Abstract
Phlebotomus duboscqi is the principle vector of Leishmania major, the causative agent of cutaneous leishmaniasis (CL), in West Africa and is the suspected vector in Mali. Although found throughout the country the seasonality and infection prevalence of P. duboscqi has not been established in Mali. We conducted a three year study in two neighboring villages, Kemena and Sougoula, in Central Mali, an area with a leishmanin skin test positivity of up to 45%. During the first year, we evaluated the overall diversity of sand flies. Of 18,595 flies collected, 12,952 (69%) belonged to 12 species of Sergentomyia and 5,643 (31%) to two species of the genus Phlebotomus, P. duboscqi and P. rodhaini. Of those, P. duboscqi was the most abundant, representing 99% of the collected Phlebotomus species. P. duboscqi was the primary sand fly collected inside dwellings, mostly by resting site collection. The seasonality and infection prevalence of P. duboscqi was monitored over two consecutive years. P. dubsocqi were collected throughout the year. Using a quasi-Poisson model we observed a significant annual (year 1 to year 2), seasonal (monthly) and village effect (Kemena versus Sougoula) on the number of collected P. duboscqi. The significant seasonal effect of the quasi-Poisson model reflects two seasonal collection peaks in May-July and October-November. The infection status of pooled P. duboscqi females was determined by PCR. The infection prevalence of pooled females, estimated using the maximum likelihood estimate of prevalence, was 2.7% in Kemena and Sougoula. Based on the PCR product size, L. major was identified as the only species found in flies from the two villages. This was confirmed by sequence alignment of a subset of PCR products from infected flies to known Leishmania species, incriminating P. duboscqi as the vector of CL in Mali.
Author Summary
Female sand flies transmit a parasite called Leishmania that causes a disease called cutaneous leishmaniasis (CL). Several species of sand flies are found in West Africa, but only one species, Phlebotomus duboscqi, has been proven to transmit the parasite. Cutaneous Leishmaniasis has also been reported from Mali, Central West Africa, but the sand fly transmitting the parasite and its annual abundance has not been established, until now. Sand flies were collected during three consecutive years from two neighboring villages in Central Mali, Kemena and Sougoula, where CL is present. P. duboscqi was collected year-round and was the dominant sand fly inside of and surrounding human dwellings. Other sand fly species, known not to be vectors of CL, were primarily found outside the village. Additionally, P. duboscqi females were found infected with L. major, the same Leishmania species identified from human CL cases in Mali. The estimated infection prevalence of P. duboscqi females was 2.7%. Interestingly, the sand fly abundance and infection prevalence was similar in the two villages despite a previous report indicating a disparate L. major exposure rate in humans. This study greatly enhances our knowledge of CL transmission in Mali, poorly studied in this country to date.
Introduction
In West Africa Phlebotomus duboscqi Neveu-Lemaire is the most important vector of Leishmania major, the causative agent of cutaneous leishmaniasis (CL) [1], [2]. P. duboscqi has been incriminated as the vector of L. major in Senegal [3] and suspected as the vector of CL in Burkina Faso [4], Niger [5], [6], The Gambia [7], Ghana [8], Cameroon [9] and Mali [10], [11], [12]. The first report of P. duboscqi in Mali was from Hombori in 1906 [13] with additional reports from Timbuctu in 1913 [14] and from Bamako and Nioro in 1943 [10]. Later work by Lariviere [11] and Desjeux [1] found P. duboscqi in all regions of the country.
Cutaneous Leishmaniasis is endemic in Mali with cases historically occurring in the districts of Nioro and Segou [11], [15]. The first published report of CL in Mali concerned two cases identified from Nioro in 1944 [16]. Later studies reported leishmanin skin test positivity rates between 10 and 61%, suggesting that Leishmania is endemic in Mali [15], [17], [18], [19]. Leishmania major was first identified as the causative agent of CL in Mali by isoenzyme analysis of parasites isolated from skin samples taken from a lesion of a tourist visiting Mopti [20] and a local resident living in the same region [21].
Despite the identification of L. major as the causative agent of CL in Mali, and although suspected as the vector, no one has identified the parasite in P. duboscqi. Here, we report on a three year survey to evaluate the diversity of sand flies and the seasonal abundance of P. duboscqi in Kemena and Sougoula, two villages endemic for CL in the District of Baroueli, Region of Segou, in Central Mali. Furthermore, we report for the first time the detection and annual prevalence of L. major parasites in P. duboscqi sand flies collected from the study sites.
Methods
Site description
Sand flies were collected from two neighboring villages, Kemena (12°33′ N–6°33′ W) and Sougoula (13°05′ N, –6°53′ W), in the Baroueli Health District, Region of Segou, Mali. Both villages have a population size of approximately 1000 inhabitants. Each village is organized into a labyrinth of adjoining compounds within which a single extended family resides in several sleeping, cooking, and storage houses. Houses are constructed of clay bricks plastered with mud and straw, and with thatched or metal roofs. Domestic animals, such as goats, sheep, and chickens are kept within the confines of a family compound while cows are maintained in corrals located around the perimeter of the village. Both villages have a limited infrastructure and lack electricity and running water. The climate consists of three distinct seasons: a dry season from March to June (temperature range 27–40°C; monthly average rainfall 5.2 mm), a rainy season from June to September (temperature range 25–35°C, monthly average rainfall 82.42 mm), and a third temperate season from October to February (temperature range 20–35°C; monthly average rainfall 3.3 mm). Vegetation is sparse and is characterized by the presence of sporadically placed trees such as shea (Vitellaria paradoxa), acacia (Faidherbia albida) and neem (Azadirachta indica) and small bushes. Most of the land surrounding each village is dedicated for agricultural use.
Sand fly collection
Sand flies were collected using 1) dark activated, CDC miniature light traps fitted with double ring fine mesh collection bags (John W. Hock Company, Gainesville, FL), 2) sticky traps consisting of single sheets of A4 paper (21×29.5 cm) coated on both sides with castor oil and mounted vertically on pegs, onto which randomly impinging sand flies would adhere (used for the sand fly diversity study only), and 3) mouth aspirators (John W. Hock Company, Gainesville, FL) for collection of resting flies inside of houses used for sleeping. All sand flies were sorted by sex, species and blood meal status, and placed in tubes containing silica gel and cotton until processed. Minimum and maximum temperatures, rainfall and relative humidity for the months of July 2006 to June 2008 were collected from the nearest available weather station in Segou, Mali. Oral informed consent was obtained from head of households for indoor collection of sand flies. Households where consent was given were listed in a written log kept by the entomological team for reference.
Sand fly diversity
To assess diversity, sand flies were collected monthly from March 2005 through June 2006 using all three collection methods. Specifically, five light traps were placed inside houses used for sleeping and two light traps were placed outside houses within the same compound in five different compounds for three consecutive nights per village (n = 2; Kemena and Sougoula) for a total of 210 trap-nights per month. Light traps were placed at dusk and collected at dawn the next day. One sticky trap was placed in each of 10 sleeping houses scattered around the village and 10 sticky traps were placed in natural holes found in trees along the perimeter of each village for three nights per month, per village. Sand flies were removed from sticky traps using soapy water with a fine-haired paint brush or dissecting needle and placed in soapy water to remove the castor oil, rinsed in pure water and transferred to vials. Resting site collections were conducted by three people, each working in one of three rooms within a compound for 20 and 15 minutes at dawn and dusk, respectively, in three compounds on two consecutive days for a monthly total of 70 min per person, per village. All sand flies collected were stored in 70% ethanol until processed.
Seasonality and Infection prevalence of P. duboscqi
To assess the seasonality and infection prevalence of P. duboscqi, light trap and resting site collections were conducted in each village on two consecutive nights per month from July 2006 through June 2008. A total of 25 light traps were placed in and around each village for a total of 100 trap-nights per month. Light traps were placed inside and outside 20 houses within five compounds for a total of four light traps per compound (Figure 1). Additionally, five light traps were placed near trees located outside each village. Resting site collections using mouth aspirators were conducted for 20 mins in two houses in each of three different compounds (six houses total) per village (Figure 1) for a total of four person hours each month (two hours at dawn and two hours at dusk) per village. Phlebotomus sand flies were grouped based on village, collection month, collection technique, location, genera and sex and were stored dry in tubes containing silica desiccant until processed. Phlebotomus females collected from individual light traps or resting sites were further separated into blood fed and non-blood fed pools of no more than 20 sand flies. Sergentomyia species were archived in 70% ethanol.
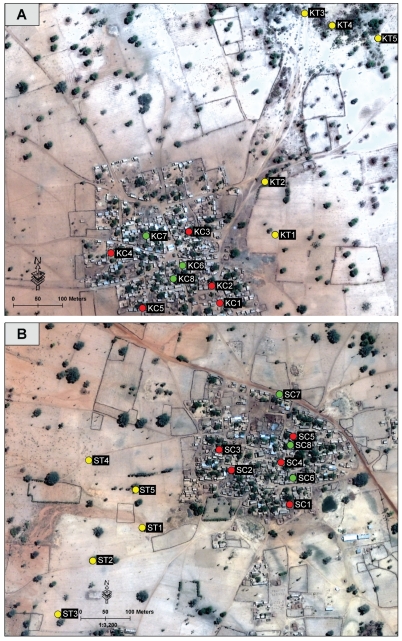
Figure 1. Satellite image of study villages in Central Mali.
Satellite image of the two study villages, Kemena (A) and Sougoula (B) illustrating the sites of monthly sand fly collections (circles) during the study of Phlebotomus duboscqi seasonality, July 2006 to June 2008. Red = compounds of indoor/outdoor house light trap collections; green = compounds of resting site collections; yellow = light trap location near trees. Image collected on May 22, 2006 by the Quickbird Satellite (DigitalGlobe, Inc. Longmont, CO USA).
Species identification
The head and terminal segments of the abdomen containing the genitalia of each sand fly were carefully removed and placed into 96-well plates containing a solution of lacto-phenol clearing solution (Bioquip). After 24 h incubation at room temperature, the head and terminalia were fixed onto a glass slide, examined using a light microscope and identified using dichotomus keys [22].
Detection of Leishmania major
From June 2006-July 2008, the abdomens of blood fed and non-blood fed Phlebotomus females from the same collection location were grouped in pools of no more than 20 individuals and placed in a microfuge tube containing lysis buffer (5.84 g/L NaCl, 68.5 g/L Sucrose, 12.10 g/L Tris, pH 9.1, 100 ml EDTA 0.5 M solution and 50 ml 10% SDS solution). After incubating overnight at 4°C the tissue was macerated using a pestle for 2 min then incubated for 30 min at 65°C. After the addition of 10 µl cold potassium acetate the samples were incubated for 30 min at 4°C and then centrifuged for 10 min at 14,000 RPM. The DNA was precipitated using 70% ethanol and resuspended in 100 µl water. The DNA concentration of each extraction was determined using a NanoDrop (Thermo Scientific Inc., Wilmington, DE). Samples with less than 4 ng/µl of DNA were removed from the sample set. Leishmania DNA was detected by PCR using forward and reverse primers for Leishmania sp. (Uni21/Lmj4) as described in [23]. PCR Primers targeting the sand fly tubulin gene were used as a control for template fidelity (PpTub-P24F 5′-GCG ATG ACT CCT TCA ACA C and PpTub-P24R 5′-TCA GCC AGC TTG CGA ATA C) [24].
A representation of PCR products was confirmed by DNA sequencing. Due to difficulties with direct sequencing of the PCR products using the Uni21 and Lmj4 primers, gel-purified PCR products were cloned into the pCR4-TOPO vector using the TOPO TA Cloning Kit for Sequencing (Invitrogen, Carlsbad CA) following the manufacturer's instructions. The clones were sequenced directly using the M13 forward and M13 reverse primers. Resulting sequences were analyzed using DNASTAR sequence analysis software (DNASTAR, Inc., Madison WI). Sequences were compared to published sequences of kDNA from L. major (Genbank Accession J04654), L. infantum (AF188701), L. tropica (Z32841), and L. donavani (AF167718) using BLAST (http://blast.ncbi.nlm.nih.gov/), aligned to known Leishmania minicircle kinetoplastic DNA using Clustal [25] and edited using BioEdit (http://www.mbio.ncsu.edu/BioEdit/page2.html).
Statistical analysis
To estimate the prevalence of infection in pooled samples of P. duboscqi females, we used the maximum likelihood estimate (MLE) of prevalence accounting for pooling with the confidence interval (CI) estimated by exact methods if the number of unique pool sizes was less than or equal to 3 [26], or otherwise by the skewness-corrected score confidence interval [27]; the estimates and both CIs were calculated using the binGroup R package [28].
To model the sand fly counts or infection rates we used a quasi-Poisson model and tested for significant effects using analysis of deviance and F test [29]. To test for seasonal effects, we tested the overall effect of months after controlling for previous counts and year. In testing for weather effects, we compared models with previous counts, year and months and tested to see if models that additionally added the previous month weather variables (including 4 weather variables at a time; selecting only one [minimum or maximum] of temperature or wild velocity variables) significantly improved the fit. For the models of rates, we estimated the number of infected flies of those tested by the MLE of prevalence and used those counts as responses in the quasi-Poisson model with an offset based on the number of flies tested so that the inferences describe effects on the rates [29]. The quasi-Poisson models were performed using R version 2.12 [30]. Graphs were made using GraphPad Prism 5 (Graphpad Software, California, USA).
Results
Sand fly species diversity
From March 2005 to June 2006, 18,595 sand flies were collected in the two villages (9,887 in Kemena and 8,708 in Sougoula) using all three collection methods. Approximately equal numbers of male and female sand flies were collected (9,221 M, 9,374 F). Sixty-nine percent (n = 12,952) of sand flies were identified as one of 12 species in the genus Sergentomyia, none of which have been implicated in the transmission of L. major (Table 1).
Table 1. Sand fly species diversity in two neighboring villages, Central Mali, March 2005-June 2006.
| Species | Males | Female | Total | Percent Total |
| Phlebotomus | 30.34 | |||
| P. duboscqi | 3028 | 2574 | 5602 | 99.28 |
| P. rodhaini | 15 | 26 | 41 | 0.72 |
| Sergentomyia | 69.66 | |||
| S. schwetzi | 3684 | 2444 | 6128 | 47.31 |
| S. antennata | 1797 | 1627 | 3424 | 26.44 |
| S. dubia | 30 | 1549 | 1579 | 12.19 |
| S. clydei | 453 | 570 | 1023 | 7.90 |
| S. africana | 187 | 228 | 415 | 3.20 |
| S. squamipleuris | 10 | 208 | 218 | 1.68 |
| S. affinis vorax | 10 | 63 | 73 | 0.56 |
| S. bedordi | 3 | 60 | 63 | 0.49 |
| S. fallax | 0 | 3 | 3 | 0.02 |
| S. buxtoni | 0 | 17 | 17 | 0.13 |
| S. darlingi | 3 | 5 | 8 | 0.06 |
| S. christopheri | 1 | 0 | 1 | 0.01 |
| Total | 9221 | 9374 | 18595 | 100% |
Of the Sergentomyia, Sergentomyia schwetzi Adler, Theodor and Parrot represented the majority with 47.3% of collected specimens, while Sergentomyia antennata Newstead was the second most abundant at 26.4%. Ten additional Sergentomyia species were collected: Sergentomyia dubia Parrot, Mornet, and Cadenat (12.2%), Sergentomyia clydei Sinton (7.9%), Sergentomyia africana Newstead (3.2%), Sergentomyia squamipleuris Newstead (1.7%), Sergentomyia affinis vorax Parrot (0.56%), Sergentomyia bedfordi Newstead (0.49%), Sergentomyia fallax Parrot (0.02%), Sergentomyia buxtoni Theodor (0.13%), Sergentomyia darlingi Lewis and Kirk (0.06%), and Sergentomyia christophersi Sinton (0.01%). The remaining 30% of sand flies collected was identified as one of two species of Phlebotomus, the overwhelming majority of which was P. duboscqi (n = 5,643, 99.3%). Only 41 Phlebotomus rodhaini Parrot (0.7%) were collected (Table 1).
Sticky traps and light traps collected sand flies in about equal numbers (n = 8,290 vs. 8,394), yet the majority of Sergentomyia (n = 7,728, 60%) were collected using sticky traps whereas only 10% of Phlebotomus (n = 562) were collected using this method. The majority of Phlebotomus (n = 3,380, 60%) were collected using light traps. Thirty percent of Phlebotomus (n = 1,701) were collected by resting site collection compared to only 1.62% (n = 210) of Sergentomyia. Comparing sticky trap and light trap collections from inside and outside houses,the majority of Phlebotomus (92%, n = 3,641) were collected inside dwellings whereas the majority of Sergentomyia (71%, n = 9,043) were collected outside dwellings.
Seasonal distribution of P. duboscqi
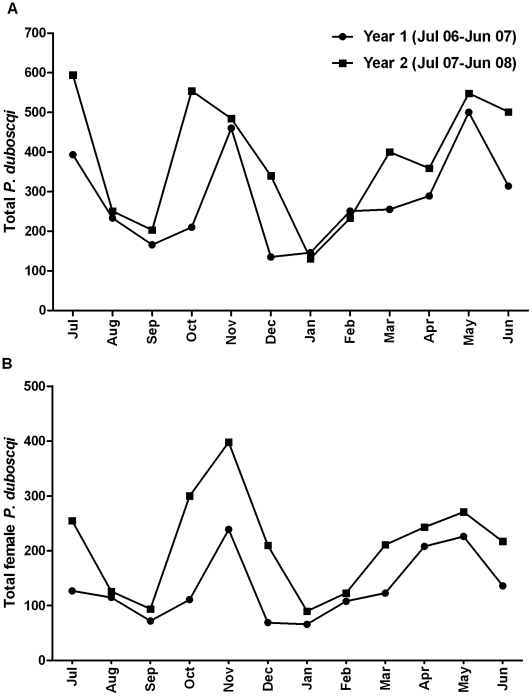
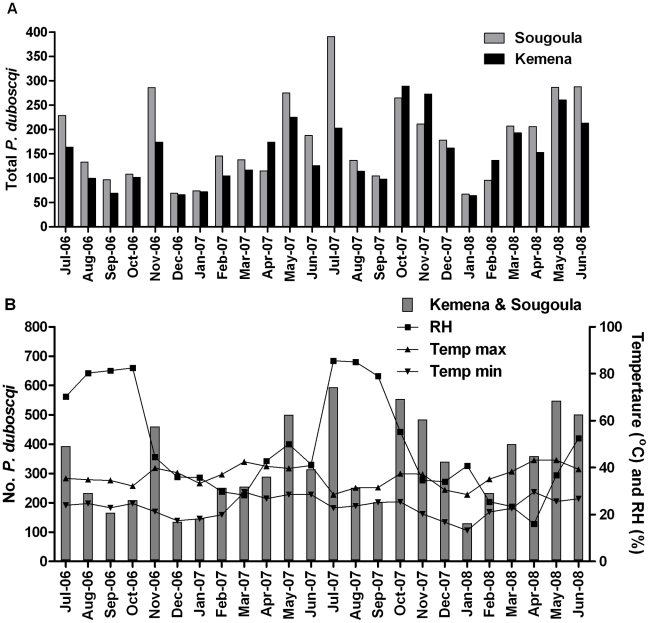
From July 2006 to June 2008, 7,950 P. duboscqi (3,998 female) were collected. Additionally, a total of 25 P. rodhaini were collected during the two years, 17 of which were collected during one month in Kemena (October 2006). Comparing the total number of P. duboscqi collected during year one (July 2006–June 2007) and year 2 (July 2006–June 2008), we found that 1.42 times more sand flies were collected in year 2 (p-value 0.0002, 95% CI: 1.19–1.69) (Figure 2A). We observed a similar effect when comparing the annual collections of female P. duboscqi (p-value 0.0003) (Figure 2B). Using the quasi-Poisson model, controlling for year and previous count, we observed a significant seasonal effect reflecting the month to month variation in the total number of sand flies collected (p-value <0.0001). A similar effect was observed when we considered only female sand flies (P-value <0.0001). Monthly collection trends were similar during both collection years. We modeled sand fly counts for each month using January, the lowest seasonal collection month, as a reference. An initial peak with a 3.9 fold change from January [FCJan] (95% CI: 2.6, 6.1) was observed in May and July (3.8 FCJan, 95% CI 2.4, 6.0). This was followed by a dip in collections in August (2.0 FCJan, 95% CI: 1.2, 3.3) and September (1.3 FCJan, 95% CI: 0.8, 2.2) and a second upward trend peaking in November (3.6 FCJan, 95% CI: 2.4, 5.8) (Figure 2A). By village, we found that 45% (n = 3,654) of all P. duboscqi (male and female) were collected in Kemena and 54% (n = 4,276) in Sougoula. Using the quasi-Poisson model, controlling for previous count, month, and year we observed a significant difference in total P. duboscqi counts between the two villages (p-value 0.0293) with the sand fly counts 1.188 times higher, on average, in Sougoula than in Kemena (95% CI: 1.025,1.377) (Figure 2A). Similar results hold when using only female sand fly counts (fold-change = 1.155, p = 0.1116, Figure 2B). The various weather variables (relative humidity, rainfall amount, maximum or minimum temperature, and maximum and minimum wind velocity) were not useful for predicting the observed total or female sand fly collections for either village (all models had p-value >0.53) (Figure 3B).
Figure 2. Monthly collections of Phlebotomus duboscqi by year in two neighboring villages in Central Mali.
The combined total number of P. duboscqi (A) and female P. duboscqi (B) collected using two collection methods (light trap and resting site collection) during two collection nights per month per village over two consecutive years in the neighboring villages of Kemena and Sougoula in Central Mali.
Figure 3. Monthly collections of Phlebotomus duboscqi by village and climatic conditions in two neighboring villages in Central Mali.
The number of P. duboscqi collected over two consecutive years using two methods (light trap and resting site collection) during two collection nights per month in each of the two neighboring villages of Kemena and Sougoula in Central Mali. (A) Comparison of the monthly collections of female P. duboscqi in Kemena and Sougoula. (B) Correlation of the main climatic conditions with the monthly collections of P. duboscqi from both villages. RH = monthly average relative humidity (%). Temp max = monthly average daily maximum temperature. Temp min = monthly average daily minimum temperature.
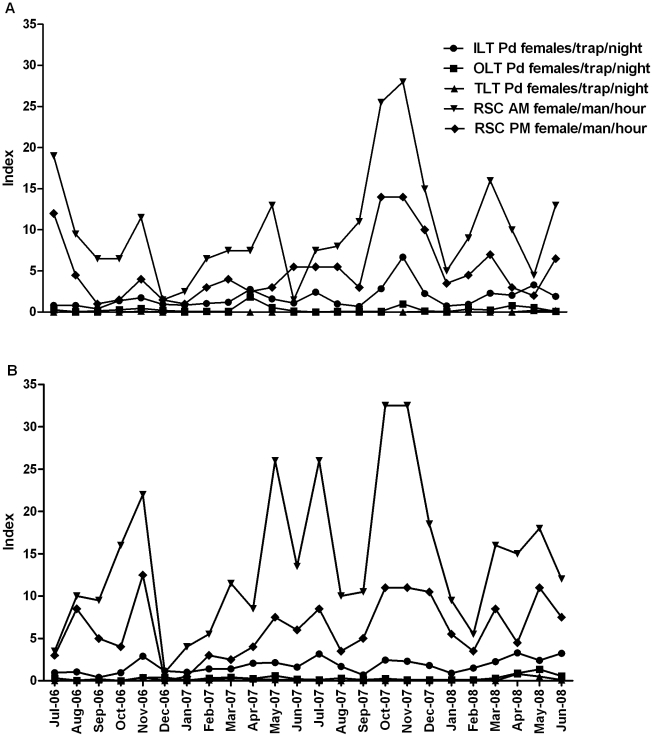
The majority of P. duboscqi was collected by resting site collection, particularly during the morning (10.54 and 14.04 female P. duboscqi/person/hour during morning collections in Kemena and Sougoula, respectively, compared to 5.12 and 6.10 P. duboscqi/person/hour during evening collections) (Figure 4). On average, five times more P. duboscqi were collected using light traps placed inside of dwellings than outside in the same compound (1.74 vs. 0.33 and 1.78 vs. 0.31 P. duboscqi females/trap/night in Kemena and Sougoula, respectively) (Figure 4). Virtually no P. duboscqi were collected in the light traps placed outside of the village near natural tree holes (0.03 and 0.12 P. duboscqi females/trap/night in Kemena and Sougoula, respectively).
Figure 4. Collection success of female Phlebotomus duboscqi using various trapping methods.
Number of female P. duboscqi collected per trapping method per night during monthly collection from July 2006 to June 2008 in two neighboring villages, Kemena (A) and Sougoula (B), in Central Mali: morning resting site collection (RSC AM) = female P. duboscqi (Pd) collected during 2 man hours, evening resting site collection (RSC PM) = female P. duboscqi collected during 2 man hours, tree light traps (TLT) = female P.duboscqi collected by 10 traps, indoor light traps (ILT) = female P duboscqi collected by 20 traps, outdoor light traps (OLT) = female P. duboscqi collected by 20 traps.
Infection of P. duboscqi with L. major
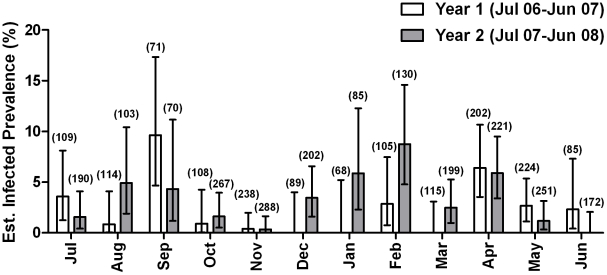
A total of 1434 pools (3706 total flies; average 2.6 flies per pool) were examined for Leishmaina infection by PCR. Ninety-seven pools were positive for L. major (Figure 5). Assuming that the sand flies are independently distributed in the pools and the size of the pools is not related to the probability of infection of the pool, we estimate the prevalence of infection to be 2.66%, 95% CI: 2.20, 3.21 (Table 2). Infected P. duboscqi were found during each month of the year, although monthly infection estimates varied greatly year to year, being the highest during September 2006 (9.64%; CI: 4.68, 17.34) and February 2008 (9.19%; 95% CI: 5.03, 15.27) (Figure 6). After controlling for sand fly count, there was no significant difference in the rates of infection from year 1 to year 2 (p-value 0.2572) and neither was there a significant month to month difference (p = 0.2085). The estimated infection prevalence of sand flies was virtually the same for both villages (2.65%, 95% CI: 1.97, 3.51 and 2.67, 95% CI: 2.02, 3.44 for Kemena and Sougoula, respectively) with no significant difference between the two villages (p-value 0.8894) (Table 2). Of the sand flies collected by light traps and resting site collections within compounds, the majority of infected sand flies were collected using light traps versus resting site collection (4.14% of vs. 0.86%). The highest estimated prevalence of infected sand flies was collected from compound 5 in Kemena (9.42%; 95% CI: 5.50, 14.92) and compound 1 in Sougoula (5.94%; 95% CI: 2.97, 10.57). Comparing the position of the light traps, the estimated infection prevalence of sand flies collected in light traps placed directly outside dwellings was higher than those placed inside dwellings (8.15% vs. 2.07%); no infected sand flies were collected from light traps placed in trees outside of either village (Table 2). The estimated infection prevalence of flies that were non-blood fed at the time of collection was 4.01% versus 1.24% for those that were blood fed.
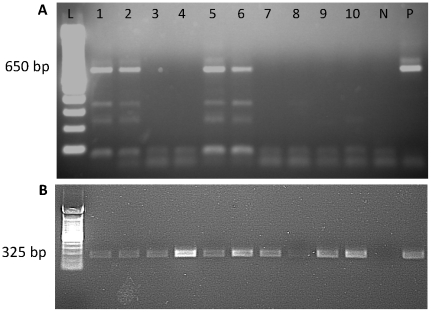
Figure 5. Leishmania specific PCR products from field collected female Phlebotomus duboscqi.
Leishmania specific (A) and tubulin loading control (B) PCR products. N = negative control, P = positive control (DNA from P. duboscqi experimentally infected with L. major). L = 100 bp ladder. Predicted band size of the PCR product for (A) L. major (650 bp) and (B) tubulin (325 bp) is indicated.
Table 2. Estimated infection prevalence of Leishmania major in female Phlebotomus duboscqi from July 2006–June 2008.
| Kemena | Sougoula | Total | |||||||||||||
| No. pools | No. Flies | No. Pools Infected | Estimated Infection Prevalence | 95% Confidence interval | No. pools | No. flies | No. Pools Infected | Estimated Infection Prevalence | 95% Confidence interval | No. pools | No. Flies | No. Pools Infected | Estimated Infection Prevalence | 95% Confidence interval | |
| Total | 674 | 1670 | 44 | 2.65 | 1.97–3.48 | 760 | 2022 | 53 | 2.67 | 2.04–3.44 | 1434 | 3706 | 97 | 2.66 | 2.20–3.21 |
| By collection method | |||||||||||||||
| LT (inside/outside houses) | 487 | 967 | 40 | 4.23 | 4.23–3.09 | 537 | 1076 | 43 | 4.05 | 3.00–5.35 | 1024 | 2043 | 83 | 4.14 | 3.34–5.06 |
| RSC | 185 | 715 | 4 | 0.56 | 0.18–1.33 | 208 | 925 | 10 | 1.10 | 0.57–1.93 | 393 | 1640 | 14 | 0.86 | 0.50–1.41 |
| LT (trees, outside village) | 2 | 2 | 0 | 0.00 | 0–84.18 | 15 | 21 | 0 | 0.00 | 1.00–16.11 | 17 | 23 | 0 | 0.00 | 0.00–14.82 |
| By location of collection | |||||||||||||||
| Interior (LT/RSC) | 551 | 1508 | 28 | 1.87 | 1.28–2.64 | 625 | 1832 | 40 | 2.23 | 1.63–2.98 | 1176 | 3340 | 68 | 2.07 | 1.63–2.59 |
| Exterior (LT outside of houses) | 123 | 176 | 16 | 9.59 | 5.80–14.83 | 135 | 190 | 13 | 6.86 | 3.91–11.10 | 258 | 366 | 29 | 8.15 | 5.65–11.33 |
| By compound | |||||||||||||||
| 1 (LT) | 119 | 282 | 10 | 3.64 | 1.90–6.35 | 93 | 155 | 9 | 5.94 | 2.97–10.57 | − | − | − | − | |
| 2 (LT) | 111 | 215 | 8 | 3.79 | 1.80–7.02 | 106 | 201 | 11 | 5.59 | 3.02–9.44 | − | − | − | − | |
| 3 (LT) | 114 | 232 | 6 | 2.59 | 1.10–5.25 | 135 | 302 | 9 | 2.99 | 1.50–5.36 | − | − | − | − | |
| 4 (LT) | 54 | 85 | 2 | 2.35 | 0.43–7.44 | 109 | 216 | 6 | 2.80 | 1.16–5.68 | − | − | − | − | |
| 5 (LT) | 91 | 155 | 14 | 9.42 | 5.50–14.92 | 109 | 223 | 8 | 3.59 | 1.72–6.61 | − | − | − | − | |
| 6 (RSC) | 58 | 210 | 2 | 0.94 | 0.17–3.00 | 58 | 164 | 3 | 1.81 | 0.49–4.75 | − | − | − | − | |
| 7 (RSC) | 72 | 336 | 2 | 0.59 | 0.11–1.90 | 77 | 399 | 2 | 0.50 | 0.09–1.60 | − | − | − | − | |
| 8 (RSC) | 55 | 169 | 0 | 0.00 | 0–2.12 | 73 | 362 | 5 | 1.45 | 0.55–3.18 | − | − | − | − | |
| By blood meal status | |||||||||||||||
| Bloodfed | 265 | 798 | 11 | 1.38 | 0.74–2.36 | 290 | 999 | 11 | 1.12 | 0.60–1.93 | 555 | 1797 | 22 | 1.24 | 0.80–1.83 |
| Non-bloodfed | 409 | 886 | 33 | 3.81 | 2.69–5.23 | 470 | 1023 | 42 | 4.17 | 3.08–5.52 | 879 | 1909 | 75 | 4.01 | 3.20–4.95 |
Figure 6. Estimated prevalence of infected sand flies collected from Kemena and Sougoula, two neighboring villages in Central Mali.
Numbers in parenthesis represent the overall number of female P. duboscqi specimens tested in pools of 1–20 flies.
Eight representative PCR products from infected wild caught P. duboscqi were sequenced using primers specific to the kinetoplast minicircle DNA of L. major. Sequence analysis confirmed that all the samples were similar to published L. major sequences based on length of the product and primer region identity. Blast analysis indicated a best match to L. major kinetoplast DNA (Genbank Accession number Z32842.1, E-value 9e-48). Further alignment of the sequences obtained from this study with known Leishmania sequences of other species in Genbank confirmed that the 650 bp fragment size, observed on gel electrophoresis of the PCR products, as characteristic of L. major strains.
Discussion
Killick-Kendrick [31] suggested the following criteria for incrimination of a vector sand fly: proven anthropophilic behavior and isolation and identification from the sand fly of the same species of Leishmania that infects man. Further evidence such as the demonstration that the sand fly feeds on the reservoir host (if known), concordance between the geographic distribution of the suspected sand fly and human disease, proof that the parasite develops in the fly and experimental transmission of the parasite by the bite of the fly can reinforce the incrimination. Based on monthly collections of sand flies over three years in two villages in central Mali, where CL is known to be endemic, we have demonstrated that P. duboscqi is the predominant Phlebotomus species; that it persists throughout the year; that females are primarily collected inside houses in both villages;and that it has an overall infection rate with L. major of 2.66% as demonstrated by PCR. This strongly points to P. duboscqi as the primary vector of L. major in Mali.
Species of the sub-genus Sergentomyia constitute the majority of sand flies collected in both villages with S. schwetzi being the most abundant. Members of the Sergentomyia genus are known to transmit Sauroleishmania among lizards. Sergentomyia schwetzi is the only Sergentomyia species known to be anthropophilic and was considered a possible vector by Parrot [5] in 1943. Later, Lawyer [32] concluded that despite the anthropophilic behavior of S. schwetzi, it was not a vector of Leishmania in humans. In this study, only 1.6% of all Sergentomyia sand flies were found during resting site collections and the overall majority (70%) was collected outside of dwellings, further supporting the exophilic nature of this sub-genus and the improbability that Sergentomyia sand flies are involved in transmission to humans in our two villages.
Two Phlebotomus species were found during the three collection years, P. duboscqi and P. rodhaini, both of which are known vectors of L. major elsewhere in West Africa. While fewer in number than Sergentomyia species, P. duboscqi was predominantly collected by resting site collection from sleeping dwellings and five times more P. duboscqi females were collected in light traps placed inside than outside of dwellings, supporting the anthropophilic nature of this fly. P. duboscqi was collected in similar numbers in both villages year round with two seasonal peaks, May-July and October-November. These results are consistent with Lariviere [11] who reported on the seasonality of 191 P. duboscqi collected in Mali. The collection of P. duboscqi throughout the year is probably the result of having constant monthly temperatures and a relative humidity that does not drop beyond 18%. However, non of the specific weather parameters tested could be significantly correlated with sand fly collections in either village (Figure 3). Few specimens of P. rodhaini were collected throughout the study period indicating that this species probably does not play a role, or plays a minor role, in the transmission of L. major in Central Mali.
To further incriminate P. duboscqi as the vector of Leishmania in our study villages, we tested 3706 specimens (in 1434 pools) for the presence of Leishmania DNA by PCR. We found that 97 of the pools tested positive for an overall estimated sand fly infection prevalence of 2.66%. None of the infected pools contained P. rodhaini. Since the infection rate in wild-caught sand flies is usually low [31], PCR was used to permit the efficient screening of a large number of specimens. Having established the infection rate of P. duboscqi in this region, we plan to isolate a viable culture of L. major, necessary to type the strain using traditional methods such as isoenzyme analysis. It is worth noting that in 2006 we established a colony of P. duboscqi collected from our two study villages. Subsequently, females from this colony were used successfully to transmit L. major to an animal model of CL [33] further supporting the status of this species as a competent vector of CL in Central Mali.
A recent study by our group [19] found that there is an unexplained discrepancy between the prevalence of leishmanin skin test (LST) positivity in our two study villages, Kemena (45% LST positive) and Sougoula (20% LST positive), despite the fact that the villages are geographically and demographically similar and are only 5 km apart. Furthermore, this discrepancy was consistent over two consecutive annual incidences (18% and 17% in Kemena vs. 5.7% in both years in Sougoula). We hypothesized that the sand fly density and infection prevalence may explain the dissimilar LST results. The two year seasonality study revealed that slightly more female P. duboscqi were collected in Sougoula than in Kemena, yet almost the same percentage of pools were infected in each village (2.67% vs. 2.66%, respectively), thus neither abundance nor infection prevalence can explain the disparate LST rates observed in the two villages [19].
Rodent species are well known reservoirs for L. major throughout its distribution range. The contribution of reservoirs to the observed disparity of LST positivity in the two villages remains to be evaluated. In West Africa, including Senegal where P. duboscqi has been incriminated as the vector of L. major, infected Mastomys erythroleucus, Tatera gambiana and Arvicanthis niloticus have been reported [34], [35], [36], [37]. All three species are found in Mali (T. Schwan, personal communications) and represent potential reservoirs of L. major in Kemena and Sougoula. Indeed, rodent burrows were observed in many of the houses where light traps were placed. Apart from the potential role of these rodents as reservoirs, their burrows also represent suitable sand fly breeding sites and a source of infected flies. Furthermore, all compounds in our study villages contain goats and chickens living in close proximity to houses used for sleeping which also represent good sand fly breeding sites for uninfected flies. A comprehensive study of the rodent population density and infection prevalence in the two villages is needed to fully understand the infection dynamics in both flies and people.
In summary, we have established, for the first time, the diversity of sand flies in two villages endemic for L. major in Central Mali and demonstrated by PCR that P. duboscqi is the primary vector. This work represents the most comprehensive analysis of P. duboscqi, to date, in Mali and further supports the endemic nature of CL in Central Mali. Further investigations of this nature are needed in West Africa.
Acknowledgments
The authors would like to thank Dr. Ryan Jochim for assistance with PCR modifications and optimization, Dr. Fabiano Oliveira for assistance with data collection and analysis, Dr. Cyril Buhler for assistance with the molecular assays in Mali, and Dr. Zaria Tatalovich and Mahamadou Toure for mapping our study sites on the satellite images and the use of ArcView imagery software. We also thank the health district authorities and the communities of Sougoula and Kemena for their support of this study.
Footnotes
The authors have declared that no competing interests exist.
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Desjeux P, Waroquy L, Dedet JP. [Human cutaneous leishmaniasis in western Africa]. Bull Soc Pathol Exot Filiales. 1981;74:414–425. [PubMed] [Google Scholar]
- 2.Lewis DJ. A taxonomic review of the genus Phlebotomus (Diptera: Psychodidae). Bulletin of the British Museum of Natural History. 1982;45:121–207. [Google Scholar]
- 3.Dedet JP, Desjeux P, Derouin F. Ecology of a focus of cutaneous leishmaniasis in the area of Thies, Senegal, West Africa. 4. Spontaneous infestation and biology of Phlebotomus duboscqi Neveu-Lemaire 1906. Bull Soc Pathol Exot Filiales. 1980;73:266–276. [PubMed] [Google Scholar]
- 4.Asimeng EJ. The distribution of Phlebotomus duboscqi with reference to the known foci of cutaneous leishmaniasis in northern Nigeria. Insect Science and its Applications. 1985;6:27–31. [Google Scholar]
- 5.Parrot L, Gougis R. Sur l'agent probable de transission du Bouton d'Orient deans la Colonie du Niger. Arch Inst Pasteur Algerie. 1943;21:268–269. [Google Scholar]
- 6.Abonnenc E. Les phlébotomes de la région éthiopienne (Diptera: Psychodidae). Mem ORSTOM. 1972;55:1–289. [Google Scholar]
- 7.Desjeux P, Bryan JH, Martin-Saxton P. Leishmaniasis in The Gambia. 2. A study of possible vectors and animal reservoirs, with the first report of a case of canine leishmaniasis in The Gambia. Trans R Soc Trop Med Hyg. 1983;77:143–148. doi: 10.1016/0035-9203(83)90052-4. [DOI] [PubMed] [Google Scholar]
- 8.Boakye D, Wilson M, Kweku M. A Review of Leishmaniasis in West Africa. Ghana Med J. 2005;39:94–97. [PMC free article] [PubMed] [Google Scholar]
- 9.Rageau J. Phlebotomus species in the Cameroons. Bull Soc Pathol Exot Filiales. 1951;44:793–800. [PubMed] [Google Scholar]
- 10.Kervran L. Description de quelques especes de Phlebotomes du Soudan Francais. Ann Parasitol Hum Comp. 1946;21:155–165. [Google Scholar]
- 11.Lariviere M, Abonnenc E, Kramer R. [Chronicle of cutaneous leishmaniasis in West Africa. The problem of the vector.]. Bull Soc Pathol Exot Filiales. 1961;54:1031–1046. [PubMed] [Google Scholar]
- 12.Kato H, Anderson J, Kamhawi S, Oliveira F, Lawyer P, et al. High degree of conservancy among secreted salivary gland proteins from two geographically distant Phlebotomus duboscqi sandflies populations (Mali and Kenya). BMC Genomics. 2006;4:226. doi: 10.1186/1471-2164-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neveu-Lemaire M. Sur un nouveau nematocere africain appartenant au genre Phlebotomus. Bull Soc Zool de Fr. 1906;20:64–67. [Google Scholar]
- 14.Roubaud E. Quelques notes sur les Phlebotomes de l'Afrique Occidentale Francaise. Bull Soc Pathol Exot. 1913;6:126–128. [Google Scholar]
- 15.Imperato PJ, Diakite S. Leishmaniasis in the Republic of Mali. Trans R Soc Trop Med Hyg. 1969;63:236–241. doi: 10.1016/0035-9203(69)90152-7. [DOI] [PubMed] [Google Scholar]
- 16.Lefrou G. La Leishmaniose cutanee au Soudan Francais. Frequence de la forme seche papulo-tuberculeuse. Bull Soc Pathol Exot. 1948;41:622–627. [Google Scholar]
- 17.Imperato PJ, Fofana B, Sow O, Diallo S. Leishmanin skin sensitivity in the inland delta of the Niger. Trop Geogr Med. 1974;26:303–306. [PubMed] [Google Scholar]
- 18.Imperato PJ, Coulibaly B, Togola T. Leishmanin skin sensitivity in northwestern Mali. Acta Trop. 1970;27:260–265. [PubMed] [Google Scholar]
- 19.Oliveira F, Doumbia S, Anderson JM, Faye O, Diarra SS, et al. Discrepant prevalence and incidence of Leishmania infection between two neighboring villages in Central Mali based on Leishmanin skin test surveys. PLoS Negl Trop Dis. 2009;3:e565. doi: 10.1371/journal.pntd.0000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garin JP, Peyramond D, Piens MA, Rioux JA, Godfrey DG, et al. [Presence of Leishmania major Yakimoff and Schokhor, 1914 in Mali. Enzymatic identification of a strain of human origin]. Ann Parasitol Hum Comp. 1985;60:93–94. doi: 10.1051/parasite/198560193. [DOI] [PubMed] [Google Scholar]
- 21.Izri MA, Doumbo O, Belazzoug S, Pratlong F. [Presence of Leishmania major MON-26 in Mali]. Ann Parasitol Hum Comp. 1989;64:510–511. doi: 10.1051/parasite/1989646510. [DOI] [PubMed] [Google Scholar]
- 22.Abonnenc E. A taxonomic reivew of the genus (Phlebotomus (Diptera: Psychodidae). Bulletin of the British Museum of Natural History. 1972;45:121–209. [Google Scholar]
- 23.Anders G, Eisenberger CL, Jonas F, Greenblatt CL. Distinguishing Leishmania tropica and Leishmania major in the Middle East using the polymerase chain reaction with kinetoplast DNA-specific primers. Trans R Soc Trop Med Hyg. 2002;96(Suppl 1):S87–92. doi: 10.1016/s0035-9203(02)90057-x. [DOI] [PubMed] [Google Scholar]
- 24.Ramalho-Ortigao J, Kamhawi S, Joshi M, Reynoso D, Lawyer P, et al. Characterization of a blood activated chitinolytic system in the midgut of the sand fly vectors Lutzomyia longipalpis and Phlebotomus papatasi. Insect Molecular Biology. 2005;14:703–712. doi: 10.1111/j.1365-2583.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- 25.Thompson JD, Gibson TJ, Higgins DG. Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinformatics Chapter 2: Unit 2. 2002;3 doi: 10.1002/0471250953.bi0203s00. [DOI] [PubMed] [Google Scholar]
- 26.Hepworth G. Exact confidence intervals for porportions estimated by group testing. Biometrics. 1996;52:1134–1146. [Google Scholar]
- 27.Hepworth G. Confidence intervals for proportions estimates by group testing with groups of unequal size. Journal of Agricultural, Biological, and Environmental Statistics. 2005;10:478–497. [Google Scholar]
- 28.Zhang B, Bilder C, Biggerstaff B, Schaarschmidt F. binGroup: Evaluation and experimental design for binomial group testing. R package version 1.0-7 2010 [Google Scholar]
- 29.Hastie TJ, Pregibon D. Chambers JM, Hastie TJ, editors. Generalized Linear Models. Statistical Models in S. Pacific Grove, CA: Wadsworth and Brooks, Cole Advanced Books and Software. 1992.
- 30.Team RDC. Vienna, Austria: R Foundation for Statistical Computing; 2010. R: A language and environment for statistical computing. [Google Scholar]
- 31.Killick-Kendrick R. Phlebotomine vectors of the leishmaniases: a review. Med Vet Entomol. 1990;4:1–24. doi: 10.1111/j.1365-2915.1990.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 32.Lawyer PG, Ngumbi PM, Anjili CO, Odongo SO, Mebrahtu YB, et al. Development of Leishmania major in Phlebotomus duboscqi and Sergentomyia schwetzi (Diptera: Psychodidae). Am J Trop Med Hyg. 1990;43:31–43. doi: 10.4269/ajtmh.1990.43.31. [DOI] [PubMed] [Google Scholar]
- 33.Peters NC, Kimblin N, Secundino N, Kamhawi S, Lawyer P, et al. Vector Transmission of Leishmania Abrogates Vaccine-Induced Protective Immunity. Plos Pathogens. 2009;5:1–11. doi: 10.1371/journal.ppat.1000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dedet JP, Derouin F, Hubert B, Schnur LF, Chance ML. Isolation of Leishmania major from Mastomys erythroleucus and Tatera gambiana in Senegal (West Africa). Ann Trop Med Parasitol. 1979;73:433–437. [PubMed] [Google Scholar]
- 35.Dedet JP, Hubert B, Desjeux P, Derouin F. [Ecology of a cutaneous leishmaniasis focus in the Thies region (Senegal, West Africa). 5. Spontaneous infection and disease reservoir role of various wild rodent species]. Bull Soc Pathol Exot Filiales. 1981;74:71–77. [PubMed] [Google Scholar]
- 36.Dedet JP, Saf'Janova VM, Desjeux P, Emelyanova LP, Schnur LF, et al. [Ecology of a reservoir of cutaneous leishmaniasis in the region of Thies (Senegal, West Africa). 6. Characterization and types of isolated Leishmania strains]. Bull Soc Pathol Exot Filiales. 1982;75:155–168. [PubMed] [Google Scholar]
- 37.Lariviere M, Camerlynck P, Ranque P, Villod MT. [Arvicanthis sp., possible natural virus reservoir of Leishmania tropica in Senegal]. C R Acad Sci Hebd Seances Acad Sci D. 1965;260:4869–4870. [PubMed] [Google Scholar]