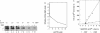Abstract
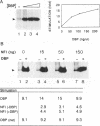
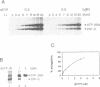
Initiation of adenovirus DNA replication in vitro minimally requires the viral TP-DNA template and the precursor terminal protein-DNA polymerase heterodimer (pTP-pol). Optimal initiation occurs in the presence of the cellular transcription factors NFI and Oct-1 and the viral DNA binding protein (DBP). We have studied the influence of these three stimulatory proteins on the kinetics of formation of the pTP-dCMP initiation complex. NFI increases the Vmax of the reaction but does not affect the apparent Km for dC-TP. This indicates that NFI acts by enlarging the amount of active initiation complex in agreement with its stabilizing effect on binding of pTP-pol to the template. Similar kinetic effects were observed for Oct-1. Since Oct-1 does not stabilize binding of pTP-pol to the origin this suggests that Oct-1 increases the rate of pTP-dCMP formation. DBP stimulates the initiation reaction in two ways. First, it moderately increases the Vmax at suboptimal NFI concentrations, which is related to its enhancing effect on binding of NFI to the origin. Second, a much larger stimulation was caused by DBP itself based on a reduction of the Km for dCTP, which was independent of the concentration of pTP-pol or NFI. The Km for dCTP during initiation is lower than during elongation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Shneidman P. S., Hurwitz J. Reconstruction of adenovirus replication origins with a human nuclear factor I binding site. J Biol Chem. 1986 Mar 5;261(7):3339–3346. [PubMed] [Google Scholar]
- Bernad A., Lázaro J. M., Salas M., Blanco L. The highly conserved amino acid sequence motif Tyr-Gly-Asp-Thr-Asp-Ser in alpha-like DNA polymerases is required by phage phi 29 DNA polymerase for protein-primed initiation and polymerization. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4610–4614. doi: 10.1073/pnas.87.12.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Bernad A., Salas M. Transition from initiation to elongation in protein-primed phi 29 DNA replication: salt-dependent stimulation by the viral protein p6. J Virol. 1988 Nov;62(11):4167–4172. doi: 10.1128/jvi.62.11.4167-4172.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Gutiérrez J., Lázaro J. M., Bernad A., Salas M. Replication of phage phi 29 DNA in vitro: role of the viral protein p6 in initiation and elongation. Nucleic Acids Res. 1986 Jun 25;14(12):4923–4937. doi: 10.1093/nar/14.12.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher J., Robinson E. C., Hay R. T. Interactions between the adenovirus type 2 DNA polymerase and the DNA binding domain of nuclear factor I. New Biol. 1990 Dec;2(12):1083–1090. [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Animal virus DNA replication. Annu Rev Biochem. 1989;58:671–717. doi: 10.1146/annurev.bi.58.070189.003323. [DOI] [PubMed] [Google Scholar]
- Chase J. W., Williams K. R. Single-stranded DNA binding proteins required for DNA replication. Annu Rev Biochem. 1986;55:103–136. doi: 10.1146/annurev.bi.55.070186.000535. [DOI] [PubMed] [Google Scholar]
- Chen M., Mermod N., Horwitz M. S. Protein-protein interactions between adenovirus DNA polymerase and nuclear factor I mediate formation of the DNA replication preinitiation complex. J Biol Chem. 1990 Oct 25;265(30):18634–18642. [PubMed] [Google Scholar]
- Cleat P. H., Hay R. T. Co-operative interactions between NFI and the adenovirus DNA binding protein at the adenovirus origin of replication. EMBO J. 1989 Jun;8(6):1841–1848. doi: 10.1002/j.1460-2075.1989.tb03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenjaerts F. E., De Vries E., Pruijn G. J., Van Driel W., Bloemers S. M., Van der Lugt N. M., Van der Vliet P. C. Enhancement of DNA replication by transcription factors NFI and NFIII/Oct-1 depends critically on the positions of their binding sites in the adenovirus origin of replication. Biochim Biophys Acta. 1991 Aug 27;1090(1):61–69. doi: 10.1016/0167-4781(91)90037-m. [DOI] [PubMed] [Google Scholar]
- Field J., Gronostajski R. M., Hurwitz J. Properties of the adenovirus DNA polymerase. J Biol Chem. 1984 Aug 10;259(15):9487–9495. [PubMed] [Google Scholar]
- Fowlkes D. M., Lord S. T., Linné T., Pettersson U., Philipson L. Interaction between the adenovirus DNA-binding protein and double-stranded DNA. J Mol Biol. 1979 Aug 5;132(2):163–180. doi: 10.1016/0022-2836(79)90389-9. [DOI] [PubMed] [Google Scholar]
- Gil G., Smith J. R., Goldstein J. L., Slaughter C. A., Orth K., Brown M. S., Osborne T. F. Multiple genes encode nuclear factor 1-like proteins that bind to the promoter for 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8963–8967. doi: 10.1073/pnas.85.23.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounari F., De Francesco R., Schmitt J., van der Vliet P., Cortese R., Stunnenberg H. Amino-terminal domain of NF1 binds to DNA as a dimer and activates adenovirus DNA replication. EMBO J. 1990 Feb;9(2):559–566. doi: 10.1002/j.1460-2075.1990.tb08143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronostajski R. M. Analysis of nuclear factor I binding to DNA using degenerate oligonucleotides. Nucleic Acids Res. 1986 Nov 25;14(22):9117–9132. doi: 10.1093/nar/14.22.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenheimer R. A., Nagata K., Kenny M., Hurwitz J. Protein-primed replication of plasmids containing the terminus of the adenovirus genome. II. Purification and characterization of a host protein required for the replication of DNA templates devoid of the terminal protein. J Biol Chem. 1984 Jun 25;259(12):7815–7825. [PubMed] [Google Scholar]
- Hay R. T. Origin of adenovirus DNA replication. Role of the nuclear factor I binding site in vivo. J Mol Biol. 1985 Nov 5;186(1):129–136. doi: 10.1016/0022-2836(85)90263-3. [DOI] [PubMed] [Google Scholar]
- Hay R. T., Russell W. C. Recognition mechanisms in the synthesis of animal virus DNA. Biochem J. 1989 Feb 15;258(1):3–16. doi: 10.1042/bj2580003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R. T. The origin of adenovirus DNA replication: minimal DNA sequence requirement in vivo. EMBO J. 1985 Feb;4(2):421–426. doi: 10.1002/j.1460-2075.1985.tb03645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Joung I., Horwitz M. S., Engler J. A. Mutagenesis of conserved region I in the DNA polymerase from human adenovirus serotype 2. Virology. 1991 Sep;184(1):235–241. doi: 10.1016/0042-6822(91)90840-8. [DOI] [PubMed] [Google Scholar]
- Kenny M. K., Hurwitz J. Initiation of adenovirus DNA replication. II. Structural requirements using synthetic oligonucleotide adenovirus templates. J Biol Chem. 1988 Jul 15;263(20):9809–9817. [PubMed] [Google Scholar]
- Kruse U., Qian F., Sippel A. E. Identification of a fourth nuclear factor I gene in chicken by cDNA cloning: NFI-X. Nucleic Acids Res. 1991 Dec 11;19(23):6641–6641. doi: 10.1093/nar/19.23.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeds J. M., Slabaugh M. B., Mathews C. K. DNA precursor pools and ribonucleotide reductase activity: distribution between the nucleus and cytoplasm of mammalian cells. Mol Cell Biol. 1985 Dec;5(12):3443–3450. doi: 10.1128/mcb.5.12.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegwater P. A., van Driel W., van der Vliet P. C. Recognition site of nuclear factor I, a sequence-specific DNA-binding protein from HeLa cells that stimulates adenovirus DNA replication. EMBO J. 1985 Jun;4(6):1515–1521. doi: 10.1002/j.1460-2075.1985.tb03811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leith I. R., Hay R. T., Russell W. C. Adenovirus subviral particles and cores can support limited DNA replication. J Gen Virol. 1989 Dec;70(Pt 12):3235–3248. doi: 10.1099/0022-1317-70-12-3235. [DOI] [PubMed] [Google Scholar]
- Lindenbaum J. O., Field J., Hurwitz J. The adenovirus DNA binding protein and adenovirus DNA polymerase interact to catalyze elongation of primed DNA templates. J Biol Chem. 1986 Aug 5;261(22):10218–10227. [PubMed] [Google Scholar]
- Mathews C. K., Moen L. K., Wang Y., Sargent R. G. Intracellular organization of DNA precursor biosynthetic enzymes. Trends Biochem Sci. 1988 Oct;13(10):394–397. doi: 10.1016/0968-0004(88)90182-x. [DOI] [PubMed] [Google Scholar]
- Meisterernst M., Gander I., Rogge L., Winnacker E. L. A quantitative analysis of nuclear factor I/DNA interactions. Nucleic Acids Res. 1988 May 25;16(10):4419–4435. doi: 10.1093/nar/16.10.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisterernst M., Rogge L., Foeckler R., Karaghiosoff M., Winnacker E. L. Structural and functional organization of a porcine gene coding for nuclear factor I. Biochemistry. 1989 Oct 3;28(20):8191–8200. doi: 10.1021/bi00446a034. [DOI] [PubMed] [Google Scholar]
- Mermod N., O'Neill E. A., Kelly T. J., Tjian R. The proline-rich transcriptional activator of CTF/NF-I is distinct from the replication and DNA binding domain. Cell. 1989 Aug 25;58(4):741–753. doi: 10.1016/0092-8674(89)90108-6. [DOI] [PubMed] [Google Scholar]
- Mul Y. M., Van der Vliet P. C. Nuclear factor I enhances adenovirus DNA replication by increasing the stability of a preinitiation complex. EMBO J. 1992 Feb;11(2):751–760. doi: 10.1002/j.1460-2075.1992.tb05108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mul Y. M., Verrijzer C. P., van der Vliet P. C. Transcription factors NFI and NFIII/oct-1 function independently, employing different mechanisms to enhance adenovirus DNA replication. J Virol. 1990 Nov;64(11):5510–5518. doi: 10.1128/jvi.64.11.5510-5518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mul Y. M., van Miltenburg R. T., De Clercq E., van der Vliet P. C. Mechanism of inhibition of adenovirus DNA replication by the acyclic nucleoside triphosphate analogue (S)-HPMPApp: influence of the adenovirus DNA binding protein. Nucleic Acids Res. 1989 Nov 25;17(22):8917–8929. doi: 10.1093/nar/17.22.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Enomoto T., Lichy J. H., Hurwitz J. Adenovirus DNA replication in vitro: identification of a host factor that stimulates synthesis of the preterminal protein-dCMP complex. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6438–6442. doi: 10.1073/pnas.79.21.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Hurwitz J. Specific binding of a cellular DNA replication protein to the origin of replication of adenovirus DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6177–6181. doi: 10.1073/pnas.80.20.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paonessa G., Gounari F., Frank R., Cortese R. Purification of a NF1-like DNA-binding protein from rat liver and cloning of the corresponding cDNA. EMBO J. 1988 Oct;7(10):3115–3123. doi: 10.1002/j.1460-2075.1988.tb03178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto I., Serrano M., Lázaro J. M., Salas M., Hermoso J. M. Interaction of the bacteriophage phi 29 protein p6 with double-stranded DNA. Proc Natl Acad Sci U S A. 1988 Jan;85(2):314–318. doi: 10.1073/pnas.85.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruijn G. J., van Driel W., van der Vliet P. C. Nuclear factor III, a novel sequence-specific DNA-binding protein from HeLa cells stimulating adenovirus DNA replication. Nature. 1986 Aug 14;322(6080):656–659. doi: 10.1038/322656a0. [DOI] [PubMed] [Google Scholar]
- Rawlins D. R., Rosenfeld P. J., Wides R. J., Challberg M. D., Kelly T. J., Jr Structure and function of the adenovirus origin of replication. Cell. 1984 May;37(1):309–319. doi: 10.1016/0092-8674(84)90327-1. [DOI] [PubMed] [Google Scholar]
- Reichard P. Interactions between deoxyribonucleotide and DNA synthesis. Annu Rev Biochem. 1988;57:349–374. doi: 10.1146/annurev.bi.57.070188.002025. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P. J., O'Neill E. A., Wides R. J., Kelly T. J. Sequence-specific interactions between cellular DNA-binding proteins and the adenovirus origin of DNA replication. Mol Cell Biol. 1987 Feb;7(2):875–886. doi: 10.1128/mcb.7.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp R. A., Kruse U., Multhaup G., Göbel U., Beyreuther K., Sippel A. E. Chicken NFI/TGGCA proteins are encoded by at least three independent genes: NFI-A, NFI-B and NFI-C with homologues in mammalian genomes. Nucleic Acids Res. 1990 May 11;18(9):2607–2616. doi: 10.1093/nar/18.9.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G., Finney M. Regulation of transcription and cell identity by POU domain proteins. Cell. 1991 Feb 8;64(3):475–478. doi: 10.1016/0092-8674(91)90227-p. [DOI] [PubMed] [Google Scholar]
- Salas M. Protein-priming of DNA replication. Annu Rev Biochem. 1991;60:39–71. doi: 10.1146/annurev.bi.60.070191.000351. [DOI] [PubMed] [Google Scholar]
- Santoro C., Mermod N., Andrews P. C., Tjian R. A family of human CCAAT-box-binding proteins active in transcription and DNA replication: cloning and expression of multiple cDNAs. Nature. 1988 Jul 21;334(6179):218–224. doi: 10.1038/334218a0. [DOI] [PubMed] [Google Scholar]
- Schechter N. M., Davies W., Anderson C. W. Adenovirus coded deoxyribonucleic acid binding protein. Isolation, physical properties, and effects of proteolytic digestion. Biochemistry. 1980 Jun 10;19(12):2802–2810. doi: 10.1021/bi00553a041. [DOI] [PubMed] [Google Scholar]
- Schneider R., Gander I., Müller U., Mertz R., Winnacker E. L. A sensitive and rapid gel retention assay for nuclear factor I and other DNA-binding proteins in crude nuclear extracts. Nucleic Acids Res. 1986 Feb 11;14(3):1303–1317. doi: 10.1093/nar/14.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Gutiérrez J., Prieto I., Hermoso J. M., Salas M. Signals at the bacteriophage phi 29 DNA replication origins required for protein p6 binding and activity. EMBO J. 1989 Jun;8(6):1879–1885. doi: 10.1002/j.1460-2075.1989.tb03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Salas M., Hermoso J. M. A novel nucleoprotein complex at a replication origin. Science. 1990 May 25;248(4958):1012–1016. doi: 10.1126/science.2111580. [DOI] [PubMed] [Google Scholar]
- Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- Stuiver M. H., Bergsma W. G., Arnberg A. C., van Amerongen H., van Grondelle R., van der Vliet P. C. Structural alterations of double-stranded DNA in complex with the adenovirus DNA-binding protein. Implications for its function in DNA replication. J Mol Biol. 1992 Jun 20;225(4):999–1011. doi: 10.1016/0022-2836(92)90100-x. [DOI] [PubMed] [Google Scholar]
- Stuiver M. H., van der Vliet P. C. Adenovirus DNA-binding protein forms a multimeric protein complex with double-stranded DNA and enhances binding of nuclear factor I. J Virol. 1990 Jan;64(1):379–386. doi: 10.1128/jvi.64.1.379-386.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temperley S. M., Hay R. T. Recognition of the adenovirus type 2 origin of DNA replication by the virally encoded DNA polymerase and preterminal proteins. EMBO J. 1992 Feb;11(2):761–768. doi: 10.1002/j.1460-2075.1992.tb05109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsernoglou D., Tsugita A., Tucker A. D., van der Vliet P. C. Characterization of the chymotryptic core of the adenovirus DNA-binding protein. FEBS Lett. 1985 Sep 2;188(2):248–252. doi: 10.1016/0014-5793(85)80381-1. [DOI] [PubMed] [Google Scholar]
- Verrijzer C. P., Kal A. J., Van der Vliet P. C. The DNA binding domain (POU domain) of transcription factor oct-1 suffices for stimulation of DNA replication. EMBO J. 1990 Jun;9(6):1883–1888. doi: 10.1002/j.1460-2075.1990.tb08314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrijzer C. P., Strating M., Mul Y. M., van der Vliet P. C. POU domain transcription factors from different subclasses stimulate adenovirus DNA replication. Nucleic Acids Res. 1992 Dec 11;20(23):6369–6375. doi: 10.1093/nar/20.23.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrijzer C. P., van Oosterhout J. A., van Weperen W. W., van der Vliet P. C. POU proteins bend DNA via the POU-specific domain. EMBO J. 1991 Oct;10(10):3007–3014. doi: 10.1002/j.1460-2075.1991.tb07851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wides R. J., Challberg M. D., Rawlins D. R., Kelly T. J. Adenovirus origin of DNA replication: sequence requirements for replication in vitro. Mol Cell Biol. 1987 Feb;7(2):864–874. doi: 10.1128/mcb.7.2.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries E., van Driel W., Tromp M., van Boom J., van der Vliet P. C. Adenovirus DNA replication in vitro: site-directed mutagenesis of the nuclear factor I binding site of the Ad2 origin. Nucleic Acids Res. 1985 Jul 11;13(13):4935–4952. doi: 10.1093/nar/13.13.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries E., van Driel W., van den Heuvel S. J., van der Vliet P. C. Contactpoint analysis of the HeLa nuclear factor I recognition site reveals symmetrical binding at one side of the DNA helix. EMBO J. 1987 Jan;6(1):161–168. doi: 10.1002/j.1460-2075.1987.tb04734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen H., van Grondelle R., van der Vliet P. C. Interaction between adenovirus DNA-binding protein and single-stranded polynucleotides studied by circular dichroism and ultraviolet absorption. Biochemistry. 1987 Jul 28;26(15):4646–4652. doi: 10.1021/bi00389a009. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Keegstra W., Jansz H. S. Complex formation between the adenovirus type 5 DNA-binding protein and single-stranded DNA. Eur J Biochem. 1978 May 16;86(2):389–398. doi: 10.1111/j.1432-1033.1978.tb12321.x. [DOI] [PubMed] [Google Scholar]