Abstract
Background
It has been known for many decades that auxin inhibits the activation of axillary buds, and hence shoot branching, while cytokinin has the opposite effect. However, the modes of action of these two hormones in branching control is still a matter of debate, and their mechanisms of interaction are equally unresolved.
Scope
Here we review the evidence for various hypotheses that have been put forward to explain how auxin and cytokinin influence axillary bud activity. In particular we discuss the roles of auxin and cytokinin in regulating each other's synthesis, the cell cycle, meristem function and auxin transport, each of which could affect branching. These different mechanisms have implications for the main site of hormone action, ranging from systemic action throughout the plant, to local action at the node or in the bud meristem or leaves. The alternative models have specific predictions, and our increasing understanding of the molecular basis for hormone transport and signalling, cell cycle control and meristem biology is providing new tools to enable these predictions to be tested.
Keywords: Shoot branching, axillary bud, dormancy, auxin, cytokinin, canalization, polar auxin transport stream, cell cycle
INTRODUCTION
Plants display enormous variation in shoot body plans, including both genetically determined differences between individuals and plastic responses of a single genotype to environmental conditions. A major element permitting diversity in shoot system architecture is the development of secondary axes of growth and their flexible regulation. During embryogenesis, the apical–basal axis is established, and this is maintained throughout the plant's life. At the upper end, the shoot apical meristem (SAM) is specified. The whole above-ground body of plants is derived from this SAM, through the iterative production of phytomers. A phytomer typically consists of a stem segment with an associated leaf and, in the leaf axil, a secondary SAM, or axillary meristem. The axillary meristems have the same potential as the primary SAM. Initially, they usually generate a small bud consisting of a few phytomers with immature leaves. The bud can subsequently remain dormant or grow out to produce a branch. In this way, new axes of growth can be added to the initial apical–basal axis of the plant. The branches can of course themselves branch, and so on, allowing ever higher orders of branching to be achieved. Furthermore, the number of axillary meristems in a phytomer can vary. Thus the formation of axillary meristems and the subsequent regulation of their activation contribute greatly to variation in shoot architecture.
Axillary meristem formation and activity depend on a plant's genotype, developmental stage and the environment in which it is growing, including factors such as daylength, light quality, temperature and nutrient availability. The integration of these multiple inputs is likely to be mediated by a network of interacting hormonal signals that move systemically through the plant. Various studies revealed antagonistic interactions between the plant hormones auxin and cytokinin (CK) in regulating bud outgrowth. More recently, strigolactones, a group of sesquiterpene lactones derived from carotenoids, or a derivative thereof, were shown to have a pivotal role in inhibiting shoot branching. It is thought that the interplay of internal and external factors regulates bud activity status through interlocked signal transduction pathways.
In this review we focus on the relationships and possible links between auxin and CK in the control of shoot branching and their possible modes and sites of action.
APICAL DOMINANCE
In many plants the growing shoot apex inhibits the outgrowth of axillary buds, a phenomenon termed ‘apical dominance’. Removal of the shoot apex leads to the release of dormant axillary buds below it to form branches. Apical dominance allows plants to focus resources into the main axis of growth, while activation of dormant buds allows for recovery after damage or loss of the main shoot.
It has been known for a long time that auxin is a major signal for apical dominance. If auxin is applied to a decapitated stump at the shoot apex, it can replace the growing apex in restoring branch inhibition (Thimann and Skoog, 1933). Auxin is mainly synthesized in the shoot apex in young leaves (Ljung et al., 2001) and subsequently transported basipetally (downwards from the tip to the base) in the polar auxin transport stream (PATS). It was shown that apical dominance depends on the PATS. For example, if auxin transport inhibitors such as 2,3,5-triiodobenzoic acid (TIBA) are applied to stems, the suppression of outgrowth is abolished (Snyder, 1949; Panigrahi and Audus, 1966). Despite its long history, the mechanism by which auxin inhibits the growth of axillary meristems is not fully understood. However, it is clear that the auxin moving in the main stem must act indirectly because it does not move upward from the stem into the bud (Sachs and Thimann, 1967; Booker et al., 2003). Therefore, a second messenger was proposed to regulate bud activity in situ.
Classical view of auxin and cytokinin antagonism in regulating shoot branching
Cytokinins represent a possible second messenger for auxin signalling in regulating bud activity. Endogenous CKs can be transported acropetally (towards the shoot apex) in the xylem sap, enter axillary buds and promote their outgrowth. Thus, CKs antagonize auxin in apical dominance. Application of CK to a bud is sufficient to trigger outgrowth even in the presence of a growing shoot apex or apically supplied auxin (Wickson and Thimann, 1958; Faiss et al., 1997). For example, experiments with excised Arabidopsis stem segments carrying a single bud showed that basally supplied CK promotes bud outgrowth even if auxin is applied apically (Chatfield et al., 2000).
Evidence that CK can act as a second messenger for auxin in bud regulation comes from the observation that auxin can negatively regulate the synthesis of CK. In Arabidopsis, the repression of CK biosynthesis by auxin was shown to be rapid, and dependent on AUXIN RESISTANT1 (AXR1)-mediated auxin signalling (Nordstrom et al., 2004). These data suggest the attractive hypothesis that auxin in the PATS acts at least in part by downregulating the synthesis of CK, restricting its availability to axillary buds, thereby repressing their growth. Although this model is widely accepted, there is considerable debate about the relevant sites for CK biosynthesis in this response and the mode and sites of its action.
Cytokinin synthesis and bud activation
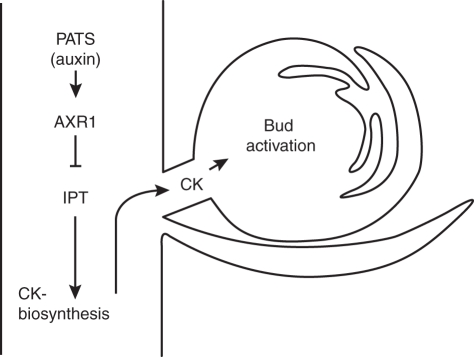
Cytokinins are synthesized throughout the plant (Nordstrom et al., 2004), and adenosine phosphate-isopentenyltransferases (IPTs), which catalyse the first step of CK biosynthesis (Hirose et al., 2008), are expressed throughout the plant (Miyawaki et al., 2004). Experiments in Pisum sativum showed that the CK biosynthetic genes ISOPENTENYLTRANSFERASE1 and ISOPENTENYLTRANSFERASE2 (PsIPT1 and PsIPT2) are expressed in the nodal regions of stems, and they are differentially expressed before and after decapitation (Tanaka et al., 2006). PsIPT1 and PsIPT2 were transiently induced 3 h after decapitation. Also treatment with TIBA in a ring around the internode resulted in activation of PsIPT1 and PsIPT2 transcription below the site of TIBA application. Based on these data, Tanaka et al. (2006) proposed a model where one role of auxin in apical dominance is to repress local biosynthesis of CK in the nodal stem. After decapitation, auxin depletion in the stem allows for local CK synthesis. The transient nature of the upregulation of PsIPT genes could be due to the re-accumulation of axuin in the main stem as the growing side shoot becomes a new auxin source and exports auxin into the main stem. The model is supported by data from experiments with excised stems. If stem segments 3 h after decapitation were kept in buffer without indole acetic acid (IAA) the PsIPT expression was maintained. If IAA was added to the buffer, PsIPT was repressed again. Tanaka et al. (2006) further found that PsIPT1 and PsIPT2 were not induced in axillary buds after decapitation. They were mainly induced in the stems and only slightly in roots. When CK levels (free nucleotide forms) were measured in the nodal stems and axillary buds before and after decapitation, increases in CK levels were first found in the nodal tissue. With a time lag, the CK levels in axillary buds also increased. From these findings, a view has emerged that a physiologically relevant site for CK synthesis in the regulation of bud activity is the nodal stem, with the CK being subsequently transported into the buds (Fig. 1).
Fig. 1.
Auxin limits CK availability. One proposed model for the regulation of shoot branching by auxin and CK is based on the assumption that CK is a second messenger of auxin. In this model, auxin limits the availability of the antagonistically acting CKs to lateral buds. IPTs in the nodal region of the stem produce CK, which is subsequently transported into the buds to promote bud activation. CK entering lateral buds is proposed to be mainly derived from biosynthesis in stems, but there may be additional relevant sites, e.g. roots. Auxin can repress CK synthesis by inhibition of IPTs, which is dependent on AXR1 function. Thus the amount of auxin in the main stem PATS can regulate bud activity without entering buds. AXR1, AUXIN RESISTANT1; CK, cytokinin; IPT, isopentenyl transferase; PATS, polar auxin transport stream.
There is considerably more controversy over the role of root-derived CK. Roots are a major source of CK in the plant, and CK synthesized in the root is transported to the shoot in the transpiration stream in the xylem. There is good evidence that the shoot can regulate the export of CK from roots. For example, CK levels are very low in the root-derived xylem sap of strigolactone-deficient mutants, and this property is controlled by the genotype of the shoot (Foo et al., 2007). In the context of apical dominance, it has been demonstrated that in bean and chickpea, CK levels in the xylem sap were higher after decapitation (Bangerth, 1994), but this increase could be prevented by application of auxin to the decapitated stump. These data led to the proposal that auxin inhibits branching partly by regulating the supply of root-derived CK to the shoot. However, the relevance of root-derived CK has been called into question.
Faiss et al. (1997) expressed a bacterial IPT gene under the control of a tetracycline-dependent 35S promoter in tobacco plants. When the gene was induced in the whole plant, trans-zeatin (tZ) and the CK metabolites zeatin riboside (ZR) and zeatin riboside O-glucoside (ZROG) concentrations rose 10- to 100-fold in roots and stems of transgenic plants, but not in leaves. Local treatment of inactive lateral buds with tetracycline resulted in bud activation. If the treatment was not continued, growth of the buds stopped. At the same time, untreated buds located in more basal or apical positions remained inhibited. Next Faiss et al. (1997) investigated whether an increase in IPT gene expression in roots was sufficient to increase CKs in the transpiration stream. The results showed that root expression of IPT leads to specific export of ZR in the xylem sap. The system was used to test whether endogenous root-produced CKs would affect growth of lateral buds in the shoot. To this end, reciprocal grafting between transgenic and wild-type tobacco plants was performed. Release of dormant buds was only detected in transgenic parts of the grafts that overproduced CK. Outgrowth of buds was observed in IPT-expressing scions, or in the basal stem nodes carried on IPT-expressing root stocks, but buds remained dormant in the non-transgenic parts of the grafted plants, either way. Interestingly, increases in ZR levels in the xylem sap of non-transgenic parts of grafts were only found close to the grafting junctions of transgenic roots, and thus high levels of CK were not reaching the shoot from the CK-overproducing roots. These experiments suggest that local production of CK rather than root-derived CK is important for shoot branching control.
On the other hand, Matsumoto-Kitano et al. (2008) demonstrated that endogenous CKs can act as long-distance signals in regulating the development of the cambium and secondary growth in stems. In grafts between CK-deficient (iP-type and tZ-type CKs) atipt1;3;5;7 quadruple mutants and Arabidopsis thaliana wild-type plants, mobile endogenous CKs rescued the mutants' defects irrespective of the grafting direction. The cambium development and secondary growth of either an atipt1;3;5;7 scion grafted to a wild-type root stock or an atipt1;3;5;7 root stock grafted to a wild-type scion was the same as in the wild-type. However, there was a difference in the rescue of iP or tZ contents depending on the direction of the graft. Wild-type root stocks rescued the ZR CK pool in the atipt1;3;5;7 scion, but not the iP pool. Conversely a wild-type scion recovered the iP pool in mutant root stocks, but not the ZR levels (Matsumoto-Kitano et al., 2008). It was therefore suggested that tZ-type CKs are the most common type transported from roots to shoots and iP-type CKs are transported from shoots to roots (Beveridge et al., 1997; Faiss et al., 1997; Corbesier et al., 2003; Matsumoto-Kitano et al., 2008). Consistent with this, the CYP735A genes, which catalyse the synthesis of tZ-type CKs, are expressed predominantly in roots (Takei et al., 2004).
To date, the physiological significance of these differences in iP- and tZ-type CK behaviour is not clear. Kudo et al. (2010) suggest that by distinguishing the different CK types plants can use iP-type CKs as acropetal messengers, whereas tZ-type CKs are perceived as basipetal messengers. Is the CK species important for bud outgrowth control? This is a plausible option because the known CK receptors in plants have similar, but not identical, affinities for different CK species. However, even if CK specificity is established, there are still conflicting data about the importance of root-derived CKs since root-derived ZR has been shown to be active in the shoot in some cases but not in others. This may simply reflect differences between plant species in the loading of CKs into the xylem, or their fate once unloaded.
MODE OF ACTION OF AUXIN AND CYTOKININ
While the origin of CKs in bud regulation is still under debate, there is even less clarity on their site and modes of action.
Bud activation and the cell cycle
Because CKs can activate buds when applied to them, and because of their known roles in promoting cell division in the SAM, an intuitively obvious mechanism for CK-mediated bud activation is in promoting the cell cycle.
In the dormant state, bud cells are mostly arrested in the G1 phase and also at the G1/S and S/G2 boundaries (Shimizu and Mori, 1998). After decapitation, bud cells in pea begin to enter the S phase after 6–9 h. Gene expression of a number of growth-specific markers correlated with bud growth and cell proliferation in the lateral buds. Histone H2A, histone H4, mitogen-activated protein (MAP) kinase, ribosomal protein gene L27 (rpL27), ribosomal protein L34 (rpL34) and CDC2 expression increased within 1 h of decapitation. rpL27 mRNA abundance was very low in dormant buds on intact pea plants, but increased significantly 2 d after decapitation. If buds became dormant again, rpL27 mRNA also declined again (Devitt and Stafstrom, 1995). In situ hybridizations revealed rpL27 mRNA distribution throughout the whole bud within 1 h of decapitation (Stafstrom and Sussex, 1992).
Cells arrested in G1 or G2 are evenly distributed in all regions of the bud (Devitt and Stafstrom, 1995). The proliferating cell nuclear antigen (PCNA/DNA polymerase auxiliary protein) gene is specifically expressed during the late G1 and S phase of the cell cycle. PCNA is involved in DNA replication and its expression marks proliferation of cells. In pea buds, its mRNA abundance correlated with the activity status of the buds. PCNA was induced after decapitation and suppressed again if buds re-entered a dormant state. RNA in situ hybridizations showed PCNA signal in all cell types of buds that were activated by decapitation, i.e. the meristem, leaf primordia and procambium (Shimizu and Mori, 1998). PCNA transcription is regulated by members of the TEOSINTE BRANCHED, CYCLOIDEA, PROLIFERATING CELL FACTORS 1 and 2 (TCP) family of transcription factors. The TCP domain consists of a non-canonical basic helix–loop–helix domain that mediates DNA binding. Based on similarities within the TCP domain they can be divided into two classes (Cubas et al., 1999). Members of the TCP gene family were shown to be involved in the regulation of growth and cell cycling, including axillary meristem activity (Kosugi and Ohashi, 1997; Tremousaygue et al., 2003; Li et al., 2005; Aguilar-Martinez et al., 2007; Finlayson, 2007).
Microarray data are consistent with a role for TCPs in bud outgrowth regulation. Cis-elements enriched in genes differentially expressed in Arabidopsis axillary buds before and after decapitation were identified. Two elements were found, GGCCCAWW and AAACCCTA, and were shown to act synergistically to drive reporter gene expression in elongating lateral bud stems (Tatematsu et al., 2005). These two motifs show similarity to site II motifs and the telo-box element, which are in vitro and in vivo binding sites of TCP proteins. TCP-binding sites were found in promoters of ribosomal proteins and they are also present in the promoters of cell-cycle-related genes such as PCNA and CYCB1;1. PROLIFERATION CELL FACTOR 1 and 2 (PCF1 and PCF2) and TCP20 were shown to bind to promoters of PCNA genes (Kosugi et al., 1995; Kosugi and Ohashi, 1997; Ito et al., 1998; Tremousaygue et al., 2003; Li et al., 2005; Tatematsu et al., 2005).
Among the cell-cycle-related genes implicated in shoot meristem function, D-type cyclins have received particular attention. D-type cyclins modulate the length of the G1 phase and are often described as rate limiting to cell division in plants (Dewitte et al., 2007). The transcription of D-type cyclins can be upregulated by CK, and their overexpression is sufficient to allow CK-independent proliferation of cells in tissue culture (Riou-Khamlichi et al., 1999). In the SAM, repression of D-cyclins can be TCP dependent (Gaudin et al., 2000).
Interestingly, overexpression of Arabidopsis cyclinD2 and cyclinD3 in tobacco plants results in a faster growth rate throughout the life cycle, including an increased rate of leaf initiation at the SAM (Boucheron et al., 2005). These data suggest that the cell cycle can limit growth, but, importantly, the overexpressing plants do not seem to show increased shoot branching. Thus, while upregulation of CK synthesis is sufficient to activate buds without generally increasing the growth rate, upregulation of the cell cycle generally increases the growth rate without activating buds. These data suggest that simply activating the cell cycle is not sufficient to activate buds, and that CKs must do more than upregulate the cell cycle.
Bud activation and auxin transport
There is increasing evidence for a central role for auxin transport in the control of bud activity. There is a tight correlation between bud outgrowth and export of auxin from the bud (Morris, 1977; Li and Bangerth, 1999; Balla et al., 2011). For example, it has been shown that upon decapitation, auxin export from previously dormant pea buds is rapidly detected as an early event in bud activation. Such export is prevented by the application of auxin to the decapitated stump. The sustained export of auxin from buds requires auxin transport canalization. The canalization hypothesis was proposed by Sachs (1981) to explain a wide range of phenomena involving the establishment of auxin transport pathways connecting auxin sources and auxin sinks. Core to canalization is a positive feedback loop between auxin flow and both the polarization and upregulation of active auxin transport. An initial auxin flow between a source and a sink upregulates auxin transport along the auxin transport pathway, culminating in the formation of narrow files of cells with high auxin transport activity from the source to the sink (Sachs, 2000). These files may later differentiate into vascular strands. Recent observations of bud activation show that inactive buds have non-polar localization of the key auxin transport protein PIN1, but upon bud activation PIN1 is both upregulated and polarized into files of cells connecting the bud to the PATS in the main stem, later allowing increased vascular connectivity between the bud and the main stem (Prusinkiewicz et al., 2009; Kalousek et al., 2010; Balla et al., 2011).
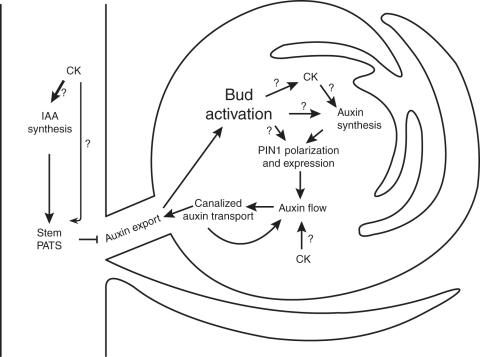
The canalization hypothesis has interesting implications when applied to shoot branching. If sustained auxin export is required for bud activity, the canalization mechanism can explain the ability of auxin moving in the main stem to inhibit the activity of axillary buds indirectly. Auxin in the main stem would lower the sink strength of the stem and thus reduce the basal level of auxin flow between the bud and the main stem. If this level is too low to trigger the positive feedback of canalization, then the bud will remain dormant. Indeed application of apical auxin to the decapitated stump of peas is sufficient to prevent auxin transport canalization from the bud to the main stem as well as to inhibit the activation of the bud (Li and Bangerth, 1999; Balla et al., 2011). There is a growing body of evidence in support of this mechanism, much of which is derived from the observations that strigolactones appear to inhibit buds principally by modulating auxin transport and its canalization (Bennett et al., 2006; Crawford et al., 2010). Therefore, an alternative mode of action for CK in bud activation could be via this canalization-based mechanism (Fig. 2). There are two ways in which CK might activate buds via this route – by modulating auxin transport and by upregulating auxin biosynthesis locally in the bud. There is some evidence to support both these modes of action.
Fig. 2.
Bud activation and auxin transport. One model for the mechanism by which auxin regulates bud outgrowth relies on establishment of auxin export out of the bud as a prerequisite to allow bud outgrowth. An initial auxin flow towards an auxin sink promotes auxin transport canalization along this path. The polarization and upregulation of auxin transport feeds back to promote auxin flow further. The canalization of auxin transport into cell files connecting the auxin source (bud) to the auxin sink (main stem) allows sustained auxin export out of the bud. This means that auxin export out of the bud can be prevented if the main stem is a weak auxin sink. This can be achieved by increasing the amount of auxin transported in the PATS in the main stem. Therefore, if CK in the primary apex promotes auxin synthesis and/or PATS, this would inhibit bud outgrowth. On the other hand, if CK promotes auxin synthesis and/or auxin flow in the bud, it would promote bud outgrowth. CK, cytokinin; PATS, polar auxin transport stream.
As described above, CK activates buds when applied directly to them. However, if it is applied to the primary apex, CK enhances the effect of apically applied auxin (Davies et al., 1966; Chatfield et al., 2000; Li and Bangerth, 2003). Davies et al. (1966) found that application of kinetin (6-furfurylaminopurine) and IAA simultaneously to the decapitated stump of bean plants (Phaseolus vulgaris) resulted in a much more pronounced apical inhibition than caused by IAA alone. Application of apical CK alone resulted in immediate bud activation, application of apical auxin alone delayed activation by up to 5 d, but simultaneous application of both still repressed growth after 14 d. One mechanism to explain this is if CK promotes auxin transport down the stem. Consistent with this idea, there was more radiolabelled auxin in the main stem when kinetin was added to the decapitated stump than with IAA alone (Davies et al., 1966).
Support for the idea that CKs might act by upregulating auxin transport comes from work investigating the effects of CK on the polarization of PIN proteins in buds. Exogenous application of benzylaminopurine (BAP) to non-growing axillary buds resulted in significant upregulation of PsPIN1 expression between 2 and 5 h after treatment. Importantly, CK treatment also results in the polarization of PIN1 24 h after the application, indicating that a PATS has been established (Kalousek et al., 2010).
Bud activation and auxin synthesis
An alternative mode of action for CK could be in upregulating auxin synthesis. Indeed recent analysis has demonstrated the ability of CK to upregulate auxin biosynthesis in roots, shoot apices and young developing leaves (Jones et al., 2010). Induction of an oestradiol-inducible transgenic line, IPT8/plant growth activator 22, was used to investigate the effects of CK on IAA biosynthesis. A stimulating effect of CK on auxin biosynthesis was found in young developing tissues and in the root system. Oestradiol induction of IPT8 in seedlings resulted in a higher concentration of iP- and Z-type CKs. Furthermore, the treatment resulted in an elevated IAA biosynthesis rate and higher IAA levels after 24 h. In roots, the increase in IAA biosynthesis rate was detected as early as 3 h after exogenous CK treatment (Jones et al., 2010).
Treatment of buds with CK has been shown to increase the amount of auxin in associated stems (Li et al., 1992; Li and Bangerth, 2003), leading to the suggestion that in addition to auxin transport, CK may upregulate auxin synthesis during bud activation. It is, however, difficult to separate synthesis and transport, because auxin synthesis is known to be under feedback control (Jones et al., 2010). Thus increased auxin export from the bud would reduce auxin levels in the bud and upregulate bud auxin biosynthesis. Computational modelling has shown that reduced feedback regulation such that auxin accumulates in the bud is alone sufficient to activate buds ectopically, because they become stronger auxin sources and are thus able to establish auxin transport into the stem even when it is a weak sink (Prusinkiewicz et al., 2009). Thus local stimulation of auxin synthesis by CK in the bud is a plausible mechanism of action for CK, but a role for CK in promoting auxin transport would have the same effect.
An important consideration here is the observation that addition of auxin directly to buds has no effect on bud activation or auxin transport canalization out of the bud, even though it can transcriptionally upregulate PINs (Blilou et al., 2005; Heisler et al., 2005; Vieten et al., 2005). This result has been used to argue against the idea that canalization from the bud is important, since according to this model increasing auxin levels in the bud are predicted to activate them. However, there is a significant difference between flooding a bud with exogenous auxin and the natural situation with auxin synthesis predominantly in young leaves. Leaf initiation at the SAM is dependent on the dynamic movement of auxin around the meristem, culminating in its transport away from each newly initiated leaf into the bud stem, which accompanies the specification of the leaf vascular midrib and its connection to the bud stem vasculature. One hypothesis for why bud activity requires auxin export is that it is essential to allow sustained leaf production because of the integral part such export plays in leaf formation. If this is the case, then flooding the meristem with auxin would not promote meristem activity because it is export from incipient leaves, as point sources of auxin, that is important, not export generically from the bud. This argues against CK acting by general upregulation of auxin synthesis, but it could plausibly act by locally upregulating auxin synthesis in young leaves. Here again, it is very difficult to distinguish between upregulation of synthesis and increased loading of auxin into the polar auxin transport stream since both would result in more auxin synthesized, more auxin transported and more bud activation.
Bud activation and the stem cell niche
Both auxin and CK play complex roles in the function and organization in the meristem itself, and in the past decade a tightly regulated network of hormone signalling within the zones of the meristem has been revealed (Dodsworth, 2009; Veit, 2009). Meristems can be divided into different zones with specific functions. The central zone at the tip of the meristem contains the stem cells and the organizing centre of the meristem. It is characterized by a low rate of cell division. All above-ground plant cells are ultimately derived from these stem cells. The peripheral as well as the rib zone beneath the organizing centre show a higher rate of cell division, and cells from these domains are released to be incorporated into growing lateral organs and the stem (Nougarede, 1967; Brand et al., 2001).
Cytokinin positively regulates meristem function and is necessary for its maintenance. High levels of CK in the central zones of the meristem are connected to the maintenance of undifferentiated cells (Riou-Khamlichi et al., 1999; Kurakawa et al., 2007). In contrast, gibberellin levels are kept lower in the central region (Jasinski et al., 2005) and, together with auxin, are associated with the initiation and outgrowth of lateral organs, with higher concentrations in developing leaves, for example.
Preventing auxin accumulation in the meristem results in diminished meristem activity. This effect is dependent on CK signalling (Zhao et al., 2010). A-type Arabidopsis response regulator (ARR) genes are primary CK response genes and implicated in negative regulation of CK signalling, thus forming a negative feedback loop (To et al., 2004). In mutants with defects in polar auxin transport and in yucca mutants with reduced auxin levels, A-type ARR genes were upregulated (Zhao et al., 2010). Auxin was shown to repress A-type ARR genes directly in the meristem. The auxin response factor ARF5/MP binds to the promoter of ARR15 to suppress gene expression, thereby allowing CK signalling. Thus, auxin and CK signalling converge on meristem function regulation by controlling A-type ARR activity. ARF5/MP is mainly present in peripheral zones of the SAM, which is complementary to the A-type ARR expression pattern (Zhao et al., 2010). In the organizing centre of the meristem, A-type ARRs are suppressed by the stem cell promoting factor WUSCHEL (Leibfried et al., 2005). Loss of A-type ARR function results in enlarged meristems (Giulini et al., 2004; Zhao et al., 2010).
Members of the class I KNOTTED1-like homeobox (KNOX) genes are required for meristem maintenance. They suppress gibberellic acid levels in the central zone (Sakamoto et al., 2001; Hay et al., 2002; Jasinski et al., 2005) and at the same time directly activate IPT genes in the central region of the meristem (Jasinski et al., 2005; Yanai et al., 2005; Sakamoto et al., 2006) and promote CK biosynthesis (Kusaba et al., 1998). Consistent with these observations, induction of IPT genes or application of CK to the shoot tip partially rescues meristem defects of shoot meristemless (stm) mutants, which are defective in a class I KNOX gene (Jasinski et al., 2005; Yanai et al., 2005). Reduction of CK levels in biosynthesis mutants or by overexpressing cytokinin oxidase (CKX) genes, which irreversibly inactivate bioactive CKs, results in reduced meristem size and leaf initiation. In extreme cases the meristems are terminated and lost (Werner et al., 2001, 2003; Miyawaki et al., 2006). Similar phenotypes are seen if CK signalling is reduced through receptor mutations (Higuchi et al., 2004).
As well as KNOX-regulated CK synthesis in the meristem, a second pathway has been implicated in local CK synthesis and meristem function. LONELY GUY (LOG) encodes a CK-activating enzyme with CK-specific phophoribohydrolase activity. LOG synthesizes active CKs, the free base forms, presumably directly from biologically inactive nucleotides (Hirose et al., 2008). The gene family was first identified in rice (Kurakawa et al., 2007). LOG mRNA shows a specific expression pattern, suggesting activation of CKs in local domains of the plant. LOG mRNA is found in meristem tips of the SAM and axillary meristems. Accordingly, log mutants show defects in the maintenance of meristems. Furthermore, inflorescence meristems are affected and mutants produce a reduced number of panicle branches and floral organs. Meristem marker expression in log mutants indicates that the defects in log are caused by impaired meristem maintenance, probably caused by low CK levels. Expression of the histone H4 gene, a marker for cells in S phase, was lower in log compared with the wild-type (Kurakawa et al., 2007).
The Arabdiopsis-genome includes a family of LOG-like genes (Kuroha et al., 2009). Members show specific but different expression patterns. Arabidopsis log mutants show similar phenotypes to the rice log mutant. In situ hybridizations revealed that LOG7 is specifically expressed at the tip of SAMs as in rice (Dodsworth, 2009; Yadav et al., 2009). This gene might be expressed in the same way in axillary meristems. Among the other Arabidopsis genes, LOG1 is expressed at the junction between the main stem and cauline leaves, marking the axillary region of the node where axillary meristems are initiated. LOG3 and LOG5 show axillary bud-specific expression (Kuroha et al., 2009).
Taking all these data together, it is clear that the stem cell niche is regulated by complex feedback loops involving both hormone and patterning systems. These loops create a highly buffered self-organizing system with active regulation of CK and auxin levels and signalling, mediated locally in the meristem by the transcription factor network that patterns the meristem. It is possible that the import of CK into buds from the stem, as proposed by the second messenger hypothesis, and the modulation of auxin export out of the axillary buds, as proposed by the auxin transport canalization hypothesis, may affect meristem activity by perturbing hormonal balance in the stem cell niche. However, given the extent to which these systems are buffered, these hormone fluxes may have relatively little effect.
THE SITE OF ACTION OF CYTOKININ
The alternative modes of action for CK described above imply alternative sites of action. A general effect on auxin transport would imply systemic action, both in the buds and in the main stem and primary apex. Upregulation of the cell cycle occurs throughout the entire bud during its activation, which would suggest uniform CK action throughout the bud. A role in auxin synthesis or auxin loading in young leaves favours local sites of action in the newly initiated leaves, and a role operating via the stem cell niche implies local action in or near the meristem central zone.
Unfortunately, because of the wide roles of CK in plant development, it is difficult to use these predictions about the sites of action to distinguish between the hypotheses. Cytokinin receptors are expressed ubiquitously, and compromising CK signalling results in highly pleiotropic phenotypes, making it difficult to investigate the role of CK in any one specific process.
There is potential for the LOG genes to provide tools to produce CK at various sites in lateral buds, including the stem cell niche as well as the whole bud. Branching analyses of such mutants might help to elucidate whether changes of CK concentration in the meristem contribute to the transition from arrested to active buds. Furthermore, synthetic CK analogues may be informative. For example, different CK analogues have different impacts on lateral shoot growth in pea, suggesting that a subset of CK receptors are involved (Li and Bangerth, 2003).
WHAT HAPPENS IN THE BUD?
Wherever and however CK and auxin act, ultimately there must be changes in the bud that regulate their activity. Release from dormancy appears to involve both the upregulation and polarization of auxin transport out of the bud, and the activation of the cell cycle and growth in the bud, including the transcriptional upregulation of genes involved in these processes.
The only bud-specific genes with proven functional importance in bud activity are a subset of genes in the TCP family mentioned above. The maize Teosinte Branched1 (TB1) gene was a founding member of this family. TB1 belongs to the class II TCP factors that are implicated in negatively regulating growth (Martin-Trillo and Cubas, 2010). Loss of function at TB1 results in a spectacular increase in shoot branching in maize (Doebley et al., 1997). TB1 is conserved among mono- and dicotyledonous plants (Martin-Trillo and Cubas, 2010). Rice and Arabidopsis loss-of-function mutants in similar genes, named FINECULM1 (FC1) and BRANCHED (BRC), respectively, have similar effects on branching (Hu and Rosenblum, 2003; Takeda et al., 2003; Aguilar-Martinez et al., 2007; Finlayson, 2007). In Arabidopsis two TCP genes are involved in branching control, named BRANCHED1 (BRC1; TCP18) and BRANCHED2 (BRC2; TCP12) (Aguilar-Martinez et al., 2007; Finalyson, 2007). Whereas brc1 mutants show a strong increase in bud activation, BRC2 was found to play a more minor role (Aguilar-Martinez et al., 2007; Finlayson, 2007).
RNA in situ analyses revealed a bud-specific expression for all these TCPs (Doebley et al., 1997; Hu and Rosenblum, 2003; Takeda et al., 2003; Kebrom et al., 2006; Aguilar-Martinez et al., 2007). Their expression is closely negatively correlated with bud activity. BRC1 is no longer detectable at the first signs of bud outgrowth, i.e. stem elongation (Aguilar-Martinez et al., 2007). Furthermore, BRC1 is quickly downregulated after decapitation and its expression is highest in buds with the least outgrowth potential in the Arabidopsis rosette. Similarly, in maize and rice, the level of outgrowth suppression generally correlates with the transcript level of the genes (Doebley et al., 1997; Hubbard et al., 2002; Takeda et al., 2003).
BRC1 expression is regulated by environmental as well as endogenous signals. For example, if plants are grown in high density there is less bud activity and BRC1 is upregulated. At the same time, brc1 mutants respond less to density signals (Aguilar-Martinez et al., 2007; Finlayson, 2007). In terms of hormonal regulation, again the correlations between bud repression and expression of these TB1-like genes are strong. A negative effect on FINECULM1 (FC1) expression by CK application was shown. FC1 transcript levels decreased in a CK dose-dependent manner. The Arabidopsis altered meristem program1 (amp1) mutants (Helliwell et al., 2001) are characterized by higher levels of CKs, more bud outgrowth, more axillary meristems and reduced BRC1 expression. Auxin-mediated bud inhibition also correlates with BRC1 activity. The auxin overproducer yucca1 (ycc1) (Zhao et al., 2001) shows enhanced bud arrest, elevated BRC1 transcripts and less branching. The double mutant combination with brc1 results in bushier plants than the wild type (Aguilar-Martinez et al., 2007; Finlayson, 2007).
These data support the suggested role of BRC1 as a local integrator of different branching signals (Aguilar-Martinez et al., 2007; Minakuchi et al., 2010). Under this scenario, the downregulation of TB1-like genes should be both necessary and sufficient to implement bud outgrowth. The bushy phenotypes of loss-of-function mutants are consistent with this idea. More evidence that TB1-like genes confer bud arrest comes from experiments where they are expressed beyond their natural domain/level. Both overexpression of OsTB1/FC1 in rice and expression of TB1 in wheat controlled by a maize ubiquitin promoter resulted in less bud outgrowth (Takeda et al., 2003; Lewis et al., 2008). Overexpression of BRC1 in an N-terminal fusion to green fluorescent protein (GFP) under the control of the cauliflower mosaic virus (CaMV) 35S promoter led to severely impaired growth of transgenic Arabidopsis plants and a reduced growth rate, resulting in plants with reduced height and smaller and misshapen rosette leaves. The SAM terminated prematurely. The more highly the transgene was expressed, the more the growth was reduced (Aguilar-Martinez et al., 2007). The known effects of TCPs on the transcription of cell cycle and growth genes described above provides a mechanistic explanation for these observations. However, there are an increasing number of examples where the correlation between TCP gene expression and bud activity breaks down, complicating the picture.
For example, in Sorghum bicolor, both simulated shade and defoliation led to a decrease of expression of cell cycling genes such as those encoding H4, PCNA, CycD2, CycB and CDKB (Kebrom et al., 2010a, b). However, in contrast to simulated shade, strong bud arrest following defoliation was not associated with increased SbTB1 expression. Thus plants can apparently inhibit bud outgrowth independently of SbTB1 function. In contrast to the gradual bud inhibition resulting from shading, defoliation triggered bud arrest immediately. Furthermore, in rice wild-type plants, lateral buds of the first formed leaf axil usually remain dormant. These buds grow out in fc1 mutants as well as in mutants defective in the biosynthesis or signalling of strigolactone. It has been suggested that FC1 and BRC1 act downstream of strigolactones because fc1 and brc1 mutants do not respond to strigolactone application (Brewer et al., 2009; Minakuchi et al., 2010). However, FC1 expression remains high in the ectopically active buds of rice strigolactone mutants (Arite et al., 2007), and overexpression of FC1 cannot fully suppress their branching phenotypes (Minakuchi et al., 2010). This could be because of post-transcriptional regulation, or it could be because high FC1 is not sufficient for bud suppression. Thus it is possible that these TCP genes are neither necessary nor sufficient to induce dormancy.
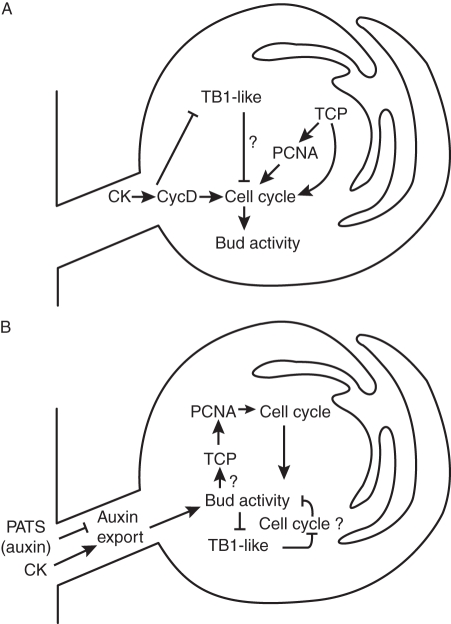
In this context it is interesting to consider the various possible modes of action for auxin and CK described above. An appealing model is that auxin in the main stem regulates CK levels at the node, and possibly also in the roots, which determines the amount of CK transported into the bud. This in turn regulates transcription of TCPs, which affects cell cycle- and growth-related genes in the bud, determining bud activity (Fig. 3A). There is good evidence supporting much of this chain of reasoning. However, there is also good evidence that it is not the only system operating. Alternatives include the auxin transport canalization-based model for bud regulation, which involves bud inhibition with no requirement for changes in hormone concentrations in the bud. Under this model, it could be that auxin export control is the integrator of bud activity, and TCP expression follows and stabilizes the effects of auxin export. This is a reasonable hypothesis because auxin transport canalization is a positive feedback process, making it inherently unstable. TCP expression may therefore be needed to stabilize the off state (Fig. 3B). It is interesting that, to date, admittedly with a rather limited set of experiments, there is complete correlation between bud activity and auxin transport out of the bud, while, as described above, the correlation with TCP expression can be broken.
Fig. 3.
(A) Bud activation and the cell cycle. This model is built on the assumption that CKs regulate TCP transcription factors and those in turn regulate cell cycling to control bud activity. CK entering buds promotes bud activity by suppressing TB1-like genes that restrict cell cycling and negatively regulate bud outgrowth. Other TCP family members have the opposite effect. They promote cell cycling during bud activation, for example by upregulating PCNA. CKs may also promote cell cycling in meristems more directly by inducing cycD transcription. (B) Bud activation and the cell cycle. Another model proposes that auxin export out of the bud is a key regulator that governs bud activity. In this scenario auxin export is a prerequisite for bud activation. It is antagonistically regulated by auxin in the primary stem PATS and by CK, which may enter the bud. Once bud auxin export enables bud activity, the cell cycle machinery gets going, which is in part mediated by TCP transcription factors via PCNA. If lack of auxin export prevents bud outgrowth, this decision is stabilized at least in part by TB1-like genes, which may suppress cell cycling and keep the bud inactive. CK, cytokinin; cycD, cyclinD; PATS, polar auxin transport stream; PCNA, proliferating cell nuclear antigen; TB1-like, Teosinte Branched1-like; TCP, Teosinte branched-Cycloidea-PCF.
FUTURE PERSPECTIVES
The relationship between auxin and CK in bud regulation has been debated and studied for many decades. Despite rapid progress in recent years, there are still many fundamental unanswered questions. Alternative hypotheses have emerged, and efforts to design experiments to distinguish between these should allow continued progress. Of particular importance are the site of action of each hormone, and the roles of auxin transport and the TCP transcription factors in the process.
ACKNOWLEDGEMENTS
D.M. was funded by an ERANet-PG grant to O.L. as part of the ACT consortium on Auxin and Cytokinin cross talk.
LITERATURE CITED
- Aguilar-Martinez JA, Poza-Carrion C, Cubas P. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. The Plant Cell. 2007;19:458–472. doi: 10.1105/tpc.106.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arite T, Iwata H, Ohshima K, et al. DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. The Plant Journal. 2007;51:1019–1029. doi: 10.1111/j.1365-313X.2007.03210.x. [DOI] [PubMed] [Google Scholar]
- Balla J, Kalousek P, Reinohl V, Friml J, Prochazka S. Competitive canalization of PIN-dependent auxin flow from axillary buds controls pea bud outgrowth. The Plant Journal. 2011;65:571–577. doi: 10.1111/j.1365-313X.2010.04443.x. [DOI] [PubMed] [Google Scholar]
- Bangerth F. Response of cytokinin concentration in the xylem exudate of bean (Phaseolus-vulgaris L) plants to decapitation and auxin treatment, and relationship to apical dominance. Planta. 1994;194:439–442. [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O. The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Current Biology. 2006;16:553–563. doi: 10.1016/j.cub.2006.01.058. [DOI] [PubMed] [Google Scholar]
- Beveridge CA, Murfet IC, Kerhoas L, Sotta B, Miginiac E, Rameau C. The shoot controls zeatin riboside export from pea roots. Evidence from the branching mutant rms4. The Plant Journal. 1997;11:339–345. [Google Scholar]
- Blilou I, Xu J, Wildwater M, et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- Booker J, Chatfield S, Leyser O. Auxin acts in xylem-associated or medullary cells to mediate apical dominance. Plant The Cell. 2003;15:495–507. doi: 10.1105/tpc.007542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucheron E, Healy JH, Bajon C, et al. Ectopic expression of Arabidopsis CYCD2 and CYCD3 in tobacco has distinct effects on the structural organization of the shoot apical meristem. Journal of Experimental Botany. 2005;56:123–134. doi: 10.1093/jxb/eri001. [DOI] [PubMed] [Google Scholar]
- Brand U, Hobe M, Simon R. Functional domains in plant shoot meristems. Bioessays. 2001;23:134–141. doi: 10.1002/1521-1878(200102)23:2<134::AID-BIES1020>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiology. 2009;150:482–493. doi: 10.1104/pp.108.134783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield SP, Stirnberg P, Forde BG, Leyser O. The hormonal regulation of axillary bud growth in Arabidopsis. The Plant Journal. 2000;24:159–169. doi: 10.1046/j.1365-313x.2000.00862.x. [DOI] [PubMed] [Google Scholar]
- Corbesier L, Prinsen E, Jacqmard A, et al. Cytokinin levels in leaves, leaf exudate and shoot apical meristem of Arabidopsis thaliana during floral transition. Journal of Experimental Botany. 2003;54:2511–2517. doi: 10.1093/jxb/erg276. [DOI] [PubMed] [Google Scholar]
- Crawford S, Shinohara N, Sieberer T, et al. Strigolactones enhance competition between shoot branches by dampening auxin transport. Development. 2010;137:2905–2913. doi: 10.1242/dev.051987. [DOI] [PubMed] [Google Scholar]
- Cubas P, Lauter N, Doebley J, Coen E. The TCP domain: a motif found in proteins regulating plant growth and development. The Plant Journal. 1999;18:215–222. doi: 10.1046/j.1365-313x.1999.00444.x. [DOI] [PubMed] [Google Scholar]
- Davies CR, Seth AK, Wareing PF. Auxin and kinetin interaction in apical dominance. Science. 1966;151:468–469. doi: 10.1126/science.151.3709.468. [DOI] [PubMed] [Google Scholar]
- Devitt ML, Stafstrom JP. Cell cycle regulation during growth–dormancy cycles in pea axillary buds. Plant Molecular Biology. 1995;29:255–265. doi: 10.1007/BF00043650. [DOI] [PubMed] [Google Scholar]
- Dewitte W, Scofield S, Alcasabas AA, et al. Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proceedings of the National Academy of Sciences, USA. 2007;104:14537–14542. doi: 10.1073/pnas.0704166104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodsworth S. A diverse and intricate signalling network regulates stem cell fate in the shoot apical meristem. Developmental Biology. 2009;336:1–9. doi: 10.1016/j.ydbio.2009.09.031. [DOI] [PubMed] [Google Scholar]
- Doebley J, Stec A, Hubbard L. The evolution of apical dominance in maize. Nature. 1997;386:485–488. doi: 10.1038/386485a0. [DOI] [PubMed] [Google Scholar]
- Faiss M, Zalubilova J, Strnad M, Schmulling T. Conditional transgenic expression of the ipt gene indicates a function for cytokinins in paracrine signaling in whole tobacco plants. The Plant Journal. 1997;12:401–415. doi: 10.1046/j.1365-313x.1997.12020401.x. [DOI] [PubMed] [Google Scholar]
- Finlayson SA. Arabidopsis Teosinte Branched1-like 1 regulates axillary bud outgrowth and is homologous to monocot Teosinte Branched1. Plant and Cell Physiology. 2007;48:667–677. doi: 10.1093/pcp/pcm044. [DOI] [PubMed] [Google Scholar]
- Foo E, Morris SE, Parmenter K, et al. Feedback regulation of xylem cytokinin content is conserved in pea and Arabidopsis. Plant Physiology. 2007;143:1418–1428. doi: 10.1104/pp.106.093708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin V, Lunness PA, Fobert PR, et al. The expression of D-cyclin genes defines distinct developmental zones in snapdragon apical meristems and is locally regulated by the Cycloidea gene. Plant Physiology. 2000;122:1137–1148. doi: 10.1104/pp.122.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulini A, Wang J, Jackson D. Control of phyllotaxy by the cytokinin-inducible response regulator homologue ABPHYL1. Nature. 2004;430:1031–1034. doi: 10.1038/nature02778. [DOI] [PubMed] [Google Scholar]
- Hay A, Kaur H, Phillips A, Hedden P, Hake S, Tsiantis M. The gibberellin pathway mediates KNOTTED1-type homeobox function in plants with different body plans. Current Biology. 2002;12:1557–1565. doi: 10.1016/s0960-9822(02)01125-9. [DOI] [PubMed] [Google Scholar]
- Heisler MG, Ohno C, Das P, et al. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Current Biology. 2005;15:1899–1911. doi: 10.1016/j.cub.2005.09.052. [DOI] [PubMed] [Google Scholar]
- Helliwell CA, Chin-Atkins AN, Wilson IW, Chapple R, Dennis ES, Chaudhury A. The Arabidopsis AMP1 gene encodes a putative glutamate carboxypeptidase. The Plant Cell. 2001;13:2115–2125. doi: 10.1105/TPC.010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Pischke MS, Mahonen AP, et al. In planta functions of the Arabidopsis cytokinin receptor family. Proceedings of the National Academy of Sciences, USA. 2004;101:8821–8826. doi: 10.1073/pnas.0402887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose N, Takei K, Kuroha T, Kamada-Nobusada T, Hayashi H, Sakakibara H. Regulation of cytokinin biosynthesis, compartmentalization and translocation. Journal of Experimental Botany. 2008;59:75–83. doi: 10.1093/jxb/erm157. [DOI] [PubMed] [Google Scholar]
- Hu MC, Rosenblum ND. Genetic regulation of branching morphogenesis: lessons learned from loss-of-function phenotypes. Pediatric Research. 2003;54:433–438. doi: 10.1203/01.PDR.0000085170.44226.DB. [DOI] [PubMed] [Google Scholar]
- Hubbard L, McSteen P, Doebley J, Hake S. Expression patterns and mutant phenotype of teosinte branched1 correlate with growth suppression in maize and teosinte. Genetics. 2002;162:1927–1935. doi: 10.1093/genetics/162.4.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Iwase M, Kodama H, Lavisse P, Komamine A, Nishihama R, Machida Y, Watanabe A. A novel cis-acting element in promoters of plant B-type cyclin genes activates M phase-specific transcription. The Plant Cell. 1998;10:331–341. doi: 10.1105/tpc.10.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski S, Piazza P, Craft J, et al. KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Current Biology. 2005;15:1560–1565. doi: 10.1016/j.cub.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Jones B, Gunneras SA, Petersson SV, et al. Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. The Plant Cell. 2010;22:2956–2969. doi: 10.1105/tpc.110.074856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalousek P, Buchtová D, Balla J, Reinöhl V, Procházka S. Cytokinins and polar transport of auxin in axillary pea buds. Magazine Acta Universitatis Agriculturae et Silviculturae Mendeleianae Brunensis. 2010;58:79–88. [Google Scholar]
- Kebrom TH, Burson BL, Finlayson SA. Phytochrome B represses Teosinte Branched1 expression and induces sorghum axillary bud outgrowth in response to light signals. Plant Physiology. 2006;140:1109–1117. doi: 10.1104/pp.105.074856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebrom TH, Brutnell TP, Finlayson SA. Suppression of sorghum axillary bud outgrowth by shade, phyB and defoliation signalling pathways. Plant, Cell and Environment. 2010a;33:48–58. doi: 10.1111/j.1365-3040.2009.02050.x. [DOI] [PubMed] [Google Scholar]
- Kebrom TH, Brutnell TP, Hays DB, Finlayson SA. Vegetative axillary bud dormancy induced by shade and defoliation signals in the grasses. Plant Signaling and Behavior. 2010b;5:317–319. doi: 10.4161/psb.5.3.11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi S, Ohashi Y. PCF1 and PCF2 specifically bind to cis elements in the rice proliferating cell nuclear antigen gene. The Plant Cell. 1997;9:1607–1619. doi: 10.1105/tpc.9.9.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi S, Suzuka I, Ohashi Y. Two of three promoter elements identified in a rice gene for proliferating cell nuclear antigen are essential for meristematic tissue-specific expression. The Plant Journal. 1995;7:877–886. doi: 10.1046/j.1365-313x.1995.07060877.x. [DOI] [PubMed] [Google Scholar]
- Kudo T, Kiba T, Sakakibara H. Metabolism and long-distance translocation of cytokinins. Journal of Integrative Plant Biology. 2010;52:53–60. doi: 10.1111/j.1744-7909.2010.00898.x. [DOI] [PubMed] [Google Scholar]
- Kurakawa T, Ueda N, Maekawa M, et al. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature. 2007;445:652–655. doi: 10.1038/nature05504. [DOI] [PubMed] [Google Scholar]
- Kuroha T, Tokunaga H, Kojima M, et al. Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in Arabidopsis. The Plant Cell. 2009;21:3152–3169. doi: 10.1105/tpc.109.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaba S, Kano-Murakami Y, Matsuoka M, et al. Alteration of hormone levels in transgenic tobacco plants overexpressing the rice homeobox gene OSH1. Plant Physiology. 1998;116:471–476. doi: 10.1104/pp.116.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibfried A, To JP, Busch W, et al. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature. 2005;438:1172–1175. doi: 10.1038/nature04270. [DOI] [PubMed] [Google Scholar]
- Lewis JM, Mackintosh CA, Shin S, et al. Overexpression of the maize Teosinte Branched1 gene in wheat suppresses tiller development. Plant Cell Reports. 2008;27:1217–1225. doi: 10.1007/s00299-008-0543-8. [DOI] [PubMed] [Google Scholar]
- Li C-H, Bangerth F. The possible role of cytokinins ethylene and indoleacetic acid in apical dominance. In: Karssen CM, van Loon LC, Vreugdenhil D, editors. Progress in plant growth regulation. Dordrecht: Kluwer Academic Publishers; 1992. pp. 431–443. [Google Scholar]
- Li C, Bangerth F. Stimulatory effect of cytokinins and interaction with IAA on the release of lateral buds of pea plants from apical dominance. Journal of Plant Physiology. 2003;160:1059–1063. doi: 10.1078/0176-1617-01042. [DOI] [PubMed] [Google Scholar]
- Li C, Potuschak T, Colon-Carmona A, Gutierrez RA, Doerner P. Arabidopsis TCP20 links regulation of growth and cell division control pathways. Proceedings of the National Academy of Sciences, USA. 2005;102:12978–12983. doi: 10.1073/pnas.0504039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CJ, Bangerth F. Autoinhibition of indoleacetic acid transport in the shoots of two-branched pea (Pisum sativum) plants and its relationship to correlative dominance. Physiologia Plantarum. 1999;106:415–420. [Google Scholar]
- Ljung K, Bhalerao RP, Sandberg G. Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. The Plant Journal. 2001;28:465–474. doi: 10.1046/j.1365-313x.2001.01173.x. [DOI] [PubMed] [Google Scholar]
- Martin-Trillo M, Cubas P. TCP genes: a family snapshot ten years later. Trends in Plant Sciences. 2010;15:31–39. doi: 10.1016/j.tplants.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Matsumoto-Kitano M, Kusumoto T, Tarkowski P, et al. Cytokinins are central regulators of cambial activity. Proceedings of the National Academy of Sciences, USA. 2008;105:20027–20031. doi: 10.1073/pnas.0805619105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakuchi K, Kameoka H, Yasuno N, et al. FINE CULM1 (FC1) works downstream of strigolactones to inhibit the outgrowth of axillary buds in rice. Plant and Cell Physiology. 2010;51:1127–1135. doi: 10.1093/pcp/pcq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki K, Matsumoto-Kitano M, Kakimoto T. Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate. The Plant Journal. 2004;37:128–138. doi: 10.1046/j.1365-313x.2003.01945.x. [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Tarkowski P, Matsumoto-Kitano M, et al. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proceedings of the National Academy of Sciences, USA. 2006;103:16598–16603. doi: 10.1073/pnas.0603522103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DA. Transport of exogenous auxin in 2-branched dwarf pea-seedlings (Pisum sativum L) – some implications for polarity and apical dominance. Planta. 1977;136:91–96. doi: 10.1007/BF00387930. [DOI] [PubMed] [Google Scholar]
- Nordstrom A, Tarkowski P, Tarkowska D, et al. Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin–cytokinin-regulated development. Proceedings of the National Academy of Sciences, USA. 2004;101:8039–8044. doi: 10.1073/pnas.0402504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nougarede A. Experimental cytology of the shoot apical cells during vegetative growth and flowering. International Review of Cytology. 1967;21:203–351. doi: 10.1016/s0074-7696(08)60815-3. [DOI] [PubMed] [Google Scholar]
- Panigrahi BM, Audus LJ. Apical dominance in Vicia faba. Annals of Botany. 1966;30:457–473. [Google Scholar]
- Prusinkiewicz P, Crawford S, Smith RS, et al. Control of bud activation by an auxin transport switch. Proceedings of the National Academy of Sciences, USA. 2009;106:17431–17436. doi: 10.1073/pnas.0906696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JA. Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science. 1999;283:1541–1544. doi: 10.1126/science.283.5407.1541. [DOI] [PubMed] [Google Scholar]
- Sachs T. The control of the patterned differentiation of vascular tissues. Advances in Botanical Research. 1981;9:151–262. [Google Scholar]
- Sachs T. Integrating cellular and organismic aspects of vascular differentiation. Plant and Cell Physiology. 2000;41:649–656. doi: 10.1093/pcp/41.6.649. [DOI] [PubMed] [Google Scholar]
- Sachs T, Thimann V. The role of auxins and cytokinins in the release of buds from dominance. American Journal of Botany. 1967;54:136–144. [Google Scholar]
- Sakamoto T, Kamiya N, Ueguchi-Tanaka M, Iwahori S, Matsuoka M. KNOX homeodomain protein directly suppresses the expression of a gibberellin biosynthetic gene in the tobacco shoot apical meristem. Genes and Development. 2001;15:581–590. doi: 10.1101/gad.867901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Sakakibara H, Kojima M, et al. Ectopic expression of KNOTTED1-like homeobox protein induces expression of cytokinin biosynthesis genes in rice. Plant Physiology. 2006;142:54–62. doi: 10.1104/pp.106.085811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Mori H. Analysis of cycles of dormancy and growth in pea axillary buds based on mRNA accumulation patterns of cell cycle-related genes. Plant and Cell Physiology. 1998;39:255–262. doi: 10.1093/oxfordjournals.pcp.a029365. [DOI] [PubMed] [Google Scholar]
- Snyder WE. Some responses of plants to 2,3,5-triiodobenzoic acid. Plant Physiology. 1949;24:195–206. doi: 10.1104/pp.24.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom JP, Sussex IM. Expression of a ribosomal protein gene in axillary buds of pea seedlings. Plant Physiology. 1992;100:1494–1502. doi: 10.1104/pp.100.3.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T, Suwa Y, Suzuki M, et al. The OsTB1 gene negatively regulates lateral branching in rice. The Plant Journal. 2003;33:513–520. doi: 10.1046/j.1365-313x.2003.01648.x. [DOI] [PubMed] [Google Scholar]
- Takei K, Yamaya T, Sakakibara H. Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-zeatin. Journal of Biological Chemistry. 2004;279:41866–41872. doi: 10.1074/jbc.M406337200. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H. Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. The Plant Journal. 2006;45:1028–1036. doi: 10.1111/j.1365-313X.2006.02656.x. [DOI] [PubMed] [Google Scholar]
- Tatematsu K, Ward S, Leyser O, Kamiya Y, Nambara E. Identification of cis-elements that regulate gene expression during initiation of axillary bud outgrowth in Arabidopsis. Plant Physiology. 2005;138:757–766. doi: 10.1104/pp.104.057984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann KV, Skoog F. Studies on the growth hormone of plants: III. The inhibiting action of the growth substance on bud development. Proceedings of the National Academy of Sciences, USA. 1933;19:714–716. doi: 10.1073/pnas.19.7.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To JP, Haberer G, Ferreira FJ, et al. Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. The Plant Cell. 2004;16:658–671. doi: 10.1105/tpc.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremousaygue D, Garnier L, Bardet C, Dabos P, Herve C, Lescure B. Internal telomeric repeats and ‘TCP domain’ protein-binding sites co-operate to regulate gene expression in Arabidopsis thaliana cycling cells. The Plant Journal. 2003;33:957–966. doi: 10.1046/j.1365-313x.2003.01682.x. [DOI] [PubMed] [Google Scholar]
- Veit B. Hormone mediated regulation of the shoot apical meristem. Plant Molecular Biology. 2009;69:397–408. doi: 10.1007/s11103-008-9396-3. [DOI] [PubMed] [Google Scholar]
- Vieten A, Vanneste S, Wisniewska J, et al. Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development. 2005;132:4521–4531. doi: 10.1242/dev.02027. [DOI] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmulling T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. The Plant Cell. 2003;15:2532–2550. doi: 10.1105/tpc.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Strnad M, Schmulling T. Regulation of plant growth by cytokinin. Proceedings of the National Academy of Sciences, USA. 2001;98:10487–10492. doi: 10.1073/pnas.171304098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickson M, Thimann KV. The antagonism of auxin and kinetin in apical dominance. Physiologia Plantarum. 1958;11:62–74. [Google Scholar]
- Yadav RK, Girke T, Pasala S, Xie M, Reddy GV. Gene expression map of the Arabidopsis shoot apical meristem stem cell niche. Proceedings of the National Academy of Sciences, USA. 2009;106:4941–4946. doi: 10.1073/pnas.0900843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai O, Shani E, Dolezal K, et al. Arabidopsis KNOXI proteins activate cytokinin biosynthesis. Current Biology. 2005;15:1566–1571. doi: 10.1016/j.cub.2005.07.060. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Christensen SK, Fankhauser C, et al. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science. 2001;291:306–309. doi: 10.1126/science.291.5502.306. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Andersen SU, Ljung K, et al. Hormonal control of the shoot stem-cell niche. Nature. 2010;465:1089–1092. doi: 10.1038/nature09126. [DOI] [PubMed] [Google Scholar]