Abstract
A series of novel β,γ-methylene-, monofluoromethylene-, and difluoromethylene-bisphosphonophosphate alkyl monoesters was synthesized. The compounds were conveniently detected during preparative HPLC using post-column derivatization with a phosphate-specific chemosensor.
Keywords: post-column derivatization, fluorescent detection, triphosphate analogues, nucleotides
Introduction
Nucleotides are ubiquitous in biological systems as essential molecules for energy storage and transfer, signaling processes, and DNA/RNA replication and processing. Whereas nucleotide derivatives incorporating a bisphosphonate in the triphosphate moiety are well known,1 corresponding analogues in which the esterifying nucleoside is replaced by a simple alkyl group have not been previously described. Such analogues may be of use as model compounds for the study of nucleotide chemistry kinetics and mechanisms.
Results and Discussion
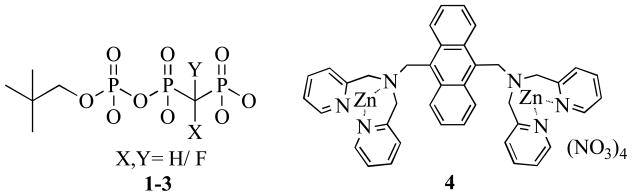
We report here the synthesis of a series of triphosphate monoalkyl esters in which the β,γ-bridging oxygen is replaced by a CXY group. These analogues were prepared by coupling a suitable phosphate alkyl monoester activated with N-methylimidazole to methylene-, monofluoromethylene- or difluoromethylenebis(phosphonic acid)1 using Jakeman’s protocol.2 Relative to the corresponding methyl monoesters, which were also investigated, the neopentyl monoesters (Figure 1) were found to offer the advantages of being easier to purify by RP HPLC while better mimicking the 5′-ester linkage of naturally occurring nucleotides and providing a convenient 1H NMR signals for monitoring hydrolysis reactions.
Figure 1.
Structures of neopentyl bisphosphonophosphate monoesters (1–3) and zinc(II)-dipicolylamine anthracene sensor (4).
Because replacing the nucleoside by a simple alkyl group eliminates the purine or pyrimidine UV/Vis chromophore, it was necessary to evolve an alternative means for detecting 1–3 during preparative HPLC. Accordingly, a post-column derivatization method was developed in which a phosphate-specific dinuclear zinc(II)-dipicolylamine-anthracene dye3 (Figure 1) is introduced into the analytical portion of the eluent, making possible sensitive fluorescence detection of eluted bisphosphonate, bisphosphonophosphate and phosphate species at 434 nm (emission). The purified compounds 1–3 were characterized by 1H and 31P NMR and by high-resolution MS.
Acknowledgments
This work was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences and by NIH program project grant 5-U19-CA105010.
References
- 1.(a) McKenna CE, Kashemirov BA, Upton TG, Batra VK, Goodman MF, Pedersen LC, Beard WA, Wilson SH. J Am Chem Soc. 2007;129:15412–15413. doi: 10.1021/ja072127v. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sucato CA, Upton TG, Kashemirov BA, Batra VK, Martínek V, Xiang Y, Beard WA, Pedersen LC, Wilson SH, McKenna CE, Florián J, Warshel A, Goodman MF. Biochemistry. 2007;46:461–467. doi: 10.1021/bi061517b. [DOI] [PubMed] [Google Scholar]; (c) Sucato CA, Upton TG, Kashemirov BA, Osuna J, Oertell K, Beard WA, Wilson SH, Florian J, Warshel A, McKenna CE, Goodman MF. Biochemistry. 2008;47:870–879. doi: 10.1021/bi7014162. [DOI] [PubMed] [Google Scholar]; (d) Batra VK, Pedersen LC, Beard WA, Wilson SH, Kashemirov BA, Upton TG, Goodman MF, McKenna CE. J Am Chem Soc. 2010;132:7616–7625. doi: 10.1021/ja909370k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohamady S, Jakeman DL. J Org Chem. 2005;70:10588–10591. doi: 10.1021/jo0518598. [DOI] [PubMed] [Google Scholar]
- 3.Ojida A, Mitooka Y, Inoue M, Hamachi I. J Am Chem Soc. 2002;124:6256–6258. doi: 10.1021/ja025761b. [DOI] [PubMed] [Google Scholar]