Abstract
Objective
Fibronectin is an important regulator of cell migration, differentiation, growth, and survival. Our data show that fibronectin also plays an important role in regulating extracellular matrix (ECM) remodeling. Fibronectin circulates in the plasma, and is also deposited into the ECM by a cell dependent process. To determine whether fibronectin affects vascular remodeling in vivo, we asked whether the fibronectin polymerization inhibitor, pUR4, inhibits intima-media thickening, and prevents excess ECM deposition in arteries using a mouse model of vascular remodeling.
Methods and Results
To induce vascular remodeling, partial ligation of the left external and internal carotid arteries was performed in mice. pUR4 and the control peptide were applied periadventitially in pluronic gel immediately after surgery. Animals were sacrificed 7 or 14 days post surgery. Morphometric analysis demonstrated that the pUR4 fibronectin inhibitor reduced carotid intima (63%), media (27%), and adventitial thickening (40%) compared to the control peptide (III-11C). Treatment with pUR4 also resulted in a dramatic decrease in leukocyte infiltration into the vessel wall (80%), decreased ICAM-1 and VCAM-1 levels, inhibited cell proliferation (60-70%), and reduced fibronectin and collagen I accumulation in the vessel wall. In addition, the fibronectin inhibitor prevented SMC phenotypic modulation, as evidence by the maintenance of smooth muscle (SM) α-actin and SM myosin heavy chain levels in medial cells.
Conclusions
These data are the first to demonstrate that fibronectin plays an important role in regulating the vascular remodeling response. Collectively, these data suggest a therapeutic benefit of periadventitial pUR4 in reducing pathologic vascular remodeling.
Keywords: extracellular matrix, fibronectin, collagen, vascular remodeling, smooth muscle cell
Extracellular matrix (ECM) molecules, including fibronectin, have direct effects on the growth and migration of endothelial cells, smooth muscle cell (SMCs), and myofibroblasts1-5. In addition, our data show that the deposition of fibronectin into the extracellular matrix (ECM) controls the deposition, organization, and stability of other matrix molecules, including collagen I, collagen III, and thrombospondin-16, 7. ECM molecules also play a critical role in stabilizing blood vessels. Mice lacking fibronectin die during embryogenesis due to cardiovascular defects8,9. SMC migration and proliferation, as well as excess deposition of ECM molecules, are major factors contributing to vessel narrowing in certain types of vascular remodeling, including intima-media thickening (IMT) of the carotid artery10. Hence, fibronectin and fibronectin deposition could play an important role in regulating vascular remodeling.
Vascular remodeling is a response of the vessel to hemodynamic changes or injury11,12. In this manuscript, vascular remodeling is defined as any change in the geometry of the vessel or vessel wall. Remodeling events can result in compensatory changes in the vessel wall that normalize wall stress12. However, these compensatory changes are frequently inadequate, and further remodeling can result in narrowing of the vessel lumen12,13. Carotid IMT in humans is a predictive indicator of cardiovascular disease, and often occurs prior to the development of atherosclerotic lesions14,15. Changes in cell growth, migration, and matrix synthesis contribute to vascular remodeling following injury or in response to changes in flow16-19. Although much attention has been focused on the contribution of medial SMC during vascular remodeling, changes in the adventitia and in adventitial fibroblasts also occur, and contribute to intimal thickening11,20.
Fibronectin is produced and secreted by numerous cell types including SMCs, fibroblasts, and myofibroblasts, and is widely distributed in ECM in vivo21. Soluble fibronectin is deposited into tissue ECM by a cell-dependent process22. In this study, we used a recombinant peptide derived from the F1 adhesin, pUR4, to inhibit fibronectin polymerization, and assessed its effects on an in vivo flow-induced vascular remodeling model in mice18. We introduced pUR4 periadventially using pluronic gel, and assessed its effects on carotid IMT, ECM deposition, and cell proliferation and differentiation. Our studies show that local delivery of a fibronectin polymerization inhibitor reduces early leukocyte infiltration and cell proliferation, and attenuates the excess deposition of fibronectin and collagen that occurs during remodeling. These studies are the first to demonstrate that fibronectin is an important regulator of vascular remodeling in vivo.
METHODS
Peptides
The pUR4 and control III-11C peptides were produced and purified as described23, 24. Details are provided in the data supplement (see http://atvb.ahajournal.org).
Carotid Ligation Surgery
Blood flow in the left carotid artery of 10 week-old FVB/NJ (FVB) mice was reduced by ligating the external and internal carotid arterial branches as described18. Mice were anesthetized with an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (5 mg/kg) as described18. The left common carotid artery was dissected free of the surrounding connective tissue and 50 μl of F-127 pluronic gel (BASF, Florham Park, NJ) containing 20 μM pUR4 or III-11C was applied around the carotid artery. Animals were separated into five groups containing 5-10 animals each: (1) sham-operated, in which the branches of the left carotid artery were exposed and dissected out, but not ligated (2) ligation (3) ligation with pluronic gel (4) ligation with pluronic gel containing III-11C (5) ligation with pluronic gel containing pUR4.
Tissue collection and processing
Blood flow in the left and right common carotid artery was measured using an ultrasonic transit-time volume flowmeter (Transonic Systems, Ithaca, NY) before sacrifice. Animals were perfusion-fixed with 10% formalin 7 or 14 days after surgery. The common carotid arteries were harvested and embedded in paraffin. Cross-sections of 5μm were cut from the bifurcation every 200 μm through the first mm length of the carotid artery as described18. Sections were stained with Verhoeff-van Gieson elastic stain then subjected to morphometric analysis.
An expanded Methods section is available in the data supplement (see http://atvb.ahajournal.org).
RESULTS
The fibronectin inhibitor, pUR4, blocks fibronectin and collagen deposition in SMC
To determine the effect of fibronectin on vascular remodeling, we used the fibronectin polymerization inhibitor, pUR4. pUR4 has been shown to inhibit fibronectin polymerization in fibroblasts, osteosarcoma cells, and endothelial cells5,23. As shown in Fig. 1D, addition of pUR4 to cultured SMCs inhibited the deposition of endogenous fibronectin into the ECM. Our published data show that fibronectin regulates the deposition of other proteins into the ECM, including type I collagen6. When SMCs were cultured in the presence of pUR4, deposition of endogenous collagen I into matrix fibrils was also inhibited (Fig. 1E). The control peptide had no effect on fibronectin or collagen I deposition (Fig. 1G,H). pUR4 binds to fibronectin25, and inhibits fibronectin deposition by interfering with the binding of fibronectin to matrix assembly sites on the cell surface23. pUR4 does not inhibit cell spreading or adhesion to collagen (unpublished data, 2008) or fibronectin23 and does not bind to other ECM proteins, including collagen I, fibrinogen, and laminin (Supplemental Fig. I, see http://atvb.ahajournal.org). In addition, pUR4 does not inhibit fibronectin or collagen mRNA synthesis in SMCs (Supplemental Table 1, see http://atvb.ahajournal.org).
Figure 1.
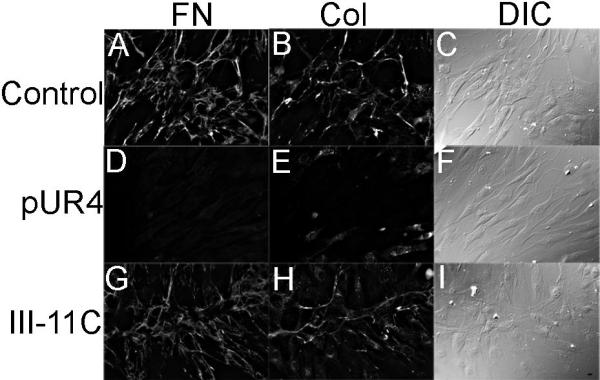
Effect of pUR4 on FN and collagen I deposition in SMCs. SMCs were grown in serum containing media in the absence (A-C) or presence of 500nM pUR4 (D-F) or control III-11C (G-I) peptides for 3 days. Cell were fixed, then incubated with a polyclonal antibody to fibronectin (A,D,G) or collagen I (B,E,H). Corresponding differential interference contrast images are shown in C,F,I. Bar, 20μm.
The pUR4 fibronectin inhibitor blocks vascular remodeling
Fibronectin is known to affect SMC growth, migration and differentiation in vitro2,26-28. Hence, fibronectin could promote vascular remodeling by multiple mechanisms. To determine whether the pUR4 fibronectin inhibitor blocks IMT, we used a flow induced model of vascular remodeling in which the internal and external branches of the common carotid artery are ligated18,29. Blood flow in the common carotid artery was significantly reduced after ligation (0.13 ± 0.01 mL/min) compared to shams (0.59 ± 0.07 mL/min). There were no differences among experimental groups treated with pluronic gel (unpublished data, 2008). Following ligation of the carotid artery, pUR4 and the control peptide were embedded in pluronic gel, and applied periadventitially. Supplemental Fig. II demonstrates that the pUR4 peptide can be readily detected in the vessel wall in both the media and adventitia 1-3 days following periadventitial application. The levels of the peptide were significantly reduced by 7 days. The control peptide could also be detected in the vessel wall (unpublished data, 2008).
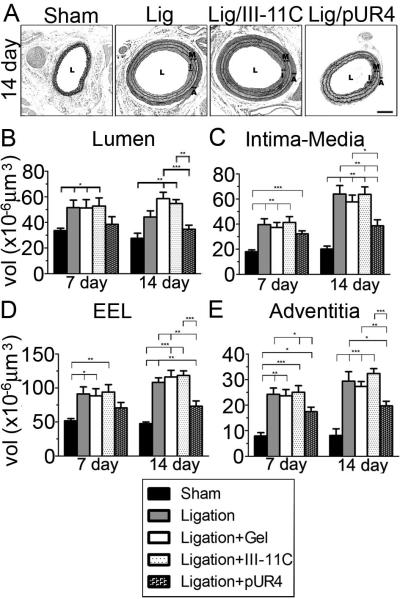
As we previously showed29,30, FVB mice exhibited significant IMT 14 days after ligation (compare sham vs. ligated, Fig. 2A). Application of pluronic gel in the absence or presence of the control peptide had no effect on vascular remodeling. Periadventitial administration of pUR4, but not the control peptide, dramatically reduced carotid remodeling (Fig. 2A). Morphometry of the carotid compartment was performed 7 and 14 days post ligation (Fig. 2B-E). Lumen volume was significantly increased after ligation in control peptide treated mice in comparison to shams (Fig. 2B), consistent with our published data in FVB mice29. This increase was prevented by pUR4 treatment. There was a dramatic effect of pUR4 on vascular wall remodeling (Fig. 2C-E). Ligation of the carotid artery resulted in a 3 fold increase in intima-media volume at 14 days (Fig. 2C). The pUR4 peptide reduced the extent of IMT by 40% in comparison with control peptide treated animals at 14 days (Fig. 2C). When vessel compartments were analyzed separately (supplemental Fig. III, see http://atvb.ahajournal.org), pUR4 was found to reduce intima thickening by 63%, and media thickening by 27%. Interestingly, there was no statistical effect of pUR4 on IMT at 7 days. The reduction of IMT by pUR4 at 14 days was due to prevention of both intimal and medial thickening compared to 7 day changes in control peptide treated animals (supplemental Fig. III). Similarly, pUR4 inhibited adventitial thickening by 38% compared to III-11C at 14 days (Fig. 2E). Finally, pUR4 treatment prevented outward remodeling over the time course, as there was no difference in EEL volume between pUR4 and shams 7 days post ligation (Fig. 2D). However, the remodeling index was not different between pUR4 and III-11C (unpublished data, 2008). These data are the first to demonstrate that fibronectin is an important regulator of vascular remodeling in vivo.
Figure 2.
The pUR4 fibronectin inhbitor decreases vascular remodeling in the carotid artery. (A) Representative photomicrographs of the left carotid artery 14 days after ligation. Lumen (L), intima (I), media (M) and adventitia (A) in ligated vessels are shown. Bar, 100μm. Morphometric analyses showing the volume (vol) of the lumen (B), intima-media (C), external elastic lamina compartment (EEL; D), and adventitia (E) 7 and 14 days after ligation. *indicates p<0.05, **p< 0.01 and ***p< 0.001.
The pUR4 inhibitor decreases ECM accumulation
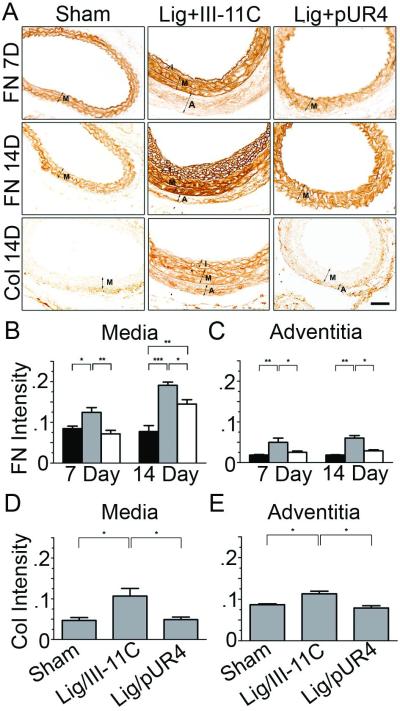
ECM accumulation is a hallmark of vascular remodeling in response to changes in blood flow or injury. Hence, we used immunohistochemistry (IHC) to determine whether pUR4 caused a reduction in fibronectin and collagen I deposition in the left carotid artery. IHC analysis indicates that there was a dramatic reduction in the accumulation of collagen I and fibronectin in the media and adventitia 7 and 14 days post surgery in pUR4 treated animals in comparison with control animals (Fig. 3A). At 7 days post surgery, pUR4 totally prevented increased fibronectin deposition (Fig 3B,C). At 14 days post surgery, fibronectin and collagen levels were still decreased in pUR4 treated animals in comparison to control peptide treated animals. The decrease in collagen deposition parallels the decrease in fibronectin deposition, and is consistent with in vitro data showing that collagen deposition depends upon fibronectin matrix polymerization6,7,31.
Figure 3.
The pUR4 fibronectin inhbitor decreases FN and collagen I deposition in the left carotid artery after ligation. (A) Immunohistochemistry of FN and collagen I 7 or 14 days post surgery. Intima (I), media (M), and adventitia (A) are shown. Quantitation of immunostaining intensity (relative unit per unit area) of FN (B,C) and collagen (D,E) in the media and adventitia 7 (fibronectin) and 14 days (fibronectin and collagen) after ligation. In panels B,C: sham (black box), ligation+III-11C (grey box), ligation+ pUR4 (open box). Bar, 25μm. *indicates p<0.05, **p< 0.01 and ***p< 0.001.
The pUR4 fibronectin inhibitor decreases SMC phenotypic modulation
To begin to define the mechanism(s) by which fibronectin regulates vascular remodeling, carotid artery sections were analyzed by IHC to determine the effect of pUR4 on SMC differentiation, cell proliferation, and leukocyte infiltration. Arterial injury is known to decrease SMC differentiation markers at early times following injury or in response to decreased blood flow16,19. This decline in SMC differentiation is thought to contribute to increased SMC migration and proliferation. Our quantitative data show that reduced flow in the left carotid artery results in decreased SMC α-actin and SM myosin heavy chain staining after treatment with III-11C peptide (Fig. 4). However, the pUR4 fibronectin inhibitor prevented SMC de-differentiation, as evidenced by the maintenance of SM α-actin and SM myosin heavy chain staining in the media (Fig. 4).
Figure 4.
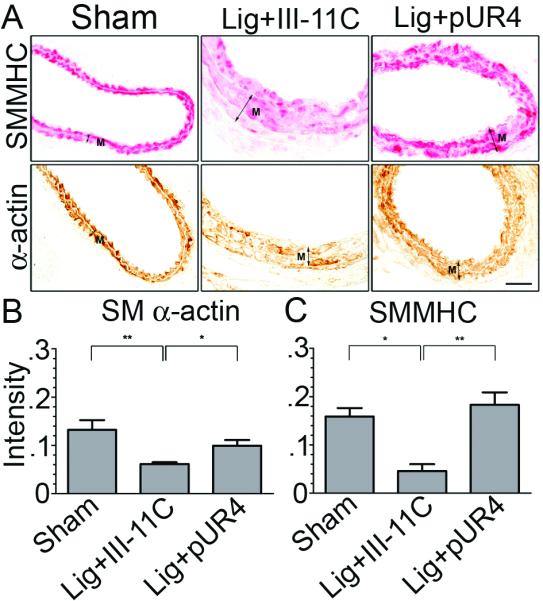
The pUR4 fibronectin inhbitor maintains SMC differentiation. (A) Immunohistochemistry for SMMHC and SM α-actin in the left carotid artery 7 days after ligation. Media=“M”. Quantitative results of immunostaining intensities (relative units per unit area) of SM α-actin (B) and SMMHC (C) in the media. Bar, 25μm. *indicates p<0.05, **p<0.01.
The pUR4 fibronectin inhibitor decreases cell proliferation
Our previous data with FVB mice showed that the greatest increase in cell proliferation and leukocyte infiltration was at 7 days30. Our data show that ligation resulted in a significant increase in cell density in the media 7 days post surgery (6.7 × 10−3 cells/mm2 in ligated animals vs. 5.0 × 10−3 vs. cells/mm2 in sham operated animals). pUR4 treatment prevented this increase in cell density by 75% (5.3 × 10−3 cells/mm2). Further, ligation of the left carotid artery increased cell proliferation in the vessel wall as shown by PCNA staining (supplemental Fig. IV, see http://atvb.ahajournal.org); this increase in cell proliferation was drastically reduced in pUR4 treated animals. There was a significant reduction in cell proliferation in the intima-media (70%) and adventitia (61%) in animals treated with pUR4 (Fig. 5A,B). These data indicate that fibronectin promotes vascular remodeling, in part, by enhancing cell proliferation.
Figure 5.
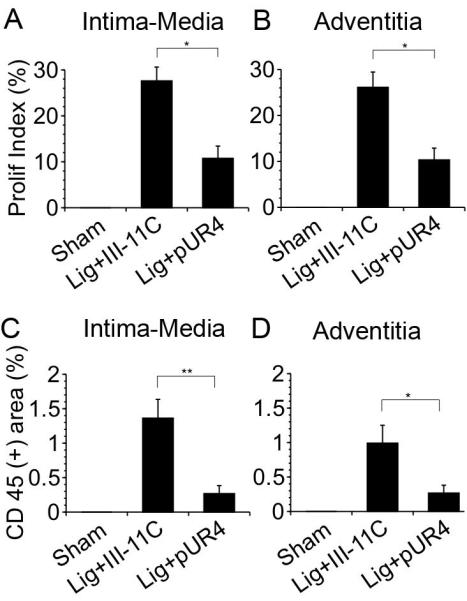
A,B) The pUR4 fibronectin inhibitor decreases cell proliferation in the left carotid artery. Sections of the left carotid artery 7 days post ligation were stained for PCNA and counterstained with hematoxylin. Quantitative IHC analysis of the proliferation index (PCNA (+) cells per total cell number) in the intima-media (A) and adventitia (B) 7 days post ligation. C,D) The pUR4 fibronectin inhibitor decreases leukocyte infiltration into the left carotid artery. Sections of the left carotid artery were stained with antibodies to CD45 7 days after ligation. Sections were counterstained with hematoxylin. Percentage of the area which is CD45 (+) was assessed in the intima-media (C) and adventitia (D) of the vessels as described in the Methods. *indicates p<0.05, **indicates p< 0.01.
The pUR4 fibronectin inhibitor dramatically reduces accumulation of inflammatory cells in the carotid artery
Inflammation plays a prominent role in vascular remodeling following injury. Flow induced vascular remodeling is also accompanied by an increase in inflammatory cells in the vessel wall19,30. Our data show that reduced flow in the left carotid artery resulted in an increase in leukocyte numbers in the intima, media and adventitia 7 days post surgery (Fig. 5C,D, and supplemental Fig. V, see http://atvb.ahajournal.org). Inhibition of fibronectin polymerization significantly decreased (80%) leukocyte infiltration into the intima, media, and adventitia (Fig. 5C,D). Further, pUR4 caused a dramatic decrease in adhesion molecule expression, as shown by the reduction in intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) levels in the vessel wall (Fig. 6, Supplemental Fig. VI, see http://atvb.ahajournal.org). These data are the first to demonstrate that fibronectin plays a critical role in regulating the inflammatory response during vascular remodeling.
Figure 6.
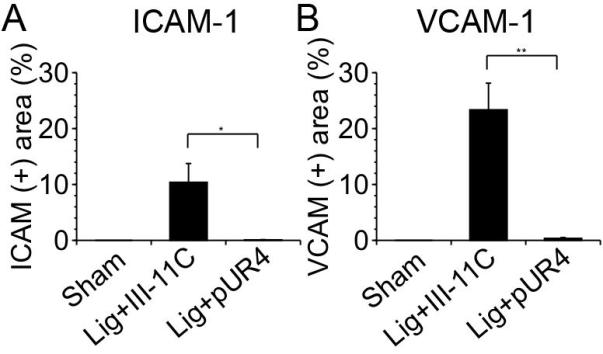
The pUR4 fibronectin inhibitor decreases ICAM-1 and VCAM-1 levels. Sections of the left carotid artery 7 days after ligation were stained with ICAM-1 or VCAM-1. Percentage of the intima-media area that was ICAM-1 (+) (A) or VCAM-1 (+) (B) was evaluated in the intima-media of the vessels. *indicates p<0.05, **indicates p< 0.01.
DISCUSSION
In this manuscript we show that periadventitial delivery the fibronectin inhibitor, pUR4, reduces IMT in response to reduced blood flow (Fig. 2). pUR4 treatment also reduced fibronectin and collagen I accumulation in the vessel wall (Fig. 3). These data are consistent with in vitro data showing that inhibition of fibronectin polymerization decreases the deposition of fibronectin and collagen I in the ECM6,7,31. Treatment with pUR4 also resulted in a dramatic decrease in leukocyte infiltration into the vessel wall (Fig. 5), reduced ICAM-1 and VCAM-1 levels (Fig. 6), inhibited cell proliferation (Fig. 5), and prevented SMC phenotypic modulation (Fig. 4). These data are the first to show that fibronectin is an important regulator of flow induced vascular remodeling.
Fibronectin is a secreted protein that is polymerized into ECM fibrils by a cell-dependent process22. There is a large literature demonstrating the importance of ECM proteins, including fibronectin and collagen, in regulating cell migration, growth, and differentiation1-4,32. Further, the ECM polymerized form of fibronectin has been shown to have distinct effects on cell behavior in comparison to protomeric fibronectin2-4,7,33,34. Similarly, polymerized collagen I has distinct effects on SMC growth in comparison to nonpolymerized collagen1. Our in vitro data indicate that fibronectin polymerization is an important regulator of cell growth, migration, and ECM deposition and stability4,6,7. These data suggest the possibility that fibronectin polymerization may be a key regulator of vascular remodeling in vivo. However, to date, in vivo evidence that fibronectin or fibronectin polymerization regulate SMC function or ECM remodeling has been lacking. In this study, we used the pUR4 peptide to inhibit fibronectin polymerization to determine the role of fibronectin matrix deposition in vascular remodeling. pUR4 binds to fibronectin, and inhibits its ability to be polymerized into ECM fibrils23. pUR4 can inhibit the polymerization of both endogenously produced (Fig. 1) and exogenously supplied fibronectin into the ECM23. pUR4 did not decrease fibronectin or collagen I mRNA production in cultured SMC (Supplemental Data, Table I, see http://atvb.ahajournal.org). However, there was a trend towards decreased fibronectin and collagen I mRNA in the carotid artery of animals treated with pUR4 in comparison with control peptide treated animals (Supplemental Data, Table II, see http://atvb.ahajournal.org). Since pUR4 does not decrease fibronectin or collagen I mRNA in vitro, it is likely that the effect of pUR4 on mRNA levels in vivo is indirect, perhaps resulting from altered cytokine production.
Fibronectin matrix has the potential to influence multiple cell properties. Fibronectin can promote SMC growth and migration in vitro2, 26, 27. Further, fibronectin matrix deposition regulates the deposition and stability of other ECM proteins6,7,31,35. Our data show that pUR4 causes a significant reduction in cell proliferation. Previous studies have shown that fibronectin polymerization can positively affect myofibroblast, SMC and endothelial cell growth2,4,5. Fibronectin polymerization can also promote myofibroblast and epithelial cell migration7,33. Hence, our in vivo data are consistent with much in vitro data that demonstrate a positive effect of fibronectin and fibronectin polymerization on cell growth and migration.
Inflammation has also been shown to play an important role in vascular remodeling in response to changes in flow36. Our data show that fibronectin plays an important role in regulating the recruitment of leukocytes into the vessel wall (Fig. 5). Fibronectin fragments are known to be chemotactic for neutrophils and monocytes21,37,38. However, most in vitro data suggest that intact fibronectin does not promote leukocyte chemotaxis21,37,38. Our data also show that the pUR4 fibronectin inhibitor causes a dramatic reduction in ICAM-1 and VCAM-1 levels in the vessel wall. It is likely that the effect of pUR4 on leukocyte infiltration is due to its ability to decrease VCAM-1 and ICAM-1 levels. Fibronectin is known to regulate the activity of nuclear factor κβ (NF–κβ) in certain cell types39-41, which in turn stimulates ICAM-1 and VCAM-1 production42. Hence, the downregulation of ICAM-1 and VCAM-1 levels in pUR4 treated animals could result from decreased NF–κβ activity.
Fibronectin is also known to promote SMC de-differentiation28,43. However, the effect of matrix fibronectin on SMC differentiation has not been previously characterized. Our in vivo data show that inhibiting fibronectin polymerization results in maintenance of the SMC differentiated phenotype (Fig. 4), indicating that fibronectin polymerization is an important regulator of SMC differentiation. Phenotypic modulation of SMC is thought to play a key role during vascular remodeling, contributing to increased SMC proliferation and migration. Hence, the ability of pUR4 to limit SMC dedifferentiation may be an important mechanism that contributes to reduced intima-media thickening following ligation.
Other ECM molecules have been shown to play important roles in vascular remodeling, including thrombospondin I44, vitronectin45 and osteopontin46. Interesting, certain ECM and cytoskeletal proteins have been shown to influence both IMT and vessel size47-49, similar to our findings with fibronectin. These data suggest that the effects of ECM proteins on outward remodeling may be an important aspect of their ability to regulate vascular remodeling. Our in vitro data show that fibronectin polymerization controls the deposition of thrombospondin 1 and collagen 1 into ECM fibrils6, 7. In addition, we previously showed that the ability of fibronectin to promote myofibroblast migration is due in large part to its ability to regulate the deposition of type I collagen7. Hence, one mechanism by which fibronectin may regulate vascular remodeling is by affecting the deposition of other matrix molecules, such as collagen I and thrombospondin 1. It is likely that ECM effects on vascular remodeling are regulated by integrins. Inhibition of integrin function using Arg-Gly-Asp (RGD) peptides, or antibody blockade of αvβ3 integrin have been shown to inhibit neotimal formation in vivo50,51.
Our data are the first to show that fibronectin is an important regulator of vascular remodeling in vivo. These results are particularly striking given that the pUR4 peptide was delivered peradventitially and that the levels of the peptide peak 1-3 days post application. The ability of pUR4 to inhibit the vascular remodeling response long term (2 weeks), coupled with the reduction in leukocyte infiltration and cell proliferation, suggest that the fibronectin inhibitor acts by blocking an early step(s) in the remodeling response. This early step is likely to involve decreased leukocyte infiltration that occurs, at least in part, as a result of decreased VCAM-1 and ICAM-1 expression. Taken together, these data suggest the possibility that pUR4, or inhibitors that act by similar mechanisms, could have therapeutic applications in treating vascular occlusive diseases.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Dr. Burns Blaxall for helpful suggestions, and for critically reading this manuscript, Mary Georger for advice on histology, Andrew Cardillo for help with qRT-PCR, and Anna Paxhia for technical assistance. This research was supported by grants from the National Institutes of Health (HL070261 and GM069729).
Footnotes
DISCLOSURES None
REFERENCES
- 1.Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of cdk2 inhibitors. Cell. 1996;87:1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- 2.Mercurius KW, Morla AO. Inhibition of vascular smooth muscle cell growth by inhibition of fibronectin matrix assembly. Circulation Research. 1998;82:548–556. doi: 10.1161/01.res.82.5.548. [DOI] [PubMed] [Google Scholar]
- 3.Bourdoulous S, Orend G, MacKenna DA, Pasqualini R, Ruoslahti E. Fibronectin matrix regulates activation of RHO and CDC42 GTPases and cell cycle progression. J.Cell Biol. 1998;143:267–276. doi: 10.1083/jcb.143.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sottile J, Hocking DC, Swiatek P. Fibronectin matrix assembly enhances adhesion-dependent cell growth. J.Cell Sci. 1998;111:2933–2943. doi: 10.1242/jcs.111.19.2933. [DOI] [PubMed] [Google Scholar]
- 5.Zhou X, Rowe RG, Hiraoka N, George JP, Wirtz D, Mosher DF, Virtanen I, Chernousov MA, Weiss SJ. Fibronectin fibrillogenesis regulates three-dimensional neovessel formation. Genes Dev. 2008;22:1231–1243. doi: 10.1101/gad.1643308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol. Biol. Cell. 2002;13:3546–3559. doi: 10.1091/mbc.E02-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sottile J, Shi F, Rublyevska I, Chiang HY, Lust J, Chandler J. Fibronectin-dependent collagen I deposition modulates the cell response to fibronectin. Am J Physiol Cell Physiol. 2007;293:C1934–1946. doi: 10.1152/ajpcell.00130.2007. [DOI] [PubMed] [Google Scholar]
- 8.George EL, Georges-Labousse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Develop. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 9.George EL, Baldwin HS, Hynes RO. Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood. 1997;90:3073–3081. [PubMed] [Google Scholar]
- 10.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 11.Strauss BH, Rabinovitch M. Adventitial fibroblasts: defining a role in vessel wall remodeling. Am J Respir Cell Mol Biol. 2000;22:1–3. doi: 10.1165/ajrcmb.22.1.f172. [DOI] [PubMed] [Google Scholar]
- 12.Berk BC. Vascular smooth muscle growth: autocrine growth mechanisms. Physiol Rev. 2001;81:999–1030. doi: 10.1152/physrev.2001.81.3.999. [DOI] [PubMed] [Google Scholar]
- 13.Pasterkamp G, de Kleijn DP, Borst C. Arterial remodeling in atherosclerosis, restenosis and after alteration of blood flow: potential mechanisms and clinical implications. Cardiovasc Res. 2000;45:843–852. doi: 10.1016/s0008-6363(99)00377-6. [DOI] [PubMed] [Google Scholar]
- 14.Cheng KS, Mikhailidis DP, Hamilton G, Seifalian AM. A review of the carotid and femoral intima-media thickness as an indicator of the presence of peripheral vascular disease and cardiovascular risk factors. Cardiovasc Res. 2002;54:528–538. doi: 10.1016/s0008-6363(01)00551-x. [DOI] [PubMed] [Google Scholar]
- 15.Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: The Muscatine Study. Circulation. 2001;104:2815–2819. doi: 10.1161/hc4601.099486. [DOI] [PubMed] [Google Scholar]
- 16.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 17.Majesky MW. Neointima formation after acute vascular injury. Role of counteradhesive extracellular matrix proteins. Tex Heart Inst J. 1994;21:78–85. [PMC free article] [PubMed] [Google Scholar]
- 18.Korshunov VA, Berk BC. Flow-induced vascular remodeling in the mouse: a model for carotid intima-media thickening. Arterioscler Thromb Vasc Biol. 2003;23:2185–2191. doi: 10.1161/01.ATV.0000103120.06092.14. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- 20.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89:1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 21.Hynes RO. Fibronectins. Springer-Verlag; New York: 1990. [Google Scholar]
- 22.McKeown-Longo PJ, Mosher DF. Interaction of the 70,000-mol-wt amino-terminal fragment of fibronectin with the matrix-assembly receptor of fibroblasts. J.Cell Biol. 1985;100:364–374. doi: 10.1083/jcb.100.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomasini-Johansson BR, Kaugman NR, Ensenberger MG, Ozeri V, Hanski E, Mosher DF. Peptide from adhesin F1 inhibits fibronectin matrix assembly. J. Biol. Chem. 2001;276:23430–23439. doi: 10.1074/jbc.M103467200. [DOI] [PubMed] [Google Scholar]
- 24.Morla A, Zhang Z, Ruoslahti E. Superfibronectin is a functionally distinct form of fibronectin. Nature. 1994;367:193–196. doi: 10.1038/367193a0. [DOI] [PubMed] [Google Scholar]
- 25.Ensenberger MG, Tomasini-Johansson BR, Sottile J, Ozeri V, Hanski E, Mosher DF. Specific interactions between F1 adhesin of Streptococcus pyogenes and N-terminal modules of fibronectin. J Biol Chem. 2001;276:35606–35613. doi: 10.1074/jbc.M105417200. [DOI] [PubMed] [Google Scholar]
- 26.DiMilla PA, Stone JA, Quinn JA, Albelda SM, Lauffenburger DA. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J Cell Biol. 1993;122:729–737. doi: 10.1083/jcb.122.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stringa E, White D, Tuan RS, Knauper V, Gavrilovic J. Role of newly synthesized fibronectin in vascular smooth muscle cell migration on matrix-metalloproteinase-degraded collagen. Biochem Soc Trans. 2002;30:102–111. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 28.Hedin U, Bottger BA, Forsberg E, Johansson S, Thyberg J. Diverse effects of fibronectin and laminin on phenotypic properties of cultured arterial smooth muscle cells. J Cell Biol. 1988;107:307–319. doi: 10.1083/jcb.107.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korshunov VA, Berk BC. Strain-dependent vascular remodeling: the “Glagov phenomenon” is genetically determined. Circulation. 2004;110:220–226. doi: 10.1161/01.CIR.0000134958.88379.2E. [DOI] [PubMed] [Google Scholar]
- 30.Korshunov VA, Solomatina MA, Plekhanova OS, Parfyonova YV, Tkachuk VA, Berk BC. Plasminogen activator expression correlates with genetic differences in vascular remodeling. J Vasc Res. 2004;41:481–490. doi: 10.1159/000081804. [DOI] [PubMed] [Google Scholar]
- 31.Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11beta 1 and alpha 2beta 1. J Biol Chem. 2002;277:37377–37381. doi: 10.1074/jbc.M206286200. [DOI] [PubMed] [Google Scholar]
- 32.Rocnik EF, Chan BM, Pickering JG. Evidence for a role of collagen synthesis in arterial smooth muscle cell migration. J Clin Invest. 1998;101:1889–1898. doi: 10.1172/JCI1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hocking DC, Chang CH. Fibronectin matrix polymerization regulates small airway epithelial cell migration. Am. J. Physiol. 2003;285:L169–179. doi: 10.1152/ajplung.00371.2002. [DOI] [PubMed] [Google Scholar]
- 34.Sechler JL, Schwarzbauer JE. Control of cell cycle progression by fibronectin matrix architecture. J.Biol.Chem. 1998;273:25533–25536. doi: 10.1074/jbc.273.40.25533. [DOI] [PubMed] [Google Scholar]
- 35.Kinsey R, Williamson MR, Chaudhry S, Mellody KT, McGovern A, Takahashi S, Shuttleworth CA, Kielty CM. Fibrillin-1 microfibril deposition is dependent on fibronectin assembly. J Cell Sci. 2008;121:2696–2704. doi: 10.1242/jcs.029819. [DOI] [PubMed] [Google Scholar]
- 36.Furukawa Y, Matsumori A, Ohashi N, Shioi T, Ono K, Harada A, Matsushima K, Sasayama S. Anti-monocyte chemoattractant protein-1/monocyte chemotactic and activating factor antibody inhibits neointimal hyperplasia in injured rat carotid arteries. Circ Res. 1999;84:306–314. doi: 10.1161/01.res.84.3.306. [DOI] [PubMed] [Google Scholar]
- 37.Clark RA, Wikner NE, Doherty DE, Norris DA. Cryptic chemotactic activity of fibronectin for human monocytes resides in the 120-kDa fibroblastic cell-binding fragment. J Biol Chem. 1988;263:12115–12123. [PubMed] [Google Scholar]
- 38.Norris DA, Clark RA, Swigart LM, Huff JC, Weston WL, Howell SE. Fibronectin fragment(s) are chemotactic for human peripheral blood monocytes. J Immunol. 1982;129:1612–1618. [PubMed] [Google Scholar]
- 39.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, Schwartz MA. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: a potential role in atherosclerosis. J Cell Biol. 2005;169:191–202. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein S, de Fougerolles AR, Blaikie P, Khan L, Pepe A, Green CD, Koteliansky V, Giancotti FG. Alpha 5 beta 1 integrin activates an NF-kappa B-dependent program of gene expression important for angiogenesis and inflammation. Mol Cell Biol. 2002;22:5912–5922. doi: 10.1128/MCB.22.16.5912-5922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren L, Blanchette JB, White LR, Clark SA, Heffner DJ, Tibbles LA, Muruve DA. Soluble fibronectin induces chemokine gene expression in renal tubular epithelial cells. Kidney Int. 2005;68:2111–2120. doi: 10.1111/j.1523-1755.2005.00667.x. [DOI] [PubMed] [Google Scholar]
- 42.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 43.Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999;250:273–283. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]
- 44.Moura R, Tjwa M, Vandervoort P, Cludts K, Hoylaerts MF. Thrombospondin-1 activates medial smooth muscle cells and triggers neointima formation upon mouse carotid artery ligation. Arterioscler Thromb Vasc Biol. 2007;27:2163–2169. doi: 10.1161/ATVBAHA.107.151282. [DOI] [PubMed] [Google Scholar]
- 45.Dufourcq P, Couffinhal T, Alzieu P, Daret D, Moreau C, Duplaa C, Bonnet J. Vitronectin is up-regulated after vascular injury and vitronectin blockade prevents neointima formation. Cardiovasc Res. 2002;53:952–962. doi: 10.1016/s0008-6363(01)00547-8. [DOI] [PubMed] [Google Scholar]
- 46.Liaw L, Lombardi DM, Almeida MM, Schwartz SM, deBlois D, Giachelli CM. Neutralizing antibodies directed against osteopontin inhibit rat carotid neointimal thickening after endothelial denudation. Arterioscler Thromb Vasc Biol. 1997;17:188–193. doi: 10.1161/01.atv.17.1.188. [DOI] [PubMed] [Google Scholar]
- 47.Li YH, Liu SL, Shi GY, Tseng GH, Liu PY, Wu HL. Thrombomodulin plays an important role in arterial remodeling and neointima formation in mouse carotid ligation model. Thromb Haemost. 2006;95:128–133. [PubMed] [Google Scholar]
- 48.Myers DL, Harmon KJ, Lindner V, Liaw L. Alterations of arterial physiology in osteopontin-null mice. Arterioscler Thromb Vasc Biol. 2003;23:1021–1028. doi: 10.1161/01.ATV.0000073312.34450.16. [DOI] [PubMed] [Google Scholar]
- 49.Schiffers PM, Henrion D, Boulanger CM, Colucci-Guyon E, Langa-Vuves F, van Essen H, Fazzi GE, Levy BI, De Mey JG. Altered flow-induced arterial remodeling in vimentin-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:611–616. doi: 10.1161/01.atv.20.3.611. [DOI] [PubMed] [Google Scholar]
- 50.Matsuno H, Stassen JM, Vermylen J, Deckmyn H. Inhibition of integrin function by a cyclic RGD-containing peptide prevents neointima formation. Circulation. 1994;90:2203–2206. doi: 10.1161/01.cir.90.5.2203. [DOI] [PubMed] [Google Scholar]
- 51.Srivatsa SS, Fitzpatrick LA, Tsao PW, Reilly TM, Holmes DR, Jr., Schwartz RS, Mousa SA. Selective alpha v beta 3 integrin blockade potently limits neointimal hyperplasia and lumen stenosis following deep coronary arterial stent injury: evidence for the functional importance of integrin alphav beta3 and osteopontin expression during neointima formation. Cardiovasc Res. 1997;36:408–428. doi: 10.1016/s0008-6363(97)00184-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.