Abstract
Recently, a small 11-amino acid amidated peptide, dopamine neuron stimulating peptide-11 (DNSP-11), was shown to exert neurotrophic-like actions on primary dopaminergic neurons and in parkinsonian rat models. This suggests smaller neurotrophic molecules may be deliverable and modifiable for therapeutic use. Here we evaluate the molecular and cellular protection properties of DNSP-11 and two other amidated-peptides, a 5-mer (DNSP-5) and a 17-mer (DNSP-17), hypothesized to be endoproteolytically processed from the pro- and mature glial cell line-derived neurotrophic factor (GDNF) protein sequence, respectively. Far-UV circular dichroism spectra show that the three DNSPs are soluble and act independently in vitro. Reverse phase HPLC and mass spectrometry analysis show that the three peptides are stable for one month at a variety of storage and experimental conditions. To gain insight into the DNSPs biodistribution properties in the brain, we used affinity chromatography to show that DNSP-17 binds heparin equally as tight as GDNF, whereas DNSP-5 and DNSP-11 do not bind heparin, which should facilitate their delivery in vivo. Finally, we present data showing that DNSP-11 provides dose-dependent protection of HEK-293 cells from staurosporine and 3-nitropropionate (3-NP) cytotoxicity, thereby supporting its broad mitochondrial-protective properties.
Keywords: propeptides, GDNF, biotherapeutic, heparin, neuroprotection
1. Introduction
Glial cell line-derived neurotrophic factor (GDNF), due to its potent and specific neurotrophic effects on dopaminergic neurons (Lin et al., 1993), has been extensively examined as a therapeutic agent for the treatment of age-related neurodegenerative diseases, such as Parkinson’s disease (PD) (Gash et al., 1996; Gill et al., 2003; Lang et al., 2006; Slevin et al., 2005). However, GDNF has not advanced beyond phase II clinical trials, primarily due to challenges attributed to the direct intracranial delivery of large proteins (Gash et al., 2005; Lang et al., 2006; Patel and Gill, 2007; Salvatore et al., 2006). Furthermore, GDNF binds heparin with high affinity (Lin et al., 1994; Lin et al., 1993), and likely other heparin-related molecules abundant in the brain matrix (Rickard et al., 2003; Sariola and Saarma, 2003), which hinders its predictable biodistribution following a direct injection (Gash et al., 2005; Lapchak et al., 1998; Patel and Gill, 2007; Piltonen et al., 2009; Salvatore et al., 2006). While additional delivery strategies have been examined to improve GDNF delivery and distribution in vivo, including: convection enhanced delivery (CED) (Fiandaca et al., 2008; Hamilton et al., 2001; Morrison et al., 2007); co-infusion with heparin during CED (Hamilton et al., 2001); removal of the GDNF N-terminal heparin binding domain (Piltonen et al., 2009); viral vector delivery (Kordower et al., 2000; Ramaswamy et al., 2009; Wang et al., 2002); and encapsulated GDNF-producing cells (Lindner et al., 1995; Lindvall and Wahlberg, 2008), an alternate approach to circumvent these delivery and distribution challenges would be to utilize small, neurotrophic-like functional molecules.
Recently, it has been hypothesized that functional, carboxy-terminally amidated peptides of 5, 11, and 17 amino acids are processed from the rodent and human GDNF precursor and mature sequences upon proteolytic cleavage by furin-like endopeptidases (Bradley et al., 2010; Immonen et al., 2008). Based on initial studies showing all three peptides possessed some dopaminergic activities, they were named dopamine neuron stimulating peptides (Bradley et al., 2009). While these peptides have not been isolated endogenously to date, initial studies in rat hippocampal CA1 pyrimidal neurons showed that the rat 11-mer sequence (named brain excitatory peptide, BEP) significantly induced synaptic excitability, while the 5- and 17-mer sequences failed to show statistical significance (Immonen et al., 2008). Furthermore, we have shown the human 11-mer sequence (named dopamine neuron stimulating peptide-11, DNSP-11) exhibits neurotrophic-like properties including (i) promoting the survival of primary fetal mesencephalic neurons; (ii) in vitro protection from 6-hydroxydopamine (6-OHDA) in primary mesencephalic and MN9D dopaminergic cell culture; (iii) improving the neurochemical resting levels of dopamine and its metabolites for up to 28 days following a single injection into the rat substantia nigra; and (iv) significantly improving apomorphine-induced rotational behavior in a severe, PD rat model (Bradley et al., 2010). Collectively, these data support the further characterization and translational evaluation of these peptides as therapeutic candidates.
Here we present the initial in vitro physical characterization of DNSP-5, DNSP-11, and DNSP-17. We show that all three peptides are soluble and stable under a variety of conditions in vitro. In addition, we show that DNSP-5 and DNSP-11 do not interact with heparin, which would enhance their in vivo biodistribution following delivery to the brain. Finally, we show that DNSP-11 offers significant protection, from both staurosporine- and 3-nitropropionate (3-NP)-induced cytotoxicity in HEK-293 cells, supporting the potential for broad beneficial effects on other, non-neuronal cell types. These data provide the basis for future evaluation and development of the dopamine neuron stimulating peptides as a disease modifying therapeutic.
2. Experimental Procedure
2.1 Materials
Unless noted, all chemicals and materials were obtained from Sigma (St. Louis, MI) and were reagent grade. Human embryonic kidney 293 (HEK-293) cells were obtained from American Type Culture Collection (Manassas, VA). DNSP-5 (sequence: Phe-Pro-Leu-Pro-Ala-amide), DNSP-11 (sequence: Pro-Pro-Glu-Ala-Pro-Ala-Glu-Asp-Arg-Ser-Leu-amide), and DNSP-17 (sequence: Glu-Arg-Asn-Arg-Gln-Ala-Ala-Ala-Ala-Asn-Pro-Glu-Asn-Ser-Arg-Gly-Lys-amide) were synthesized by AC Scientific (Duluth, GA) and the W.M. Keck Foundation Biotechnology Resource Laboratory at Yale University and purified to >98% by reverse phase-high pressure liquid chromatography (RP-HPLC). Recombinant human GDNF, expressed in Escherichia coli, was generously provided from Dr. Barry Hoffer, NIDA.
2.2 Stability Study
Individual (0.3 and 1.0 mg/mL) and combination solutions of DNSP-5, DNSP-11, and DNSP-17 were made in sterile citrate buffer (10 mM Citrate + 150 mM NaCl, pH 5.0). Samples were then stored at −80 °C and 37 °C for 0, 3, 7, 10, 14, 17, 21, 25, 28, or 31 days. At these time points, aliquots were analyzed for degradation using RP-HPLC (Waters Breeze System) with dH20 (HPLC grade) + 0.1% trifluoroacetic acid (TFA) as the aqueous mobile phase. Samples were loaded to a C4 column (4.6 mm × 75 mm, 300 Å pore size, GRACE/Vydac 214TP54, Deerfield, IL) at a flow rate of 1 mL/min and the column flow through was monitored at 214 nm with a Waters 2486 dual-wavelength UV/VIS detector. Samples were eluted with a linear gradient of the organic mobile phase (acetonitrile + 0.1% TFA), to a final aqueous:organic phase ratio of 75:25 after 30 minutes. All solvents were HPLC grade, degassed and filtered prior to use. At 31 days, aliquots were subjected to LC-MS analysis.
2.3 Far-UV circular dichroism spectroscopy
CD measurements were performed for each purified peptide sample (DNSP-5, 130 μM; DNSP-11, 21 μM; DNSP-17, 13 μM) in 50 mM sodium phosphate buffer, pH 7.0. Measurements were made in a 1 mm quartz cuvette using a Jasco J-810 spectrophotometer. Spectra were recorded as the average of four far-UV wavelength scans from 250 to 190 nm with 0.5 nm steps and 8 second averaging time.
2.4 Heparin affinity chromatography
10 μM peptide and GDNF samples in 10 mM sodium citrate, pH 5.6 were loaded to a 1 mL HiTrap™ Heparin HP Column (GE Healthcare) at 1 mL/min. Column elutant was simultaneously monitored for peptide/protein (λ =215 nm) and salt concentration using an AKTA Explorer 100 equipped with UV/Vis detector and conductivity monitor. Following column loading and washing, heparin-binding samples were eluted with a high-salt linear gradient (10 mM sodium citrate + 2 M NaCl, pH 5.6). All buffers were freshly prepared, filtered and degassed prior to use.
2.5 Caspase-3 Activity Assay
HEK-293 cells were plated to 100,000 cells/well. Cell cultures were exposed to defined dosages of DNSP-5, DNSP-11, or DNSP-17 and either 1 μM staurosporine or 8 mM 3-nitropropionate exposure. The Enz Chek (Invitrogen) caspase-3 kit was used to monitor caspase-3 activity. Fluorescence measurements were made after 12 hours of treatment (λex/λem 496/520nm) using a Molecular Devices Spectramax M5 plate reader. Protein levels of lysed cells were measured by BCA assay (BioRad) and normalized for every experiment. Data are expressed as percent of control and were repeated a minimum of 3 times.
3. Results
3.1 RP-HPLC analysis and long-term stability of the DNSPs
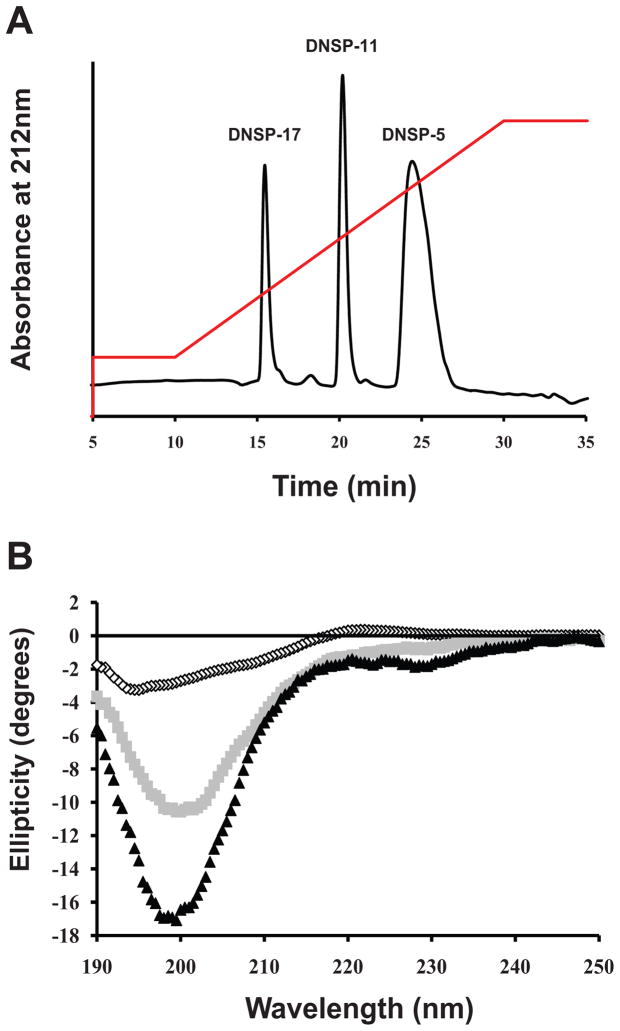
Reverse phase HPLC (RP-HPLC) was used to isolate and purify DNSP-5, DNSP-11, and DNSP-17 from an aqueous tripeptide mixture solution (Figure 1A). The individual DNSPs were separated from an aqueous tripeptide mixture solution on a C4 reverse phase column with increasing concentrations of acetonitrile, in an inverse relationship to their size (Table 1). The identification of each well-resolved peak was confirmed by liquid chromatography mass spectrometry (LC-MS) (Table 1).
Figure 1.
Physical characterization of the DNSPs. (A) A tripeptide mixture of DNSP-5, DNSP-11, and DNSP-17 was loaded to a C4 column in dH2O and 0.1% TFA. After ten minutes of wash (1 mL/min), samples were eluted with a linear gradient (red) of the organic mobile phase (acetonitrile + 0.1% TFA), to a final aqueous:organic phase ratio of 75:25 after 30 minutes. Elution of the peptides was monitored at 214 nm. (B) Far-UV CD analysis of DNSP-5 (open circles), DNSP-11 (gray squares), and DNSP-17 (back triangles) shows that the peptides have backbone characteristics of small, soluble peptides.
Table 1.
Characterization of the DNSPs in vitro stability after incubation at 37°C for 31 days. Integration of the RP-HPLC elution peaks for each of the DNSPs were used to calculate the percent of peptide remaining after incubation at 37 °C for 31 days. These samples were then submitted to mass spectrometry, in which the determined molecular weight following extended incubation was in agreement with the calculated sequence molecular weight.
| Propeptide | RP-HPLC Retention Time (minutes) | % Remaining after 30 days (37°C) | Calculated Molecular Weight | Molecular Weight after 31 days (37°C) |
|---|---|---|---|---|
| DNSP-5 | 24.4 | 93.6% | 542 | 542.1 |
| DNSP-11 | 20.2 | 97.6% | 1180 | 1180.3 |
| DNSP-17 | 15.5 | 94.6% | 1868 | 1868.1 |
RP-HPLC and LC-MS were used to monitor the stability of DNSP-5, DNSP-11, and DNSP-17. The peptides were stored in citrate buffer (10 mM Citrate + 150 mM NaCl, pH 5.0) at −80 °C and 37 °C for 31 days. These conditions were chosen based on their relevance to long-term storage and use in future in vivo translational studies. The peptides were stable at all temperatures tested, thus allowing for the confident use of the individual peptides for further investigation, when stored in vitro at these temperatures (Table 1, supplemental figure 1). Furthermore, LC-MS and amino acid sequencing data confirmed there was no intrinsic degradation within these sequences (i.e. deamidation) upon storage: Each peptide sequence was as originally synthesized (data not shown). Additional studies performed at 4°C found no degradation at one week as determined by RP-HPLC (data not shown).
3.2 Far-UV circular dichroism structural analysis
The peptide backbone structure of DNSP-5, DNSP-11, and DNSP-17 were examined using circular dichroism (CD) spectroscopy in the far-UV region. All three peptides exhibited a minimum ellipticity value between 196–200 nm, with small spectral signatures observed (shoulders between 208–230 nm), typical of small, soluble peptides of similar length sampling multiple backbone conformations including: random coil, turn, polyproline II and α-helix (Figure 1B). Furthermore, CD was also utilized to evaluate if there are intermolecular interactions between DNSP-5, DNSP-11, and DNSP-17 in vitro. The tripeptide mixture has similar far-UV CD spectra as the additive spectra of the individual peptides, with minor differences at 200 nm that are within experimental error (supplemental figure 2), thus demonstrating that the three peptides do not interact in vitro.
3.3 Heparin binding analysis
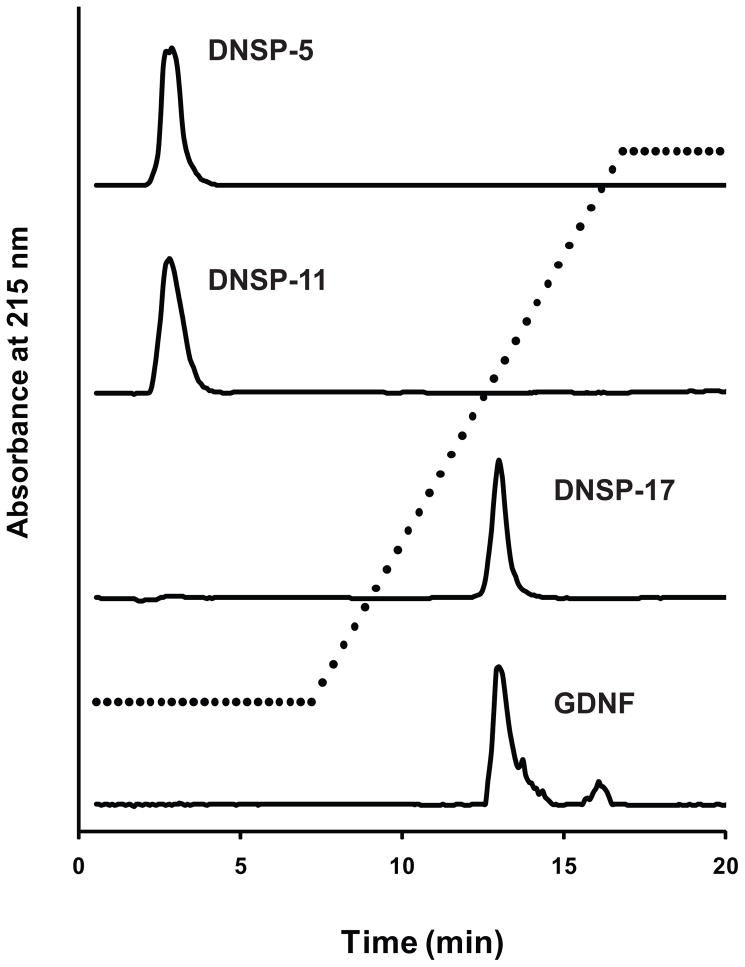
We investigated the heparin-binding properties of the DNSPs using heparin affinity chromatography. Human GDNF binds tightly to the heparin sepharose column, eluting at high salt concentrations [~ 0.8 M NaCl] in 10 mM sodium citrate buffer (pH 5.6), consistent with earlier data (Lin et al., 1994). DNSP-17, which sequence is present within the heparin binding N-terminus of mature GDNF, binds strongly to the heparin column with an identical elution profile to mature GDNF (Figure 2). However, the peptides derived from the GDNF prosequence, DNSP-5 and DNSP-11, have no affinity for the heparin column, eluting in the column flow through (Figure 2).
Figure 2.
Heparin affinity chromatography of the DNSPs and GDNF. 10 μM samples of the synthetic DNSPs and GDNF (in 10 mM sodium citrate, pH 5.6) were applied to a HiTrap™ Heparin HP affinity column and elutant was monitored at 215 nm. After sample loading and wash, a high salt (10 mM sodium citrate + 2 M NaCl) linear gradient was applied (dashed line) to elute heparin-binding samples.
3.4 Protection from staurosporine and 3-nitropropionate in HEK-293 cells
While DNSP-11 has analogous in vivo neurotrophic-like properties, it likely functions differently than GDNF. We showed that DNSP-11 does not directly interact with the physiological receptor of mature GDNF, GFRα1 (Bradley et al., 2010). Furthermore, treatment with 10 ng/mL (~ 10 nM) DNSP-11 was shown to block 1 μM staurosporine-induced cytotoxicity in nutrient-deprived dopaminergic B65 cells, and its neuroprotective effects included preventing the release of cytochrome c from mitochondria (Bradley et al., 2010). DNSP-11 proteomic pull down studies identified 16 proteins by MALDI-TOF mass spectrometry, 11 of which possess metabolic functions (Bradley et al., 2010). Collectively, these data support our hypothesis that DNSP-11’s neurotrophic effects are mediated through the mitochondria.
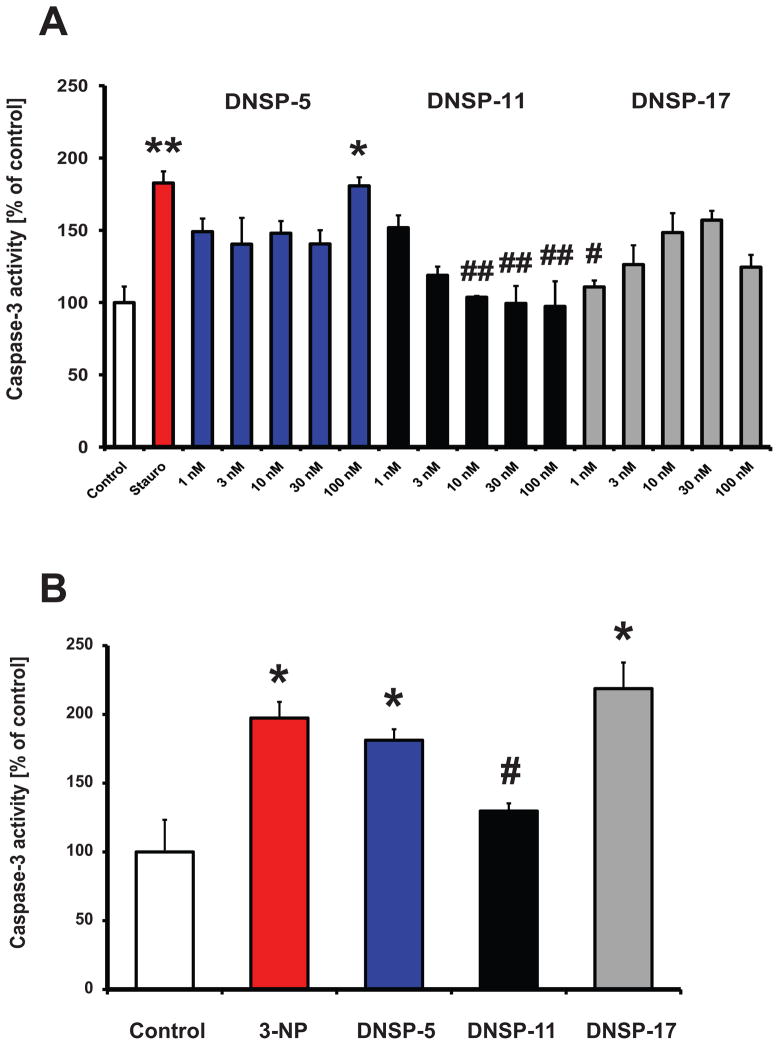
To extend this line of investigation, we examined the protective roles of the DNSPs from the activation of caspase-3, a pro-apoptotic protein, in cultured non-neuronal HEK-293 cells. Following 12 hours of staurosporine (1 μM) exposure, caspase-3 activity was significantly increased by approximately 80%, consistent with initiation of apoptosis (Figure 3A). Between 10 to 100 nM, DNSP-11 provided significant protection (return to control values) from staurosporine-induced activation of caspase-3 (Figure 3A). At dosages lower than 10 nM, the protective effects of DNSP-11 were not significant. Both DNSP-5 and DNSP-17 did not provide any significant protection from staurosporine-induced activation of caspase-3 at the tested dosages in the non-neuronal cell line, with the exception of the lowest (1 nM) DNSP-17 dosage (Figure 3A).
Figure 3.
Protective effects of the DNSPs. (A) Dose responses (1 nM – 100 nM) for DNSP-5 (blue bars), DNSP-11 (black bars), and DNSP-17 (gray bars) protection from 1 μM staurosporine-induced cytotoxicity (red bar), were measured by caspase-3 activity 12 hours after treatment in HEK-293 cells. Stauro-staurosporine. (B) Protection from 8 mM 3-nitropropionate (3-NP) was measured by caspase-3 activity assay 12 hours after treatment with 10 nM treatment with the DNSPs. For both experiments, the control (open bar) was citrate buffer alone. One-way ANOVA was used to test for significance amongst groups, followed by Tukey’s post-hoc analysis (*p<0.05, **p<0.01 vs control; #p<0.05, ##<0.01 vs toxin).
To determine if the protective effects were specific for mitochondria, we examined the protection afforded by the DNSPs against 3-NP activation of caspase-3 activity in HEK-293 cells. 3-NP is an apoptosis-inducing, mitochondrial specific toxin that irreversibly inhibits succinate dehydrogenase of the Kreb’s cycle and complex II of respiration, resulting in mitochondria membrane permeabilization and caspase-3 activation (Beal, 1994; Ludolph et al., 1991; Nasr et al., 2009; Palfi et al., 1996). Exposure of 8 mM 3-NP to HEK-293 cells for 12 hours significantly increased caspase-3 activity approximately 100% over control (Figure 3B). At a same concentration (10 nM) that provided staurosporine protection, DNSP-11 provided significant protection from 3-NP induced activation of caspase-3 activity in HEK-293 cells, whereas equimolar concentrations of DNSP-5 and DNSP-17 provided no significant protection (Figure 3B).
4. Discussion
Neurotrophic factors have received considerable attention as potential therapeutic agents for neurodegenerative disorders, including PD. However, the clinical application of these native molecules has been unrealized due to the lack of successful clinical trials and prolonged patent protection/litigation. Large trophic factors, such as GDNF, have inherent pharmacological disadvantages and challenges: they must be delivered to the CNS by invasive procedures (Thorne and Frey, 2001). Thus, smaller molecules like the DNSPs, which are relatively easy to synthesize and modify to improve bioavailability, have the potential for more widespread use in the clinic.
Because of the stringent requirements of biotherapeutics for in vitro stability and solubility, the DNSPs were evaluated for these properties (Bell, 1997; Powell, 1994). RP-HPLC and LC-MS data showed that each of the DNSPs were stable at −80 °C and 37 °C for one month without any appreciable loss or intrinsic modification of the peptides. Other common storage and experimental temperatures tested had similar results, supporting that the DNSPs are stable for long-term storage and delivery, such as an internal implanted pump stored in the abdominal cavity holding one month’s supply of peptide. Thus, the DNSPs are stable, which is essential if they are to be used in clinical applications.
It was hypothesized that the processed DNSPs might have endogenous/physiological function. It is possible that the DNSPs form an intermolecular complex for bioactivity. We show that the far-UV CD spectra of the individual DNSPs are additive for the tripeptide mixture thereby suggesting that these peptides do not interact in vitro. Furthermore, these spectra show dynamic structural characteristics that would be expected of small, soluble peptides of 5, 11, and 17 amino acids.
To gain insight into the biodistribution properties of the DNSPs, we examined their heparin binding properties by affinity chromatography. Heparin binding has been shown to limit the biodistribution of GDNF, thereby affecting its therapeutic targeting following an intracranial injection (Gash et al., 2005; Lapchak et al., 1998). Unlike mature GDNF, both DNSP-5 and DNSP-11 do not bind heparin (Figure 2), thus suggesting that the GDNF prosequence-derived peptides would have enhanced volume of distribution properties when delivered intracranially. Although the apparent lack of heparin binding might make it difficult to control the diffusion of these peptides into non-targeted/undesired regions of the brain, our previous immunohistochemical staining data with DNSP-11 showed rapid uptake into neurons found both in the substantia nigra, pars reticulata and substantia nigra, pars compacta within 30 minutes following a direct injection (Bradley et al., 2010). Furthermore, increased resting levels of dopamine and its metabolites were observed for up to 28 days in normal and 6-OHDA- lesioned Fischer 344 rats following a single injection of DNSP-11 into the substantia nigra, with no adverse effects observed under these conditions (Bradley et al., 2010). In vivo studies with DNSP-5 are currently ongoing.
Heparin affinity chromatography of DNSP-17, an amidated 17 amino acid sequence putatively derived from amino acids 13–29 within the N-terminal region of mature GDNF, exhibited an equal binding profile as GDNF (Figure 2). These data are consistent with earlier studies, which have demonstrated that heparin binding activity is located within the GDNF N-terminal domain, however, the residues primarily responsible for heparin binding are unresolved (Alfano et al., 2007; Parkash et al., 2008; Piltonen et al., 2009). Complete removal of the highly-basic, 38 amino acid residue N-terminal domain resulted in an elimination of heparin binding and increased GDNF biodistribution in vivo (Piltonen et al., 2009). Recent data from the crystal structure of the GDNF-GFRα1 complex, show basic GDNF residues Arg35, Lys37, and Arg39 to be interacting with sucrose octasulfate, a heparin mimic (Parkash et al., 2008). However, GDNF residues 1–34 were unresolved in the receptor-complex crystal structure (Parkash et al., 2008) to rule out additional binding sites. In an earlier study, ELISA and affinity chromatography data showed that removal of the N-terminal domain residues 24–39 (NΔ2) resulted in a weakening of heparin binding, whereas deletion of residues 4–23 (NΔ1) had little change relative to full-length GDNF (Alfano et al., 2007). Given DNSP-17’s strong heparin-binding properties, this suggests that a significant portion of the N-terminal GDNF heparin binding domain is provided by this sequence, likely the two dibasic BXB clusters at the N- and C-terminal ends of the peptide sequence (corresponding to mature GDNF residues Arg14, Arg16, Arg27, and Lys29). Thus, DNSP-17 could provide a further tool towards understanding of the unresolved role of heparin binding and GDNF signaling (Alfano et al., 2007; Piltonen et al., 2009). Additionally, DNSP-17 may serve as a co-infusate with GDNF to improve its distribution following a direct injection or CED.
DNSP-11 appears to be a functional proGDNF-derived peptide. Initial studies of the rat homolog of DNSP-11, BEP, showed a significant increase in synaptic excitability of rat CA1 pyramidal neurons, as well as broad-binding within the adult rat brain (Immonen et al., 2008). Additional studies demonstrated that DNSP-11 exhibits neurotrophic-like effects in vivo, including long-lived increases in rat resting dopamine levels following a single nigral injection (Bradley et al., 2010). Furthermore, DNSP-11 was shown to be protective at a single dose from 6-OHDA, staurosporine and gramicidin cytotoxins in dopaminergic cell lines (Bradley et al., 2010). Consistent with these prior observations, DNSP-11 provides significant dose-dependent protection from staurosporine and 3-NP in the non-neuronal, HEK-293 cell line (Figure 3A, 3B). While staurosporine is a broad-based cytotoxin, 3-NP targets succinate dehydrogenase of the respiratory complex II of the mitochondrial electron transport chain, supporting that DNSP-11’s protective mechanism of action involves mitochondria.
Unlike DNSP-11, DNSP-5 did not provide significant protection from staurosporine and 3-NP in HEK-293 cells. In addition, DNSP-17 showed limited protection from staurosporine (only at 1 nM), while failing to produce 3-NP protection. This limited functionality is also supported by previous work demonstrating that the DNSP-5 and the DNSP-17 peptide sequences failed to produce significant neuronal excitability (Immonen et al., 2008). Collectively, these early findings support a limited mitochondrial protective role for DNSP-5 and DNSP-17. However, given the findings with DNSP-11, further studies are warranted to establish the possible functional roles of these relatively unexplored sequences.
5. Conclusion
The emergence of naturally occurring, physiologically functional propeptides from the neurotrophic factor family provides a wealth of untapped sequences for exploration and evaluation. As these newly characterized peptides undergo further therapeutic evaluation, it is necessary to conduct studies with molecules characterized under a variety of experimental and storage conditions for reproducibility and translation. Here we show that the DNSPs are inherently stable and soluble under these conditions. Of the three peptides, DNSP-5 and DNSP-11 do not bind heparin, which would facilitate their biodistribution properties when delivered in the brain. Finally, we show that DNSP-11 exhibits protection from the cytotoxins staurosporine and 3-NP, in HEK-293 cells, supporting a potentially broad role as a disease altering therapeutic.
Supplementary Material
Acknowledgments
The authors thank Raymond Bartus for his helpful discussion. The authors acknowledge Stewart Surgener, Jack Schmidt, Martin Chow and Louis Hersh for their discussion and use of HPLC instrumentation. FPLC and CD spectroscopy experiments were performed in the University of Kentucky Center of Structural Biology. Mass spectrometry was performed at the University of Kentucky Mass Spectrometry Facility. Peptide amino acid sequencing analysis was performed at the University of Nebraska-Lincoln Center of Biotechnology. T.L.T. conducted research as part of her University of Kentucky Undergraduate Honors Thesis project. This research was supported by training grants to K.A.K. (T32 DA022738), J.T-C. (T32 AG000242) and funds from NIH COBRE Pilot (P20RR20171 - L.H.B.), PhRMA Foundation (L.H.B.), NINDS (NS039787 - L.H.B., D.M.G., G.A.G.), Columbus Foundation (L.H.B.), and University of Kentucky College of Medicine Startup Funds (L.H.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfano I, Vora P, Mummery RS, Mulloy B, Rider CC. The major determinant of the heparin binding of glial cell-line-derived neurotrophic factor is near the N-terminus and is dispensable for receptor binding. Biochemical Journal. 2007;404:131–140. doi: 10.1042/BJ20061747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF. Neurochemistry and toxin models in Huntington’s disease. Current Opinion in Neurology. 1994;7:542–547. doi: 10.1097/00019052-199412000-00012. [DOI] [PubMed] [Google Scholar]
- Bell LN. Peptide stability in solids and solutions. Biotechnology Progress. 1997;13:342–346. [Google Scholar]
- Bradley LH, Fuqua J, Richardson A, Turchan-Cholewo J, Ai Y, Kelps KA, Glass JD, He X, Zhang Z, Grondin R, Littrell OM, Huettl P, Pomerleau F, Gash DM, Gerhardt GA. Dopamine neuron stimulating actions of a GDNF propeptide. PLoS ONE. 2010;5:e9752. doi: 10.1371/journal.pone.0009752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley LH, Gash DM, Gerhardt GA, Glass JD. Amidated dopamine neuron stimulating peptides for CNS dopaminergic upregulation. 12/508,916. US Patent Application. 2009
- Fiandaca MS, Forsayeth JR, Dickinson PJ, Bankiewicz KS. Image-guided convection-enhanced delivery platform in the treatment of neurological diseases. Neurotherapeutics. 2008;5:123–127. doi: 10.1016/j.nurt.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gash DM, Zhang Z, Ai Y, Grondin R, Coffey R, Gerhardt GA. Trophic factor distribution predicts functional recovery in parkinsonian monkeys. Ann Neurol. 2005;58:224–233. doi: 10.1002/ana.20549. [DOI] [PubMed] [Google Scholar]
- Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D, Lapchak PA, Collins F, Hoffer BJ, Gerhardt GA. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- Hamilton JF, Morrison PF, Chen MY, Harvey-White J, Pernaute RS, Phillips H, Oldfield E, Bankiewicz KS. Heparin coinfusion during convection-enhanced delivery (CED) increases the distribution of the glial-derived neurotrophic factor (GDNF) ligand family in rat striatum and enhances the pharmacological activity of neurturin. Experimental Neurology. 2001;168:155–161. doi: 10.1006/exnr.2000.7571. [DOI] [PubMed] [Google Scholar]
- Immonen T, Alakuijala A, Hytonen M, Sainio K, Poteryaev D, Saarma M, Pasternack M, Sariola H. A proGDNF-related peptide BEP increases synaptic excitation in rat hippocampus. Exp Neurol. 2008;210:793–796. doi: 10.1016/j.expneurol.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VG, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Hilt DC, Jiao S, Collin F, Miyoshi Y, Yi A, Zhang Z, Gash DM. Topographical distribution of [125I]-glial cell line-derived neurotrophic factor in unlesioned and MPTP-lesioned rhesus monkey brain following a bolus intraventricular injection. Brain Res. 1998;789:9–22. doi: 10.1016/s0006-8993(97)01495-9. [DOI] [PubMed] [Google Scholar]
- Lin L-FH, Zhang TJ, Collins F, Armes LG. Purification and initial characterization of rat B49 glial cell line-derived neurotrophic factor. Journal of Neurochemistry. 1994;63:758–768. doi: 10.1046/j.1471-4159.1994.63020758.x. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lindner MD, Winn SR, Baetge EE, Hammang JP, Gentile FT, Doherty E, McDermott PE, Frydel B, Ullman MD, Schallert T, Emerich DF. Implantation of encapsulated catecholamine and GDNF-producing cells in rats with unilateral dopamine depletions and parkinsonian symptoms. Experimental Neurology. 1995;132:62–76. doi: 10.1016/0014-4886(95)90059-4. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Wahlberg LU. Encapsulated cell biodelivery of GDNF: A novel clinical strategy for neuroprotection and neuroregeneration in Parkinson’s disease? Experimental Neurology. 2008;209:82–88. doi: 10.1016/j.expneurol.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Ludolph AC, He F, Spencer PS, Hammerstad J, Sabri M. 3-Nitropropionic acid-exogenous animal neurotoxin and possible human striatal toxin. Can J Neurol Sci. 1991;18:492–498. doi: 10.1017/s0317167100032212. [DOI] [PubMed] [Google Scholar]
- Morrison PF, Lonser RR, Oldfield EH. Convective delivery of glial cell line-derived neurotrophic factor in the human putamen. Journal of Neurosurgery. 2007;107:74–83. doi: 10.3171/JNS-07/07/0074. [DOI] [PubMed] [Google Scholar]
- Nasr P, Carbery T, Geddes J. N-Methyl-d-aspartate receptor antagonists have variable affect in 3-nitropropionic acid toxicity. Neurochemical Research. 2009;34:490–498. doi: 10.1007/s11064-008-9809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfi S, Ferrante RJ, Brouillet E, Beal MF, Dolan R, Guyot MC, Peschanski M, Hantraye P. Chronic 3-Nitropropionic acid treatment in baboons replicates the cognitive and motor deficits of Huntington’s disease. J Neurosci. 1996;16:3019–3025. doi: 10.1523/JNEUROSCI.16-09-03019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkash V, Leppanen VM, Virtanen H, Jurvansuu JM, Bespalov MM, Sidorova YA, Runeberg-Roos P, Saarma M, Goldman A. The structure of the glial cell line-derived neurotrophic factor-coreceptor complex: insights into RET signaling and heparin binding. J Biol Chem. 2008;283:35164–35172. doi: 10.1074/jbc.M802543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NK, Gill SS. GDNF delivery for Parkinson’s disease. In: Sakas DE, Simpson BA, editors. Operative Neuromodulation. Springer; Vienna: 2007. pp. 135–154. [DOI] [PubMed] [Google Scholar]
- Piltonen M, Bespalov MM, Ervasti D, Matilainen T, Sidorova YA, Rauvala H, Saarma M, Männistö PT. Heparin-binding determinants of GDNF reduce its tissue distribution but are beneficial for the protection of nigral dopaminergic neurons. Experimental Neurology. 2009;219:499–506. doi: 10.1016/j.expneurol.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Powell MF. Peptide Stability in Aqueous Parenteral Formulations. In: Cleland JL, Langer R, editors. Formulation and delivery of proteins and peptides. American Chemical Society; 1994. pp. 100–117. [Google Scholar]
- Ramaswamy S, Soderstrom KE, Kordower JH. Trophic factors therapy in Parkinson’s disease. In: Verhaagen J, Hol EM, Huitenga I, Wijnholds J, Bergen AB, Boer GJ, Swaab DF, editors. Progress in Brain Research. Elsevier; 2009. pp. 201–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard SM, Mummery RS, Mulloy B, Rider CC. The binding of human glial cell line-derived neurotrophic factor to heparin and heparan sulfate: importance of 2-O-sulfate groups and effect on its interaction with its receptor, GFRa1. Glycobiology. 2003;13:419–426. doi: 10.1093/glycob/cwg046. [DOI] [PubMed] [Google Scholar]
- Salvatore MF, Ai Y, Fischer B, Zhang AM, Grondin RC, Zhang Z, Gerhardt GA, Gash DM. Point source concentration of GDNF may explain failure of phase II clinical trial. Exp Neurol. 2006;202:497–505. doi: 10.1016/j.expneurol.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 2003;116:3855–3862. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg. 2005;102:216–222. doi: 10.3171/jns.2005.102.2.0216. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Frey WHI. Delivery of neurotrophic factors to the central nervous system: Pharmacokinetic considerations. Clinical Pharmacokinetics. 2001;40:907–946. doi: 10.2165/00003088-200140120-00003. [DOI] [PubMed] [Google Scholar]
- Wang L, Muramatsu S, Lu Y, Ikeguchi K, Fujimoto K, Okada T, Mizukami H, Hanazono Y, Kume A, Urano F, Ichinose H, Nagatsu T, Nakano I, Ozawa K. Delayed delivery of AAV-GDNF prevents nigral neurodegeneration and promotes functional recovery in a rat model of Parkinson’s disease. Gene Ther. 2002;9:381–389. doi: 10.1038/sj.gt.3301682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.