Abstract
The ventral hippocampus modulates anxiety-like behavior in rats, and serotonergic transmission within the hippocampus facilitates adaptation to stress. Chronic amphetamine treatment results in anxiety-like behavior in rats and reduced monoamine concentrations in the ventral hippocampus. Since reduced hippocampal serotonergic transmission in response to stress is observed in rats that display high anxiety-like behavior, anxiety states in amphetamine-treated rats may be associated with reduced stress-related serotonergic transmission in the hippocampus. Therefore, using in vivo microdialysis in anesthetized rats, we investigated the effect of corticosterone infused locally into the ventral hippocampus on serotonergic transmission, and the effect of chronic amphetamine pretreatment on corticosteroid receptor protein expression and the corticosterone-induced serotonergic response. Extracellular serotonin in the ventral hippocampus was increased by corticosterone in drug naïve rats, and this corticosterone-induced serotonin augmentation was blocked by the glucocorticoid receptor antagonist mifepristone. Furthermore, chronic pretreatment with amphetamine abolished the serotonin response to physiologically relevant corticosterone levels and reduced glucocorticoid receptor protein expression. Together, our results suggest that chronic amphetamine exposure reduces serotonergic neurotransmission, in part via alterations to glucocorticoid receptor-facilitation of serotonin release in the rat ventral hippocampus. Reduced serotonergic activity in the ventral hippocampus may contribute to altered stress responses and adaptive coping following repeated drug exposure.
Keywords: addiction, psychostimulant, anxiety, hippocampus, serotonin, glucocorticoid
1. Introduction
The hippocampus is important for the processing of sensory information and the generation of adaptive behavior in response to environmental stimuli (Bast, 2007; Bast et al., 2009), including stressors (Herman and Mueller, 2006). A high density of corticosteroid receptors are concentrated in the hippocampus (de Kloet et al., 1975; Reul and de Kloet, 1985; Chao et al., 1989), and it receives a robust serotonergic innervation from the raphe nuclei in the brain stem (Azmitia and Segal, 1978, Sharp et al., 1990; Mokler et al., 1998). Therefore, the hippocampus is an important site in the interaction between the serotonergic and neuroendocrine responses to stress.
Stress stimulates serotonin (5-HT) release in the ventral hippocampus of rats (Wright et al., 1992; Rex et al., 2005) and activation of 5-HT1A receptors in the hippocampus inhibits LTP and impairs consolidation of stress-related memories (Sakai and Tanaka, 1993; Carli et al., 1992; Stiedl et al., 2000). Therefore, hippocampal 5-HT acting through 5-HT1A receptors is thought to reduce the anxiogenic effects of stressful stimuli (Joca et al., 2007). This hypothesis is supported by the fact that 5-HT and 5-HT1A receptor agonists administered immediately following stress experience facilitate stress adaptation (Guimaraes et al., 1993; Joca et al., 2003; McBlane and Handley, 1994). Also, rat lines selectively bred for high anxiety-like behavior have lower stress-induced 5-HT release in the hippocampus compared to those bred for low anxiety-like behavior (Umriukhin et al., 2002; Keck et al., 2005), and chronic paroxetine treatment restores serotonin transmission and stress-coping behavior in these animals (Keck et al., 2005; Keck et al., 2003). These findings combined with others showing that tolerance to chronic stress is related to serotonergic activity in the hippocampus (Kennett et al., 1985, 1987; Storey et al., 2006), all suggest that dysregulation of serotonergic function may contribute to the pathogenesis of affective disorders.
Stress also increases corticosterone levels in the hippocampus to about 200–300% of baseline (Keeney et al., 2006; Thoeringer et al., 2007; Droste et al., 2008; Chauveau et al., 2010). The effects of corticosterone are mediated by mineralocorticoid receptors (MR) and glucocorticoid receptors (GR), which are co-expressed by hippocampal neurons (de Kloet and McEwen, 1976; Velduis et al., 1982; Han et al, 2005). Corticosterone is known to regulate tryptophan hydroxylase activity (Azmitia and McEwen, 1974; Sze, 1976; Singh et al., 1994), monoamine oxidase expression (Morsink et al., 2007), and serotonin turnover in the hippocampus (Van loon et al., 1981; De Kloet et al., 1982; Korte-bouws et al., 1996) via the GR. Direct corticosterone stimulation of 5-HT release has been demonstrated in the hippocampus of the lizard Anolis carolinensis (Summers et al., 2003). However, it is unknown if corticosterone directly stimulates serotonin release in the mammalian hippocampus, and through what receptor mechanism. Given that measures of serotonergic activity that are indirectly related to transmitter release are influenced by GRs, we hypothesized that GR receptors in the hippocampus would also mediate 5-HT release in this region.
Chronic stress decreases hippocampal corticosteroid receptor expression in the hippocampus (Sapolsky et al., 1984) and influences susceptibility for psychostimulant sensitization and self-administration (Prasad et al., 1998; Covington and Miczek, 2001). Also, reduced corticosteroid receptor function in the hippocampus is associated with an increased propensity for amphetamine self-administration (Maccari et al., 1991). Furthermore, chronic amphetamine treatment of rats results in heightened anxiety states (Barr et al., 2010; Vuong et al., 2010) which may be a result of altered GR-5-HT interactions in the hippocampus (Zhou et al., 2008). However, it is unknown if chronic amphetamine exposure reduces glucocorticoid receptor protein expression and thus alters corticosterone-mediated serotonergic activity within the hippocampus. Therefore, we investigated the effect of chronic amphetamine administration on corticosteroid receptor protein expression and the relationship between corticosteroid receptors and serotonin release in the hippocampus in drug naïve and chronically amphetamine-treated animals. It was hypothesized that amphetamine treatment would reduce GR expression in the ventral hippocampus leading to reduced 5-HT transmission, which could have implications for the neurobiology underlying stress sensitivity and impaired stress coping following chronic exposure to amphetamine.
2. Experimental Procedures
2.1 Animals
Male Sprague-Dawley rats (3 weeks old) were purchased from the University of South Dakota Animal Resource Center. Rats were housed in pairs, maintained at 22°C, on a reverse 12 h light 12 h dark cycle with free access to food and water. Rats were used in the following studies once they reached early adulthood (8 weeks of age). The procedures were approved by the Institutional Animal Care and Use Committee of the University of South Dakota, and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 Experiment 1: Effects of Corticosterone on 5-HT Release in the Ventral Hippocampus
The purpose of this experiment was twofold; first to confirm the release of 5-HT in response to corticosterone in the ventral hippocampus of the rat since this hippocampal region is most often associated with affective states (McHugh et al., 2004; Degroot and Treit, 2004; Kjelstrup et al., 2002; Bannerman et al., 2003) and second, to determine whether GRs mediate corticosterone-stimulated 5-HT release. Extracellular 5-HT in response to local infusion of corticosterone was measured from the ventral hippocampus using in vivo microdialysis as described in detail by Forster et al. (2008).
Surgery
Drug naïve rats were anesthetized with urethane (1.8g kg, i.p., Sigma) and placed within a small mammal stereotaxic frame (Kopf, Tujunga, CA, USA). Animals remained under anesthesia throughout the course of the experiment, with body temperature held at 37°C by a temperature-controlled heating pad (Harvard Apparatus, Holliston, MA, USA). Previous studies from our laboratory have shown comparable levels of basal circulating corticosterone (~100 ng/ml) in urethane-anesthetized rats (Forster et al., 2008) and non-stressed awake rats (Lukkes et al., 2009). A laboratory-made microdialysis probe (2.5 mm exposed membrane length, average recovery for 5-HT was 19.6%) was inserted into the ventral hippocampus (AP, −5.2 mm from bregma; ML, 4.5 mm from midline;−8.7 mm from dura; Paxinos & Watson, 1997). Artificial cerebrospinal fluid (aCSF) was continuously perfused through the probe at a rate of 0.4 μl min.
Microdialysis
Dialysate (8 μl) collection began 4 hrs following probe insertion at 20 min intervals (Forster et al., 2008) and 5-HT levels were measured using high-performance liquid chromatography with electrochemical detection (see below for details). Following collection of at least three comparable baseline samples, perfusion with aCSF was changed to perfusion with either the GR antagonist mifepristone (10 mg/ml, 1.25 ng or 2.9 nmol total delivered, Bitran et al., 1998) or vehicle (5% ethanol, 5% camphor) followed by corticosterone-HBC (200 ng/ml, Summers et al., 2003; n=10 for vehicle-corticosterone, n=8 for mifepristone-corticosterone) or vehicle (11.2% HBC; n=9 for vehicle-vehicle, n=8 for mifepristone-vehicle) for 20 min, after which perfusion with aCSF alone was re-established. Delivery of corticosterone through the probe was assessed by measuring the percent of a known corticosterone concentration delivered into an in vitro solution of aCSF via the probe. Delivery of hormone via the dialysis probe was approximately 15% effective, producing an actual concentration of 30 ng/ml (0.24 ng or 0.69 nmol total delivered) corticosterone, which has been previously shown to increase 5-HT release in the lizard brain (Summers et al., 2003), and is similar to levels of corticosterone measured from the hippocampus during stress (Droste et al., 2008). Following drug delivery, dialysates were collected until 5-HT returned to baseline levels. In the case of rats where no changes in 5-HT were observed, eight post-drug samples were collected.
High-performance liquid chromatography measurement of 5-HT
The detection of 5-HT in dialysates was accomplished using high performance liquid chromatography with electrochemical detection (Bradberry et al., 1991; Forster et al., 2008). The mobile phase (containing per liter: 300 mg EDTA, 432 mg sodium octanesulfonate, 4.8 g NaH2PO4, 300 μl triethylamine and 122 ml acetonitrile, pH 5.35; all obtained from Sigma, St Louis, MO, USA) was pumped through a UniJet 3 μm C18 microbore column (Bioanalytical Systems; West Lafayette, IN, USA) under nitrogen gas pressure (2000 psi). Dialysates were injected onto the chromatographic system using a rheodyne injector via a 5 μl loop (Bioanalytical Systems). The perfusate rate of 0.4μL min resulted in the collection of approximately 8 μL of dialysate 20 min to ensure that the loop was overfilled with each sample. Following separation by the column, 5-HT was detected by a glassy carbon electrode (Bioanalytical Systems), which was maintained at +0.5 V with respect to an Ag AgCl2 reference electrode using an LC-4C potentiostat (Bioanalytical Systems). The voltage output was recorded by Clarity v2.4 Chromatography Station for Windows (DataApex, Prague, Czech Republic). 5-HT peaks were identified by comparison to a 5-HT standard (7.9 pg 5 μL 5-HT). The 2:1 signal to noise detection limit for 5-HT using this system was 0.06 +/− 0.01 pg.
Histology
Upon completion of experiments, rats were killed by overdose with sodium pentobarbital (0.5 mL Fatal Plus, i.p.; Vortech, Dearborn, MI, USA) and the brains were removed and fixed in 10% buffered formalin (Fisher Scientific). Brains were sectioned frozen at 60 μm on a sliding microtome and then examined under a light microscope to determine placements of probes. Only data from rats with correct probe placements in the ventral hippocampus were included in the analyses.
2.3 Experiment 2: Effects of Amphetamine Treatment on Corticosteroid Receptor Levels in the Hippocampus
The following experiment aimed to determine the effects of chronic amphetamine treatment on GRs in the ventral hippocampus since Experiment 1 demonstrated that GRs mediate corticosterone-induced 5-HT release in this region. The levels of MRs in the ventral hippocampus were also determined to establish the specificity of the effects of amphetamine on receptors bound by corticosterone. Also, MR and GR levels were determined in the dorsal hippocampus to establish the specificity of the effects of amphetamine along the dorsoventral axis of the hippocampus.
Amphetamine Treatment
Male adult rats (n = 10 per treatment) were treated with amphetamine (2.5 mg/kg, ip. daily) or saline for two weeks. This treatment schedule induces long-lasting anxiety states that emerge the day following last treatment (Vuong et al., 2010; Barr et al., 2010). Rats were decapitated the day following last treatment and the brains were rapidly removed. Brains were frozen and stored at −80°C, until sectioning.
Western immunoblotting
Brains were sectioned frozen (300 μm) within a cryostat (Lecia Jung CM 1800; North Central Instruments, Plymouth, MN) at −10 °C. The dorsal and ventral hippocampus (as defined by Barr et al., 2010) were dissected from frozen sections on a freezing stage (Physiotemp; North Central Instruments) using a 23 gauge cannula, and homogenized in 40 μl of HEPES buffer. Protein concentrations were determined within 5 μl sample duplicates using a Bradford Kit (BioRad Laboratories, Hercules, CA) and a microplate reader (Bio-Tek Instruments, Winooski, TV, USA). Samples (50 μg/lane) were loaded on a 7.5% SDS polyacrylamide gel (PAGE) and electroblotted onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad Laboratories) from the gel by a semi-dry blotting apparatus (Bio-Rad Laboratories). The blotted membrane was then blocked with 5% skim milk at 4°C overnight and then incubated with rabbit polyclonal antibody against MR (Santa Cruz, Santa Cruz, CA; 1:500) or rabbit polyclonal antibody against GR (Santa Cruz,; 1:200) in Tris-buffered saline containing 0.1% Tween-20 (TBST) at 4°C for 24 hr. Membranes were washed three times with TBST, and then incubated with IRDye 800-conjugated goat anti-rabbit IgG second antibody (Rockland Inc., Gilbertsville, PA 1:2,000) for 2 hr at room temperature. After the incubation, membranes were washed three times with TBST before visualization. Control for protein loading was achieved by using primary antibodies to actin (1:2000; Chemicon International) and IRDye 800-conjugated affinity purified anti-mouse IgG as secondary antibodies (1:5000; H&L; Rockland Inc.). Proteins were detected using the Odyssey infrared imaging system (excitation/emission filters at 780 nm/820 nm range, LI-COR Biosciences, Lincoln, NE). Optical densities from each individual sample were corrected against actin levels.
2.4 Experiment 3: Effects of Chronic Amphetamine Pre-treatment on Corticosterone-Elicited 5-HT Release in the Ventral Hippocampus
The following experiment was performed to assess the effects of chronic amphetamine pre-treatment on corticosterone-elicited 5-HT release in the ventral hippocampus. Given that it was previously determined that GRs mediate corticosterone-induced 5-HT release in this region (Experiment 1) and GRs in the ventral hippocampus of amphetamine pre-treated rats were significantly reduced (Experiment 2), it was hypothesized that corticosterone-induced 5-HT release would be reduced in amphetamine pretreated rats.
Amphetamine Treatment and Microdialysis Experiments
A separate cohort of rats was treated with amphetamine (2.5 mg/kg, ip. daily) or saline daily for two weeks as described for Experiment 2. The morning following the last injection, a microdialysis probe was inserted into the ventral hippocampus and dialystes collected as for Experiment 1. To ensure that endogenous levels of corticosterone were similar between amphetamine and saline pre-treated rats prior to corticosterone infusion, a dialysate sample was collected for 40 min prior to baseline 5-HT samples for analysis of basal hippocampal corticosterone concentrations (see below for details). After collection of at least three comparable 5-HT baseline samples, perfusion with aCSF was changed to perfusion with either corticosterone-HBC (200 ng/ml or 2000ng/ml corticosterone; 0.24ng or 2.4ng total delivered, equal to 0.69 and 6.92 nmol) or vehicle (11.2% HBC) for 20 min, after which perfusion with aCSF alone was re-established (n=6 for saline + vehicle, n=8 for saline + 200ng/ml corticosterone, n=7 for saline+ 2000ng/ml corticosterone, n=7 for amphetamine + vehicle, n=8 for amphetamine + 200ng/ml corticosterone, n=5 for amphetamine + 2000ng/ml corticosterone). Following drug delivery, dialysates were collected until 5-HT returned to baseline levels, with rats euthanized and brains sectioned for probe placement as described for Experiment 1.
Measurement of corticosterone levels in dialysates
Measurement of corticosterone from dialysates was performed using a corticosterone enzyme-linked immunoassay kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions with slight modifications (Forster et al., 2008). Briefly, 40 μl of dialysate and 0.5 μl of steroid displacement reagent were diluted with 960 μl of assay buffer for a 25-fold dilution. Duplicates of samples, controls and corticosterone standards (0–2000 ng ml) were assayed. Corticosterone levels were detected by absorbance of samples at 405 nm (wavelength correction set at 595 nm), using an automated plate reader and Gen5 software (Bio-Tek Instruments, Winooski, VT, USA). Absorbance values from samples were applied to the standard curve generated (r2 = 0.99) and corticosterone levels were expressed as ng ml. Absorbance values were also used to calculate the percentage of maximum binding (16.9%) and percentage of non-specific binding (2.9%). Both of these values were within the manufacturer’s range. Levels of corticosterone in the dialysates were low, but well above the detection limit of the assay (22 pg/ml).
2.5 Data analysis
For microdialysis experiments, the height of the 5-HT peaks in three baseline dialysis samples were averaged and post-drug 5-HT peak heights were calculated as a percentage change from mean baseline levels for each animal (Forster et al., 2008). For each experiment, 5-HT levels were analyzed with respect to time (within-subject factor) and treatment group (between-subject factor) using two-way ANOVA with one repeated measure. Significant effects of treatment at a given time-point were further analyzed by Student–Newman Keul’s (SNK) multiple comparison procedure. When a significant effect of time was noted, a one-way ANOVA with one repeated measure was performed across time for each given treatment. Resulting significant time-points were identified by Holm-Sidak post-hoc test for multiple comparisons with a single control, where the sample collected immediately before the first drug infusion served as the control sample. Separate one-way ANOVAs were used to measure the effect of drug treatment on hippocampal GR and MR receptor optical density (corrected for actin) and on hippocampal corticosterone levels. All analyses were performed using SigmaStat v.3.5, with the alpha level set at 0.05.
3. Results
3.1 Experiment 1: Effects of Corticosterone on 5-HT Release in the Ventral Hippocampus
To establish whether intrahippocampal corticosterone administration produced an effect on extracellular 5-HT levels in the rat ventral hippocampus, corticosterone was infused into the ventral hippocampus for 20 min through a microdialysis probe (Figure 1A). There were significant effects of drug treatment (F1,16 = 10.30; P = 0.005), time (F11,150 = 3.41; P<0.001), and a significant interaction between treatment and time (F11,150 = 3.67; P<0.001). Infusion of corticosterone into the ventral hippocampus following vehicle infusion resulted in a significant increase in 5-HT levels over time (F11,80 = 4.55; P < 0.001). Levels of 5-HT were significantly increased at 20–80 min. and 120 min. post-infusion when compared with pre-infusion levels (Holm-Sidak, P < 0.05), suggesting that corticosterone infusion in the ventral hippocampus results in a rapid and prolonged increased in 5-HT levels in this region (Figure 1B). Furthermore, vehicle-corticosterone treated rats showed significantly greater corticosterone-induced 5-HT levels at 20–120 min post infusion (SNK, P < 0.05) when compared to mifepristone-corticosterone treated rats (Figure 1B), suggesting that this GR receptor antagonist blocked the effects of corticosterone on 5-HT release. Mifepristone pre-treatment in the absence of corticosterone had no effect on 5-HT levels, as there was no effect of drug, time, or a significant interaction between treatment and time.
Figure 1.
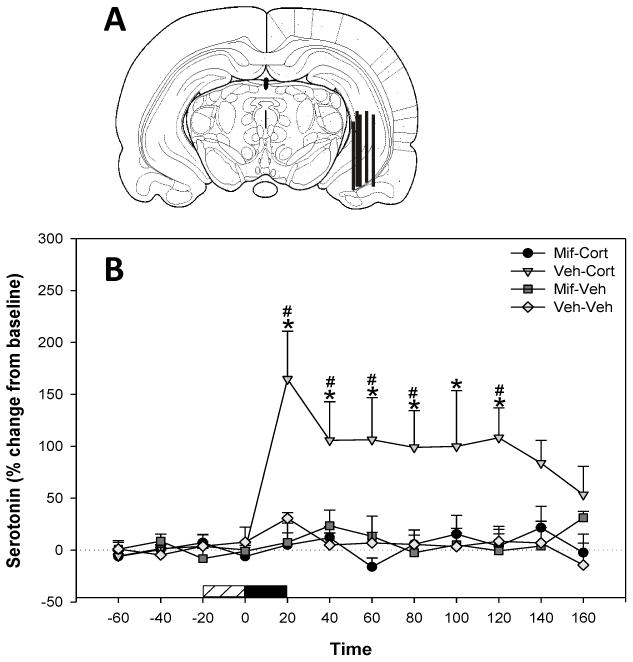
Effects of mifepristone in the ventral hippocampus on basal and corticosterone-induced 5-HT release. (A) Representative coronal diagrams of microdialysis probe membrane placements. Figure adapted from Paxinos & Watson (1997, bregma −5.30mm). (B) Corticosterone (200 ng ml) infused into the ventral hippocampus produced a long term increase in extracellular 5-HT. Mifeprisone (10 mg ml) infusion blocked corticosterone-induced increases in 5-HT. Data represent mean ± SEM. Horizontal bar: coarse=mifepristone or vehicle infusion, filled=corticosterone or vehicle infusion. *Significantly different from pre-infusion levels. # Significant differences between Veh – Cort/Mif-Cort and Veh-Veh/Mif-Veh treatment groups (P < 0.05).
3.2 Experiment 2: Effects of Amphetamine Treatment on Corticosteroid Receptor Levels in the Hippocampus
Western immunoblotting revealed the presence/levels of GR and MR in the dorsal and ventral hippocampus following pretreatment with amphetamine or saline for 2 weeks (Figure 2). Amphetamine produced a significant down-regulation of ventral hippocampal GR protein in comparison with control (F(1,16) = 4.98, P = 0.040) with a trend toward significance in the dorsal hippocampus (F(1,18) = 2.59, P = 0.125; Figure 2A). There was no effect of treatment on dorsal or ventral MR expression (Figure 2B). The balance of the responses mediated by the two types of corticosteroid receptor in the hippocampus is critical for stress responses and adaptive behavior, thus dysregulation of GR/MR balance enhances vulnerability to stress and disease (de Kloet et al., 1998; Velicković et al.,2008). Therefore, we examined the GR/MR ratios in dorsal and ventral hippocampus. The GR/MR ratio in both the dorsal (F(1,17) = 4.97, P = 0.040) and ventral (F(1, 16) = 4.94, P = 0.041) hippocampus of amphetamine pretreated rats was decreased compared to controls (Fig. 2C).
Figure 2.
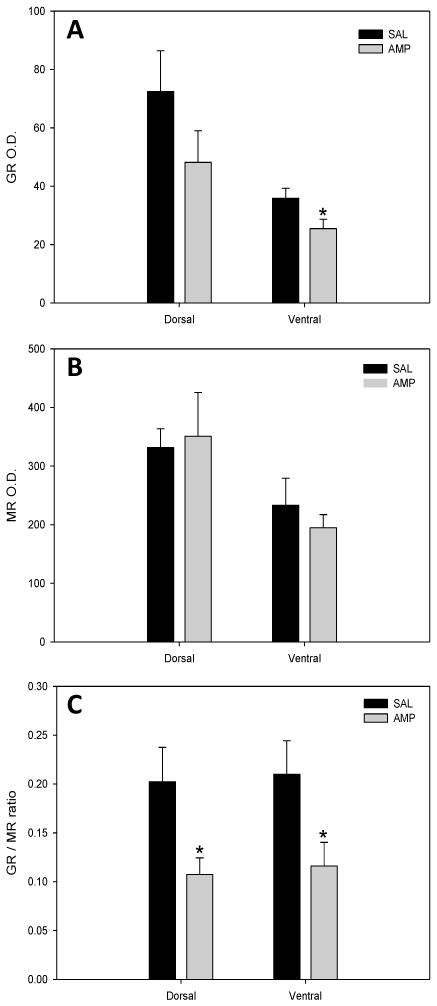
Corticosteroid receptor levels in dorsal and ventral hippocampal tissue of saline- and amphetamine-treated rats. Levels of GR and MR protein was determined by western immunoblot. (A) Amphetamine-treated rats exhibited significantly reduced levels of GRs within ventral hippopcampal tissue as compared to saline-treated controls. (B) No effects of treatment were observed on MR levels within dorsal or ventral hippocampal tissue. (C) Amphetamine-treated rats exhibited significantly reduced levels of GR/MR protein ratio within dorsal and ventral hippopcampus when compared to saline-treated controls. O.D. = Optical density. Means ± S.E.M. are shown for all groups.
3.3 Experiment 3: Effects of Chronic Amphetamine Pre-treatment on Corticosterone-Elicited 5-HT Release in the Ventral Hippocampus
In order to determine whether chronic amphetamine treatment altered ventral hippocampal corticosterone levels, corticosterone levels were measured in the dialysate collected from this region prior to corticosterone or vehicle perfusion. Chronic amphetamine treatment had no effect on basal corticosterone (mean ± SEM: 5.41 ± 0.60) in the ventral hippocampus compared to saline controls (mean ± SEM: 6.39 ± 0.73).
To investigate the effects of chronic amphetamine on corticosterone-elicited serotonin levels, corticosterone (0, 200 or 2000 ng/ml) was infused for 20 min into the ventral hippocampus through a microdialysis probe in animals pretreated with amphetamine or saline. Microdialysis probe membranes were located within the ventral hippocampus in similar locations as observed for Experiment 1 (Figure 3A), and the placement of microdialysis probes was similar in all treatment groups. Basal extracellular 5-HT levels were not significantly different between saline pre-treated rats (1.87+/− 0.73 pg/5 μl; uncorrected for recovery) and amphetamine pretreated rats (2.02+/− 0.33 pg/5μl; uncorrected for recovery).
Figure 3.
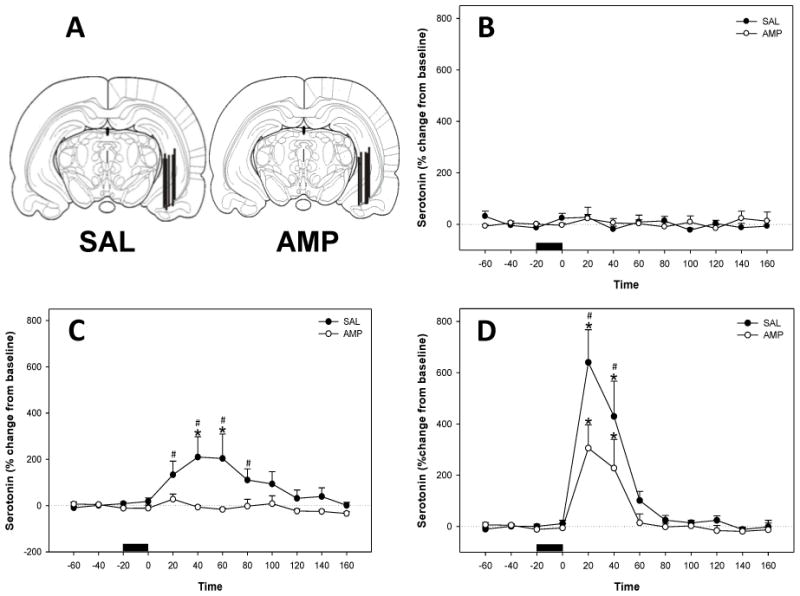
Effects of amphetamine pretreatment on corticosterone-induced 5-HT release in the ventral hippocampus. (A) Representative coronal diagrams of microdialysis probe membrane placements in both treatment groups. Figure adapted from Paxinos & Watson (1997, bregma −5.30mm). (B) Vehicle (11.2% HBC) infused into the ventral hippocampus did not effect extracellular 5-HT in saline or amphetamine (2.5 mg kg, ip.) pretreated animals. Data represent mean ± SEM. Horizontal bar= vehicle infusion (C) Corticosterone (200 ng ml) infused into the ventral hippocampus produced a long term increase in extracellular 5-HT in saline pretreated animals. Amphetamine pretreatment (2.5 mg kg, ip.) abolished corticosterone-induced increases in ventral hippocampal 5-HT. Data represent mean ± SEM. Horizontal bar = corticosterone infusion *Significantly different from pre-infusion levels. # Significant differences between treatment groups (P < 0.05). (D) Corticosterone (2000 ng ml) infused into the ventral hippocampus increased extracellular 5-HT in saline and amphetamine (2.5 mg kg, ip.) pretreated animals. Data represent mean ± SEM. Horizontal bar = corticosterone infusion *Significantly different from pre-infusion levels. # Significant differences between treatment groups (P < 0.05).
When the vehicle for corticosterone (HBC) was infused into the ventral hippocampus of saline and amphetamine pretreated animals there was no significant effect on extracellular 5-HT levels (Figure 3B). When 200ng/ml corticosterone was infused into the ventral hippocampus of saline treated animals, there was an increase in extracellular 5-HT levels, and this effect was absent in animals pretreated with amphetamine (Figure 3C). Significant main effects of drug treatment (F1, 14 = 6.45; P = 0.024), time (F11,144 = 3.09; P < 0.001) and a significant interaction between treatment and time (F11,144 = 2.88; P = 0.002) were evident. Infusion of 200ng/ml corticosterone into the ventral hippocampus of saline treated rats resulted in increased 5-HT levels over time (F11,70 = 3.06; P = 0.002). Post-hoc tests revealed that 5-HT levels were increased at 40–60 min post-infusion in saline pre-treated rats when compared with pre-infusion levels (Holm-Sidak; P<0.05). Conversely, 5-HT levels were not significantly altered over time following 200 ng/ml corticosterone infusion in rats pretreated with amphetamine. When saline and amphetamine groups were compared at each time point following 200 ng/ml corticosterone, saline pretreated rats showed significantly greater corticosterone-induced 5-HT levels at 20–80 min post infusion (SNK, P < 0.05). Infusion of 2000 ng/ml corticosterone into the ventral hippocampus of saline and amphetamine treated animals also increased extracellular 5-HT levels (Figure 3D). There was a significant effect of time (F11,141 = 20.43; P < 0.001) and a significant interaction between treatment and time (F11,141 = 2.17; P = 0.021). Infusion of 2000 ng/ml corticosterone significantly increased 5-HT levels in saline (F8,62 = 15.15; P < 0.001) and in amphetamine (F4,44 = 8.90; P < 0.001) pretreated rats. Post-hoc tests revealed that 5-HT levels were increased at 20–40 min post-infusion in both saline and amphetamine pretreated rats when compared with pre-infusion levels (Holm-Sidak; P < 0.05). However, when saline and amphetamine groups were compared at each time point following 2000 ng/ml corticosterone, saline pretreated rats showed significantly greater corticosterone-induced 5-HT levels at 20–40 min post infusion (SNK, P < 0.05).
4. Discussion
These studies examined the relationship between chronic amphetamine exposure, corticosterone, and ventral hippocampal serotonergic neurotransmission. Local administration of stress-relevant levels of corticosterone evoked an increase in extracellular ventral hippocampal 5-HT levels in the rat, as has been observed in the hippocampus of the lizard Anolis carolinensis (Summers et al., 2003). We have added to this by demonstrating that corticosterone-induced 5-HT release in the ventral hippocampus is mediated by GRs in this region. Furthermore, chronic amphetamine administration reduced GR levels in the ventral hippocampus, abolished the 5-HT response to stress relevant levels of exogenous corticosterone (200 ng/ml) and attenuated 5-HT release in response to extra-physiological corticosterone levels (2000 ng/ml) without affecting endogenous levels of corticosterone in this region. These results show that the 5-HT system of the ventral hippocampus is altered by chronic amphetamine administration via alterations to GR-mediated facilitation of 5-HT release, suggesting that chronic amphetamine disrupts stress-related serotonergic activity in the hippocampus.
In the present in vivo experiments, the facilitation of 5-HT release by corticosterone was prevented by the GR antagonist mifeprisone (RU486). Therefore, the effect of corticosterone on 5-HT release in this area is mediated through classical GRs. The GR can be membrane associated (Orchinik et al., 1997; Liposits and Bohn, 1993; Johnson et al., 2005) and has recently been shown to mediate rapid effects of corticosterone on hippocampal neurotransmission and memory processes (Wang and Wang, 2009; Venero and Borrell, 1999; Prager and Johnson, 2009; Chauveau et al., 2010; Roozendaal et al., 2010). Future studies utilizing corticosterone-BSA or protein synthesis inhibitors will confirm the effect of corticosterone on 5-HT release as a non-genomic, classical glucocorticoid receptor–mediated process (Zheng and Ramierez, 1996; Liu et al., 1995). Interestingly, the GR antagonist mifepristone alone had no effect on hippocampal 5-HT release. Therefore, while GRs mediate corticosterone-elicited 5-HT release in the ventral hippocampus, they do not seem to play a role in the regulation of extracellular levels of hippocampal 5-HT under basal conditions.
In the present study, GR protein levels were reduced in the ventral hippocampus of amphetamine pretreated animals with a trend towards reduction in the dorsal hippocampus. The lack of observed effect of amphetamine pretreatment on MRs in either region suggests a specific effect of chronic amphetamine treatment on GRs in the hippocampus. Previous studies have shown that repeated amphetamine administration also selectively down-regulates GR mRNA in the dorsal hippocampus (Budziszewska et al., 1995, 1996; Yau et al., 1994; Shilling et al., 1996), whereas studies that investigated GR protein expression showed no effect of cocaine administration in the dorsal hippocampus or ventral subiculum (Mantsch et al., 2007), perhaps revealing a dissociation between mRNA measures and protein expression. The current findings support the lack of psychostimulant effects on GR protein expression in the dorsal hippocampus, and add to this by demonstrating reduced GR levels and function in the ventral hippocampus of amphetamine pretreated rats.
While the mechanisms by which chronic amphetamine administration selectively decreases GRs in the ventral hippocampus are not known, it is recognized that amphetamine activates the hypothalamic-pituitary-adrenal axis resulting in acute increases in the levels of glucocorticoids in the bloodstream and in the brain (Knych and Eisenberg, 1979; Swerdlow et al., 1993). We have found that basal circulating corticosterone (Barr et al., 2010) or basal corticosterone levels in the ventral hippocampus (current study) are not altered by chronic amphetamine treatment when measured one day following last treatment in the absence of amphetamine injection. However, hypersecretion of corticosterone occurs in response to a challenge injection of psychostimulant in rats sensitized to amphetamine or cocaine (Schmidt et al., 1995, 1999). Therefore, one mechanism by which chronic amphetamine treatment could reduce GR expression in the ventral hippocampus is via repeated acute and sensitized elevations of corticosterone during the actual treatment regime. This is supported by studies showing an inverse relationship between corticosterone and GR levels. For example, hippocampal GRs are up regulated by adrenalectomy, (Tornello et al., 1982; Reul et al., 1989; Herman et al., 1989), whereas stress and elevated glucocorticoid treatment down-regulates hippocampal GR (Sapolsky et al., 1984; Sapolsky and McEwen, 1985). However, some studies measuring GR mRNA levels in response to glucocorticoids or stress have been less clear (Herman et al., 1999; Herman and Spencer, 1998; Reul et al., 1989), suggesting GR expression may be dose-dependent. Like GRs, adrenalectomy-induced increases in MR mRNA and down-regulation of MR mRNA following chronic treatment with high doses of corticosterone have been observed (Kalman and Spencer, 2002). However, GR expression is more sensitive to corticosterone-induced down-regulation (Sarabdjitsingh et al., 2010). This may be because the expression of MR is mainly autoregulated, whereas GR expression can be inhibited through activity at both the MR and GR (Chao et al., 1998; Herman and Spencer, 1998). Consequently, the differential regulation of GR versus MR expression by amphetamine may be due to the greater sensitivity to corticosterone-induced down-regulation demonstrated by the GR.
The current study showed that chronic amphetamine treatment attenuated corticosterone-induced 5-HT release in the ventral hippocampus. Basal hippocampal corticosterone was unaltered by chronic amphetamine exposure when measured the day following last treatment. As a result, the exogenously applied corticosterone would be competing with similar endogenous corticosterone levels in the ventral hippocampus of both treatment groups. Furthermore, repeated amphetamine does not alter the firing rate of the dorsal raphe serotonergic neurons that provide the serotonergic innervation to the ventral hippocampus (Heidenreich et al., 1987, Heidenreich and Rebec, 1989), suggesting that the hippocampal serotonergic system would not be generally sub-sensitive to stimulation. We have previously demonstrated that 5-HT tissue concentrations are lower in the ventral dentate gyrus of rats the day following chronic amphetamine treatment (Barr et al., 2010). However, 5-HT concentration in ventral CA regions was not affected by amphetamine treatment (Barr et al., 2010) and the microdialysis probe in the current experiment sampled from both subregions of the ventral hippocampus. Furthermore, basal extracellular 5-HT levels as measured by in vivo microdialysis in the current study were not different between saline and amphetamine pretreated rats. Also, extra-physiological levels of corticosterone were able to induce an attenuated increase in extracellular 5-HT suggesting that amphetamine treated rats have reduced but functional GRs that can be activated by high levels corticosterone. Overall, given that GRs mediate corticosterone-elicited 5-HT release in the ventral hippocampus, the lack of responsiveness to stress-relevant levels of corticosterone is likely a result of the reduction of GR availability in this region by amphetamine pretreatment.
The corticosteroid receptor balance hypothesis asserts that the balance in MR- and GR-mediated responses is necessary for homeostasis, adaptation, and health (de Kloet, 1991; de Kloet et al., 1998). Activity of MRs serves to evaluate the nature and severity of stressors, whereas GR activation promotes recovery from stress and adaptation. Therefore, balance in corticosteroid receptor activation is necessary for the determination of an appropriate coping strategy (de Kloet and Derijk, 2004). Amphetamine treatment reduced GR with no effect on MR expression, leading to a reduction in GR/MR balance evident in both the dorsal and ventral hippocampus. Although not studied here, this reduction in the GR/MR ratio in the dorsal hippocampus may result in reduced corticosterone-induced 5-HT activity in this region (Korte-Bouws et al., 1996), like we have observed for the ventral hippocampus. Since 5-HT impairs the processing of stress-related memories in the dorsal hippocampus (Guimaraes et al., 1993), reduced activity in this region could disrupt stress adaptation. Overall, the results suggest suboptimal stress reactivity and reduced stress coping ability in animals exposed to repeated amphetamine. We have already determined that the exact amphetamine treatment schedule used here induces long-lasting anxiety states that are evident the day following last treatment and at 4 weeks of withdrawal (Barr et al., 2010; Vuong et al., 2010). However, further studies are necessary to determine the duration of the effects of amphetamine induced corticosteroid receptor imbalance and whether these are related to neuroendocrine and behavioral responses to stressors.
In conclusion, the present studies suggest that stress-relevant increases in corticosterone within the ventral hippocampus increases 5-HT release via the GR in this region. Also, GR-5-HT interactions are reduced by chronic amphetamine exposure, providing, in part, a neurobiological mechanism by which amphetamine withdrawal is characterized by alterations to affect and behavior.
Acknowledgments
This work was supported by grants NIH R01 DA019921 & NIH P20 RR015567 which is a designated Center of Biomedical Research Excellence (COBRE). We thank Jamie L. Scholl for valuable assistance with these experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azmitia EC, Jr, McEwen BS. Adrenalcortical influence on rat brain tryptophan hydroxylase activity. Brain Res. 1974;78:291–302. doi: 10.1016/0006-8993(74)90553-8. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Barr JL, Renner KJ, Forster GL. Withdrawal from chronic amphetamine produces persistent anxiety-like behavior but temporally-limited reductions in monoamines and neurogenesis in the adult rat dentate gyrus. Neuropharmacology. 2010;59:395–405. doi: 10.1016/j.neuropharm.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast T. Toward an integrative perspective on hippocampal function: from the rapid encoding of experience to adaptive behavior. Rev Neurosci. 2007;18:253–281. doi: 10.1515/revneuro.2007.18.3-4.253. [DOI] [PubMed] [Google Scholar]
- Bast T, Wilson IA, Witter MP, Morris RG. From rapid place learning to behavioral performance: a key role for the intermediate hippocampus. PLoS Biol. 2009;7:e1000089. doi: 10.1371/journal.pbio.1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, Dowd JA, Dugan MM, Renda P. Corticosterone is permissive to the anxiolytic effect that results from the blockade of hippocampal mineralocorticoid receptors. Pharmacol Biochem Behav. 1998;60:879–887. doi: 10.1016/s0091-3057(98)00071-9. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Sprouse JS, Sheldon PW, Aghajanian GK, Roth RH. In vitro microdialysis: a novel technique for stimulated neurotransmitter release measurements. J Neurosci Methods. 1991;36:85–90. doi: 10.1016/0165-0270(91)90141-l. [DOI] [PubMed] [Google Scholar]
- Budziszewska B, Jaworska-Feil L, Lason W. Repeated amphetamine administration down-regulates glucocorticoid, but not mineralocorticoid, receptors in the rat hippocampus. Pol J Pharmacol. 1995;47:401–406. [PubMed] [Google Scholar]
- Budziszewska B, Leskiewicz M, Jaworska-Feil L, Lason W. Repeated cocaine administration down-regulates glucocorticoid receptors in the rat brain cortex and hippocampus. Pol J Pharmacol. 1996;48:575–581. [PubMed] [Google Scholar]
- Carli M, Tatarczynska E, Cervo L, Samanin R. Stimulation of hippocampal 5-HT1A receptors causes amnesia and anxiolytic-like but not antidepressant-like effects in the rat. Eur J Pharmacol. 1993;234:215–221. doi: 10.1016/0014-2999(93)90956-i. [DOI] [PubMed] [Google Scholar]
- Chao HM, Choo PH, McEwen BS. Glucocorticoid and mineralocorticoid receptor mRNA expression in rat brain. Neuroendocrinology. 1989;50:365–371. doi: 10.1159/000125250. [DOI] [PubMed] [Google Scholar]
- Chao HM, Ma LY, McEwen BS, Sakai RR. Regulation of glucocorticoid receptor and mineralocorticoid receptor messenger ribonucleic acids by selective agonists in the rat hippocampus. Endocrinology. 1998;139:1810–1814. doi: 10.1210/endo.139.4.5896. [DOI] [PubMed] [Google Scholar]
- Chauveau F, Tronche C, Pierard C, Liscia P, Drouet I, Coutan M, Beracochea D. Rapid stress-induced corticosterone rise in the hippocampus reverses serial memory retrieval pattern. Hippocampus. 2010;20:196–207. doi: 10.1002/hipo.20605. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology (Berl) 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Derijk R. Signaling pathways in brain involved in predisposition and pathogenesis of stress-related disease: genetic and kinetic factors affecting the MR/GR balance. Ann N Y Acad Sci. 2004;1032:14–34. doi: 10.1196/annals.1314.003. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Oitzl M, Sutanto W. Implication of brain corticosteroid receptor diversity for the adaptation syndrome concept. Methods Achiev Exp Pathol. 1991;14:104–132. [PubMed] [Google Scholar]
- De Kloet ER, Kovacs GL, Szabo G, Telegdy G, Bohus B, Versteeg DH. Decreased serotonin turnover in the dorsal hippocampus of rat brain shortly after adrenalectomy: selective normalization after corticosterone substitution. Brain Res. 1982;239:659–663. doi: 10.1016/0006-8993(82)90546-7. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, McEwen BS. Differences between cytosol receptor complexes with corticosterone and dexamethasone in hippocampal tissue from rat brain. Biochim Biophys Acta. 1976;421:124–132. doi: 10.1016/0304-4165(76)90176-8. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- De Kloet R, Wallach G, McEwen BS. Differences in corticosterone and dexamethasone binding to rat brain and pituitary. Endocrinology. 1975;96:598–609. doi: 10.1210/endo-96-3-598. [DOI] [PubMed] [Google Scholar]
- Degroot A, Treit D. Anxiety is functionally segregated within the septo-hippocampal system. Brain Res. 2004;1001:60–71. doi: 10.1016/j.brainres.2003.10.065. [DOI] [PubMed] [Google Scholar]
- Droste SK, de Groote L, Atkinson HC, Lightman SL, Reul JM, Linthorst AC. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology. 2008;149:3244–3253. doi: 10.1210/en.2008-0103. [DOI] [PubMed] [Google Scholar]
- Forster GL, Pringle RB, Mouw NJ, Vuong SM, Watt MJ, Burke AR, Lowry CA, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity. Eur J Neurosci. 2008;28:299–310. doi: 10.1111/j.1460-9568.2008.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes FS, Del Bel EA, Padovan CM, Netto SM, de Almeida RT. Hippocampal 5-HT receptors and consolidation of stressful memories. Behav Brain Res. 1993;58:133–139. doi: 10.1016/0166-4328(93)90098-b. [DOI] [PubMed] [Google Scholar]
- Han F, Ozawa H, Matsuda K, Nishi M, Kawata M. Colocalization of mineralocorticoid receptor and glucocorticoid receptor in the hippocampus and hypothalamus. Neurosci Res. 2005;51:371–381. doi: 10.1016/j.neures.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Heidenreich BA, Basse-Tomusk AE, Rebec GV. Serotonergic dorsal raphe neurons: subsensitivity to amphetamine with long-term treatment. Neuropharmacology. 1987;26:719–724. doi: 10.1016/0028-3908(87)90233-4. [DOI] [PubMed] [Google Scholar]
- Heidenreich BA, Rebec GV. Serotonergic dorsal raphe neurons: changes in spontaneous neuronal activity and responsiveness to 5-MeODMT following long-term amphetamine administration. Neurosci Lett. 1989;103:81–86. doi: 10.1016/0304-3940(89)90489-8. [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–224. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Herman JP, Patel PD, Akil H, Watson SJ. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol Endocrinol. 1989;3:1886–1894. doi: 10.1210/mend-3-11-1886. [DOI] [PubMed] [Google Scholar]
- Herman JP, Spencer R. Regulation of hippocampal glucocorticoid receptor gene transcription and protein expression in vivo. J Neurosci. 1998;18:7462–7473. doi: 10.1523/JNEUROSCI.18-18-07462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Watson SJ, Spencer RL. Defense of adrenocorticosteroid receptor expression in rat hippocampus: effects of stress and strain. Endocrinology. 1999;140:3981–3991. doi: 10.1210/endo.140.9.6962. [DOI] [PubMed] [Google Scholar]
- Joca SR, Ferreira FR, Guimaraes FS. Modulation of stress consequences by hippocampal monoaminergic, glutamatergic and nitrergic neurotransmitter systems. Stress. 2007;10:227–249. doi: 10.1080/10253890701223130. [DOI] [PubMed] [Google Scholar]
- Joca SR, Padovan CM, Guimaraes FS. Activation of post-synaptic 5-HT(1A) receptors in the dorsal hippocampus prevents learned helplessness development. Brain Res. 2003;978:177–184. doi: 10.1016/s0006-8993(03)02943-3. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Farb C, Morrison JH, McEwen BS, LeDoux JE. Localization of glucocorticoid receptors at postsynaptic membranes in the lateral amygdala. Neuroscience. 2005;136:289–299. doi: 10.1016/j.neuroscience.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Kalman BA, Spencer RL. Rapid corticosteroid-dependent regulation of mineralocorticoid receptor protein expression in rat brain. Endocrinology. 2002;143:4184–4195. doi: 10.1210/en.2002-220375. [DOI] [PubMed] [Google Scholar]
- Keck ME, Sartori SB, Welt T, Muller MB, Ohl F, Holsboer F, Landgraf R, Singewald N. Differences in serotonergic neurotransmission between rats displaying high or low anxiety/depression-like behaviour: effects of chronic paroxetine treatment. J Neurochem. 2005;92:1170–1179. doi: 10.1111/j.1471-4159.2004.02953.x. [DOI] [PubMed] [Google Scholar]
- Keck ME, Welt T, Muller MB, Uhr M, Ohl F, Wigger A, Toschi N, Holsboer F, Landgraf R. Reduction of hypothalamic vasopressinergic hyperdrive contributes to clinically relevant behavioral and neuroendocrine effects of chronic paroxetine treatment in a psychopathological rat model. Neuropsychopharmacology. 2003;28:235–243. doi: 10.1038/sj.npp.1300040. [DOI] [PubMed] [Google Scholar]
- Keeney A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice. J Neuroendocrinol. 2006;18:330–338. doi: 10.1111/j.1365-2826.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Dickinson SL, Curzon G. Central serotonergic responses and behavioural adaptation to repeated immobilization: the effect of the corticosterone synthesis inhibitor metyrapone. Eur J Pharmacol. 1985;119:143–152. doi: 10.1016/0014-2999(85)90290-0. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Dourish CT, Curzon G. Antidepressant-like action of 5-HT1A agonists and conventional antidepressants in an animal model of depression. Eur J Pharmacol. 1987;134:265–274. doi: 10.1016/0014-2999(87)90357-8. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knych ET, Eisenberg RM. Effect of amphetamine on plasma corticosterone in the conscious rat. Neuroendocrinology. 1979;29:110–118. doi: 10.1159/000122912. [DOI] [PubMed] [Google Scholar]
- Korte-Bouws GA, Korte SM, De Kloet ER, Bohus B. Blockade of corticosterone synthesis reduces serotonin turnover in the dorsal hippocampus of the rat as measured by microdialysis. J Neuroendocrinol. 1996;8:877–881. doi: 10.1046/j.1365-2826.1996.05389.x. [DOI] [PubMed] [Google Scholar]
- Liposits Z, Bohn MC. Association of glucocorticoid receptor immunoreactivity with cell membrane and transport vesicles in hippocampal and hypothalamic neurons of the rat. J Neurosci Res. 1993;35:14–19. doi: 10.1002/jnr.490350103. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang CA, Chen YZ. Nongenomic effect of glucocorticoid on the release of arginine vasopressin from hypothalamic slices in rats. Neuroendocrinology. 1995;62:628–633. doi: 10.1159/000127059. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Mokin MV, Scholl JL, Forster GL. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm Behav. 2009;55:248–256. doi: 10.1016/j.yhbeh.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Maccari S, Piazza PV, Deminiere JM, Angelucci L, Simon H, Le Moal M. Hippocampal type I and type II corticosteroid receptor affinities are reduced in rats predisposed to develop amphetamine self-administration. Brain Res. 1991;548:305–309. doi: 10.1016/0006-8993(91)91137-p. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Cullinan WE, Tang LC, Baker DA, Katz ES, Hoks MA, Ziegler DR. Daily cocaine self-administration under long-access conditions augments restraint-induced increases in plasma corticosterone and impairs glucocorticoid receptor-mediated negative feedback in rats. Brain Res. 2007;1167:101–111. doi: 10.1016/j.brainres.2007.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBlane JW, Handley SL. Effects of two stressors on behaviour in the elevated X-maze: preliminary investigation of their interaction with 8-OH-DPAT. Psychopharmacology (Berl) 1994;116:173–182. doi: 10.1007/BF02245060. [DOI] [PubMed] [Google Scholar]
- McHugh SB, Deacon RM, Rawlins JN, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci. 2004;118:63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- Mokler DJ, Lariviere D, Johnson DW, Theriault NL, Bronzino JD, Dixon M, Morgane PJ. Serotonin neuronal release from dorsal hippocampus following electrical stimulation of the dorsal and median raphe nuclei in conscious rats. Hippocampus. 1998;8:262–273. doi: 10.1002/(SICI)1098-1063(1998)8:3<262::AID-HIPO8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Morsink MC, Van Gemert NG, Steenbergen PJ, Joels M, De Kloet ER, Datson NA. Rapid glucocorticoid effects on the expression of hippocampal neurotransmission-related genes. Brain Res. 2007;1150:14–20. doi: 10.1016/j.brainres.2007.02.083. [DOI] [PubMed] [Google Scholar]
- Orchinik M, Hastings N, Witt D, McEwen BS. High-affinity binding of corticosterone to mammalian neuronal membranes: possible role of corticosteroid binding globulin. J Steroid Biochem Mol Biol. 1997;60:229–236. doi: 10.1016/s0960-0760(96)00191-4. [DOI] [PubMed] [Google Scholar]
- Prager EM, Johnson LR. Stress at the synapse: signal transduction mechanisms of adrenal steroids at neuronal membranes. Sci Signal. 2009;2:re5. doi: 10.1126/scisignal.286re5. [DOI] [PubMed] [Google Scholar]
- Prasad BM, Ulibarri C, Sorg BA. Stress-induced cross-sensitization to cocaine: effect of adrenalectomy and corticosterone after short- and long-term withdrawal. Psychopharmacology (Berl) 1998;136:24–33. doi: 10.1007/s002130050535. [DOI] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Reul JM, Pearce PT, Funder JW, Krozowski ZS. Type I and type II corticosteroid receptor gene expression in the rat: effect of adrenalectomy and dexamethasone administration. Mol Endocrinol. 1989;3:1674–1680. doi: 10.1210/mend-3-10-1674. [DOI] [PubMed] [Google Scholar]
- Rex A, Voigt JP, Fink H. Anxiety but not arousal increases 5-hydroxytryptamine release in the rat ventral hippocampus in vivo. Eur J Neurosci. 2005;22:1185–1189. doi: 10.1111/j.1460-9568.2005.04251.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, Haettig J, Wood MA. Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. J Neurosci. 2010;30:5037–5046. doi: 10.1523/JNEUROSCI.5717-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N, Tanaka C. Inhibitory modulation of long-term potentiation via the 5-HT1A receptor in slices of the rat hippocampal dentate gyrus. Brain Res. 1993;613:326–330. doi: 10.1016/0006-8993(93)90921-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Stress down-regulates corticosterone receptors in a site-specific manner in the brain. Endocrinology. 1984;114:287–292. doi: 10.1210/endo-114-1-287. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, McEwen BS. Down-regulation of neural corticosterone receptors by corticosterone and dexamethasone. Brain Res. 1985;339:161–165. doi: 10.1016/0006-8993(85)90638-9. [DOI] [PubMed] [Google Scholar]
- Sarabdjitsingh RA, Isenia S, Polman A, Mijalkovic J, Lachize S, Datson N, de Kloet ER, Meijer OC. Disrupted corticosterone pulsatile patterns attenuate responsiveness to glucocorticoid signaling in rat brain. Endocrinology. 2010;151:1177–1186. doi: 10.1210/en.2009-1119. [DOI] [PubMed] [Google Scholar]
- Schmidt ED, Tilders FJ, Binnekade R, Schoffelmeer AN, De Vries TJ. Stressor- or drug-induced sensitization of the corticosterone response is not critically involved in the long-term expression of behavioural sensitization to amphetamine. Neuroscience. 1999;92:343–352. doi: 10.1016/s0306-4522(98)00725-8. [DOI] [PubMed] [Google Scholar]
- Schmidt ED, Tilders FJ, Janszen AW, Binnekade R, De Vries TJ, Schoffelmeer AN. Intermittent cocaine exposure causes delayed and long-lasting sensitization of cocaine-induced ACTH secretion in rats. Eur J Pharmacol. 1995;285:317–321. doi: 10.1016/0014-2999(95)00540-2. [DOI] [PubMed] [Google Scholar]
- Sharp T, Bramwell SR, Grahame-Smith DG. Release of endogenous 5-hydroxytryptamine in rat ventral hippocampus evoked by electrical stimulation of the dorsal raphe nucleus as detected by microdialysis: sensitivity to tetrodotoxin, calcium and calcium antagonists. Neuroscience. 1990;39:629–637. doi: 10.1016/0306-4522(90)90247-2. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Kelsoe JR, Segal DS. Hippocampal glucocorticoid receptor mRNA is up-regulated by acute and down-regulated by chronic amphetamine treatment. Brain Res Mol Brain Res. 1996;38:156–160. doi: 10.1016/0169-328x(96)00009-5. [DOI] [PubMed] [Google Scholar]
- Singh VB, Corley KC, Krieg RJ, Phan TH, Boadle-Biber MC. Sound stress activation of tryptophan hydroxylase blocked by hypophysectomy and intracranial RU 38486. Eur J Pharmacol. 1994;256:177–184. doi: 10.1016/0014-2999(94)90243-7. [DOI] [PubMed] [Google Scholar]
- Stiedl O, Misane I, Spiess J, Ogren SO. Involvement of the 5-HT1A receptors in classical fear conditioning in C57BL/6J mice. J Neurosci. 2000;20:8515–8527. doi: 10.1523/JNEUROSCI.20-22-08515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey JD, Robertson DA, Beattie JE, Reid IC, Mitchell SN, Balfour DJ. Behavioural and neurochemical responses evoked by repeated exposure to an elevated open platform. Behav Brain Res. 2006;166:220–229. doi: 10.1016/j.bbr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Summers TR, Matter JM, McKay JM, Ronan PJ, Larson ET, Renner KJ, Summers CH. Rapid glucocorticoid stimulation and GABAergic inhibition of hippocampal serotonergic response: in vivo dialysis in the lizard anolis carolinensis. Horm Behav. 2003;43:245–253. doi: 10.1016/s0018-506x(02)00014-4. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Koob GF, Cador M, Lorang M, Hauger RL. Pituitary-adrenal axis responses to acute amphetamine in the rat. Pharmacol Biochem Behav. 1993;45:629–637. doi: 10.1016/0091-3057(93)90518-x. [DOI] [PubMed] [Google Scholar]
- Sze PY. Glucocorticoid regulation of the serotonergic system of the brain. Adv Biochem Psychopharmacol. 1976;15:251–265. [PubMed] [Google Scholar]
- Thoeringer CK, Sillaber I, Roedel A, Erhardt A, Mueller MB, Ohl F, Holsboer F, Keck ME. The temporal dynamics of intrahippocampal corticosterone in response to stress-related stimuli with different emotional and physical load: an in vivo microdialysis study in C57BL/6 and DBA/2 inbred mice. Psychoneuroendocrinology. 2007;32:746–757. doi: 10.1016/j.psyneuen.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Tornello S, Orti E, De Nicola AF, Rainbow TC, McEwen BS. Regulation of glucocorticoid receptors in brain by corticosterone treatment of adrenalectomized rats. Neuroendocrinology. 1982;35:411–417. doi: 10.1159/000123429. [DOI] [PubMed] [Google Scholar]
- Umriukhin AE, Wigger A, Singewald N, Landgraf R. Hypothalamic and hippocampal release of serotonin in rats bred for hyper- or hypo-anxiety. Stress. 2002;5:299–305. doi: 10.1080/1025389021000061200. [DOI] [PubMed] [Google Scholar]
- Van Loon GR, Shum A, Sole MJ. Decreased brain serotonin turnover after short term (two-hour) adrenalectomy in rats: a comparison of four turnover methods. Endocrinology. 1981;108:1392–1402. doi: 10.1210/endo-108-4-1392. [DOI] [PubMed] [Google Scholar]
- Veldhuis HD, Van Koppen C, Van Ittersum M, De Kloet ER. Specificity of the adrenal steroid receptor system in rat hippocampus. Endocrinology. 1982;110:2044–2051. doi: 10.1210/endo-110-6-2044. [DOI] [PubMed] [Google Scholar]
- Velicković N, Djordjević A, Matić G, Horvat A. Radiation-induced hyposuppression of the hypothalamic-pituitary-adrenal axis is associated with alterations of hippocampal corticosteroid receptor expression. Radiation Research. 2008;169(4):397–407. doi: 10.1667/RR1200.1. [DOI] [PubMed] [Google Scholar]
- Venero C, Borrell J. Rapid glucocorticoid effects on excitatory amino acid levels in the hippocampus: a microdialysis study in freely moving rats. Eur J Neurosci. 1999;11:2465–2473. doi: 10.1046/j.1460-9568.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- Vuong SM, Oliver HA, Scholl JL, Oliver KM, Forster GL. Increased anxiety-like behavior of rats during amphetamine withdrawal is reversed by CRF2 receptor antagonism. Behav Brain Res. 2010;208:278–281. doi: 10.1016/j.bbr.2009.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Wang SJ. Modulation of presynaptic glucocorticoid receptors on glutamate release from rat hippocampal nerve terminals. Synapse. 2009;63:745–751. doi: 10.1002/syn.20654. [DOI] [PubMed] [Google Scholar]
- Wright IK, Upton N, Marsden CA. Effect of established and putative anxiolytics on extracellular 5-HT and 5-HIAA in the ventral hippocampus of rats during behaviour on the elevated X-maze. Psychopharmacology (Berl) 1992;109:338–346. doi: 10.1007/BF02245882. [DOI] [PubMed] [Google Scholar]
- Yau JL, Kelly PA, Sharkey J, Seckl JR. Chronic 3,4-methylenedioxymethamphetamine administration decreases glucocorticoid and mineralocorticoid receptor, but increases 5-hydroxytryptamine1C receptor gene expression in the rat hippocampus. Neuroscience. 1994;61:31–40. doi: 10.1016/0306-4522(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Zheng J, Ali A, Ramirez VD. Steroids conjugated to bovine serum albumin as tools to demonstrate specific steroid neuronal membrane binding sites. J Psychiatry Neurosci. 1996;21:187–197. [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Li L, Tang S, Cao X, Li Z, Li W, Li C, Zhang X. Effects of serotonin depletion on the hippocampal GR/MR and BDNF expression during the stress adaptation. Behav Brain Res. 2008;195:129–138. doi: 10.1016/j.bbr.2008.06.009. [DOI] [PubMed] [Google Scholar]


