Abstract
The 5' splice junction (5'SJ) of Group II intron transcripts is subject to a specific hydrolysis reaction (SJH). This reaction occurs either within a single transcript containing intron sequences through domain 5 (D5) or by cooperation of two separate transcripts, one bearing the 5'SJ and another contributing D5 (1). In this report we describe the latter reaction in terms of its kinetic parameters. A minimal D5 RNA of 36 nts (GGD5) was sufficient to promote SJH of a second transcript containing the 5' exon plus intron domains 1, 2, and 3 (E1:123). Equimolar production of two RNAs, the 5' exon (E1) and an intron fragment containing domains 1, 2, and 3 (123) was observed. The kinetic coefficients were evaluated by an excess GGD5 approach. The apparent Km was complex, varying with GGD5 concentration. This behavior indicates heterogeneity in E1:123 with respect to GGD5 binding. The binding heterogeneity may result from formation of E1:123 dimers or from nicks in some molecules of each E1:123 preparation. The heterogeneity was always evident, but to a variable degree, regardless of the procedure by which E1:123 was isolated. The system may be described in terms of parameters analogous to kcat and Km. At infinite dilution of GGD5, the characterizing values were: k2 degrees (the analog of kcat) = 0.0055 min-1 and Km degrees = 0.22 microM. In the limit of GGD5 saturation, the values were: k2 infinity = 0.012 min-1 and Km infinity = 4.5 microM. A natural variant D5, representing the sequence from intron 1 of the yeast cytochrome-b gene, was also functional in SJH. This GGD5b1 was governed by similar Km degrees and Km infinity values, but was only one-third as active over the entire D5 concentration range. A different D5 isomer was entirely ineffective for SJH.
Full text
PDF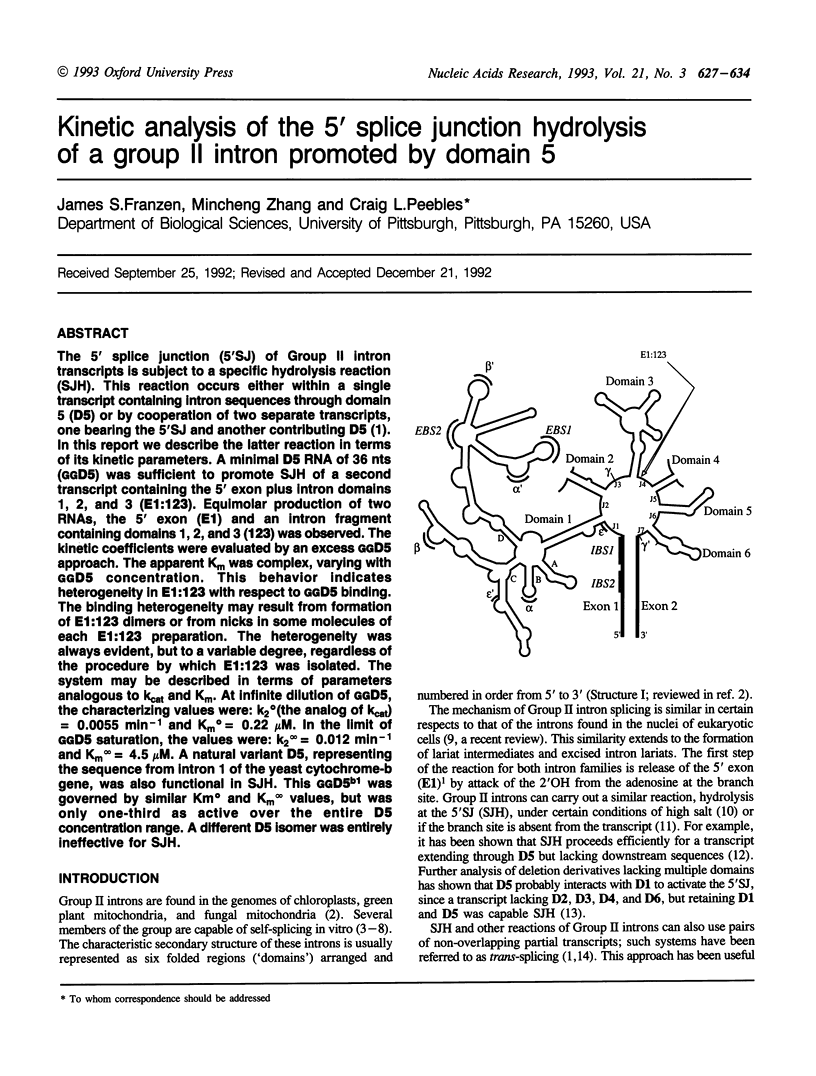



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Green M. R. Biochemical mechanisms of constitutive and regulated pre-mRNA splicing. Annu Rev Cell Biol. 1991;7:559–599. doi: 10.1146/annurev.cb.07.110191.003015. [DOI] [PubMed] [Google Scholar]
- Hebbar S. K., Belcher S. M., Perlman P. S. A maturase-encoding group IIA intron of yeast mitochondria self-splices in vitro. Nucleic Acids Res. 1992 Apr 11;20(7):1747–1754. doi: 10.1093/nar/20.7.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschlag D., Cech T. R. Catalysis of RNA cleavage by the Tetrahymena thermophila ribozyme. 1. Kinetic description of the reaction of an RNA substrate complementary to the active site. Biochemistry. 1990 Nov 6;29(44):10159–10171. doi: 10.1021/bi00496a003. [DOI] [PubMed] [Google Scholar]
- Heus H. A., Pardi A. Structural features that give rise to the unusual stability of RNA hairpins containing GNRA loops. Science. 1991 Jul 12;253(5016):191–194. doi: 10.1126/science.1712983. [DOI] [PubMed] [Google Scholar]
- Jacquier A., Michel F. Multiple exon-binding sites in class II self-splicing introns. Cell. 1987 Jul 3;50(1):17–29. doi: 10.1016/0092-8674(87)90658-1. [DOI] [PubMed] [Google Scholar]
- Jacquier A., Rosbash M. Efficient trans-splicing of a yeast mitochondrial RNA group II intron implicates a strong 5' exon-intron interaction. Science. 1986 Nov 28;234(4780):1099–1104. doi: 10.1126/science.2430332. [DOI] [PubMed] [Google Scholar]
- Jarrell K. A., Dietrich R. C., Perlman P. S. Group II intron domain 5 facilitates a trans-splicing reaction. Mol Cell Biol. 1988 Jun;8(6):2361–2366. doi: 10.1128/mcb.8.6.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell K. A., Peebles C. L., Dietrich R. C., Romiti S. L., Perlman P. S. Group II intron self-splicing. Alternative reaction conditions yield novel products. J Biol Chem. 1988 Mar 5;263(7):3432–3439. [PubMed] [Google Scholar]
- Klotz I. M., Hunston D. L. Properties of graphical representations of multiple classes of binding sites. Biochemistry. 1971 Aug 3;10(16):3065–3069. doi: 10.1021/bi00792a013. [DOI] [PubMed] [Google Scholar]
- Koch J. L., Boulanger S. C., Dib-Hajj S. D., Hebbar S. K., Perlman P. S. Group II introns deleted for multiple substructures retain self-splicing activity. Mol Cell Biol. 1992 May;12(5):1950–1958. doi: 10.1128/mcb.12.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kück U., Godehardt I., Schmidt U. A self-splicing group II intron in the mitochondrial large subunit rRNA (LSUrRNA) gene of the eukaryotic alga Scenedesmus obliquus. Nucleic Acids Res. 1990 May 11;18(9):2691–2697. doi: 10.1093/nar/18.9.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel F., Umesono K., Ozeki H. Comparative and functional anatomy of group II catalytic introns--a review. Gene. 1989 Oct 15;82(1):5–30. doi: 10.1016/0378-1119(89)90026-7. [DOI] [PubMed] [Google Scholar]
- Milligan J. F., Uhlenbeck O. C. Synthesis of small RNAs using T7 RNA polymerase. Methods Enzymol. 1989;180:51–62. doi: 10.1016/0076-6879(89)80091-6. [DOI] [PubMed] [Google Scholar]
- Peebles C. L., Perlman P. S., Mecklenburg K. L., Petrillo M. L., Tabor J. H., Jarrell K. A., Cheng H. L. A self-splicing RNA excises an intron lariat. Cell. 1986 Jan 31;44(2):213–223. doi: 10.1016/0092-8674(86)90755-5. [DOI] [PubMed] [Google Scholar]
- Pyle A. M., McSwiggen J. A., Cech T. R. Direct measurement of oligonucleotide substrate binding to wild-type and mutant ribozymes from Tetrahymena. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8187–8191. doi: 10.1073/pnas.87.21.8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer C., Schweyen R. J. Self-splicing of group II introns in vitro: mapping of the branch point and mutational inhibition of lariat formation. Cell. 1986 Aug 15;46(4):557–565. doi: 10.1016/0092-8674(86)90881-0. [DOI] [PubMed] [Google Scholar]
- Schmidt U., Riederer B., Mörl M., Schmelzer C., Stahl U. Self-splicing of the mobile group II intron of the filamentous fungus Podospora anserina (COI I1) in vitro. EMBO J. 1990 Jul;9(7):2289–2298. doi: 10.1002/j.1460-2075.1990.tb07400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walstrum S. A., Uhlenbeck O. C. The self-splicing RNA of Tetrahymena is trapped in a less active conformation by gel purification. Biochemistry. 1990 Nov 20;29(46):10573–10576. doi: 10.1021/bi00498a022. [DOI] [PubMed] [Google Scholar]
- van der Veen R., Arnberg A. C., van der Horst G., Bonen L., Tabak H. F., Grivell L. A. Excised group II introns in yeast mitochondria are lariats and can be formed by self-splicing in vitro. Cell. 1986 Jan 31;44(2):225–234. doi: 10.1016/0092-8674(86)90756-7. [DOI] [PubMed] [Google Scholar]
- van der Veen R., Kwakman J. H., Grivell L. A. Mutations at the lariat acceptor site allow self-splicing of a group II intron without lariat formation. EMBO J. 1987 Dec 1;6(12):3827–3831. doi: 10.1002/j.1460-2075.1987.tb02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




