Abstract
The brain’s energy economy excessively favors intrinsic, spontaneous neural activity over extrinsic, evoked activity, presumably to maintain its internal organization. Emerging hypotheses capable of explaining such an investment posit that the brain’s intrinsic functional architecture encodes a blueprint for its repertoire of responses to the external world. Yet, there is little evidence directly linking intrinsic and extrinsic activity in the brain. Here we relate differences among individuals in the magnitude of task-evoked activity during performance of an Eriksen flanker task, to spontaneous oscillatory phenomena observed during rest. Specifically, we focused on the amplitude of low-frequency oscillations (LFO, 0.01–0.1Hz) present in the BOLD signal. LFO amplitude measures obtained during rest successfully predicted the magnitude of task-evoked activity in a variety of regions that were all activated during performance of the flanker task. In these regions, higher LFO amplitude at rest predicted higher task-evoked activity. LFO amplitude measures obtained during rest were also found to have robust predictive value for behavior. In midline cingulate regions, LFO amplitudes not only predicted the speed and consistency of performance, but also the magnitude of the behavioral congruency effect embedded in the flanker task. These results support the emerging hypothesis that the brain’s repertoire of responses to the external world are represented and updated in the brain’s intrinsic functional architecture.
Keywords: resting state, intrinsic, extrinsic, functional networks, fALFF
1. INTRODUCTION
As much as 95% of the brain’s total energy consumption is devoted to maintaining and updating its internal organization (Raichle, 2010). The magnitude of this investment in intrinsic operations is puzzling given the importance of responding to environmental inputs and external demands. One possibility is that intrinsic brain activity may provide a functional framework for the brain’s moment-to-moment responses to the external world (Fox et al., 2006; Raichle, 2010). Support for this hypothesis comes from a recent demonstration (Smith et al., 2009) of striking correspondence between the functional systems revealed by task-based and task-independent (i.e., resting state) studies. This led Smith et al. (2009, pg. 13040) to conclude: “the full repertoire of functional networks utilized by the brain in action is continuously and dynamically ‘active’ even when at rest.”
Building upon this notion, we recently described brain regions in which the magnitude of fMRI activations during Eriksen flanker task performance was predicted by the strength of resting state functional connectivity (RSFC) between those regions and the default mode and task-positive networks (Mennes et al., 2010). Regions exhibiting significant RSFC/task-evoked activity relationships were primarily located in transition zones between task-activated and task-deactivated regions. Those transition zones coincided with the boundaries between the task-positive and default mode resting state networks. Together with the observations of Smith et al. (2009), these findings support the hypothesis that the functional architecture employed by the brain to respond to the external world is effectively represented in patterns of intrinsic activity.
Here, we shift our focus from measures of functional connectivity during rest, which index synchronization of low-frequency BOLD oscillations (LFO; 0.01–0.1Hz) between spatially distinct brain regions, to non-relational, regional properties of the brain’s intrinsic functional dynamics. The value of focusing on the temporal dynamics of the BOLD signal at a given voxel is increasingly appreciated in studies using a variety of approaches, including standard deviation based measures (Biswal et al., 1995; Garrett et al., 2010), Fourier-based frequency domain measures (Zang et al., 2007; Zou et al., 2009; Zou et al., 2008), and wavelet and fractal analyses (Barnes et al., 2009; Maxim et al., 2005). Frequency-based approaches have the advantage of providing frequency specific indices of oscillatory phenomena, thus allowing the investigation of BOLD signal variability in specific frequencies or frequency bands. Accordingly, we employed two increasingly popular voxel-wise, frequency-based measures of low-frequency BOLD oscillations: amplitude of low-frequency fluctuations (ALFF) and fractional ALFF (fALFF)(Zang et al., 2007; Zou et al., 2009; Zou et al., 2008). ALFF is defined as the total power in the low-frequency range (0.01Hz-0.1Hz). By definition, ALFF is equivalent to the standard deviation within that specific low-frequency band. In contrast, fractional ALFF (fALFF) is defined as the total power in the low-frequency range (0.01Hz–0.1Hz) relative to the total power across all measurable frequencies (0.01Hz–0.25Hz). As such, fALFF is a normalized version of ALFF, and has been shown to be less susceptible to artifactual signals in regions located within the vicinity of vessels and/or significant pulsatile motion (e.g., ventricles, brainstem; Zuo et al., 2010; Zou et al., 2008). Accordingly, we focused on fALFF in the present analyses. Both ALFF and fALFF are test-retest reliable across time (Zuo et al., 2010) and are promising potential biomarkers of psychiatric disorders (Hoptman et al., 2010; Huang et al., 2010; Lui et al., 2010; Yang et al., 2010; Zang et al., 2007; Zhang et al., 2010). Importantly, ALFF/fALFF measures can be used to study the dynamics of the BOLD signal at the local, voxel-wise level, without assessing the relationship between regions. In addition, like independent component analysis (Beckmann et al., 2005; Calhoun et al., 2001; Damoiseaux et al., 2006; McKeown et al., 1998; Smith et al., 2009) and clustering approaches (Bellec et al., 2010; Cohen et al., 2008; Cordes et al., 2002; Kelly et al., 2010; van den Heuvel et al., 2008), local amplitude measures do not require the a priori selection of regions of interest. Using fALFF as a local index of intrinsic brain activity, we tested whether we could predict inter-individual differences in the magnitude of BOLD activity evoked by an Eriksen flanker task based on inter-individual differences in intrinsic brain activity. By employing the same dataset used in our previous study of the relationship between RSFC and task-evoked activity (Mennes et al., 2010), we can contrast findings obtained with regional (ALFF, fALFF) vs. relational resting state measures (RSFC).
Given our hypothesis that common neural mechanisms underlie intrinsic and extrinsic BOLD activity, we predicted that for a given region, participants exhibiting higher LFO amplitudes during rest would also exhibit greater task-evoked BOLD responses during task-performance. This hypothesis corroborates with the idea that extrinsic activity builds on underlying intrinsic activity (Fox et al., 2006; Smith et al., 2009). Alternatively, extrinsic and intrinsic activity may be in competition with one another. This would lead to the prediction that participants with higher LFO amplitudes will show lower task-evoked BOLD activity, as the LFO may act as a source of noise in the measurement of extrinsic activity (i.e., decrease the signal to noise ratio), and may even interfere with extrinsic phenomena more directly (e.g., due to competition for resources). Additionally, we took the opportunity to explore possible relationships between resting state LFO amplitude measures and behavioral performance on the Eriksen flanker task.
2. METHODS
2.1. Participants and experimental paradigm
We used a dataset comprising 26 participants (mean age 20.5 ± 4.8 years, 11 males), previously included in other studies by our lab (Kelly et al., 2008; Mennes et al., 2010). All participants were without a history of psychiatric or neurological illness as confirmed by psychiatric assessment. Written informed consent was obtained prior to participation as approved by the institutional review boards of New York University (NYU) and the NYU School of Medicine.
Two 5-minute fMRI scans were acquired while participants completed a slow event-related Eriksen flanker task (inter-trial interval varied between 8 and 14 s with mean = 12 s). On each trial, participants had to indicate the direction of a central arrow in an array of 5 arrows. In congruent trials all arrows pointed in the same direction as the central arrow (e.g., > > > > >). In contrast, in incongruent trials the flanking arrows pointed in the opposite direction (e.g., > > < > >). Each run contained 12 congruent and 12 incongruent trials, presented in a pseudorandom order. Participants responded using the index- and middle finger of the right hand. In addition, all participants completed a brief (6.5 min) resting state scan during which they were asked to relax with eyes open. The order of the resting state and task scans was counterbalanced across participants. The two task runs were always administered consecutively. Finally, the scanning session was completed with a 25-minute long task assessing reward processing and a 12-minute MPRAGE anatomical scan.
2.2. Data acquisition
All scans were acquired using a standard Siemens head coil on a Siemens Allegra 3.0T scanner. During each of the two flanker task blocks we obtained 146 contiguous echo planar imaging (EPI) whole-brain volumes (TR = 2000 ms; TE = 30 ms; flip angle = 80°: 40 slices: matrix = 64 × 64; FOV = 192 mm; acquisition voxel size = 3 × 3 × 4 mm). The resting state scan consisted of 197 contiguous EPI volumes (TR = 2000 ms; TE = 25 ms; flip angle = 90°: 39 slices: matrix = 64 × 64; FOV = 192 mm; acquisition voxel size = 3 × 3 × 3 mm). For spatial normalization and localization, we obtained a high-resolution T1-weighted magnetization prepared gradient echo sequence (MPRAGE: TR = 2500 ms; TE = 4.35 ms; TI = 900 ms; flip angle = 8°; 176 slices: FOV = 256mm).
2.3. Image preprocessing
Both the flanker task (two runs concatenated) and the resting state data were preprocessed as follows: slice timing correction for interleaved acquisition (using Fourier-space time-series phase-shifting), motion correction (by aligning each volume to the mean image using Fourier interpolation) and despiking (detection and reduction of extreme time series outliers) were carried out using AFNI (http://afni.nimh.nih.gov/afni/). Further preprocessing was performed using FSL (www.fmrib.ox.ac.uk) and comprised spatial smoothing using a Gaussian kernel of FWHM 6mm, and mean-based intensity normalization of all volumes by the same factor (i.e., all volumes are scaled by the same amount). No temporal filtering was implemented during preprocessing of the resting state scans, thus assuring that the entire frequency spectrum below the Nyquist frequency (0.25Hz) could be examined in subsequent LFO amplitude analyses (ALFF/fALFF) (see ALFF and fALFF section below for details on specific frequencies assessed by each measure). The flanker task scans were temporally filtered using both a high-pass (Gaussian-weighted least-squares straight line fitting, with sigma = 100.0 s) and low-pass filter (Gaussian low-pass temporal filtering: HWHM 2.8 s) for task analysis.
Registration of each participant s high-resolution anatomical image to a common stereotaxic space (the Montreal Neurological Institute 152-brain template (MNI152); 2×2×2mm resolution) was accomplished using a two-step process (Andersson et al., 2007). First, a 12 degrees of freedom linear affine transformation was computed using FLIRT (Jenkinson et al., 2002; Jenkinson and Smith, 2001). Subsequently, the registration was refined using FNIRT nonlinear registration (Andersson et al., 2007). All analyses up to and including the generation of individual participant ALFF/fALFF and task-evoked activity maps were completed in native space. For group comparisons, participant-level images were first registered to MNI152 2×2×2mm space by applying the nonlinear warp parameters calculated for the anatomical image after coregistering each participant s functional and anatomical images using a 6 degrees of freedom linear affine transformation.
2.4. ALFF and fALFF
2.4.1. Participant-level calculation
For each participant, we calculated ALFF and fractional ALFF (fALFF) for the resting state scan (for details see Zuo et al., 2010) and task scan after removal of task-related activity (with multiple regression, see below) (Fair et al., 2007; Kelly et al., 2008). Both ALFF and fALFF provide a characterization of local, voxel-wise BOLD signal dynamics. Specifically, they index the contribution of fluctuations within the low frequency range to the variability of the BOLD signal. ALFF is calculated as the sum of amplitudes within the low frequency range (0.01 – 0.1 Hz), and indexes the overall strength or intensity of LFO (i.e., the variance of the signal within that range). fALFF is ALFF expressed as a fraction of the sum of amplitudes across the entire frequency range detectable in the signal. fALFF thus represents the contribution of fluctuations within the low frequency range to the variability of the BOLD signal, relative to the contribution of fluctuations within the whole detectable frequency range. In other words, fALFF is a normalization of ALFF with respect to all available frequencies in the measured signal. Participant-level voxel-wise ALFF/fALFF maps were transformed into Z-score maps by subtracting at each voxel the mean obtained for the entire brain, and dividing by the whole brain’standard deviation (Zuo et al., 2010). Given that fALFF has been shown to be less susceptible to artifactual signals in regions located within the vicinity of vessels and/or significant pulsatile motion, as well as for the sake of clarity, all results regarding ALFF are presented in the Supplementary Materials accompanying this manuscript.
2.5. Flanker task analysis
2.5.1. Participant-level flanker task analysis
Using FSL FEAT, we performed a multiple regression analysis regressing each participant s 4-D flanker task volume on four task regressors coding for correct congruent trials, correct incongruent trials, errors across all trials, and a block regressor (which coded for the task blocks). This analysis produced participant-level maps of all voxels exhibiting task-related activation and deactivation in the congruent (Congruent > Baseline) or incongruent trials (Incongruent > Baseline), as well as those voxels exhibiting differential activity for congruent and incongruent trials (Incongruent > Congruent). Finally, we also calculated overall task-related activation and deactivation across congruent and incongruent trials (i.e., Congruent + Incongruent > Baseline).
2.6. Testing the relationship between LFO and BOLD activity evoked by the flanker task
To test the presence of a relationship between ALFF or fALFF measures and indices of BOLD activity evoked by flanker task performance, we conducted a voxel-matched linear regression analysis for each voxel in the brain (see also Mennes et al., 2010). Specifically, we modeled the LFO values obtained at each voxel as a predictor in the regression model (separately for ALFF and fALFF). The parameter estimates for the flanker task at that same voxel were entered as dependent variables. This resulted in a unique linear regression model for each voxel in which the LFO value predicted BOLD activity evoked by the flanker task. To account for the effect of scan order on the resting state LFO amplitude/task-evoked activity relationship, we included scan order (rest/flanker vs. flanker/rest) as covariate in all models. Separate analyses were conducted for overall task-evoked activity (Congruent + Incongruent > Baseline), and task activity associated with congruent trials (Congruent > Baseline), incongruent trials (Incongruent > Baseline), and the congruency effect (Incongruent > Congruent). Participant-level maps for fALFF, ALFF and task-evoked responses were first transformed to MNI152 space by applying the transformation to MNI152 standard space (2×2×2mm resolution) computed during preprocessing. All voxel-matched analyses were carried out using FSL FEAT.
In practice, we regressed a 26 volume 4-D image (1 volume per participant) containing the flanker task parameter estimates on a 26 volume 4-D image containing each participant s ALFF or fALFF map (called “voxel-dependent EV”). Cluster-based statistical correction for multiple comparisons was performed applying Gaussian random field theory (Z > 2.3; cluster significance: p < 0.05, corrected). Since ALFF and fALFF are highly represented in gray matter (Biswal et al., 1995) we masked the results with a gray matter mask derived from the MNI152 average gray matter tissue prior using a liberal threshold of 25% tissue probability. This analysis produced thresholded Z-statistic maps of those voxels that showed a significant linear relationship across participants between ALFF or fALFF obtained during rest and their BOLD activity measured during a given condition of the flanker task. Peak voxels shown in the tables represent the voxel with the highest Z-statistic for each significant cluster.
2.7. Brain/behavior relationships
To investigate the relationship between behavioral performance during the flanker task and resting state LFO amplitude measures we entered mean reaction time (RT) and coefficient of variation (CV; [standard deviation of RT/mean RT]) as factors of interest in separate group-level fALFF analyses. Given the inherent relationship between mean RT and standard deviation, we used CV as our measure of intra-individual variability in order to maximize our ability to detect brain/behavior relationships uniquely related to performance variability. Separate analyses were conducted for mean RT and CV of the congruent and incongruent flanker trials. In addition, we investigated the behavioral effect of congruency on mean RT (i.e., [mean RT incongruent - mean RT congruent] / mean RT congruent). The same procedures were applied to investigate the relationship between behavioral performance and task-evoked activity. Behavioral performance was entered as a factor of interest in separate group-level analyses for the task-evoked parameter estimates associated with the congruent (Congruent > Baseline) and incongruent (Incongruent > Baseline) trials and the congruency effect (Incongruent > Congruent). Behavioral performance for the respective trials was matched with its specific parameter estimates (e.g., congruent mean RT was entered in a model using Congruent > Baseline parameter estimates). In addition to behavioral performance we included scan order (rest/flanker vs. flanker/rest) as covariate in the model. All resulting Z-statistic maps were corrected for multiple comparisons using Gaussian random field theory (Z > 2.3; cluster significance: p < 0.05, corrected). The resulting thresholded Z-statistic maps indicated regions showing a significant linear relationship between ALFF, fALFF or task-evoked activity and mean RT or CV for each trial type. Peak voxels shown in the tables represent the voxel with the highest Z-statistic for each significant cluster.
3. RESULTS
3.1. LFO amplitude during rest predicts magnitude of task-evoked BOLD response
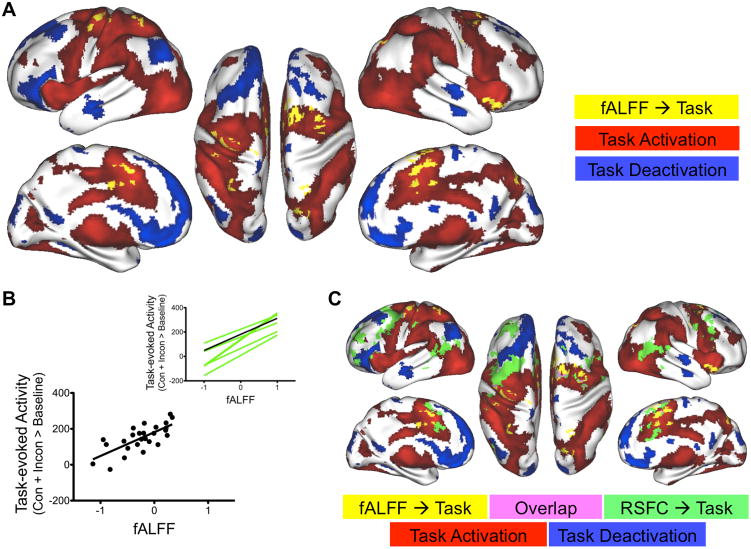
Voxel-matched regression analyses identified several brain regions where fALFF obtained for a participant during rest predicted the magnitude of event-related BOLD responses during flanker task performance, regardless of trial type (congruent, incongruent, overall; Figure 1, Table 1 and Supplementary Figure S1). Highly similar results for ALFF are described in the Supplementary Material accompanying this manuscript. Moreover, regions exhibiting significant relationships were centered within clusters of significant task-evoked activation (Figure 1). This observation stands in contrast to our prior work, in which most regions showing a significant RSFC/task-evoked activity relationship were in transition zones between task-activated and task-deactivated regions (Mennes et al., 2010) (Figure 1C).
Figure 1. Amplitude of low-frequency oscillations (LFO) observed in the BOLD signal during rest predicted task-evoked activation.
A. Regions exhibiting a significant relationship between resting state fractional amplitude of low-frequency fluctuations (fALFF) and overall (Congruent + Incongruent > Baseline) activity evoked by the flanker task. Yellow: Regions exhibiting a significant resting state fALFF/task-evoked activity relationship. Red: regions exhibiting significant overall (Congruent + Incongruent > Baseline) task-evoked activation. Blue: regions exhibiting significant overall task-evoked deactivation. B. Regression lines for all clusters exhibiting a significant linear relationship between resting state fALFF and overall task-evoked activity. Inset shows the regression lines for all significant clusters, while the scatter plot illustrates the actual relationship for the regression line shown in black in the inset. C. Comparison of regions in which either resting state fALFF or resting state functional connectivity (RSFC) significantly predicted overall task-evoked activity. Regions (shown in yellow) in which resting state fALFF predicted overall (Congruent + Incongruent > Baseline) task-evoked activity were located within regions of significant overall task-evoked activation. Regions (shown in green) in which RSFC predicted overall task-evoked activity were mainly located in transition zones between significant overall task-evoked activation and deactivation (see Mennes et al., 2010). We observed only 7% overlap (shown in violet) between the results obtained for fALFF and those observed for RSFC. Red: Significant overall flanker task activation (Congruent + Incongruent > Baseline). Blue: Significant overall flanker task deactivation.
Table 1.
Peak voxels for fALFF predicting task-evoked activity
| Anatomical Structure | Hemisphere | Clustersize | max Z-stat | Peak Coordinates MNI (mm) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| fALFF → task-evoked activity: Congruent > Baselinea | ||||||
| Positive Relationship | ||||||
| Precentral Gyrus | L | 445 | 4.33 | −46 | −10 | 54 |
| Anterior Cingulate Cortex | R | 416 | 4.53 | 6 | 8 | 50 |
| Superior Frontal Gyrus | R | 229 | 4.34 | 20 | 6 | 72 |
| Middle Frontal Gyrus | R | 217 | 4.8 | 34 | −4 | 42 |
| Cerebellum | L | 167 | 3.83 | −36 | −46 | −48 |
| Precuneus | R | 151 | 3.9 | 16 | −68 | 40 |
| fALFF → task-evoked activity: Incongruent > Baselinea | ||||||
| Positive Relationship | ||||||
| Anterior Cingulate Cortex | R | 429 | 4.39 | 12 | 12 | 32 |
| Precentral Gyrus | L | 357 | 3.86 | −38 | −16 | 60 |
| Precuneus | R | 297 | 4.33 | 16 | −70 | 40 |
| Superior Frontal Gyrus | R | 291 | 4.83 | 16 | 10 | 72 |
| Precentral Gyrus | R | 209 | 4.21 | 34 | −8 | 48 |
| Paracingulate Gyrus | R | 171 | 4.33 | 8 | 38 | 24 |
| fALFF → task-evoked activity: Congruent + Incongruent > Baselineb | ||||||
| Positive Relationship | ||||||
| Superior Frontal Gyrus | R | 843 | 4.71 | 16 | 10 | 72 |
| Lateral Occipital Cortex | R | 330 | 4.69 | 26 | −58 | 68 |
| Precentral Gyrus | L | 290 | 4.27 | −46 | −10 | 52 |
| Middle Frontal Gyrus | R | 221 | 4.81 | 34 | −4 | 42 |
| Postcentral Gyrus | L | 186 | 4.21 | −58 | −26 | 52 |
| Cerebellum | L | 184 | 3.79 | −42 | −48 | −44 |
| Insula | R | 157 | 4.55 | 38 | 14 | −12 |
| fALFF → task-evoked activity: Incongruent > Congruentc | ||||||
| Positive Relationship | ||||||
| Superior Parietal Lobule | L | 128 | 3.98 | −24 | −56 | 48 |
| Negative Relationship | ||||||
| Precuneus | L | 226 | 3.93 | 2 | −66 | 24 |
| Middle Frontal Gyrus | R | 220 | 4.21 | −40 | 14 | 30 |
Regions correspond to those shown in Supplementary Figure 1B;
Regions correspond to those shown in Figure 1A;
Regions correspond to those shown in Supplementary Figure 1D.
All regions exhibiting a significant LFO/task-evoked activity relationship showed a positive relationship between the LFO amplitude measure and overall task-evoked activity (Congruent + Incongruent > Baseline). Participants showing higher fALFF during rest showed higher BOLD activity during task performance. Significant regions included dorsal and ventral anterior cingulate cortex, bilateral frontal eye fields, bilateral middle frontal gyrus, bilateral insula (operculum), left precentral sulcus, and right precuneus.
Our results did not vary by trial type. Similar results were found for activity evoked by the congruent (Congruent > Baseline) and incongruent (Incongruent > Baseline) flanker trials (Supplementary Figure S1). In addition, 60–75% of the voxels associated with a specific trial type were also found when using overall task-evoked activity (Congruent + Incongruent > Baseline). This is not surprising given that the analyses for overall task-evoked activity included both congruent and incongruent trials.
Beyond predicting general task-evoked activity (Congruent > Baseline, Incongruent > Baseline or Congruent + Incongruent > Baseline), fALFF measured during rest also predicted task-evoked activity associated with the congruency effect (Incongruent vs. Congruent). We observed both positive and negative relationships between resting-state fALFF and task-evoked activity associated with the congruency effect (Supplementary Figure S1; Table 1). Of special interest was a significant cluster in left lateral occipital cortex that exhibited a greater congruency effect in participants with higher fALFF and overlapped with significant task-evoked congruency related activation (Incongruent > Congruent).
3.2. Brain/behavior relationships
We tested for the presence of relationships for fALFF and task-evoked activity with mean RT and coefficient of variation (CV) for each trial type (congruent, incongruent) separately, as well as for the magnitude of the congruency effect measured by [(mean RT incongruent – mean RT congruent)/mean RT congruent].
Using fALFF as a predictor, robust brain/behavior relationships were observed for both incongruent and congruent trials (Figure 2; Table 2), as well as for the congruency effect (Figure 4). Interestingly, in cingulate and precuneus, areas typically implicated in higher order integration processes, greater fALFF was associated with faster (lower mean RT) and more consistent behavior (lower CV). Among these, a region in dorsal anterior cingulate cortex (ACC) showed a significant fALFF/task-evoked activity relationship for both congruent and incongruent trials (Figure 3). In addition, greater fALFF in medial and dorsal motor areas was associated with less consistent behavior (higher CV). As shown in Supplementary Figure S3 and Supplementary Table S2, ALFF/behavior relationships were less robust.
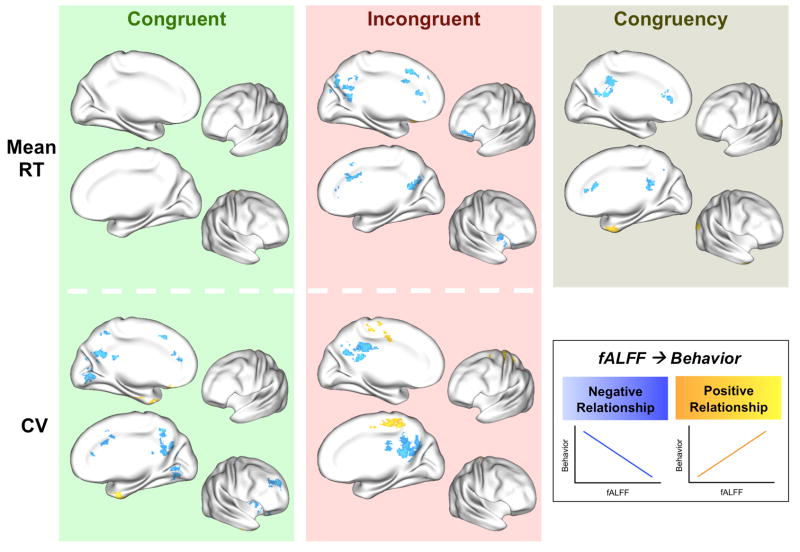
Figure 2. Resting state fALFF predicted behavioral performance during the Eriksen flanker task.
For both congruent (green background) and incongruent (rose background) trials in the flanker task, areas depicted in blue indicate regions in which higher resting state fALFF was associated with better performance, i.e., lower mean reaction time (mean RT; Top) or smaller coefficient of variation (CV; [standard deviation/mean RT]; Bottom). Areas depicted in yellow/orange indicate regions in which higher resting state fALFF was associated with more variable performance (higher CV). We observed no regions that showed a significant relationship between resting state fALFF and mean RT for the congruent trials. Resting state fALFF also predicted behavioral performance associated with the congruency effect (brown background; [mean RT incongruent – mean RT congruent]/mean RT congruent; see also Figure 4). Areas depicted in blue indicate regions in which higher resting state fALFF was associated with a smaller congruency effect. Areas depicted in yellow/orange indicate regions in which higher resting state fALFF was associated with a larger effect of congruency on mean RT. Results for resting state ALFF predicting behavioral performance are shown in Supplementary Figure S3. Results for task-evoked activity predicting behavioral performance are shown in Supplementary Figure S4. Results for fALFF predicting behavioral performance when mean RT and CV were included in one model are shown in Supplementary Figure S7.
Table 2.
Peak voxels for fALFF predicting behavioral performance
| Anatomical Structure | Hemisphere | Clustersize | max Z-stat | Peak Coordinates MNI (mm) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| fALFF → Behavior: Incongruent RTa | ||||||
| Positive Relationship | ||||||
| Cerebellum | L | 956 | 4.12 | 0 | −70 | −44 |
| Inferior Temporal Gyrus | L | 396 | 4.23 | −46 | −22 | −34 |
| Orbitofrontal Cortex | L | 357 | 4.15 | −10 | 22 | −24 |
| Postcentral Gyrus | L | 349 | 3.77 | −32 | −34 | 46 |
| Negative Relationship | ||||||
| Precuneus | R | 815 | 4.06 | 8 | −64 | 22 |
| Anterior Cingulate Cortex | R | 721 | 3.95 | 2 | 14 | 32 |
| Insula | R | 407 | 3.88 | 42 | 20 | −10 |
| Insula | L | 404 | 3.54 | −36 | 22 | −8 |
| fALFF → Behavior: Congruent CVa | ||||||
| Positive Relationship | ||||||
| Cerebellum | R | 2303 | 4.88 | 6 | −66 | −46 |
| Temporal Fusiform Cortex | R | 849 | 4.09 | 34 | −16 | −40 |
| Inferior Temporal Gyrus | L | 718 | 4.66 | −44 | −20 | −34 |
| Orbitofrontal Cortex | L | 380 | 4.43 | −14 | 38 | −22 |
| Negative Relationship | ||||||
| Precuneus | R | 1890 | 4.32 | 4 | −64 | 28 |
| Paracingulate Gyrus | L | 451 | 3.48 | −4 | 18 | 44 |
| Middle Frontal Gyrus | R | 380 | 4.38 | 24 | 30 | 34 |
| Orbitofrontal Cortex | R | 333 | 4.36 | 42 | 20 | −12 |
| fALFF → Behavior: Incongruent CVa | ||||||
| Positive Relationship | ||||||
| Postcentral Gyrus | L | 2391 | 4.74 | −26 | −36 | 56 |
| Negative Relationship | ||||||
| Precuneus | R | 1642 | 4.77 | 14 | −62 | 24 |
| fALFF → Behavior: Congruency Effectb,c | ||||||
| Positive Relationship | ||||||
| Inferior Temporal Gyrus | R | 796 | 4.03 | 40 | 0 | −42 |
| Cerebellum | R | 682 | 4.19 | 48 | −70 | −44 |
| Cerebellum | L | 533 | 3.81 | −38 | −60 | −46 |
| Lateral Occipital Cortex | R | 358 | 4.6 | 44 | −68 | 26 |
| Lateral Occipital Cortex | L | 280 | 4.02 | −36 | −78 | 4 |
| Negative Relationship | ||||||
| Precuneus | L | 544 | 3.64 | −2 | −46 | 42 |
| Anterior Cingulate Cortex | R | 287 | 4.13 | 6 | 24 | 16 |
Figure 4. Intrinsic properties of medial wall structures, but extrinsic properties of lateral prefrontal cortical areas predicted behavioral performance associated with the congruency effect.
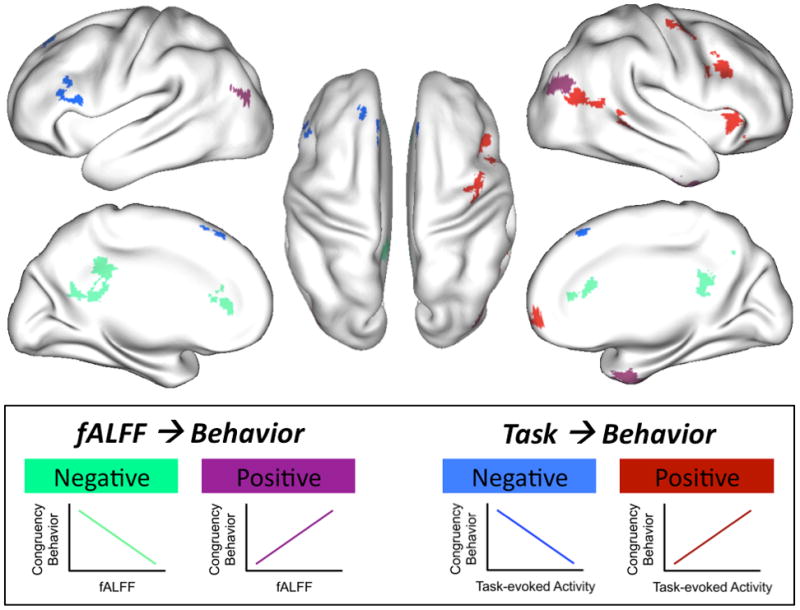
Regions exhibiting a significant positive (violet) or negative (green) relationship between resting state fALFF and the behavioral congruency effect ([mean RT incongruent – mean RT congruent]/mean RT congruent) were mainly found in the medial wall. In contrast, regions exhibiting a significant relationship (positive, red; negative, blue) between task-evoked activity observed for the congruency effect (Incongruent > Congruent) and the behavioral congruency effect were mainly located in lateral frontal-parietal cortex.
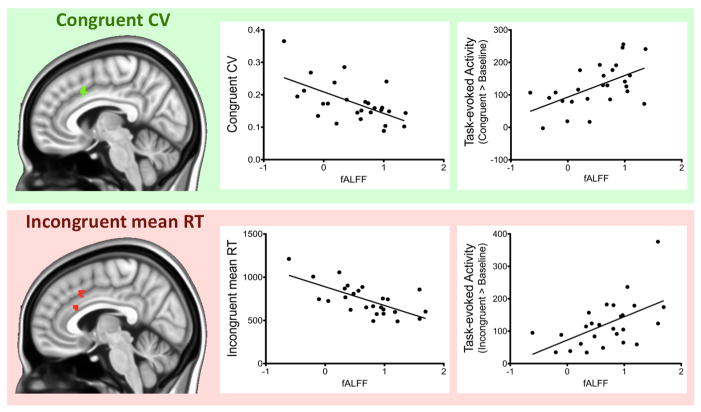
Figure 3. Overlap between regions exhibiting a resting state fALFF/behavior relationship as well as a resting state fALFF/task-evoked activity relationship.
Top: Regions exhibiting both a relationship between resting state fALFF and coefficient of variation (CV) for the congruent trials as well as a relationship between resting state fALFF and task-evoked activity associated with the congruent trials of the flanker task (Congruent > Baseline). Bottom: Regions exhibiting both a relationship between resting state fALFF and mean reaction time (mean RT) for the incongruent trials as well as a relationship between resting state fALFF and task-evoked activity associated with the incongruent trials of the flanker task (Incongruent > Baseline). MNI coordinates for the region overlapping between the top and bottom surface maps are x=2, y=20, z=38. Graphs on the right illustrate the linear relationships between resting state fALFF and behavior and resting state fALFF and task-evoked activity for the regions shown on the brains on the left. Given the marked difference in values between behavior and task-evoked activity, graphs are shown separately for each measure. However, fALFF, shown on the x-axis, is the same for the behavior and the task-evoked activity graphs.
When task-evoked activity was used as predictor, brain-behavior relationships for the individual trial types were limited (see Supplementary Figure S4; Supplementary Table S3). An exception was left primary motor cortex, in which higher task-evoked activity on congruent trials was associated with faster behavioral performance. By contrast, the relationship between task-evoked activity and performance associated with the congruency effect were much more compelling (see Figure 4). Task-evoked activity in right lateral prefrontal cortical areas was positively associated with the behavioral congruency effect. In other words, the larger the difference in activity between congruent and incongruent trials in these regions, the more participants slowed down on the incongruent trials, relative to congruent trials. The opposite pattern was found for left dorsolateral prefrontal cortex, in which larger differences in BOLD activity were associated with less slowing on the incongruent trials, relative to the congruent trials.
Finally, although using CV instead of standard deviation avoids potentially artifactual relationships between mean performance and variability across participants, significant relationships can still exist. In the present work, we found a marginal relationship between mean RT and CV across participants for each of the trial types (for congruent trials: r(RT,CV)=0.44; p=0.03; for incongruent trials: r(RT,CV)=0.52; p=0.006; overall: r(RT,CV)=0.38; p=0.051). This prompted us to repeat our analyses with mean RT and CV included in the same model in order to determine the specificity of the findings reported for the two measures. Overall, for both mean RT and CV, brain/behavior relationships remain significant in posterior regions (e.g., precuneus, occipital cortex; see Supplementary Figure S7 and Supplementary Table S4). In contrast, regions in dorsal anterior cingulate cortex and insula did not reach significance in the joint mean RT and CV regression model, suggesting that these areas might represent a neural mechanism underlying both speed and variability. Alternatively, our sample size might have limited our statistical power to detect differences in those areas.
4. DISCUSSION
Our findings substantiate the notion that the intrinsic functional architecture of the brain provides a framework for its repertoire of extrinsic responses (Braun and Mattia, 2010; Fox et al., 2006; Raichle, 2010; Smith et al., 2009; Steyn-Ross et al., 2009). Voxel-matched regression analyses revealed an array of regions in which an individual s LFO amplitude measures obtained during rest predicted the magnitude of task-evoked BOLD activations. For all regions exhibiting significant fALFF/task-evoked activity relationships, individuals showing higher LFO amplitude also exhibited stronger task-evoked activity during the congruent and incongruent trials of the flanker task. Additionally, we found that inter-individual differences in LFO amplitude measures in midline cingulate regions observed during rest were predictive of differences in behavioral performance.
4.1. RSFC and fALFF Predict Different Aspects of Task-Evoked Activity
Regions highlighted by voxel-matched regression as showing a significant relationship between an individual s resting state LFO amplitude and their respective task-evoked activity were primarily located within task-activated regions. This finding contrasts with what we observed in our prior work relating resting state functional connectivity (RSFC) to task-evoked activity using the same dataset (Mennes et al., 2010). In those analyses, regions exhibiting a significant relationship between RSFC and task-evoked activity were primarily located in transition zones between task activation and deactivation regions (see Figure 1c). In fact, these transition zones showed considerable overlap with a frontoparietal control network (Spreng et al., 2010; Vincent et al., 2008). Moreover, we found that for regions showing significant RSFC/task-evoked activity relationships, the magnitude of task-activation observed was linked to the percentage of voxels belonging to the task-positive resting state network as opposed to the default mode network. As such, these findings suggested that RSFC has predictive value for the spatial extent of activations evoked by task performance. In contrast, the present work found that fALFF, an index of the local amplitude of the BOLD oscillations, has predictive value for the actual height of the BOLD response observed in regions showing significant task-evoked activity.
4.2. The neurophysiological bases of intrinsic/extrinsic relationships
A key question is whether the current BOLD effects reflect interactions between intrinsic and extrinsic activity at the neural level, or are possibly a byproduct of inter-individual differences in cerebral blood flow or neurovascular coupling. LFO amplitude and task-evoked responses might be equally affected by inter-individual differences in physiological parameters, including cerebral blood flow and blood volume, or in neurovascular coupling underlying the regulation of these parameters in response to either intrinsic or extrinsic activity (Raichle and Mintun, 2006). Yet, we recently demonstrated that regional and inter-individual differences in LFO measures (ALFF, fALFF) were maintained during breath-holding, which markedly perturbs the vascular system (see supplemenatery materials in Zuo et al., 2010). Here, we observed regional specificity for the relationships between inter-individual differences in LFO amplitude measures and task-evoked responses. For instance, we did not observe resting state LFO/task-evoked activity relationships for regions that showed task-evoked deactivations. Furthermore, we observed differences in the resting-state LFO/task-evoked activity relationship depending on whether we examined loose (Congruent > Baseline, Incongruent > Baseline, Congruent + Incongruent > Baseline) or tight (Incongruent > Congruent) task contrasts. If a simple neurovascular transfer function could explain inter-individual differences in the BOLD response, it is unlikely that such regional and contrast-related specificity would emerge. Multimodal imaging approaches will be required to disentangle neural from vascular contributions to intrinsic and extrinsic BOLD activity.
Considering the sources of network stability across resting state fMRI measures, it is important to keep structural underpinnings in mind (Hagmann et al., 2008; Honey et al., 2009). Functional and structural connectivity exhibit robust correspondence, with functional connections that have a structural correlate showing the greatest strength (Honey et al., 2009). However, caution is needed when considering structure/function relationships. Numerous studies have demonstrated that functional connectivity transcends structural connectivity, (Di Martino et al., 2008; Hagmann et al., 2008; Margulies et al., 2009; Raichle, 2010; Roy et al., 2009; van den Heuvel et al., 2009; Vincent et al., 2007) and is likely modulated by a variety of factors, such as fiber properties (Ghosh et al., 2008).
4.3. Inter-individual differences in behavior relate to the brain’s functional architecture
Brain/behavior relationships observed with fALFF were more robust than those obtained with task-evoked activity for the incongruent and congruent trial types. Across congruent and incongruent trials, only one region in left primary motor cortex (precentral gyrus) was found to show a robust relationship between task-evoked activity and mean reaction time. In contrast, numerous regions exhibited significant relationships between an individual s fALFF and behavior (compare Figure 2 to Supplementary Figure S4).
The ability of resting state fALFF measures to predict inter-individual differences in the speed and consistency of behavioral performance underscores the merits of exploring the brain’s intrinsic functional architecture. The intrinsic properties of medial wall structures commonly implicated in the regulation of behavior may be of particular importance (MacDonald et al., 2000; Ridderinkhof et al., 2004; Rushworth et al., 2004). This was evident for speeded performance on attentionally demanding trials (i.e., incongruent), as well as for the maintenance of consistent task performance during less challenging trials (i.e., congruent).
Medial and lateral cortices exhibited an interesting dissociation with respect to the relative contributions of their intrinsic (fALFF) and extrinsic (task-evoked activity) properties to the behavioral congruency effect (a common index of attentional function). The magnitude of the behavioral congruency effect observed for an individual was only related to the magnitude of task-evoked responses for lateral frontal cortices, and only to the resting state fALFF measures for medial wall regions. Such a distinction may suggest that the contributions of anterior and posterior cingulate regions to the recruitment and regulation of attentional resources are governed by internally generated trait and state factors (e.g., arousal, alertness, vigilance, anxiety). In contrast, the contributions of lateral prefrontal regions to contextual processing and attentional control (MacDonald et al., 2000; Milham et al., 2003) are determined by the responsivity to external stimuli.
Finally, we highlight a dorsal ACC region showing a relationship between resting state fALFF and task-evoked activity as well as a relationship between resting state fALFF and behavioral performance, regardless of trial type. This region is typically implicated in cognitive control and conflict monitoring (Botvinick et al., 2004; Ridderinkhof et al., 2004), and might represent a hub for monitoring and modulating the interplay between intrinsic and extrinsic activity as well as behavioral performance.
4.4. Implications
The robust predictive power of intrinsic activity raises the question of whether examinations of intrinsic architecture alone may be sufficient for identifying biomarkers of development, genetics, aging or disease. At the present time, such suggestions are not yet warranted. Rather, resting state approaches have great potential to become a powerful paradigm for the investigation of functional brain networks in a manner complementary to task-based approaches, without replacing them. For instance, resting state/task-evoked relationships were more robust for overall task performance (Incongruent + Congruent > Baseline) than for tight, task specific comparisons isolating specific cognitive constructs (Incongruent > Congruent). Future work should extend the examination of resting state/task-evoked relationships across a number of tasks to determine the specificity of our findings to the employed task paradigm.
With such caveats in mind the current methods may be particularly fruitful when investigating clinical phenotypes that may be characterized by irregularities in the relationship between intrinsic and extrinsic brain activity. Such studies will advance our understanding of how disease-related abnormalities in the brain’s intrinsic architecture impact activations observed with task probes. Given the strong relationship between resting state and task-based LFO measures, it should be possible to begin to address this question in existing task-based datasets.
4.5. Limitations
Our slow event-related flanker paradigm included a limited number of trials (n=48), precluding investigation of trial-to-trial effects of intrinsic activity on task activations (Fox et al., 2007; Fox et al., 2006; Sadaghiani et al., 2009). Additionally, attentional demands differ notably between slow and rapid event-related designs (e.g., a higher degree of vigilant attention is required for slow designs). Future studies can determine the extent to which our findings generalize to alternative experimental designs. Parametric manipulations of task-demands (e.g., n-back task) may be particularly useful for providing more comprehensive characterizations of the relationship between resting state fMRI measures and task activations. We counter balanced the order of rest and task scans across participants, minimizing the contributions of order effects. As resting state fMRI measures are likely impacted by fatigue and arousal, future work should determine the extent to which rest/task relationships may be affected by when a rest scan occurs in a session, as well as possible interactions between scans.
Finally, we did not collect physiological measures (respiration, heart-rate), which can contribute to low-frequency BOLD fluctuations. However, recent work suggests that the contribution of these signals to the resting state signal is relatively small (< 5%) (Petridou et al., 2009; see Zuo et al., (2010) for a more extensive discussion of this issue).
4.6. Conclusions
The amplitude of low-frequency oscillations measured during rest can predict the magnitude of task-evoked, extrinsic BOLD activations observed during an Eriksen flanker task. Intrinsic activity, represented by low-frequency oscillations detected during a separate resting state fMRI scan, had the same predictive power as intrinsic activity detected during task-performance. Resting state intrinsic activity was also robustly associated with behavioral performance in medial cingulate regions. These results support the notion that intrinsic brain activity serves as a framework for extrinsic, evoked brain activity and behavior. They also emphasize the potential utility of resting state fMRI for the investigation of functional brain organization.
Supplementary Material
Acknowledgments
The authors thank all participants for their cooperation. This research was partially supported by grants from NIMH (R01MH083246 and K23MH087770), Autism Speaks, the Stavros Niarchos Foundation, the Leon Levy Foundation, and gifts from Joseph P. Healy, Linda and Richard Schaps, Jill and Bob Smith, and the endowment provided by Phyllis Green and Randolph Cōwen. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson JLR, Jenkinson M, Smith SM. TR07JA2 : Non-linear registration, aka Spatial normalisation. FMRIB Analysis Group Technical Reports. 2007 http://www.fmrib.ox.ac.uk/analysis/techrep/
- Barnes A, Bullmore ET, Suckling J. Endogenous human brain dynamics recover slowly following cognitive effort. PLoS One. 2009;4:e6626. doi: 10.1371/journal.pone.0006626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellec P, Rosa-Neto P, Lyttelton OC, Benali H, Evans AC. Multi-level bootstrap analysis of stable clusters in resting-state fMRI. Neuroimage. 2010;51:1126–1139. doi: 10.1016/j.neuroimage.2010.02.082. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Braun J, Mattia M. Attractors and noise: twin drivers of decisions and multistability. Neuroimage. 2010;52:740–751. doi: 10.1016/j.neuroimage.2009.12.126. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AL, Fair DA, Dosenbach NU, Miezin FM, Dierker D, Van Essen DC, Schlaggar BL, Petersen SE. Defining functional areas in individual human brains using resting functional connectivity MRI. Neuroimage. 2008;41:45–57. doi: 10.1016/j.neuroimage.2008.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton V, Carew JD, Arfanakis K, Maravilla K. Hierarchical clustering to measure connectivity in fMRI resting-state data. Magn Reson Imaging. 2002;20:305–317. doi: 10.1016/s0730-725x(02)00503-9. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006;9:23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- Garrett DD, Kovacevic N, McIntosh AR, Grady CL. Blood oxygen level-dependent signal variability is more than just noise. J Neurosci. 2010;30:4914–4921. doi: 10.1523/JNEUROSCI.5166-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Rho Y, McIntosh AR, Kotter R, Jirsa VK. Noise during rest enables the exploration of the brain’s dynamic repertoire. PLoS Comput Biol. 2008;4:e1000196. doi: 10.1371/journal.pcbi.1000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, Zuo XN, Butler PD, Javitt DC, D’Angelo D, Mauro CJ, Milham MP. Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophr Res. 2010;117:13–20. doi: 10.1016/j.schres.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XQ, Lui S, Deng W, Chan RC, Wu QZ, Jiang LJ, Zhang JR, Jia ZY, Li XL, Li F, Chen L, Li T, Gong QY. Localization of cerebral functional deficits in treatment-naive, first-episode schizophrenia using resting-state fMRI. Neuroimage. 2010;49:2901–2906. doi: 10.1016/j.neuroimage.2009.11.072. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister PR, Brady JM, Smith SM. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith SM. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kelly C, Uddin LQ, Shehzad Z, Margulies DS, Castellanos FX, Milham MP, Petrides M. Broca’s region: linking human brain functional connectivity data and non-human primate tracing anatomy studies. Eur J Neurosci. 2010;32:383–398. doi: 10.1111/j.1460-9568.2010.07279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S, Li T, Deng W, Jiang L, Wu Q, Tang H, Yue Q, Huang X, Chan RC, Collier DA, Meda SA, Pearlson G, Mechelli A, Sweeney JA, Gong Q. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67:783–792. doi: 10.1001/archgenpsychiatry.2010.84. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci U S A. 2009;106:20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxim V, Sendur L, Fadili J, Suckling J, Gould R, Howard R, Bullmore E. Fractional Gaussian noise, functional MRI and Alzheimer’s disease. Neuroimage. 2005;25:141–158. doi: 10.1016/j.neuroimage.2004.10.044. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Makeig S, Brown GG, Jung TP, Kindermann SS, Bell AJ, Sejnowski TJ. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp. 1998;6:160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Kelly C, Zuo XN, Di Martino A, Biswal B, Xavier Castellanos F, Milham MP. Inter-Individual Differences in Resting State Functional Connectivity Predict Task-Induced BOLD Activity. Neuroimage. 2010;50:1690–1701. doi: 10.1016/j.neuroimage.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Claus ED, Cohen NJ. Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. Neuroimage. 2003;18:483–493. doi: 10.1016/s1053-8119(02)00050-2. [DOI] [PubMed] [Google Scholar]
- Petridou N, Schafer A, Gowland P, Bowtell R. Phase vs. magnitude information in functional magnetic resonance imaging time series: toward understanding the noise. Magn Reson Imaging. 2009;27:1046–1057. doi: 10.1016/j.mri.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Raichle ME. Two views of brain function. Trends Cogn Sci. 2010;14:180–190. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annual Review of Neuroscience. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, Hesselmann G, Kleinschmidt A. Distributed and antagonistic contributions of ongoing activity fluctuations to auditory stimulus detection. J Neurosci. 2009;29:13410–13417. doi: 10.1523/JNEUROSCI.2592-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyn-Ross ML, Steyn-Ross DA, Wilson MT, Sleigh JW. Modeling brain activation patterns for the default and cognitive states. Neuroimage. 2009;45:298–311. doi: 10.1016/j.neuroimage.2008.11.036. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M, Mandl R, Hulshoff Pol H. Normalized cut group clustering of resting-state FMRI data. PLoS One. 2008;3:e2001. doi: 10.1371/journal.pone.0002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30:3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Yang H, Li OQ, Zhang MM, Long XY. Spontaneous brain activity in medication- naive ADHD boys revealed by ALFF analysis. Brain Dev. 2010 doi: 10.1016/j.braindev.2009.12.007. in press. [DOI] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain & Development. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lu G, Zhong Y, Tan Q, Chen H, Liao W, Tian L, Li Z, Shi J, Liu Y. fMRI study of mesial temporal lobe epilepsy using amplitude of low-frequency fluctuation analysis. Hum Brain Mapp. 2010 doi: 10.1002/hbm.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q, Wu CW, Stein EA, Zang Y, Yang Y. Static and dynamic characteristics of cerebral blood flow during the resting state. Neuroimage. 2009;48:515–524. doi: 10.1016/j.neuroimage.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172:137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, Castellanos FX, Biswal BB, Milham MP. The oscillating brain: complex and reliable. Neuroimage. 2010;49:1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.