Abstract
Background
Human leukocyte antigen (HLA)-B27 is strongly associated with the development of reactive arthritis (ReA) in humans after salmonellosis. Human monocytic U937 cells transfected with HLA-B27 are less able to eliminate intracellular Salmonella enterica serovar Enteritidis than those transfected with control HLA antigens (e.g. HLA-A2). To investigate further the mechanisms by which HLA-B27-transfected cells allow increased replication of these bacteria, a DNA-based microarray was used for comparative genomic analysis of S. Enteritidis grown in HLA-B27- or HLA-A2-transfected cells. The microarray consisted of 5080 oligonucleotides from different serovars of Salmonella including S. Enteritidis PT4-specific genes. Bacterial RNA was isolated from the infected HLA-B27- or HLA-A2-transfected cells, reverse-transcribed to cDNA, and hybridized with the oligonucleotides on the microarrays. Some microarray results were confirmed by RT-PCR.
Results
When gene expression was compared between Salmonella grown in HLA-B27 cells and in HLA-A2 cells, 118 of the 4610 S. Enteritidis-related genes differed in expression at 8 h after infection, but no significant difference was detectable at 2 h after infection. These differentially expressed genes are mainly involved in Salmonella virulence, DNA replication, energy conversion and metabolism, and uptake and metabolism of nutrient substances, etc. The difference suggests HLA-B27-dependent modulation of Salmonella gene expression, resulting in increased Salmonella replication in HLA-B27-positive cells. Among the up-regulated genes were those located in Salmonella pathogenicity island (SPI)-2, which play a central role in intracellular survival and replication of Salmonella.
Conclusions
This is the first report to show the regulation of Salmonella gene expression by HLA-B27 during infection of host cells. This regulation probably leads to increased Salmonella survival and replication in HLA-B27-positive cells. SPI-2 genes seem to contribute significantly to the increased replication.
Background
The clinical outcomes of non-typhoidal salmonellosis range from self-limiting gastroenteritis to life-threatening systemic infections [1]. Many serovars of Salmonella enterica cause these infections, serovar Enteritidis being among the most common [2,3]. The acute gastrointestinal infection caused by Salmonella may result in complications such as reactive arthritis (ReA) [4-6]. Originally, ReA was described as an aseptic inflammation that develops after an infection elsewhere in the body [7]. ReA is an asymmetric polyarthritis and the outcome of the disease ranges from mild symptoms to severe and chronic clinical manifestations. Up to 80% of patients with ReA express the HLA-B27 antigen [8,9].
Macrophages are important in the pathogenesis of Salmonella infections. They are an integral part of the immune response as they present antigens to the innate defence system and communicate with the adaptive immune system to resist the bacterial infection [10-12]. However, unlike many other pathogens, Salmonella can survive inside macrophages by adapting to this particular intracellular environmental niche. After Salmonella uptake into macrophages, the intracellular bacteria reside in large membrane-bound phagosomes, called spacious phagosomes (SP), which develop into Salmonella-containing vacuoles (SCV) [13]. The formation of SP or SCV favours the survival and replication of Salmonella in macrophages. Even so, Salmonella encounter intracellular host defence mechanisms, including reactive oxygen and nitrogen species (ROS and RNS), antimicrobial peptides, lysosomal enzymes, and adaptive immune responses [10,12]. In order to survive in the host and to avoid clearance by the host immune system, Salmonella express virulence factors to deal with this stressful environment [14].
Many virulence genes of pathogenic bacteria are located in large multigene chromosome regions termed pathogenicity islands (PAIs) [15]. In Salmonella, they are called Salmonella pathogenicity islands (SPIs) [16]. Two SPIs, SPI-1 and SPI-2, encode structurally similar but functionally distinct type III secretion systems (T3SS), specialized protein export machineries that Salmonella uses to deliver virulence proteins into the cytosol of host cells [17]. The SPI-1-encoded T3SS is active extracellularly. SPI-1 mediates invasion into non-phagocytic cells [18], and it is required for the intestinal inflammatory responses [19]. The SPI-2 virulence genes are expressed intracellularly and are required for the survival of bacteria in macrophages and systemic infections. SPI-2 mutant strains are dramatically attenuated, showing a 104-fold reduction in virulence in LD50 in the murine salmonellosis model [16] and impaired intracellular replication and survival in macrophages [20,21].
HLA-B27 confers a very strong genetic predisposition towards the development of a group of rheumatic disorders called spondyloarthropathies (SpA), including ankylosing spondylitis (AS) and ReA. HLA-B27-positive individuals have a five-fold higher incidence of ReA than the general population [22,23]. ReA occurs following certain infections, e.g. those caused by Salmonella and Yersinia pathogens [7,8,24,25]. Expression of HLA-B27 also increases the risk that the patient will suffer a more severe and prolonged disorder [6,8]. The interaction between ReA-triggering bacteria and HLA-B27-positive subjects is abnormal and leads to increased persistence of the causative microbes/microbial antigens in HLA-B27-positive patients [26-28]. The interaction between HLA-B27 molecules and arthritogenic microbes was investigated more thoroughly using in vitro infected cells. Experiments investigating the invasion of HLA-B27 cells by Gram-negative bacteria, including Salmonella, are inconclusive. Studies have shown either decreased [29] or similar [30] levels of invasion of HLA-B27-transfected murine L fibroblasts compared with control L cell lines (L cells transfected with other MHC class I genes) or increased invasion of HLA-B27-transfected intestinal epithelial Henle-407 cells by Salmonella [31]. Once inside the host cells, Salmonella is able to replicate more quickly [32,33], and is eliminated more slowly, in HLA-B27-positive cells [30,34] than transfected control cells. The survival and persistence of Salmonella in the intracellular environment is associated with bacterial gene expression [35]. However, little is known about Salmonella gene expression in association with HLA-B27 during bacterial infection and persistence.
Results and Discussion
Cell surface expression of HLA-B27 and HLA-A2 molecules
The transfected HLA-B27 and HLA-A2 were always expressed on the surface of the respective cells, as detected by immunofluorescence in new batches of the cell lines (data not shown). The level of expression of the transfected molecules in U937 cells was similar to that of HLA-B51, one of the MHC class I molecules endogenously expressed by U937 cells [34]. In addition, the surface expression levels of the transfected molecules corresponded to the levels of those molecules endogenously expressed on peripheral blood monocytes [34].
Increased replication of Salmonella in HLA-B27-positive U937 cells
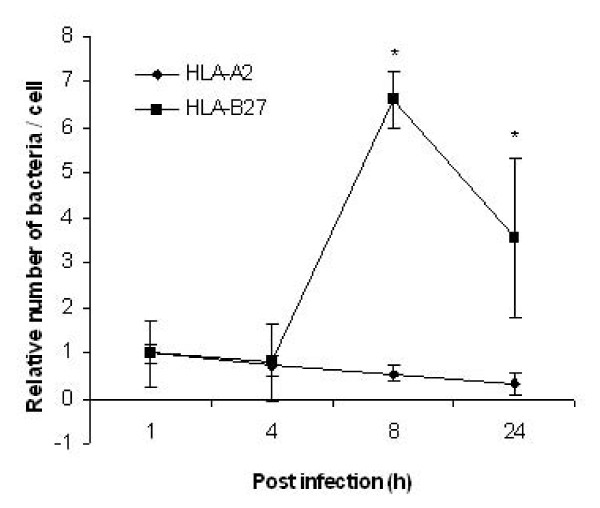
We used the in vitro model of infection established earlier in our laboratory to monitor growth of S. Enteritidis in macrophage-like U937 cells [32-34]. Cells transfected with HLA-B27 or HLA-A2 were infected with complement-opsonized salmonellae. HLA-A2 transfected cells were used as a negative control since it is a common tissue antigen that is not related to the development of ReA [34]. At 1, 4, 8, and 24 h post infection, the host cells were lysed and the number of living intracellular bacteria per cell was determined by counting the number of colony forming units (CFU) (Figure. 1). Consistent with our previous results, no difference in the uptake of Salmonella was observed between the two cell lines (1 h post infection) [34]. However, more bacteria were recovered from HLA-B27-expressing cells than HLA-A2-expressing cells at 8 h and even up to 24 h post infection, suggesting that HLA-B27-transfected cells become permissive for intracellular Salmonella survival and replication, which is also consistent with our previous studies [32-34].
Figure 1.
Relative number of intracellular bacteria in each living U937 cell. The cultured HLA-B27 or HLA-A2-transfected U937 cells were infected with complement-opsonized S. Enteritidis PT4 KS8822/88 and the incubation was continued at 37°C for 1, 4, 8 or 24 h after infection. At the indicated time points, the host cells were lysed and the number of bacteria per cell was counted and reported as colony forming units (CFU). For both cell lines, the number of intracellular bacteria at 1 h after infection was designated 1. The numbers of intracellular bacteria at the following indicated time points are shown as fold changes relative to the number at 1 h after infection. Values are mean ± standard deviation of duplicate samples from a representative experiment out of three with similar results. * P < 0.05 versus S. Enteritidis-infected HLA-A2 transfectants. Data were compared using Student's paired 2-tailed t-test.
Global gene expression profiles of S. Enteritidis in HLA-B27- and HLA-A2-transfected U937 cells
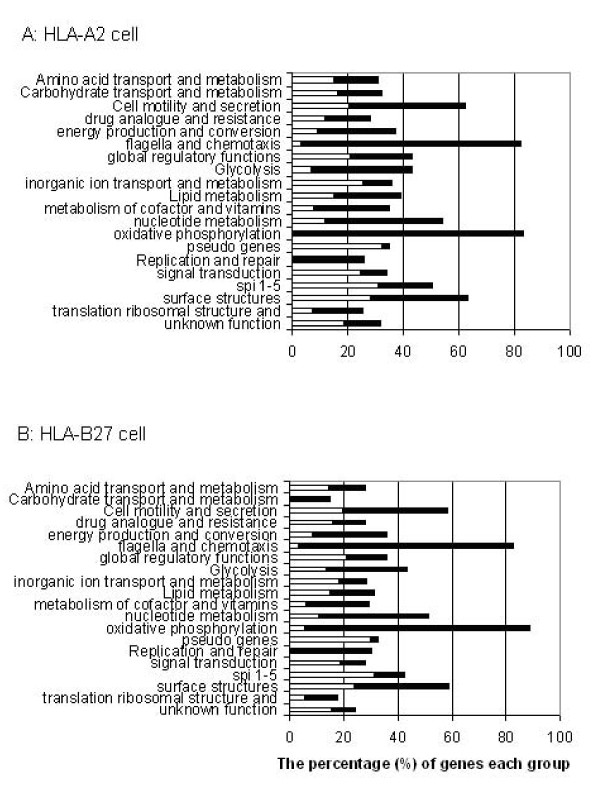
The 'SALSA' genomic Salmonella serovar microarray containing 5080 Salmonella oligonucleotides was used to monitor gene expression in Salmonella grown in the host cells [36]. Gene expression was compared between Salmonella grown in U937 cells and in LB broth. In total, 1,388 genes of Salmonella grown in HLA-B27 cells and 1,049 genes of Salmonella grown in HLA-A2 cells showed statistically significant differences in expression at 2 h post infection when compared to the transcriptome in LB broth culture. At 8 h post infection, the expression of 1,352 genes of Salmonella grown in HLA-B27 cells and of 1,559 genes of Salmonella grown in HLA-A2 cells showed statistically significant differences from Salmonella grown in LB broth. The genes that showed significant expression differences and were up- or down-regulated more than two-fold for each individual comparison are listed in Additional file 1: Supplemental Tables 1-6. The categories of Salmonella genes showing differential expression between HLA-B27 cells and LB and between HLA-A2 cells and LB at 2 h post infection can be found in Figure. 2. In particular, the functional categories analysis showed large differences in the expression of genes required for cell motility and secretion, flagellae and chemotaxis and oxidative phosphorylation. These differences in gene expression reflect bacterial adaptation to the intracellular environments.
Figure 2.
Differential expression of S. Enteritidis genes divided into functional groups at 2 h time point. Bars indicate percentages of genes in each group that showed significant changes in expression in HLA-A2 cells (A) and HLA-B27 cells (B), which were compared to cells grown in LB. The white bars indicate the proportion of up-regulated genes and the black bars the percentage of down-regulated genes for each group.
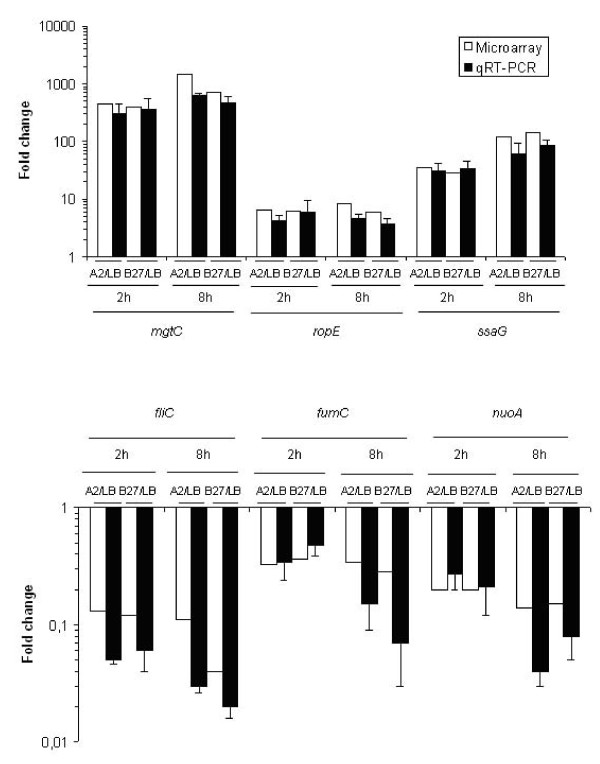
To confirm the microarray data, quantitative real time RT-PCR was performed on six genes selected from different functional categories and showing differential up- or down-regulated expression between HLA-B27 and A2 cells: mgtC, rpoE and ssaG (up-regulated); fliC, fumC and nuoA (down-regulated) (Figure. 3). RT-PCR was performed using the same bacterial RNA from HLA-B27 cells, A2 cells and LB broth, as was used in microarray experiments. It is important that the genes selected covered the whole scale of the microarray data from high expression level to medium and to low expression level, as well as in differential expression from high fold changes to low fold changes in U937 cells vs. in LB, among up-regulated and down-regulated genes, respectively. The quantity of cDNA for each gene was obtained after normalization to the levels of rfaH cDNA, which was chosen as a control since rfaH is expressed stably at moderate levels under most of the conditions tested (see Additional file 2: Supplemental Table S8). The RT-PCR results of the six genes studied were consistent with the microarray data (Figure. 3), but due to the small number of genes studied with RT-PCR, data may be considered preliminary.
Figure 3.
Validation of microarray results by quantitative real time RT-PCR. The fold changes in gene expression are shown when S. Enteritidis growth in HLA-A2 or HLA-B27 cells was compared to growth in LB broth. The expression levels of selected up-regulated genes (A) or down-regulated genes (B) are shown in logarithmic scale. The experiments were repeated at least three times with similar results. Values are mean numbers ± standard deviations generated from three independent experiments. Correlation coefficients (R) for comparisons of two techniques are: up-regulated genes; 0.977 (A2/LB) and 0.959 (B27/LB), down-regulated genes 0.530 (A2/LB) and 0.620 (B27/LB) (see Additional file 2: Supplemental Table S7).
Salmonella gene expression is regulated by HLA-B27 at the 8 h time point
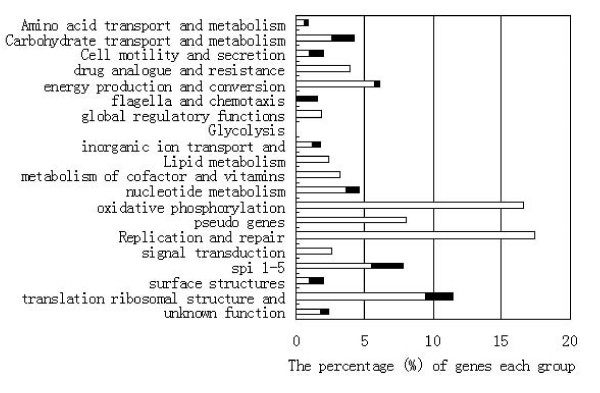
To determine whether bacterial gene expression plays a role in Salmonella replication/persistence in HLA-B27 cells, we assessed the transcriptome of S. Enteritidis in the two cell lines. Differential expression of the genes betweeen HLA-B27 cells and HLA-A2 cells is based on the comparison of the gene expression in HLA-B27 cells to LB and the gene expression in HLA-A2 cells to LB. For example, for the gene whose expression is 6-fold in HLA-B27 cells compared to LB, and 2-fold in HLA-A2 cells compared to LB, the expression is 3-fold in HLA-B27 cells compared to HLA-A2 cells. No genes showed significant differences when the transcriptomes of Salmonella grown in HLA-B27- and HLA-A2-transfected cell lines were compared at 2 h post infection. This is consistent with the similarity observed in the intracellular growth of Salmonella at this time point. In contrast, 118 genes showed significant differences in expression between Salmonella grown in HLA-B27 cells and in HLA-A2 cells at 8 h post infection (86 genes were up-regulated and 32 genes down-regulated (see Additional file 1: Supplemental Tables S6-1 and -2, Figure 4 and Table 1). Bacterial gene expression reflects the environmental conditions in which the bacteria reside. The difference between bacteria grown in HLA-B27 and HLA-A2 cells suggests that HLA-B27 modulates the intracellular environment and causes changes in Salmonella gene expression during macrophage infection, resulting in increased survival and replication in HLA-B27 cells. This modulation may be due to changes in host cell signalling pathways [33,37]. This is the first observation showing that Salmonella gene expression is influenced by HLA-B27. The analysis of functional categories revealed that the groups of genes most altered in the presence of HLA-B27 included those involved in Salmonella virulence, DNA replication, energy conversion and metabolism, and uptake and metabolism of nutrients.
Figure 4.
Differential expression of S. Enteritidis genes between HLA-B27 and HLA-A2 cells divided into functional groups at 8 h time point. Bars indicate percentages of genes in each group that showed significant changes in S. Enteritidis gene expression in HLA-B27 cells compared to in HLA-A2 cells. The white bars indicate the proportion of up-regulated genes and the black bars the percentage of down-regulated genes for each group.
Table 1.
The leading genes that were differentially expressed between the two cell lines
| Name | Genbank | Function and product | Fold changesα |
|---|---|---|---|
| ssaN | AAL20339 | Secretion system apparatus | 3.97 |
| ssaO | AAL20340 | Secretion system apparatus | 2.23 |
| ssaR | AAL20343 | Secretion system apparatus | 4.09 |
| ssaS | AAL20344 | Secretion system apparatus | 3.25 |
| ssaV | AAL20338 | Secretion system apparatus | 2.56 |
| sscA | AAL20323 | Secretion system apparatus | 2.93 |
| sse I | AAL19985 | Gifsy-2 prophage; putative type III secreted protein | 4.67 |
| sspH2 | AAL21143 | Leucine-rich repeat protein, induced by the SPI-2 regulator ssrA/B | 3.89 |
| dnaE | AAL19195 | DNA polymerase III, alpha subunit | 2.68 |
| gyrA | AAL21173 | DNA gyrase, subunit A, type II topoisomerase | 2.09 |
| gyrB | AAL22694 | DNA gyrase, subunit B, type II topoisomerase | 2.39 |
| cyoC | AAL19396 | Cytochrome o ubiquinol oxidase subunit III | 3.52 |
| cyoD | AAL19395 | Cytochrome o ubiquinol oxidase subunit IV | 3.50 |
| nuoJ | AAL21221 | NADH dehydrogenase I chain J | 2.19 |
| nuoK | AAL21220 | NADH dehydrogenase I chain K | 2.42 |
| nuoM | AAL21218 | NADH dehydrogenase I chain M | 2.39 |
| nuoN | AAL21217 | NADH dehydrogenase I chain N | 2.27 |
| glnG | AAL22844 | Response regulator in two-component regulatory system with GlnL (EBP family) | 2.88 |
| glnL | AAL22845 | Sensory kinase (phosphatase) in two-component regulatory system with GlnG | 2.41 |
| glnA | AAL22846 | Glutamine synthetase | 2.53 |
| gltB | AAL22199 | Glutamate synthase, large subunit | 2.21 |
| gltD | AAL22200 | Glutamate synthase, small subunit | 2.18 |
| aceF | AAL19117 | Pyruvate dehydrogenase, dihydrolipoyltransacetylase component | 2.66 |
| fruA | AAL21108 | Sugar specific PTS system, fructose-specific transport | 3.05 |
| fruF | AAL21110 | Phosphoenolpyruvate-dependent | 2.67 |
| sugarphosphotransferase system, EIIA | |||
| pykF | AAL20302 | Pyruvate kinase I (formerly F), fructose stimulated | 3.33 |
| pstC | AAL22714 | ABC superfamily (membrane), high affinity phosphate | 0.48 |
α FDR (P-value < 0.05) was set up during microarray data analysis.
Up-regulation of SPI-2 genes may prevent damage by ROS
SPI-2 genes have been shown to make Salmonella capable of growing intracellularly by avoiding killing by reactive oxygen species (ROS) [38]. During Salmonella replication in macrophages, the innate defence systems of the host cells are triggered to limit the infection. ROS produced by phagocyte NADPH oxidase (phox) are the most effective anti-bacterial agents [39]. NADPH oxidase catalyzes the univalent reduction of molecular oxygen and produces superoxide, which has modest antibacterial activity but serves as a precursor for more toxic substrates, such as hydrogen peroxide and hydroxyl radicals [40]. Compared to those grown in HLA-A2 cells, Salmonella grown in HLA-B27 cells showed increased expression of SPI-2 genes including ssaN, ssaO, ssaR, ssaS, ssaV, sscA, sseI and sspH2 (Additional file 1: Supplemental Table S6-1). SPI-2 deficient mutants have impaired ability to grow in macrophages but are able to survive and are virulent within gp91phox knockout mice [39]. Patients with X-linked gp91phox mutations (chronic granulomatous disease, CGD) or gp91phox knockout mice that are unable to produce ROS are extremely susceptible to bacterial infection, including those caused by salmonellae [41,42]. Taken together, these findings indicated that SPI-2 is required for survival in the presence of ROS. The up-regulation of SPI-2 genes in HLA-B27 cells contributed to the increased intracellular growth and replication of Salmonella.
Intracellular growth of Salmonella SPI-2 mutants affected by HLA-B27
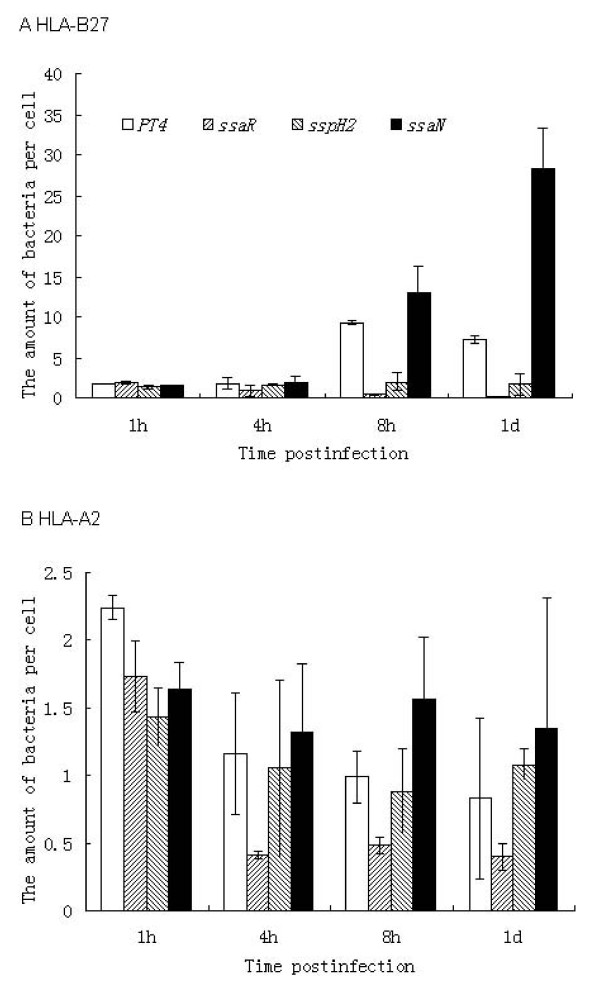
To verify the significance of SPI-2 genes in Salmonella replication in HLA-B27 cells, three genes most markedly up-regulated in HLA-B27 cells - ssaN, ssaR and sspH2 - were deleted; ssaN and ssaR belong to the SPI-2 secretion apparatus and sspH2 is an effector gene. The intracellular growth of the mutants was compared with that of wild-type S. Enteritidis PT4 KS8822/88. Mutation of ssaR dramatically impaired intracellular Salmonella growth in HLA-B27 cells, decreasing it 19-fold at the 8 h and 33-fold at the 24 h time points (Figure. 5A). It has previously been shown that ΔssaR mutants cannot grow intracellularly in murine macrophages or form the elongated Salmonella-induced filaments (Sifs) in epithelial cells [43] required for the formation of Salmonella-containing vacuoles (SCV) [44]. The formation and maintenance of SCV promotes the survival and proliferation of bacteria in macrophages [45] and the lack of formation of Sifs by ssaR mutants may account for the attenuation of this strain. The effector protein SspH2 is co-localized with vacuole-associated actin polymerization involved in the maintenance of SCV membrane integrity [46]. The sspH2 mutant was also attenuated more than 4-fold in HLA-B27 cells at both 8 and 24 hours after infection (Figure. 5A). Unexpectedly, the mutant carrying the ssaN deletion showed dramatically increased proliferation in HLA-B27 cells: 1.4-fold at the 8 h and 3.9-fold at the 24 h time points (Figure. 5A). The increased growth of the ssaN mutant in HLA-B27 cells might indicate that this mutant has good fitness in HLA-B27-positive U937 cells. The previous study by Eriksson et al [47] showed that the selection for S. Typhimurium mutants performed in murine macrophage-like J774-A.1 cells provided bacterial variants capable of a selective downregulation of nitric oxide (NO) expression. These variants showed increased host fitness. This is especially interesting, as in our model with murine L cells [30], iNOS activity and NO production were decreased in HLA-B27 positive cells, which correlated with increased S. Enteritidis growth. However, human U937 cells show very low iNOS activity and produce only small amounts of NO in general, and no difference between HLA-B27-positive and control cells was detected in this respect [48]. So the increased proliferation of the ssaN mutant in HLA-B27 cells, and no effect on growth in HLA-A2 cells (Figure 5B), perhaps indicates another specific and delicate relationship between ssaN and HLA-B27. These observations demonstrated a delicate balance between bacterial growth and pathogenesis.
Figure 5.
Intracellular survival and replication of SPI-2 mutant Salmonella strains in U937 cells. The cultured HLA-B27 or HLA-A2-transfected U937 cells were infected with complement-opsonized S. Enteritidis PT4 KS8822/88 wild type or mutants and incubation of the bacteria-infected cells was continued at 37°C for 1, 4, 8 or 24 h after infection. At the defined time points, the host cells were lysed and the number of bacteria per cell was reported as colony forming units (CFU). The numbers of bacteria in each HLA-B27 cell (A) or HLA-A2 cell (B) at the indicated time points are shown. Values are mean ± standard deviation from three independent experiments with duplicate samples. * P < 0.05, statistically significant differences between the wild type and mutant strains. Data were compared using Student's paired 2-tailed t-test.
Regulation of bacterial DNA synthesis by HLA-B27
As Salmonella replicates more in HLA-B27-transfected cells than in HLA-A2 transfectants, it is not surprising that genes involved in DNA synthesis are up-regulated in HLA-B27 cells compared to HLA-A2 cells. DNA polymerase III (pol III) holoenzyme is a major replicase responsible for DNA synthesis [49]. It is a multiprotein complex containing over 10 distinct subunits. The α subunit encoded by dnaE is one component of the catalytic core (αεθ) of the pol III holoenzyme and mutations affecting the α unit result in reduced growth of S. enteritica serovar Typhimurium [50]. The expression of the gene dnaE was induced 2.7-fold in HLA-B27 cells compared to HLA-A2 cells at 8 h time point (Table 1). Other dna genes including dnaA encoding DNA replication initial protein, dnaB encoding putative replicative DNA helicases, and dnaN encoding β-subunit of DNA polymerase III were up-regulated 1.9-, 1.4-, and 2.0-fold, respectively. However, dnaQ encoding ε-subunit of DNA polymerase III was expressed at the similar level in two cell lines (see Additional file 1: Supplemental Table S1). The up-regulation of most dna genes in HLA-B27 cells compared to HLA-A2 cells might indicate more DNA replication in HLA-B27 cells. Two genes (gyrA and gyrB) required for the production of the heterotetramer-DNA gyrase were also up-regulated in HLA-B27 cells compared with the control cells. DNA gyrase is an essential enzyme for bacterial DNA synthesis; it introduces negative supercoils into DNA during replication [51]. The induction of gyrA and gyrB perhaps indicates that more DNA was synthesized by Salmonella resulting in increased bacterial proliferation in HLA-B27-transfected cells.
Salmonella substrate and energy metabolism
Growth in a nutrient-limited environment may be echoed in changes in gene expression. Expression of genes involved in substrate and energy metabolism, including nuoJKMN encoding NADH dehydrogenase I chains (J, K, M and N components), cyoCD encoding cytochrome O ubiquinol oxidase subunits III and IV, and aceF encoding a putative dehydrogenase was up-regulated in HLA-B27 cells compared to HLA-A2 cells. This suggests that more energy was produced in HLA-B27 cells under aerobic conditions, which could be connected to the enhanced bacterial replication observed in HLA-B27 cells [52,53].
Up-regulation of amino acid metabolism by Salmonella growth in HLA-B27 cells compared to HLA-A2 cells was evident from the induction of gltB, gltD and glnA encoding glutamate synthase/glutamine synthetase, and glnGL encoding a two-component regulator of glutamine synthetase [54]. Glutamate is an important precursor of other amino acids and of pyrimidine and purine synthesis in bacteria [55]. Moreover, sugar catabolism genes such as aceF, fruA, fruF and pykF were elevated in HLA-B27 cells. Collectively, these data suggest that HLA-B27 affects intracellular substrate metabolism and energy production by Salmonella during infection of U937 cells, probably due to the increased bacterial proliferation in these cells.
HLA-B27 modified ionic transport systems
Magnesium is involved in the stabilisation of the cellular membrane and functions as a coenzyme. MgtA and MgtB are two inducible transporters, and high up-regulation of mgtB and mgtA in both HLA-B27 and HLA-A2 macrophages might suggest that Mg2+ is extremely limited within the SCV of infected U937 cells [56,57]. From 1 to 8 h after infection, the degree to which these two genes were up-regulated was increased in both cell lines, indicating that the amounts of magnesium decreased during the course of infection. MgtC is also involved in Mg2+ transport in the intracellular survival of Salmonella and other bacteria in macrophages [58,59]. The two genes mgtC and mgtB are located in one operon, mgtCB, which belongs to SPI-3 on the Salmonella chromosome [58]. Our data showed that both mgtC and mgtB were less induced in HLA-B27-positive cells than in HLA-B27-negative cells, suggesting that HLA-B27 modulates the acquisition of Mg2+ in macrophages.
Phosphate availability is limited in macrophage vacuoles [60]. These observations are supported by our microarray data showing that the phoBR regulon, the pstC and pstS genes responsible for phosphate uptake and/or transport, were up-regulated during intracellular growth. The genes were induced to similar levels in both cell lines at 2 h after infection but the expression of pstC was down-regulated in HLA-B27 cells compared to HLA-A2 cells at 8 h postinfection. Taken together, these results suggest that HLA-B27 affects the intracellular ionic status of the SCV.
Conclusions
We have elucidated the global gene expression profile of S. Enteritidis PT4 KS8822/88 during intracellular growth in human monocyte/macrophages for the first time. The gene expression profile shown by S. Enteritidis during intracellular growth is very different from that exhibited in LB broth. However, it is similar to the patterns of gene expression reported for S. Typhimurium bacterial intracellular growth in macrophages [52]. Approximately one quarter of S. Enteritidis genes showed up- or down-regulation in host cells compared to LB broth. Among the genes up-regulated intracellularly were SPI-2 virulence genes, nutrient acquisition system genes, and ionic uptake and/or synthesis genes. Similar differences in intracellular S. Typhimurium gene expression were also seen between macrophages and medium in vitro [52]. SPI-2 genes are broadly induced to evade immune responses by host cells. Acquisition and transport system genes were elevated to take up the necessary elements. Among the down-regulated genes, some were related to central metabolism, such as the tricarboxylic acid cycle and oxidative phosphorylation, indicating that the level of oxygen is extremely low in the SCV intracellular environment, which seems contrary to the observation of intracellular S. Typhimurium growth [52]. Also, Salmonella metabolism and energy production were slower intracellularly than during growth in LB medium. These changes reflect the intracellular environment that the bacteria encounter during infection of macrophages [61].
The survival and proliferation of pathogens within macrophages is critical for establishing systemic infection, and this process is related to bacterial gene expression [62,63]. The expression of HLA-B27 in the U937 cells appeared to generate a favourable environment for intracellular Salmonella growth compared with HLA-A2-transfected cells, resulting in more bacteria in HLA-B27-transfected cells. The increased ability of Salmonella to survive is probably due to the altered gene expression. The expression of 118 genes at 8 h after infection differed significantly between HLA-B27 cells and HLA-A2 cells, suggesting that HLA-B27 modified Salmonella gene expression and/or affected the intracellular bacterial growth environment during the infection of macrophages. Expression of SPI-2 genes, which play a key role in the increased persistence of Salmonella in macrophages, was up-regulated in HLA-B27 cells compared to HLA-A2 cells. DNA replication, energy production and nutrient metabolism were also increased, suggesting that more bacterial physiological activities occurred in HLA-B27 cells. This is consistent with the increased replication and survival of Salmonella in HLA-B27-transfected macrophages. Interestingly, expression of many SPI-2 genes, energy production and nutrient metabolism are also increased in epithelial cells where Salmonella replicates intracellularly [64], whereas e.g. Cyo and Cyd terminal oxidases, which use oxygen exclusively as terminal acceptor, are strongly downregulated in Salmonella inhabiting restrictive cells, fibroblasts [65] and HLA-A2 cells (Additional file 1: Supplemental Table S2-2 and S4-2).
Mutagenesis of the SPI-2 genes demonstrated the crucial role of SPI-2 in the survival and replication of Salmonella in HLA-B27 cells, as has been seen in other macrophages [18,19]. The intracellular replication and survival of the strains bearing deletion of either ssaR or sspH2 was impaired in comparison with wild-type PT4 (Figure. 5A). Both proteins participate in the maintenance of the SCV membrane, which is important for the survival of Salmonella in infected macrophages. Increased growth of the ssaN mutant in HLA-B27-positive cells might indicate a special connection between this mutant and HLA-B27-positive cells, and may even be relevant to the pathogenesis of HLA-B27-associated ReA.
HLA-B27 modifies the host cell's signalling pathways during Salmonella infection. Our group recently demonstrated that mitogen activated protein kinase (MAPK) p38 is involved in Salmonella replication in U937 cells, and that the p38 MAPK pathway is deregulated in HLA-B27-transfected cells [33]. Salmonella uses type III secretion systems (T3SS) encoded in SPI-1 or -2 to deliver virulence proteins into host cells, which subsequently interfere with the host cell's signalling pathways [15,66,67]. Further experiments will be required to determine how Salmonella genes, particularly SPI-2 genes, affect intracellular signalling pathways and cause increased replication of bacteria in HLA-B27 cells. Nevertheless, these findings give new insights into the disturbed microbe-host interaction in HLA-B27-positive cells.
Methods
Cell lines and transfections
The human monocytic U937 cell line was obtained from ATCC (Rockville, USA). The full-length 6-kb genomic clone of human HLA-B*2705 DNA [68] in the vector pUC19 and the full-length 5.1-kb genomic clone of human HLA-A2 DNA [69] in the vector pUC9 were kind gifts from Dr. Joel D. Taurog. For transfection, U937 cells were suspended in RPMI 1640 containing 1.8 mM L-glutamine and 1 mM sodium pyruvate (Gibco, Paisley, Scotland), and were then cotransfected by electroporation with the vectors carrying either HLA-B*2705 or HLA-A2 DNA with the plasmid pSV2neo (to provide resistance to geneticin [G-418]) (CalBiochem, Darmstadt, Germany). Stable transfectants were selected with 0.5 mg/ml geneticin and the expression of transfected HLA-B27 or -A2 molecules on the surface of U937 cells was examined by immunofluorescence (IF) and flow cytometry as described previously [34]. The transfected cell lines were maintained in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS; PAA laboratories, Linz, Austria), 1.8 mM L-glutamine and 50 μg/ml gentamicin (Gm) (both from Biological Institutes, Kibbutz Beit Herennek, Israel) at 37°C in a humidified atmosphere of 5% CO2/95% air. Cell cultures were tested to confirm freedom from mycoplasma contamination.
Bacterial strains, plasmids and culture conditions
Bacterial strains and plasmids used in this study are listed in Table 2. The wild type S. Enteritidis strain was originally isolated from the stool of a patient with Salmonella-triggered ReA [34], and was typed as phage type 4 (PT4) and named S. Enteritidis PT4 KS8822/88. The S. Enteritidis and Escherichia coli strains were routinely grown in Luria-Bertani (LB) broth at 37°C. Bacteria carrying a temperature-sensitive plasmid (pKOBEGA) were grown at 30°C. Media were supplemented with 100 μg/ml ampicillin (Amp) (Sigma) and 30 μg/ml kanamycin (Km) (Sigma) as required.
Table 2.
Bacterial strains and plasmids used in this study
| Strains and plasmids | Characteristics | Source/reference |
|---|---|---|
| Strains | ||
| S. Enteritidis PT4 | Wild-type clinical isolate | 34α |
| PT4 (pKOBEGA) | PT4 containing helper plasmid pKOBEGA, AmpR | This study |
| PT4ΔssaN | PT4 ΔssaN::Km, in-frame deletion, KmR | This study |
| PT4ΔssaR | PT4 ΔssaR::Km, in-frame deletion, KmR | This study |
| PT4ΔsspH2 | PT4 ΔsspH2::Km, in-frame deletion, KmR | This study |
| E. coli MC4100 | Used as template for amplication of kanamycin resistance gene | Gift from J. M. Ghigo |
| Plasmid pKOBEGA | Vector for recombination experiments, AmpR | Gift from J. M.Ghigo |
α : S. Enteritidis strain was typed as PT4 and named as the strain KS8822/88 in this study.
Cell infection model
For infection, the U937 cells were diluted to 1.0 × 106 cells/ml and then seeded in tissue culture flasks (75 cm2) or 24-well plates (Greiner, Germany). Prior to bacterial infection, the cells were cultured with phorbol myristate acetate (PMA; Sigma) for 24 h to differentiate them toward more mature macrophage-like cells. Two hours before infection, the adherent cells were washed with Hank's balanced salt solution (HBSS) and then overlaid with prewarmed RPMI 1640 supplemented with 10% human AB serum (Finnish Red Cross, Helsinki, Finland). The cells were then co-cultured with Salmonella at a multiplicity of infection (MOI) around 50:1. After 2 h of infection, the cells were washed three times with HBSS to remove non-adherent bacteria and overlaid with fresh RPMI 1640 containing 50 μg/ml Gm to kill extracellular bacteria. The Salmonella-infected cells were then incubated at 37°C for 1, 4, 8 or 24 hours as indicated. To determine the number of living intracellular bacteria, the infected cells were scraped using a cell scraper and the amount of living host cells was counted under a microscope after staining with Trypan blue. Host cells were lysed with 1% Triton X-100 in 1 × phosphate-buffered saline (PBS) at room temperature for 5-10 min, 20 μl of the lysate in ten-fold serial dilutions in PBS were added to LB agar plates, and the numbers of bacteria were reported as CFU (colony forming units).
RNA extraction
Two methods were used to extract bacterial RNA from infected cells. At 1 or 8 h post infection, the intracellular bacteria were recovered from infected macrophages and lysed with 1% Triton X-100 [34]. The lysate was first centrifuged at 250 × g at 4°C to remove pieces of broken host cells and then the supernatant was centrifuged at 12,000 × g for 5 min to precipitate bacterial cells. The approach developed for recovering bacteria from infected cells ensured the minimum contamination of extracted bacterial RNA with eukaryotic RNA. Otherwise, infected macrophages were lysed in 0.1% SDS, 1% acidic phenol, 19% ethanol in water on ice for 30 min [60] and similar results were obtained. Total bacterial RNA was extracted and purified using the Promega SV total RNA purification kit according to the manufacturer's instructions (Promega). Control RNAs from bacteria grown in LB broth to mid-logarithmic phase and from eukaryotic cells were isolated using the same RNA purification kit. RNA integrity was monitored by agarose gel electrophoresis and using the Agilent 2100 system (Agilent). The concentration and purity of RNA was measured by spectrophotometry Bio-RAD SmartSpee™3000 (Bio-Rad).
Microarray hybridisation
The 'SALSA' Salmonella serovar microarray was developed with 5080 Salmonella genes from different serovars of Salmonella including 196 S. Enteritidis PT4 specific genes. More details are available at http://www.ifr.bbsrc.ac.uk/safety/microarrays/#protocols. A type II experiment design was used for hybridisation with labelled bacterial genomic DNA as a reference channel in each experiment [70]. The protocol for hybridisation was as previously described [52]. Briefly, total bacterial RNA (5 μg) from Salmonella grown in host cells or in LB broth was converted into cDNA using Superscript II reverse transcriptase and random primer as recommended by the manufacturer. The cDNA produced from the reverse transcription reaction and genomic DNA was subsequently fluorescently labelled using Cy5 or Cy3 dyes by random priming, with increasing labelling efficiency using Klenow enzyme. Labelled cDNA and genomic DNA were hybridized with the microarray slides overnight at 65°C. After hybridization, the slides were carefully washed, dried and scanned. All hybridizations were performed with at least three biological replicates.
Microarray data analysis
Fluorescent intensity data from each array were collected using a GenePix 4000A scanner (Axon Instruments). To compensate for unequal dye incorporation, data were centred by bringing the median natural logarithm of the ratios for each group of spots printed by the same pin to zero. The data were analyzed using GENE-SPRING™6 software (Silicon Genetics). The significance of the centred data at P = 0.05 was determined using a parametric-based statistical test, adjusting the individual P-value by the Benjamini and Hochberg false discovery rate multiple test correction.
Microarray data accession number
The supporting microarray data have been deposited in the Array Express database http://www.ebi.ac.uk/arrayexpress with accession number E-MEXP-1438.
Quantitative real time RT-PCR
Primer3 software http://frodo.wi.mit.edu[71] was used to design the primers for quantitative real time RT-PCR and purchased from Sigma (Table 3). The primers were designed to have similar melting temperatures and lengths of PCR products. About 1 μg of total RNA from the bacteria after 2 or 8 h infection of U937 cells or growth in LB medium was reverse-transcribed to 1st strand cDNA using AMV reverse transcriptase and the random primer provided in the kit following the manufacturer's instructions (1st Strand cDNA Synthesis Kit for RT-PCR (AMV)+, Roche). The resultant cDNA was used as a template during PCR, which was performed on a Roche LightCycler instrument and using the LightCycler FastStart DNA Master SYBR green I kit (Roche). The protocol for the PCR reaction was an initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 5 s, annealing at 56-64°C for 5 s and extension at 72°C for 10 s. For certain pairs of primers the annealing temperatures might be changed to optimize the yield of products. The amplified specific products were examined by melting temperature curves and agarose gel electrophoresis. Relative gene expression was quantified using a standard curve method plotted from ten-fold serial dilutions of known quantities of cDNA samples. Each standard curve for each gene was then used to transform threshold cycle (Ct) to the relative amount of unknown cDNA of the gene. The analysis was based on the Ct values of the specific genes, with rfaH as a normalizer. The amount of the specific gene was divided by the amount of rfaH in the same cDNA preparation to calculate the normalized value. Relative gene expression was presented as ratios of the normalized values of cDNA from bacterial growth in U937 cells to the growth in LB broth. All experiments were performed in triplicate using independent RNA preparations (biological replicates).
Table 3.
Primers used in the real-time RT-PCR in this study
| Genes | Primers | Sequences |
|---|---|---|
| mgtC | mgtC-F | 5'-GTCTCTGGTATTGGCTTTCTGG-3' |
| mgtC-R | 5'-TTGGCACAAAGAATAATGATCG-3' | |
| rpoE | rpoE-K | 5'-GACGCGATTGAAGCAGAAA-3' |
| rpoE-R | 5'-CCAGCTCCCGTAAGGTGAT-3' | |
| ssaG | ssaG-F | 5'-TTAGTGGATATGCTCTCCCACA-3' |
| ssaG-R | 5'-TCATTTTGATCAGTGAACTTTCG-3' | |
| fliC | fliC-F | 5'-AGCCTGTCGCTGTTGACC-3' |
| fliC-R | 5'-CGCTGCAGGTTGTGGTTG-3' | |
| fumC | fumC-F | 5'-CGGTATGGAACGCAAAGTG-3' |
| fumC-R | 5'-CTCCTGGCCTAAGGTGAGC-3' | |
| nuoA | nuoA-F | 5'-TGCTGCCTGATGCTGGTA-3' |
| unoA-R | 5'-CGCTTTCGCGGATAGAAG-3' | |
| rfaH | rfaH-F | 5'-ACTTCAGCGTGCTCAGGAA-3' |
| rfaH-R | 5'-GCGTGGCGTTGATTGTAGT-3' |
DNA manipulations and preparation of electrocompetent cells
Plasmid DNA from E. coli was purified with a Quantum Prep plasmid miniprep kit (Bio-Rad). Plasmids were transformed into the strain S. Enteritidis PT4 KS8822/88 by electroporation. Transformants carrying the Red helper plasmid were made electrocompetent as described previously [72] with some modifications. Cells were grown overnight in LB broth Amp at 30°C and then 1 ml of overnight cultures were used to inoculate 100 ml of LB broth Amp and incubation was continued to an OD600 of 0.15-0.2. L-arabinose (Sigma) was then added to a final concentration of 10 mM and incubation was continued until the OD600 reached 0.7. The suspension was cooled on ice for 20 min, and the cells were made electrocompetent by washing twice with the same volume of ice-cold water and then once with 40 ml of ice-cold 10% glycerol. The cells were finally resuspended in 1 ml of ice-cold 10% glycerol, then divided into 50 μl working aliquots and kept at -70°C for up to two months. Total genomic DNA was isolated according to the manufacturer's instructions (High Pure PCR template Preparation kit, Roche).
Construction of gene mutants by the λ Red mutagenesis method
Salmonella genes were disrupted by the method described previously [72,73]. Briefly, purified plasmid pKOBEGA was introduced into Salmonella by electroporation, and transformants were selected on LB agar Amp after incubation for 24 h at 30°C. The λ Red helper plasmid pKOBEGA is a low-copy-number plasmid that contains an ampicillin resistance gene, a temperature-sensitive origin of replication and the Red system, including three genes expressing the Exo, Bet and Gam functions of phage λ, which helps allelic exchanges between linear DNA and the corresponding region on the chromosome [74]. The Salmonella strains carrying the λ Red helper plasmid were made electrocompetent as described above. PCR linear DNA fragments were generated with specific hybrid primers and high-fidelity thermophilic DNA polymerase (Dynazyme Ext; Finnzymes). The primer pairs shown in Table 4 were used. PCR products were purified with a QIAquick PCR Purification kit (QIAGEN) and 5 μg of purified product (5-10 μl) was electroporated (25 μF, 200 Ω, 2.5 kV) into 50 μl of eletrocompetent cells according to the manufacturer's instructions (Bio-Rad), then 950 μl of LB broth was added to the shocked cells and the solution was transferred into clear eppendorf tubes and incubated for 2 h at 37°C, and then spread on to LB agar Km to select Km transformants after overnight growth at 37°C. Selected antibiotic resistance colonies were then grown on LB broth Km at 43°C for 24 h and then spread on LB agar Km or Amp at 37°C to check the loss of the helper plasmid. The mutated genes were confirmed by PCR using the primers shown in Table 4, which are external to the site of mutagenesis with the internal deletion replaced by the kanamycin cassette.
Table 4.
Primers used in the gene mutagenesis in this study
| Genes | Primers | Sequences |
|---|---|---|
| ssaN | ssaN-Km-F | 5'-CTTTGCTATCTCCTTTTACGAGTACAATCGGGCTTCACTGC |
| GGGCAGCAAGTGATGGCCTAAAGCCACGTTGTGTCTCAA-3'α | ||
| ssaN-Km-R | 5'-CTTCAGACAGTGTAAAATCGATGAATTCGCGGACTTCTCGT | |
| CCACGTTCACCAATTAACAGCGCTGAGGTCTGCCTCGTG-3'α | ||
| ssaN-F | 5'-CGTTGTTAAATGCGTGGTTG-3' | |
| ssaN-R | 5'-CCCATTCCCGTACGTTCTAA-3' | |
| ssaR | ssaR-Km-F | 5'-GCGCTTGTACTTTCCTTATTCATTATGGGGCCGACGCTATT |
| AGCTGTAAAAGAGCGCTGGAAAGCCACGTTGTGTCTCAA-3'α | ||
| ssaR-Km-R | 5'-ATTAATATGAGCAAAGAATCAGGTTTTATCTTTCTTTTTAT | |
| GTTCTTCAGGCCAGGTTCGGCGCTGAGGTCTGCCTCGTG-3'α | ||
| ssaR-F | 5'-TATCGCACTGTATGGCCTTG-3' | |
| ssaR-R | 5'-ACCGCCTGCCAGTAAAAATA-3' | |
| sspH2 | sspH2-Km-F | 5'-CTTTATGAAGTTTTCCGTCTCACTCAGTCTGTCCAGGAAGA |
| GGCTGAATGCGTCGGCGTTAAAGCCACGTTGTGTCTCAA-3'α | ||
| sspH2-Km-R | 5'-ACACTGGTTATTCCTGATAATAATCTGACCAGCCTGCCGGC | |
| GCTGCCGCCAGAACTGCGGGCGCTGAGGTTGTGTCTCAA-3'α | ||
| sspH2-F | 5'-TCATCTTCAGCCAGTTGTGC-3' | |
| sspH2-R | 5'-GTAATCGCCGCATTTATCGT-3' |
α: 80-nucleotide (nt)-long primers including 60 nt homology extensions complementary to the targeted regions of the genes and 20 nt priming sequences (underlined) for the synthesis of the kanamycin resistance cassette gene from E. coli MC4100 ybeW::Km.
Authors' contributions
SCG designed and carried out the experiments, and wrote the manuscript. VD performed the microarray tests and analyzed the data. QSH designed the experiments and analyzed the data. JCDH conceived and designed the experiments. KG conceived and designed the experiments, and wrote the manuscript. All authors reviewed the manuscript.
Supplementary Material
Supplemental Tables S1- 6 - Global gene expression of S. Enteritidis PT4 in HLA-B27- or HLA-A2-transfected cells using microarray assay. The Supplemental Tables S1-6 contain gene expression profiles of S. Enteritidis PT4 strain during infection of U937 cells (Table S1), genes up- or down-regulated more than two-fold in HLA-A2-transfected U937 cells compared to LB culture at 2 h after infection (Tables S2-1, -2), in HLA-B27-transfected cells compared to LB culture at 2 h after infection (Table S3-1,-2), in HLA-A2-transfected U937 cells compared to LB culture at 8 h after infection (Tables S4-1, -2), in HLA-B27-transfected cells compared to LB culture at 8 h after infection (Table S5-1,-2) and in HLA-B27-transfected cells compared to HLA-A2-transfected cells at 8 h after infection (Tables S6-1, -2).
Supplemental Tables S7 and S8 - The correlation analysis between PT-PCR and array data and the expression of the gene rfaH in different conditions. The Supplemental Tables S7 and S8 contain the correlation analysis between PT-PCR and array data (Table S7) and the expression of the gene rfaH in different conditions (Table S8).
Contributor Information
Shichao Ge, Email: shichaoge2004@yahoo.com.cn.
Vittoria Danino, Email: v.danino@uea.ac.uk.
Qiushui He, Email: qiuhe@utu.fi.
Jay CD Hinton, Email: jay.hinton@tcd.ie.
Kaisa Granfors, Email: kaisa.granfors@utu.fi.
Acknowledgements
We are grateful for useful discussions and advice from Jari Jalava, Yangyang Wang, Sanna Vähämiko, Anna Sahlberg, Isabelle Hautefort and Arthur Thompson. We thank Tuija Turjas, Tuula Rantasalo, Päivi Haaranen, and Erkki Nieminen for their technical assistance. We also thank Anja Siitonen for typing our S. Enteritidis strain, Joel D. Taurog for providing the HLA-B27 and HLA-A2 genomic DNA, and Jean-Marc Ghigo and Iñigo Lasa for providing E. coli MC4100 ybeW::Km, plasmid pKOBEGA and bacteriophage P22. Biomedes is acknowledged for copyediting of the manuscript.
This study was supported by grants from the Academy of Finland and the Sigrid Jusélius Foundation to Kaisa Granfors and the BBSRC core strategic grant to Jay C. D. Hinton and the DEFRA VTRI VT104 grant to Vittoria Danino.
References
- Fierer J, Guiney DG. Diverse virulence traits underlying different clinical outcomes of Salmonella infection. J Clin Invest. 2001;107:775–780. doi: 10.1172/JCI12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishu B, Koehler J, Lee LA, Rodrigue DF, Brenner H. et al. Outbreaks of S. Enteritidis infections in the United States, 1985-1991. J Infect Dis. 1994;169:547–552. doi: 10.1093/infdis/169.3.547. [DOI] [PubMed] [Google Scholar]
- Rabsch W, Tschäpe H, Bäumler AJ. Non-typhoidal salmonellosis: emerging problems. Microbes and Infect. 2001;3:237–247. doi: 10.1016/S1286-4579(01)01375-2. [DOI] [PubMed] [Google Scholar]
- Burmester GR, Daser A, Kamradt T, Krause A, Mitchison NA. et al. Immunology of reactive arthritis. Annu Rev Immunol. 1995;13:229–250. doi: 10.1146/annurev.iy.13.040195.001305. [DOI] [PubMed] [Google Scholar]
- Locht H, Molbak K, Krogfelt KA. High frequency of reactive joint symptoms after an outbreak of Salmonella Enteritidis. J Rheumatol. 2002;29:767–77. [PubMed] [Google Scholar]
- Ekman P, Kiveskari J, Granfors K. Modification of disease outcome in Salmonella-infected patients by HLA-B27. Arthritis Rheum. 2000;43:1527–34. doi: 10.1002/1529-0131(200007)43:7<1527::AID-ANR17>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Ahvonen P, Sievers K, Aho K. Arthritis associated with Yersinia enterocolitica infection. Acta Rheumatol Scand. 1969;15:232–253. doi: 10.3109/rhe1.1969.15.issue-1-4.32. [DOI] [PubMed] [Google Scholar]
- Leirisalo M, Skylv G, Kousa M, Voipio-Pulkki L, Suoranta H. et al. Followup study on patients with Reiter's disease and reactive arthritis, with special reference to HLA-B27. Arthritis Rheum. 1982;25:249–259. doi: 10.1002/art.1780250302. [DOI] [PubMed] [Google Scholar]
- Aho K, Ahvonen P, Lassus A, Sievers K, Yiilikainen A. HL-A in reactive arthritis: a study of Yersinia arthritis and Reiter's disease. Arthritis Rheum. 1974;17:521–526. doi: 10.1002/art.1780170505. [DOI] [PubMed] [Google Scholar]
- Mastroeni P. Immunity to systemic Salmonella infections. Curr Mol Med. 2002;2:393–406. doi: 10.2174/1566524023362492. [DOI] [PubMed] [Google Scholar]
- Nicolas P, Mor A. Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Annu Rev Microbiol. 1995;49:277–304. doi: 10.1146/annurev.mi.49.100195.001425. [DOI] [PubMed] [Google Scholar]
- Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang FC. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J Exp Med. 2000;192:227–236. doi: 10.1084/jem.192.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpuche-Aranda CM, Racoosin EL, Swanson JA, Miller SI. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J Exp Med. 1994;179:601–608. doi: 10.1084/jem.179.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst RK, Guina T, Miller SI. How intracellular bacteria survive: surface modification that promotes resistance to host innate immune responses. J Infect Dis. 1999;179:S326–330. doi: 10.1086/513850. [DOI] [PubMed] [Google Scholar]
- Hacker J, Blum-Oehler G, Muhldorfer I, Tschäpe H. Pathogenicity island of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- Shea JE, Hensel M, Gleeson C, Holden DW. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:2593–2597. doi: 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueck CJ. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán JE. Molecular genetic bases of Salmonella entry into host cells. Mol Microbiol. 1996;20:263–271. doi: 10.1111/j.1365-2958.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- Wallis TS, Galyov EE. Molecular basis of Salmonella-induced enteritis. Mol Microbiol. 2000;36:997–1005. doi: 10.1046/j.1365-2958.2000.01892.x. [DOI] [PubMed] [Google Scholar]
- Cirillo DM, Valdivia RH, Monack DM, Falkow S. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol Microbiol. 1998;30:175–188. doi: 10.1046/j.1365-2958.1998.01048.x. [DOI] [PubMed] [Google Scholar]
- Hensel M, Shea JE, Waterman SR, Mundy R, Nikolaus T, Banks G, Gleeson C, Fang FC, Holden DW. Genes encoding putative effector proteinsof the type III secretion systems of Salmonella pathogenicity islands 2 are required for bacterialvirulence and proliferation in macrophages. Mol Microbiol. 1998;30:163–174. doi: 10.1046/j.1365-2958.1998.01047.x. [DOI] [PubMed] [Google Scholar]
- Colmegna I, Cuchacovich R, Espinoza LR. HLA-B27-associated reactive arthritis: pathogenetic and clinical considerations. Clin Microbiol Rev. 2004;17:348–369. doi: 10.1128/CMR.17.2.348-369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltkamp TEW. Factors involved in the pathogenesis of HLA-B27 associated arthritis. Scand J Rheumatol. 1995;24(Suppl 101):213–217. doi: 10.3109/03009749509100931. [DOI] [PubMed] [Google Scholar]
- Rohekar S, Tsui FW, Tsui HW, Xi N, Riarh R, Bilotta R, Inman RD. Symptomatic acute reactive arthritis after an outbreak of salmonella. J Rheumatol. 2008;35:1599–1602. [PubMed] [Google Scholar]
- Mäki-Ikola O, Granfors K. Salmonella-triggered reactive arthiritis. Lancet. 1992;339:1096–1098. doi: 10.1016/0140-6736(92)90675-S. [DOI] [PubMed] [Google Scholar]
- Granfors K, Merilahti-Palo R, Luukkainen R, Möttönen T, Lahesmaa R. et al. Persistence of Yersinia antigens in peripheral blood cells from patients with Yersinia enterocolitica O:3 infection with or without reactive arthritis. Arthritis Rheum. 1998;41:855–862. doi: 10.1002/1529-0131(199805)41:5<855::AID-ART12>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Granfors K, Jalkanen S, Mäki-Ikola O, Lahesmaa-Rantala R, Saario R. et al. Salmonella lipopolysaccharide in synovial cells from patients with reactive arthritis. Lancet. 1990;335:685–688. doi: 10.1016/0140-6736(90)90804-E. [DOI] [PubMed] [Google Scholar]
- Granfors K, Jalkanen S, von Essen R, Lahesmaa-Rantala R, Isomaki O. et al. Yersinia antigens in synovial-fluid from patients with reactive arthritis. N Engl J Med. 1989;320:216–221. doi: 10.1056/NEJM198901263200404. [DOI] [PubMed] [Google Scholar]
- Kapasi K, Inman RD. HLA-B27 expression modulates gram-negative bacterial invasion into transfected L cells. J Immunol. 1992;148:3554–3559. [PubMed] [Google Scholar]
- Virtala M, Kirveskari J, Granfors K. HLA-B27 modulates the survival of Salmonella enteritidis in transfected L cells, possibly by impaired nitric oxide production. Infect Immun. 1997;65:4236–4242. doi: 10.1128/iai.65.10.4236-4242.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarinen M, Ekman P, Ikeda M, Virtala M, Grönberg A. et al. Invasion of Salmonella into human intestinal epithelial cells is modulated by HLA-B27. Rheumatology. 2002;41:651–657. doi: 10.1093/rheumatology/41.6.651. [DOI] [PubMed] [Google Scholar]
- Penttinen MA, Heiskanen KM, Mohapatra R, DeLay ML, Colbert RA. et al. Enhanced intracellular replication of Salmonella Enteritidis in HLA-B27-expressing human monocytic cells: dependency on glutamic acid 45 in the B pocket of HLA-B27. Arthritis Rheum. 2004;50:2255–2263. doi: 10.1002/art.20336. [DOI] [PubMed] [Google Scholar]
- Sahlberg A, Penttinen MA, Keiskanen KM, Colbert RA, Sistonen L. et al. Evidence that the p38 MAP kinase pathway is dysregualted in HLA-B27-expressing human monocytic cells: correlation with HLA-B27 misfolding. Arthritis Rheum. 2007;56:2652–2662. doi: 10.1002/art.22746. [DOI] [PubMed] [Google Scholar]
- Laitio P, Virtala M, Salmi M, Pelliniemi LJ, Yu DT. et al. HLA-B27 modulates intracellular survival of Salmonella enteritidis in human monocytic cells. Eur J Immunol. 1997;27:1331–1338. doi: 10.1002/eji.1830270606. [DOI] [PubMed] [Google Scholar]
- Buchmeier NA, Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990;248:730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- Karavolos MH, Bulmer DM, Thompson A, Winzer K, Williams P, Hinton JC, Khan CM. Adrenaline modulates the global transcriptional profiles of Salmonella revealing a role in the antimicrobial peptide and oxidative stress resistance responses. BMC Genomics. 2008;9:458–471. doi: 10.1186/1471-2164-9-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa T, Ikeda M, Yamaguchi A, Tsai WC, Tamura N. et al. Expression of arthritis-causing HLA-B27 on Hela cells promotes induction of c-fos in response to in vitro invasion by Salmonella typhimurium. J Clin Invest. 1998;101:263–272. doi: 10.1172/JCI471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Torres A, Xu Y, Jones-Carson J, Holden DW, Lucia SM. et al. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADHP oxidase. Science. 2000;287:1655–1658. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- Vazquez-Torres A, Fang FC. Salmonella evasion of the NADPH phagocyte oxidase. Microbes Infect. 2001;3:1313–1320. doi: 10.1016/S1286-4579(01)01492-7. [DOI] [PubMed] [Google Scholar]
- Babior BM. The respiratory burst oxidase. Curr Opin Hematol. 1995;2:55–60. doi: 10.1097/00062752-199502010-00008. [DOI] [PubMed] [Google Scholar]
- Mouy R, Fischer A, Vilmer E, Seger R, Griscelli C. Incidence, severity, and prevention of infections in chronic granulomatous disease. J Pediatr. 1989;114:555–560. doi: 10.1016/S0022-3476(89)80693-6. [DOI] [PubMed] [Google Scholar]
- Shiloh MU, MacMicking JD, Nicholson S, Brause JE, Potter S. et al. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/S1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- Brumell JH, Rosenberger CM, Gotto GT, Marcus SL, Finlay BB. SifA permits survival and replication of Salmonella typhimurium in murine macrophages. Cell Microbiol. 2001;3:75–84. doi: 10.1046/j.1462-5822.2001.00087.x. [DOI] [PubMed] [Google Scholar]
- Garcia-del Portillo F, Zwick MB, Leung KY, Finlay BB. Salmonella induces the formation of filamentous structures containing lysosomal membrane glycoproteins in epithelial cells. Proc Natl Acad Sci USA. 1993;90:10544–10548. doi: 10.1073/pnas.90.22.10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler LA, Steele-Mortimer O. Taking possession: biogenesis of the Salmonella-containing vacuole. Traffic. 2003;4:587–599. doi: 10.1034/j.1600-0854.2003.00118.x. [DOI] [PubMed] [Google Scholar]
- Méresse S, Unsworth KE, Habermann A, Griffiths G, Fang FC. et al. Remodelling of the actin cytoskeleton is essential for replication of intravacuolar Salmonella. Cell Microbiol. 2001;3:567–577. doi: 10.1046/j.1462-5822.2001.00141.x. [DOI] [PubMed] [Google Scholar]
- Eriksson S, Björkman J, Borg S, Syk A, Pettersson S, Andersson DI, Rhen M. Salmonella typhimurium mutants that downregulate phagocyte nitric oxide production. Cell Microbiol. 2000;2:239–250. doi: 10.1046/j.1462-5822.2000.00051.x. [DOI] [PubMed] [Google Scholar]
- Ekman P, Saarinen M, He Q, Virtala M, Salmi M, Granfors K. Human mnocytic U937 cells kill Salmonella in vitro by NO-independent mechanism. Infect Immun. 1999;67:3670–3673. doi: 10.1128/iai.67.7.3670-3673.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgers PM, Kornberg A, Sakakibara Y. The dnaN gene codes for the β subunit of DNA polymerase III holoenzyme of Escherichia coli. Proc Natl Acad Sci USA. 1981;78:5391–5395. doi: 10.1073/pnas.78.9.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancy ED, Lifsics MR, Munson P, Maurer R. Nucleotide sequences of dnaE, the gene for the polymerase subunit of DNA polymerase III in Salmonella typhimurium, and a variant that facilitates growth in the absence of another polymerase subunit. J Bacteriol. 1989;171:5581–5586. doi: 10.1128/jb.171.10.5581-5586.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd KE, Menzel R. his operons of Escherichia coli and Salmonella typhimurium are regulated by DNA supercoiling. Proc Natl Acad Sci USA. 1987;84:517–521. doi: 10.1073/pnas.84.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson S, Lucchini S, Thompson A, Rhen M, Hinton JCD. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol Microbiol. 2003;47:103–118. doi: 10.1046/j.1365-2958.2003.03313.x. [DOI] [PubMed] [Google Scholar]
- Garcia-del Protillo F. Salmonella intracellular proliferation: where, when and how? Microbes infect. pp. 1305–1311. [DOI] [PubMed]
- Reitzer LJ, Magasanil B. Asparagine synthetases of Klebsiella aerogenes: properties and regulation of synthesis. J Bacteriol. 1982;151:1299–1313. doi: 10.1128/jb.151.3.1299-1313.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce JD, Wilkie I, Harper M, Paustian ML, Kapur V. et al. Genomic scale analysis of Pasteurella multocida gene expression during growth within the natural chicken host. Infect Immun. 2002;70:6871–6879. doi: 10.1128/IAI.70.12.6871-6879.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RL, Kaczmarek MT, Kucharski LM, Maguire ME. Magnesuim transport in Salmonella typhimurium: regulation of mgtA and mgtCB during invasion of epithelial and macrophage cells. Microbiology. 1998;144:1835–1843. doi: 10.1099/00221287-144-7-1835. [DOI] [PubMed] [Google Scholar]
- Snavely MD, Miller CG, Maguire ME. The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. J Biol Chem. 1991;266:815–823. [PubMed] [Google Scholar]
- Blanc-Potard AB, Groisman EA. The Salmonella selC locus contains a pathogenicity island mediating intramacrophage survival. EMBO J. 1997;16:5376–5385. doi: 10.1093/emboj/16.17.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney KE, Valvano MA. The mgtC gene of Burkholderia cenocepacia is required for growth under magnesium limitation conditions and intracellular survival in macrophages. Infect Immun. 2006;74:5477–5486. doi: 10.1128/IAI.00798-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiwick J, Nikolaus T, Erdogan S, Hensel M. Environmental regulation of Salmonella pathogenicity island 2 gene expression. Mol Micobiol. 1999;31:1759–1773. doi: 10.1046/j.1365-2958.1999.01312.x. [DOI] [PubMed] [Google Scholar]
- Buchmeier NA, Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990;248:730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- Fields PI, Swanson RV, Haidaris CG, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl ME, Miller SI. Salmonella: a model for bacterial pathogenesis. Annu Rev Med. 2001;52:259–274. doi: 10.1146/annurev.med.52.1.259. [DOI] [PubMed] [Google Scholar]
- Hautefort I, Thompson A, Eriksson-Ygberg S, Parker ML, Lucchini S, Danino V, Bongaerts RJ, Ahmad N, Rhen M, Hinton JC. During infection of epithelial cells Salmonella enterica serovar Typhimurium undergoes a time-dependent transcriptional adaptation that results in simultaneous expression of three type 3 secretion systems. Cell Microbiol. 2008;10:958–984. doi: 10.1111/j.1462-5822.2007.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-del Portillo F, Núñez-Hernández C, Eisman B, Ramos-Vivas J. Growth control in the Salmonella -containing vacuole. Curr Opin Microbiol. 2008;11:46–52. doi: 10.1016/j.mib.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Mecsas J, Strauss EJ. Molecular mechanisms of bacterial virulence: Type III secretion and pathogenicity islands. Emerg Infect Dis. 1996;30:271–288. doi: 10.3201/eid0204.960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiya K, Groisman EA, Nikai T. Involvement of Salmonella pathogenicity island 2 in the up-regulation of interleukin-10 expression in macrophages: role of protein kinase A signal pathway. Infect Immun. 2004;72:1964–1973. doi: 10.1128/IAI.72.4.1964-1973.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurog JD, Lowen L, Forman J, Hammer RE. HLA-B27 in inbred and non-inbred transgenic mice: cell surface expression and recognition as an alloantigen in the absence of human β2-microglobulin. J Immunol. 1988;141:4020–4023. [PubMed] [Google Scholar]
- Koller BH, Orr HT. Cloning and complete sequence of an HLA-A2 gene: analysis of two HLA-A alleles at the nucleotide level. Immunol. 1985;134:727–2733. [PubMed] [Google Scholar]
- Yang YH, Speed T. Design issues for cDNA microarray experiments. Nat Rew Genet. 2002;3:579–588. doi: 10.1038/nrg863. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biological programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Latasa C, Roux A, Toledo-Arana A, Ghigo JM, Gamazo C. et al. BapA, a large secreted protein required for biofilm formation and host colonization of Salmonella enterica serovar Enteritidis. Mol Microbiol. 2005;58:1322–1339. doi: 10.1111/j.1365-2958.2005.04907.x. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaveroche MK, Ghigo JM, d'Enfert C. A rapid method for efficient gene replacement in the filamentous fungus Aspergillus nidulans. Nucleic Acids Res. 2000;28:E97. doi: 10.1093/nar/28.22.e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables S1- 6 - Global gene expression of S. Enteritidis PT4 in HLA-B27- or HLA-A2-transfected cells using microarray assay. The Supplemental Tables S1-6 contain gene expression profiles of S. Enteritidis PT4 strain during infection of U937 cells (Table S1), genes up- or down-regulated more than two-fold in HLA-A2-transfected U937 cells compared to LB culture at 2 h after infection (Tables S2-1, -2), in HLA-B27-transfected cells compared to LB culture at 2 h after infection (Table S3-1,-2), in HLA-A2-transfected U937 cells compared to LB culture at 8 h after infection (Tables S4-1, -2), in HLA-B27-transfected cells compared to LB culture at 8 h after infection (Table S5-1,-2) and in HLA-B27-transfected cells compared to HLA-A2-transfected cells at 8 h after infection (Tables S6-1, -2).
Supplemental Tables S7 and S8 - The correlation analysis between PT-PCR and array data and the expression of the gene rfaH in different conditions. The Supplemental Tables S7 and S8 contain the correlation analysis between PT-PCR and array data (Table S7) and the expression of the gene rfaH in different conditions (Table S8).