Abstract
HIV sexual transmission via the male genital tract remains poorly defined. Male circumcision was shown to reduce female-to-male transmission in Africa, providing a clue that the foreskin plays a role in the route of transmission. Scientific data in four categories relating to how the foreskin might affect HIV transmission is summarized: (i) surface area, (ii) microbiologic environment, (iii) HIV-1-susceptible cells, and (iv) tissue structure. The relative contribution of each of these areas is yet unknown, and further studies will be crucial in understanding how male circumcision affects HIV transmission in men.
Keywords: Circumcision, foreskin, HIV, transmission
Male circumcision has been shown to be effective in substantially reducing female-to-male HIV sexual transmission in Africa1–3. While many interesting theories have been proposed regarding how circumcision works, few are adequately supported by published data4,5. Additional clinical results have revealed that the protection is unfortunately one-sided—that is, male circumcision does not appear to protect female partners against HIV infection6. A meta-analysis of studies enrolling men who have sex with men also failed to establish a protective role for male circumcision in this population; though, newer data does support protection in men who report only insertive roles7,8. These conflicting results are difficult to fully explain, given the unknown role of the male foreskin in HIV sexual transmission. In this review, we highlight existing data regarding the potential role of the foreskin and mechanisms behind the observed effects of male circumcision. Figure 1 depicts four major categories of proposed mechanisms, although their relative contributions are yet unknown. We also identify areas that need to be further explored in each category to fully understand how HIV is transmitted in men.
Fig. 1.
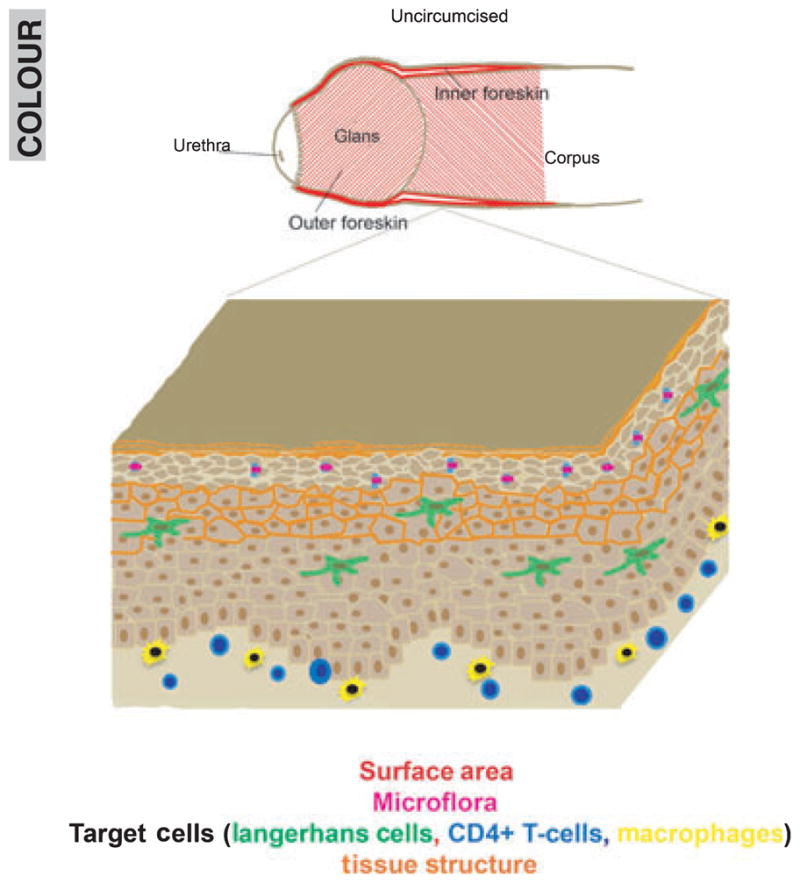
The uncircumcised penile model and potential mechanisms behind increased HIV transmission in this model. Potential areas of infection (red) are increased with the presence of the foreskin. Surface microflora (purple) can change with hygiene practices and removal of the foreskin. Systemic or external stimuli can alter potential HIV-1 target cell populations (green, blue, yellow) in the tissue. Circumcision may change tissue permeability and structural elements (orange).
Surface area
In a brief report, Kigozi et al.9 observed that the size of foreskins excised from 965 men enrolled in the Rakai Community Cohort Study significantly correlated with HIV incidence rates. That is, subjects whose measured foreskin surface areas were in the upper quartile (45.6–99.8 cm2) had over a twofold increased risk of HIV infection compared to those in the lowest quartile (adjusted IRR, 2.37, 95% CI 1.05–5.31). One explanation for this finding is that a greater surface area would be associated with more resident HIV immune cells (Langerhans cells, CD4+ T cells, CD8+ T cells, and macrophages), and hence greater rates of infection. Although published data regarding relative target cell densities in the penis have been conflicting to date (discussed in further detail below), the mere presence of a greater epithelial surface containing a greater absolute number of cells might provide enough of a selective advantage for the virus. This phenomenon may also contribute to the decreased efficiency of female-to-male HIV transmission relative to either male-to-female or male-to-male routes of sexual transmission10,11.
Microflora
Once it became clear that male circumcision could reduce HIV transmission to men, additional studies originating from the African circumcision trials were undertaken to determine whether the prevalence of other sexually transmitted infections (STIs) were affected. Two groups showed that prevalence rates for human papillomavirus infections were significantly lower in circumcised men over a 2-year period12,13. However, both studies were limited by the inclusion of only two time points or samples collected per subject. In addition, the collection method employed by both groups (superficial swabs of either the urethra or coronal sulcus) could not control for contamination from recent sexual partners. Tobian et al. also reported decreased herpes simplex virus type 2 (HSV-2) incidence rates among circumcised men, as determined by HSV-2 serologies. In contrast, male circumcision had no effect on either Treponema palli-dum (syphilis) or Neisseria gonorrhoeae infection rates. Similarly, a report from Kenya saw no effect in prevalence rates of either Trichomonas vaginalis, Chlamydia trachomatis, or N. gonorrhoeae infections after male circumcision14. The reason for the disparity seen between the effect of male circumcision on viral and bacterial pathogens is not entirely clear, but likely relate to differences in routes taken during transmission (i.e., the squamous epithelia found in foreskin, glans, and shaft tissue versus the columnar epithelium of the urethra).
In addition to infectious pathogens, male circumcision might also affect commensal bacteria that naturally colonize the penile surface. To study this, the Ugandan group swabbed the coronal sulci of 12 HIV-seronegative men both before and 12 months after circumcision15. Using 16S rRNA sequencing, Price et al. reported that different bacterial families were found after circumcision. Anaerobic bacterial species, some associated with bacterial vaginosis in women, were found in greater abundance on the uncircumcised penile surface. How exactly the type of bacteria found on the surface relates to HIV transmission is unknown; one possibility is that the microbiological shift away from an anaerobic environment after circumcision decreases nascent inflammation and thereby reduces the likelihood that an invading HIV particle would encounter an immune cell to initiate infection.
The penile surface’s microflora may also be affected by personal hygiene (i.e., different levels of hygiene might allow different types of bacterial species to populate), which has been shown to correlate with HIV seroprevalence16. O’Farrell et al. used clinician’s assessments of ‘wetness’ around the glans or coronal sulcus to show that uncircumcised men had significantly higher rates of wetness when compared to circumcised men. Importantly, they also found a 66.3% HIV seroprevalence in men with any level of penile wetness when compared to 45.9% in those with no wetness (P < 0.001). These results together suggest that the presence of the foreskin can substantially influence the microenvironment on or near the surface of the penis and that this may in turn affect HIV susceptibility.
HIV-1-susceptible cells
Prior to the widely publicized clinical benefit of male circumcision, Hussain et al.17 published a report analyzing immune cells in the genital tract. They found no difference in the number of Langerhans (LCs) or CD4+ T cells between the inner and outer foreskin of adult men. Later reports have found conflicting results (Table I): one found more HIV-susceptible cells in the outer when compared to either inner foreskin or glans tissue, and another reported more cells in the inner than the outer foreskin4,18. A study published by our own group, in collaboration with Dr. Robin Shattock’s group, showed that initial differences in LCs and CD4+ T-cell (glans ≫ inner > outer) densities were not seen after the tissues were allowed to culture for a few days4,5,18. Therefore, it is possible that some of the previously observed differences were a result of surgically induced trauma to the tissues and may not accurately reflect normal tissues.
Table I.
Summary of Target Cell Densities in the Foreskin and Glans Penile Tissue
| Langerhans cell densities (relative ratio) | CD4+ T-cell lymphocyte densities (relative ratio) | |
|---|---|---|
| Outer Foreskin: Inner Foreskin | ||
| Hussain et al.a | 1.0 | 1.0 |
| Patterson et al.b | 0.2 | 0.2 |
| McCoombe and Shortc | 1.4 | 1.2 |
| Fischetti et al.d | 0.7 | 0.4 |
| Fahrbach et al.e | 1.4 | 0.6 |
| Range | 0.2–3.4 | 0.2–1.2 |
| Glans: Inner Foreskin | ||
| McCoombe and Short | 0.9 | 1.0 |
| Fischetti et al. | 1.4 | 1.4 |
| Range | 0.9–1.4 | 1.0–1.4 |
Hussain et al.16: n = 3, reported as cells/mm2.
Patterson et al.17. n = 14 (inner) and 6 (outer), reported as percentage of total cells seen.
McCoombe and Short4. n = 21 and 9 (fresh and cadaveric specimens, respectively), reported as cells/mm2.
Fischetti et al.5 n = NA, reported as cells/um2.
Fahrbach et al.18. n = 11, reported as cells/um2.
To further understand the dynamics of the immunologic environment in the male genital tract, Fahrbach et al. 19 examined target cell activity in the inner and outer foreskin in response to inflammatory cytokines. Using long-term tissue explant cultures and fluorescent microscopy, they showed that LCs and CD4+ T cells in the inner foreskin were significantly more responsive to certain cytokines than those in the outer foreskin. One possible explanation for these findings is that the inner foreskin is more permeable to external agents and stimuli than the outer foreskin. This increased permeability may then relate to increased viral susceptibility in the inner foreskin when compared to other penile surfaces.
Tissue structure
An appealing early theory proposed that the inner foreskin’s keratin, or cornified, layer was thinner than that of other penile surfaces. A thinner keratin layer potentially allows HIV to reach resident target cells more easily and hence makes uncircumcised men more susceptible to infection. To support this, a study using penile tissue from cadaveric donors reported that the keratin of the inner foreskin was approximately 1.5 subjective units thinner than that of the outer foreskin or glans penis4. However, subsequent reports by our group and others have found no significant difference between the inner and outer foreskin keratinization after repeated measurements 20,21. This superficial layer is also easily sloughed, so an intact layer is unlikely to be found after sexual intercourse or to play a key role in protection against HIV infection. Another argument against this primary role is that the keratinization of the oral mucosa is relatively non-existent, yet oral transmission of HIV remains the most inefficient route of transmission22.
Beyond the keratin layers, the skin’s barrier function relies on other components such as intercellular junctions. These cell-to-cell junctions serve to regulate cell and epidermal growth, but also to protect the integrity of the epidermis23,24. Expression of these proteins can vary between epithelial strata in different areas of the body, which may influence how well protected some areas are when compared to others. Early work in our laboratory has shown subtle differences in protein expression patterns of foreskin and cervical tissues, which may contribute to differences in HIV movement between the female and male genital tract. We have also investigated skin characteristics relating to barrier function and permeability and found that these may lend insight into how the presence of the foreskin may lead to greater HIV transmission (data not shown).
Conclusions
Female-to-male HIV sexual transmission is the least well-described route of transmission, perhaps because of its relative inefficiency. However, many men initially acquire HIV from heterosexual sex with infected female partners, and they in turn infect others unknowingly. Male circumcision has only been shown to protect the men themselves against HIV acquisition, not their female partners6. The lack of a fundamental understanding in how circumcision works to prevent against infections precludes our ability to understand why it protects in certain routes and not others.
In 2007, the Merck Adenovirus 5 (Ad5)-HIV-1 gag/nef/pol vaccine (STEP) trial was halted because of significantly increased HIV acquisition rates in vaccine when compared to placebo recipients25. Furthermore, uncircumcised vaccinated men were at up to a fourfold increased risk for HIV infection relative to the other cohorts. Longer-term follow-up showed that only circumcision status (and not baseline Ad5 titers, as initially believed) correlated with HIV incidence rates. The reasons for these findings remain unknown even after several years of ad hoc studies. Overly simplistic theories, such as keratin thicknesses or sheer numbers of resident target cells, do not sufficiently explain these observations. Instead, it is likely that a more complex interplay of all of the above factors exists: the greater epithelial surface area provided by the foreskin, differences in the existing microbiologic and immunologic environment between the circumcised and uncircumcised male genital tract (enhanced by the vaccine), and differences in structural characteristics that allowed for greater stimuli exposures (particularly repeated ones). Unfortunately, no studies have been conducted to address these localized factors, and no answers have been found in serology-based studies26.
From the relatively few studies we do have available which have explored HIV transmission in the male genital tract, we are left with even more questions: how exactly does HIV use a greater epithelial surface area to its advantage? How does HIV cause infection through penile epithelia? How does an anaerobic or aerobic flora affect virus movement into the epithelium or nascent immune cells? How does the penile skin’s structure and barrier function change after circumcision, and how does this affect HIV transmission? Lack of specimen availability and known working models will certainly make finding these answers difficult. Nonetheless, these hard-sought answers will serve to broaden our knowledge of HIV sexual transmission and allow us to apply what we have found in male circumcision to all at-risk populations.
References
- 1.Bailey RC, Moses S, Parker CB, Agot K, Maclean I, Krieger JN, Williams CF, Campbell RT, Ndinya-Achola JO. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 2.Auvert BTD, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, Controlled Intervention Trial of Male Circumcision for Reduction of HIV Infection Risk. The ANRS 1265 Trial. PLos Medicine. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray RHKG, Serwadda D, Makumbi F, Watya S, Nalugoda F, Kiwanuka N, Moulton LH, Chaudhary MA, Chen MZ, Sewankambo NK, Wabwire-Mangen F, Bacon MC, Williams CF, Opendi P, Reynolds SJ, Laeyendecker O, Quinn TC, Wawer MJ. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 4.McCoombe SG, Short RV. Potential HIV-1 target cells in the human penis. AIDS. 2006;20:1491–1495. doi: 10.1097/01.aids.0000237364.11123.98. [DOI] [PubMed] [Google Scholar]
- 5.Fischetti L, Barry SM, Hope TJ, Shattock RJ. HIV-1 infection of human penile explant tissue and protection by candidate microbicides. AIDS. 2009;23:319–328. doi: 10.1097/QAD.0b013e328321b778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wawer MJMF, Kigozi G, Serwadda D, Watya S, Nalugoda F, Buwembo D, Ssempijja V, Kiwanuka N, Moulton LH, Sewankambo NK, Reynolds SJ, Quinn TC, Opendi P, Iga B, Ridzon R, Laeyendecker O, Gray RH. Circumcision in HIV-infected men and its effect on HIV transmission to female partners in Rakai, Uganda: a randomised controlled trial. Lancet. 2009;374:229–237. doi: 10.1016/S0140-6736(09)60998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millett GA, Flores SA, Marks G, Reed JB, Herbst JH. Circumcision status and risk of HIV and sexually transmitted infections among men who have sex with men: a meta-analysis. JAMA. 2008;300:1674–1684. doi: 10.1001/jama.300.14.1674. [DOI] [PubMed] [Google Scholar]
- 8.Templeton DJJF, Mao L, Prestage GP, Donovan B, Imrie K, Kippax S, Kaldor JM, Grulich AE. Circumcision and risk of HIV infection in Australian homosexual men. AIDS. 2009;23:2347–2351. doi: 10.1097/QAD.0b013e32833202b8. [DOI] [PubMed] [Google Scholar]
- 9.Kigozi GWM, Ssettuba A, Kagaayi J, Nalugoda F, Watya S, Mangen FW, Kiwanuka N, Bacon MC, Lutalo T, Serwadda D, Gray RH. Foreskin surface area and HIV acquisition in Rakai, Uganda (size matters) AIDS. 2009;23:2209–2213. doi: 10.1097/QAD.0b013e328330eda8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varghese BMJ, Peterman TA, Branson BM, Steketee RW. Reducing the risk of sexual HIV transmission. quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis. 2002;29:38–43. doi: 10.1097/00007435-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 11.European Study Group on Heterosexual Transmission of HIV. comparison of female to male and male to female transmission of HIV in 563 stable couples. BMJ. 1992;304:809–813. doi: 10.1136/bmj.304.6830.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auvert BS-TJ, Cutler E, Nieuwoudt M, Lissouba P, Puren A, Taljaard D. Effect of male circumcision on the prevalence of high-risk human papillomavirus in young men. results of a randomized controlled trial conducted in Orange Farm, South Africa. J Infect Dis. 2009;199:14–19. doi: 10.1086/595566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tobian AASD, Quinn TC, Kigozi G, Gravitt PE, Laeyendecker O, Charvat B, Ssempijja V, Riedesel M, Oliver AE, Nowak RG, Moulton LH, Chen MZ, Reynolds SJ, Wawer MJ, Gray RH. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med. 2009;360:1298–1309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta SDMS, Agot K, Parker C, Ndinya-Achola JO, Maclean I, Bailey RC. Adult male circumcision does not reduce the risk of incident Neisseria gonorrhoeae, Chlamydia trachomatis, or Trichomonas vaginalis infection. results from a randomized, controlled trial in Kenya. J Infect Dis. 2009;200:370–378. doi: 10.1086/600074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price LB, Liu CM, Johnson KE, Aziz M, Lau MK, Bowers J, Ravel J, Keim PS, Serwadda D, Wawer MJ, Gray RH. The effects of circumcision on the penis microbiome. PLoS ONE. 2010;5:e8422. doi: 10.1371/journal.pone.0008422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Farrell NML, Moodley P, Pillay K, Vanmali T, Quigley M, Hayes R, Sturm AW. Association Between HIV and Subpreputial Penile Wetness in Uncircumcised Men in South Africa. Journal of Acquired Immune Deficiency Syndrome. 2006;43:69–77. doi: 10.1097/01.qai.0000225014.61192.98. [DOI] [PubMed] [Google Scholar]
- 17.Hussain LS, Lehner T. Comparative investigation of Langerhans’ cells and potential receptors for HIV in oral, genitourinary and rectal epithelia. Immunology. 1995;85:475–484. [PMC free article] [PubMed] [Google Scholar]
- 18.Patterson BK, Landay A, Siegel JN, Flener ZPD, Chaviano A, Bailey RC. Susceptibility of Human Immunodeficicency Virus-1 Infection of Human Foreskin and Cervical Tissue Grown in Explant Culture. Americal Journal of Pathology. 2002;161:867– 873. doi: 10.1016/S0002-9440(10)64247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fahrbach KMBS, Anderson MR, Hope TJ. Enhanced cellular responses and environmental sampling within inner foreskin explants. implications for the foreskin’s role in HIV transmission. Mucosal Immunol. 2010;3:410–418. doi: 10.1038/mi.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinh MH, McRaven MD, Kelley Z, Penugonda S, Hope TJ. Keratinization of the adult male foreskin and implications for male circumcision. AIDS. 2010;24:899–906. doi: 10.1097/QAD.0b013e3283367779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin QZX, Wang Y, Shen H, Sun F, Ding W. Langerhans’ cell density and degree of keratinization in foreskins of Chinese preschool boys and adults. Int Urol Nephrol. 2009;41:747–753. doi: 10.1007/s11255-008-9521-x. [DOI] [PubMed] [Google Scholar]
- 22.Chenine ALSN, Kramer VG, Sciaranghella G, Rasmussen RA, Lee SJ, Santosuosso M, Poznansky MC, Velu V, Amara RR, Souder C, Anderson DC, Villinger F, Else JG, Novembre FJ, Strobert E, O’Neil SP, Secor WE, Ruprecht RM. Relative transmissibility of an R5 clade C simian-human immunodeficiency virus across different mucosae in macaques parallels the relative risks of sexual HIV-1 transmission in humans via different routes. J Infect Dis. 2010;201:1155–1163. doi: 10.1086/651274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schluter H, Wepf R, Moll I. Sealing the live part of the skin. the integrated meshwork of desmosome, tight junctions and curvilinear ridge structures in the cells of the uppermost granular layer of the human epidermis. Eur J Cell Biol. 2004;83:655–665. doi: 10.1078/0171-9335-00434. [DOI] [PubMed] [Google Scholar]
- 24.El-Domyati M, Attia S, Saleh F, Brown D, Birk DE, Gasparro F, Ahmad H, Uitto J. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11:398–405. doi: 10.1034/j.1600-0625.2002.110502.x. [DOI] [PubMed] [Google Scholar]
- 25.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN Step Study Protocol Team. Efficacy assessment of a cellmediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-ofconcept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson M, Mehrotra D, Fitzgerald D, et al. Efficacy Results from the STEP Study (Merck V520 Protocol 023/HVTN 502). A Phase II Test-of-Concept Trial of the MRKAd5 HIV-1 Gag/Pol/Nef Trivalent Vaccine. 15th Conference on Retroviruses and Opportunistic Infections; 2008. [Google Scholar]


