The term “publication planning” can refer to a research group’s organizational timetable and plan. Within the pharmaceutical industry, however, the term describes the finely calibrated process by which clinical trials, commentaries and other articles supporting the efficacy of particular products are written and released into the biomedical literature. This article describes how industry uses publication planning to sway medical and public opinion through the medium of medical journals.
Industry publications describe the utility of publication planning in the following terms: it can “provide essential, appropriate sources for other communications, whether promotional or scientific.”1 It may also “influence regulatory authorities globally” and “influence disease perception and management through citation, discussion, and recommendation.”2
The controlled production and release of pre-clinical studies, clinical trials, reviews and commentaries may begin years before a drug is launched (Fig. 1). Peer-reviewed clinical efficacy studies supporting a new drug or a new indication for a commercially available drug are considered “primary” or “core” publications. According to an industry article, “For a pharma company, getting research published in a peer-reviewed medical journal is like winning a stamp of approval from its most influential audience. It’s an automatic validation unmatched by any other medium.”2 Primary articles “[p]rovide authoritative sources for marketing communications and other promotional materials,” “[s]upport the positioning and selling platform, and coordinate with the overall marketing plan” and “[a]ccelerate the adoption of a new chemical entity or new indication.”1 In other words, they provide the foundation for subsequent “secondary” or “derivative” publications, which include journal advertisements, promotional materials used by sales representatives, and reviews and opinion pieces published in medical journals.
Figure 1.
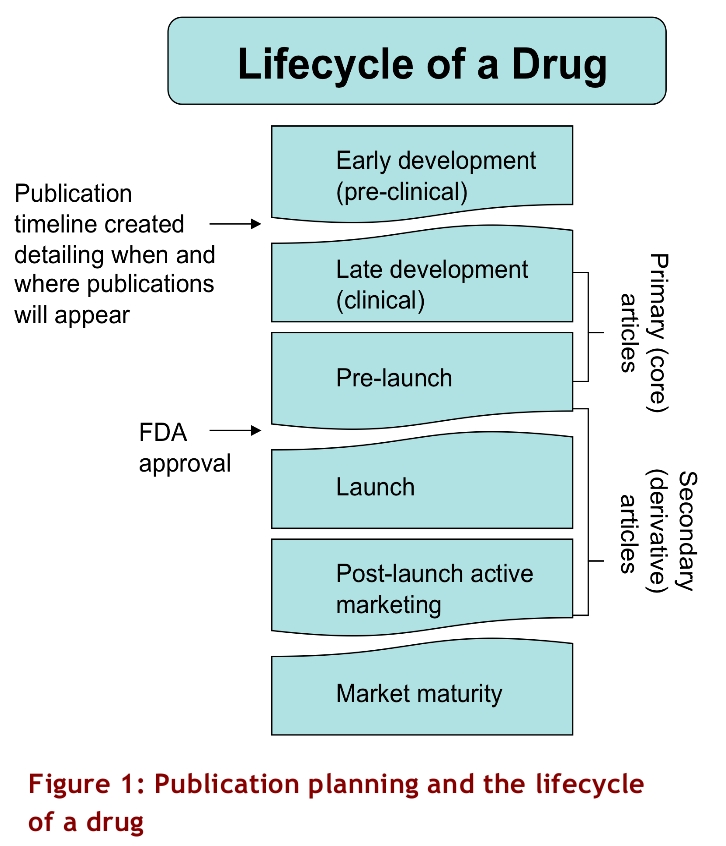
Publication planning and the lifecycle of a drug
Pharmaceutical companies cannot legally promote a drug before it has been approved by a regulatory authority, nor can they legally promote a marketed drug for off-label use (i.e., for indications other than those approved). However, the US Food and Drug Administration (FDA does not consider articles in the medical literature as promotional. As one industry article states, “Peer-reviewed publications offer pharma companies shelter from often-stormy regulatory waters. FDA views published articles as protected commercial speech so doesn’t regulate their content.”2
For this reason, the generation of subtly persuasive opinion pieces that can be distributed to prescribers in the pages of medical journals is an extremely important component of publication planning. Sponsored articles can be difficult for journal editors and readers to spot. In many cases, the drug that the article is meant to promote is not even mentioned. For example, if a drug is the only treatment for a given condition, articles that review the prevalence, severity or complications of that condition will prepare the market by raising physician awareness of specific issues. Articles that highlight the inconvenience or risks of competing therapies increase receptivity to a new drug that is more convenient or safer (e.g., has once-a-day dosing or is not associated with an adverse effect that may occur with competing drugs).
Reviews and commentaries are the Trojan horses bearing these messages. To ensure that articles are well written and contain suitably subtle marketing messages, a pharmaceutical company may enlist the assistance of a professional medical writer. Such assistance ranges from editing to ghostwriting (i.e., writing contributed by authors who are not acknowledged when the article is published).
Medical writers, who are often scientists or health professionals, are crucial to publication planning. They ensure that manuscripts are scientifically correct, professional, organized, readable, persuasive and submitted on time. Medical writers prepare primary and secondary publications, including clinical trial reports and reviews; they may also prepare meeting materials and abstracts.3 They may work directly for pharmaceutical companies, most often as freelancers, or they may be employed by medical education and communications companies (MECCs), which derive most of their income from pharmaceutical companies.4
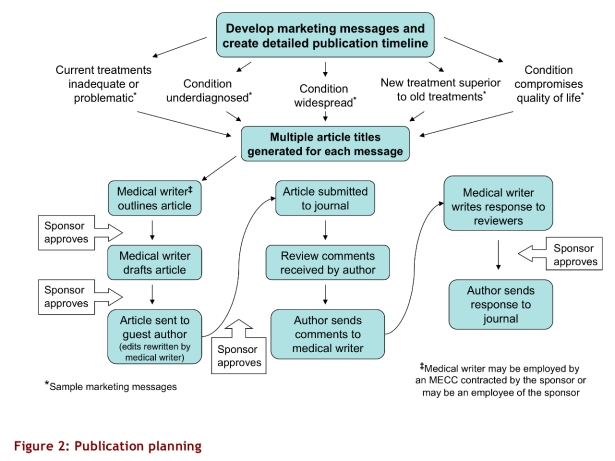
A pharmaceutical company may create a publication plan internally or work with a MECC on the plan. The process described here is used to create secondary publications. Typically, a publication plan includes a timeline and lists of articles, grouped under specific messages, with proposed titles and journals to target for submission (Fig. 2). Potential guest authors may also be listed.
Figure 2.
Publication planning
Once the topic of an article is chosen, a medical writer generates an outline, which is approved by the sponsoring pharmaceutical company. The writer then researches and writes the paper, incorporating the appropriate marketing message; an experienced writer may be able to communicate messages that align with a sponsor’s marketing objectives even when specific messages are not provided. After the completed article is approved, a guest author, usually an academically affiliated physician, is approached by the sponsor. Guest authors (who may receive payment through a MECC) are generally offered the option to contribute to or amend the article; they usually realize that edits disadvantageous to a sponsoring company’s marketing goals will result in the article not being published – or being published under another physician’s name.5
After approving the article, the guest author submits the manuscript as his or her original work to a journal specified by the pharmaceutical company. If the journal asks for revisions or clarifications, the medical writer writes the response, again for the guest author’s signature.
The extent of medical writers’ contributions to the medical literature is unknown. A limited study of 1000 research articles in 10 international journals found that a medical writer was acknowledged in about 6% of the articles.7 In a recent case study, researchers examined internal documents belonging to Merck that were revealed during litigation related to rofecoxib, and linked these documents to clinical trials and review articles published in medical journals. Of 20 rofecoxib clinical trial manuscripts that were, according to internal documents, authored by Merck scientists or medical writers, 16 gave an external, academically affiliated investigator first authorship position when they were subsequently published in journals. Furthermore, 50 of 72 review articles discussed within Merck prior to publication were published as solo-authored articles by academic physicians, and only 2 of the coauthored articles attributed authorship to a Merck employee.6
Two organizations support those who work in publication planning. The mission of the International Publication Planning Association “is to foster excellence in medical publications and communications within the biopharmaceutical industry”; according to its website, the association “provides practical strategies for developing, implementing and executing an effective publication and communication plan as a critical component of the clinical biopharmaceutical development process. Our aim is to help biopharmaceutical communication executives and their agencies produce ethical and targeted publications and clinical data throughout the product lifecycle.”8 The vision of the International Society for Medical Publication Professionals (ISMPP) “is to be the recognized and respected authority for the pharmaceutical, biotech, and device industries medical publication profession.“9
Conclusion
Publication planning, as it is currently practised by pharmaceutical companies, can undermine the medical literature. Industry control over the timing, content and authorship of studies and opinion pieces including reviews and commentaries distorts medical discourse. That academic health professionals (physicians, nurses, pharmacists) lend their names to articles to which they may have contributed nothing is ironic, considering that such behaviour by students in the same academic institutions would be considered plagiarism.
The International Committee of Medical Journal Editors has published important criteria for authorship.10 Medical writers are highly skilled professionals who certainly should be acknowledged when they meet the criteria for authorship. Disclosure, however, does not remove commercial influence. Sponsored writing reflects sponsored messages. Even the most vigilant editor could not uncover all of the marketing messages embedded in manuscripts by publication planning professionals.
We suggest that clinical trials sponsored by industry should be clearly labelled as such and sequestered from independent studies in medical journals. Industry-sponsored reviews and commentaries, including those sponsored through MECC intermediaries, should not be published in peer-reviewed medical literature.
Most peer-reviewed journals already require the disclosure of industry sponsorship for the publication of clinical trials and reviews. However, as we have discussed, it is difficult to discern when medical writers have been involved.
The infiltration of the medical literature by undisclosed sponsors using ghostwritten articles raises serious ethical issues. The medical literature should be a repository of reliable, unbiased scientific studies and considered opinion. Invisible industry influences on publications and presentations undermine a vital foundation for clinical decision-making.
Perhaps further education of the academic and medical community about this practice and its ethical implications will lead to more critical review of manuscripts, and refusal of guest authorship invitations by academics and clinicians.
Acknowledgments
We thank Alicia Bell, MS, for helpful comments and for creating the figures.
Biographies
Adriane Fugh-Berman, MD, is an associate professor in the Department of Physiology and Biophysics, Georgetown University Medical Center, Washington, DC.
Susanna J. Dodgson, PhD, is Adjunct Professor of Pharmacy, University of Lagos, Nigeria, and COO and President of Ustawi International, Inc., Haddonfield, NJ.
Footnotes
Competing interests: Dr. Fugh-Berman is the principal investigator on a grant from the Attorney General Consumer and Prescriber Education grant program, created as part of a 2004 settlement between Warner-Lambert, a division of Pfizer, Inc., and the Attorneys General of 50 States and the District of Columbia, to settle allegations that Warner-Lambert conducted an unlawful marketing campaign for the drug Neurontin (gabapentin) that violated state consumer protection laws. Dr. Fugh-Berman has also been an expert witness on behalf of plaintiffs in litigation regarding pharmaceutical marketing practices. Dr. Dodgson is the Editor-in-Chief of Medical Journal of Therapeutics Africa, and Chief Operating Officer of Ustawi International Inc., a global health resources company, and was Director of the MS in Biomedical Writing Program at the University of the Sciences in Philadelphia.
Funding source: This work received no specific funding.
References
- 1.Beebe FA. Publication planning. Med Mark Media. 2004:41–6. [Google Scholar]
- 2.Balter W, Skelton M, Safir PO. The P's and Q's of publication planning. Pharm Executive. 2003:130–6. [Google Scholar]
- 3.Moghadam Reza G. Scientific writing: a career for pharmacists. Am J Health Syst Pharm. 2003;60(18):1899–900. doi: 10.1093/ajhp/60.18.1899. [DOI] [PubMed] [Google Scholar]
- 4.Accreditation Council for Continuing Medical Education. ACCME Annual Report Data 2006. ACCME; 2007. [accessed 2008 Dec 18]. http://www.accme.org/dir_docs/doc_upload/f51ed7d8-e3b4-479a-a9d8-57b6efedc27a_uploaddocument.pdf. [Google Scholar]
- 5.Healy D. Let them eat Prozac: The unhealthy relationship between the pharmaceutical industry and depression. New York: New York University Press; 2004. [Google Scholar]
- 6.Woolley Karen L, Ely Julie A, Woolley Mark J, Findlay Leigh, Lynch Felicity A, Choi Yoonah, McDonald Jane M. Declaration of medical writing assistance in international peer-reviewed journals. JAMA. 2006;296(80):932–3. doi: 10.1001/jama.296.8.932-b. http://jama.ama-assn.org/cgi/content/full/296/8/932-a. [DOI] [PubMed] [Google Scholar]
- 7.Ross Joseph S, Hill Kevin P, Egilman David S, Krumholz Harlan M. Guest authorship and ghostwriting in publications related to Rofecoxib: A case study of industry documents from Rofecoxib litigation. JAMA. 2008;299(15):1800. doi: 10.1001/jama.299.15.1800. http://jama.ama-assn.org/cgi/doi/10.1001/jama.299.15.1800. [DOI] [PubMed] [Google Scholar]
- 8.The International Publication Planning Association. Welcome to TIPPA. [accessed 2008 Dec 17]. http://www.publicationplanningassociation.org.
- 9.International Society for Medical Publishing Professionals. [accessed 2008 Dec 19]. http://www.ismpp.org.
- 10.International Committee of Medical Journal Editors. Uniform requirements for manuscripts submitted to medical journals: writing and editing for biomedical publication. [accessed 2008 Dec 17]. http://www.icmje.org. [Google Scholar]