Abstract
The prevalence of tobacco smoking in HIV-1 positive individuals is 3-fold greater than that in the HIV-1 negative population; however, whether HIV-1 viral proteins and nicotine together produce molecular changes in mesolimbic structures that mediate psychomotor behavior has not been studied. This study determined whether HIV-1 viral proteins changed nicotine-induced behavioral sensitization in HIV-1 transgenic (HIV-1Tg) rats. Further, we examined cAMP response element binding protein (CREB) and extracellular regulated kinase (ERK1/2) signaling in the prefrontal cortex (PFC), nucleus accumbens (NAc) and ventral tegmental area (VTA). HIV-1Tg rats exhibited a transient decrease of activity during habituation, but showed attenuated nicotine (0.35 mg/kg, s.c.)-induced behavioral sensitization compared to Fisher 344 (F344) rats. The basal levels of phosphorylated CREB and ERK2 were lower in the PFC of HIV-1Tg rats, but not in the NAc and VTA, relative to the controls. In the nicotine-treated groups, the levels of phosphorylated CREB and ERK2 in the PFC were increased in HIV-1Tg rats, but decreased in F344 animals. Moreover, repeated nicotine administration reduced phosphorylated ERK2 in the VTA of HIV-1Tg rats and in the NAc of F344 rats, but had no effect on phosphorylated CREB, indicating a region-specific change of intracellular signaling. These results demonstrate that HIV-1 viral proteins produce differences in basal and nicotine-induced alterations in CREB and ERK signaling that may contribute to the alteration in psychomotor sensitization. Thus, HIV-1 positive smokers are possibly more vulnerable to alterations in CREB and ERK signaling and this has implications for motivated behavior, including tobacco smoking, in HIV-1 positive individuals who self-administer nicotine.
1. Introduction
Tobacco smoking prevalence among HIV-1 positive population is 3-fold greater than that in HIV-1 negative population, in HIV-1 negative population (Burkhalter et al., 2005; CDC, 2007; Gritz et al., 2004; Nahvi and Cooperman, 2009; Niaura et al., 2000). HIV-1 infected patients are more likely to become dependent on nicotine, and less likely to quit than HIV-1 negative individuals (Fuster et al., 2009; Hershberger et al., 2004; Nahvi and Cooperman, 2009). There is an increasing body of clinical and experimental evidence that tobacco smoking is associated with a more rapid progression to AIDS (Crothers et al., 2005; Furber et al., 2007; Nieman et al., 1993; Zhao et al., 2010) and HIV-1 associated dementia (Burns et al., 1996; Manda et al., 2010). Considering that the HIV-1 positive population exhibits a greater risk for tobacco-associated morbidity and mortality (Palella et al., 2006; Triant et al., 2007), there is a critical need to define the molecular mechanisms underlying the enhanced susceptibility to nicotine dependence in this population.
HIV-1 infection is associated with a variety of neurological impairments that result, in part, from the presence of HIV-1 viral proteins, such as Tat and gp120. Some of the neurological deficits caused by these viral proteins reflect an apparent dysfunction of the mesocorticolimbic dopamine (DA) system (Berger and Arendt, 2000; Koutsilieri et al., 2002; Nath et al., 1987), the motivation pathway of the brain (Berridge, 2007; Everitt and Robbins, 2005; Wise and Bozarth, 1987). Indeed, long-term viral protein exposure can accelerate damage in this DA system (Del Valle et al., 2000; Ferris et al., 2008; Hudson et al., 2010; Nath, 2010). For example, a significant reduction in DA transporter (DAT) density in striatum was observed in HIV-1 positive patients (Chang et al., 2008; Wang et al., 2004), and in vitro Tat or gp120 decreased the specific [3H]DA uptake in rat striatum (Wallace et al., 2006; Zhu et al., 2009). Importantly, the use of addictive drugs by HIV-1 positive individuals results in greater neurological impairments relative to individuals who are infected with HIV-1 but do not abuse drugs (Del Valle et al., 2000; Ferris et al., 2008; Hudson et al., 2010; Nath, 2010). Furthermore, the mesocorticolimbic DA pathway is compromised in HIV-1 positive individuals that exhibit co-morbid drug abuse (Kumar et al., 2009; Norman et al., 2009; Obermann et al., 2009). The extent to which HIV-1 related viral proteins and drugs of abuse alter motivation in humans is not well understood.
Our laboratory uses a rodent model to investigate the neurobehavioral and neurochemical changes induced by the combination of HIV-1 viral proteins and abused drugs. Several approaches are utilized to study these viral proteins because experimental rodents cannot be infected with HIV-1: 1) in vitro exposure to Tat (Zhu et al., 2009), 2) direct microinjection of Tat into rat brain (Fitting et al., 2008; Harrod et al., 2008), 3) transgenic mice that express Tat protein (Duncan et al., 2008; Kim et al., 2003), and 4) HIV-1 transgenic (HIV-1Tg) rats, which express HIV-1 viral proteins (Reid et al., 2001). These models mimic different aspects of viral protein-induced neurotoxicity, although none of these models fully represent the spectrum of HIV-1 viral protein insult in humans (Nath, 2010). We are using the HIV-1Tg model in combination with basic behaviors that are mediated by the mesocorticolimbic system. This pathway organizes motivated behaviors that range various levels of complexity. For example, psychomotor behavior, such as locomotor/exploratory activity, represents the integration of sensory and motor information (Berridge, 2007; Wise and Bozarth, 1987), whereas drug maintained responding is a combination of Pavlovian and operant conditioning processes (Everitt and Robbins, 2005; Rescorla, 1991; Robinson and Berridge, 2003), and is a relatively more complex form of motivated behavior. Insult to this pathway produces significant changes in both types of responding (Corrigall et al., 1992; Fink and Smith, 1980; Joyce and Koob, 1981; Kelly and Iversen, 1976; Koob et al., 1981; Kubos et al., 1987; Roberts et al., 1977).
Nicotine activates nicotinic acetylcholine receptors (nAChRs) located throughout the mesocorticolimbic DA system, specifically in the prefrontal cortex (PFC), nucleus accumbens (NAc) and ventral tegmental area (VTA) (Kita et al., 1992; Laviolette and van der Kooy, 2004; Mansvelder et al., 2002; Panagis and Spyraki, 1996). Nicotine increases DA levels in the NAc (Nisell et al., 1994a, b), and repeated nicotine treatment induces a progressive increase in psychomotor behavior, which represents the initiation of behavioral sensitization (Clarke and Kumar, 1983a, b; Kalivas, 1995; Post, 1980). Accordingly, the locomotor stimulant properties of nicotine are blocked by lesion of mesolimbic DA neurons (Louis and Clarke, 1998) or by nicotinic receptor antagonists (Clarke and Kumar, 1983a; Corrigall et al., 1994). The behavioral sensitization procedure is sensitive to behavioral changes produced by the psychostimulant effects of abused drugs, however, it is not a measure of drug reward (Berridge and Robinson, 1998; Robinson and Berridge, 1993; Wise and Bozarth, 1987). This procedure was used in the present experiment to determine whether HIV-1 Tg rats exhibited deficits in locomotor sensitization to nicotine. Furthermore, HIV-1 viral proteins alter dopaminergic pathways that mediate behavioral sensitization. For example, intra-accumbal or striatal Tat rats show decreased DAT activity in striatum (Maragos et al., 2002), decreased DA levels (Cass et al., 2003), and attenuated behavioral sensitization to cocaine (Harrod et al., 2008). Moreover, HIV-1Tg rats show enhanced behavioral sensitization to methamphetamine (Kass et al., 2010; Liu et al., 2009). However, whether the combination of nicotine and HIV-1 viral proteins alters psychomotor behavior has not been investigated.
The extracellular regulated protein kinase (ERK) and its downstream transcriptional signaling protein, the cyclic AMP response element binding protein (CREB), appear critical for long-term adaptations in individuals who exhibit drug abuse (Berhow et al., 1996; Carlezon et al., 1998; Girault et al., 2007; Nestler, 2001). ERK is one of the mitogen-activated protein kinases involved in numerous cellular processes, including long-term neuronal plasticity and survival (Hetman and Gozdz, 2004; Subramaniam and Unsicker, 2010). Abundant evidence suggests that ERK is an essential component of the signaling pathways involved in synaptic plasticity and the long-term effects of abused drugs (Berhow et al., 1996; Girault et al., 2007; Lu et al., 2009; Valjent et al., 2006). Two major isoforms of ERK, ERK1 and ERK2, are very similar in sequence (Yoon and Seger, 2006), but have distinct functions (Lloyd, 2006; Lu et al., 2009). It has implicated that ERK2 is more strikingly changed than ERK1 in the long-term effects of drugs of abuse (Girault et al., 2007; Iniguez et al., 2010; Valjent et al., 2005). Moreover, acute nicotine treatment increases CREB phosphorylation in the NAc, striatum and VTA (Jackson et al., 2009; Walters et al., 2005). Chronic nicotine exposure in mice decreases CREB phosphorylation in the NAc, whereas nicotine withdrawal increases CREB phosphorylation in the VTA (Brunzell et al., 2003). In addition, the levels of CREB and phosphorylated CREB are decreased in the cortex and amygdala after withdrawal from repeated nicotine administration (Pandey et al., 2001). Thus, long-term nicotine exposure leads to neural adaptations in intracellular signaling through the changes of ERK and CREB signaling (Brunzell et al., 2009; Brunzell et al., 2003; Mineur et al., 2009). To date, the effects of HIV-1 viral proteins on ERK and CREB signaling are unknown.
It was hypothesized that the combination of HIV-1 viral proteins and nicotine would alter nicotine-induced behavioral sensitization, and would also produce changes in the expression of intracellular signaling proteins. To test these hypotheses, HIV-1Tg rats and Fischer 344/NHsd (F344) non-transgenic, wild-type control rats were used to determine if genetically expressed HIV-1 viral proteins produce altered nicotine-induced behavioral sensitization. To investigate a potential mechanism, the modulation of ERK and CREB signaling following repeated nicotine exposure was determined in the PFC, NAc and VTA regions of the mesocorticolimbic DA system.
2. Methods
2.1. Subjects
Male HIV-1Tg Fisher 344/NHsd rats and age-matched male nontransgenic Fisher 344/NHsd rats were obtained from Harlan Laboratories, Inc. (Indianapolis, IN). The HIV-1Tg rat model carries a gag-pol-deleted HIV-1 provirus regulated by the viral promoter expressing seven of the nine HIV-1 viral proteins (Reid et al., 2001). Since the HIV-1Tg rat model is developed from F344 strain, F344 rats were used as the control animals. Rats at age of 7–9 weeks arrived in the animal care facilities and were pair housed throughout the experiment. Rodent food (ProLab Rat/Mouse/Hamster Chow 3000) and water were provided ad libitum. The colony was maintained at 21 ± 2 °C, 50 ± 10% relative humidity and a 12L:12D cycle with lights on at 0700 h (EST). The animals were weighed daily. The animals were maintained according to the National Institute of Health (NIH) guidelines in AAALAC accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of South Carolina.
2.2. Drugs
Nicotine hydrogen tartrate salt was purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in sterile saline (0.9% sodium chloride). Nicotine was prepared immediately prior to injection. The nicotine solution was neutralized to pH 7.0 with NaHCO3. Nicotine (0.35 mg/kg, freebase) was administered subcutaneously (s.c.) in a volume of 1 ml/kg once daily for 20 days.
2.3. Locomotor activity procedure
2.3.1. Behavioral apparatus
The activity monitors were square (40 × 40 cm) locomotor activity chambers (Hamilton-Kinder Inc., Poway, CA) that detect free movement of animals by infrared photocell interruptions. This equipment uses an infrared photocell grid (32 emitter/detector pairs) to measure locomotor activity. The chambers were converted into round (~ 40 cm diameter) compartments by adding clear Plexiglas inserts; photocell emitter/detector pairs were tuned by the manufacturer to handle the extra perspex width. Total horizontal activity represents all beam breaks in the horizontal plane. All activity monitors were located in an isolated room.
2.3.2. Habituation
Rats in the HIV-1Tg-Saline (HIV1Tg-Sal; n = 8/group), HIV-1Tg-Nicotine (HIV-1Tg-Nic; n = 8/group), F344-Saline (F344-Sal; n = 8/group) and the F344-Nicotine (F344-Nic; n = 8/group) groups were habituated to the locomotor activity chambers for two 60-min sessions, once/day. No injections were administered on the habituation days. Twenty four hours after the second habituation session, all rats were habituated to the chambers for 30 min prior to injection, and then injected (s.c.) with saline and placed into the activity chambers for 60-min to measure baseline activity.
2.3.3. Pre-injection habituation and nicotine-induced behavioral sensitization
The behavioral sensitization procedure began 24 hours after the saline baseline measurement. First, all rats received a 30-minute habituation period in the testing chamber prior to nicotine (0.35 mg/kg) or saline injection as previously reported (Addy et al., 2007). This was done so that the onset of nicotine’s effects did not overlap with the period that rats showed the most exploratory behavior in the chamber, which was during the first 15 min. Previous research indicates that control rats exhibit asymptotic levels of within-session habituation by 20 to 30 min, according to similar procedures and use of the same automated chambers (Harrod et al., 2008; Harrod and Van Horn, 2009). Rats were administered nicotine or saline subcutaneously every day for a total of 20 days. Locomotor activity was assessed every other day, i.e., on days 1, 3, 5, 7, 9, 11, 13, 15, 17, and 19, for 60 min. On alternate days, rats were administered nicotine or saline in the home cage.
2.4. Western blot analysis
Following completion of the behavioral study, brains were removed by rapid decapitation 4 hours after the last injection on day 20. Brains were placed in ice-cold PBS and dissected in a chilled matrix. PFC, NAc and VTA were dissected and immediately sonicated on ice in a homogenization buffer containing 20 mM HEPES, 0.5 mM EDTA, 0.1 mM EGTA, 0.4 M NaCI, 5 mM MgCI2, 20% glycerol, 1 mM PMSF, phosphatase inhibitor cocktails I (Sigma, P2850) and protease inhibitors (Sigma, P8340). Samples were centrifuged at 12000 g for 15 min. The supernatant was stored at −80°C. Protein concentrations were determined in duplicate using Bio-Rad DC protein detection reagent. Proteins (30, 10 or 15 μg per sample in the PFC, NAc or VTA) were loaded for ERK, phosphorylated ERK (pERK), CREB, phosphorylated CREB (pCREB) and Tyrosine Hydroxylase (TH) immunoreactivity.
Proteins were separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) for 60 min at 150 V, and subsequently transferred to Immobilon-P transfer membranes (IPVH00010, 0.45 μm pore size; Millipore Co., Bedford, MA) in transfer buffer (50 mM Tris, 250 mM glycine, 3.5 mM SDS) using a Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad, Hercules, CA) for 110 min at 72 V. Transfer membranes were incubated with blocking buffer (5% dry milk powder in PBS containing 0.5% Tween 20) for 1 h at room temperature followed by incubation with primary antibodies diluted in blocking buffer overnight at 4 °C. Antisera against ERK½ (V114A, Promega, Madison, WI) and pERK½ (SC-16982R, Santa cruz biotechnology, inc, Santa Cruz, CA) were used at a dilution of 1:2000 and 1:1000, respectively. Anti-CREB (9104, Cell signaling, Danvers, MA) and anti-pCREB (9196L, Cell signaling, Danvers, MA) antibodies were used at a dilution of 1:1000 and 1:500, respectively. Anti-TH (2792) was diluted 1:2000 (Cell signaling, Danvers, MA). Blots were washed 5 min × 5 times with wash buffer (PBS containing 0.5% Tween 20) at room temperature, and then incubated for 1 h in affinity-purified, peroxidase-labeled, anti-rabbit IgG (1:10000 for ERK½, 1:5000 for pERK½, 1:20000 for TH, Jackson ImmunoResearch, West Grove, PA) and 1:2000 anti-mouse IgG (7076, Bio-Rad, Hercules, CA) in blocking buffer for 1 h at room temperature. Blots on the transfer membranes were detected using enhanced chemiluminescence and developed on Hyperfilm (ECL-plus; Amersham Biosciences UK Ltd., Little Chalfont Buckinghamshire UK). After detection and quantification of these proteins, each blot was stripped in 10% of Re-blot plus mild antibody stripping solution (CHEMICON, Temecula, CA) for 20 min at room temperature and reprobed for detection of β-tubulin (sc-9104, Santa cruz biotechnology, inc, Santa Cruz, CA). β-tubulin was used to monitor protein loading among samples. Multiple autoradiographs were obtained using different exposure times, and immunoreactive bands within the linear range of detection were quantified by densitometric scanning using Scion image software (Scion Corp., Frederick, MD).
2.5. Data analyses
The data are presented as mean values ± standard error of the mean (S.E.M.). In order to analyze the effects of nicotine exposure on body weight gain, the body weights of the rats were expressed as a percentage of the body weights on the day prior to nicotine injection. The effect of nicotine administration on body weight gain was analyzed with a (2 × 2 × 20) mixed factorial analysis of variance (ANOVA), with genotype (HIV1-Tg or F344) and treatment (nicotine or saline) as the between-subjects factors, and day as the within-subjects factor. A genotype × day × time (2 × 2 × 12) mixed factorial ANOVA was used to analyze data from the 2 habituation days, and a genotype × time (2 × 12) factorial ANOVA was conducted on the saline baseline day. The pre-injection habituation part of the experiment was analyzed using a genotype × treatment × day × time (2 × 2 × 10 × 12) ANOVA. The effect of repeated nicotine injection on total horizontal activity was analyzed using a genotype × treatment × day × time (2 × 2 × 10 × 12) factorial ANOVA, with genotype and treatment as between-subjects factors, and day and time as within-subjects factors. To determine the effects of repeated nicotine administration on the activity of signaling proteins (ERK, CREB and TH), separate genotype × treatment (2 × 2) factorial ANOVAs were performed on the data from the PFC, NAc, and VTA. Simple effect comparisons were made for post hoc analyses. All statistical analyses were performed using SPSS (standard version 18.0, Chicago, IL) and differences were considered significant at p < 0.05.
3. Results
3.1. Effect of nicotine on body weight
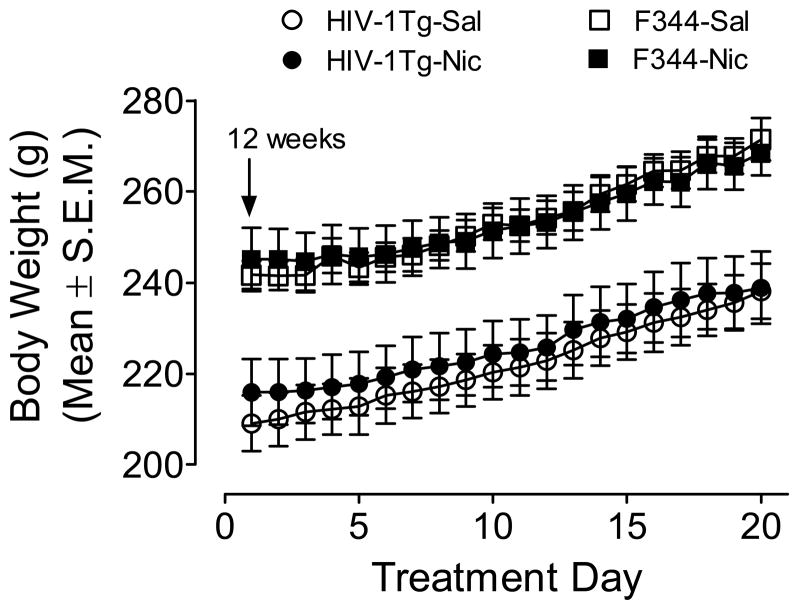
Daily body weights were analyzed using a genotype × treatment × day ANOVA. There were significant main effects of genotype (F(1,28) = 25.71; p < 0.001) and day (F(19,532) = 384.28; p < 0.001), indicating that HIV-1Tg rats weighed less than F344 controls, and that all animals gained weight over days. Body weight gain across days was not different between HIV-1Tg rats and F344 rats (F(19,532) = 1.24; p = 0.22): these groups showed 11 and 12% increase in weight gain from day 1 to day 20, respectively (Figure 1).
Figure 1.
Body weight of HIV-1Tg and F344 rats during the nicotine or saline treatment period. Beginning at 12 weeks of age, rats were injected subcutaneously with nicotine or saline prior to locomotor measurement. Data are presented as the mean ± S.E.M. n=8 rats per group.
3.2. Habituation, pre-injection habituation, and nicotine-induced locomotor activity in HIV-1Tg and F344 rats
3.2.1. Habituation and Saline Baseline
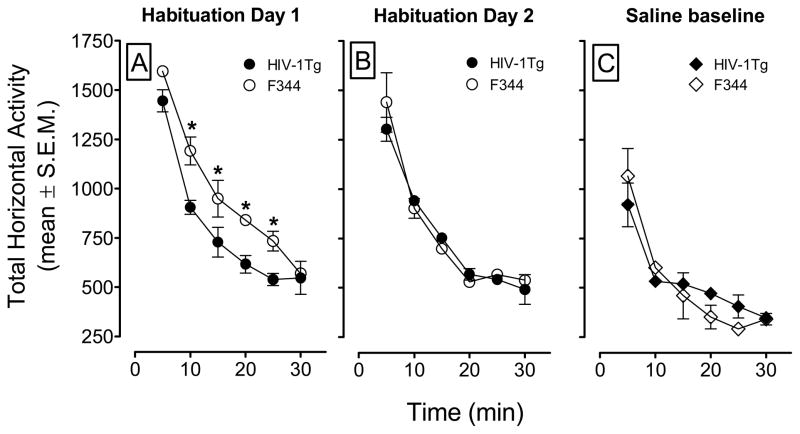
Animals were habituated to the chambers for two days, 60 min per day. The total horizontal activity that occurred during the two habituation days is shown on Figures 2A and 2B. A genotype × day × time ANOVA (2 × 2 × 12) revealed main effects of day (F(1, 30) = 55.95, p < 0.001) and time (F(11, 330) = 147.6, p < 0.001), and a significant genotype × day × time interaction (F(11, 330) = 2.14, p < 0.05). Both genotypes showed the most activity at the beginning of the habituation session, and the activity decreased over the 30-min period, and both groups of rats were at asymptote for the remaining 30 min of the session. HIV-1Tg rats exhibited less locomotor activity than did F344 rats in the first 30 min of the first habituation session (p < 0.01 Bonferroni t-test), and this is observed as a downward, and leftward shift in the habituation curve (Figure 2B). No significant differences in total horizontal activity during second habituation session were detected (Fig. 2B). On the third day, total horizontal activity was recorded for all groups after a saline injection to determine baseline activity prior to the induction of sensitization phase of the experiment. The genotype × time ANOVA revealed a main effect of time (F(11, 330) = 41.77, p < 0.001), and a genotype × time interaction (F(11, 330) = 2.41, p < 0.05). No main effect of genotype was found. In general, both genotypes showed lower activity during the first 5 min of the saline baseline day, acquired asymptotic levels of activity more quickly, and showed a lower asymptote compared to that of the habituation sessions (Fig. 2C). Within the first 30 min period, the F344 habituation curve crossed and slightly went below that of the HIV-1Tg curve. The habituation curve crossed again at minute 30, and this was observed again at the end of the session within the last 30 min (data not shown). None of the comparisons indicated differences between the HIV-1Tg and F344 rats (all p >.05).
Figure 2.
The time-course data during the habituation and the saline baseline sessions. Panels A and B show the total horizontal activity (mean ± S.E.M.) during the first 30 min of the habituation period. Panel C shows the total horizontal activity (mean ± S.E.M.) across the first 30 min of the session following saline injection. * p < 0.05, difference between HIV-1Tg and F344 rats at the corresponding time interval. n=8 rats per group.
3.2.2. Pre-injection habituation
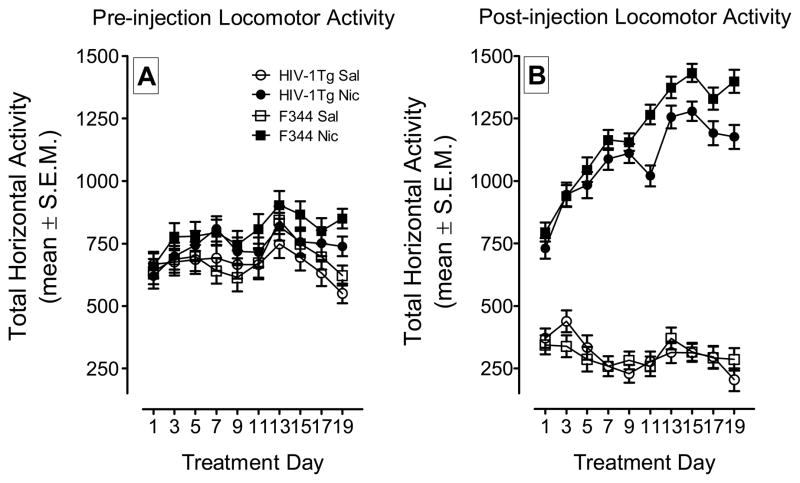
Animals were placed into locomotor chambers for 30 min prior to the activity measurement to produce within-session habituation of activity prior to nicotine or saline injection. Total horizontal activity during the 30 min habituation period across the 19-day treatment was recorded and is shown in Figure 3A. A mixed-factor genotype × treatment × day × time ANOVA (2 × 2 × 10 × 12) revealed main effects of treatment (F(1, 28) = 4.76, p < 0.05), day (F(9, 252) = 9.15, p < 0.05), time (F(5, 140) = 705.56, p < 0.05) and a significant day × treatment interaction (F(9, 252) = 2.74, p < 0.01). There was no main effect of genotype and there were no significant interactions containing this factor. The treatment × day interaction indicates that, regardless of genotype, animals treated with nicotine show increased activity during the pre-injection habituation measures as the number of habituation/injection days increased. To test this, activity from the first and last pre-injection habituation days were compared using a within-subjects comparison of the saline (HIV-1Tg Sal and F344 Sal) and nicotine (HIV-1Tg Nic and F344 Nic) treated groups. The saline treated rats showed activity counts of 652.6 (± 39.3) and 586.4 (± 20.3) on days 1 and 19, respectively; no change in pre-injection habituation activity was observed (F(1, 15) = 2.6, p > 0.05). The nicotine treated animals exhibited 640.3 (± 29.9) and 764.6 (± 35.5) activity counts on days 1 and 19, respectively, and this increase in locomotor activity during pre-injection habituation sessions was significant (F(1, 15) = 25.0, p < 0.001). These data indicate that animals injected with nicotine, but not saline, exhibit a significant increase in activity during the pre-injection observation.
Figure 3.
The time-course data during the behavioral sensitization phase. HIV-1Tg and F344 rats were administered nicotine (Nic; 0.35 mg/kg; s.c.) or saline (Sal) on Days 1–19. Panel A shows the total horizontal activity (mean ± S.E.M.) during the 30 min pre-injection habituation period. Panel B shows the total horizontal activity (mean ± S.E.M.) during the 60 min following nicotine or saline injection. n=8 rats per group.
3.2.3. Nicotine-induced behavioral sensitization
To determine the effect of HIV-1 viral proteins on nicotine-mediated locomotor sensitization, we measured horizontal activity following administration of nicotine (0.35 mg/kg, s.c.) or saline in HIV-1Tg and F344 rats (Fig. 3B). A genotype × treatment × day × time ANOVA revealed significant main effects of genotype (F(1, 27) = 4.37, p < 0.05), treatment (F(1, 27) = 965.71, p < 0.001), day (F(9, 243) = 23.52, p < 0.001) and time (F(11, 297) = 536.04, p < 0.001). A significant treatment × day interaction (F(9, 243) = 37.40, p < 0.05) was found, indicating that repeated nicotine injection produced behavioral sensitization. Rats treated with saline exhibited decreased activity across treatment days, from a mean (±S.E.M.) of 357 (±27) activity counts on day 1, to 245 (±32) on day 19. Nicotine treated rats exhibited 763 (±28) activity counts on day 1, but showed increased locomotor counts of 1287 (±33) on day 19. The genotype × treatment (F(1, 27) = 4.40, p < 0.05) and genotype × day (F(9, 243) = 2.76, p < 0.05) interactions indicate that genotype significantly interacted with the effects of repeated nicotine administration. The pattern of nicotine-induced sensitization was similar for the HIV-1Tg and F344 rats following the first ten nicotine injections; however, the nicotine-induced behavioral sensitization was attenuated in HIV-1Tg rats compared to F344 rats during treatment days 11–19. The HIV-1Tg rats showed decreased nicotine-induced activity on four of the remaining five nicotine behavioral assessments, thus suggesting that transgenic animals do not acquire the same magnitude of nicotine-induced behavioral sensitization.
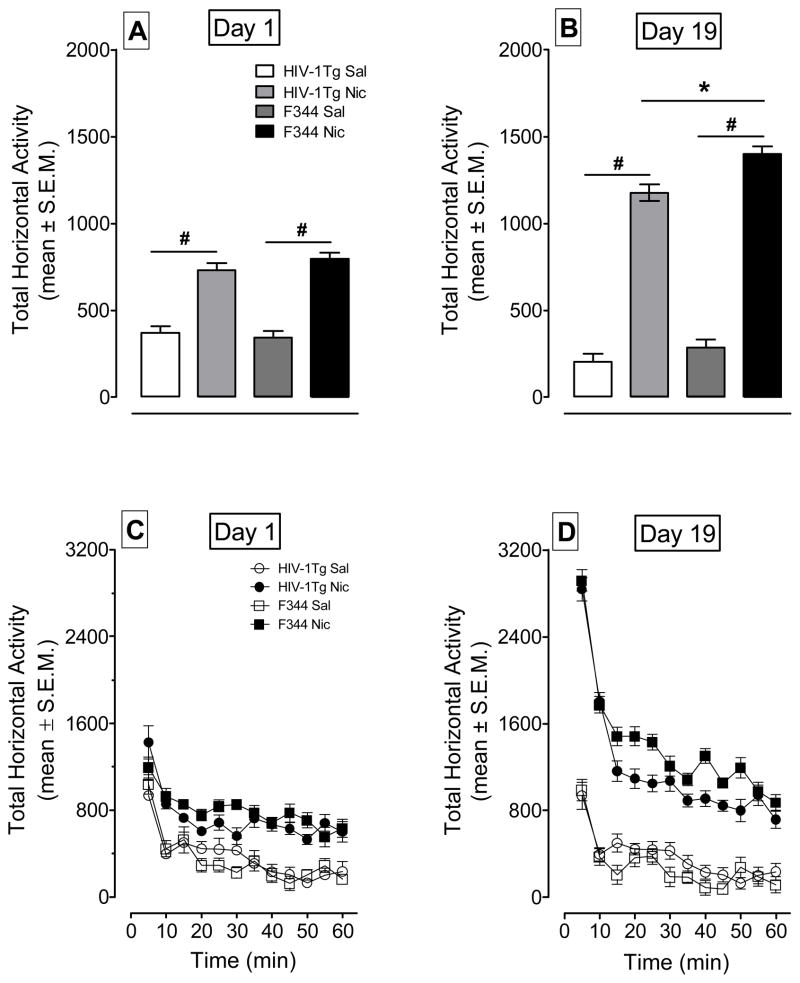
A genotype × treatment × day ANOVA was conducted on the first and final injection days to determine if HIV-1Tg rats exhibited attenuated nicotine-induced behavioral sensitization (Figures. 4A and 4B). There were significant main effects of genotype (F(1,28) = 6.27; p < 0.05), treatment (F(1,28) = 192.05; p < 0.001) and day (F(1,28) = 14.97; p < 0.01). A significant genotype × day interaction (F(1,28) = 5.29; p < 0.05) and a significant treatment × day interaction (F(1,28) = 40.96; p < 0.001) were found. On day 1, the HIV-1Tg and F344 rats in nicotine-treated groups exhibited more activity than their saline controls (p < 0.001; p < 0.001, Bonferroni t-test, respectively). No differences between the HIV-1Tg-Nic and F344-Nic groups were observed (p > 0.05). Similarly, there were no differences between HIV-1Tg-Sal and F344-Sal groups on day 1 (p > 0.05). On day 19, HIV-1Tg and F344 rats injected with nicotine displayed greater activity compared to their saline controls (p < 0.001); however, both groups showed enhanced activity following repeated nicotine injection relative to day 1 (F(1,6) = 136.5; p< 0.001), and day 19 (F(1,7) = 98.8; p < 0.001, respectively). The HIV-1Tg-Nic group exhibited less locomotor activity relative to the F344-Nic group (p < 0.01), suggesting that the HIV-1Tg rats exhibited attenuated psychomotor sensitization relative to the F344 rats.
Figure 4.
The time-course data for total horizontal activity during day 1 and day 19 of the behavioral sensitization phase. Panels A and B show the total horizontal activity (mean ± S.E.M.) across the 60-min session. Panels C and D show the time course of the total horizontal activity (mean ± S.E.M.) during each 5-min time interval. * p < 0.05 difference between HIV-1Tg and F344 rats. # p < 0.05 difference between nicotine- and saline-treatment group. n=8 rats per group.
The time course data from Day 1 and Day 19 are illustrated in Figures 4C and 4D. Figure 4C shows that rats treated with nicotine and saline had the same amount of activity in the first five min of the session, and that nicotine injected rats showed more activity than did the rats administered saline. Figure 4D, which represents day 19, clearly shows that animals treated with nicotine exhibited higher activity counts in the first 5 min relative to the saline groups, and although both groups showed within-session habituation, animals in the nicotine conditions exhibited higher activity counts throughout the remainder of the hour. Furthermore, HIV-1Tg rats treated with nicotine showed less activity than the F344 rats treated with nicotine throughout the remainder of the hour measurement.
3.3. Levels and activity of ERK and CREB signaling proteins in HIV-1Tg and F344 rats
To determine whether the nicotine-induced behavioral change is associated with mesocorticolimbic DA signaling, we examined the effects of repeated nicotine administration on the levels and the phosphorylation state of CREB and ERK in the PFC, NAc and VTA from the HIV-1Tg and F344 rats used in the behavioral experiment.
3.3.1. PFC
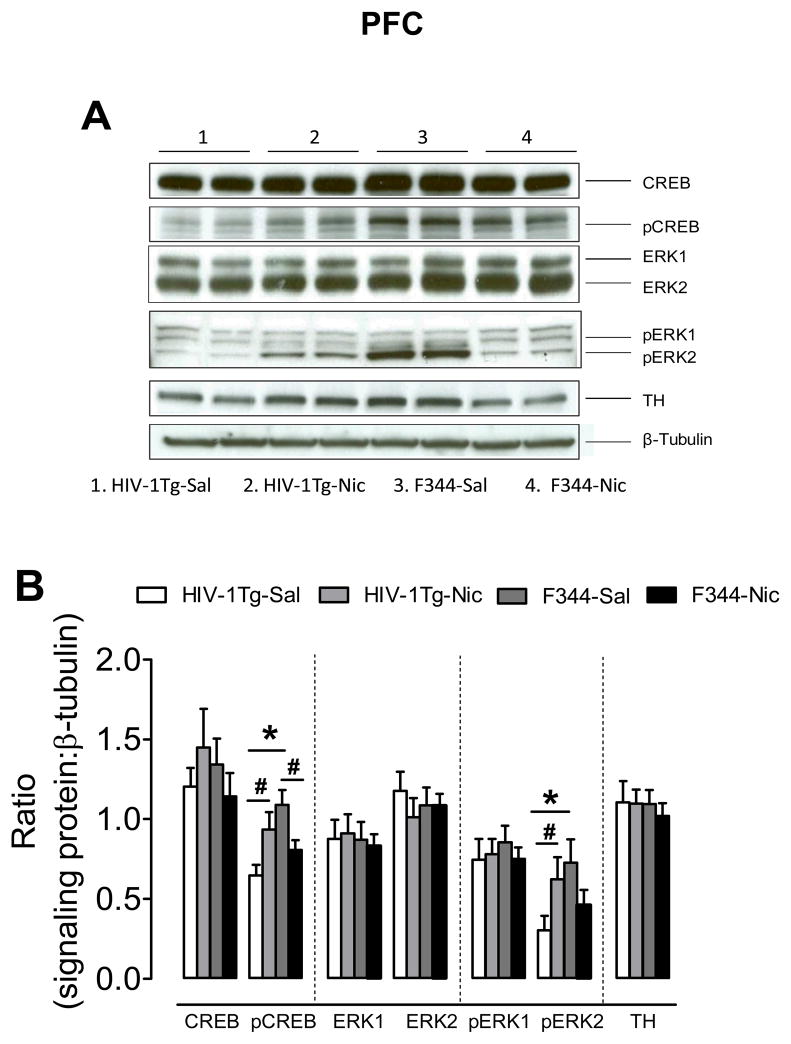
Separate two-way ANOVAs were performed to determine the effect of nicotine on the levels and phosphorylation state of the signaling proteins. As shown in Figure 5, no significant differences in total CREB, ERK1, ERK2 and TH were found in the PFC among the groups. With respect to the ratio of pCREB/β-tubulin, a main effect of genotype (F(1, 27) = 4.34, p < 0.05) and a significant genotype × treatment interaction (F(1, 27) = 9.47, p < 0.05) were found. Post hoc analysis revealed that the ratio of pCREB/β-tubulin was lower in the HIV-1Tg rats than that in the F344 rats in saline-control groups (F(1, 13) = 5.64, p < 0.05). The level of pCREB was greater in the nicotine-treated HIV-1Tg group than that in the saline-treated HIV-1Tg group (F(1, 13) = 4.69, p < 0.05). In contrast, pCREB levels were decreased in the nicotine-treated F344 group compared to the saline-treated F344 group (F(1, 13) = 4.31, p < 0.05).
Figure 5.
Levels of ERK, CREB and TH proteins in the PFC in HIV-1Tg and F344 rats. (A) Representative western blots showing the protein density of CREB, pCREB, ERK1/2, pERK1/2, TH and β-tubulin in nicotine or saline treated HIV-1Tg (HIV-1Tg-Nic, HIV-1Tg-Sal) and F344 rats (F344-Nic, F344-Sal). (B) Total and phosphorylated protein levels of ERK1, ERK2 and CREB along with levels of TH after chronic nicotine or saline injection. Ratios are presented as the mean percentage of β-tubulin ± S.E.M. * p < 0.05 difference between HIV-1Tg and F344 rats. # p < 0.05 difference between the nicotine- and saline-treatment groups. n=8 rats per group.
With respect to the ratio of pERK2/β-tubulin in the PFC, a main effect of genotype (F(1, 27) = 4.32, p < 0.05) and a significant genotype × treatment interaction were found (F(1, 27) = 8.81, p < 0.05). Post hoc analysis revealed that the ratio of pERK2/β-tubulin in the PFC was lower in HIV-1Tg rats than that in F344 rats in saline-treated group (F(1, 13) = 13.2, p < 0.05). Nicotine increased the ratio in HIV-1Tg rats compared to the saline-treated group (F(1, 13) = 6.64, p < 0.05). There was a trend toward a decrease in the ratio of pERK2/β-tubulin in F344 rats after repeated nicotine injection (F(1,13) = 3.31; p = 0.07). The ratio of pERK2/β-tubulin was greater in HIV-1Tg-Nic group than that in F344-Nic group (p < 0.05).
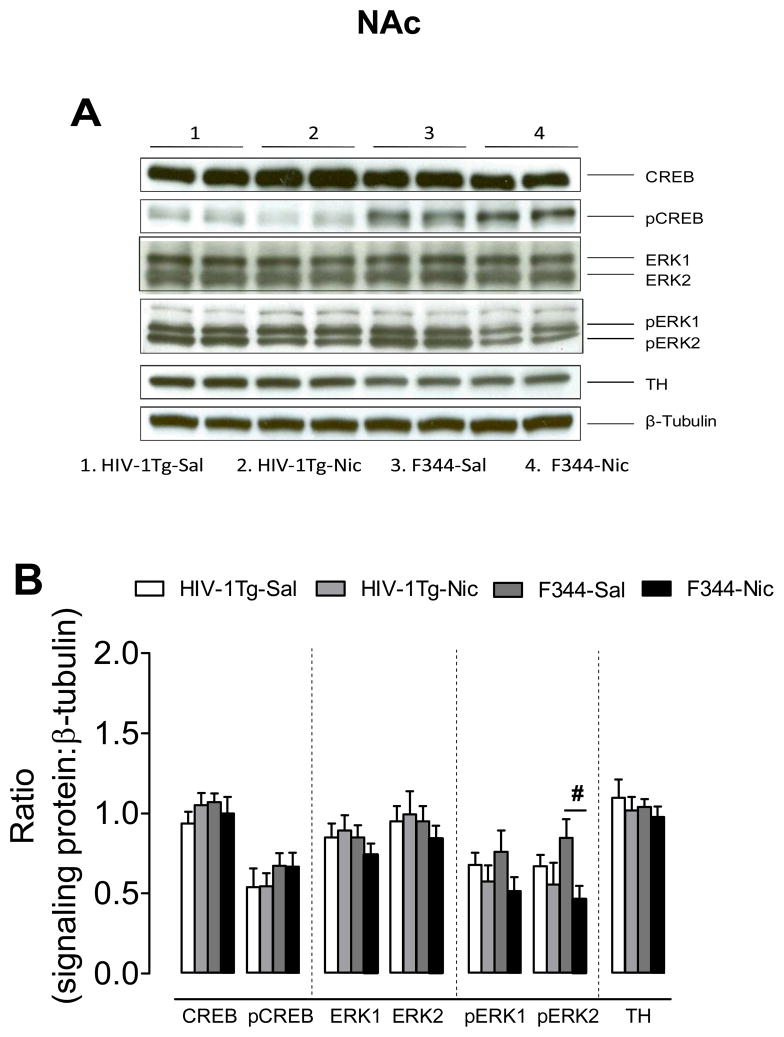
3.3.2. NAc
There were no changes in total and phosphorylated CREB, in total ERK and pERK1, or in the levels of TH observed in NAc of HIV-1Tg and F344 rats following nicotine or saline injection. With respect to ratio of pERK2/β-tubulin, the two-way ANOVA revealed a significant main effect of treatment (F(1, 27) = 5.31, p < 0.05), but neither the main effect of genotype nor the genotype × treatment interaction was significant (Fig. 6). Repeated nicotine administration decreased the ratio of pERK2/β-tubulin in F344 rats (F(1, 14) = 7.05, p < 0.05), but not in the HIV-1Tg rats (F(1.14) = 0.51, p > 0.05).
Figure 6.
Levels of ERK, CREB and TH proteins in the NAc in HIV-1Tg and F344 rats. (A) Representative western blots showing the protein density of CREB, pCREB, ERK1/2, pERK1/2, TH and β-tubulin in the nicotine or saline treated HIV-1Tg (HIV-1Tg-Nic, HIV-1Tg-Sal) and F344 rats (F344-Nic, F344-Sal). (B) Total and phosphorylated protein levels of ERK1, ERK2 and CREB along with levels of TH after chronic nicotine or saline injection. Ratios are presented as the mean percentage of β-tubulin ± S.E.M. # p < 0.05 difference between the nicotine- and saline-treatment groups. n=8 rats per group.
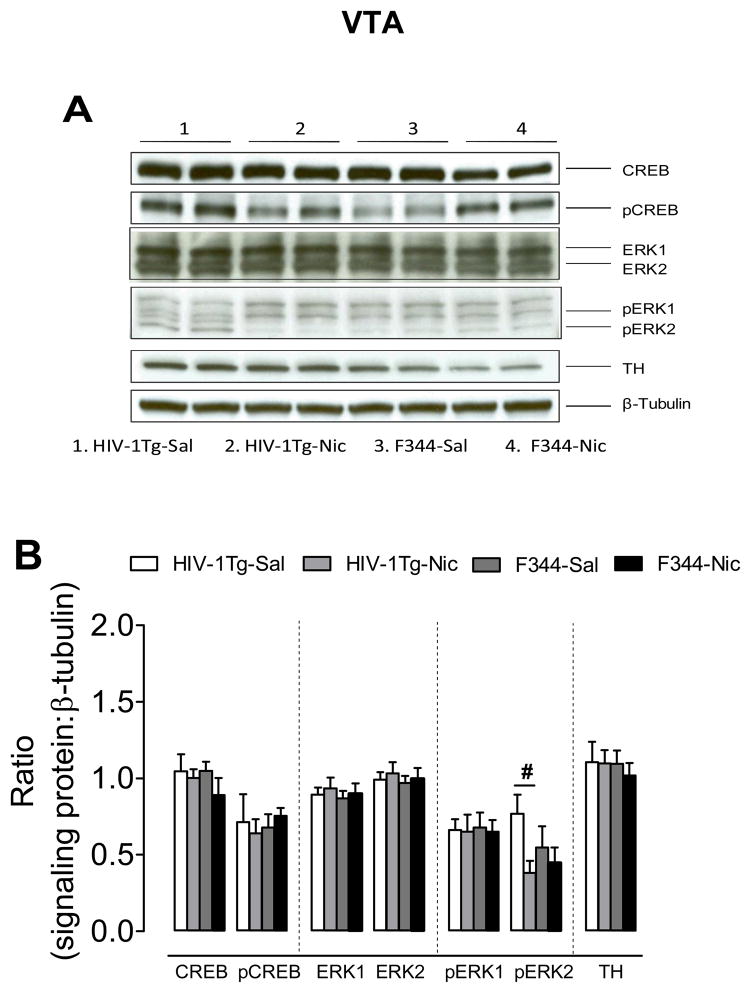
3.3.3. VTA
There was no change in total and phosphorylated ERK and CREB, or in the levels of TH observed in the VTA following nicotine administration. Regarding the ratio of pERK2/β-tubulin, two-way ANOVA revealed a significant main effect of treatment (F(1, 27) = 4.45, p < 0.05), but neither the main effect of genotype nor the genotype × treatment interaction was significant (Fig. 7). Repeated nicotine administration produced a decreased ratio of pERK2/β-tubulin in HIV-1Tg rats (F(1, 14) = 6.74, p < 0.05), but not in the F344 rats (F(1.14) = 0.34, p > 0.05).
Figure 7.
Levels of ERK, CREB and TH proteins in the VTA in HIV-1Tg and F344 rats (A) Representative western blots showing the protein density of CREB, pCREB, ERK1/2, pERK1/2, TH and β-tubulin in the nicotine or saline treated HIV-1Tg (HIV-1Tg-Nic, HIV-1Tg-Sal) and F344 rats (F344-Nic, F344-Sal). (B) Total and phosphorylated protein levels of ERK1, ERK2 and CREB along with levels of TH after chronic nicotine or saline injection. Ratios are expressed as the mean percentage of β-tubulin ± S.E.M. # p < 0.05 difference between the nicotine- and saline-treatment group. n=8 rats per group.
4. Discussion
The present findings demonstrate that genetically expressed HIV-1 viral proteins alter the sensitivity of the locomotor effects of repeated nicotine administration. HIV-1Tg rats exhibited diminished locomotor activity during habituation to a novel context and showed an attenuation of nicotine-induced behavioral sensitization. Importantly, the basal levels of pCREB and pERK2 in the PFC were lower in the HIV-1Tg saline group compared to F344 saline controls. Following repeated nicotine administration, the levels of pCREB and pERK2 in PFC were decreased in F344 rats, but increased in HIV-1Tg rats, suggesting opposite effects of nicotine on these phosphorylated signaling proteins. In addition, repeated nicotine administration decreased pERK2 levels in the NAc of F344 rats and this effect was also observed in the VTA of HIV-1Tg rats. Thus, HIV-1 viral protein-induced alterations in the CREB and ERK signaling pathway in the mesocorticolimbic DA system appear to have played a role in the locomotor effects of repeated nicotine in HIV-1Tg rats.
Regarding the behavioral portion of the experiment, we observed that HIV-1Tg rats exhibited alterations in locomotor activity during both the habituation and behavioral sensitization phases of the experiment. First, the transgenic rats showed less activity during day 1 of habituation compared to F344 rats, indicating that the novelty of the initial context exposure produced less activity in the transgenic rats relative to control rats. This difference in habituation between the HIV-1Tg and F344 rats was transient, as the transgenic rats did not continue to exhibit the blunted locomotor response, relative to F344 controls, on either day 2 of habituation or on the saline baseline measure. A recent study reported HIV-1Tg rats exhibited less rearing and head movement activity compared to F344 rats following repeated saline injections (Liu et al., 2009), but these differences did not reach significance in another report (Kass et al., 2010). The lower baseline activity exhibited by HIV-1Tg rats in the present study and reported by Liu et al. (2009) appear to be the result of manipulation of dopaminergic system by genetically expressed viral proteins (Fink and Smith, 1980). Indeed, HIV-1Tg rats exhibited increased expression of D1 receptors in the PFC (Liu et al., 2009) and a decrease in DAT mRNA (Webb et al., 2010). These studies are consistent with clinical studies showing a significant reduction of DAT density in the putamen and ventral striatum in HIV-1 infected patients (Chang et al., 2008; Wang et al., 2004). In addition, D1 expression has been reported to be negatively correlated with baseline locomotor activity observed in D1 receptor-deficient mice (El-Ghundi et al., 2010). Thus, our behavioral data indicate that the attenuated habituation curve in HIV-1Tg rats is related to neural adaptations produced by HIV-1 viral proteins, and that this transient effect represents an attenuation of activity in response to the novelty of the locomotor activity chambers.
The induction of nicotine-induced behavioral sensitization was altered in transgenic rats as well. In this study, enhanced locomotor activity was observed in both genotypes across days following repeated nicotine administration. Although no difference in acute nicotine-induced activity between the two genotypes was observed on day 1, the HIV-1Tg group displayed reduced nicotine-induced locomotor activity during the later days, i.e., 13–19, relative to the nicotine-treated F344 group. Thus, although transgenic rats showed a blunted response to repeated nicotine exposure during the induction of sensitization, those animals did not exhibit a deficit in developing behavioral sensitization. Rather, HIV-1Tg rats exhibited less sensitivity to the repeated effects of nicotine, and this deficit may contribute to an alteration in nAChR-mediated dopamine neurotransmission. In accord with these behavioral data, we recently found that HIV-1Tg rats had lower IC50 values for [3H]nicotine binding with 5-fold rightward shift of the nicotine concentration curve, compared to F344 controls (unpublished data). Similarly, previous research showed that intra-accumbal Tat infusion attenuated cocaine-induced behavioral sensitization in rats (Harrod et al., 2008), suggesting that the viral protein Tat is involved in the altered behavioral response to psychostimulant drugs. Together, the results indicate that the HIV-1Tg rats are a pertinent model to investigate how chronic exposure to viral proteins and nicotine alter dopaminergic pathways that mediate motivated behavior.
Notably, although behavioral sensitization is a sensitive measure for the influence of psychostimulants on the mesocorticolimbic system (Berridge, 2007), it does not measure drug reward. Thus, predictions regarding cigarette smoking in HIV-1 positive individuals are limited. Given that two behavioral models of viral protein exposure produced diminished psychostimulant-induced behavioral sensitization (Harrod et al., 2008), it is suggested that cigarette smoking by HIV-1 positive individuals will produce alterations in motivated behavior due to the interplay of nicotine exposure and HIV viral proteins within the mesocorticolimbic DA system.
Previous research suggests the possibility that the sensitization of one type of behavior, like rearing, could result in the decrease of another behavior, such as horizontal activity (Iwamoto, 1984; Jerome and Sanberg, 1987; Ksir, 1994; Reid et al., 1998). That the attenuation of total horizontal activity exhibited by the HIV-1Tg rats was diminished in response to the emergence of a competing behavior like rearing or stereotypy, however, is not likely. First, our automated activity chambers measure rearing as all beam breaks in the vertical plane. In the present experiment, there were no effects or interactions with the factor of genotype so the data are not shown; however, animals progressively exhibited sensitization and both genotypes showed asymptotic levels by day 7. Thus, rearing did not emerge on days 11–19, which corresponds to the treatment days that transgenic rats exhibited less nicotine-induced total horizontal activity. Although nicotine-induced sensitization of stereotypy has been reported (Reid et al., 1998), it is not a consistent finding (Harrod et al., 2004; Harrod et al., 2008; Jerome and Sanberg, 1987; Ksir, 1994). We did not use observational procedures in the present experiment, so the levels of nicotine-induced stereotypy were not determined. Our previous studies, which used a combination of automated and observational procedures, show that repeated nicotine or cocaine injection induced sensitization of horizontal activity and rearing incidence, but not of stereotypy (Harrod et al., 2004; Harrod et al., 2008). It is unlikely that the attenuation of total horizontal activity observed for the HIV-1Tg rats is attributable to an emergence of stereotypic behavior.
The present results show that animals injected with nicotine, regardless of genotype, exhibited increased locomotor activity during pre-injection habituation, which was particularly evident on days 13–15. Rats in the saline control groups showed steady activity across the 19 day period. The increase in activity in the nicotine treated groups likely represents drug-induced conditioned hyperactivity, which is well documented to be mediated by Pavlovian conditioning processes (Anagnostaras and Robinson, 1996; Bevins and Palmatier, 2003). Repeated psychostimulant injection within the same context allows for contextual cues to function as a conditional stimulus, and the drug effect, e.g., hyperactivity, to act as an unconditional stimulus (Anagnostaras and Robinson, 1996). In the present experiment, repeated context-nicotine pairings support the standard associative model described above, and exposure to the chamber prior to daily drug injection represents presentation of the conditional stimulus, or the context alone, without the influence of the unconditional stimulus. Our results indicate that after being placed in the context, hyperactivity, which is similar to the unconditional stimulus effects of repeated nicotine, was observed and there were no effects of genotype on this effect.
The second part of the experiment determined levels of transcriptional factors throughout the mesocorticolimbic DA system in nicotine sensitized HIV-1 rats relative to F344 controls. First, HIV-1Tg-saline rats exhibited lower basal levels of pCREB and pERK2 in the PFC, but not in the VTA or NAc, compared to F344-saline controls. These findings suggest that viral proteins produced a neurobiological adaptation in ERK and CREB signaling in the PFC. The observed changes in signaling have implications for the functionality of the mesocorticolimbic DA system. For example, neuronal firing elicits ERK activity in the brain of rats (Davis et al., 2000; Thiels et al., 2002; Ying et al., 2002), whereas blocking ERK activity decreases the firing rate of DA neurons (Iniguez et al., 2010). Increased tonic release of DA enhances ERK activity, which is attenuated in DA D1 receptor mutant mice (Chen and Xu, 2010). Further, deletion of DA D1 receptors in mice produces higher pCREB levels in the striatum (El-Ghundi et al., 2010), suggesting that CREB phosphorylation is stimulated by DA D1 receptor activation. ERK activation is coupled to activation of CREB (Nakayama et al., 2001; Ying et al., 2002) and, in turn, supports adaptive processes such as long-term potentiation and psychostimulant-induced sensitization (DiRocco et al., 2009; Ying et al., 2002). It has been reported that Tat, gp120 and other viral proteins have higher expression in the PFC compared to other brain regions of HIV-1Tg rats (Peng et al., 2010). Thus, the lower basal levels of pERK2 and pCREB could contribute to the differences in DA D1 receptor expression that was previously reported between HIV-1Tg rats and the F344 controls (Liu et al., 2009). Moreover, although the regional high expression of these viral proteins may contribute to the PFC-specific changes of pERK2 and pCREB, it is also possible that these signaling proteins in PFC are more sensitive to HIV-1 viral protein insult. Together, the molecular data show that the basal levels of particular transcriptional factors, which are implicated in the regulation of mesocorticolimbic function, are altered in HIV-1Tg animals.
Repeated nicotine administration significantly decreased pCREB in the PFC of F344-nicoitne rats compared to F344-saline group. Although delivering chronic nicotine through drinking water increased the ratio of pCREB/CREB in the PFC in C57BL/6J mice (Brunzell et al., 2003), another report showed that the levels of CREB and pCREB were decreased in the cortex of rats 18 h after withdrawal from repeated administration of 2 mg/kg of nicotine (Pandey et al., 2001). Thus, nicotine-mediated regulation of CREB activity is largely dependent on the species, dosage, route of administration, and the time needed to harvest brains (Brunzell et al., 2003; Pandey et al., 2001). The current results show that repeated nicotine increased pCREB in cortical tissue of HIV-1Tg rats, with no change in CREB. This finding is interesting for two reasons. First, F344 rats exhibited decreased pCREB following repeated nicotine administration, and second, the nicotine-induced increase in pCREB occurred despite lower basal levels of this transcription factor in HIV-1Tg rats relative to F344 controls. This suggests that the processes that mediate the lower basal levels of pCREB in the transgenic rats do not prevent repeated nicotine from regulating CREB signaling. Rather, the current results suggest that nicotine and HIV-1 viral proteins act synergistically to alter CREB signaling in the PFC. Decreased CREB activity is associated with an increase in drug reward and food preference (Carlezon et al., 1998). In general, this suggests that an aberrant decrease in CREB activity, as is shown in the present experiment, may negatively impact normal function of the mesocorticolimbic DA system. Determining if PFC CREB activity is also implicated in the reduced rate of nicotine-induced reward in HIV-1Tg rats is of future interest.
Regarding the ERK experiments, we observed significant alterations in pERK2 levels in the PFC, NAc, and VTA with no change in ERK1, ERK2, or pERK1 in either HIV-1Tg or F344 rats. Notably, the overall levels of pERK2 in the PFC were similar to those changes observed with pCREB in the PFC. Basal levels of pERK2 from the HIV-1Tg-Saline rats were lower than that of the F344-Saline rats, which indicates that the presence of viral proteins reduces pERK2 in the PFC. Following repeated nicotine injection, F344 animals exhibited a trend for decreased pERK2 relative to F344-Saline rats, whereas HIV-1Tg showed increased pERK2 relative to their saline controls. Regarding the VTA, basal pERK2 levels did not differ by genotype in the saline control group, but repeated nicotine decreased this transcriptional factor in HIV-1Tg rats relative to the saline controls. In the NAc, however, there were no differences between levels of pERK2 in the HIV-1Tg-Nic and HIV-1Tg-Saline rats, but the nicotine sensitized F344 rats exhibited decreased pERK2 relative to controls. This is consistent with the diminished pCREB levels observed in the PFC of HIV-1Tg rats, and this result indicates that viral proteins manipulate ERK signaling in a region specific manner.
Our results suggest that viral protein-induced changes in ERK phosphorylation may exacerbate the plasticity related to the magnitude of the attenuated nicotine-induced sensitization observed in the behavioral part of our experiment. This conclusion is supported by recent reports (Girault et al., 2007; Iniguez et al., 2010; Valjent et al., 2006; Valjent et al., 2005). For example, blocking ERK1/2 activity by SL327, a selective inhibitor of mitogen-activated protein kinase, prevented the induction of locomotor sensitization by repeated injection of cocaine or amphetamine (Valjent et al., 2006; Valjent et al., 2005). Further, discrete manipulation of ERK2 within the VTA, using a viral-mediated dominant negative mutant of ERK2, blunted the expression of cocaine-induced behavioral sensitization (Iniguez et al., 2010). The current findings suggest that low levels of prefrontal pERK2 may contribute to the blunted nicotine-induced locomotor activity observed in transgenic rats. Thus, the present study provides evidence that HIV-1 viral proteins impair ERK signaling, thereby contributing to the long-term behavioral changes induced by repeated nicotine. Additional research is needed to elucidate the role of HIV-1 viral proteins on ERK and CREB signaling in the PFC on nicotine reward. As mentioned above, whether the combined effects of nicotine and HIV-1 viral proteins alter the rewarding effects of nicotine cannot be inferred from the present experiment. Nonetheless, these findings further indicate that HIV-1 positive individuals who smoke cigarettes may experience a synergistic effect of viral proteins and nicotine on transcriptional factors that regulate mesocorticolimbic function.
The current study found no differences in basal TH levels regardless of region or genotype, which is consistent with the findings of a recent report showing no change in protein levels of TH in the striatum of naive HIV-1Tg rats (Webb et al., 2010). Notably, regardless of the nicotine-induced changes in pCREB and pERK in the PFC, repeated nicotine injection did not alter the level of TH in any region. This is in contrast to the findings of Brunzell et al. who reported that chronic nicotine exposure in drinking water increased TH levels in the PFC in mice 1.5 h after the last nicotine injection; an increase that returned to normal levels 24 h after withdrawal (Brunzell et al., 2003). The discrepant findings between the current and previous experiments may be related to differences in species and in the route of nicotine administration. However, a recent report showed that in vitro exposure to nicotine only increased TH mRNA levels of mouse midbrain slices within 1 hour, but did not change TH protein for different periods of time up to 48 hours (Radcliffe et al., 2009). Hence, it is possible that nicotine stimulation transiently changes transcriptional TH levels, without changing the protein levels of TH.
In conclusion, the current results suggest that genetically expressed HIV-1 viral proteins in rats diminish basal expression levels of pERK2 and pCREB in the PFC, which may explain, at least in part, the low baseline locomotor activity of HIV-1Tg rats. The opposite effects of nicotine on pERK2 and pCREB in the PFC between HIV-1Tg rats and F344 rats may play a role in the blunted locomotor response to repeated administration of nicotine noted in HIV-1Tg rats. Determining how HIV-1 viral proteins and nicotine influence ERK and CREB signaling in the mesocorticolimbic system will be important to understand why HIV-1 positive individuals exhibit increased vulnerability for nicotine addiction.
Acknowledgments
This research was supported by grants from the National Institute on Drug Abuse to Jun Zhu (DA024275 and DA026721) and to Steven B. Harrod (DA021287) and by an Award of Research and Productive Scholarship from the University of South Carolina to Jun Zhu.
References
- Addy NA, Fornasiero EF, Stevens TR, Taylor JR, Picciotto MR. Role of calcineurin in nicotine-mediated locomotor sensitization. J Neurosci. 2007;27:8571–8580. doi: 10.1523/JNEUROSCI.2601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110:1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Berger JR, Arendt G. HIV dementia: the role of the basal ganglia and dopaminergic systems. J Psychopharmacol. 2000;14:214–221. doi: 10.1177/026988110001400304. [DOI] [PubMed] [Google Scholar]
- Berhow MT, Hiroi N, Nestler EJ. Regulation of ERK (extracellular signal regulated kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J Neurosci. 1996;16:4707–4715. doi: 10.1523/JNEUROSCI.16-15-04707.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Palmatier MI. Nicotine-conditioned locomotor sensitization in rats: assessment of the US-preexposure effect. Behav Brain Res. 2003;143:65–74. doi: 10.1016/s0166-4328(03)00009-3. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Mineur YS, Neve RL, Picciotto MR. Nucleus accumbens CREB activity is necessary for nicotine conditioned place preference. Neuropsychopharmacology. 2009;34:1993–2001. doi: 10.1038/npp.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell DH, Russell DS, Picciotto MR. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. J Neurochem. 2003;84:1431–1441. doi: 10.1046/j.1471-4159.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- Burkhalter JE, Springer CM, Chhabra R, Ostroff JS, Rapkini BD. Tobacco use and readiness to quit smoking in low-income HIV-infected persons. Nicotine Tob Res. 2005;7:511–22. doi: 10.1080/14622200500186064. [DOI] [PubMed] [Google Scholar]
- Burns DN, Hillman D, Neaton JD, Sherer R, Mitchell T, Capps L, Vallier WG, Thurnherr MD, Gordin FM. Cigarette smoking, bacterial pneumonia, and other clinical outcomes in HIV-1 infection. Terry Beirn Community Programs for Clinical Research on AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:374–383. doi: 10.1097/00042560-199612010-00012. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Cass WA, Harned ME, Peters LE, Nath A, Maragos WF. HIV-1 protein Tat potentiation of methamphetamine-induced decreases in evoked overflow of dopamine in the striatum of the rat. Brain Res. 2003;984:133–142. doi: 10.1016/s0006-8993(03)03122-6. [DOI] [PubMed] [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage. 2008;42:869–878. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Xu M. Dopamine D1 and D3 receptors are differentially involved in cue-elicited cocaine seeking. J Neurochem. 2010;114:530–541. doi: 10.1111/j.1471-4159.2010.06775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Cigarette smoking among adults — United States, 2006. Morbidity and Mortality Weekly Report. 2007;56(44):1157–1161. [PubMed] [Google Scholar]
- Clarke PB, Kumar R. Characterization of the locomotor stimulant action of nicotine in tolerant rats. Br J Pharmacol. 1983a;80:587–594. doi: 10.1111/j.1476-5381.1983.tb10733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB, Kumar R. The effects of nicotine on locomotor activity in non-tolerant and tolerant rats. Br J Pharmacol. 1983b;78:329–337. doi: 10.1111/j.1476-5381.1983.tb09398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Crothers K, Griffith TA, McGinnis KA, Rodriguez-Barradas MC, Leaf DA, Weissman S, Gibert CL, Butt AA, Justice AC. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. J Gen Intern Med. 2005;20:1142–1145. doi: 10.1111/j.1525-1497.2005.0255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20:4563–4572. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle L, Croul S, Morgello S, Amini S, Rappaport J, Khalili K. Detection of HIV-1 Tat and JCV capsid protein, VP1, in AIDS brain with progressive multifocal leukoencephalopathy. J Neurovirol. 2000;6:221–228. doi: 10.3109/13550280009015824. [DOI] [PubMed] [Google Scholar]
- DiRocco DP, Scheiner ZS, Sindreu CB, Chan GC, Storm DR. A role for calmodulin-stimulated adenylyl cyclases in cocaine sensitization. J Neurosci. 2009;29:2393–2403. doi: 10.1523/JNEUROSCI.4356-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MJ, Bruce-Keller AJ, Conner C, Knapp PE, Xu R, Nath A, Hauser KF. Effects of chronic expression of the HIV-induced protein, transactivator of transcription, on circadian activity rhythms in mice, with or without morphine. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1680–1687. doi: 10.1152/ajpregu.90496.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghundi MB, Fan T, Karasinska JM, Yeung J, Zhou M, O’Dowd BF, George SR. Restoration of amphetamine-induced locomotor sensitization in dopamine D1 receptor-deficient mice. Psychopharmacology (Berl) 2010;207:599–618. doi: 10.1007/s00213-009-1690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neurosci Biobehav Rev. 2008;32:883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink JS, Smith GP. Mesolimbic and mesocortical dopaminergic neurons are necessary for normal exploratory behavior in rats. Neurosci Lett. 1980;17:61–65. doi: 10.1016/0304-3940(80)90062-2. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Hasselrot U, Mactutus CF. Differential long-term neurotoxicity of HIV-1 proteins in the rat hippocampal formation: a design-based stereological study. Hippocampus. 2008;18:135–147. doi: 10.1002/hipo.20376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furber AS, Maheswaran R, Newell JN, Carroll C. Is smoking tobacco an independent risk factor for HIV infection and progression to AIDS? A systemic review. Sex Transm Infect. 2007;83:41–46. doi: 10.1136/sti.2005.019505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster M, Estrada V, Fernandez-Pinilla MC, Fuentes-Ferrer ME, Tellez MJ, Vergas J, Serrano-Villar S, Fernandez-Cruz A. Smoking cessation in HIV patients: rate of success and associated factors. HIV Med. 2009;10:614–619. doi: 10.1111/j.1468-1293.2009.00735.x. [DOI] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Gritz ER, Vidrine DJ, Lazev AB, Amick BC, 3rd, Arduino RC. Smoking behavior in a low-income multiethnic HIV/AIDS population. Nicotine Tob Res. 2004;6:71–7. doi: 10.1080/14622200310001656885. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Mactutus CF, Bennett K, Hasselrot U, Wu G, Welch M, Booze RM. Sex differences and repeated intravenous nicotine: behavioral sensitization and dopamine receptors. Pharmacol Biochem Behav. 2004;78:581–592. doi: 10.1016/j.pbb.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Mactutus CF, Fitting S, Hasselrot U, Booze RM. Intra-accumbal Tat1–72 alters acute and sensitized responses to cocaine. Pharmacol Biochem Behav. 2008;90:723–729. doi: 10.1016/j.pbb.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrod SB, Van Horn ML. Sex differences in tolerance to the locomotor depressant effects of lobeline in periadolescent rats. Pharmacology Biochemistry and Behavior. 2009;94:296–304. doi: 10.1016/j.pbb.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershberger SL, Fisher DG, Reynolds GL, Klahn JA, Wood MM. Nicotine dependence and HIV risk behaviors among illicit drug users. Addict Behav. 2004;29:623–625. doi: 10.1016/j.addbeh.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Hetman M, Gozdz A. Role of extracellular signal regulated kinases 1 and 2 in neuronal survival. Eur J Biochem. 2004;271:2050–2055. doi: 10.1111/j.1432-1033.2004.04133.x. [DOI] [PubMed] [Google Scholar]
- Hudson LG, Gale JM, Padilla RS, Pickett G, Alexander BE, Wang J, Kusewitt DF. Microarray analysis of cutaneous squamous cell carcinomas reveals enhanced expression of epidermal differentiation complex genes. Mol Carcinog. 2010;49:619–629. doi: 10.1002/mc.20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez SD, Warren BL, Neve RL, Russo SJ, Nestler EJ, Bolanos-Guzman CA. Viral-mediated expression of extracellular signal-regulated kinase-2 in the ventral tegmental area modulates behavioral responses to cocaine. Behav Brain Res. 2010;214:460–464. doi: 10.1016/j.bbr.2010.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto ET. An assessment of the spontaneous activity of rats administered morphine, phencyclidine, or nicotine using automated and observational methods. Psychopharmacology (Berl) 1984;84:374–382. doi: 10.1007/BF00555216. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI. The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. J Pharmacol Exp Ther. 2009;331:547–554. doi: 10.1124/jpet.109.155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerome A, Sanberg PR. The effects of nicotine on locomotor behavior in non-tolerant rats: a multivariate assessment. Psychopharmacology (Berl) 1987;93:397–400. doi: 10.1007/BF00187264. [DOI] [PubMed] [Google Scholar]
- Joyce EM, Koob GF. Amphetamine-, scopolamine- and caffeine-induced locomotor activity following 6-hydroxydopamine lesions of the mesolimbic dopamine system. Psychopharmacology (Berl) 1981;73:311–313. doi: 10.1007/BF00426456. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Interactions between dopamine and excitatory amino acids in behavioral sensitization to psychostimulants. Drug Alcohol Depend. 1995;37:95–100. doi: 10.1016/0376-8716(94)01063-q. [DOI] [PubMed] [Google Scholar]
- Kass MD, Liu X, Vigorito M, Chang L, Chang SL. Methamphetamine-Induced Behavioral and Physiological Effects in Adolescent and Adult HIV-1 Transgenic Rats. J Neuroimmune Pharmacol. 2010;5:566–73. doi: 10.1007/s11481-010-9221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PH, Iversen SD. Selective 6OHDA-induced destruction of mesolimbic dopamine neurons: abolition of psychostimulant-induced locomotor activity in rats. Eur J Pharmacol. 1976;40:45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T, Okamoto M, Nakashima T. Nicotine-induced sensitization to ambulatory stimulant effect produced by daily administration into the ventral tegmental area and the nucleus accumbens in rats. Life Sci. 1992;50:583–590. doi: 10.1016/0024-3205(92)90370-5. [DOI] [PubMed] [Google Scholar]
- Koob GF, Stinus L, Le Moal M. Hyperactivity and hypoactivity produced by lesions to the mesolimbic dopamine system. Behav Brain Res. 1981;3:341–359. doi: 10.1016/0166-4328(81)90004-8. [DOI] [PubMed] [Google Scholar]
- Koutsilieri E, Sopper S, Scheller C, ter Meulen V, Riederer P. Involvement of dopamine in the progression of AIDS Dementia Complex. J Neural Transm. 2002;109:399–410. doi: 10.1007/s007020200032. [DOI] [PubMed] [Google Scholar]
- Ksir C. Acute and chronic nicotine effects on measures of activity in rats: a multivariate analysis. Psychopharmacology (Berl) 1994;115:105–109. doi: 10.1007/BF02244758. [DOI] [PubMed] [Google Scholar]
- Kubos KL, Moran TH, Robinson RG. Differential and asymmetrical behavioral effects of electrolytic or 6-hydroxydopamine lesions in the nucleus accumbens. Brain Res. 1987;401:147–151. doi: 10.1016/0006-8993(87)91174-7. [DOI] [PubMed] [Google Scholar]
- Kumar AM, Fernandez JB, Singer EJ, Commins D, Waldrop-Valverde D, Ownby RL, Kumar M. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J Neurovirol. 2009;15:257–274. doi: 10.1080/13550280902973952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- Liu X, Chang L, Vigorito M, Kass M, Li H, Chang SL. Methamphetamine-induced behavioral sensitization is enhanced in the HIV-1 transgenic rat. J Neuroimmune Pharmacol. 2009;4:309–316. doi: 10.1007/s11481-009-9160-8. [DOI] [PubMed] [Google Scholar]
- Lloyd AC. Distinct functions for ERKs? J Biol. 2006;5:13. doi: 10.1186/jbiol46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis M, Clarke PB. Effect of ventral tegmental 6-hydroxydopamine lesions on the locomotor stimulant action of nicotine in rats. Neuropharmacology. 1998;37:1503–1513. doi: 10.1016/s0028-3908(98)00151-8. [DOI] [PubMed] [Google Scholar]
- Lu L, Wang X, Wu P, Xu C, Zhao M, Morales M, Harvey BK, Hoffer BJ, Shaham Y. Role of ventral tegmental area glial cell line-derived neurotrophic factor in incubation of cocaine craving. Biol Psychiatry. 2009;66:137–145. doi: 10.1016/j.biopsych.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manda VK, Mittapalli RK, Geldenhuys WJ, Lockman PR. Chronic exposure to nicotine and saquinavir decreases endothelial Notch-4 expression and disrupts blood-brain barrier integrity. J Neurochem. 2010;115:515–525. doi: 10.1111/j.1471-4159.2010.06948.x. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Maragos WF, Young KL, Turchan JT, Guseva M, Pauly JR, Nath A, Cass WA. Human immunodeficiency virus-1 Tat protein and methamphetamine interact synergistically to impair striatal dopaminergic function. J Neurochem. 2002;83:955–963. doi: 10.1046/j.1471-4159.2002.01212.x. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Brunzell DH, Grady SR, Lindstrom JM, McIntosh JM, Marks MJ, King SL, Picciotto MR. Localized low-level re-expression of high-affinity mesolimbic nicotinic acetylcholine receptors restores nicotine-induced locomotion but not place conditioning. Genes Brain Behav. 2009;8:257–266. doi: 10.1111/j.1601-183X.2008.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahvi S, Cooperman NA. Review: the need for smoking cessation among HIV-positive smokers. AIDS Educ Prev. 2009;21:14–27. doi: 10.1521/aeap.2009.21.3_supp.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Numakawa T, Ikeuchi T, Hatanaka H. Nicotine-induced phosphorylation of extracellular signal-regulated protein kinase and CREB in PC12h cells. J Neurochem. 2001;79:489–498. doi: 10.1046/j.1471-4159.2001.00602.x. [DOI] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus-associated neurocognitive disorder: pathophysiology in relation to drug addiction. Ann N Y Acad Sci. 2010;1187:122–128. doi: 10.1111/j.1749-6632.2009.05277.x. [DOI] [PubMed] [Google Scholar]
- Nath A, Jankovic J, Pettigrew LC. Movement disorders and AIDS. Neurology. 1987;37:37–41. doi: 10.1212/wnl.37.1.37. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Morrow K, Tashima K, Flanigan T, Abrams DB. Human immunodeficiency virus infection, AIDS, and smoking cessation: the time is now. Clin Infect Dis. 2000;31:808–12. doi: 10.1086/314048. [DOI] [PubMed] [Google Scholar]
- Nieman RB, Fleming J, Coker RJ, Harris JR, Mitchell DM. The effect of cigarette smoking on the development of AIDS in HIV-1-seropositive individuals. AIDS. 1993;7:705–710. doi: 10.1097/00002030-199305000-00015. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Infusion of nicotine in the ventral tegmental area or the nucleus accumbens of the rat differentially affects accumbal dopamine release. Pharmacol Toxicol. 1994a;75:348–352. doi: 10.1111/j.1600-0773.1994.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994b;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Norman LR, Basso M, Kumar A, Malow R. Neuropsychological consequences of HIV and substance abuse: a literature review and implications for treatment and future research. Curr Drug Abuse Rev. 2009;2:143–156. doi: 10.2174/1874473710902020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermann M, Kuper M, Kastrup O, Yaldizli O, Esser S, Thiermann J, Koutsilieri E, Arendt G, Diener HC, Maschke M. Substantia nigra hyperechogenicity and CSF dopamine depletion in HIV. J Neurol. 2009;256:948–953. doi: 10.1007/s00415-009-5052-3. [DOI] [PubMed] [Google Scholar]
- Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- Panagis G, Spyraki C. Neuropharmacological evidence for the role of dopamine in ventral pallidum self-stimulation. Psychopharmacology (Berl) 1996;123:280–288. doi: 10.1007/BF02246582. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Xu T, Mittal N. Effects of protracted nicotine exposure and withdrawal on the expression and phosphorylation of the CREB gene transcription factor in rat brain. J Neurochem. 2001;77:943–952. doi: 10.1046/j.1471-4159.2001.00309.x. [DOI] [PubMed] [Google Scholar]
- Peng J, Vigorito M, Liu X, Zhou D, Wu X, Chang SL. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol. 2010;218:94–101. doi: 10.1016/j.jneuroim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Post RM. Intermittent versus continuous stimulation: effect of time interval on the development of sensitization or tolerance. Life Sci. 1980;26:1275–1282. doi: 10.1016/0024-3205(80)90085-5. [DOI] [PubMed] [Google Scholar]
- Radcliffe PM, Sterling CR, Tank AW. Induction of tyrosine hydroxylase mRNA by nicotine in rat midbrain is inhibited by mifepristone. J Neurochem. 2009;109:1272–1284. doi: 10.1111/j.1471-4159.2009.06056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Ho LB, Berger SP. Behavioral and neurochemical components of nicotine sensitization following 15-day pretreatment: studies on contextual conditioning. Behav Pharmacol. 1998;9:137–148. [PubMed] [Google Scholar]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Jr, Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O’Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Associative Relations in Instrumental Learning - the 18th Bartlett Memorial Lecture. Quarterly Journal of Experimental Psychology Section B-Comparative and Physiological Psychology. 1991;43:1–23. [Google Scholar]
- Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6:615–620. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Subramaniam S, Unsicker K. ERK and cell death: ERK1/2 in neuronal death. FEBS J. 2010;277:22–29. doi: 10.1111/j.1742-4658.2009.07367.x. [DOI] [PubMed] [Google Scholar]
- Thiels E, Kanterewicz BI, Norman ED, Trzaskos JM, Klann E. Long-term depression in the adult hippocampus in vivo involves activation of extracellular signal-regulated kinase and phosphorylation of Elk-1. J Neurosci. 2002;22:2054–2062. doi: 10.1523/JNEUROSCI.22-06-02054.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Trzaskos JM, Girault JA, Herve D. Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci. 2006;7:20. doi: 10.1186/1471-2202-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuates gp120- and tat1–72-induced oxidative stress and prevents loss of dopamine transporter function. Synapse. 2006;59:51–60. doi: 10.1002/syn.20214. [DOI] [PubMed] [Google Scholar]
- Walters CL, Cleck JN, Kuo YC, Blendy JA. Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron. 2005;46:933–943. doi: 10.1016/j.neuron.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127:2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Webb KM, Aksenov MY, Mactutus CF, Booze RM. Evidence for developmental dopaminergic alterations in the human immunodeficiency virus-1 transgenic rat. J Neurovirol. 2010;16:168–173. doi: 10.3109/13550281003690177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- Zhao L, Li F, Zhang Y, Elbourkadi N, Wang Z, Yu C, Taylor EW. Mechanisms and genes involved in enhancement of HIV infectivity by tobacco smoke. Toxicology. 2010;278:242–248. doi: 10.1016/j.tox.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Zhu J, Mactutus CF, Wallace DR, Booze RM. HIV-1 Tat protein-induced rapid and reversible decrease in [3H]dopamine uptake: dissociation of [3H]dopamine uptake and [3H]2beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. J Pharmacol Exp Ther. 2009;329:1071–1083. doi: 10.1124/jpet.108.150144. [DOI] [PMC free article] [PubMed] [Google Scholar]