Abstract
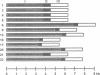
The maxicircle of the parasitic protozoan Trypanosoma brucei, one component of the mitochondrial genome, has size differences among isolates that localize to the variable region (VR) between the ND5 and 12S rRNA genes. We present here the nucleotide sequence of this entire region, thus completing the sequence of the maxicircle genome. We also find heterogeneously sized transcripts from throughout most of the VR. The VR has three distinct sections, each with characteristic repeated sequences. The repeated sequences in two sections are short and highly reiterated; the intraspecies size variation occurs within this region. The third section contains non-repetitive sequences and a large duplication immediately upstream of the 12S rRNA gene. Two repeat units within section I contain a sequence that has homology to the DNA replication origin of minicircles. This region also contains sequences with homology to topoisomerase II binding and cleavage sites. These findings suggest a role for the VR in DNA replication of the maxicircle.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benne R. RNA editing in trypanosomes: is there a message? Trends Genet. 1990 Jun;6(6):177–181. doi: 10.1016/0168-9525(90)90173-4. [DOI] [PubMed] [Google Scholar]
- Blum B., Bakalara N., Simpson L. A model for RNA editing in kinetoplastid mitochondria: "guide" RNA molecules transcribed from maxicircle DNA provide the edited information. Cell. 1990 Jan 26;60(2):189–198. doi: 10.1016/0092-8674(90)90735-w. [DOI] [PubMed] [Google Scholar]
- Borst P., Fase-Fowler F., Hoeijmakers J. H., Frasch A. C. Variations in maxi-circle and mini-circle sequences in kinetoplast DNAs from different Trypanosoma brucei strains. Biochim Biophys Acta. 1980 Dec 11;610(2):197–210. doi: 10.1016/0005-2787(80)90001-5. [DOI] [PubMed] [Google Scholar]
- Borst P., Weijers P. J., Brakenhoff G. J. Analysis by electron microscopy of the variable segment in the maxi-circle of kinetoplast DNA from Trypanosoma brucei. Biochim Biophys Acta. 1982 Dec 31;699(3):272–280. doi: 10.1016/0167-4781(82)90117-8. [DOI] [PubMed] [Google Scholar]
- Cabot E. L., Beckenbach A. T. Simultaneous editing of multiple nucleic acid and protein sequences with ESEE. Comput Appl Biosci. 1989 Jul;5(3):233–234. doi: 10.1093/bioinformatics/5.3.233. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. A novel endoribonuclease cleaves at a priming site of mouse mitochondrial DNA replication. EMBO J. 1987 Feb;6(2):409–417. doi: 10.1002/j.1460-2075.1987.tb04770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Feagin J. E., Jasmer D. P., Stuart K. Apocytochrome b and other mitochondrial DNA sequences are differentially expressed during the life cycle of Trypanosoma brucei. Nucleic Acids Res. 1985 Jun 25;13(12):4577–4596. doi: 10.1093/nar/13.12.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagin J. E., Stuart K. Differential expression of mitochondrial genes between life cycle stages of Trypanosoma brucei. Proc Natl Acad Sci U S A. 1985 May;82(10):3380–3384. doi: 10.1073/pnas.82.10.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. W. Origin and evolution of mitochondrial DNA. Annu Rev Cell Biol. 1989;5:25–50. doi: 10.1146/annurev.cb.05.110189.000325. [DOI] [PubMed] [Google Scholar]
- Hajduk S. L., Klein V. A., Englund P. T. Replication of kinetoplast DNA maxicircles. Cell. 1984 Feb;36(2):483–492. doi: 10.1016/0092-8674(84)90241-1. [DOI] [PubMed] [Google Scholar]
- Hajduk S., Adler B., Bertrand K., Fearon K., Hager K., Hancock K., Harris M., Le Blanc A., Moore R., Pollard V. Molecular biology of African trypanosomes: development of new strategies to combat an old disease. Am J Med Sci. 1992 Apr;303(4):258–270. doi: 10.1097/00000441-199204000-00011. [DOI] [PubMed] [Google Scholar]
- Hancock K., Hajduk S. L. The mitochondrial tRNAs of Trypanosoma brucei are nuclear encoded. J Biol Chem. 1990 Nov 5;265(31):19208–19215. [PubMed] [Google Scholar]
- Higgins D. G., Bleasby A. J., Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992 Apr;8(2):189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- Hughes D., Simpson L., Kayne P. S., Neckelmann N. Autonomous replication sequences in the maxicircle kinetoplast DNA of Leishmania tarentolae. Mol Biochem Parasitol. 1984 Nov;13(3):263–275. doi: 10.1016/0166-6851(84)90118-x. [DOI] [PubMed] [Google Scholar]
- Jasmer D. P., Feagin J. E., Payne M., Stuart K. Variation of G-rich mitochondrial transcripts among stocks of Trypanosoma brucei. Mol Biochem Parasitol. 1987 Jan 15;22(2-3):259–272. doi: 10.1016/0166-6851(87)90057-0. [DOI] [PubMed] [Google Scholar]
- Jasmer D. P., Feagin J. E., Stuart K. Diverse patterns of expression of the cytochrome c oxidase subunit I gene and unassigned reading frames 4 and 5 during the life cycle of Trypanosoma brucei. Mol Cell Biol. 1985 Nov;5(11):3041–3047. doi: 10.1128/mcb.5.11.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. J., Hill G. C., Fox T. D., Stuart K. The maxicircle of Trypanosoma brucei kinetoplast DNA hybridizes with a mitochondrial gene encoding cytochrome oxidase subunit II. Mol Biochem Parasitol. 1982 Jun;5(6):381–390. doi: 10.1016/0166-6851(82)90011-1. [DOI] [PubMed] [Google Scholar]
- Kim R., Ray D. S. Conservation of a 29-base-pair sequence within maxicircle ARS fragments from six species of trypanosomes. Gene. 1985;40(2-3):291–299. doi: 10.1016/0378-1119(85)90052-6. [DOI] [PubMed] [Google Scholar]
- Kusukawa N., Uemori T., Asada K., Kato I. Rapid and reliable protocol for direct sequencing of material amplified by the polymerase chain reaction. Biotechniques. 1990 Jul;9(1):66-8, 70, 72. [PubMed] [Google Scholar]
- Maslov D. A., Kolesnikov A. A., Zaitseva G. N. Conservative and divergent base sequence regions in the maxicircle kinetoplast DNA of several trypanosomatid flagellates. Mol Biochem Parasitol. 1984 Jul;12(3):351–364. doi: 10.1016/0166-6851(84)90091-4. [DOI] [PubMed] [Google Scholar]
- Michelotti E. F., Harris M. E., Adler B., Torri A. F., Hajduk S. L. Trypanosoma brucei mitochondrial ribosomal RNA synthesis, processing and developmentally regulated expression. Mol Biochem Parasitol. 1992 Aug;54(1):31–41. doi: 10.1016/0166-6851(92)90092-x. [DOI] [PubMed] [Google Scholar]
- Muhich M. L., Neckelmann N., Simpson L. The divergent region of the Leishmania tarentolae kinetoplast maxicircle DNA contains a diverse set of repetitive sequences. Nucleic Acids Res. 1985 May 10;13(9):3241–3260. doi: 10.1093/nar/13.9.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhich M. L., Simpson L., Simpson A. M. Comparison of maxicircle DNAs of Leishmania tarentolae and Trypanosoma brucei. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4060–4064. doi: 10.1073/pnas.80.13.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myler P. J., Allison J., Agabian N., Stuart K. Antigenic variation in African trypanosomes by gene replacement or activation of alternate telomeres. Cell. 1984 Nov;39(1):203–211. doi: 10.1016/0092-8674(84)90206-x. [DOI] [PubMed] [Google Scholar]
- Ntambi J. M., Englund P. T. A gap at a unique location in newly replicated kinetoplast DNA minicircles from Trypanosoma equiperdum. J Biol Chem. 1985 May 10;260(9):5574–5579. [PubMed] [Google Scholar]
- Ray D. S. Conserved sequence blocks in kinetoplast minicircles from diverse species of trypanosomes. Mol Cell Biol. 1989 Mar;9(3):1365–1367. doi: 10.1128/mcb.9.3.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read L. K., Myler P. J., Stuart K. Extensive editing of both processed and preprocessed maxicircle CR6 transcripts in Trypanosoma brucei. J Biol Chem. 1992 Jan 15;267(2):1123–1128. [PubMed] [Google Scholar]
- Ryan K. A., Shapiro T. A., Rauch C. A., Englund P. T. Replication of kinetoplast DNA in trypanosomes. Annu Rev Microbiol. 1988;42:339–358. doi: 10.1146/annurev.mi.42.100188.002011. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L., Neckelmann N., de la Cruz V. F., Simpson A. M., Feagin J. E., Jasmer D. P., Stuart K. Comparison of the maxicircle (mitochondrial) genomes of Leishmania tarentolae and Trypanosoma brucei at the level of nucleotide sequence. J Biol Chem. 1987 May 5;262(13):6182–6196. [PubMed] [Google Scholar]
- Stohl L. L., Clayton D. A. Saccharomyces cerevisiae contains an RNase MRP that cleaves at a conserved mitochondrial RNA sequence implicated in replication priming. Mol Cell Biol. 1992 Jun;12(6):2561–2569. doi: 10.1128/mcb.12.6.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart K. D., Gelvin S. B. Localization of kinetoplast DNA maxicircle transcripts in bloodstream and procyclic form Trypanosoma brucei. Mol Cell Biol. 1982 Jul;2(7):845–852. doi: 10.1128/mcb.2.7.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart K., Gelvin S. R. Kinetoplast DNA of normal and mutant Trypanosoma brucei. Am J Trop Med Hyg. 1980 Sep;29(5 Suppl):1075–1081. doi: 10.4269/ajtmh.1980.29.1075. [DOI] [PubMed] [Google Scholar]
- Stuart K., Gobright E., Jenni L., Milhausen M., Thomashow L., Agabian N. The IsTaR 1 serodeme of Trypanosoma brucei: development of a new serodeme. J Parasitol. 1984 Oct;70(5):747–754. [PubMed] [Google Scholar]
- Stuart K. Kinetoplast DNA OF Trypanosoma brucei: physical map of the maxicircle. Plasmid. 1979 Oct;2(4):520–528. doi: 10.1016/0147-619x(79)90051-9. [DOI] [PubMed] [Google Scholar]
- Stuart K. Kinetoplast DNA, mitochondrial DNA with a difference. Mol Biochem Parasitol. 1983 Oct;9(2):93–104. doi: 10.1016/0166-6851(83)90103-2. [DOI] [PubMed] [Google Scholar]
- Stuart K. Mitochondrial DNA of an African trypanosome. J Cell Biochem. 1983;23(1-4):13–26. doi: 10.1002/jcb.240230103. [DOI] [PubMed] [Google Scholar]
- Stuart K. RNA editing in trypanosomatid mitochondria. Annu Rev Microbiol. 1991;45:327–344. doi: 10.1146/annurev.mi.45.100191.001551. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper J. N., Bennett J. L., Clayton D. A. A role for RNAase MRP in mitochondrial RNA processing. Cell. 1992 Jul 10;70(1):16–20. doi: 10.1016/0092-8674(92)90529-l. [DOI] [PubMed] [Google Scholar]
- de Vries B. F., Mulder E., Brakenhoff J. P., Sloof P., Benne R. The variable region of the Trypanosoma brucei kinetoplast maxicircle: sequence and transcript analysis of a repetitive and a non-repetitive fragment. Mol Biochem Parasitol. 1988 Jan 1;27(1):71–82. doi: 10.1016/0166-6851(88)90026-6. [DOI] [PubMed] [Google Scholar]
- van der Spek H., Arts G. J., Zwaal R. R., van den Burg J., Sloof P., Benne R. Conserved genes encode guide RNAs in mitochondria of Crithidia fasciculata. EMBO J. 1991 May;10(5):1217–1224. doi: 10.1002/j.1460-2075.1991.tb08063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]