Abstract
Background
Behavior is a complex process resulting from the integration of genetic and environmental information. Drosophila melanogaster rely on multiple sensory modalities for reproductive success, and mating causes physiological changes in both sexes that affect reproductive output or behavior. Some of these effects are likely mediated by changes in gene expression. Courtship and mating alter female transcript profiles, but it is not known how mating affects male gene expression.
Results
We used Drosophila genome arrays to identify changes in gene expression profiles that occur in mated male heads. Forty-seven genes differed between mated and control heads 2 hrs post mating. Many mating-responsive genes are highly expressed in non-neural head tissues, including an adipose tissue called the fat body. One fat body-enriched gene, female-specific independent of transformer (fit), is a downstream target of the somatic sex-determination hierarchy, a genetic pathway that regulates Drosophila reproductive behaviors as well as expression of some fat-expressed genes; three other mating-responsive loci are also downstream components of this pathway. Another mating-responsive gene expressed in fat, Juvenile hormone esterase (Jhe), is necessary for robust male courtship behavior and mating success.
Conclusions
Our study demonstrates that mating causes changes in male head gene expression profiles and supports an increasing body of work implicating adipose signaling in behavior modulation. Since several mating-induced genes are sex-determination hierarchy target genes, additional mating-responsive loci may be downstream components of this pathway as well.
Background
Behavior involves the perception and processing of sensory information into a signaling cascade that mediates physiological and motor outputs. This complex process is influenced by an organism's environment, genetic make-up and nervous system function. Social interactions influence an organism's behavior [1-5], and these behavioral changes are associated with alterations in morphology [6-9] and gene expression [6,10-17]. However, the mechanisms mediating the changes are unclear. As we work to understand responses to behavior at the transcript level, we can clarify the regulatory and intracellular processes governing nervous system function and behavior.
Therefore, we are studying reproductive behaviors in the genetically tractable Drosophila melanogaster, which exhibit stereotypical mating behaviors [reviewed in [18,19]] regulated by genetics [reviewed in [20,21]] and social interactions [[1,22,23]; reviewed in [19,24,25]]. The sex-determination gene hierarchy is the major regulator of Drosophila reproduction [reviewed in [26,27]]. Components of this pathway affect sexually dimorphic development, including the neural circuitries necessary for sex-specific courtship behaviors [28-32]. However, the behavioral functions of only a few of the downstream target genes of the hierarchy are known [33-43].
Although the potential for performing courtship behavior is under genetic control, experience with other individuals alters behavior, particularly in the context of courtship learning [19,24,25]. During courtship and mating, the male is inundated with sensory information that must be interpreted so that the appropriate signals are sent throughout the body for a successful mating. Therefore, it is reasonable to expect that a more experienced male would be better at performing some aspect of courtship to improve his mating success. In support of this idea, Drosophila males experienced at courting females initiate courtship toward novel, receptive females more quickly than do inexperienced males [44,45]. In a natural setting where many flies are competing for mates, rapid courtship initiation may give an experienced male a competitive advantage that increases his mating success. Simply observing courtship and mating behavior of other flies is not sufficient to decrease the male's own mating latency, indicating that this learning behavior requires active participation [45]. It is possible that changes in courting and mated male gene expression underlie this decreased courtship latency in subsequent interactions.
By combining behavioral assays with microarray technology, it is possible to assess behaviorally-responsive gene expression changes on a genome-wide scale [12,22,46-51] to find loci regulating or regulated by behavior, including sex-determination hierarchy target genes. Prior work in our lab demonstrated that males rapidly alter gene expression at the whole-animal level during courtship [12,22]. Next, we focused on changes occurring in the male head as a result of mating since these changes likely affect function of the nervous system and other reproductively important tissues to promote reproductive success. Our study demonstrates that courtship culminating in mating affects gene expression patterns in male heads and that many of the gene products are expressed in non-neural adipose tissue that may play an important modulatory role in neural function and behavior.
Results and Discussion
Mating causes expression changes in male heads
Gene expression levels change rapidly as males court females [12,22]. To determine the effects of courtship culminating in mating on male gene expression, we compared transcriptional profiles of males that mated with a female to those that were not presented with a female (control). Labeled samples from control and treatment groups were hybridized to Drosophila Genome 2.0 Arrays (Affymetrix, Santa Clara, CA, USA), which are based on the Flybase 3.1 annotation, targeting nearly 18,500 transcripts.
In the current study we focused on head expression, rather than whole body expression [12,22], to identify gene expression changes in the nervous system and other tissues within the head (such as sensory systems and fat body) that likely modulate reproduction. We isolated male heads (rather than dissecting out the brains) since accumulating evidence from our lab [[12,22]; L.L. Ellis and G.E. Carney, unpublished results] as well as from other published studies [[37,39,40,43]; reviewed in [52]] indicates that head tissues, such as the fat body surrounding the brain (Fig. 1), likely also have important modulatory functions in behavior. To have the potential to identify gene expression changes in these tissues as well, we elected to assay the entire male head for alterations in gene expression patterns in response to mating.
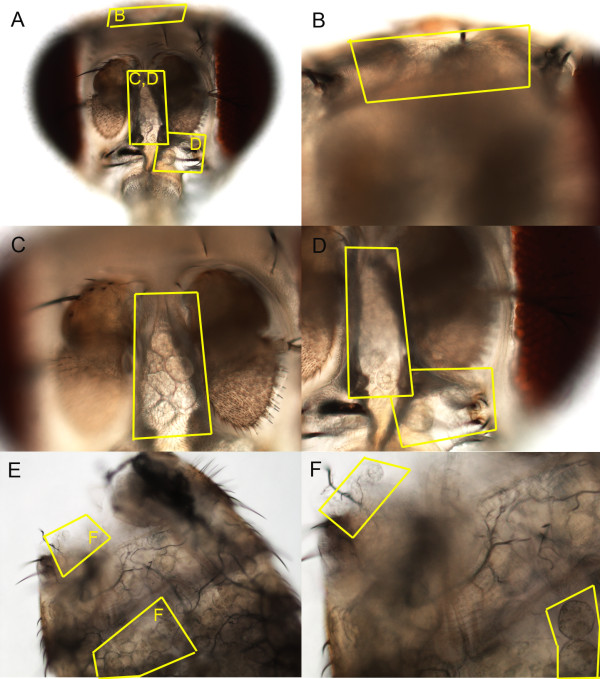
Figure 1.
Fat body tissue in the adult male. Low magnification image (10×) from the front of an adult male head (A) or dorsal abdominal cuticle (E). Boxed areas indicate adipose tissue magnified at 20× in 3 areas of the head (B-D) and 2 areas along the abdominal cuticle (F).
We used five algorithms to extract expression values from each array and performed paired t-test comparisons between the expression values derived from mated male head arrays and control male head arrays. Using this strategy we identified 47 mating-responsive genes (See Methods). Two hours after mating with a female, males significantly up regulated 25 genes (Table 1) and down regulated 22 genes (Table 2). Such changes are not likely to be activity-dependent since control males had locomotor levels similar to males that courted females (two-tailed t-test, p > 0.05).
Table 1.
Candidate genes up regulated 2 hrs after mating
| Gene identifier | Gene name | Avg. fold change | GO Molecular function | GO Biological process |
|---|---|---|---|---|
| CG2163 | polyA-binding protein II (Pabp2) | 1.4 | Poly(A) binding | mRNA polyadenylation |
| CG4288 | 1.28 | High affinity inorganic phosphate: sodium symporter activity | Transport | |
| CG4501 | bubblegum (bgm) | 1.38 | Long-chain-fatty acid-CoA ligase activity | Long-chain fatty acid metabolic process |
| CG4825 | Phosphatidyl-serine synthase | 1.22 | CDP-diacylglycerol-serine O-phosphatidyltrans-ferase activity | Phosphatidyl-serine biosynthetic process |
| CG5527 | 1.23 | Endothelin-converting enzyme activity | Proteolysis | |
| CG5618 | 1.14 | Dipeptidyl-peptidase III activity | Proteolysis | |
| CG6188 | 1.64 | Glycine N-methyltransferase activity | Unknown | |
| CG6342 | Iron regulatory protein 1B (Irp-1B) | 1.26 | Iron ion binding | Regulation of translational initiation by iron |
| CG8425 | Juvenile hormone esterase (Jhe) | 1.86 | Juvenile-hormone esterase activity | Juvenile hormone catabolic process |
| CG8449 | 1.28 | Rab GTPase activator activity | Regulation of Rab GTPase activity | |
| CG9989 | 1.52 | Endonuclease activity | Unknown | |
| CG11765 | Peroxiredoxin 2540 (Prx2540-2) | 1.2 | Antioxidant activity | Unknown |
| CG12116 | 1.22 | Sepiapterin reductase activity | Metabolic process | |
| CG13360 | 1.28 | Unknown | Unknown | |
| CG13607 | 1.23 | Unknown | Unknown | |
| CG13965 | 1.35 | Unknown | Unknown | |
| CG16772 | 1.5 | Unknown | Unknown | |
| CG16901 | squid (sqd) | 1.25 | mRNA binding | Oocyte axis determination |
| CG17364 | 1.67 | GTP binding | Microtubule-based process | |
| CG17820 | female-specific independent of transformer (fit) | 1.4 | Unknown | Unknown |
| CG18262 | 1.3 | Zinc ion binding | Unknown | |
| CG30026 | 1.42 | Unknown | Unknown | |
| CG30095 | 1.86 | Oxidoreductase activity | Metabolic process | |
| CG30084 | Z band alternatively spliced PDZ-motif protein 52 (Zasp52) | 1.38 | Protein binding | Unknown |
| CG33486 | asparagine synthetase | 1.28 | Asparagine synthetase (glutamine-hydrolyzing) activity | Asparagine biosynthetic process |
Twenty-five genes are significantly (p < 0.001) up regulated in male heads 2 hrs after mating when compared to control male heads.
Table 2.
Candidate genes down regulated 2 hrs after mating
| Gene identifier | Gene name | Avg. fold change | GO Molecular function | GO Biological process |
|---|---|---|---|---|
| CG1897 | Drop (Dr) | -1.5 | DNA binding | Central nervous system development |
| CG2505 | α-Esterase-2 (α-Est2) | -1.3 | Carboxylesterase activity | Unknown |
| CG3200 | Rhythmically expressed gene 2 (Reg-2) | -1.27 | Phosphoglycolate phosphatase activity | Metabolic process |
| CG3926 | Serine pyruvate aminotrans-ferase (Spat) | -1.34 | Serine-pyruvate transamine activity | Glyoxylate catabolic process |
| CG4105 | Cytochrome P450-4e3 (Cyp4e3) | -1.3 | Electron carrier activity | Unknown |
| CG5840 | -1.34 | Pyrroline-5-carboxylate reductase activity | Proline biosynthetic process | |
| CG6806 | Larval serum protein 2 (Lsp2) | -1.28 | Nutrient reservoir activity | Transport |
| CG7224 | -1.16 | Unknown | Unknown | |
| CG7390 | senescence marker protein-30 (smp-30) | -1.36 | Unknown | Unknown |
| CG8112 | -1.42 | Sterol O-acyltransferase activity | Unknown | |
| CG8846 | Thor | -1.26 | Eukaryotic initation factor 4E binding | Immune response |
| CG9416 | -1.25 | Sequence-specific DNA binding | Regulation of transcription | |
| CG9733 | -1.6 | Trypsin activity | Proteolysis | |
| CG11909 | target of brain insulin (tobi) | -1.42 | α-glucosidase activity | Carbohydrate metabolic process |
| CG11919 | -1.36 | ATP binding | Peroxisome organization and biogenesis | |
| CG16898 | -1.68 | Unknown | Unknown | |
| CG18003 | -1.36 | Glycolate oxidase activity | Metabolic process | |
| CG30489 | Cyp12d1-p | -1.3 | Electron carrier activity | Unknown |
| CG31075 | -1.26 | Aldehyde dehydrogenase (NAD) activity | Pyruvate metabolic process | |
| CG31628 | adenosine 3 (ade3) | -1.28 | Phosphoribo-sylamine-glycine ligase activity | Purine base biosynthetic process |
| CG31689 | -1.25 | ATPase activity | Unknown | |
| CG33462 | -4.08 | Trypsin activity | Proteolysis |
Average fold changes, molecular functions and biological processes are shown for 22 genes that are significantly (p < 0.001) down regulated in male heads 2 hrs after mating.
Verification of microarray results by independent qPCR
To confirm the microarray results, we performed qPCR analysis on independently collected mated and control male head RNA samples. We tested a subset of genes whose expression levels changed significantly in mated male heads compared to control male heads. Eight out of 10 up-regulated genes and 2 out of 3 down-regulated genes had the expected directional change (Table 3). We did not verify up regulation of CG4825 and fit or down regulation of CG8112 by qPCR. However, increased fit expression in the fat body lining the brain was confirmed by in situ hybridization (see below).
Table 3.
Confirmation of microarray results by qPCR
| Gene identifier | Gene symbol | Microarray Fold change | qPCR Relative fold change ± SEM | Avg. relative expression level in control male heads ± SEM | Avg. relative expression level in mated male heads ± SEM |
|---|---|---|---|---|---|
| CG5618 | 1.14 | 2.02 ± 0.49* | 0.36 ± 0.09 | 0.74 ± 0.18 | |
| CG6188 | 1.64 | 1.94 ± 0.26* | 2.25 ± 0.42 | 4.38 ± 0.58 | |
| CG8449 | 1.28 | 1.35 ± 0.15* | 1.24 ± 0.26 | 1.68 ± 0.18 | |
| CG16772 | 1.5 | 4.07 ± 1.55 | 6.86 ± 1.72 | 27.94 ± 10.61 | |
| CG30026 | 1.42 | 2.23 ± 0.35* | 4.36 ± 0.84 | 9.74 ± 1.53 | |
| CG4501 | bgm | 1.38 | 4.47 ± 1.11* | 1.42 ± 0.31 | 6.37 ± 1.57 |
| CG6342 | Irp-1B | 1.26 | 1.42 ± 0.12 | 1.09 ± 0.18 | 1.55 ± 0.13 |
| CG11765 | Prx2540-2 | 1.2 | 1.23 ± 0.12* | 0.47 ± 0.08 | 0.58 ± 0.06 |
| CG2505 | αEst2 | -1.3 | -1.26 ± 0.15 | 2.84 ± 0.59 | 2.25 ± 0.42 |
| CG7390 | smp-30 | -1.36 | -1.69 ± 0.16* | 1.28 ± 0.45 | 0.77 ± 0.2 |
* Indicates a significant (p < 0.05) difference between the average relative expression level in control male heads and mated male heads. SEM = Standard error of the mean.
Expression of candidate genes is not restricted to the brain
We hypothesized that examining gene expression in head tissue instead of whole bodies would uncover genes that function in reproduction by regulating nervous system signaling. This could be via direct effects on neural gene expression or by effects on other tissues in the head that receive or respond to courtship and mating signals. We found that expression of many mating-responsive genes is enriched in the head but not the brain (Table 4) [53], indicating expression occurs outside of the brain. While some of the genes are expressed in the eye, others appear enriched in tissues other than the brain and eye.
Table 4.
Candidate genes are enriched in head tissue other than the brain, including adult adipose tissue
| Total no. of genes | Head | Brain | Eye | Fat body | |
|---|---|---|---|---|---|
| Up regulated | 25 | 18 | 4 | 9 | 16 |
| Down regulated | 22 | 20 | 2 | 12 | 18 |
Data was compiled from FlyAtlas [53].
One possibility is that they are expressed in an adipose tissue called the fat body that lines the head cavity surrounding the brain (Fig. 1a-d) and is implicated in courtship behavior modulation [[37,39,40,43]; reviewed in [52]]. Data showing that mating-responsive genes enriched in the head are also enriched in the adult fat body (Table 4) [53] support this hypothesis. In situ hybridization confirmed that several mating-responsive loci (CG13360, bubblegum (bgm), Prx2540-2, CG8449 and CG4825) are expressed in male fat body tissue (Fig. 2).
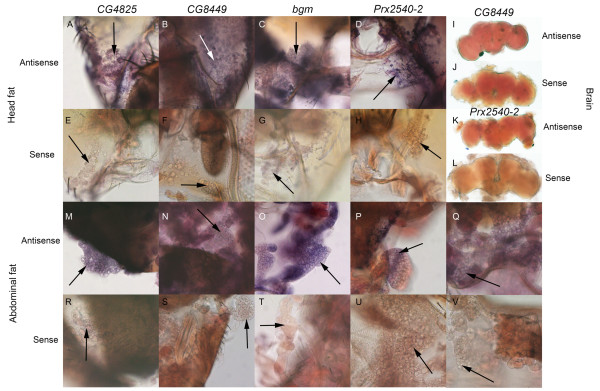
Figure 2.
Candidate genes are expressed in fat tissue. Antisense (A-D,I,K,M-Q) or sense (E-H,J,L,R-V) RNA probes were designed to cDNA clones for CG4825 (A,E,M,R), CG8449 (B,F,I,J,N,S), bgm (C,G,O,T), Prx2540-2 (D,H,K,L,P,U), and CG13360 (Q,V). In situ hybridization to whole-mount tissue showed candidate gene expression in male CS fat body tissue (arrows) present on head (A-H) and abdominal (M-V) cuticle. Purple reactivity indicates presence of transcripts. Brains (I-L) showed light pink background staining but lacked detectable expression of either CG8449 (I,J) or Prx2540-2 (K,L).
FlyAtlas data indicate that the fat-expressed genes CG13360, bgm, and Prx2540-2 are expressed at very low levels in brains, while CG8449 and CG4825 are expressed at low to moderate levels in the brain [53]. By in situ we did not detect brain expression of these five transcripts (Fig. 2 and data not shown), although we cannot rule out the possibility that low levels of message are present.
To test the hypothesis that mating-induced changes in gene expression occur in the fat body, we compared fit expression in the heads of mated and control heads. fit expression increased in the adipose tissue surrounding the male brain after courtship culminating in mating (Fig. 3).
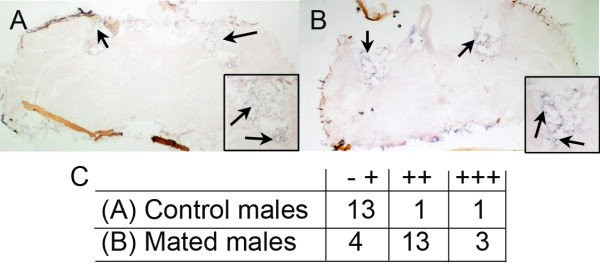
Figure 3.
fit expression in the fat body is increased after courtship culminating in mating. In situ hybridization was performed on cryosectioned male heads and confirmed that fit transcript levels were up regulated in adipose tissue (arrows) of mated males (panel B) compared to control males (panel A) as indicted by increased intensity of purple staining in mated male fat body. Insets in panels A and B show magnified views of head fat. Qualitative assessment of signal intensity in both treatment groups indicates that fit expression increased in mated male heads (panel C).
Juvenile hormone esterases are important for male reproductive behaviors
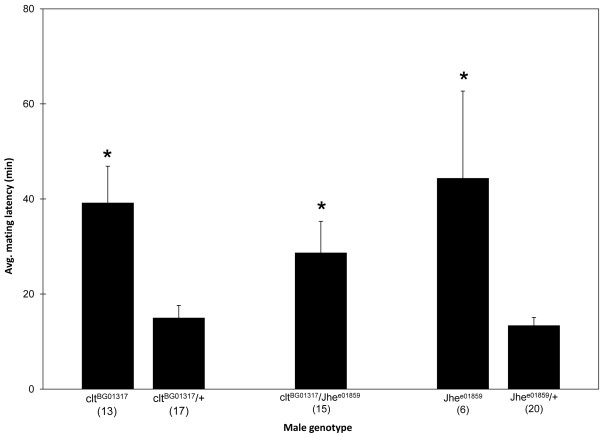
We hypothesized that if a gene is up regulated after mating, that gene likely affects some aspect of reproductive behavior. Therefore, we assayed the percentage of time a male spent courting a female, known as the courtship index (CI), of candidate mating-responsive gene mutants. A Jhe P-element insertion, Jhee01859, resulted in significantly reduced CI values in homozygous mutant males compared to heterozygous or wild-type controls (Fig. 4). Heterozygous males showed similar courtship activity compared to wild-type males, ruling out heterozygous effects on CI levels. Though Jhe males court females less vigorously, they perform standard courtship steps, eventually culminating in copulation.
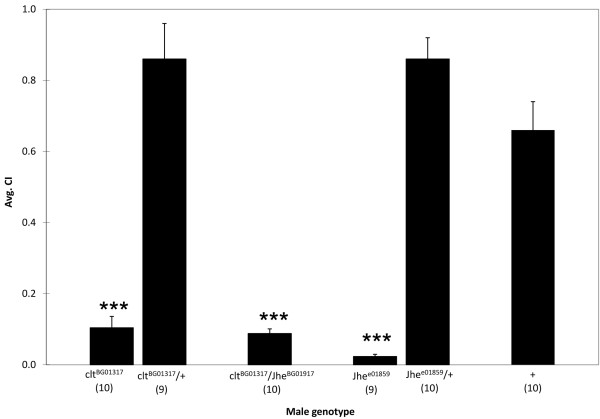
Figure 4.
Jhe and clt mutants reduce courtship toward females. Mutant males homozygous for P-element insertions in Jhe or clt showed reduced courtship (***p < 0.001) under red light compared to sibling heterozygous and wild-type controls. Jhee01859 +/+ cltBG01317 mutant males showed significant reductions in courtship compared to either heterozygote or the wild-type control. (N) reflects sample size. Error bars are SEM.
In addition to Jhe there are three other candidate juvenile hormone esterase genes in the Drosophila genome [54]. One of the genes, cricklet (clt), also had an available P-element insertion, so we tested cltBG01317 mutants to see if they had a similar phenotype to Jhe mutants. We found that clt mutants also have decreased CIs relative to controls (Fig. 4). There is also a strong genetic interaction between Jhe and clt. Transheterozygous mutant males had significantly reduced courtship compared to controls (Fig. 4).
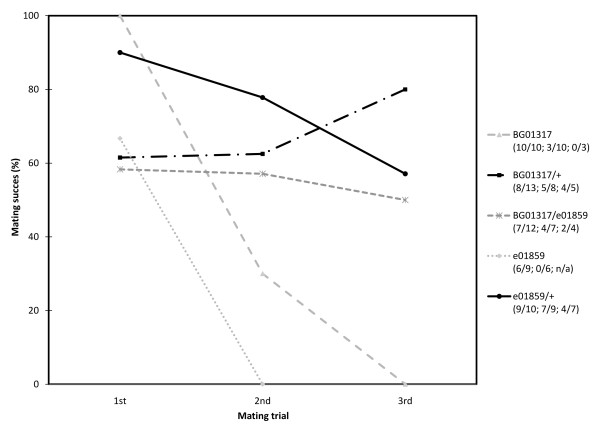
We predicted that mating-responsive loci would function to prime the male for subsequent mating encounters by regulating courtship or mating latency and duration. Therefore, we predicted that decreasing Jhe, which was up regulated in mated male heads, would increase courtship or mating latency. To test this hypothesis we examined the courtship and mating kinetics of mutants for Jhe and the related esterase clt. Courtship latency (time to initiation of courtship) did not differ among mutants and controls. Though Jhe and clt males mated with females, they had a significant (p < 0.05) increase in mating latency (Fig. 5) (ANOVA, genotype p < 0.05, trial p > 0.05), while mating duration was unaffected. The increased mating latency was not dependent on the mating trial (1st, 2nd or 3rd). However, as we increased the number of mating attempts, the mating success (as measured by the act of copulation) of Jhe and clt mutant males was significantly reduced (Fig. 6) (Binary Logistic Regression, genotype p < 0.01, trial p < 0.0001, interaction p < 0.0001) compared to heterozygous controls. Since we ruled out a heterozygous effect on CI values we did not test for heterozygous effects on mating latency or mating success. Females mated to Jhe or clt mutant males laid equivalent numbers of eggs regardless of the mating trial and day of egg laying (ANOVA, genotype p > 0.05, trial p > 0.05, day p > 0.05), and neither Jhe nor clt mutant females had detectable fertility defects.
Figure 5.
Mating latency is increased in Jhe and clt mutants. Under red light conditions, homozygous and transheterozygous mutant males had significantly (ANOVA p < 0.01, Tukey's *p < 0.05) increased mating latencies toward CS virgin females regardless of the mating bout (1st, 2nd, or 3rd); therefore, overall average mating latencies are shown. (N) reflects sample size. Error bars are SEM.
Figure 6.
Mating success decreases in Jhe and clt mutants. Jhe/+ and clt/+ control males successfully mated with 3 females in succession, while experimental Jhe and clt mutant males significantly (Binary Logistic Regression, genotype p < 0.01, trial p < 0.0001, interaction p < 0.0001) decreased their mating success with the 2nd and 3rd females. No. of successful matings/Total no. of pairings for 1st; 2nd; 3rd trials are shown.
Conclusions
Changes in gene expression upon mating
The complex reproductive behaviors exhibited by Drosophila require the interaction between genetics and environment. Courtship is an innate and stereotypical process under control of the somatic sex-determination hierarchy and is influenced by social interactions. Courtship and mating elicit gene expression changes in females [49,55-57], and courtship affects transcript profiles in males [12,22]. The female post-mating effects occur rapidly (within minutes) or can be detected several hours after mating [49,55-57]. Within 5 min of courtship, whole-male gene expression profiles also change rapidly [12,22]. In this study we expanded on our earlier studies in whole males to show that courtship culminating in mating causes changes in gene expression in the male head as well. Expression levels likely change rapidly in response to sensory cues received during courtship, while the physiological changes from mating [58] may mediate long-term expression level changes in the nervous system or elsewhere in the fly that can feed back to the nervous system.
The expression profile of a 5 min courting male differs from that of a 2 hr post-mating male. This is not surprising since we expected that the process of mating would have major effects on male physiology that would be reflected in altered transcriptional profiles. Of the 47 genes with altered expression 2 hrs after mating (Tables 1 and 2), only 1 gene, fit, is also up regulated in males after 5 min of courtship [12]. CG16772 is up regulated 2 hrs after mating but is down regulated during 5 min of courtship [12]. CG16772 is one of several fat body-expressed immune response genes down regulated during courtship, possibly to allow energetic resources to be directed toward offspring production rather than immunity [12,22]. After mating, expression of CG16772 may increase because contact with a female increases the likelihood of encountering a pathogen.
The fact that few genes overlap between these data sets is not surprising since we assayed different time points (5 min or 2 hrs), different tissues (whole bodies in previous studies versus heads in this study) and different behaviors (courtship alone versus courtship culminating in mating). We also used different approaches for analyzing the data due to the differences in experimental design for each test. The analysis strategies provide us a conservative estimate of the transcripts affected by courtship and mating.
We predict that some mating-responsive genes facilitate an increased male mating efficiency for future encounters. Little is known about how repeated matings affect male mating latency, duration or fecundity. After his first mating, the male may perceive and process female stimuli more rapidly, may be more appealing to the female, or may be physiologically primed for subsequent matings by replenishment of Acps, sperm or other seminal proteins, resulting in decreased courtship or mating latencies. Alterations in gene expression, such as those described here and in our earlier work [12,22], may contribute to these expected behavioral and physiological changes.
Gene expression in adipose tissue
The fat body is a secretory tissue [reviewed in [59]] whose effects on fly reproductive behavior have previously been described [[37,39,40,43]; reviewed in [52]]. The majority of mating-responsive genes are expressed in adult adipose tissue (fat body) (Table 4), and we analyzed a subset of six up-regulated genes to show that they are expressed in adipose tissue surrounding the brain (Figs. 2 and 3). Furthermore, we observed increased expression of fit in male adipose tissue after courtship followed by mating (Fig. 3). fit also is expressed in the head fat of females and originally was named based upon its high expression in females under the control of Sex-lethal [39], which is the initial regulatory gene in the somatic sex-determination hierarchy.
Other studies also indicated that several mating-responsive genes identified in our study are expressed in the fat body surrounding the brain. Larval serum protein 2 (Lsp2) is expressed in the head fat of both sexes [60]. Of the 25 genes up regulated by courtship and mating, 14 are detectable (signal strength greater than 20) in brain and 21 genes are detectable in fat body based upon a microarray analysis of adult mRNA expression levels [53]. Of these 25 up regulated genes, 16 are enriched in the fat body relative to other adult tissues (Table 4).
Taken together, these results imply that the brain is not the only tissue responding to or regulating post-mating behavior, but that adipose tissue plays a role in this process as well. In response to mating, a signaling cascade initiated by neurosecretory cells may transmit the signal to the surrounding fat body. The fat body then could perpetuate the signal by secreting factors that influence neuronal or non-neuronal tissues. We hypothesize that expression level changes in the brain alter neuronal signaling either directly or indirectly, which impacts the processing of sensory cues and targets other reproductively important tissues.
Juvenile hormone esterases and male reproductive behavior
Another mating-responsive gene, Jhe, is also expressed in adipose tissue [61-64] and functions in reproductive behavior (Figs. 4, 5, 6). Jhe and three closely related esterase genes (clt, Jhedup, and CG7529) have juvenile hormone esterase (JHE) activity in vitro. JHEs together with juvenile hormone epoxide hydrolases hydrolyze Juvenile hormone (JH) to regulate JH levels [65,66]. Since Jhe expression is positively regulated by JH [67], the mating-induced increase in Jhe expression identified in our study may be JH dependent.
Much of our understanding of physiological functions of JH comes from studies investigating its function during development [reviewed in [68]]. However, JH also has important post-developmental functions such as promoting accessory gland protein (Acp) synthesis [69]. During mating Acps are transferred, along with sperm, to the female [70], and the transfer of Acps triggers male synthesis of new Acps [58]. Males also transfer Sex-peptide to the female during mating [71-73]. Sex-peptide increases JH levels in females [74], which stimulates egg development [75]. However, possible mating-induced changes in male JH levels have not been evaluated. Since ejaculate components must be replenished after mating, we hypothesize that male JH levels increase after mating to stimulate Acp synthesis. The increase in JH would up regulate Jhe expression which would, in turn, reduce JH levels once the ejaculate components have been replenished.
JH also has a role in modulating behavior since males with reduced JH court females less intensely [76], Our data suggest that an increase in JH, caused by reduction of Jhe or clt, may also disrupt courtship (Figs. 4, 5, 6). Jhe and clt deficient males, which likely have increased levels of JH, court less vigorously (Fig. 4), have increased mating latencies (Fig. 5), and have reduced mating success (Fig. 6). This situation exemplifies the complex regulation governing behavior and implies that JH levels must be tightly regulated in order to ensure appropriate behavioral and physiological responses.
Gene expression in the brain
Although we are particularly interested in the large number of fat-expressed genes that were identified in this and earlier screens [12,22], we also note that several of the identified transcripts are expressed in brains as would be expected for genes that function in behavior. Proper function of the nervous system relies on the appropriate cellular architecture, connections and signaling. Behavior requires the sensory systems to perceive the information accurately and transmit such information to the brain for processing. The brain can then transmit the signal to the appropriate output pathways which can modify signaling in tissues such as the fat body or the brain itself. Therefore the establishment and maintenance of the brain (and sensory systems) is vital to the organism's ability to respond to its environment and experiences. It is possible that mating-responsive genes act in the development or maintenance of a mated male brain as opposed to a naïve male brain.
Thirteen of the 21 fat-expressed genes up regulated in mated males are also expressed in brains at detectable levels [53]; a single transcript, CG4288 is detected in brains but not fat [53]. None of these genes have known function in behavior, but their reported mutant phenotypes or molecular functions indicate that several of the loci may have important neural maintenance functions.
For example, mutants for bgm, an enzyme involved in fatty acid metabolism that is expressed in both the brain and fat, have a neurodegeneration phenotype in response to accumulation of long chain fatty acids [77]. Another gene that potentially functions in a neurodegeneration pathway is Phosphatidyl-serine synthase, which responds to changes in polygluatmate (polyQ) levels [78]. polyQ diseases, including Huntington's Disease, are adult on-set progressive neural degeneration diseases caused by the accumulation of glutamate repeats [79].
Cellular homeostasis is important in the maintenance and function of the Drosophila brain. One gene that helps maintain this homeostasis is Iron regulatory protein 1B (Irp-1B) which encodes a protein that binds to iron-responsive elements (IREs) to regulate iron metabolism [80]. In addition to affecting cell survival and homeostasis, neural morphology might also be regulated by mating-responsive candidates. Mutants of Pabp2 show pathfinding and targeting defects in the larval neuromuscular junction [81].
Mating-responsive genes and the sex-determination hierarchy
This genome-wide analysis identified known sex-determination hierarchy target genes such as fit. Three other mating-responsive genes (CG16772, Prx2540-2, and CG16898) (Tables 1 and 2) are also regulated by the sex-determination hierarchy [41]. Transcriptional profiling of mutants for a variety of sex-determination hierarchy genes indicates that Prx2540-2 and CG16898 are regulated by fruitless (fru), while fit is downstream of transformer (tra). CG16772 may also function downstream of tra [41].
The splicing factor squid (sqd) is up regulated in mated male heads (Table 1). Interestingly, primary transcripts of the sqd locus are sex-specifically spliced in the head as well as the germline, although it is not known if sqd splicing is regulated by the sex-determination hierarchy [82]. It is possible that sqd and other mating-responsive loci function as downstream targets of the sex-determination hierarchy to regulate morphological and behavioral differences between male and female Drosophila. Alternatively, there may be other pathways (such as those that regulate alternative splicing) that function together with the sex-determination hierarchy to regulate reproductive behavior.
We predict that mating-responsive genes also function in other aspects of reproduction and behavior; therefore, we propose this genome-wide approach as a powerful tool for determining the genetic pathways and intracellular processes regulating reproduction, both at the behavioral and physiological levels.
Methods
Microarray Analysis
The wild-type Canton-S (CS) strain was isogenized to reduce genetic variation and the isoline was kept at 25°C on a 12-hr light/dark cycle. Twenty or fewer virgin CS males were aged collectively for 3 days at 25°C. On day 4, individual males were aspirated into vials. Virgin females were collected and aged in groups of 20 or fewer flies for 4 days at 25°C.
On day 5, males were equally divided into two treatment groups. One group (referred to as "mated males") consisted of individual males that were placed with a female for courtship and mating, while the second group of males (referred to as "control males") was mock exposed to a female. We tested both groups at the same time to allow for paired microarray and Cyber-T analyses (see below). For the mated male group, a single, aged virgin female was aspirated into each male's vial. The control males were treated identically except that no female was transmitted during the aspiration process.
Upon completion of mating, females were removed from the vials. Males from both treatment groups were quick frozen 2 hrs later and stored at -80°C for future RNA extraction; only pairs for which the mated male had a mating latency less than 30 min and mating duration of 18-30 min were collected for RNA extraction. Seventy-four percent of mated males tested met this requirement. All procedures were conducted at the same time each day to control for circadian effects.
Head tissue was separated from the remaining body by vortexing quick-frozen flies. Male heads were assigned to one of 20 groups (30 heads in each group; 10 mated and 10 control RNA preparations) so that control and mated samples collected together could be analyzed by paired statistical comparisons. Following standard protocols, total RNA from head tissue was extracted in Trizol (Invitrogen, Carlsbad, CA, USA). Total head RNA preparations from 10 groups (5 control groups and their corresponding mated groups) were sent to the University of Kentucky MicroArray Core Facility for labeling and hybridization to Affymetrix Drosophila 2.0 Genome Arrays following standard Affymetrix (Santa Clara, CA, USA) protocols.
Expression values were generated similarly to previous experiments [12,22] using five algorithms (PM, PM-MM, MAS 5.0, GCRMA, and GeneSpring). Multiple expression value algorithms were used to control for variation among the algorithms and to generate a statistically stronger candidate gene set. We used dChip's PM (perfect match between the probe and target sequence) and PM-MM (one nucleotide between the probe and target sequence is mismatched) algorithms [83], as well as those implemented by GCOS (MAS 5.0, Affymetrix), R (GCRMA) [84], and GeneSpring (Agilent, Santa Clara, CA, USA). For the dChip algorithms, expression values were only considered if greater than 50; for the other 3 methods, expression values were required to be greater than 100. To test for significance, we used Cyber-T's Bayesian t-test analysis [85]. Candidate mating-responsive genes included those whose expression differed significantly (p < 0.001) between control male heads and mated male heads in at least 3 expression value data sets and had a false discovery rate less than 0.05 [86]. With such stringent criteria, we did not specify a particular fold change cut-off value.
qPCR
To confirm the microarray results, qPCR was performed on 10 independent samples (5 mated and 5 control RNA preparations) that were collected as described above but were not used in the microarray analysis. polyA+ RNA was isolated from each of the 10 samples using the Oligotex mRNA mini kit (Qiagen, Netherlands). cDNA was synthesized using the SuperScript First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). We designed primers to amplify 10 up-regulated and 3 down-regulated genes, choosing genes that are predicted to be enriched in brain, fat body or both tissues based upon FlyAtlas expression data [53]. When possible, primer pairs were designed across introns to control for amplification specificity. Genes that are expressed at low levels in the head [[53]; L. L. Ellis and G. E. Carney, unpublished results] were not tested.
Using the SYBR Green PCR Mastermix (Applied Biosystems, Foster City, CA, USA), 2 μL of a 1:4 dilution of each template was run in triplicate in the ABI7500 (Applied Biosystems, Foster City, CA, USA) using default parameters. Control reactions lacking template and controls with template but without Reverse Transcriptase were used. Primer-specific amplification was determined by analyzing dissociation curves for each primer pair.
mRNA levels were determined by the Relative Standard Curve Method (Applied Biosystems, Foster City, CA, USA), and candidate gene transcript levels were normalized to rp49 transcript levels. Normalizing the mated male transcript levels to the control male transcript levels generated a relative fold change. We also analyzed trends in the average relative transcript levels of each treatment (control and mated) using the two-tailed t-test. Secondary qPCR analysis confirmed increased expression of CG6188 and decreased expression of alpha Esterase-2.
Regression of mean expression microarray analysis fold changes compared to independent qPCR fold changes indicated a highly significant positive correlation between results obtained by the two methods (r = 0.51, N = 10, p = 0.021).
In situ hybridization
Digoxigenin (DIG)-labeled sense and antisense RNA probes were made from cDNA clones for six candidate genes with predicted fat body expression following the manufacturer's standard protocol (Roche, Nutley, NJ, USA). The genes and their corresponding cDNA clones were CG4825 (LD10327), CG8449 (GH10459), CG13360 (LP09811), bubblegum (bgm) (GM14009), fit (RH40291) and Prx2540-2 (RH69586). Expression of fit is regulated by tra while expression of Prx2540-2 is regulated by fruitless (fru) [41]; tra and fru are regulatory components of the sex-determination hierarchy. Antisense and sense probes were hydrolyzed into 200 bp fragments and in situ hybridization to male brains, head carcass and abdominal cuticle was performed as described in [33]. Antisense probes detected expressed transcripts in each case, while sense probes served as negative controls for expression.
To verify the increased expression of fit in male head tissue after courtship followed by mating, virgin CS males were collected 2 hrs after mating and compared to virgin CS control males that did not mate with a female. After treatment, males were cryosectioned in OCT compound and in situ hybridization was performed on the sections as described previously [37]. Control and mated tissues were placed on the same slide to control for histochemical reaction time. We qualitatively assessed fit expression in adipose tissue lining the brain from non-existent (-) to highly expressed (+++).
Courtship assays
All flies were kept on a 12-hr light/dark cycle at 25°C. P-element insertion mutations in Jhe and cricklet (clt) were obtained from the Bloomington Drosophila Stock Center (cltBG01317) and the Exelixis Collection at Harvard Medical School (Jhee01859). These insertions are likely hypomorphs since they are located in proximal promoter regions. Each P-element was backcrossed into the CS background to generate a genetically similar control that had one wild-type copy of Jhe or clt. To test for a genetic interaction between Jhe and clt, the two insertion strains, Jhee01859 and cltBG01317, were crossed to generate transheterozygous flies containing a single P-element insertion in each gene (Jhee01859 +/+ cltBG01317). Virgin P-insertion or control males were collected and stored individually for 4 to 5 days; virgin CS females were aged collectively for 3 to 5 days.
Behavioral assays were conducted at 22°C under red light conditions to diminish the effect of eye color on vision and courtship. We video recorded the interactions with a digital camcorder so that subsequent analyses could be performed. To analyze courtship behavior, a male was aspirated into a mating chamber (diameter = 1 cm) and a virgin CS female was introduced 2 min later. The pair was video recorded for 10 min. The courtship index (CI; percentage of time the male spent performing courtship during the initial 10 min of observation) and courtship latency (time until courtship occurs) were calculated. CI values were arcsine transformed for statistical analysis. Two-tailed t-test comparisons between homozygous mutants and controls were calculated to determine significance (p < 0.05). Jhee01859 +/+ cltBG01317 males were compared to both control genotypes (two-tailed t-test).
Fertility Assays
The ability of a male to mate with multiple CS females and the fecundity of these matings was also assessed. Jhe and clt mutants and heterozygous controls, as well as CS virgin females, were collected and aged as described for the courtship assay. Under red light, a male was aspirated into a mating chamber, followed by a CS virgin female. The male was given 2 hrs to mate with the female. If mating occurred, the female was placed in a vial with food to measure fecundity (number of eggs laid and number of adult offspring) and the male was placed in a new mating chamber. A second CS virgin female was aspirated into the new chamber and the pair was given 2 hrs to mate. If the second mating occurred, the female was placed in a vial for later progeny counts, and the male was moved to another chamber for mating with a third and final female. The third mated female was also kept for further analysis. For the first mating bout, all 10 cltBG01317 males mated, while only three of the ten males mated with the second female and none of the 3 males mated with the third female. Eight out of 13 cltBG01317/+ males mated with the first female, five of those eight males mated with the second female and four of the remaining five males mated with the third female. Jhee01859 males only mated with the first female (six out of nine males). However, nine of ten Jhee01859/+ males mated with the first female, seven of those nine males mated with the second female and four of the seven males mated with the third female. For the transheterozygous cltBG01317/Jhee01859 males, seven of 12 mated with the first female, four of seven males mated with the second female and two of the four males mated with the third female.
The mating latencies and durations for each of the three possible matings were measured and significance was determined by Univariate ANOVA analysis using genotype and mating trial as fixed variables with Tukey's post-hoc analysis (SPSS). Males that did not mate within the 2 hr window were scored as being unsuccessful. Using linear regression, we assessed the significance (p < 0.05) of genotype and mating bout on mating success.
For 6 days following the assay, the female was transferred to a new vial and the number of eggs laid in each vial was determined. Vials were maintained at 25°C for 18 days to allow for a count of the total number of adult progeny. Significant effects of genotype and trial on mating latency or duration were measured by the Univariate ANOVA and Tukey's post-hoc analysis. We also measured the significance of genotype, mating bout and day of egg laying on the male's fecundity (Univariate ANOVA and Tukey's post-hoc analysis). Fecundity was measured by the total number of eggs laid and by the arcsine transformed ratio of adult offspring to eggs laid.
Authors' contributions
LLE designed and executed experiments, analyzed the data, and helped write the manuscript. GEC conceived and designed experiments and helped write the manuscript. All authors read and approved the final draft.
Contributor Information
Lisa L Ellis, Email: lellis@tamu.edu.
Ginger E Carney, Email: gcarney@mail.bio.tamu.edu.
Acknowledgements
We thank Ms. Donna Wall and Dr. Kuey-Chu Chen at the University of Kentucky MicroArray Core Facility for microarray processing. Mr. Bruce Ellis created the electrical probes used for ablations, and Dr. Adam G. Jones provided helpful discussions regarding the statistical analyses. We also thank two anonymous reviewers for their helpful comments on the manuscript. Texas A&M University provided funding to G.E.C. The microarray data from this study are available through the GEO database, accession number GSE24156.
References
- Dukas R, Mooers AO. Environmental enrichment improves mating success in fruit flies. Anim Behav. 2003;66:741–749. doi: 10.1006/anbe.2002.2261. [DOI] [Google Scholar]
- Gailey DA, Hall JC, Siegel RW. Reduced reproductive success for a conditioning mutant in experimental populations of Drosophila melanogaster. Genetics. 1985;111:795–804. doi: 10.1093/genetics/111.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RW, Hall JC. Conditioned responses in courtship behavior of normal and mutant Drosophila. Proc Natl Acad Sci USA. 1979;76:3430–3434. doi: 10.1073/pnas.76.7.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Kidokoro Y. Aggressive behaviors of female Drosophila melanogaster are influenced by their social experience and food resources. Physiol Entomol. 2002;27:21–28. doi: 10.1046/j.1365-3032.2002.00262.x. [DOI] [Google Scholar]
- Yurkovic A, Wang O, Basu AC, Kravitz EA. Learning and memory associated with aggression in Drosophila melanogaster. Proc Natl Acad Sci USA. 2006;103:17519–17524. doi: 10.1073/pnas.0608211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Hughes M, Lee K, Gould E. Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nat Neurosci. 2006;9:1094–1095. doi: 10.1038/nn1753. [DOI] [PubMed] [Google Scholar]
- Mori T, Hiraka I, Kurata Y, Kawachi H, Kishida O, Nishimura K. Genetic basis of phenotypic plasticity for predator-induced morphological defenses in anuran tadpole, Rana pirica, using cDNA subtraction and microarray analysis. Biochem Biophys Res Commun. 2005;330:1138–1145. doi: 10.1016/j.bbrc.2005.03.091. [DOI] [PubMed] [Google Scholar]
- Stewart BA, McLean JR. Population density regulates Drosophila synaptic morphology in a Fasciclin-II-dependent manner. J Neurobiol. 2004;61:392–399. doi: 10.1002/neu.20096. [DOI] [PubMed] [Google Scholar]
- Technau GM. Fiber number in the mushroom bodies of adult Drosophila melanogaster depends on age, sex, and experience. J Neurogenet. 2007;21:183–196. doi: 10.1080/01677060701695359. [DOI] [PubMed] [Google Scholar]
- Anseloni VCZ, He F, Novikova SI, Robbins MT, Lidow IA, Ennis M, Lidow MS. Alterations in stress-associated behaviors and neurochemical markers in adult rats after neonatal short-lasting local inflammatory insult. Neuroscience. 2005;131:635–645. doi: 10.1016/j.neuroscience.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Burmeister SS, Jarvis ED, Fernald RD. Rapid behavioral and genomic responses to social opportunity. PLoS Biol. 2005;3:1996–2004. doi: 10.1371/journal.pbio.0030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney GE. A rapid genome-wide response to Drosophila melanogaster social interactions. BMC Genomics. 2007;8:288. doi: 10.1186/1471-2164-8-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Funes P, Dowse HB, Hall JC. Resetting the circadian clock by social experience in Drosophila melanogaster. Science. 2002;298:2010–2012. doi: 10.1126/science.1076008. [DOI] [PubMed] [Google Scholar]
- Mehren JE, Griffith LC. Calcium-independent calcium/calmodulin-dependent protein kinase II in the adult Drosophila CNS enhances the training of pheromonal cues. J Neurosci. 2004;24:10584–10593. doi: 10.1523/JNEUROSCI.3560-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata S, Yoshiara T, Lim CR, Sugino M, Kogure M, Ohnuki T, Komurasaki T, Matsubara K. Psychophysiological stress-regulated gene expression in mice. FEBS Lett. 2005;579:2137–2142. doi: 10.1016/j.febslet.2005.02.069. [DOI] [PubMed] [Google Scholar]
- Reiser M, Poeggel G, Schnabel R, Schroder H, Braun K. Effect of social experience on dopamine-stimulated adenylyl cyclase activity and G protein composition in chick forebrain. J Neurochem. 1999;73:1293–1299. doi: 10.1046/j.1471-4159.1999.0731293.x. [DOI] [PubMed] [Google Scholar]
- Shen CP, Tsimberg Y, Salvadore C, Meller E. Activation of Erk and JNK MAPK pathways by acute swim stress in rat brain regions. BMC Neurosci. 2004;5:36. doi: 10.1186/1471-2202-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan RJ. Understanding the genetic construction of behavior. Sci Am. 1995;272:72–78. doi: 10.1038/scientificamerican0495-72. [DOI] [PubMed] [Google Scholar]
- Greenspan RJ, Ferveur J-F. Courtship in Drosophila. Annu Rev Genet. 2000;34:205–232. doi: 10.1146/annurev.genet.34.1.205. [DOI] [PubMed] [Google Scholar]
- Billeter J-C, Goodwin SF, O'Dell KM. Genes mediating sex-specific behaviors in Drosophila. Adv Genet. 2002;47:87–116. doi: 10.1016/s0065-2660(02)47003-4. full_text. [DOI] [PubMed] [Google Scholar]
- Tompkins L. Genetic analysis of sex appeal in Drosophila. Behav Genet. 1984;14:411–440. doi: 10.1007/BF01065443. [DOI] [PubMed] [Google Scholar]
- Ellis LL, Carney GE. Drosophila melanogaster males respond differently at the behavioral and genome-wide levels to Drosophila melanogaster and Drosophila simulans females. J Evol Biol. 2009;22:2183–2191. doi: 10.1111/j.1420-9101.2009.01834.x. [DOI] [PubMed] [Google Scholar]
- Siwicki KK, Ladewski L. Associative learning and memory in Drosophila: beyond olfactory conditioning. Behav Processes. 2003;64:225–238. doi: 10.1016/S0376-6357(03)00137-2. [DOI] [PubMed] [Google Scholar]
- Ewing AW. Functional-aspects of Drosophila courtship. Biol Rev. 1983;58:275–292. doi: 10.1111/j.1469-185X.1983.tb00390.x. [DOI] [Google Scholar]
- Mehren JE, Ejima A, Griffith LC. Unconventional sex: Fresh approaches to courtship learning. Curr Opin Neurobiol. 2004;14:745–750. doi: 10.1016/j.conb.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Cline TW. Reflections on a path to sexual commitment. Genetics. 2005;169:1179–1185. doi: 10.1093/genetics/169.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirangi TR, McKeown M. Sex in flies: What 'body-mind' dichotomy? Dev Biol. 2007;306:10–19. doi: 10.1016/j.ydbio.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Demir E, Dickson BJ. fruitless splicing specifies male courtship behavior in Drosophila. Cell. 2005;121:785–794. doi: 10.1016/j.cell.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Finley KD, Taylor BJ, Milstein M, McKeown M. dissatisfaction, a gene involved in sex-specific behavior and neural development of Drosophila melanogaster. Proc Natl Acad Sci USA. 1997;94:913–918. doi: 10.1073/pnas.94.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Ote M, Tazawa T, Yamamoto D. Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature. 2005;438:229–233. doi: 10.1038/nature04229. [DOI] [PubMed] [Google Scholar]
- Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS. Male-specific fruitless specifies the neural substrates of Drosophila courtship behavior. Nature. 2005;436:395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- Stockinger P, Kvitsiani D, Rotkopf S, Tirian L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Arbeitman MN, Fleming AA, Siegal ML, Null BH, Baker BS. A genomic analysis of Drosophila somatic sexual differentation and its regulation. Development. 2004;131:2007–2021. doi: 10.1242/dev.01077. [DOI] [PubMed] [Google Scholar]
- Burtis KC, Coschigano KT, Baker BS, Wensink PC. The Doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer. EMBO J. 1991;10:2577–2582. doi: 10.1002/j.1460-2075.1991.tb07798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann MJ, Chung E, Levin LR. A new family of adenylyl cyclase genes in the male germline of Drosophila melanogaster. Dev Genes Evol. 2000;210:200–206. doi: 10.1007/s004270050304. [DOI] [PubMed] [Google Scholar]
- Dalton JE, Lebo MS, Sanders LE, Sun F, Arbeitman MN. Ecdysone receptor acts in fruitless- expressing neurons to mediate Drosophila courtship behaviors. Curr Biol. 2009;19:1447–1452. doi: 10.1016/j.cub.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauwalder B, Tsujimoto S, Moss J, Mattox W. The Drosophila takeout gene is regulated by the somatic sex-determination pathway and affects male courtship behavior. Genes Dev. 2002;16:2879–2892. doi: 10.1101/gad.1010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau MD, Radovic A, Wittkopp PJ, Long AD. A gene necessary for normal male courtship, yellow, acts downstream of fruitless in the Drosophila melanogaster larval brain. J Neurobiol. 2003;55:53–72. doi: 10.1002/neu.10196. [DOI] [PubMed] [Google Scholar]
- Fujii S, Amrein H. Genes expressed in the Drosophila head reveal a role for fat cells in sex-specific physiology. EMBO J. 2002;21:5353–5363. doi: 10.1093/emboj/cdf556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Toyama A, Amrein H. A male-specific fatty acid omega-hydroxylase, SXE1, is necessary for efficient male mating in Drosophila melanogaster. Genetics. 2008;180:179–90. doi: 10.1534/genetics.108.089177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman TD, Arbeitman MN. Genomic and functional studies of Drosophila sex hierarchy regulated gene expression in adult head and nervous system tissues. PLoS Genet. 2007;3:e216. doi: 10.1371/journal.pgen.0030216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp A, Duncan I, Godt D, Carroll SB. Genetic control and evolution of sexually dimorphic characters in Drosophila. Nature. 2000;408:553–559. doi: 10.1038/35046017. [DOI] [PubMed] [Google Scholar]
- Lazareva AA, Roman G, Mattox W, Hardin PE, Dauwalder B. A role for the adult fat body in Drosophila male courtship behavior. PLoS Genet. 2007;3:e16. doi: 10.1371/journal.pgen.0030016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukas R. Experience improves courtship in male fruit flies. Anim Behav. 2005;69:1203–1209. doi: 10.1016/j.anbehav.2004.08.012. [DOI] [Google Scholar]
- Polejack A, Tidon R. Learning of courtship components in Drosophila mercatorum (Paterson & Wheller) (Diptera, Drosophilidae) Rev Bras Entomol. 2007;51:82–86. doi: 10.1590/S0085-56262007000100014. [DOI] [Google Scholar]
- Anholt RRH, Dilda CL, Chang S, Fanara JJ, Kulkarni NH, Ganguly I, Rollmann SM, Kamdar KP, Mackay TFC. The genetic architecture of odor-guided behavior in Drosophila: Epistasis and the transcriptome. Nat Genet. 2003;35:180–184. doi: 10.1038/ng1240. [DOI] [PubMed] [Google Scholar]
- Ceriani MF, Hogenesch JB, Yanovsky M, Panda S, Straume M, Kay SA. Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. J Neurosci. 2002;22:9305–9319. doi: 10.1523/JNEUROSCI.22-21-09305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau J, Chiang AS, Grady L, Barditch J, Gossweiler S, McNeil J, Smith P, Buldoc F, Scott R, Certa U, Broger C, Tully T. The staufen/pumilio pathway is involved in Drosophila long-term memory. Curr Biol. 2003;13:286–296. doi: 10.1016/S0960-9822(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Lawniczak MK, Begun DJ. A genome-wide analysis of courting and mating responses in Drosophila melanogaster females. Genome. 2004;47:900–910. doi: 10.1139/g04-050. [DOI] [PubMed] [Google Scholar]
- Mackay TFC, Heinsohn SL, Lyman RF, Moehring AJ, Morgan TJ, Rollmann SM. Genetics and genomics of Drosophila mating behavior. Proc Natl Acad Sci USA. 2005;102:6622–6629. doi: 10.1073/pnas.0501986102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma DP, White KP, Hirsch J, Greenspan RJ. Identification of genes involved in Drosophila melanogaster geotaxis, a complex behavioral trait. Nat Genet. 2002;31:349–353. doi: 10.1038/ng893. [DOI] [PubMed] [Google Scholar]
- Dauwalder B. Systems behavior: Of male courtship, the nervous system and beyond in Drosophila. Curr Genomics. 2008;9:517–524. doi: 10.2174/138920208786847980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- Campbell PM, Harcourt RL, Crone EJ, Claudianos C, Hammock BD, Russell RJ, Oakeshott JG. Identification of a juvenile hormone esterase gene by matching its peptide mass fingerprint with a sequence from the Drosophila genome project. Inesct Biochem Mol Biol. 2001;31:513–520. doi: 10.1016/S0965-1748(01)00035-2. [DOI] [PubMed] [Google Scholar]
- Mack PD, Kapelnikov A, Heifetz Y, Bender M. Mating-responsive genes in reproductive tissues of female Drosophila melanogaster. Proc Natl Acad Sci USA. 2006;103:10358–10363. doi: 10.1073/pnas.0604046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw LA, Clark AG, Wolfner MF. Post-mating gene expression profiles of female Drosophila melanogaster in response to time and to four male accessory gland proteins. Genetics. 2008;179:1395–1408. doi: 10.1534/genetics.108.086934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw LA, Gibson G, Clark AG, Wolfner MF. Genes regulated by mating, sperm, or seminal proteins in mated female Drosophila melanogaster. Curr Biol. 2004;14:1509–1514. doi: 10.1016/j.cub.2004.08.028. [DOI] [PubMed] [Google Scholar]
- DiBenedetto AJ, Harada HA, Wolfner MF. Structure, cell-specific expression, and mating-induced regulation of a Drosophila melanogaster male accessory gland gene. Dev Biol. 1990;139:134–148. doi: 10.1016/0012-1606(90)90284-P. [DOI] [PubMed] [Google Scholar]
- Schlegel A, Stainier DYR. Lessons from "lower" organisms: What worms, flies, and zebrafish can teach us about human energy metabolism. PLoS Genet. 2007;3:2037–2048. doi: 10.1371/journal.pgen.0030199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes H, Edmondson RG, Fink P, Keizlarova-Lepesant J, Lepesant JA, Miles JP, Spivey DW. Adult expression of the Drosophila Lsp-2 gene. Dev Biol. 1990;142:138–146. doi: 10.1016/0012-1606(90)90157-E. [DOI] [PubMed] [Google Scholar]
- Anand A, Crone EJ, Zera AJ. Tissue and stage-specific juvenile hormone (JHE) and epoxide hydrolase (JHEH) enzyme activities and Jhe transcript abundance in lines of the cricket Gryllus assimilis artificially selected for plasma JHE activity: Implications for JHE microevolution. J Insect Physiol. 2008;54:1323–1331. doi: 10.1016/j.jinsphys.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Kamimura M, Takahashi M, Kikuchi K, Reza AMS, Kiuchi M. Tissue-specific regulation of Juvenile hormone esterase gene expression by 20-hydroxyecdysone and Juvenile hormone in Bombyx mori. Arch Insect Biochem. 2007;65:143–151. doi: 10.1002/arch.20186. [DOI] [PubMed] [Google Scholar]
- Klages G, Emmerich H. Juvenile hormone metabolism and juvenile hormone esterase titer in hemolymph and peripheral tissues of Drosophila hydei. J Comp Physiol. 1979;132:319–325. [Google Scholar]
- Shanmugavelu S, Baytan AR, Chesnut JD, Bonning BC. A novel protein that binds juvenile hormone esterase in fat body tissue and pericardial cells of the tobacco hornworm Manduca sexta. J Biol Chem. 2000;275:1802–1806. doi: 10.1074/jbc.275.3.1802. [DOI] [PubMed] [Google Scholar]
- Campbell PM, Healy MJ, Oakeshott JG. Characterization of juvenile hormone esterase in Drosophila melanogaster. Insect Biochem Mol Biol. 1992;22:665–677. doi: 10.1016/0965-1748(92)90045-G. [DOI] [PubMed] [Google Scholar]
- Campbell PM, Oakeshott JG, Healy MJ. Purification and kinetic characterization of juvenile hormone esterase from Drosophila melanogaster. Insect Biochem Mol Biol. 1998;28:501–515. doi: 10.1016/S0965-1748(98)00037-X. [DOI] [PubMed] [Google Scholar]
- Kethidi DR, Xi ZY, Palli SR. Developmental and hormonal regulation of juvenile hormone esterase gene in Drosophila melanogaster. J Insect Physiol. 2005;51:393–400. doi: 10.1016/j.jinsphys.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Flatt T, Tu MP, Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. Bioessays. 2005;27:999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- Wolfner MF, Partridge L, Lewin S, Kalb JM, Chapman T, Herndon LA. Mating and hormonal triggers regulate accessory gland gene expression in male Drosophila. J Insect Physiol. 1997;43:1117–1123. doi: 10.1016/S0022-1910(97)00062-0. [DOI] [PubMed] [Google Scholar]
- Wolfner MF. Tokens of love: Functions and regulation of Drosophila male accessory gland products. Insect Biochem Mol Biol. 1997;27:179–192. doi: 10.1016/S0965-1748(96)00084-7. [DOI] [PubMed] [Google Scholar]
- Chen PS. The accessory gland proteins in male Drosophila: Structural, reproductive, and evolutionary aspects. Experientia. 1996;52:503–510. doi: 10.1007/BF01969718. [DOI] [PubMed] [Google Scholar]
- Kubli E. The sex-peptide. Bioessays. 1992;14:779–784. doi: 10.1002/bies.950141111. [DOI] [PubMed] [Google Scholar]
- Wolfner MF, Harada HA, Bertram MJ, Stelick TJ, Kraus KW, Kalb JM, Lung YO, Neubaum DM, Park M, Tram U. New genes for male accessory gland proteins in Drosophila melanogaster. Insect Biochem Mol Biol. 1997;27:825–834. doi: 10.1016/S0965-1748(97)00056-8. [DOI] [PubMed] [Google Scholar]
- Moshitzky P, Fleischmann I, Chaimov N, Saudan P, Klauser S, Kubli E, Applebaum SW. Sex-peptide activates juvenile hormone biosynthesis in the Drosophila melanogaster corpus allatum. Arch Insect Biochem Physiol. 1996;32:363–374. doi: 10.1002/(SICI)1520-6327(1996)32:3/4<363::AID-ARCH9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Soller M, Bownes M, Kubli E. Control of oocyte maturation in sexually mature Drosophila females. Dev Biol. 1999;208:337–351. doi: 10.1006/dbio.1999.9210. [DOI] [PubMed] [Google Scholar]
- Wilson TG, DeMoor S, Lei J. Juvenile hormone involvement in Drosophila melanogaster male reproduction as suggested by the methoprene-tolerant(27) mutant phenotype. Insect Biochem Mol Biol. 2003;33:1167–1175. doi: 10.1016/j.ibmb.2003.06.007. [DOI] [PubMed] [Google Scholar]
- Min KT, Benzer S. Preventing neurodegeneration in the Drosophila mutant bubblegum. Science. 1999;248:1958. doi: 10.1126/science.284.5422.1985. [DOI] [PubMed] [Google Scholar]
- Nelson B, Nishimura S, Kanuka H, Kuranaga E, Inoue M, Hori G, Nakahara H, Miura M. Isolation of gene sets affected specifically by polyglutamine expression: Implication of the TOR signaling pathway in neurodegeneration. Cell Death Diffn. 2005;12:1115–1123. doi: 10.1038/sj.cdd.4401635. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY, Orr HT. Glutamine repeats and neurodegeneration. Ann Rev Neurosci. 2000;23:217–247. doi: 10.1146/annurev.neuro.23.1.217. [DOI] [PubMed] [Google Scholar]
- Muckenthaler M, Gunkel N, Frishman D, Cyrklaff A, Tomancak P, Hentze MW. Iron-regulatory protein-1 (IRP-1) is highly conserved in two invertebrate species-characterization of IRP-1 homologues in Drosophila melanogaster and Caenorhabditis elegans. Europ J Biochem. 1998;254:230–237. doi: 10.1046/j.1432-1327.1998.2540230.x. [DOI] [PubMed] [Google Scholar]
- Liebl FLW, Werner KM, Sheng Q, Karr JE, McCabe BD, Featherstone DE. Genome-wide P-element screen for Drosophila synaptogenesis mutants. J Neurobiol. 2006;66:332–347. doi: 10.1002/neu.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telonis-Scott M, Kopp A, Wayne ML, Nuzhdin SV, McIntyre LM. Sex-specific splicing in Drosophila: Widespread occurrence, tissue specificity and evolutionary conservation. Genetics. 2009;181:421–434. doi: 10.1534/genetics.108.096743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wong WH. Model-based analysis of oligonucleotide arrays: Expression index computation and outlier detection. Proc Natl Acad Sci USA. 2001;98:31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna; 2008. [Google Scholar]
- Baldi P, Long AD. A bayesian framework for the analysis of microarray expression data: Regularized t-test and statistical inferences of gene changes. Bioinformatics. 2001;17:509–519. doi: 10.1093/bioinformatics/17.6.509. [DOI] [PubMed] [Google Scholar]
- Storey JD, Tibshiriani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]