Abstract
Background
PCNA (proliferating cell nuclear antigen) has been found in the nuclei of yeast, plant and animal cells that undergo cell division, suggesting a function in cell cycle regulation and/or DNA replication. It subsequently became clear that PCNA also played a role in other processes involving the cell genome.
Scope
This review discusses eukaryotic PCNA, with an emphasis on plant PCNA, in terms of the protein structure and its biochemical properties as well as gene structure, organization, expression and function. PCNA exerts a tripartite function by operating as (1) a sliding clamp during DNA synthesis, (2) a polymerase switch factor and (3) a recruitment factor. Most of its functions are mediated by its interactions with various proteins involved in DNA synthesis, repair and recombination as well as in regulation of the cell cycle and chromatid cohesion. Moreover, post-translational modifications of PCNA play a key role in regulation of its functions. Finally, a phylogenetic comparison of PCNA genes suggests that the multi-functionality observed in most species is a product of evolution.
Conclusions
Most plant PCNAs exhibit features similar to those found for PCNAs of other eukaryotes. Similarities include: (1) a trimeric ring structure of the PCNA sliding clamp, (2) the involvement of PCNA in DNA replication and repair, (3) the ability to stimulate the activity of DNA polymerase δ and (4) the ability to interact with p21, a regulator of the cell cycle. However, many plant genomes seem to contain the second, probably functional, copy of the PCNA gene, in contrast to PCNA pseudogenes that are found in mammalian genomes.
Keywords: Proliferating cell nuclear antigen, PCNA, cyclin, DNA replication, DNA repair, cell cycle
INTRODUCTION
Proliferating cell nuclear antigen (PCNA) is an evolutionarily well-conserved protein found in all eukaryotic species as well as in Archaea. PCNA was first shown to act as a processivity factor of DNA polymerase δ, which is required for DNA synthesis during replication (Tan et al., 1986; Bravo et al., 1987; Prelich et al., 1987). However, besides DNA replication, PCNA functions are associated with other vital cellular processes such as chromatin remodelling, DNA repair, sister-chromatid cohesion and cell cycle control (Maga and Hubscher, 2003). The complexity of PCNA functions are reflected by the history of its discovery and subsequent investigation. This protein was identified over 30 years ago as an antigen for an autoimmune disease in the serum of patients with systemic lupus erythematosus (Miyachi et al., 1978). Two years later, another group found a 36-kDa protein that was differentially expressed during the cell cycle (Bravo and Celis, 1980) and named it ‘cyclin’ (Bravo et al., 1982). Later, it was shown that expression levels of PCNA are associated with proliferation or neoplastic transformation (Bravo et al., 1982; Celis et al., 1984). Further experiments revealed that PCNA and ‘cyclin’ were the same protein (Mathews et al., 1984).
Genes encoding PCNA and/or its products have been identified in a wide variety of diverse organisms such as animals, yeast and higher plants, the plants investigated including Arabidopsis, bean (Strzalka and Ziemienowicz, 2007; Strzalka et al., 2010), carrot (Hata et al., 1992), maize (Lopez et al., 1995, 1997), pea (Shimizu and Mori, 1998a), periwinkle (Kodama et al., 1991), rape (Markley et al., 1993), rice (Suzuka et al., 1989), soybean (Daidoji et al., 1992) and tobacco (Park et al., 1999). Analysis of all known PCNAs suggests that this protein is conserved in sequence, structure and function. Yeast and Drosophila PCNAs were able to substitute for mammalian PCNA in a DNA replication assay (Bauer and Burgers, 1988; Ng et al., 1990), plant PCNAs stimulated the activity of human DNA polymerase δ (Matsumoto et al., 1994a; Strzalka et al., 2010) and mammalian PCNA stimulated the activity and processivity of two δ-like polymerases isolated from wheat embryos (Laquel et al., 1993). An additional important finding that highlighted the conservation of PCNA described the ability of purified pea, bean and Arabidopsis PCNA to interact and form a stable complex with human p21/WAF1 (Ball and Lane, 1996; Strzalka et al., 2009, 2010), a p53-dependent protein involved in cell cycle regulation and stress response. Finally, the human anti-PCNA auto-antibody reacts not only with the nuclei of proliferating cells of all experimental animals examined so far (Celis et al., 1987) but also with the nuclei of plant cells (Daidoji et al., 1992). Although a number of reviews on PCNA have appeared in recent years (e.g. Naryzhny, 2008; Stoimenov and Helleday, 2009), all of them aim to describe the properties of yeast and/or animal PCNAs. This review will highlight most aspects of the structure and function of plant PCNA against the background of knowledge on yeast/animal PCNAs.
PCNA STRUCTURE
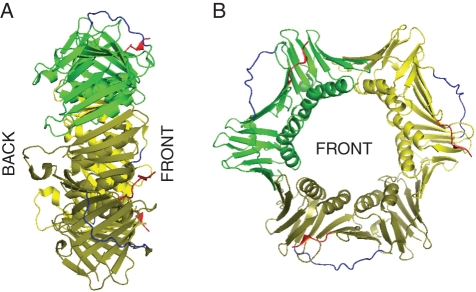
Despite the continuous evolution of genetic material initiating from the origin of life on Earth, a protein encoded by the proliferating cell nuclear antigen gene (PCNA) has retained its characteristic structure and function over millions of years among all analysed eukaryotes and Archaea. PCNA belongs to the DNA sliding clamp family, which also includes the Escherichia coli DNA polymerase (pol) III β subunit and the T4 phage, gene45 protein. The name ‘sliding clamp family’ was proposed based on the observation that a DNA pol III β subunit loaded on circular DNA was stably bound to the nucleic acid, while cleavage of the DNA molecule led to its efficient dissociation. This observation suggested a closed ring structure of the DNA pol III β subunit that possessed the ability to slide along a DNA molecule (Stukenberg et al., 1991). Electron microscopy studies proposed a similar model for the gp45 protein of a T4 bacteriophage (Gogol et al., 1992). These previously proposed ring structure models were ultimately confirmed by X-ray diffraction of the first crystallized protein homologue of PCNA, namely the bacterial DNA pol III β subunit of E. coli (Kong et al., 1992). The DNA pol III β subunit was shown to form a homodimer with a pseudo-six-fold symmetry axis in which each monomer was composed of three repeated domains. Asymmetric charge distribution on the surface of the DNA pol III β subunit facilitates interaction between the monomers and is mediated by opposite-charged poles in a head-to-tail orientation. The diameter of a central cavity ring in which the double helix of DNA is placed is 3·5 nm. Such spatial arrangement around the double helix allows for free and smooth sliding movements along the DNA molecule, thus preserving the physiological activity of this protein. Although the calculated molecular weight of the DNA pol III β subunit (41 kDa) differs from that of PCNA and gp45 monomers (29 and 25 kDa, respectively), the observed molecular weight under native conditions is similar for all three proteins. The lower molecular weights of PCNA and phage T4 gp45 proteins is a result of a shorter amino acid chain. However, the comparison of secondary structures between the DNA pol III β subunit and those predicted for the gp45 protein, yeast and human PCNA suggested spatial congruencies between these proteins (Kong et al., 1992). Taking into account the lack of significant similarities between the amino acid sequences of the proteins analysed, this was a surprising conclusion. The first detailed crystal structure of PCNA was revealed for the yeast protein (Krishna et al., 1994), followed two years later by the structure of human PCNA in complex with a p21 fragment (Gulbis et al., 1996). In 2001, a crystal structure of archaea PCNA was determined (Matsumiya et al., 2001), and finally 21 years after the initial discovery, the Arabidopsis PCNA structure was presented (Strzalka et al., 2009). Structural data obtained from the analysis of human, yeast, archaea and plant PCNA confirmed the similarity between PCNA and the E. coli DNA pol III β subunit, illustrating that PCNA also forms a pseudo-six-fold symmetry ring around DNA with an internal diameter of 3·4 nm. Additionally, one can distinguish two different, front (with protruding C-terminal end) and back, sides of the PCNA ring (Krishna et al., 1994; Gulbis et al., 1996; Matsumiya et al., 2001; Strzalka et al., 2009). In contrast to the DNA pol III β ring with two subunits, the PCNA ring consists of three conjoined, identical monomers (Fig. 1). PCNA is loaded onto DNA with the front side (C terminus) positioned towards the 3′ end of the elongating DNA strand. This ensures that DNA polymerases, which bind to the back side of PCNA, are orientated towards the growing end (Moldovan et al., 2007). The inner surface of the PCNA ring, which is in close proximity to DNA, consists of a positively charged helical region, whereas the outer surface has a negatively charged β structure (Krishna et al., 1994; Gulbis et al., 1996; Matsumiya et al., 2001; Strzalka et al., 2009). The recently reported first low-resolution crystal structure of yeast PCNA complexed with a DNA fragment shed more light on the nature of PCNA–DNA interactions (McNally et al., 2010). Mutational analysis of yeast PCNA suggested that positively charged residues in the centre of the clamp create a binding surface that makes contact with DNA (McNally et al., 2010). Note that although the DNA pol III β subunit is composed of three domains and the PCNA subunit possesses two globular domains, both of them form a ring structure with a total of six globular domains. The interaction between PCNA monomers is similar to that of DNA pol III β subunits and is characterized by a head-to-tail orientation. Each PCNA monomer is composed of an inter-domain connecting loop (IDCL) which makes significant contribution to the biological activity of PCNA. The IDCL serves to connect the N- and C-terminal domains of each monomer, although this is not its sole function. Studies on PCNA showed that IDCL is an important docking site for different interacting proteins such as DNA pol δ, p21, DNA ligase 1 (DNA lig1), flap endonuclease (Fen1) and DNA-(cytosine-5) methyl transferase. Other important regions are located both in the N terminus where interaction between PCNA α-helices and cyclin-D was found, and in the C-terminal region, which is involved in the interaction with proteins such as DNA pol ɛ, replication factor C (RFC), cyclin-dependent kinase 2, and growth arrest and DNA damage 45 protein (Maga and Hubscher, 2003).
Fig. 1.
Structure of Arabidopsis thaliana PCNA1. The three-dimensional model of AtPCNA1 was adapted from the Protein Data Bank (pdb number: 2ZVV). Different colours were used for each subunit: green, yellow and olive. The inter-domain connecting loop and C terminus were coloured in blue and red, respectively. (A) and (B) present the side and front views of the PCNA ring, respectively. The model was generated using PyMol software.
In the amino acid sequence of PCNA several conserved motifs and residues were identified. The most important motifs are: (1) the D41 residue responsible for stimulation of DNA pol δ and efficient stimulation of RFC ATPase activity (Ayyagari et al., 1995; Fukuda et al., 1995); (2) Q125L126G127I128, which is essential for binding of p21 and DNA pol δ (Gulbis et al., 1996; Jonsson et al., 1998; Zhang et al., 1998); (3) V188D189K190, which is conserved within plants and vertebrates (Jonsson et al., 1998); and (4) L251A252P253K254 responsible for proper folding of PCNA (Jonsson et al., 1998).
FUNCTION OF PCNA IN DNA REPLICATION
The control of DNA replication is a key element in the proper functioning of a cell, and it may influence genome stability. Duplication of the genetic material that occurs in S phase of the cell cycle must be coordinated with other cellular processes, for example mitosis. DNA replication is regulated mainly at the initiation step as a result of cooperation between different signalling pathways controlling the cell cycle (Waga and Stillman, 1998).
DNA synthesis and maturation of the Okazaki fragments
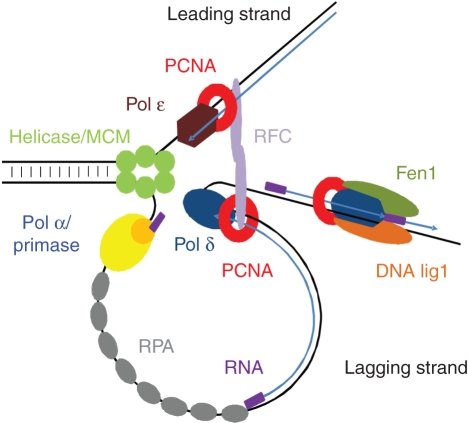
DNA replication in plants (for a review see Bryant, 2010), as in mammals, is initiated by phosphorylation of some replication initiation factors that form the origin recognition complex (ORC) at the origin of replication (ori). This phosphorylation occurs upon transition from the G1 to S phase of the cell cycle and leads to the activation of the ori by dissociation and degradation of the CDC6 protein. Next, other proteins, including the initiating DNA polymerase, are loaded stepwise onto the ori. Strand separation by a DNA helicase stimulated by the single-stranded DNA binding protein RPA (Ishibashi et al., 2006) is the last step of the pre-initiation stage (Fig. 2). DNA synthesis is initiated at both the leading and the lagging strands by the DNA polymerase α/primase complex that synthesizes short RNA primers. These primers are then elongated by DNA pol α, an enzyme that has been extensively studied in plants (Bryant et al., 1992, 2000; Benedetto et al., 1996; Garcia-Maya and Buck, 1998). In the next step, the initiatory RNA–DNA primer is recognized by the RFC protein complex (Furukawa et al., 2003) that binds to the 3′ end, thus leading to dissociation of the DNA pol α/primase complex. RFC acts as a DNA-dependent ATPase, and plays a role in DNA replication by loading the trimeric PCNA protein on DNA at the end of the RNA–DNA primer synthesized by DNA pol α/primase. Biochemical analyses using a model system of the virus SV40 (Simian vacuolating virus 40) reconstituted from purified mammalian proteins revealed the involvement of other DNA polymerases in addition to DNA pol α, namely DNA pol δ and DNA pol ɛ, in DNA synthesis (Burgers, 1998). PCNA functions as a platform for DNA pol δ/ɛ and other replication proteins that interact with PCNA. It is important to note that only the presence of PCNA at the replication fork (Fig. 2) enables the exchange of DNA pol α for the other polymerases continuing DNA synthesis. Binding of PCNA to the 3′ end of the primer prevents re-loading of DNA pol α and acts as a recruitment signal for replicases with higher processivity: DNA pol δ/ɛ (Maga and Hubscher, 2003). After loading polymerases on DNA, the DNA pol δ/ɛ–PCNA complex moves along the template strand with DNA synthesis occurring concomitantly on the leading and lagging strand, in a continuous and discontinuous manner, respectively. On the leading strand, the primer is extended by DNA pol ɛ, whereas DNA pol δ completes the synthesis of each Okazaki fragment on the lagging strand template (Burgers, 2009). The latter polymerase requires a processivity factor, PCNA. In plants, PCNA was identified (Suzuka et al., 1989) prior to the characterization of DNA polymerase δ as a replicative enzyme in wheat, the activity of which was stimulated by PCNA (Richard et al., 1991). Genes encoding subunits of DNA pol δ were identified in the genomes of other plant species such as soybean (Collins et al., 1997), rice (Uchiyama et al., 2002) and Arabidopsis (Shultz et al., 2007). Not surprisingly, genes coding for DNA pol δ and PCNA are both expressed in dividing cells (Garcia et al., 2006; Kosugi and Ohashi, 2002).
Fig. 2.
Model of eukaryotic DNA replication. PCNA function in DNA synthesis (a cofactor of DNA pol δ/ɛ) and in maturation of the Okazaki fragments is shown (modified from Burges, 2009).
The last stage of DNA replication is maturation of the Okazaki fragments, during which removal of the primers occurs followed by gap filling and ligation of the adjacent ends. The primer is displaced by the replicating polymerase (DNA pol δ) and the single-stranded fragment consisting of the RNA–DNA primer is removed by the flap endonuclease Fen1, with or without the help of RNase H (Kao and Bambara, 2003; Shultz et al., 2007). Finally, the Okazaki fragments of the lagging strand and the replicon-sized pieces of DNA of the leading strand are joined together by DNA lig1 (Taylor et al., 1998; Bray et al., 2008). Both Fen1 and DNA lig1 cooperate with PCNA during DNA replication in mammals, but in plants the interaction with PCNA was only shown for Fen1 (Kimura et al., 2001). The sequential binding of proteins involved in maturation of the Okazaki fragments ensures the directionality of this process. Of note, the localization of the PCNA ring on DNA is ideal for allowing this protein to function in stabilization, recruitment and dynamic exchange (due to differences in the affinities and the interaction constants) of various replication proteins, thus making PCNA a key coordinator of the replication process.
Translesion synthesis
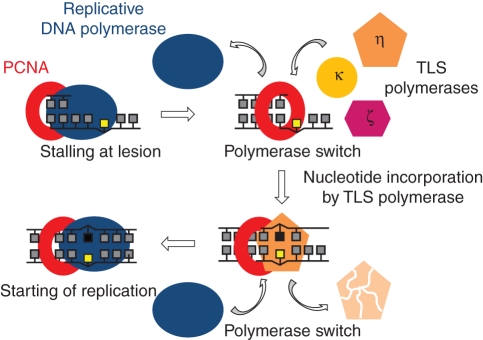
During replication of eukaryotic DNA, synthesis across damaged templates is achieved by specialized DNA polymerases in a process called TLS (translesion synthesis). The list of TLS polymerases identified in mammalian, yeast and plant cells increased dramatically over the last decade, and now includes DNA pol η, ι, κ, ζ, Rev1 and Rev7 (Burgers et al., 2001; Prakash et al., 2005; Takahashi et al., 2005). Synthesis of DNA from a damaged template enables completion of DNA replication by overcoming the stalled replication fork at the damage site (replicative polymerases are unable to replicate damaged DNA). In such a situation, DNA polymerases δ/ɛ are temporarily replaced by TLS polymerases, which incorporate a correct or incorrect nucleotide (Fig. 3). The initial recruitment of TLS polymerases to the replication fork occurs via their interaction with PCNA, as shown for yeast and human proteins (Haracska et al., 2001a, b, 2002), and is stimulated by monoubiquitination of PCNA (see below; Soria and Gottifredi, 2010). PCNA does not change the processivity or fidelity of TLS polymerases, but its presence stimulates their activity resulting in increased efficiency of nucleotide incorporation at sites opposite to the DNA damage (Prakash et al., 2005; Shcherbakova and Fijalkowska, 2006). TLS polymerases act either alone or in pairs depending on the type of damage. These enzymes must be tightly controlled as, due to their permissive active site and lack of proofreading activity, they are prone to errors and thus can induce mutations. One of the proteins that can control TLS polymerases is p21, another interacting partner of PCNA with involvement in cell cycle control (see below). Finally, DNA synthesis must be resumed later by a replicative polymerase that first needs to be reloaded on DNA template. Although PCNA is likely to be involved in this second polymerase switch, an additional mechanism for removal of the TLS polymerase is required as well.
Fig. 3.
Translesion DNA synthesis (TLS). Switch of DNA polymerases is mediated by PCNA (modified from Lehmann, 2003).
THE ROLE OF PCNA IN DNA REPAIR
During the life cycle of plants cells undergo a certain number of divisions. Due to the continuous exposure of plants to harmful factors (endogenous and environmental), the genetic material may be damaged in different ways. Accumulation of spontaneous or induced mutations over numerous cell divisions threatens to perturb the proper functioning of the organism. The biological effect of mutagenic agents depends on the type of DNA damage they cause, and may result in uncontrolled cell proliferation or cell death. DNA-damaging agents may be chemical or physical factors and there are a multitude of the two. Endogenous mutagenic factors mainly include reactive free radicals such as the hydroxyl radical, nitric oxide and superoxide anion, while environmental mutagenic factors include UVB, ionizing radiation and chemicals. Stable inheritance of undamaged genetic information by progeny required the development of different types of DNA repair systems such as base excision repair (BER), nucleotide excision repair (NER), mismatch repair (MMR) and double-strand break repair (DSBR). Knowledge of DNA repair systems in eukaryotes comes mainly from studies conducted on yeast and animals.
Besides the role of PCNA in DNA replication it also performs other important functions in some of the aforementioned DNA repair systems (Tuteja et al., 2001; Maga and Hubscher, 2003; Stoimenov and Helleday, 2009). Unfortunately, the role of plant PCNA in DNA repair is poorly characterized and existing data demonstrating its importance during repair are indirect. The suspected role of plant PCNA during repair has been implied mainly from the identification and analysis of plant proteins whose yeast and animal homologues, involved in DNA repair systems, were shown to interact with PCNA. These homologous studies suggest that the key role of PCNA in DNA repair is exerted via its interaction, not only with DNA pol δ/ɛ during DNA re-synthesis but also with other DNA repair proteins.
Base excision repair
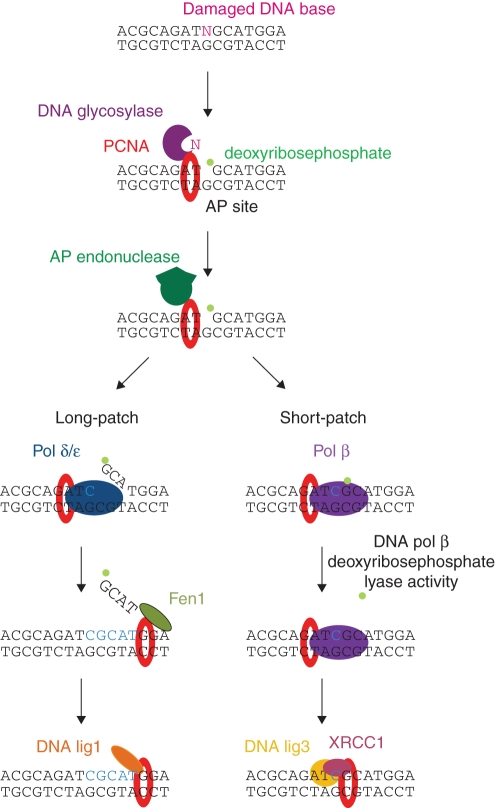
BER is responsible for replacing chemically altered nucleotide bases in DNA. The first step is the release of the damaged base from the DNA chain by DNA glycosylase, resulting in formation of an AP site. Next, AP endonuclease cleaves the phosphodiester bond of the damaged nucleotide and DNA re-synthesis is performed by a DNA pol δ/ɛ- (long-patch BER) or DNA pol β- (short-patch BER) dependent mechanism. In long-patch BER, Fen1 and DNA lig1 are required to complete the process, whereas the DNA pol β pathway involves DNA lig3 and XRCC1 (Fig. 4; Sancar et al., 2004; Bray and West, 2005; Kimura and Sakaguchi, 2006). The importance of PCNA in BER was documented by Matsumoto and colleagues who found that the PCNA-dependent DNA pol δ/ɛ-mediated repair pathway of abasic sites in Xenopus laevis (long-patch BER) was employed as an alternative mechanism to the DNA pol β-mediated pathway (short-patch BER; Matsumoto et al. 1994b, 1999). Next, yeast and mammalian enzymes involved in BER such as AP endonuclease 1 (Dianova et al., 2001), AP endonuclease 2 (Tsuchimoto et al., 2001; Unk et al., 2002), uracil-DNA glycosylase 2 (Krokan et al., 2001; Ko and Bennett, 2005), 3-methyladenine-DNA glycosylase (Xia et al., 2005), human homologue of E. coli endonuclease III (Oyama et al., 2004) and MutY homologue (Parker et al., 2001) were shown to interact with PCNA. This litany of interacting partners supports a role of PCNA not only in the DNA re-synthesis step, but also in other steps of BER. Additional, new evidence supporting the role of PCNA in short-patch BER was provided by experiments showing recruitment of XRCC1 protein by PCNA to replication foci and interaction of PCNA with DNA pol β (Kedar et al., 2002; Fan et al., 2004). XRCC1 is believed to work as a scaffolding protein in both BER and single-strand break repair, as it interacts with several proteins participating in these related pathways (Fan et al., 2004). The mechanism of BER in plants is not well analysed; however, some key enzymes taking part in this process have been discovered and characterized. The first, uracil-DNA glycosylase, was found in carrot cells (Talpaert-Borle and Liuzzi, 1982; Talpaert-Borle, 1987). Next, the cDNA coding for 3-methyl glycosylase was isolated from Arabidopsis (Santerre and Britt, 1994) followed by isolation of other DNA glycosylases (Dany and Tissier, 2001; Gao and Murphy, 2001; García-Oritz et al., 2001; Murphy and Gao, 2001; Morales-Ruiz et al., 2003; Murphy and George, 2005). In addition, sequencing of the rice genome revealed the presence of ten types of DNA glycosylases and three AP endonucleases (Kimura and Sakaguchi, 2006). Moreover, other factors required for plant DNA re-synthesis such as DNA pol δ (Uchiyama et al., 2002), DNA pol ɛ (Ronceret et al., 2005), DNA lig1 (Wu et al., 2001) and Fen1 (Kimura et al., 2003) have been characterized. These findings suggest that the mechanisms of BER are similar in all eukaryotes. Therefore, although the interaction of plant PCNA with BER factors has not been reported so far, we may assume that such interactions exist in nature.
Fig. 4.
Model of base excision repair in eukaryotic cells (modified from Kimura and Sakaguchi, 2006).
Nucleotide excision repair
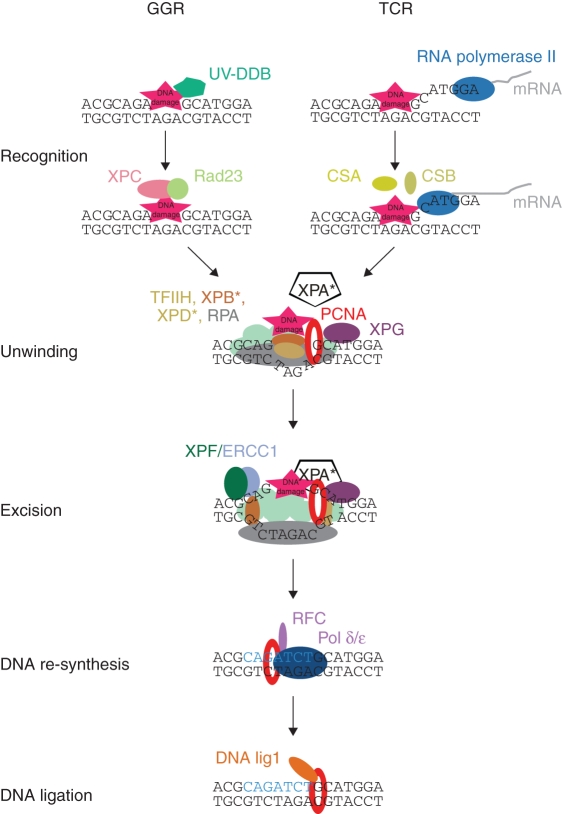
NER recognizes and repairs different types of DNA damage, including bulky DNA lesions. It is classified into two groups, global genome repair (GGR) and transcription-coupled repair (TCR). GGR works at any region of the genome whereas TCR is limited to actively transcribed sites. In contrast to the short- and long-patch BER, GGR- and TCR-NER require different proteins/mechanisms for DNA damage recognition. For damage recognition in the GGR pathway, UV-DDB and XPC/Rad23 complexes are required whereas in the TCR mechanism RNA polymerase II together with CSA and CSB proteins play a key role. After damage recognition, GGR and TCR use the same repair pathway by recruiting the TFIIH transcription factor (including XPB, XPD), XPA and RPA that are required for DNA unwinding. Next, XPF/ERCC1- and XPG-dependent excision of a DNA segment containing the damaged site occurs, followed by the PCNA-, RFC-, DNA pol δ/ɛ- and DNA lig1-dependent re-synthesis step (Fig. 5; Sancar et al., 2004; Bray and West, 2005; Kimura and Sakaguchi, 2006). The first information about the involvement of PCNA in NER came from studies of human cells that showed PCNA is one of the proteins required for efficient reconstitution of the NER system (Shivji et al., 1992; Aboussekhra et al., 1995). The main role of PCNA in NER is during new DNA fragment re-synthesis, and is achieved through PCNA interaction with DNA pol δ/ɛ, RFC, DNA lig1 and Fen1: similar to its role in BER. However, the interaction between PCNA and XPG endonuclease suggests other additional roles for PCNA in NER (Gary et al., 1997). Currently, it is known that PCNA is recruited specifically at the site of XPG incision. Additionally, immunofluorescence studies on wild-type and XPA mutant cells showed that after UV treatment, PCNA localization to the damaged sites in the nucleus is dependent on functional XPA protein. These findings clearly indicate that PCNA is important not only in the DNA re-synthesis step but also in other steps of NER (Aboussekhra and Wood, 1995; Li et al., 1996; Miura and Sasaki, 1996). PCNA located on DNA seems first to coordinate the recruitment of proteins essential for proper DNA repair (XPG, XPA), followed by the action of replication fork enzymes completing the repair of the damaged site. In plant cells, NER-related genes were extensively studied in Arabidopsis, mainly by isolation of UV-sensitive mutants. Among isolated mutants, UVH1, UVH3 and UVH6 were found to carry mutations in the homologues of human XPF(AtRad1), XPG(AtRad2) and XPD(AtRad3) genes, respectively (Fidantsef et al., 2000; Gallego et al., 2000; Liu et al., 2000, 2001, 2003). In addition, sequencing of the Arabidopsis thaliana genome resulted in identification of genes coding for proteins similar to the mammalian proteins involved in NER such as XPC, XPB1, XPB2, XPD, XPF, XPG, ERCC1, CEN2, CSA, CSB and Rad23 (Bray and West, 2005). Surprisingly, a homologue of the XPA gene has not yet been found in plants. Although this suggests that the mechanism of NER in plants and animals is not entirely the same, it does not exclude a role for plant PCNA in NER that is analogous to mammalian/yeast PCNA.
Fig. 5.
Model of nucleotide excision repair in eukaryotic cells (modified from Kimura and Sakaguchi, 2006). Asterisks mark proteins for which homologues have not yet been found in plants. GGR, global genome repair; TCR, transcription coupled repair.
Mismatch repair
MMR corrects misincorporated bases using DNA methylation patterns as a marker of the template strand. The base mismatch can occur due to DNA polymerase errors or small insertions/deletions loops developed during replication; it may also arise during recombination. Recognition of the mismatched site is dependent on its type. In Arabidopsis, either MutSα (MSH2/MSH6 heterodimer) or MutSγ (MSH2/MSH7 heterodimer) recognizes incorrectly matched bases and single-nucleotide additions, whereas MutSβ (MSH2/MSH3 heterodimer) recognizes a region with redundant DNA loops (Culligan and Hays, 2000; Wu et al., 2003). Although the next steps of MMR in plants have not been well characterized it is believed that they employ a mechanism similar to that observed in animals and yeasts. After DNA damage recognition, other components such as MutLα (MLH1/PMS2 heterodimer), EXO1, RPA, HMGB1 and DNA replication enzymes are required to repair the damaged site (Fig. 6; Bray and West, 2005; Jiricny, 2006; Kimura and Sakaguchi, 2006; Moldovan et al., 2007). In MMR, PCNA works not only as a DNA pol δ processivity factor but also in early events preceding DNA synthesis (Umar et al., 1996). PCNA is involved in the mismatch recognition and DNA incision processes. During studies on yeast and mammalian proteins, PCNA was found to interact with MSH3 and MSH6, and to enhance their specific binding to mismatched sites (Clark et al., 2000; Flores-Rozas et al., 2000; Kleczkowska et al., 2001; Lee and Alani, 2006). Moreover, PCNA and MSH2-MSH6 were shown to form a stable ternary complex stimulating the preferential binding of MSH2-MSH6 to the mispaired DNA bases (Lau and Kolodner, 2003). Next, other members of the MMR pathway, such as MutL homologue 1 (MLH1) and exonuclease 1 (EXO1; Lee and Alani, 2006), were identified to interact with PCNA. Recent studies suggest that the recruitment of PCNA, RFC and EXO1 is necessary to activate the latent endonuclease activity of the MutLα complex (MLH1-PMS2; Kadyrov et al., 2006). Isolation of plant MSH and MLH genes (MSH2, MSH3, MSH6, MSH7, MLH1) was reported first for Arabidopsis (Ade et al., 1999, 2001; Culligan and Hays, 1997, 2000; Pelletier et al., 1999). Moreover, Mus1 and Mus2 homologues of MutS were isolated from Zea mays (Horwath et al., 2002). These findings suggest that the MMR mechanisms in mammalian/yeast and plants cells are similar, and thus that plant PCNA is likely to be involved in MMR in plant cells as well.
Fig. 6.
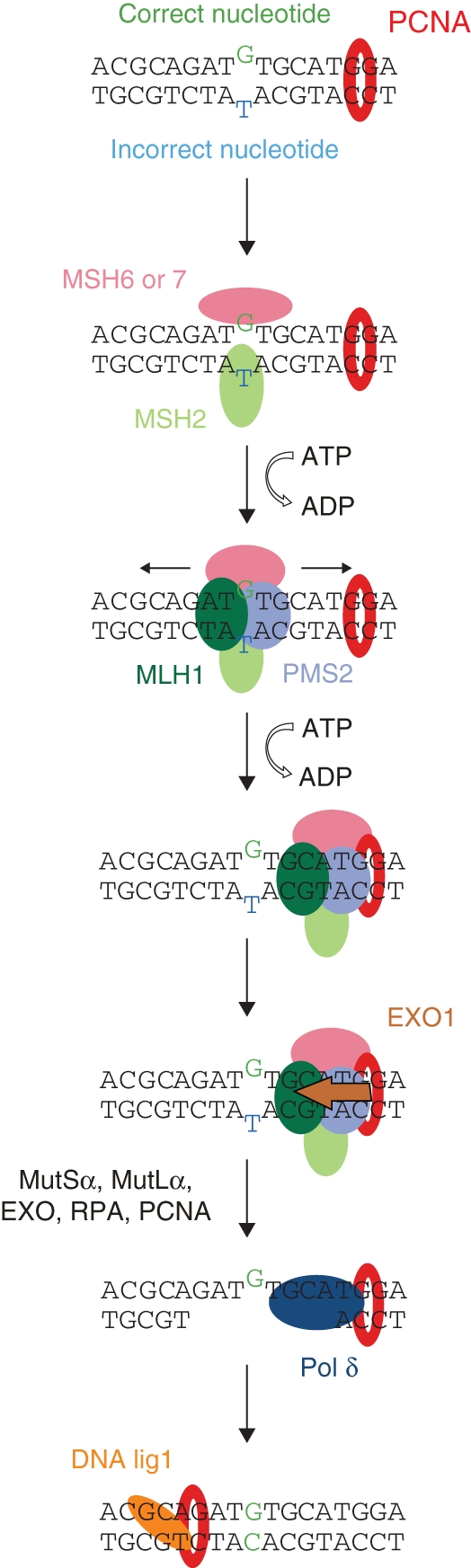
Model of mismatch repair in plant cells (modified from Stojic et al., 2004).
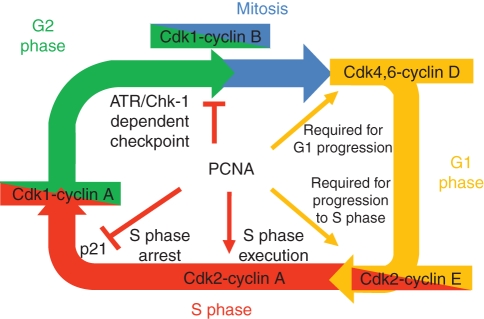
ROLE FOR PCNA IN CELL CYCLE CONTROL
Proper regulation of the cell cycle is a key element controlling cell division. Many proteins are involved in this process including cyclins and cyclin-dependent kinases that regulate accurate transition of the cell through subsequent phases of the cell cycle: G1, where cells grow in size, assess their metabolic status and prepare for division; S, where genome duplication occurs; G2, where cells check for completion of DNA replication and get ready to divide; and M, where mitosis takes place (Fig. 7). PCNA interacts with several eukaryotic cell cycle proteins. Biochemical analyses of animal PCNA showed interaction of this protein with the cyclin A–Cdk2 complex. This suggests that PCNA acts as a link between Cdk2 and its substrates, for example RFC and DNA lig1, which are phosphorylated by the Cdk2 kinase (Koundrioukoff et al., 2000).
Fig. 7.
Model for interactions of PCNA with proteins regulating the cell cycle, based on studies of mammalian PCNA (adapted from Maga and Hubscher, 2003).
Under normal conditions progression of the cell cycle is undisturbed. However, DNA damage and cell ageing lead to production of the p21 protein, which blocks transition from G1 to S phase. p21 achieves this cell cycle arrest between G1 and S phase by inhibiting the activity of cyclin-dependent kinases (Sherr and Roberts, 1995). The p21 protein has been identified in a complex formed by PCNA, cyclins and cyclin-dependent kinases (Xiong et al., 1992, 1993). Studies performed on terminally differentiated cardiomyocytes showed that cell cycle arrest was dependent on maintaining a high concentration of p21, which in turn reduced the level of PCNA (Engel et al., 2003). Regulation of p21 expression is modulated by various factors such as p53 (el-Deiry et al., 1993), MyoD (Guo et al., 1995), STAT (Chin et al., 1996) and C/EBPα (Timchenko et al., 1996). In vitro studies on the interaction between p21 and PCNA suggest that binding of p21 to the sliding clamp formed by DNA-associated PCNA may block its interaction with RFC and DNA pol δ (Flores-Rozas et al., 1994; Waga et al., 1994), thus stopping DNA synthesis. PCNA seems to act as an important mediator of p21 function, which raises the mechanistic question of how p21 is able to regulate both the cell cycle and DNA replication. A likely explanation of this phenomenon lies in the number of PCNA-interacting partners. Competition between p21 and DNA pol δ for the same binding site within the PCNA trimer induces a particular cell response at the molecular level, for example stalling of the replication fork and cell division arrest (Maga and Hubscher, 2003). As PCNA acts as a cofactor of DNA pol δ for DNA synthesis during not only DNA replication but also DNA repair, its interaction with p21 might affect the latter process as well. However, published data are equivocal: some results indicate that p21–PCNA interaction inhibits NER and MMR (Pan et al., 1995; Umar et al., 1996), whereas other groups did not observe any inhibitory effect of p21 binding to PCNA on PCNA-dependent NER (Li et al., 1994; Shivji et al., 1998). More recent reports indicate another aspect of the p21–PCNA interaction in DNA replication and TLS. p21 was shown to be degraded in a CRL4CDT2- (type E3 ligase) dependent manner in response to DNA damage caused by some genotoxic factors (e.g. UV radiation and methylmethane sulfonate), and the interaction of p21 with PCNA was a prerequisite for this proteolysis (for a review see Soria and Gottifredi, 2010). PCNA-coupled degradation of p21 bound to the replication complex stalled at DNA lesions was proposed as a requirement for polymerase switch (DNA pol δ → DNA pol η), thus allowing for TLS. Next, CRL4CDT2-driven degradation of DNA pol η leads to reloading of DNA pol δ by PCNA, restarting the replicative DNA synthesis (Soria and Gottifredi, 2010).
A plant homologue of p21 protein has not been identified so far. Interestingly, plant PCNA proteins are able to interact with human p21 (Ball and Lane, 1996; Strzalka et al., 2009, 2010). As cell division is regulated by molecular machinery that has been evolutionary conserved from fungi to mammals (including plants), this interaction not only indicates high structural and functional similarity between animal and plant PCNAs, but may also suggest the existence of a protein similar and analogous in function to animal p21 that may be involved in regulation of the plant cell cycle (Boruc et al., 2010).
POST-TRANSLATIONAL MODIFICATIONS OF PCNA
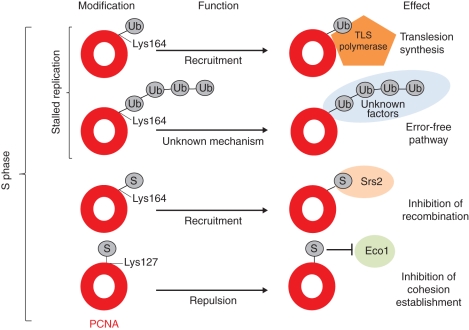
Post-translational modifications regulate proteome activity in the mediation of complex, hierarchical, regulatory processes that are crucial to eukaryotic cell function. Among the myriad of modifications capable of changing and regulating the function of a protein, ubiquitination and sumoylation were found to modify the functions of PCNA (Lee and Myung, 2008). These modifications consist of a covalent attachment of ubiquitin (Ub) or SUMO (small ubiquitin-like modifier) peptides. The Ub modifier is most widely known for targeting its protein substrates for proteosomal degradation (Konstantinova et al., 2008). For example, target proteins polyubiquitinated at Lys48 are recognized by specific receptors within the 26S proteasome or within adaptor proteins associated with the proteasome (Konstantinova et al., 2008). In addition, mono- and polyubiquitination of protein targets using different Ub lysine residue linkages facilitate regulation of subcellular localization, chromatin structure, signal transduction, ribosomal protein synthesis and DNA damage repair (Ikeda and Dikic, 2008). DNA damage that is not repaired prior to DNA replication in S phase blocks the progression of the DNA replication fork. Stalled DNA replication forks activate a specific DNA repair mechanism called post-replication repair (PRR). There are two PRR pathways known in eukaryotic cells: (1) TLS (described above) that simply bypasses DNA damage and (2) damage avoidance pathway that probably uses the replicated undamaged sister chromatid for template (Ulrich, 2007). The second pathway is error-free, whereas the first pathway is error-prone as TLS polymerases may incorporate an incorrect nucleotide opposite the DNA lesion. These errors are later repaired by BER, NER or homologous recombination. Different PRR pathways are determined by different modifications of PCNA (Fig. 8). PCNA can be modified either by sumoylation or ubiquitination at the same Lys164 residue in response to the stalling DNA replication fork (Lee and Myung, 2008); in addition, the SUMO modifier can be attached to PCNA at the Lys127 residue (Bergink and Jentch, 2009). In yeasts and mammals, PCNA monoubiquitination promotes the recruitment of TLS polymerases that facilitate DNA damage bypass, whereas PCNA polyubiquitination is thought to promote the error-free damage avoidance through template switching, although the molecular mechanism is not clearly understood (Lee and Myung, 2008). PCNA sumoylation has been reported only in yeast cells. Lys164-sumoylated PCNA recruits the Srs2 helicase to block the formation of Rad51-ssDNA filament to prevent inappropriate homologous recombination (Watts, 2006; Lee and Myung, 2008). Sumoylation of PCNA on Lys127 inhibits interaction with certain PCNA-binding proteins, e.g. Eco1, which is responsible for establishing sister-chromatid cohesion in S phase (Bergink and Jentch, 2009). Intriguingly, none of the above-mentioned post-translational modifications of PCNA has been reported so far for plants, although the ubiquitination and sumoylation processes, along with their factors, are well known (Miura and Hasegawa, 2010).
Fig. 8.
PCNA modifications during S phase and their effects on the genome (adapted from Bergink and Jentsch, 2009).
PCNA GENES
Gene number and their function
Genomes of all eukaryotic organisms contain at least one copy of the PCNA gene. In animals, one copy of the PCNA gene was found in the rat genome (Matsumoto et al., 1987), whereas one PCNA gene and several pseudogenes are present in mouse and human (Almendral et al., 1987; Ku et al., 1989; Travali et al., 1989; Yamaguchi et al., 1991). Interestingly, the genomes of Drosophila and Toxoplasma contain two PCNA genes (Ruike et al., 2006; Guerini et al., 2000). In plants, a single copy of the PCNA gene was found in genomes of Oryza sativa (rice), Catharanthus roseus (rose), Pinus nigra (black poplar), Pisum sativum, P. vulgaris, Brassica napus and Glycine max, whereas species such as A. thaliana, Z. mays, Daucus carota, Nicotiana tabacum and Phaseolus coccineus contain at least two PCNA genes. The presence of three functional PCNA genes was shown for archaeal genomes: Sulfolobus solfataricus and Aeropyrum pernix (Dionne et al., 2003; Imamura et al., 2007). Despite low sequence similarities (<25 %), PCNAs from S. solfataricus and A. pernix showed some analogous features. In both species, PCNAs formed a heterotrimeric ring structure. However, in S. solfataricus, none of these proteins could itself form a homotrimer (trimer formation occurred only in the presence of three different PCNA proteins; Dionne et al., 2003), whereas A. pernix PCNA2 was able to form a trimeric structure both by itself (a homotrimer) or with PCNA1 and PCNA3 proteins (a heterotrimer), while neither PCNA1 nor PCNA3 of A. pernix was able to form a homotrimer (Imamura et al., 2007). Moreover, it was shown that archaeal PCNA monomers exhibited different substrate interaction specificities, indicating that each PCNA is responsible for attracting different replication-related proteins to the replication fork (Dionne et al., 2003; Imamura et al., 2007). Drosophila melanogaster DmPCNA2 shows 51·7 % similarity to DmPCNA1 (Ruike et al., 2006), which possesses features typical of all PCNAs (Henderson et al., 1994). DmPCNA2 contains D41 and motif III, but its motifs I and II are incomplete. Nevertheless, DmPCNA2 was capable of forming a homotrimer and stimulating DNA pol δ activity. Differences in the expression pattern of DmPCNA1 and DmPCNA2 genes in response to UV treatment suggested that DmPCNA2 may function as an independent sliding clamp of DmPCNA1 during DNA repair (Ruike et al., 2006). In another organism containing two PCNA genes, Toxoplasma gondii, both gene products also contain D41 and motif III and are able to form homotrimers (Guerini et al., 2000). However, only TgPCNA1 probably serves as the major replisomal PCNA, whereas TgPCNA2 probably exhibits a different function (Guerini et al., 2005). In fact, no actual (specific) function could be shown for TgPCNA2, as disruption of this gene did not influence DNA polymerase activity, response to chemical mutagens or recombination frequency (Guerini et al., 2000).
In most known cases of plants containing two PCNA genes, a high level of amino acid sequence identity was observed: A. thaliana, 96·6 %; N. tabacum, 97·0 %; Z. mays, 98·5 %. Lower identity was observed for D. carota, 63·0 %, but this is mainly due to the presence of an additional 100 amino-acid-long C-terminal tail in DcPCNA2, with the identity level between the first 264 amino acids of DcPCNA1 and DcPCNA2 being 87·6 %. Recent studies on Arabidopsis PCNA1 and PCNA2 revealed that both proteins showed ability to interact with Arabidopsis DNA polymerase η and human p21 (Anderson et al., 2008; Strzalka et al., 2009). However, only AtPCNA2 was able to trigger restoration of normal UV resistance and mutation kinetics in a yeast rad30 mutant expressing the Arabidopsis POLH gene (yeast Rad30 and Arabidopsis POLH genes encode DNA polymerase η; Anderson et al., 2008). In addition, AtPCNA1 and AtPCNA2 genes showed slightly different expression patterns in response to the exposure of Arabidopsis plants to heavy metal cadmium ions, which cause genotoxic effects (Liu et al., 2009). These findings indicate that in eukaryotic cells the second PCNA protein may indeed exert functions different from those of PCNA1.
Intriguingly, proteins encoded by the PCNA1 and PCNA-like1 genes identified in the P. coccineus genome shared only 54 % of their amino acid sequence (Strzalka et al., 2010). PcPCNA1 behaved like a typical PCNA protein: it formed a homotrimer and stimulated the activity of human DNA polymerase δ. Additionally, PcPCNA1 interacted with a p21 peptide and was recognized by an anti-human PCNA monoclonal antibody. The second PCNA protein of runner bean, PcPCNA-like1, does not possess any of the motifs that are crucial for typical activity of PCNA and, consequently, none of the features of PcPCNA1 was observed in PcPCNA-like1. Interestingly, both the genetic organization of PcPCNA1 and PcPCNA-like1 genes and their expression patterns were similar. As the PcPCNA-like1 gene most likely encodes a functional protein, the PcPCNA-like1 protein must exert yet unknown functions, different from those of PcPCNA1. However, it cannot be excluded that PcPCNA-like1 requires PcPCNA1 for formation of the trimeric ring and/or for exerting its function. Alternatively, the PcPCNA-like1 gene may represent an expressed pseudogene.
Gene expression
Expression of the PCNA genes in all organisms is correlated with cell proliferation and thus with DNA synthesis during genome replication in S phase of the cell cycle. In plants, maize PCNA1 and PCNA2 genes were expressed in root and shoot tips as well as in young spikeletes and cobs but not in leaves, old spikeletes or pollen. Furthermore, it has been shown that the PCNA transcript was intensively produced in rice roots and root tips but not in mature leaves (Kimura et al., 2001). Also, the data presented by Shimizu and Mori, who studied levels of PCNA transcripts in dormant auxiliary buds, confirmed the correlation between PCNA gene expression and cell proliferation. They demonstrated that before decapitation, the level of the transcript in dormant auxiliary buds was minuscule, whereas after decapitation, PCNA gene expression in rice was remarkably up-regulated, which correlated with bud growth and thus with cell proliferation (Shimizu and Mori, 1998a, b).
A correlation between PCNA gene expression and cell proliferation was also observed in other plant species. It was found in runner bean that at the beginning of seed germination, PcPCNA1 transcripts were present at low levels, followed by up-regulation of the PcPCNA1 gene during the first stage of germination and then down-regulation during the late phase of germination (Strzalka et al., 2010). By contrast, in root, stem and leaf tissues, the level of PcPCNA1 transcript was low, except in the micropylar region of seeds, where this gene was actively expressed. The observed increase in PcPCNA1 expression in the embryonic axis during seed germination and in the developing embryo from the micropylar region of developing seeds as well as the increase in ZmPCNA expression in root tips and young tissues clearly relates to intensive cell proliferation. As cell proliferation is accompanied by DNA replication, an increase in expression of plant PCNA genes is most likely due to the resumption of DNA replication. The observed decrease in the level of the PcPCNA1 transcript at later stages of germination when young seedlings are formed is most likely due to a shift of the ratio between dividing (meristematic) and non-dividing cells towards the latter. Low expression levels of the PcPCNA1 and ZmPCNA genes in mature plant organs confirm a correlation between PCNA expression and cell proliferation/DNA replication as these organs predominantly contain non-dividing cells. Direct proof that PCNA expression correlates with cell proliferation and DNA replication was provided by studies performed on synchronized plant cell culture that showed PCNA expression is mainly confined to S phase (Kodama et al., 1991).
Regulatory elements of PCNA gene expression were studied in a few organisms such as rice and tobacco. Upstream sequences of the rice PCNA gene were shown to mediate expression of the PCNA–GUS chimeric gene in meristems of transgenic tobacco plants (Kosugi et al., 1991). Moreover, two PCNA gene promoter elements essential for meristematic-tissue-specific expression were identified (Kosugi et al., 1995). Continuation of this work resulted in the identification of two proteins, PCF1 and PCF2, which specifically bind to cis elements in the rice PCNA gene (Kosugi and Ohashi, 1997). E2F-like sites of the rice and tobacco PCNA promoter were shown to be required for meristematic-tissue-specific expression of this gene in actively dividing cells (Kosugi and Ohashi, 2002). Engagement of the E2F site of the tobacco PCNA gene promoter was presented by Hanley-Bowdoin's group who found that the E2F1 + 2 sites contribute to repression of the PCNA promoter in mature tissues, whereas the E2F1 site with transcription activators positively regulates PCNA gene expression in young leaves (Egelkrout et al., 2002). Presence of the E2F binding sites in the promoter of the PCNA gene provides additional proof for correlation of PCNA gene expression with DNA replication, as these regulatory elements are found in promoter regions of other genes encoding proteins involved in DNA replication (Bryant, 2010).
CONCLUSIONS
Although our knowledge of plant PCNA lags far behind that of animal and yeast PCNA, the picture emerging from the data gathered so far clearly indicates that eukaryotic PCNA has remained conserved in structure and function. Expression of the PCNA genes correlates with cell proliferation and DNA replication and, not surprisingly, the protein was shown to be involved in both processes. In addition, PCNA plays a key role in three DNA repair pathways, BER, NER and MMR, and exerts its function via direct interaction with various proteins involved in these processes. Besides DNA polymerases engaged in DNA synthesis during DNA replication and repair, these interactions also occur between factors required in the steps prior to DNA synthesis. Furthermore, the interaction of PCNA with other factors prevents inappropriate homologous recombination, allows for sister-chromatid cohesion and positively or negatively controls progression of the cell cycle. Moreover, in some processes involving DNA synthesis PCNA acts as a polymerase switch to ensure replication of damaged DNA. Many of the above-described functions of PCNA are regulated by post-translational modifications of PCNA and its interaction partners.
Why genomes of some eukaryotes, including plants, contain more than one PCNA gene is intriguing. Duplicated PCNA genes seem to have slightly different functions with one gene encoding the main PCNA protein showing all, or nearly all, the features characteristic of PCNA while a second gene is most likely involved in some aspects of the cell response to DNA-damaging factors. The exact function of the second PCNA gene in both animal and plant cells remains to be discovered. In a few cases, animal genomes contain PCNA pseudogenes, whereas the presence of PCNA pseudogenes in plant genomes has not yet been proven.
ACKNOWLEDGEMENTS
This work was partly supported by an internal grant from the University of Lethbridge and by Grant N N301 474438 from the Polish Ministry of Science and Higher Education. We thank Dr Jody Filkowski for English revision of the manuscript.
LITERATURE CITED
- Aboussekhra A, Wood RD. Detection of nucleotide excision repair incisions in human fibroblasts by immunostaining for PCNA. Experimental Cell Research. 1995;221:326–332. doi: 10.1006/excr.1995.1382. [DOI] [PubMed] [Google Scholar]
- Aboussekhra A, Biggerstaff M, Shivji MK, et al. Mammalian DNA nucleotide excision repair reconstituted with purified protein components. Cell. 1995;80:859–868. doi: 10.1016/0092-8674(95)90289-9. [DOI] [PubMed] [Google Scholar]
- Ade J, Belzile F, Philippe H, Doutriaux MP. Four mismatch repair paralogues coexist in Arabidopsis thaliana: AtMSH2, AtMSH3, AtMSH6-1 and AtMSH6-2. Molecular and General Genetics. 1999;262:239–249. doi: 10.1007/pl00008640. [DOI] [PubMed] [Google Scholar]
- Ade J, Haffani Y, Beizile FJ. Functional analysis of the Arabidopsis thaliana mismatch repair gene MSH2. Genome. 2001;44:651–657. [PubMed] [Google Scholar]
- Almendral JM, Huebsch D, Blundell PA, Macdonald-Bravo H, Bravo R. Cloning and sequence of the human nuclear protein cyclin: homology with DNA-binding proteins. Proceedings of the National Academy of Sciences of the USA. 1987;84:1575–1579. doi: 10.1073/pnas.84.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson H, Vonarx EJ, Pastushok L, et al. Arabidopsis thaliana Y-family DNA polymerase η catalyses translesion synthesis and interacts functionally with PCNA2. The Plant Journal. 2008;55:895–908. doi: 10.1111/j.1365-313X.2008.03562.x. [DOI] [PubMed] [Google Scholar]
- Ayyagari R, Impellizzeri KJ, Yoder BL, Gary SL, Burgers PM. A mutational analysis of the yeast proliferating cell nuclear antigen indicates distinct roles in DNA replication and DNA repair. Molecular and Cellular Biology. 1995;15:4420–4429. doi: 10.1128/mcb.15.8.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KL, Lane DP. Human and plant proliferating-cell nuclear antigens have a highly conserved binding site for the p53-inducible gene product p21WAF1. European Journal of Biochemistry. 1996;237:854–861. doi: 10.1111/j.1432-1033.1996.0854p.x. [DOI] [PubMed] [Google Scholar]
- Bauer GA, Burgers PM. The yeast analog of mammalian cyclin/proliferating-cell nuclear antigen interacts with mammalian DNA polymerase delta. Proceedings of the National Academy of Sciences of the USA. 1988;85:7506–7510. doi: 10.1073/pnas.85.20.7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetto JP, Ech-Chaoui R, Plissonneau J, Laquel P, Litvak S, Castroviejo M. Changes of enzymes and factors involved in DNA synthesis during wheat embryo germination. Plant Molecular Biology. 1996;31:1217–1225. doi: 10.1007/BF00040838. [DOI] [PubMed] [Google Scholar]
- Bergink S, Jentch S. Principles of ubiquitin and SUMO modifications in DNA repair. Nature. 2009;458:461–467. doi: 10.1038/nature07963. [DOI] [PubMed] [Google Scholar]
- Boruc J, Van den Daele H, Hollunder J, et al. Functional modules in Arabidopsis core cell cycle binary protein–protein interaction network. The Plant Cell. 2010;22:1264–1280. doi: 10.1105/tpc.109.073635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, Celis JE. A search for differential polypeptide synthesis throughout the cell cycle of HeLa cells. The Journal of Cell Biology. 1980;84:795–802. doi: 10.1083/jcb.84.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, Fey SJ, Bellatin J, Larsen PM, Celis JE. Identification of nuclear polypeptide (‘cyclin’) whose relative proportion is sensitive to changes in the rate of cell proliferation and to transformation. Progress in Clinical and Biological Research. 1982;85 (part A):235–248. [PubMed] [Google Scholar]
- Bravo R, Frank R, Blundell PA, Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987;326:515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Bray C, West C. DNA repair mechanisms in plants: crucial sensors and effectors for the maintenance of genome integrity. New Phytologist. 2005;168:511–528. doi: 10.1111/j.1469-8137.2005.01548.x. [DOI] [PubMed] [Google Scholar]
- Bray CM, Sunderland PA, Waterworth WM, West CE. DNA ligase: a means to an end joining. In: Bryant JA, Francis D, editors. The eukaryotic cell cycle. Abingdon: Taylor and Francis; 2008. pp. 203–217. [Google Scholar]
- Bryant JA. Replication of nuclear DNA. Progress in Botany. 2010;71:25–60. [Google Scholar]
- Bryant JA, Fitchett PN, Hughes SG, Sibson DR. DNA polymerase-α in pea is part of large multiprotein complex. Journal of Experimental Botany. 1992;43:31–40. [Google Scholar]
- Bryant JA, Brice DC, Fitchett PN, Anderson LE. A novel DNA-binding protein associate with DNA polymerase-α in pea stimulates polymerase activity on infrequently primed templates. Journal of Experimental Botany. 2000;51:1945–1947. doi: 10.1093/jexbot/51.352.1945. [DOI] [PubMed] [Google Scholar]
- Burgers PM. Eukaryotic DNA polymerases in DNA replication and DNA repair. Chromosoma. 1998;107:218–227. doi: 10.1007/s004120050300. [DOI] [PubMed] [Google Scholar]
- Burgers PM. Polymerase dynamics at the replication fork. The Journal of Biological Chemistry. 2009;284:4041–4045. doi: 10.1074/jbc.R800062200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgers PM, Koonin EV, Bruford E, et al. Eukaryotic DNA polymerases: proposal for revised nomenclature. The Journal of Biological Chemistry. 2001;276:43487–43490. doi: 10.1074/jbc.R100056200. [DOI] [PubMed] [Google Scholar]
- Celis JE, Bravo R, Larsen PM, Fey SJ. Cyclin: a nuclear protein whose level correlates directly with the proliferative state of normal as well as transformed cells. Leukemia Research. 1984;8:143–157. doi: 10.1016/0145-2126(84)90135-8. [DOI] [PubMed] [Google Scholar]
- Celis JE, Madsen P, Celis A, Hielsen HV, Gesser B. Cyclin (PCNA, auxiliary protein of DNA polymerase delta) is a central component of the pathway(s) leading to DNA replication and cell division. FEBS Letters. 1987;220:1–7. doi: 10.1016/0014-5793(87)80865-7. [DOI] [PubMed] [Google Scholar]
- Chin YE, Kitagawa M, Su WC, You ZH, Iwamoto Y, Fu XY. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science. 1996;272:719–722. doi: 10.1126/science.272.5262.719. [DOI] [PubMed] [Google Scholar]
- Clark AB, Valle F, Drotschmann K, Gary RK, Kunkel TA. Functional interaction of proliferating cell nuclear antigen with MSH2-MSH6 and MSH2-MSH3 complexes. The Journal of Biological Chemistry. 2000;275:36498–36501. doi: 10.1074/jbc.C000513200. [DOI] [PubMed] [Google Scholar]
- Collins JTB, Heinhorst S, Cannon GC. Characterization of DNA polymerases and a replication accessory protein from the Glycine max cell line SB-1, and detection of a delta-like DNA polymerase gene in soybean cDNA. Plant Physiology. 1997;114 (Suppl. 3):156. [Google Scholar]
- Culligan KM, Hays JB. DNA mismatch repair in plants (an Arabidopsis thaliana gene that predicts a protein belonging to the MSH2 subfamily of eukaryotic MutS homologs) Plant Physiology. 1997;115:833–839. doi: 10.1104/pp.115.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culligan KM, Hays JB. Arabidopsis MutS homologs – AtMSH2, AtMSH3, AtMSH6, and a novel AtMSH7 – form three distinct protein heterodimers with different specificities for mismatched DNA. The Plant Cell. 2000;12:991–1002. doi: 10.1105/tpc.12.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daidoji H, Takasaki Y, Nakene PK. Proliferating cell nuclear antigen (PCNA/cyclin) in plant proliferating cells: immunohistochemical and quantitative analysis using autoantibody and murine monoclonal antibodies to PCNA. Cell Biochemistry and Function. 1992;10:123–132. doi: 10.1002/cbf.290100209. [DOI] [PubMed] [Google Scholar]
- Dany AL, Tissier A. A functional OGG1 homologue from Arabidopsis thaliana. Molecular Genetics and Genomics. 2001;265:293–301. doi: 10.1007/s004380000414. [DOI] [PubMed] [Google Scholar]
- el-Deiry WS, Tokino T, Velculescu VE, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- Dianova II, Bohr VA, Dianov GL. Interaction of human AP endonuclease 1 with flap endonuclease 1 and proliferating cell nuclear antigen involved in long-patch base excision repair. Biochemistry. 2001;40:12639–12644. doi: 10.1021/bi011117i. [DOI] [PubMed] [Google Scholar]
- Dionne I, Nookala RK, Jackson SP, Doherty AJ, Bell SD. A heterotrimeric PCNA in the hyperthermophilic archaeon Sulfolobus solfataricus. Molecular Cell. 2003;11:275–282. doi: 10.1016/s1097-2765(02)00824-9. [DOI] [PubMed] [Google Scholar]
- Egelkrout EM, Mariconti L, Settlage SB, Cella R, Robertson D, Hanley-Bowdoin L. Two E2F elements regulate the proliferating cell nuclear antigen promoter differently during leaf development. The Plant Cell. 2002;14:3225–3236. doi: 10.1105/tpc.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel FB, Hauck L, Boehm M, Nabel EG, Dietz R, von Harsdorf R. p21 (CIP1) controls proliferating cell nuclear antigen level in mature cardiomyocytes. Molecular and Cellular Biology. 2003;23:555–565. doi: 10.1128/MCB.23.2.555-565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Otterlei M, Wong HK, Tomkinson AE, Wilson DM. XRCC1 co-localizes and physically interacts with PCNA. Nucleic Acids Research. 2004;32:2193–2201. doi: 10.1093/nar/gkh556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidantsef AL, Mitchell DL, Britt AB. The Arabidopsis UVH1 gene is a homolog of the yeast repair endonuclease RAD.1. Plant Physiology. 2000;124:579–586. doi: 10.1104/pp.124.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Rozas H, Kelman Z, Dean FB, et al. Cdk-interacting protein 1 directly binds with proliferating cell nuclear antigen and inhibits DNA replication catalyzed by the DNA polymerase holoenzyme. Proceedings of the National Academy of Sciences of the USA. 1994;91:8655–8659. doi: 10.1073/pnas.91.18.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Rozas H, Clark D, Kolodner RD. Proliferating cell nuclear antigen and Msh2p-Msh6p interact to form an active mispair recognition complex. Nature Genetics. 2000;26:375–378. doi: 10.1038/81708. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Morioka H, Imajou S, Ikeda S, Ohtsuka E, Tsurimoto T. Structure-function relationship of the eukaryotic DNA replication factor, proliferating cell nuclear antigen. The Journal of Biological Chemistry. 1995;270:22527–22534. doi: 10.1074/jbc.270.38.22527. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Ishibashi T, Kimura S, Tanaka H, Hashimoto J, Sakaguchi K. Characterization of all the subunits of replication factor C from a higher plant, rice (Oryza sativa L.), and their relation to development. Plant Molecular Biology. 2003;53:15–25. doi: 10.1023/B:PLAN.0000009258.04711.62. [DOI] [PubMed] [Google Scholar]
- Gallego F, Fleck O, Li A, Wyrzykowska J, Tinland B. AtRAD1, a plant homologue of human and yeast nucleotide excision repair endonucleases, is involved in dark repair of UV damages and recombination. The Plant Journal. 2000;21:507–518. doi: 10.1046/j.1365-313x.2000.00694.x. [DOI] [PubMed] [Google Scholar]
- Gao MJ, Murphy TM. Alternative forms of formamidopyrimidine-DNA glycosylase from Arabidopsis thaliana. Photochemistry and Photobiology. 2001;73:128–134. doi: 10.1562/0031-8655(2001)073<0128:afofdg>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Garcia E, Quiroz F, Uchiyama Y, Sakaguchi K, Vazquez-Ramos JM. Expression of maize delta-type polymerase during seed germination. Physiologia Plantarum. 2006;127:268–276. [Google Scholar]
- Garcia-Maya MM, Buck KW. Purification and properties of a DNA primase from Nicotiana tabacum. Planta. 1998;204:93–99. doi: 10.1007/s004250050234. [DOI] [PubMed] [Google Scholar]
- García-Ortiz MV, Ariza RR, Roldán-Arjona T. An OGG1 orthologue encoding a functional 8-oxoguanine DNA glycosylase/lyase in Arabidopsis thaliana. Plant Molecular Biology. 2001;47:795–804. doi: 10.1023/a:1013644026132. [DOI] [PubMed] [Google Scholar]
- Gary R, Ludwig DL, Cornelius HL, MacInnes MA, Park MS. The DNA repair endonuclease XPG binds to proliferating cell nuclear antigen (PCNA) and shares sequence elements with the PCNA-binding regions of FEN-1 and cyclin-dependent kinase inhibitor p21. The Journal of Biological Chemistry. 1997;272:24522–24529. doi: 10.1074/jbc.272.39.24522. [DOI] [PubMed] [Google Scholar]
- Gogol EP, Young MC, Kubasek WL, Jarvis TC, von Hippel PH. Cryoelectron microscopic visualization of functional subassemblies of the bacteriophage T4 DNA replication complex. Journal of Molecular Biology. 1992;224:395–412. doi: 10.1016/0022-2836(92)91003-8. [DOI] [PubMed] [Google Scholar]
- Guerini MN, Que X, Reed SL, White MW. Two genes encoding unique proliferating-cell-nuclear-antigens are expressed in Toxoplasma gondii. Molecular and Biochemical Parasitology. 2000;109:121–131. doi: 10.1016/s0166-6851(00)00240-1. [DOI] [PubMed] [Google Scholar]
- Guerini MN, Behnke MS, White MW. Biochemical and genetic analysis of the distinct proliferating cell nuclear antigens of Toxoplasma gondii. Molecular and Biochemical Parasitology. 2005;142:56–65. doi: 10.1016/j.molbiopara.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Gulbis JM, Kelman Z, Hurwitz J, O'Donnell M, Kuriyan J. Structure of the C-terminal region of p21(WAF1/CIP1) complexed with human PCNA. Cell. 1996;87:297–306. doi: 10.1016/s0092-8674(00)81347-1. [DOI] [PubMed] [Google Scholar]
- Guo K, Wang J, Anders V, Smith RC, Walsh K. MyoD-induced expression of p21 inhibits cyclin-dependent kinase activity upon myocyte terminal differentiation. Molecular and Cellular Biology. 1995;15:3823–3829. doi: 10.1128/mcb.15.7.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haracska L, Johnson RE, Unk I, et al. Physical and functional interactions of human DNA polymerase eta with PCNA. Molecular and Cellular Biology. 2001a;21:7199–7206. doi: 10.1128/MCB.21.21.7199-7206.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haracska L, Johnson RE, Unk I, et al. Targeting of human DNA polymerase iota to the replication machinery via interaction with PCNA. Proceedings of the National Academy of Sciences of the USA. 2001b;98:14256–14261. doi: 10.1073/pnas.261560798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haracska L, Unk I, Johnson RE, et al. Stimulation of DNA synthesis activity of human DNA polymerase kappa by PCNA. Molecular and Cellular Biology. 2002;22:784–791. doi: 10.1128/MCB.22.3.784-791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata S, Kouchi H, Tanaka Y, et al. Identification of carrot cDNA clones encoding a second putative proliferating cell-nuclear antigen, DNA polymerase delta auxiliary protein. European Journal of Biochemistry. 1992;203:367–371. doi: 10.1111/j.1432-1033.1992.tb16559.x. [DOI] [PubMed] [Google Scholar]
- Henderson DS, Banga SS, Grigliatti TA, Boyd JB. Mutagen sensitivity and suppression of position effect variegation result from mutation in mus209, the Drosophila gene encoding PCNA. The EMBO Journal. 1994;13:1450–1459. doi: 10.1002/j.1460-2075.1994.tb06399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwath M, Kramer W, Kunze R. Structure and expression of the Zea mays MutS-homologs Mus1 and Mus2. Theoretical and Applied Genetics. 2002;105:423–430. doi: 10.1007/s00122-002-0955-8. [DOI] [PubMed] [Google Scholar]
- Ikeda F, Dikic I. Atypical ubiquitin chain: new molecular signals. EMBO Reports. 2008;9:536–542. doi: 10.1038/embor.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K, Fukunaga K, Kawarabayasi Y, Ishino Y. Specific interactions of three proliferating cell nuclear antigens with replication-related proteins in Aeropyrum pernix. Molecular Microbiology. 2007;64:308–318. doi: 10.1111/j.1365-2958.2007.05645.x. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Kimura S, Sakaguchi K. A higher plant has three different types of RPA heterotrimeric complex. The Journal of Biochemistry. 2006;139:99–104. doi: 10.1093/jb/mvj014. [DOI] [PubMed] [Google Scholar]
- Jiricny J. The multifaceted mismatch-repair system. Nature Reviews Molecular Cell Biology. 2006;7:335–346. doi: 10.1038/nrm1907. [DOI] [PubMed] [Google Scholar]
- Kadyrov FA, Dzantiev L, Constantin N, Modrich P. Endonucleolytic function of MutL alpha in human mismatch repair. Cell. 2006;126:297–308. doi: 10.1016/j.cell.2006.05.039. [DOI] [PubMed] [Google Scholar]
- Kao HI, Bambara RA. The protein components and mechanism of eukaryotic Okazaki fragment maturation. Critical Reviews in Biochemistry and Molecular Biology. 2003;38:433–452. doi: 10.1080/10409230390259382. [DOI] [PubMed] [Google Scholar]
- Kedar PS, Kim SJ, Robertson A, et al. Direct interaction between mammalian DNA polymerase β and proliferating cell nuclear antigen. The Journal of Biological Chemistry. 2002;277:31115–31123. doi: 10.1074/jbc.M201497200. [DOI] [PubMed] [Google Scholar]
- Kimura S, Sakaguchi K. DNA repair in plants. Chemical Reviews. 2006;106:753–766. doi: 10.1021/cr040482n. [DOI] [PubMed] [Google Scholar]
- Kimura S, Suzuki T, Yanagawa Y, et al. Characterization of plant proliferating cell nuclear antigen (PCNA) and flap endonuclease-1 (FEN-1), and their distribution in mitotic and meiotic cell cycles. The Plant Journal. 2001;28:643–653. doi: 10.1046/j.1365-313x.2001.01184.x. [DOI] [PubMed] [Google Scholar]
- Kimura S, Furukawa T, Kasai N, et al. Functional characterization of two flap endonuclease-1 (FEN-1) homologues in rice. Gene. 2003;314:63–71. doi: 10.1016/s0378-1119(03)00694-2. [DOI] [PubMed] [Google Scholar]
- Kleczkowska HE, Marra G, Lettieri T, Jiricny J. hMSH3 and hMSH6 interact with PCNA and colocalize with it to replication foci. Genes and Development. 2001;15:724–736. doi: 10.1101/gad.191201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko R, Bennett SE. Physical and functional interaction of human nuclear uracil-DNA glycosylase with proliferating cell nuclear antigen. DNA Repair. 2005;4:1421–1431. doi: 10.1016/j.dnarep.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama H, Ito M, Ohnishi N, Suzuka I, Komamine A. Molecular cloning of the gene for plant proliferating-cell nuclear antigen and expression of this gene during the cell cycle in synchronized cultures of Catharanthus roseus cells. European Journal of Biochemistry. 1991;197:495–503. doi: 10.1111/j.1432-1033.1991.tb15937.x. [DOI] [PubMed] [Google Scholar]
- Kong XP, Onrust R, O'Donnell M, Kuriyan J. Three-dimensional structure of the beta subunit of E. coli DNA polymerase III holoenzyme: a sliding DNA clamp. Cell. 1992;69:425–437. doi: 10.1016/0092-8674(92)90445-i. [DOI] [PubMed] [Google Scholar]
- Konstantinova IM, Tsimokha AS, Mittenberg AG. Role of proteasomes in cellular regulation. International Review of Cell and Molecular Biology. 2008;267:59–124. doi: 10.1016/S1937-6448(08)00602-3. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Ohashi Y. PCF1 and PCF2 specifically bind to cis elements in the rice proliferating cell nuclear antigen gene. The Plant Cell. 1997;9:1607–1619. doi: 10.1105/tpc.9.9.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi S, Ohashi Y. E2F sites that can interact with E2F proteins cloned from rice are required for meristematic tissue-specific expression of rice and tobacco proliferating cell nuclear antigen promoters. The Plant Journal. 2002;29:45–59. doi: 10.1046/j.1365-313x.2002.01196.x. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Suzuka I, Ohashi Y, Murakami T, Arai Y. Upstream sequences of rice proliferating cell nuclear antigen (PCNA) gene mediate expression of PCNA-GUS chimeric gene in meristems of transgenic tobacco plants. Nucleic Acids Research. 1991;19:1571–1576. doi: 10.1093/nar/19.7.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi S, Suzuka I, Ohashi Y. Two of three promoter elements identified in a rice gene for proliferating cell nuclear antigen are essential for meristematic tissue-specific expression. The Plant Journal. 1995;7:877–886. doi: 10.1046/j.1365-313x.1995.07060877.x. [DOI] [PubMed] [Google Scholar]
- Koundrioukoff S, Jonsson ZO, Hasan S, et al. A direct interaction between proliferating cell nuclear antigen (PCNA) and Cdk2 targets PCNA-interacting proteins for phosphorylation. The Journal of Biological Chemistry. 2000;275:22882–22887. doi: 10.1074/jbc.M001850200. [DOI] [PubMed] [Google Scholar]
- Krishna TS, Kong XP, Gary S, Burgers PM, Kuriyan J. Crystal structure of the eukaryotic DNA polymerase processivity factor PCNA. Cell. 1994;79:1233–1243. doi: 10.1016/0092-8674(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Krokan HE, Otterlei M, Nilsen H, et al. Properties and functions of human uracil-DNA glycosylase from the UNG gene. Progress in Nucleic Acid Research and Molecular Biology. 2001;68:365–386. doi: 10.1016/s0079-6603(01)68112-1. [DOI] [PubMed] [Google Scholar]
- Ku DH, Travali S, Calabretta B, Huebner K, Baserga R. Human gene for proliferating cell nuclear antigen has pseudogenes and localizes to chromosome 20. Somatic Cell and Molecular Genetics. 1989;15:297–307. doi: 10.1007/BF01534969. [DOI] [PubMed] [Google Scholar]
- Laquel P, Litvak S, Castroviejo M. Mammalian proliferating cell nuclear antigen stimulates the processivity of two wheat embryo DNA polymerases. Plant Physiology. 1993;102:107–114. doi: 10.1104/pp.102.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau PJ, Kolodner RD. Transfer of the MSH2/MSH6 complex from proliferating cell nuclear antigen to mispaired bases in DNA. The Journal of Biological Chemistry. 2003;278:14–17. doi: 10.1074/jbc.C200627200. [DOI] [PubMed] [Google Scholar]
- Lee KY, Myung K. PCNA modifications for regulation of post-replication repair pathways. Molecules and Cells. 2008;26:5–11. [PMC free article] [PubMed] [Google Scholar]
- Lee SD, Alani E. Analysis of interactions between mismatch repair initiation factors and the replication processivity factor PCNA. Journal of Molecular Biology. 2006;355:175–184. doi: 10.1016/j.jmb.2005.10.059. [DOI] [PubMed] [Google Scholar]
- Li R, Waga S, Hannon GJ, Beach D, Stillman B. Differential effects by the p21 CDK inhibitor on PCNA-dependent DNA replication and repair. Nature. 1994;371:534–537. doi: 10.1038/371534a0. [DOI] [PubMed] [Google Scholar]
- Li R, Hannon GJ, Beach D, Stillman B. Subcellular distribution of p21 and PCNA in normal and repair-deficient cells following DNA damage. Current Biology. 1996;6:189–199. doi: 10.1016/s0960-9822(02)00452-9. [DOI] [PubMed] [Google Scholar]
- Liu W, Zhou Q, Li P, et al. DNA mismatch repair related gene expression as potential biomarkers to assess cadmium exposure in Arabidopsis seedlings. Journal of Hazardous Materials. 2009;167:1007–1013. doi: 10.1016/j.jhazmat.2009.01.093. [DOI] [PubMed] [Google Scholar]
- Liu Z, Showkat Hossain G, Islas-Osuna MA, Mitchell DL, Mount DW. Repair of UV damage in plants by nucleotide excision repair: Arabidopsis UVH1 DNA repair gene is a homolog of Saccharomyces cerevisiae Rad1. The Plant Journal. 2000;21:519–528. doi: 10.1046/j.1365-313x.2000.00707.x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Hall JD, Mount DW. Arabidopsis UVH3 gene is a homolog of the Saccharomyces cerevisiae RAD2 and human XPG DNA repair genes. The Plant Journal. 2001;26:329–338. doi: 10.1046/j.1365-313x.2001.01031.x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Hong SW, Escobar M, et al. Arabidopsis UVH6, a homolog of human XPD and yeast RAD3 DNA repair genes, functions in DNA repair and is essential for plant growth. Plant Physiology. 2003;132:1405–1415. doi: 10.1104/pp.103.021808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez I, Khan S, Vazquez-Ramos J, Hussey PJ. Molecular cloning of a maize cDNA clone encoding a putative proliferating cell nuclear antigen. Biochimica et Biophysica Acta. 1995;1260:119–121. doi: 10.1016/0167-4781(94)00192-6. [DOI] [PubMed] [Google Scholar]
- Lopez I, Khan S, Vazquez J, Hussey PJ. The proliferating cell nuclear antigen (PCNA) gene family in Zea mays is composed of two members that have similar expression programmes. Biochimica et Biophysica Acta. 1997;1353:1–6. doi: 10.1016/s0167-4781(97)00072-9. [DOI] [PubMed] [Google Scholar]
- Jonsson ZO, Hindges R, Hubscher U. Regulation of DNA replication and repair proteins through interaction with the front side of proliferating cell nuclear antigen. The EMBO Journal. 1998;17:2412–2425. doi: 10.1093/emboj/17.8.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maga G, Hubscher U. Proliferation cell nuclear antigen (PCNA): a dancer with many partners. Journal of Cell Science. 2003;116:3051–3060. doi: 10.1242/jcs.00653. [DOI] [PubMed] [Google Scholar]
- Markley NA, Bonham-Smith PC, Moloney MM. Molecular cloning and expression of a cDNA encoding the proliferating cell nuclear antigen from Brassica napus (oilseed rape) Genome. 1993;36:459–466. doi: 10.1139/g93-063. [DOI] [PubMed] [Google Scholar]
- Matsumiya S, Ishino Y, Morikawa K. Crystal structure of an archaeal DNA sliding clamp: proliferating cell nuclear antigen from Pyrococcus furiosus. Protein Science. 2001;10:17–23. doi: 10.1110/ps.36401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Moriuchi T, Koji T, Nakane PK. Molecular cloning of cDNA coding for rat proliferating cell nuclear antigen (PCNA)/cyclin. The EMBO Journal. 1987;6:637–642. doi: 10.1002/j.1460-2075.1987.tb04802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Hata S, Suzuka I, Hashimoto J. Expression of functional proliferating-cell nuclear antigen from rice (Oryza sativa) in Escherichia coli. Activity in association with human DNA polymerase delta. European Journal of Biochemistry. 1994a;223:179–187. doi: 10.1111/j.1432-1033.1994.tb18981.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Kim K, Bogenhagen DF. Proliferating cell nuclear antigen-dependent abasic site repair in Xenopus laevis oocytes: an alternative pathway of base excision DNA repair. Molecular and Cellular Biology. 1994b;14:6187–6197. doi: 10.1128/mcb.14.9.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Kim K, Hurwitz J, Gary R, Park MS, Tomkinson AE. Reconstitution of PCNA-dependent repair of apurinic/apyrimidinic sites with purified human enzymes. The Journal of Biological Chemistry. 1999;274:33703–33708. doi: 10.1074/jbc.274.47.33703. [DOI] [PubMed] [Google Scholar]
- Mathews MB, Bernstein RM, Franza BR, Jr, Garrels JI. Identity of the proliferating cell nuclear antigen and cyclin. Nature. 1984;309:374–376. doi: 10.1038/309374a0. [DOI] [PubMed] [Google Scholar]
- McNally R, Bowman GD, Goedken ER, O'Donnell M, Kuriyan J. Analysis of the role of PCNA-DNA contacts during clamp loading. BMC Structural Biology. 2010;10:3. doi: 10.1186/1472-6807-10-3. doi:10.1186/1472-6807-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Hasegawa PM. Sumoylation and other ubiquitin-like post-translational modifications in plants. Trends in Cell Biology. 2010;20:223–232. doi: 10.1016/j.tcb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Miura M, Sasaki T. Effect of XPA gene mutations on UV-induced immunostaining of PCNA in fibroblasts from xeroderma pigmentosum group A patients. Mutation Research. 1996;364:51–56. doi: 10.1016/0921-8777(96)00021-3. [DOI] [PubMed] [Google Scholar]
- Miyachi K, Fritzler MJ, Tan EM. Autoantibody to a nuclear antigen in proliferating cells. The Journal of Immunology. 1978;121:2228–2234. [PubMed] [Google Scholar]
- Moldovan G-L, Pfander B, Jentsch S. PCNA, the maestro of replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Morales-Ruiz T, Birincioglu M, Jaruga P, Rodriguez H, Roldan-Arjona T, Dizdaroglu M. Arabidopsis thaliana Ogg1 protein excises 8-hydroxyguanine and 2,6-diamino-4-hydroxy-5-formamidopyrimidine from oxidatively damaged DNA containing multiple lesions. Biochemistry. 2003;42:3089–3095. doi: 10.1021/bi027226u. [DOI] [PubMed] [Google Scholar]
- Murphy TM, Gao MJ. Multiple forms of formamidopyrimidine-DNA glycosylase produced by alternative splicing in Arabidopsis thaliana. Journal of Photochemistry and Photobiology B. 2001;61:87–93. doi: 10.1016/s1011-1344(01)00172-5. [DOI] [PubMed] [Google Scholar]
- Murphy TM, George A. A comparison of two DNA base excision repair glycosylases from Arabidopsis thaliana. Biochemical and Biophysical Research Communications. 2005;329:869–872. doi: 10.1016/j.bbrc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- Naryzhny SN. Proliferating cell nuclear antigen: a proteomics view. Cellular and Molecular Life Sciences. 2008;65:3789–3808. doi: 10.1007/s00018-008-8305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Prelich G, Anderson CW, Stillman B, Fisher PA. Drosophila proliferating cell nuclear antigen. Structural and functional homology with its mammalian counterpart. The Journal of Biological Chemistry. 1990;265:11948–11954. [PubMed] [Google Scholar]
- Oyama M, Wakasugi M, Hama T, et al. Human NTH1 physically interacts with p53 and proliferating cell nuclear antigen. Biochemical and Biophysical Research Communications. 2004;321:183–191. doi: 10.1016/j.bbrc.2004.06.136. [DOI] [PubMed] [Google Scholar]
- Pan ZQ, Reardon JT, Li L, et al. Inhibition of nucleotide excision repair by the cyclin-dependent kinase inhibitor p21. The Journal of Biological Chemistry. 1995;270:22008–22016. doi: 10.1074/jbc.270.37.22008. [DOI] [PubMed] [Google Scholar]
- Park S-C, Park E-H, Cho JW. Nucleotide sequence of a tobacco cDNA clone encoding a homolog of proliferating cell nuclear antigen (Accession No. AF038875) Plant Physiology. 1999;119:806. [Google Scholar]
- Parker A, Gu Y, Mahoney W, Lee SH, Singh KK, Lu AL. Human homolog of the MutY repair protein (hMYH) physically interacts with proteins involved in long patch DNA base excision repair. The Journal of Biological Chemistry. 2001;276:5547–5555. doi: 10.1074/jbc.M008463200. [DOI] [PubMed] [Google Scholar]
- Pelletier JM, Hilpert M, Belzile F, Kunze R. Isolation and characterization of AtMLH1, a MutL homologue from Arabidopsis thaliana. Molecular and General Genetics. 1999;262:633–642. doi: 10.1007/s004380051126. [DOI] [PubMed] [Google Scholar]
- Prelich G, Tan CK, Kostura M, et al. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 1987;326:517–520. doi: 10.1038/326517a0. [DOI] [PubMed] [Google Scholar]
- Prakash S, Johnson RE, Prakash L. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annual Review of Biochemistry. 2005;74:317–353. doi: 10.1146/annurev.biochem.74.082803.133250. [DOI] [PubMed] [Google Scholar]
- Richard MC, Litvak S, Castroviejo M. DNA polymerase B from wheat embryos: a plant delta-like DNA polymerase. Archives of Biochemistry and Biophysics. 1991;287:141–150. doi: 10.1016/0003-9861(91)90399-4. [DOI] [PubMed] [Google Scholar]
- Ronceret A, Guilleminot J, Lincker F, et al. Genetic analysis of two Arabidopsis DNA polymerase epsilon subunits during early embryogenesis. The Plant Journal. 2005;44:223–236. doi: 10.1111/j.1365-313X.2005.02521.x. [DOI] [PubMed] [Google Scholar]
- Ruike T, Takeuchi R, Takata K, et al. Characterization of a second proliferating cell nuclear antigen (PCNA2) from Drosophila melanogaster. The FEBS Journal. 2006;273:5062–5073. doi: 10.1111/j.1742-4658.2006.05504.x. [DOI] [PubMed] [Google Scholar]
- Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annual Review of Biochemistry. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- Santerre A, Britt AB. Cloning of a 3-methyladenine-DNA glycosylase from Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the USA. 1994;91:2240–2244. doi: 10.1073/pnas.91.6.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcherbakova PV, Fijalkowska IJ. Translesion synthesis DNA polymerases and control of genome stability. Frontiers in Bioscience. 2006;11:2496–2517. doi: 10.2741/1985. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes and Development. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Mori H. Analysis of cycles of dormancy and growth in pea axillary buds based on mRNA accumulation patterns of cell cycle-related genes. Plant and Cell Physiology. 1998a;39:255–262. doi: 10.1093/oxfordjournals.pcp.a029365. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Mori H. Changes in protein interactions of cell cycle-related genes during the dormancy-to-growth transition in pea axillary buds. Plant and Cell Physiology. 1998b;39:1073–1079. doi: 10.1093/oxfordjournals.pcp.a029304. [DOI] [PubMed] [Google Scholar]
- Shivji KK, Kenny MK, Wood RD. Proliferating cell nuclear antigen is required for DNA excision repair. Cell. 1992;69:367–374. doi: 10.1016/0092-8674(92)90416-a. [DOI] [PubMed] [Google Scholar]
- Shivji MK, Ferrari E, Ball K, Hubscher U, Wood RD. Resistance of human nucleotide excision repair synthesis in vivo to p21Cdn1. Oncogene. 1998;17:2827–2838. doi: 10.1038/sj.onc.1202352. [DOI] [PubMed] [Google Scholar]
- Shultz RW, Tatineni VM, Hanley-Bowdoin L, Thompson WF. Genome-wide analysis of the core DNA replication machinery in the higher plants Arabidopsis and rice. Plant Physiology. 2007;144:1697–1714. doi: 10.1104/pp.107.101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria G, Gottifredi V. PCNA-coupled p21 degradation after DNA damage: the exception that confirms the rule? DNA Repair. 2010;9:358–364. doi: 10.1016/j.dnarep.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojic L, Brun R, Jiricny J. Mismatch repair and DNA damage signalling. DNA Repair. 2004;3:1091–1101. doi: 10.1016/j.dnarep.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Stoimenov I, Helleday T. PCNA on the crossroad of cancer. Biochemical Society Transactions. 2009;37:605–613. doi: 10.1042/BST0370605. [DOI] [PubMed] [Google Scholar]
- Strzalka W, Ziemienowicz A. Molecular cloning of Phaseolus vulgaris cDNA encoding proliferating cell nuclear antigen. Journal of Plant Physiology. 2007;164:209–213. doi: 10.1016/j.jplph.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Strzalka W, Oyama T, Tori K, Morikawa K. Crystal structures of the Arabidopsis thaliana proliferating cell nuclear antigen 1 and 2 proteins complexed with the human p21 C-terminal segment. Protein Science. 2009;18:1072–1080. doi: 10.1002/pro.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzalka W, Kaczmarek A, Naganowska B, Ziemienowicz A. Identification and functional analysis of PCNA1 and PCNA-like1 genes of Phaseolus coccineus. Journal of Experimental Botany. 2010;61:873–888. doi: 10.1093/jxb/erp354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukenberg PT, Studwell-Vaughan PS, O'Donnell M. Mechanism of the sliding beta-clamp of DNA polymerase III holoenzyme. The Journal of Biological Chemistry. 1991;266:11328–11334. [PubMed] [Google Scholar]
- Suzuka I, Daidoji H, Matsuoka M, et al. Gene for proliferating-cell nuclear antigen (DNA polymerase delta auxiliary protein) is present in both mammalian and higher plant genomes. Proceedings of the National Academy of Sciences of the USA. 1989;86:3189–3193. doi: 10.1073/pnas.86.9.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Sakamoto A, Sato S, Kato T, Tabata S, Tanaka A. Roles of Arabidopsis AtREV1 and AtREV7 in translesion synthesis. Plant Physiology. 2005;138:870–881. doi: 10.1104/pp.105.060236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpaert-Borle M. Formation, detection and repair of AP sites. Mutation Research. 1987;181:45–56. doi: 10.1016/0027-5107(87)90286-7. [DOI] [PubMed] [Google Scholar]
- Talpaert-Borle M, Liuzzi M. Base-excision repair in carrot cells. Partial purification and characterization of uracil-DNA glycosylase and apurinic/apyrimidinic endodeoxyribonuclease. European Journal of Biochemistry. 1982;124:435–440. [PubMed] [Google Scholar]
- Tan CK, Castillo C, So AG, Downey KM. An auxiliary protein for DNA polymerase-delta from fetal calf thymus. The Journal of Biological Chemistry. 1986;261:12310–12316. [PubMed] [Google Scholar]
- Taylor RM, Hamer MJ, Rosamond J, Bray CM. Molecular cloning and functional analysis of the Arabidopsis thaliana DNA ligase I homologue. The Plant Journal. 1998;14:75–81. doi: 10.1046/j.1365-313x.1998.00094.x. [DOI] [PubMed] [Google Scholar]
- Timchenko NA, Wilde M, Nakanishi M, Smith JR, Darlington GJ. CCAAT/enhancer-binding protein alpha (C/EBP alpha) inhibits cell proliferation through the p21 (WAF-1/CIP-1/SDI-1) protein. Genes and Development. 1996;10:804–815. doi: 10.1101/gad.10.7.804. [DOI] [PubMed] [Google Scholar]
- Travali S, Ku DH, Rizzo MG, Ottavio L, Baserga R, Calabretta B. Structure of the human gene for the proliferating cell nuclear antigen. The Journal of Biological Chemistry. 1989;264:7466–7472. [PubMed] [Google Scholar]
- Tsuchimoto D, Sakai Y, Sakumi K, et al. Human APE2 protein is mostly localized in the nuclei and to some extent in the mitochondria, while nuclear APE2 is partly associated with proliferating cell nuclear antigen. Nucleic Acids Research. 2001;29:2349–2360. doi: 10.1093/nar/29.11.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja N, Singh MB, Misra MK, Bhalla PL, Tuteja R. Molecular mechanisms of DNA damage and repair: progress in plants. Critical Reviews in Biochemistry and Molecular Biology. 2001;36:337–397. doi: 10.1080/20014091074219. [DOI] [PubMed] [Google Scholar]
- Uchiyama Y, Hatanaka M, Kimura S, et al. Characterization of DNA polymerase δ from a higher plant, rice (Oryza sativa L.) Gene. 2002;295:19–26. doi: 10.1016/s0378-1119(02)00822-3. [DOI] [PubMed] [Google Scholar]
- Ulrich HD. Conservation of DNA damage tolerance pathways from yeast to humans. Biochemical Society Transactions. 2007;35:1334–1337. doi: 10.1042/BST0351334. [DOI] [PubMed] [Google Scholar]
- Umar A, Buermeyer AB, Simon JA, et al. Requirement for PCNA in DNA mismatch repair at a step preceding DNA resynthesis. Cell. 1996;87:65–73. doi: 10.1016/s0092-8674(00)81323-9. [DOI] [PubMed] [Google Scholar]
- Unk I, Haracska L, Gomes XV, Burgers PM, Prakash L, Prakash S. Stimulation of 3′>5' exonuclease and 3'-phosphodiesterase activities of yeast apn2 by proliferating cell nuclear antigen. Molecular and Cellular Biology. 2002;22:6480–6486. doi: 10.1128/MCB.22.18.6480-6486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waga S, Stillman B. The DNA replication fork in eukaryotic cells. Annual Review of Biochemistry. 1998;67:721–751. doi: 10.1146/annurev.biochem.67.1.721. [DOI] [PubMed] [Google Scholar]
- Waga S, Hannon GJ, Beach D, Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994;369:574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- Watts F. Sumoylation of PCNA: wrestling with recombination at stalled replication forks. DNA Repair. 2006;5:399–403. doi: 10.1016/j.dnarep.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wu SY, Culligan K, Lamers M, Hays J. Dissimilar mispair-recognition spectra of Arabidopsis DNA-mismatch-repair proteins MSH2·MSH6 (MutSα) and MSH2·MSH7 (MutSγ) Nucleic Acids Research. 2003;31:6027–6034. doi: 10.1093/nar/gkg780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YQ, Hohn B, Ziemienowicz Z. Characterization of an ATP-dependent type I DNA ligase from Arabidopsis thaliana. Plant Molecular Biology. 2001;46:161–170. doi: 10.1023/a:1010679901911. [DOI] [PubMed] [Google Scholar]
- Xia L, Zheng L, Lee HW, et al. Human 3-methyladenine-DNA glycosylase: effect of sequence context on excision, association with PCNA, and stimulation by AP endonuclease. Journal of Molecular Biology. 2005;346:1259–1274. doi: 10.1016/j.jmb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Zhang H, Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell. 1992;71:505–514. doi: 10.1016/0092-8674(92)90518-h. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Hayashi Y, Hirose F, et al. Molecular cloning and structural analysis of mouse gene and pseudogenes for proliferating cell nuclear antigen. Nucleic Acids Research. 1991;19:2403–2410. doi: 10.1093/nar/19.9.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Sun Y, Hsu H, Zhang L, Zhang Y, Lee MY. The interdomain connector loop of human PCNA is involved in a direct interaction with human polymerase delta. The Journal of Biological Chemistry. 1998;273:713–719. doi: 10.1074/jbc.273.2.713. [DOI] [PubMed] [Google Scholar]