed] [Google Scholar]
- 71.Berlanga O, Bori-Sanz T, James JR, Frampton J, Davis SJ, Tomlinson MG, et al. Glycoprotein VI oligomerization in cell lines and platelets. J Thromb Haemost. 2007;5:1026–1033. doi: 10.1111/j.1538-7836.2007.02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Symer DE, Dintzis RZ, Diamond DJ, Dintzis HM. Inhibition or activation of human T cell receptor transfectants is controlled by defined, soluble antigen arrays. J Exp Med. 1992;176:1421–1430. doi: 10.1084/jem.176.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schweitzer-Stenner R, Tamir I, Pecht I. Analysis of Fc(epsilon)RI-mediated mast cell stimulation by surface-carried antigens. Biophys J. 1997;72:2470–2478. doi: 10.1016/S0006-3495(97)78891-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patrick SM, Kim S, Braunstein NS, Thomas JL, Leonard EF. Dependence of T cell activation on area of contact and density of a ligand-coated surface. J Immunol Methods. 2000;241:97–108. doi: 10.1016/s0022-1759(00)00216-7. [DOI] [PubMed] [Google Scholar]
- 75.Germain RN. T-cell signaling: the importance of receptor clustering. Curr Biol. 1997;7:640–644. doi: 10.1016/s0960-9822(06)00323-x. [DOI] [PubMed] [Google Scholar]
- 76.Alam SM, Davies GM, Lin CM, Zal T, Nasholds W, Jameson SC, et al. Qualitative and quantitative differences in T cell receptor binding of agonist and antagonist ligands. Immunity. 1999;10:227–237. doi: 10.1016/s1074-7613(00)80023-0. [DOI] [PubMed] [Google Scholar]
- 77.Cadena DL, Gill GN. Receptor tyrosine kinases. Faseb J. 1992;6:2332–2337. doi: 10.1096/fasebj.6.6.1312047. [DOI] [PubMed] [Google Scholar]
- 78.Sigalov A, Aivazian D, Stern L. Homooligomerization of the cytoplasmic domain of the T cell receptor zeta chain and of other proteins containing the immunoreceptor tyrosine-based activation motif. Biochemistry. 2004;43:2049–2061. doi: 10.1021/bi035900h. [DOI] [PubMed] [Google Scholar]
- 79.Sigalov AB, Aivazian DA, Uversky VN, Stern LJ. Lipid-binding activity of intrinsically unstructured cytoplasmic domains of multichain immune recognition receptor signaling subunits. Biochemistry. 2006;45:15731–15739. doi: 10.1021/bi061108f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sigalov AB, Hendricks GM. Membrane binding mode of intrinsically disordered cytoplasmic domains of T cell receptor signaling subunits depends on lipid composition. Biochem Biophys Res Commun. 2009;389:388–393. doi: 10.1016/j.bbrc.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sigalov AB, Zhuravleva AV, Orekhov VY. Binding of intrinsically disordered proteins is not necessarily accompanied by a structural transition to a folded form. Biochimie. 2007;89:419–421. doi: 10.1016/j.biochi.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mittag T, Kay LE, Forman-Kay JD. Protein dynamics and conformational disorder in molecular recognition. J Mol Recognit. 2009 doi: 10.1002/jmr.961. [DOI] [PubMed] [Google Scholar]
- 83.Tompa P, Fuxreiter M. Fuzzy complexes: polymorphism and structural disorder in protein-protein interactions. Trends Biochem Sci. 2008;33:2–8. doi: 10.1016/j.tibs.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 84.Finger C, Escher C, Schneider D. The single transmembrane domains of human receptor tyrosine kinases encode self-interactions. Sci Signal. 2009;2:56. doi: 10.1126/scisignal.2000547. [DOI] [PubMed] [Google Scholar]
- 85.Li E, Hristova K. Role of receptor tyrosine kinase transmembrane domains in cell signaling and human pathologies. Biochemistry. 2006;45:6241–6251. doi: 10.1021/bi060609y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li E, You M, Hristova K. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and forster resonance energy transfer suggest weak interactions between fibroblast growth factor receptor 3 (FGFR3) transmembrane domains in the absence of extracellular domains and ligands. Biochemistry. 2005;44:352–360. doi: 10.1021/bi048480k. [DOI] [PubMed] [Google Scholar]
- 87.Smith SO, Smith C, Shekar S, Peersen O, Ziliox M, Aimoto S. Transmembrane interactions in the activation of the Neu receptor tyrosine kinase. Biochemistry. 2002;41:9321–9332. doi: 10.1021/bi012117l. [DOI] [PubMed] [Google Scholar]
- 88.Fantl WJ, Johnson DE, Williams LT. Signalling by receptor tyrosine kinases. Annu Rev Biochem. 1993;62:453–481. doi: 10.1146/annurev.bi.62.070193.002321. [DOI] [PubMed] [Google Scholar]
- 89.Bormann BJ, Engelman DM. Intramembrane helixhelix association in oligomerization and transmembrane signaling. Annu Rev Biophys Biomol Struct. 1992;21:223–242. doi: 10.1146/annurev.bb.21.060192.001255. [DOI] [PubMed] [Google Scholar]
- 90.Finger C, Volkmer T, Prodohl A, Otzen DE, Engelman DM, Schneider D. The stability of transmembrane helix interactions measured in a biological membrane. J Mol Biol. 2006;358:1221–1228. doi: 10.1016/j.jmb.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 91.Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, et al. Ligand recognition by αβ T cell receptors. Annu Rev Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 92.Andersen PS, Geisler C, Buus S, Mariuzza RA, Karjalainen K. Role of the T cell receptor ligand affinity in T cell activation by bacterial superantigens. J Biol Chem. 2001;276:33452–33457. doi: 10.1074/jbc.M103750200. [DOI] [PubMed] [Google Scholar]
- 93.Garcia KC, Tallquist MD, Pease LR, Brunmark A, Scott CA, Degano M, et al. αβ T cell receptor interactions with syngeneic and allogeneic ligands: affinity measurements and crystallization. Proc Natl Acad Sci USA. 1997;94:13838–13843. doi: 10.1073/pnas.94.25.13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Torigoe C, Inman JK, Metzger H. An unusual mechanism for ligand antagonism. Science. 1998;281:568–572. doi: 10.1126/science.281.5376.568. [DOI] [PubMed] [Google Scholar]
- 95.Miura Y, Takahashi T, Jung SM, Moroi M. Analysis of the interaction of platelet collagen receptor glycoprotein VI (GPVI) with collagen. A dimeric form of GPVI, but not the monomeric form, shows affinity to fibrous collagen. J Biol Chem. 2002;277:46197–46204. doi: 10.1074/jbc.M204029200. [DOI] [PubMed] [Google Scholar]
- 96.Haefner J. Modeling Biological Systems: Principles and Applications. New York: Springer; 2005. [Google Scholar]
- 97.Sigalov A. Multi-chain immune recognition receptors: spatial organization and signal transduction. Semin Immunol. 2005;17:51–64. doi: 10.1016/j.smim.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 98.Sigalov AB. Transmembrane interactions as immunotherapeutic targets: lessons from viral pathogenesis. Adv Exp Med Biol. 2007;601:335–344. doi: 10.1007/978-0-387-72005-0_36. [DOI] [PubMed] [Google Scholar]
- 99.Tanner KG, Kyte J. Dimerization of the extracellular domain of the receptor for epidermal growth factor containing the membrane-spanning segment in response to treatment with epidermal growth factor. J Biol Chem. 1999;274:35985–35990. doi: 10.1074/jbc.274.50.35985. [DOI] [PubMed] [Google Scholar]
- 100.Hubbard SR. Structural analysis of receptor tyrosine kinases. Prog Biophys Mol Biol. 1999;71:343–358. doi: 10.1016/s0079-6107(98)00047-9. [DOI] [PubMed] [Google Scholar]
- 101.Schlessinger J. Signal transduction. Autoinhibition control. Science. 2003;300:750–752. doi: 10.1126/science.1082024. [DOI] [PubMed] [Google Scholar]
- 102.Jiang G, Hunter T. Receptor signaling: when dimerization is not enough. Curr Biol. 1999;9:568–571. doi: 10.1016/s0960-9822(99)80357-1. [DOI] [PubMed] [Google Scholar]
- 103.Marianayagam NJ, Sunde M, Matthews JM. The power of two: protein dimerization in biology. Trends Biochem Sci. 2004;29:618–625. doi: 10.1016/j.tibs.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 104.Siegel RM, Muppidi JR, Sarker M, Lobito A, Jen M, Martin D, et al. SPOTS: signaling protein oligomeric transduction structures are early mediators of death receptor-induced apoptosis at the plasma membrane. J Cell Biol. 2004;167:735–744. doi: 10.1083/jcb.200406101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chan FK. Three is better than one: pre-ligand receptor assembly in the regulation of TNF receptor signaling. Cytokine. 2007;37:101–107. doi: 10.1016/j.cyto.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chan KF, Siegel MR, Lenardo JM. Signaling by the TNF receptor superfamily and T cell homeostasis. Immunity. 2000;13:419–422. doi: 10.1016/s1074-7613(00)00041-8. [DOI] [PubMed] [Google Scholar]
- 107.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 108.Hays JL, Watowich SJ. Oligomerization-induced modulation of TPR-MET tyrosine kinase activity. J Biol Chem. 2003;278:27456–27463. doi: 10.1074/jbc.M210648200. [DOI] [PubMed] [Google Scholar]
- 109.Hays JL, Watowich SJ. Oligomerization-dependent changes in the thermodynamic properties of the TPR-MET receptor tyrosine kinase. Biochemistry. 2004;43:10570–10578. doi: 10.1021/bi0363275. [DOI] [PubMed] [Google Scholar]
- 110.Moriki T, Maruyama H, Maruyama IN. Activation of preformed EGF receptor dimers by ligand-induced rotation of the transmembrane domain. J Mol Biol. 2001;311:1011–1026. doi: 10.1006/jmbi.2001.4923. [DOI] [PubMed] [Google Scholar]
- 111.Yu X, Sharma KD, Takahashi T, Iwamoto R, Mekada E. Ligand-independent dimer formation of epidermal growth factor receptor (EGFR) is a step separable from ligand-induced EGFR signaling. Mol Biol Cell. 2002;13:2547–2557. doi: 10.1091/mbc.01-08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bell CA, Tynan JA, Hart KC, Meyer AN, Robertson SC, Donoghue DJ. Rotational coupling of the transmembrane and kinase domains of the Neu receptor tyrosine kinase. Mol Biol Cell. 2000;11:3589–3599. doi: 10.1091/mbc.11.10.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ballinger MD, Wells JA. Will any dimer do? Nat Struct Biol. 1998;5:938–940. doi: 10.1038/2911. [DOI] [PubMed] [Google Scholar]
- 114.Livnah O, Johnson DL, Stura EA, Farrell FX, Barbone FP, You Y, et al. An antagonist peptide-EPO receptor complex suggests that receptor dimerization is not sufficient for activation. Nat Struct Biol. 1998;5:993–1004. doi: 10.1038/2965. [DOI] [PubMed] [Google Scholar]
- 115.Syed RS, Reid SW, Li C, Cheetham JC, Aoki KH, Liu B, et al. Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature. 1998;395:511–516. doi: 10.1038/26773. [DOI] [PubMed] [Google Scholar]
- 116.Simpson RJ, Hammacher A, Smith DK, Matthews JM, Ward LD. Interleukin-6: structure-function relationships. Protein Sci. 1997;6:929–955. doi: 10.1002/pro.5560060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Greiser JS, Stross C, Heinrich PC, Behrmann I, Hermanns HM. Orientational constraints of the gp130 intracellular juxtamembrane domain for signaling. J Biol Chem. 2002;277:26959–26965. doi: 10.1074/jbc.M204113200. [DOI] [PubMed] [Google Scholar]
- 118.Muller-Newen G, Kuster A, Wijdenes J, Schaper F, Heinrich PC. Studies on the interleukin-6-type cytokine signal transducer gp130 reveal a novel mechanism of receptor activation by monoclonal antibodies. J Biol Chem. 2000;275:4579–4586. doi: 10.1074/jbc.275.7.4579. [DOI] [PubMed] [Google Scholar]
- 119.Autissier P, De Vos J, Liautard J, Tupitsyn N, Jacquet C, Chavdia N, et al. Dimerization and activation of the common transducing chain (gp130) of the cytokines of the IL-6 family by mAb. Int Immunol. 1998;10:1881–1889. doi: 10.1093/intimm/10.12.1881. [DOI] [PubMed] [Google Scholar]
- 120.Lee HK, Dunzendorfer S, Tobias PS. Cytoplasmic domain-mediated dimerizations of toll-like receptor 4 observed by β-lactamase enzyme fragment complementation. J Biol Chem. 2004;279:10564–10574. doi: 10.1074/jbc.M311564200. [DOI] [PubMed] [Google Scholar]
- 121.Weber AN, Moncrieffe MC, Gangloff M, Imler JL, Gay NJ. Ligand-receptor and receptor-receptor interactions act in concert to activate signaling in the Drosophila toll pathway. J Biol Chem. 2005;280:22793–22799. doi: 10.1074/jbc.M502074200. [DOI] [PubMed] [Google Scholar]
- 122.Gomes MM, Herr AB. IgA and IgA-specific receptors in human disease: structural and functional insights into pathogenesis and therapeutic potential. Springer Semin Immunopathol. 2006;28:383–395. doi: 10.1007/s00281-006-0048-x. [DOI] [PubMed] [Google Scholar]
- 123.Honda Z. Fcε- and Fcγ-receptor signaling in diseases. Springer Semin Immunopathol. 2006;28:365–375. doi: 10.1007/s00281-006-0051-2. [DOI] [PubMed] [Google Scholar]
- 124.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 125.Clemetson KJ. Platelet receptors and their role in diseases. Clin Chem Lab Med. 2003;41:253–260. doi: 10.1515/CCLM.2003.039. [DOI] [PubMed] [Google Scholar]
- 126.Reth M. Oligomeric antigen receptors: a new view on signaling for the selection of lymphocytes. Trends Immunol. 2001;22:356–360. doi: 10.1016/s1471-4906(01)01964-0. [DOI] [PubMed] [Google Scholar]
- 127.Langlet C, Bernard AM, Drevot P, He HT. Membrane rafts and signaling by the multichain immune recognition receptors. Curr Opin Immunol. 2000;12:250–255. doi: 10.1016/s0952-7915(00)00084-4. [DOI] [PubMed] [Google Scholar]
- 128.Dykstra M, Cherukuri A, Pierce SK. Rafts and synapses in the spatial organization of immune cell signaling receptors. J Leukoc Biol. 2001;70:699–707. [PubMed] [Google Scholar]
- 129.Sigalov AB. SCHOOL model and new targeting strategies. Adv Exp Med Biol. 2008;640:268–311. doi: 10.1007/978-0-387-09789-3_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sigalov AB. Signaling chain homooligomerization (SCHOOL) model. Adv Exp Med Biol. 2008;640:121–163. doi: 10.1007/978-0-387-09789-3_12. [DOI] [PubMed] [Google Scholar]
- 131.Berko D, Carmi Y, Cafri G, Ben-Zaken S, Sheikhet HM, Tzehoval E, et al. Membrane-anchored β2-microglobulin stabilizes a highly receptive state of MHC class I molecules. J Immunol. 2005;174:2116–2123. doi: 10.4049/jimmunol.174.4.2116. [DOI] [PubMed] [Google Scholar]
- 132.Pribluda VS, Pribluda C, Metzger H. Transphosphorylation as the mechanism by which the high-affinity receptor for IgE is phosphorylated upon aggregation. Proc Natl Acad Sci USA. 1994;91:11246–11250. doi: 10.1073/pnas.91.23.11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schamel WW, Arechaga I, Risueno RM, van Santen HM, Cabezas P, Risco C, et al. Coexistence of multivalent and monovalent TCRs explains high sensitivity and wide range of response. J Exp Med. 2005;202:493–503. doi: 10.1084/jem.20042155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jung SM, Tsuji K, Moroi M. Glycoprotein (GP) VI dimer as a major collagen-binding site of native platelets: direct evidence obtained with dimeric GPVI-specific Fabs. J Thromb Haemost. 2009;7:1347–1355. doi: 10.1111/j.1538-7836.2009.03496.x. [DOI] [PubMed] [Google Scholar]
- 135.Pitcher LA, van Oers NS. T-cell receptor signal transmission: who gives an ITAM? Trends Immunol. 2003;24:554–560. doi: 10.1016/j.it.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 136.Pike KA, Baig E, Ratcliffe MJ. The avian B-cell receptor complex: distinct roles of Igα and Igβ in B-cell development. Immunol Rev. 2004;197:10–25. doi: 10.1111/j.0105-2896.2004.0111.x. [DOI] [PubMed] [Google Scholar]
- 137.Storch B, Meixlsperger S, Jumaa H. The Ig-α ITAM is required for efficient differentiation but not proliferation of pre-B cells. Eur J Immunol. 2007;37:252–260. doi: 10.1002/eji.200636667. [DOI] [PubMed] [Google Scholar]
- 138.Gazumyan A, Reichlin A, Nussenzweig MC. Igβ tyrosine residues contribute to the control of B cell receptor signaling by regulating receptor internalization. J Exp Med. 2006;203:1785–1794. doi: 10.1084/jem.20060221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lin S, Cicala C, Scharenberg AM, Kinet JP. The Fc(ε)RIbeta subunit functions as an amplifier of Fc(ε)RIγ-mediated cell activation signals. Cell. 1996;85:985–995. doi: 10.1016/s0092-8674(00)81300-8. [DOI] [PubMed] [Google Scholar]
- 140.Sanchez-Mejorada G, Rosales C. Signal transduction by immunoglobulin Fc receptors. J Leukoc Biol. 1998;63:521–533. doi: 10.1002/jlb.63.5.521. [DOI] [PubMed] [Google Scholar]
- 141.Lysechko TL, Ostergaard HL. Differential Src family kinase activity requirements for CD3ζ phosphorylation/ZAP70 recruitment and CD3ε phosphorylation. J Immunol. 2005;174:7807–7814. doi: 10.4049/jimmunol.174.12.7807. [DOI] [PubMed] [Google Scholar]
- 142.Kuhns MS, Davis MM. Disruption of extracellular interactions impairs T cell receptor-CD3 complex stability and signaling. Immunity. 2007;26:357–369. doi: 10.1016/j.immuni.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 143.Chau LA, Bluestone JA, Madrenas J. Dissociation of intracellular signaling pathways in response to partial agonist ligands of the T cell receptor. J Exp Med. 1998;187:1699–1709. doi: 10.1084/jem.187.10.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jensen WA, Pleiman CM, Beaufils P, Wegener AM, Malissen B, Cambier JC. Qualitatively distinct signaling through T cell antigen receptor subunits. Eur J Immunol. 1997;27:707–716. doi: 10.1002/eji.1830270320. [DOI] [PubMed] [Google Scholar]
- 145.Pitcher LA, Mathis MA, Young JA, Deford LM, Purtic B, Wulfing C, et al. The CD3γε/δε signaling module provides normal T cell functions in the absence of the TCRzeta immunoreceptor tyrosine-based activation motifs. Eur J Immunol. 2005;35:3643–3654. doi: 10.1002/eji.200535136. [DOI] [PubMed] [Google Scholar]
- 146.Kesti T, Ruppelt A, Wang JH, Liss M, Wagner R, Tasken K, et al. Reciprocal regulation of SH3 and SH2 domain binding via tyrosine phosphorylation of a common site in CD3{ε} J Immunol. 2007;179:878–885. doi: 10.4049/jimmunol.179.2.878. [DOI] [PubMed] [Google Scholar]
- 147.Chae WJ, Lee HK, Han JH, Kim SW, Bothwell AL, Morio T, et al. Qualitatively differential regulation of T cell activation and apoptosis by T cell receptor ζ chain ITAMs and their tyrosine residues. Int Immunol. 2004;16:1225–1236. doi: 10.1093/intimm/dxh120. [DOI] [PubMed] [Google Scholar]
- 148.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 149.Rojo JM, Bello R, Portoles P. T-cell receptor. Adv Exp Med Biol. 2008;640:1–11. doi: 10.1007/978-0-387-09789-3_1. [DOI] [PubMed] [Google Scholar]
- 150.Wines BD, Trist HM, Monteiro RC, Van Kooten C, Hogarth PM. Fc receptor γ chain residues at the interface of the cytoplasmic and transmembrane domains affect association with FcαRI, surface expression and function. J Biol Chem. 2004;279:26339–26345. doi: 10.1074/jbc.M403684200. [DOI] [PubMed] [Google Scholar]
- 151.Kikuchi-Maki A, Catina TL, Campbell KS. Cutting edge: KIR2DL4 transduces signals into human NK cells through association with the Fc receptor γ protein. J Immunol. 2005;174:3859–3863. doi: 10.4049/jimmunol.174.7.3859. [DOI] [PubMed] [Google Scholar]
- 152.DeFranco AL, Mittelstadt PR, Blum JH, Stevens TL, Law DA, Chan VW, et al. Mechanism of B cell antigen receptor function: transmembrane signaling and triggering of apoptosis. Adv Exp Med Biol. 1994;365:9–22. doi: 10.1007/978-1-4899-0987-9_2. [DOI] [PubMed] [Google Scholar]
- 153.Morton HC, van den Herik-Oudijk IE, Vossebeld P, Snijders A, Verhoeven AJ, Capel PJ, et al. Functional association between the human myeloid immunoglobulin A Fc receptor (CD89) and FcRγ chain. Molecular basis for CD89/FcRγ chain association. J Biol Chem. 1995;270:29781–29787. doi: 10.1074/jbc.270.50.29781. [DOI] [PubMed] [Google Scholar]
- 154.Kim MK, Huang ZY, Hwang PH, Jones BA, Sato N, Hunter S, et al. Fcγ receptor transmembrane domains: role in cell surface expression, γ chain interaction and phagocytosis. Blood. 2003;101:4479–4484. doi: 10.1182/blood.V101.11.4479. [DOI] [PubMed] [Google Scholar]
- 155.Singleton TE, Platzer B, Dehlink E, Fiebiger E. The first transmembrane region of the β-chain stabilizes the tetrameric FcεRI complex. Mol Immunol. 2009;46:2333–2339. doi: 10.1016/j.molimm.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Angelov GS, Guillaume P, Cebecauer M, Bosshard G, Dojcinovic D, Baumgaertner P, et al. Soluble MHC-peptide complexes containing long rigid linkers abolish CTL-mediated cytotoxicity. J Immunol. 2006;176:3356–3365. doi: 10.4049/jimmunol.176.6.3356. [DOI] [PubMed] [Google Scholar]
- 157.Rotzschke O, Falk K, Strominger JL. Superactivation of an immune response triggered by oligomerized T cell epitopes. Proc Natl Acad Sci USA. 1997;94:14642–14647. doi: 10.1073/pnas.94.26.14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tamir I, Schweitzer-Stenner R, Pecht I. Immobilization of the type I receptor for IgE initiates signal transduction in mast cells. Biochemistry. 1996;35:6872–6883. doi: 10.1021/bi952556i. [DOI] [PubMed] [Google Scholar]
- 159.Yang H, Parkhouse RM. Differential activation requirements associated with stimulation of T cells via different epitopes of CD3. Immunology. 1998;93:26–32. doi: 10.1046/j.1365-2567.1998.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Niederberger N, Buehler LK, Ampudia J, Gascoigne NR. Thymocyte stimulation by anti-TCR-beta, but not by anti-TCR-alpha, leads to induction of developmental transcription program. J Leukoc Biol. 2005;77:830–841. doi: 10.1189/jlb.1004608. [DOI] [PubMed] [Google Scholar]
- 161.Carreno LJ, Gonzalez PA, Kalergis AM. Modulation of T cell function by TCR/pMHC binding kinetics. Immunobiology. 2006;211:47–64. doi: 10.1016/j.imbio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 162.Holler PD, Lim AR, Cho BK, Rund LA, Kranz DM. CD8(−) T cell transfectants that express a high affinity T cell receptor exhibit enhanced peptide-dependent activation. J Exp Med. 2001;194:1043–1052. doi: 10.1084/jem.194.8.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ortega E, Schweitzer-Stenner R, Pecht I. Possible orientational constraints determine secretory signals induced by aggregation of IgE receptors on mast cells. EMBO J. 1988;7:4101–4109. doi: 10.1002/j.1460-2075.1988.tb03304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pecht I, Ortega E, Jovin TM. Rotational dynamics of the Fcε receptor on mast cells monitored by specific monoclonal antibodies and IgE. Biochemistry. 1991;30:3450–3458. doi: 10.1021/bi00228a015. [DOI] [PubMed] [Google Scholar]
- 165.Gil D, Schamel WW, Montoya M, Sanchez-Madrid F, Alarcon B. Recruitment of Nck by CD3ε reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell. 2002;109:901–912. doi: 10.1016/s0092-8674(02)00799-7. [DOI] [PubMed] [Google Scholar]
- 166.Tamir I, Cambier JC. Antigen receptor signaling: integration of protein tyrosine kinase functions. Oncogene. 1998;17:1353–1364. doi: 10.1038/sj.onc.1202187. [DOI] [PubMed] [Google Scholar]
- 167.Sulzer B, Perelson AS. Immunons revisited: binding of multivalent antigens to B cells. Mol Immunol. 1997;34:63–74. doi: 10.1016/s0161-5890(96)00096-x. [DOI] [PubMed] [Google Scholar]
- 168.Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu Rev Immunol. 1997;15:453–479. doi: 10.1146/annurev.immunol.15.1.453. [DOI] [PubMed] [Google Scholar]
- 169.Nimmerjahn F. Activating and inhibitory FcγRs in autoimmune disorders. Springer Semin Immunopathol. 2006;28:305–319. doi: 10.1007/s00281-006-0052-1. [DOI] [PubMed] [Google Scholar]
- 170.Maurer D, Fiebiger E, Reininger B, Ebner C, Petzelbauer P, Shi GP, et al. Fcε receptor I on dendritic cells delivers IgE-bound multivalent antigens into a cathepsin S-dependent pathway of MHC class II presentation. J Immunol. 1998;161:2731–2739. [PubMed] [Google Scholar]
- 171.Lara M, Ortega E, Pecht I, Pfeiffer JR, Martinez AM, Lee RJ, et al. Overcoming the signaling defect of Lyn-sequestering, signal-curtailing FcεRI dimers: aggregated dimers can dissociate from Lyn and form signaling complexes with Syk. J Immunol. 2001;167:4329–4337. doi: 10.4049/jimmunol.167.8.4329. [DOI] [PubMed] [Google Scholar]
- 172.Metzger H. The high affinity receptor for IgE, FcεRI. Novartis Found Symp. 2004;257:51–59. [PubMed] [Google Scholar]
- 173.Metzger H, Eglite S, Haleem-Smith H, Reischl I, Torigoe C. Quantitative aspects of signal transduction by the receptor with high affinity for IgE. Mol Immunol. 2002;38:1207–1211. doi: 10.1016/s0161-5890(02)00065-2. [DOI] [PubMed] [Google Scholar]
- 174.Holowka D, Sil D, Torigoe C, Baird B. Insights into immunoglobulin E receptor signaling from structurally defined ligands. Immunol Rev. 2007;217:269–279. doi: 10.1111/j.1600-065X.2007.00517.x. [DOI] [PubMed] [Google Scholar]
- 175.Radaev S, Sun PD. Structure and function of natural killer cell surface receptors. Annu Rev Biophys Biomol Struct. 2003;32:93–114. doi: 10.1146/annurev.biophys.32.110601.142347. [DOI] [PubMed] [Google Scholar]
- 176.Natarajan K, Dimasi N, Wang J, Mariuzza RA, Margulies DH. Structure and function of natural killer cell receptors: multiple molecular solutions to self, nonself discrimination. Annu Rev Immunol. 2002;20:853–885. doi: 10.1146/annurev.immunol.20.100301.064812. [DOI] [PubMed] [Google Scholar]
- 177.Turnbull IR, Colonna M. Activating and inhibitory functions of DAP12. Nat Rev Immunol. 2007;7:155–161. doi: 10.1038/nri2014. [DOI] [PubMed] [Google Scholar]
- 178.Posner RG, Paar JM, Licht A, Pecht I, Conrad DH, Hlavacek WS. Interaction of a monoclonal IgE-specific antibody with cell-surface IgE-FcεRI: characterization of equilibrium binding and secretory response. Biochemistry. 2004;43:11352–11360. doi: 10.1021/bi049686o. [DOI] [PubMed] [Google Scholar]
- 179.Schweitzer-Stenner R, Ortega E, Pecht I. Kinetics of FcεRI dimer formation by specific monoclonal antibodies on mast cells. Biochemistry. 1994;33:8813–8825. doi: 10.1021/bi00195a025. [DOI] [PubMed] [Google Scholar]
- 180.Kent UM, Mao SY, Wofsy C, Goldstein B, Ross S, Metzger H. Dynamics of signal transduction after aggregation of cell-surface receptors: studies on the type I receptor for IgE. Proc Natl Acad Sci USA. 1994;91:3087–3091. doi: 10.1073/pnas.91.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yamasaki S, Ishikawa E, Kohno M, Saito T. The quantity and duration of FcRγ signals determine mast cell degranulation and survival. Blood. 2004;103:3093–3101. doi: 10.1182/blood-2003-08-2944. [DOI] [PubMed] [Google Scholar]
- 182.Gibbs BF, Rathling A, Zillikens D, Huber M, Haas H. Initial FcεRI-mediated signal strength plays a key role in regulating basophil signaling and deactivation. J Allergy Clin Immunol. 2006;118:1060–1067. doi: 10.1016/j.jaci.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 183.Pasquier B, Launay P, Kanamaru Y, Moura IC, Pfirsch S, Ruffie C, et al. Identification of FcαRI as an inhibitory receptor that controls inflammation: dual role of FcRγ ITAM. Immunity. 2005;22:31–42. doi: 10.1016/j.immuni.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 184.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mescher MF, Agarwal P, Casey KA, Hammerbeck CD, Xiao Z, Curtsinger JM. Molecular basis for checkpoints in the CD8 T cell response: Tolerance versus activation. Semin Immunol. 2007;19:153–161. doi: 10.1016/j.smim.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Colombetti S, Benigni F, Basso V, Mondino A. Clonal anergy is maintained independently of T cell proliferation. J Immunol. 2002;169:6178–6186. doi: 10.4049/jimmunol.169.11.6178. [DOI] [PubMed] [Google Scholar]
- 187.Choi S, Schwartz RH. Molecular mechanisms for adaptive tolerance and other T cell anergy models. Semin Immunol. 2007;19:140–152. doi: 10.1016/j.smim.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chiodetti L, Choi S, Barber DL, Schwartz RH. Adaptive tolerance and clonal anergy are distinct biochemical states. J Immunol. 2006;176:2279–2291. doi: 10.4049/jimmunol.176.4.2279. [DOI] [PubMed] [Google Scholar]
- 189.Gu H. Anergic signals: the action is upstream. Immunity. 2008;28:598–600. doi: 10.1016/j.immuni.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 190.Teague RM, Greenberg PD, Fowler C, Huang MZ, Tan X, Morimoto J, et al. Peripheral CD8+ T cell tolerance to self-proteins is regulated proximally at the T cell receptor. Immunity. 2008;28:662–674. doi: 10.1016/j.immuni.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Srinivasan M, Frauwirth KA. Peripheral tolerance in CD8+ T cells. Cytokine. 2009;46:147–159. doi: 10.1016/j.cyto.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 192.Recio MJ, Moreno-Pelayo MA, Kilic SS, Guardo AC, Sanal O, Allende LM, et al. Differential biological role of CD3 chains revealed by human immunodeficiencies. J Immunol. 2007;178:2556–2564. doi: 10.4049/jimmunol.178.4.2556. [DOI] [PubMed] [Google Scholar]
- 193.Gil D, Schrum AG, Alarcon B, Palmer E. T cell receptor engagement by peptide-MHC ligands induces a conformational change in the CD3 complex of thymocytes. J Exp Med. 2005;201:517–522. doi: 10.1084/jem.20042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Risueno RM, Gil D, Fernandez E, Sanchez-Madrid F, Alarcon B. Ligand-induced conformational change in the T-cell receptor associated with productive immune synapses. Blood. 2005;106:601–608. doi: 10.1182/blood-2004-12-4763. [DOI] [PubMed] [Google Scholar]
- 195.Risueno RM, van Santen HM, Alarcon B. A conformational change senses the strength of T cell receptor-ligand interaction during thymic selection. Proc Natl Acad Sci USA. 2006;103:9625–9630. doi: 10.1073/pnas.0601785103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vilen BJ, Nakamura T, Cambier JC. Antigen-stimulated dissociation of BCR mIg from Ig-α/Ig-β: implications for receptor desensitization. Immunity. 1999;10:239–248. doi: 10.1016/s1074-7613(00)80024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Vilen BJ, Famiglietti SJ, Carbone AM, Kay BK, Cambier JC. B cell antigen receptor desensitization: disruption of receptor coupling to tyrosine kinase activation. J Immunol. 1997;159:231–243. [PMC free article] [PubMed] [Google Scholar]
- 198.Cambier JC, Fisher CL, Pickles H, Morrison DC. Dual molecular mechanisms mediate ligand-induced membrane Ig desensitization. J Immunol. 1990;145:13–19. [PubMed] [Google Scholar]
- 199.Vilen BJ, Burke KM, Sleater M, Cambier JC. Transmodulation of BCR signaling by transduction- incompetent antigen receptors: implications for impaired signaling in anergic B cells. J Immunol. 2002;168:4344–4351. doi: 10.4049/jimmunol.168.9.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Arnon TI, Achdout H, Levi O, Markel G, Saleh N, Katz G, et al. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat Immunol. 2005;6:515–523. doi: 10.1038/ni1190. [DOI] [PubMed] [Google Scholar]
- 201.Levit MN, Liu Y, Stock JB. Stimulus response coupling in bacterial chemotaxis: receptor dimers in signalling arrays. Mol Microbiol. 1998;30:459–466. doi: 10.1046/j.1365-2958.1998.01066.x. [DOI] [PubMed] [Google Scholar]
- 202.Sourjik V, Berg HC. Functional interactions between receptors in bacterial chemotaxis. Nature. 2004;428:437–441. doi: 10.1038/nature02406. [DOI] [PubMed] [Google Scholar]
- 203.Sourjik V. Receptor clustering and signal processing in E. coli chemotaxis. Trends Microbiol. 2004;12:569–576. doi: 10.1016/j.tim.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 204.Kawaguchi M, Eckels DD. Differential activation through the TCR-CD3 complex affects the requirement for costimulation of human T cells. Hum Immunol. 1995;43:136–148. doi: 10.1016/0198-8859(94)00160-r. [DOI] [PubMed] [Google Scholar]
- 205.Lanier LL, Ruitenberg JJ, Allison JP, Weiss A. Distinct epitopes on the T cell antigen receptor of HPB-ALL tumor cells identified by monoclonal antibodies. J Immunol. 1986;137:2286–2292. [PubMed] [Google Scholar]
- 206.Schlitt HJ, Kurrle R, Wonigeit K. T cell activation by monoclonal antibodies directed to different epitopes on the human T cell receptor/CD3 complex: evidence for two different modes of activation. Eur J Immunol. 1989;19:1649–1655. doi: 10.1002/eji.1830190920. [DOI] [PubMed] [Google Scholar]
- 207.Schwinzer R, Franklin RA, Domenico J, Renz H, Gelfand EW. Monoclonal antibodies directed to different epitopes in the CD3-TCR complex induce different states of competence in resting human T cells. J Immunol. 1992;148:1322–1328. [PubMed] [Google Scholar]
- 208.Yoon ST, Dianzani U, Bottomly K, Janeway CA., Jr Both high and low avidity antibodies to the T cell receptor can have agonist or antagonist activity. Immunity. 1994;1:563–569. doi: 10.1016/1074-7613(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 209.Arkin M. Protein-protein interactions and cancer: small molecules going in for the kill. Curr Opin Chem Biol. 2005;9:317–324. doi: 10.1016/j.cbpa.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 210.Ryan DP, Matthews JM. Protein-protein interactions in human disease. Curr Opin Struct Biol. 2005;15:441–446. doi: 10.1016/j.sbi.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 211.Veselovsky AV, Ivanov YD, Ivanov AS, Archakov AI, Lewi P, Janssen P. Protein-protein interactions: mechanisms and modification by drugs. J Mol Recognit. 2002;15:405–422. doi: 10.1002/jmr.597. [DOI] [PubMed] [Google Scholar]
- 212.Archakov AI, Govorun VM, Dubanov AV, Ivanov YD, Veselovsky AV, Lewi P, et al. Protein-protein interactions as a target for drugs in proteomics. Proteomics. 2003;3:380–391. doi: 10.1002/pmic.200390053. [DOI] [PubMed] [Google Scholar]
- 213.Pagliaro L, Felding J, Audouze K, Nielsen SJ, Terry RB, Krog-Jensen C, et al. Emerging classes of protein-protein interaction inhibitors and new tools for their development. Curr Opin Chem Biol. 2004;8:442–449. doi: 10.1016/j.cbpa.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 214.Loregian A, Palu G. Disruption of protein-protein interactions: towards new targets for chemotherapy. J Cell Physiol. 2005;204:750–762. doi: 10.1002/jcp.20356. [DOI] [PubMed] [Google Scholar]
- 215.Toogood PL. Inhibition of protein-protein association by small molecules: approaches and progress. J Med Chem. 2002;45:1543–1558. doi: 10.1021/jm010468s. [DOI] [PubMed] [Google Scholar]
- 216.Fry DC. Protein-protein interactions as targets for small molecule drug discovery. Biopolymers. 2006;84:535–552. doi: 10.1002/bip.20608. [DOI] [PubMed] [Google Scholar]
- 217.Fletcher S, Hamilton AD. Targeting protein-protein interactions by rational design: mimicry of protein surfaces. J R Soc Interface. 2006;3:215–233. doi: 10.1098/rsif.2006.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hershberger SJ, Lee SG, Chmielewski J. Scaffolds for blocking protein-protein interactions. Curr Top Med Chem. 2007;7:928–942. doi: 10.2174/156802607780906726. [DOI] [PubMed] [Google Scholar]
- 219.Che Y, Brooks BR, Marshall GR. Development of small molecules designed to modulate protein-protein interactions. J Comput Aided Mol Des. 2006;20:109–130. doi: 10.1007/s10822-006-9040-8. [DOI] [PubMed] [Google Scholar]
- 220.Sillerud LO, Larson RS. Design and structure of peptide and peptidomimetic antagonists of protein-protein interaction. Curr Protein Pept Sci. 2005;6:151–169. doi: 10.2174/1389203053545462. [DOI] [PubMed] [Google Scholar]
- 221.Sigalov AB. Interaction between HIV gp41 fusion peptide and T cell receptor: putting the puzzle pieces back together. Faseb J. 2007;21:1633–1634. doi: 10.1096/fj.07-0603ltr. [DOI] [PubMed] [Google Scholar]
- 222.Sigalov AB. New therapeutic strategies targeting transmembrane signal transduction in the immune system. Cell Adh Migr. 2010:4. doi: 10.4161/cam.4.2.10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sigalov AB. More on: glycoprotein VI oligomerization: a novel concept of platelet inhibition. J Thromb Haemost. 2007;5:2310–2312. doi: 10.1111/j.1538-7836.2007.02714.x. [DOI] [PubMed] [Google Scholar]
- 224.Reich Z, Boniface JJ, Lyons DS, Borochov N, Wachtel EJ, Davis MM. Ligand-specific oligomerization of T-cell receptor molecules. Nature. 1997;387:617–620. doi: 10.1038/42500. [DOI] [PubMed] [Google Scholar]
- 225.Garboczi DN, Ghosh P, Utz U, Fan QR, Biddison WE, Wiley DC. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- 226.Abastado JP, Lone YC, Casrouge A, Boulot G, Kourilsky P. Dimerization of soluble major histocompatibility complex-peptide complexes is sufficient for activation of T cell hybridoma and induction of unresponsiveness. J Exp Med. 1995;182:439–447. doi: 10.1084/jem.182.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lindstedt R, Monk N, Lombardi G, Lechler R. Amino acid substitutions in the putative MHC class II “dimer of dimers” interface inhibit CD4+ T cell activation. J Immunol. 2001;166:800–808. doi: 10.4049/jimmunol.166.2.800. [DOI] [PubMed] [Google Scholar]
- 228.Hayball JD, Lake RA. The immune function of MHC class II molecules mutated in the putative superdimer interface. Mol Cell Biochem. 2005;273:1–9. doi: 10.1007/s11010-005-5281-4. [DOI] [PubMed] [Google Scholar]
- 229.Watts TH. T cell activation by preformed, longlived Ia-peptide complexes. Quantitative aspects. J Immunol. 1988;141:3708–3714. [PubMed] [Google Scholar]
- 230.Rubin B, Knibiehler M, Gairin JE. Allosteric Changes in the TCR/CD3 Structure Upon Interaction With Extra- or Intra-cellular Ligands. Scand J Immunol. 2007;66:228–237. doi: 10.1111/j.1365-3083.2007.01979.x. [DOI] [PubMed] [Google Scholar]
- 231.Minguet S, Schamel WW. Permissive geometry model. Adv Exp Med Biol. 2008;640:113–120. doi: 10.1007/978-0-387-09789-3_11. [DOI] [PubMed] [Google Scholar]
- 232.Schamel WW, Reth M. Clustering models. Adv Exp Med Biol. 2008;640:64–73. doi: 10.1007/978-0-387-09789-3_6. [DOI] [PubMed] [Google Scholar]
- 233.Cochran JR, Cameron TO, Stone JD, Lubetsky JB, Stern LJ. Receptor proximity, not intermolecular orientation, is critical for triggering T-cell activation. J Biol Chem. 2001;276:28068–28074. doi: 10.1074/jbc.M103280200. [DOI] [PubMed] [Google Scholar]
- 234.Cebecauer M, Guillaume P, Hozak P, Mark S, Everett H, Schneider P, et al. Soluble MHC-peptide complexes induce rapid death of CD8+ CTL. J Immunol. 2005;174:6809–6819. doi: 10.4049/jimmunol.174.11.6809. [DOI] [PubMed] [Google Scholar]
- 235.Yamasaki S, Ishikawa E, Sakuma M, Ogata K, Sakata-Sogawa K, Hiroshima M, et al. Mechanistic basis of pre-T cell receptor-mediated autonomous signaling critical for thymocyte development. Nat Immunol. 2006;7:67–75. doi: 10.1038/ni1290. [DOI] [PubMed] [Google Scholar]
- 236.Yamasaki S, Saito T. Molecular basis for pre-TCR-mediated autonomous signaling. Trends Immunol. 2007;28:39–43. doi: 10.1016/j.it.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 237.Geisberger R, Crameri R, Achatz G. Models of signal transduction through the B-cell antigen receptor. Immunology. 2003;110:401–410. doi: 10.1111/j.1365-2567.2003.01770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yamashita T, Mao SY, Metzger H. Aggregation of the high-affinity IgE receptor and enhanced activity of p53/56lyn protein-tyrosine kinase. Proc Natl Acad Sci USA. 1994;91:11251–11255. doi: 10.1073/pnas.91.23.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kane P, Erickson J, Fewtrell C, Baird B, Holowka D. Cross-linking of IgE-receptor complexes at the cell surface: synthesis and characterization of a long bivalent hapten that is capable of triggering mast cells and rat basophilic leukemia cells. Mol Immunol. 1986;23:783–790. doi: 10.1016/0161-5890(86)90090-8. [DOI] [PubMed] [Google Scholar]
- 240.Kanamaru Y, Blank U, Monteiro RC. IgA Fc receptorI Is a molecular switch that determines IgA activating or inhibitory functions. Contrib Nephrol. 2007;157:148–152. doi: 10.1159/000102459. [DOI] [PubMed] [Google Scholar]
- 241.Powell MS, Hogarth PM. Fc receptors. Adv Exp Med Biol. 2008;640:22–34. doi: 10.1007/978-0-387-09789-3_3. [DOI] [PubMed] [Google Scholar]
- 242.Li P, Morris DL, Willcox BE, Steinle A, Spies T, Strong RK. Complex structure of the activating immunoreceptor NKG2D and its MHC class I-like ligand MICA. Nat Immunol. 2001;2:443–451. doi: 10.1038/87757. [DOI] [PubMed] [Google Scholar]
- 243.Strong RK. Asymmetric ligand recognition by the activating natural killer cell receptor NKG2D, a symmetric homodimer. Mol Immunol. 2002;38:1029–1037. doi: 10.1016/s0161-5890(02)00032-9. [DOI] [PubMed] [Google Scholar]
- 244.Farndale RW. Collagen-induced platelet activation. Blood Cells Mol Dis. 2006;36:162–165. doi: 10.1016/j.bcmd.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 245.Smethurst PA, Onley DJ, Jarvis GE, O'Connor MN, Knight CG, Herr AB, et al. Structural basis for the platelet-collagen interaction: the smallest motif within collagen that recognizes and activates platelet Glycoprotein VI contains two glycine-proline-hydroxyproline triplets. J Biol Chem. 2007;282:1296–1304. doi: 10.1074/jbc.M606479200. [DOI] [PubMed] [Google Scholar]
- 246.Lu Q, Navdaev A, Clemetson JM, Clemetson KJ, Snake veno. C-type lectins interacting with platelet receptors. Structure-function relationships and effects on haemostasis. Toxicon. 2005;45:1089–1098. doi: 10.1016/j.toxicon.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 247.Kato K, Furihata K, Cheli Y, Radis-Baptista G, Kunicki TJ. Effect of multimer size and a natural dimorphism on the binding of convulxin to platelet glycoprotein (GP)VI. J Thromb Haemost. 2006;4:1107–1113. doi: 10.1111/j.1538-7836.2006.01874.x. [DOI] [PubMed] [Google Scholar]
- 248.Horii K, Brooks MT, Herr AB. Convulxin forms a dimer in solution and can bind eight copies of glycoprotein VI: implications for platelet activation. Biochemistry. 2009;48:2907–2914. doi: 10.1021/bi801820q. [DOI] [PubMed] [Google Scholar]
- 249.Singer KL, Mostov KE. Dimerization of the polymeric immunoglobulin receptor controls its transcytotic trafficking. Mol Biol Cell. 1998;9:901–915. doi: 10.1091/mbc.9.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mocsai A, Abram CL, Jakus Z, Hu Y, Lanier LL, Lowell CA. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat Immunol. 2006;7:1326–1333. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Li R, Mitra N, Gratkowski H, Vilaire G, Litvinov R, Nagasami C, et al. Activation of integrin αIIbβ3 by modulation of transmembrane helix associations. Science. 2003;300:795–798. doi: 10.1126/science.1079441. [DOI] [PubMed] [Google Scholar]
- 252.Hantgan RR, Lyles DS, Mallett TC, Rocco M, Nagaswami C, Weisel JW. Ligand binding promotes the entropy-driven oligomerization of integrin αIIbβ3. J Biol Chem. 2003;278:3417–3426. doi: 10.1074/jbc.M208869200. [DOI] [PubMed] [Google Scholar]
- 253.Iber D, Gruhn T. Organisation of B-cell receptors on the cell membrane. Syst Biol (Stevenage) 2006;153:401–404. doi: 10.1049/ip-syb:20060015. [DOI] [PubMed] [Google Scholar]
- 254.Matsuuchi L, Gold MR. New views of BCR structure and organization. Curr Opin Immunol. 2001;13:270–277. doi: 10.1016/s0952-7915(00)00215-6. [DOI] [PubMed] [Google Scholar]
- 255.Schamel WW, Reth M. Monomeric and oligomeric complexes of the B cell antigen receptor. Immunity. 2000;13:5–14. doi: 10.1016/s1074-7613(00)00003-0. [DOI] [PubMed] [Google Scholar]
- 256.Kim ST, Takeuchi K, Sun ZY, Touma M, Castro CE, Fahmy A, et al. The αβ T cell receptor is an anisotropic mechanosensor. J Biol Chem. 2009;284:31028–31037. doi: 10.1074/jbc.M109.052712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Moss WC, Irvine DJ, Davis MM, Krummel MF. Quantifying signaling-induced reorientation of T cell receptors during immunological synapse formation. Proc Natl Acad Sci USA. 2002;99:15024–15029. doi: 10.1073/pnas.192573999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Minguet S, Swamy M, Alarcon B, Luescher IF, Schamel WW. Full activation of the T cell receptor requires both clustering and conformational changes at CD3. Immunity. 2007;26:43–54. doi: 10.1016/j.immuni.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 259.Kato Z, Stern JN, Nakamura HK, Kuwata K, Kondo N, Strominger JL. Positioning of autoimmune TCR-Ob.2F3 and TCR-Ob.3D1 on the MBP85-99/HLA-DR2 complex. Proc Natl Acad Sci USA. 2008;105:15523–15528. doi: 10.1073/pnas.0807338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Ding YH, Baker BM, Garboczi DN, Biddison WE, Wiley DC. Four A6-TCR/peptide/HLA-A2 structures that generate very different T cell signals are nearly identical. Immunity. 1999;11:45–56. doi: 10.1016/s1074-7613(00)80080-1. [DOI] [PubMed] [Google Scholar]
- 261.Ortega E, Lara M, Lee I, Santana C, Martinez AM, Pfeiffer JR, et al. Lyn dissociation from phosphorylated FcεRI subunits: a new regulatory step in the FcεRI signaling cascade revealed by studies of FcεRI dimer signaling activity. J Immunol. 1999;162:176–185. [PubMed] [Google Scholar]
- 262.Siegers GM, Yang J, Duerr CU, Nielsen PJ, Reth M, Schamel WW. Identification of disulfide bonds in the Ig-α/Ig-β component of the B cell antigen receptor using the Drosophila S2 cell reconstitution system. Int Immunol. 2006;18:1385–1396. doi: 10.1093/intimm/dxl072. [DOI] [PubMed] [Google Scholar]
- 263.Su Z, Wang H, Wan Y, Bi Z. In vitro preparation and characterization of the human CD3εε homodimer and CD3εγ and CD3εδ heterodimers. Int J Mol Med. 2009;24:437–444. [PubMed] [Google Scholar]
- 264.Asai K, Fujimoto K, Harazaki M, Kusunoki T, Korematsu S, Ide C, et al. Distinct aggregation of β- and γ-chains of the high-affinity IgE receptor on cross-linking. J Histochem Cytochem. 2000;48:1705–1716. doi: 10.1177/002215540004801213. [DOI] [PubMed] [Google Scholar]
- 265.Kishimoto H, Kubo RT, Yorifuji H, Nakayama T, Asano Y, Tada T. Physical dissociation of the TCR-CD3 complex accompanies receptor ligation. J Exp Med. 1995;182:1997–2006. doi: 10.1084/jem.182.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kosugi A, Saitoh S, Noda S, Yasuda K, Hayashi F, Ogata M, et al. Translocation of tyrosine-phosphorylated TCRzeta chain to glycolipid-enriched membrane domains upon T cell activation. Int Immunol. 1999;11:1395–1401. doi: 10.1093/intimm/11.9.1395. [DOI] [PubMed] [Google Scholar]
- 267.La Gruta NL, Liu H, Dilioglou S, Rhodes M, Wiest DL, Vignali DA. Architectural changes in the TCR:CD3 complex induced by MHC:Peptide ligation. J Immunol. 2004;172:3662–3669. doi: 10.4049/jimmunol.172.6.3662. [DOI] [PubMed] [Google Scholar]
- 268.Liu H, Rhodes M, Wiest DL, Vignali DA. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity. 2000;13:665–675. doi: 10.1016/s1074-7613(00)00066-2. [DOI] [PubMed] [Google Scholar]
- 269.D'Oro U, Munitic I, Chacko G, Karpova T, McNally J, Ashwell JD. Regulation of constitutive TCR internalization by the ζ-chain. J Immunol. 2002;169:6269–6278. doi: 10.4049/jimmunol.169.11.6269. [DOI] [PubMed] [Google Scholar]
- 270.Ouchida R, Yamasaki S, Hikida M, Masuda K, Kawamura K, Wada A, et al. A lysosomal protein negatively regulates surface T cell antigen receptor expression by promoting CD3ζ-chain degradation. Immunity. 2008;29:33–43. doi: 10.1016/j.immuni.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 271.McKay DB, Irie HY, Hollander G, Ferrara JL, Strom TB, Li Y, et al. Antigen-induced unresponsiveness results in altered T cell signaling. J Immunol. 1999;163:6455–6461. [PubMed] [Google Scholar]
- 272.Willems F, Andris F, Xu D, Abramowicz D, Wissing M, Goldman M, et al. The induction of human T cell unresponsiveness by soluble anti-CD3 mAb requires T cell activation. Int Immunol. 1995;7:1593–1598. doi: 10.1093/intimm/7.10.1593. [DOI] [PubMed] [Google Scholar]
- 273.Wang XM, Djordjevic JT, Kurosaka N, Schibeci S, Lee L, Williamson P, et al. T-cell antigen receptor peptides inhibit signal transduction within the membrane bilayer. Clin Immunol. 2002;105:199–207. doi: 10.1006/clim.2002.5270. [DOI] [PubMed] [Google Scholar]
- 274.Kim JH, Cramer L, Mueller H, Wilson B, Vilen BJ. Independent trafficking of Ig-α/Ig-β and mu-heavy chain is facilitated by dissociation of the B cell antigen receptor complex. J Immunol. 2005;175:147–154. doi: 10.4049/jimmunol.175.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Kremyanskaya M, Monroe JG. Ig-independent Igβ expression on the surface of B lymphocytes after B cell receptor aggregation. J Immunol. 2005;174:1501–1506. doi: 10.4049/jimmunol.174.3.1501. [DOI] [PubMed] [Google Scholar]
- 276.Gascoigne NR, Zal T, Alam SM. T-cell receptor binding kinetics in T-cell development and activation. Expert Rev Mol Med. 2001;2001:1–17. doi: 10.1017/S1462399401002502. [DOI] [PubMed] [Google Scholar]
- 277.Germain RN, Stefanova I. The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation. Annu Rev Immunol. 1999;17:467–522. doi: 10.1146/annurev.immunol.17.1.467. [DOI] [PubMed] [Google Scholar]
- 278.Engelhardt JJ, Krummel MF. The importance of prolonged binding to antigen-presenting cells for T cell fate decisions. Immunity. 2008;28:143–145. doi: 10.1016/j.immuni.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 279.Scholer A, Hugues S, Boissonnas A, Fetler L, Amigorena S. Intercellular adhesion molecule-1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory. Immunity. 2008;28:258–270. doi: 10.1016/j.immuni.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 280.Rosette C, Werlen G, Daniels MA, Holman PO, Alam SM, Travers PJ, et al. The impact of duration versus extent of TCR occupancy on T cell activation: a revision of the kinetic proofreading model. Immunity. 2001;15:59–70. doi: 10.1016/s1074-7613(01)00173-x. [DOI] [PubMed] [Google Scholar]
- 281.Goldstein B, Coombs D, Faeder JR, Hlavacek WS. Kinetic proofreading model. Adv Exp Med Biol. 2008;640:82–94. doi: 10.1007/978-0-387-09789-3_8. [DOI] [PubMed] [Google Scholar]
- 282.Werlen G, Hausmann B, Naeher D, Palmer E. Signaling life and death in the thymus: timing is everything. Science. 2003;299:1859–1863. doi: 10.1126/science.1067833. [DOI] [PubMed] [Google Scholar]
- 283.Love PE, Lee J, Shores EW. Critical relationship between TCR signaling potential and TCR affinity during thymocyte selection. J Immunol. 2000;165:3080–3087. doi: 10.4049/jimmunol.165.6.3080. [DOI] [PubMed] [Google Scholar]
- 284.Mirshahidi S, Ferris LC, Sadegh-Nasseri S. The magnitude of TCR engagement is a critical predictor of T cell anergy or activation. J Immunol. 2004;172:5346–5355. doi: 10.4049/jimmunol.172.9.5346. [DOI] [PubMed] [Google Scholar]
- 285.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 286.Langenkamp A, Casorati G, Garavaglia C, Dellabona P, Lanzavecchia A, Sallusto F. T cell priming by dendritic cells: thresholds for proliferation, differentiation and death and intraclonal functional diversification. Eur J Immunol. 2002;32:2046–2054. doi: 10.1002/1521-4141(200207)32:7<2046::AID-IMMU2046>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 287.Varma R. TCR triggering by the pMHC complex: valency, affinity and dynamics. Sci Signal. 2008;1:21. doi: 10.1126/stke.119pe21. [DOI] [PubMed] [Google Scholar]
- 288.Naeher D, Daniels MA, Hausmann B, Guillaume P, Luescher I, Palmer E. A constant affinity threshold for T cell tolerance. J Exp Med. 2007;204:2553–2559. doi: 10.1084/jem.20070254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Batista FD, Neuberger MS. Affinity dependence of the B cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity. 1998;8:751–759. doi: 10.1016/s1074-7613(00)80580-4. [DOI] [PubMed] [Google Scholar]
- 290.Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, et al. B cell receptor signal strength determines B cell fate. Nat Immunol. 2004;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- 291.Benschop RJ, Cambier JC. B cell development: signal transduction by antigen receptors and their surrogates. Curr Opin Immunol. 1999;11:143–151. doi: 10.1016/s0952-7915(99)80025-9. [DOI] [PubMed] [Google Scholar]
- 292.George J, Penner SJ, Weber J, Berry J, Claflin JL. Influence of membrane Ig receptor density and affinity on B cell signaling by antigen. Implications for affinity maturation. J Immunol. 1993;151:5955–5965. [PubMed] [Google Scholar]
- 293.Singh DK, Kumar D, Siddiqui Z, Basu SK, Kumar V, Rao KV. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell. 2005;121:281–293. doi: 10.1016/j.cell.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 294.Kinet JP. The high-affinity IgE receptor (FcεRI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 295.Yamasaki S, Saito T. Regulation of mast cell activation through FcεRI. Chem Immunol Allergy. 2005;87:22–31. doi: 10.1159/000087568. [DOI] [PubMed] [Google Scholar]
- 296.Hlavacek WS, Redondo A, Wofsy C, Goldstein B. Kinetic proofreading in receptor-mediated transduction of cellular signals: receptor aggregation, partially activated receptor, and cytosolic messengers. Bull Math Biol. 2002;64:887–911. doi: 10.1006/bulm.2002.0306. [DOI] [PubMed] [Google Scholar]
- 297.Iwashima M. Kinetic perspectives of T cell antigen receptor signaling. A two-tier model for T cell full activation. Immunol Rev. 2003;191:196–210. doi: 10.1034/j.1600-065x.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- 298.Hlavacek WS, Redondo A, Metzger H, Wofsy C, Goldstein B. Kinetic proofreading models for cell signaling predict ways to escape kinetic proofreading. Proc Natl Acad Sci USA. 2001;98:7295–7300. doi: 10.1073/pnas.121172298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Ono S, Ohno H, Saito T. Rapid turnover of the CD3ζ chain independent of the TCR-CD3 complex in normal T cells. Immunity. 1995;2:639–644. doi: 10.1016/1074-7613(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 300.Holst J, Wang H, Eder KD, Workman CJ, Boyd KL, Baquet Z, et al. Scalable signaling mediated by T cell antigen receptor-CD3 ITAMs ensures effective negative selection and prevents autoimmunity. Nat Immunol. 2008;9:658–666. doi: 10.1038/ni.1611. [DOI] [PubMed] [Google Scholar]
- 301.Malissen B. CD3 ITAMs count! Nat Immunol. 2008;9:583–584. doi: 10.1038/ni0608-583. [DOI] [PubMed] [Google Scholar]
- 302.McKeithan TW. Kinetic proofreading in T-cell receptor signal transduction. Proc Natl Acad Sci USA. 1995;92:5042–5046. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Rabinowitz JD, Beeson C, Lyons DS, Davis MM, McConnell HM. Kinetic discrimination in T-cell activation. Proc Natl Acad Sci USA. 1996;93:1401–1405. doi: 10.1073/pnas.93.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Rabinowitz JD, Beeson C, Wulfing C, Tate K, Allen PM, Davis MM, et al. Altered T cell receptor ligands trigger a subset of early T cell signals. Immunity. 1996;5:125–135. doi: 10.1016/s1074-7613(00)80489-6. [DOI] [PubMed] [Google Scholar]
- 305.Kersh GJ, Kersh EN, Fremont DH, Allen PM. High- and low-potency ligands with similar affinities for the TCR: the importance of kinetics in TCR signaling. Immunity. 1998;9:817–826. doi: 10.1016/s1074-7613(00)80647-0. [DOI] [PubMed] [Google Scholar]
- 306.Schamel WW, Risueno RM, Minguet S, Ortiz AR, Alarcon B. A conformation- and avidity-based proofreading mechanism for the TCR-CD3 complex. Trends Immunol. 2006;27:176–182. doi: 10.1016/j.it.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 307.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 308.Itoh Y, Hemmer B, Martin R, Germain RN. Serial TCR engagement and down-modulation by peptide:MHC molecule ligands: relationship to the quality of individual TCR signaling events. J Immunol. 1999;162:2073–2080. [PubMed] [Google Scholar]
- 309.Borovsky Z, Mishan-Eisenberg G, Yaniv E, Rachmilewitz J. Serial triggering of T cell receptors results in incremental accumulation of signaling intermediates. J Biol Chem. 2002;277:21529–21536. doi: 10.1074/jbc.M201613200. [DOI] [PubMed] [Google Scholar]
- 310.Sousa J, Carneiro J. A mathematical analysis of TCR serial triggering and downregulation. Eur J Immunol. 2000;30:3219–3227. doi: 10.1002/1521-4141(200011)30:11<3219::AID-IMMU3219>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 311.Friedl P, Gunzer M. Interaction of T cells with APCs: the serial encounter model. Trends Immunol. 2001;22:187–191. doi: 10.1016/s1471-4906(01)01869-5. [DOI] [PubMed] [Google Scholar]
- 312.Davis MM. A new trigger for T cells. Cell. 2002;110:285–287. doi: 10.1016/s0092-8674(02)00865-6. [DOI] [PubMed] [Google Scholar]
- 313.Alarcon B, Gil D, Delgado P, Schamel WW. Initiation of TCR signaling: regulation within CD3 dimers. Immunol Rev. 2003;191:38–46. doi: 10.1034/j.1600-065x.2003.00017.x. [DOI] [PubMed] [Google Scholar]
- 314.Reth M, Wienands J, Schamel WW. An unsolved problem of the clonal selection theory and the model of an oligomeric B-cell antigen receptor. Immunol Rev. 2000;176:10–18. doi: 10.1034/j.1600-065x.2000.00610.x. [DOI] [PubMed] [Google Scholar]
- 315.Aivazian D, Stern LJ. Phosphorylation of T cell receptor ζ is regulated by a lipid dependent folding transition. Nat Struct Biol. 2000;7:1023–1026. doi: 10.1038/80930. [DOI] [PubMed] [Google Scholar]
- 316.Rojo JM, Janeway CA., Jr The biologic activity of anti-T cell receptor V region monoclonal antibodies is determined by the epitope recognized. J Immunol. 1988;140:1081–1088. [PubMed] [Google Scholar]
- 317.Krogsgaard M, Prado N, Adams EJ, He XL, Chow DC, Wilson DB, et al. Evidence that structural rearrangements and/or flexibility during TCR binding can contribute to T cell activation. Mol Cell. 2003;12:1367–1378. doi: 10.1016/s1097-2765(03)00474-x. [DOI] [PubMed] [Google Scholar]
- 318.Kjer-Nielsen L, Dunstone MA, Kostenko L, Ely LK, Beddoe T, Mifsud NA, et al. Crystal structure of the human T cell receptor CD3εγ heterodimer complexed to the therapeutic mAb OKT3. Proc Natl Acad Sci USA. 2004;101:7675–7680. doi: 10.1073/pnas.0402295101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Levin SE, Weiss A. Twisting tails exposed: the evidence for TCR conformational change. J Exp Med. 2005;201:489–492. doi: 10.1084/jem.20050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Anton van der Merwe P, Davis SJ, Shaw AS, Dustin ML. Cytoskeletal polarization and redistribution of cell-surface molecules during T cell antigen recognition. Semin Immunol. 2000;12:5–21. doi: 10.1006/smim.2000.0203. [DOI] [PubMed] [Google Scholar]
- 321.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 322.Davis SJ, van der Merwe PA. The kinetic-segregation model: TCR triggering and beyond. Nat Immunol. 2006;7:803–809. doi: 10.1038/ni1369. [DOI] [PubMed] [Google Scholar]
- 323.Kuhns MS, Davis MM. The safety on the TCR trigger. Cell. 2008;135:594–596. doi: 10.1016/j.cell.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Xu C, Gagnon E, Call ME, Schnell JR, Schwieters CD, Carman CV, et al. Regulation of T cell receptor activation by dynamic membrane binding of the CD3ε cytoplasmic tyrosine-based motif. Cell. 2008;135:702–713. doi: 10.1016/j.cell.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Manolios N, Ali M, Amon M, Bender V. Therapeutic application of transmembrane T and natural killer cell receptor peptides. Adv Exp Med Biol. 2008;640:208–219. doi: 10.1007/978-0-387-09789-3_16. [DOI] [PubMed] [Google Scholar]
- 326.Manolios N, Collier S, Taylor J, Pollard J, Harrison LC, Bender V. T-cell antigen receptor transmembrane peptides modulate T-cell function and T cell-mediated disease. Nat Med. 1997;3:84–88. doi: 10.1038/nm0197-84. [DOI] [PubMed] [Google Scholar]
- 327.Ali M, De Planque MRR, Huynh NT, Manolios N, Separovic F. Biophysical studies of a transmembrane peptide derived from the T cell antigen receptor. Lett Pept Sci. 2002;8:227–233. [Google Scholar]
- 328.Bender V, Ali M, Amon M, Diefenbach E, Manolios N. T cell antigen receptor peptide-lipid membrane interactions using surface plasmon resonance. J Biol Chem. 2004;279:54002–54007. doi: 10.1074/jbc.M403909200. [DOI] [PubMed] [Google Scholar]
- 329.Gerber D, Quintana FJ, Bloch I, Cohen IR, Shai Y. D-enantiomer peptide of the TCRα transmembrane domain inhibits T-cell activation in vitro and in vivo. Faseb J. 2005;19:1190–1192. doi: 10.1096/fj.04-3498fje. [DOI] [PubMed] [Google Scholar]
- 330.Enk AH, Knop J. T cell receptor mimic peptides and their potential application in T-cell-mediated disease. Int Arch Allergy Immunol. 2000;123:275–281. doi: 10.1159/000053639. [DOI] [PubMed] [Google Scholar]
- 331.Gollner GP, Muller G, Alt R, Knop J, Enk AH. Therapeutic application of T cell receptor mimic peptides or cDNA in the treatment of T cell-mediated skin diseases. Gene Ther. 2000;7:1000–1004. doi: 10.1038/sj.gt.3301183. [DOI] [PubMed] [Google Scholar]
- 332.Manolios N, Huynh NT, Collier S. Peptides in the treatment of inflammatory skin disease. Australas J Dermatol. 2002;43:226–227. doi: 10.1046/j.1440-0960.2002.00603.x. [DOI] [PubMed] [Google Scholar]
- 333.Kurosaka N, Bolte A, Ali M, Manolios N. T-cell antigen receptor assembly and cell surface expression is not affected by treatment with T-cell antigen receptor-α chain transmembrane Peptide. Protein Pept Lett. 2007;14:299–303. doi: 10.2174/092986607780090865. [DOI] [PubMed] [Google Scholar]
- 334.Collier S, Bolte A, Manolios N. Discrepancy in CD3-transmembrane peptide activity between in vitro and in vivo T-cell inhibition. Scand J Immunol. 2006;64:388–391. doi: 10.1111/j.1365-3083.2006.01806.x. [DOI] [PubMed] [Google Scholar]
- 335.Amon MA, Ali M, Bender V, Chan YN, Toth I, Manolios N. Lipidation and glycosylation of a T cell antigen receptor (TCR) transmembrane hydrophobic peptide dramatically enhances in vitro and in vivo function. Biochim Biophys Acta. 2006 doi: 10.1016/j.bbamcr.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 336.Ali M, Salam NK, Amon M, Bender V, Hibbs DE, Manolios N. T-Cell Antigen Receptor-alpha Chain Transmembrane Peptides: Correlation between Structure and Function. Int J Pept Res Ther. 2006;12:261–267. [Google Scholar]
- 337.Vandebona H, Ali M, Amon M, Bender V, Manolios N. Immunoreceptor transmembrane peptides and their effect on natural killer (NK) cell cytotoxicity. Protein Pept Lett. 2006;13:1017–1024. doi: 10.2174/092986606778777452. [DOI] [PubMed] [Google Scholar]
- 338.Sigalov AB. Novel mechanistic concept of platelet inhibition. Expert Opin Ther Targets. 2008;12:677–692. doi: 10.1517/14728222.12.6.677. [DOI] [PubMed] [Google Scholar]
- 339.Sigalov AB. Novel mechanistic insights into viral modulation of immune receptor signaling. PLoS Pathog. 2009;5:1000404. doi: 10.1371/journal.ppat.1000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Kim WM, Sigalov AB. Viral pathogenesis, modulation of immune receptor signaling and treatment. Adv Exp Med Biol. 2008;640:325–349. doi: 10.1007/978-0-387-09789-3_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Spencer DM, Wandless TJ, Schreiber SL, Crabtree GR. Controlling signal transduction with synthetic ligands. Science. 1993;262:1019–1024. doi: 10.1126/science.7694365. [DOI] [PubMed] [Google Scholar]
- 342.Soldevila G, Castellanos C, Malissen M, Berg LJ. Analysis of the individual role of the TCRζ chain in transgenic mice after conditional activation with chemical inducers of dimerization. Cell Immunol. 2001;214:123–138. doi: 10.1006/cimm.2001.1892. [DOI] [PubMed] [Google Scholar]
- 343.Crabtree GR, Schreiber SL. Three-part inventions: intracellular signaling and induced proximity. Trends Biochem Sci. 1996;21:418–422. doi: 10.1016/s0968-0004(96)20027-1. [DOI] [PubMed] [Google Scholar]
- 344.Buferne M, Luton F, Letourneur F, Hoeveler A, Couez D, Barad M, et al. Role of CD3δ in surface expression of the TCR/CD3 complex and in activation for killing analyzed with a CD3δ-negative cytotoxic T lymphocyte variant. J Immunol. 1992;148:657–664. [PubMed] [Google Scholar]
- 345.Luton F, Buferne M, Legendre V, Chauvet E, Boyer C, Schmitt-Verhulst AM. Role of CD3γ and CD3δ cytoplasmic domains in cytolytic T lymphocyte functions and TCR/CD3 downmodulation. J Immunol. 1997;158:4162–4170. [PubMed] [Google Scholar]
- 346.Haks MC, Cordaro TA, van den Brakel JH, Haanen JB, de Vries EF, Borst J, et al. A redundant role of the CD3γ-immunoreceptor tyrosine-based activation motif in mature T cell function. J Immunol. 2001;166:2576–2588. doi: 10.4049/jimmunol.166.4.2576. [DOI] [PubMed] [Google Scholar]
- 347.Haks MC, Pepin E, van den Brakel JH, Smeele SA, Belkowski SM, Kessels HW, et al. Contributions of the T cell receptor-associated CD3γ-ITAM to thymocyte selection. J Exp Med. 2002;196:1–13. doi: 10.1084/jem.20020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.de Saint Basile G, Geissmann F, Flori E, Uring-Lambert B, Soudais C, Cavazzana-Calvo M, et al. Severe combined immunodeficiency caused by deficiency in either the δ or the ε subunit of CD3. J Clin Invest. 2004;114:1512–1517. doi: 10.1172/JCI22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Roifman CM. CD3δ immunodeficiency. Curr Opin Allergy Clin Immunol. 2004;4:479–484. doi: 10.1097/00130832-200412000-00002. [DOI] [PubMed] [Google Scholar]
- 350.Teixeiro E, Daniels MA, Hausmann B, Schrum AG, Naeher D, Luescher I, et al. T cell division and death are segregated by mutation of TCRβ chain constant domains. Immunity. 2004;21:515–526. doi: 10.1016/j.immuni.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 351.Teixeiro E, Daniels MA, Hamilton SE, Schrum AG, Bragado R, Jameson SC, et al. Different T cell receptor signals determine CD8+ memory versus effectordevelopment. Science. 2009;323:502–505. doi: 10.1126/science.1163612. [DOI] [PubMed] [Google Scholar]
- 352.Torres PS, Zapata DA, Pacheco-Castro A, Rodriguez-Fernandez JL, Cabanas C, Regueiro JR. Contribution of CD3γ to TCR regulation and signaling in human mature T lymphocytes. Int Immunol. 2002;14:1357–1367. doi: 10.1093/intimm/dxf095. [DOI] [PubMed] [Google Scholar]
- 353.Rodriguez-Tarduchy G, Sahuquillo AG, Alarcon B, Bragado R. Apoptosis but not other activation events is inhibited by a mutation in the transmembrane domain of T cell receptor β that impairs CD3ζ association. J Biol Chem. 1996;271:30417–30425. doi: 10.1074/jbc.271.48.30417. [DOI] [PubMed] [Google Scholar]
- 354.Sutton BJ, Gould HJ. The human IgE network. Nature. 1993;366:421–428. doi: 10.1038/366421a0. [DOI] [PubMed] [Google Scholar]
- 355.Gould HJ, Sutton BJ, Beavil AJ, Beavil RL, McCloskey N, Coker HA, et al. The biology of IGE and the basis of allergic disease. Annu Rev Immunol. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 356.Kraft S, Novak N. Fc receptors as determinants of allergic reactions. Trends Immunol. 2006;27:88–95. doi: 10.1016/j.it.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 357.Adamczewski M, Paolini R, Kinet JP. Evidence for two distinct phosphorylation pathways activated by high affinity immunoglobulin E receptors. J Biol Chem. 1992;267:18126–18132. [PubMed] [Google Scholar]
- 358.Dombrowicz D, Lin S, Flamand V, Brini AT, Koller BH, Kinet JP. Allergy-associated FcRβ is a molecular amplifier of IgE- and IgG-mediated in vivo responses. Immunity. 1998;8:517–529. doi: 10.1016/s1074-7613(00)80556-7. [DOI] [PubMed] [Google Scholar]
- 359.Eiseman E, Bolen JB. Signal transduction by the cytoplasmic domains of FcεRI-γ and TCR-ζ in rat basophilic leukemia cells. J Biol Chem. 1992;267:21027–21032. [PubMed] [Google Scholar]
- 360.Jouvin MH, Adamczewski M, Numerof R, Letourneur O, Valle A, Kinet JP. Differential control of the tyrosine kinases Lyn and Syk by the two signaling chains of the high affinity immunoglobulin E receptor. J Biol Chem. 1994;269:5918–5925. [PubMed] [Google Scholar]
- 361.Scharenberg AM, Lin S, Cuenod B, Yamamura H, Kinet JP. Reconstitution of interactions between tyrosine kinases and the high affinity IgE receptor which are controlled by receptor clustering. EMBO J. 1995;14:3385–3394. doi: 10.1002/j.1460-2075.1995.tb07344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Shiue L, Green J, Green OM, Karas JL, Morgenstern JP, Ram MK, et al. Interaction of p72syk with the γ and β subunits of the high-affinity receptor for immunoglobulin E, FcεRI. Mol Cell Biol. 1995;15:272–281. doi: 10.1128/mcb.15.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Wilson BS, Kapp N, Lee RJ, Pfeiffer JR, Martinez AM, Platt Y, et al. Distinct functions of the FcεR1γ and β subunits in the control of FcεR1-mediated tyrosine kinase activation and signaling responses in RBL-2H3 mast cells. J Biol Chem. 1995;270:4013–4022. doi: 10.1074/jbc.270.8.4013. [DOI] [PubMed] [Google Scholar]
- 364.Goldstein B, Faeder JR, Hlavacek WS. Mathematical and computational models of immune-receptor signalling. Nat Rev Immunol. 2004;4:445–456. doi: 10.1038/nri1374. [DOI] [PubMed] [Google Scholar]
- 365.Goldstein B, Faeder JR, Hlavacek WS, Blinov ML, Redondo A, Wofsy C. Modeling the early signaling events mediated by FcεR1. Mol Immunol. 2002;38:1213–1219. doi: 10.1016/s0161-5890(02)00066-4. [DOI] [PubMed] [Google Scholar]
- 366.Faeder JR, Hlavacek WS, Reischl I, Blinov ML, Metzger H, Redondo A, et al. Investigation of early events in FcεR1-mediated signaling using a detailed mathematical model. J Immunol. 2003;170:3769–3781. doi: 10.4049/jimmunol.170.7.3769. [DOI] [PubMed] [Google Scholar]
- 367.Hlavacek WS, Faeder JR, Blinov ML, Perelson AS, Goldstein B. The complexity of complexes in signal transduction. Biotechnol Bioeng. 2003;84:783–794. doi: 10.1002/bit.10842. [DOI] [PubMed] [Google Scholar]
- 368.Nadler MJ, Matthews SA, Turner H, Kinet JP. Signal transduction by the high-affinity immunoglobulin E receptor FcεR1: coupling form to function. Adv Immunol. 2000;76:325–355. doi: 10.1016/s0065-2776(01)76022-1. [DOI] [PubMed] [Google Scholar]
- 369.Liu KJ, Parikh VS, Tucker PW, Kim BS. Role of the B cell antigen receptor in antigen processing and presentation. Involvement of the transmembrane region in intracellular trafficking of receptor/ligand complexes. J Immunol. 1993;151:6143–6154. [PubMed] [Google Scholar]
- 370.Pleiman CM, D'Ambrosio D, Cambier JC. The B-cell antigen receptor complex: structure and signal transduction. Immunol Today. 1994;15:393–399. doi: 10.1016/0167-5699(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 371.Cambier JC. Signal transduction by T- and B-cell antigen receptors: converging structures and concepts. Curr Opin Immunol. 1992;4:257–264. doi: 10.1016/0952-7915(92)90074-o. [DOI] [PubMed] [Google Scholar]
- 372.Cambier JC, Pleiman CM, Clark MR. Signal transduction by the B cell antigen receptor and its coreceptors. Annu Rev Immunol. 1994;12:457–486. doi: 10.1146/annurev.iy.12.040194.002325. [DOI] [PubMed] [Google Scholar]
- 373.Kraus M, Pao LI, Reichlin A, Hu Y, Canono B, Cambier JC, et al. Interference with immunoglobulin (Ig)α immunoreceptor tyrosine-based activation motif (ITAM) phosphorylation modulates or blocks B cell development, depending on the availability of an Igβ cytoplasmic tail. J Exp Med. 2001;194:455–469. doi: 10.1084/jem.194.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Batista FD, Iber D, Neuberger MS. B cells acquire antigen from target cells after synapse formation. Nature. 2001;411:489–494. doi: 10.1038/35078099. [DOI] [PubMed] [Google Scholar]
- 375.Bannish G, Fuentes-Panana EM, Cambier JC, Pear WS, Monroe JG. Ligand-independent signaling functions for the B lymphocyte antigen receptor and their role in positive selection during B lymphopoiesis. J Exp Med. 2001;194:1583–1596. doi: 10.1084/jem.194.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Kato K, Kanaji T, Russell S, Kunicki TJ, Furihata K, Kanaji S, et al. The contribution of glycoprotein VI to stable platelet adhesion and thrombus formation illustrated by targeted gene deletion. Blood. 2003;102:1701–1707. doi: 10.1182/blood-2003-03-0717. [DOI] [PubMed] [Google Scholar]
- 377.Gawaz M. Role of platelets in coronary thrombosis and reperfusion of ischemic myocardium. Cardiovasc Res. 2004;61:498–511. doi: 10.1016/j.cardiores.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 378.Gibbins JM, Okuma M, Farndale R, Barnes M, Watson SP. Glycoprotein VI is the collagen receptor in platelets which underlies tyrosine phosphorylation of the Fc receptor γ-chain. FEBS Lett. 1997;413:255–259. doi: 10.1016/s0014-5793(97)00926-5. [DOI] [PubMed] [Google Scholar]
- 379.Sigalov AB. Inhibiting Collagen-induced Platelet Aggregation and Activation with Peptide Variants. US 12/001,258 and PCT PCT/US2007/025389 patent applications filed on 12/11/2007 and 12/12/2007, respectively, claiming a priority to US 60/874,694 provisional patent application filed on 12/13/2006. [Google Scholar]
- 380.Leitenberg D, Balamuth F, Bottomly K. Changes in the T cell receptor macromolecular signaling complex and membrane microdomains during T cell development and activation. Semin Immunol. 2001;13:129–138. doi: 10.1006/smim.2000.0304. [DOI] [PubMed] [Google Scholar]
- 381.Janeway CA, Jr, Bottomly K. Responses of T cells to ligands for the T-cell receptor. Semin Immunol. 1996;8:108–115. doi: 10.1006/smim.1996.0013. [DOI] [PubMed] [Google Scholar]
- 382.Janeway CA, Jr, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275–285. doi: 10.1016/0092-8674(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 383.Niedergang F, Dautry-Varsat A, Alcover A. Peptide antigen or superantigen-induced downregulation of TCRs involves both stimulated and unstimulated receptors. J Immunol. 1997;159:1703–1710. [PubMed] [Google Scholar]
- 384.Bonefeld CM, Rasmussen AB, Lauritsen JP, von Essen M, Odum N, Andersen PS, et al. TCR comodulation of nonengaged TCR takes place by a protein kinase C and CD3γ di-leucine-based motif-dependent mechanism. J Immunol. 2003;171:3003–3009. doi: 10.4049/jimmunol.171.6.3003. [DOI] [PubMed] [Google Scholar]
- 385.San Jose E, Borroto A, Niedergang F, Alcover A, Alarcon B. Triggering the TCR complex causes the downregulation of nonengaged receptors by a signal transduction-dependent mechanism. Immunity. 2000;12:161–170. doi: 10.1016/s1074-7613(00)80169-7. [DOI] [PubMed] [Google Scholar]
- 386.von Essen M, Nielsen MW, Bonefeld CM, Boding L, Larsen JM, Leitges M, et al. Protein kinase C (PKC)α and PKCτ are the major PKC isotypes involved in TCR downregulation. J Immunol. 2006;176:7502–7510. doi: 10.4049/jimmunol.176.12.7502. [DOI] [PubMed] [Google Scholar]
- 387.Quintana FJ, Gerber D, Kent SC, Cohen IR, Shai Y. HIV-1 fusion peptide targets the TCR and inhibits antigen-specific T cell activation. J Clin Invest. 2005;115:2149–2158. doi: 10.1172/JCI23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Bloch I, Quintana FJ, Gerber D, Cohen T, Cohen IR, Shai Y. T-Cell inactivation and immunosuppressive activity induced by HIV gp41 via novel interacting motif. Faseb J. 2007;21:393–401. doi: 10.1096/fj.06-7061com. [DOI] [PubMed] [Google Scholar]
- 389.Alarcon B, Swamy M, van Santen HM, Schamel WW. T-cell antigen-receptor stoichiometry: preclustering for sensitivity. EMBO Rep. 2006;7:490–495. doi: 10.1038/sj.embor.7400682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Kim YM, Pan JY, Korbel GA, Peperzak V, Boes M, Ploegh HL. Monovalent ligation of the B cell receptor induces receptor activation but fails to promote antigen presentation. Proc Natl Acad Sci USA. 2006;103:3327–3332. doi: 10.1073/pnas.0511315103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Eynon EE, Parker DC. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992;175:131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Gahring LC, Weigle WO. The induction of peripheral T cell unresponsiveness in adult mice by monomeric human γ-globulin. J Immunol. 1989;143:2094–2100. [PubMed] [Google Scholar]