Abstract
Background
In this study, we assess how effective pandemic and trivalent 2009-2010 seasonal vaccines were in preventing influenza-like illness (ILI) during the 2009 A(H1N1) pandemic in France. We also compare vaccine effectiveness against ILI versus laboratory-confirmed pandemic A(H1N1) influenza, and assess the possible bias caused by using non-specific endpoints and observational data.
Methodology and Principal Findings
We estimated vaccine effectiveness by using the following formula: VE = (PPV-PCV)/(PPV(1-PCV)) × 100%, where PPV is the proportion vaccinated in the population and PCV the proportion of vaccinated influenza cases. People were considered vaccinated three weeks after receiving a dose of vaccine. ILI and pandemic A(H1N1) laboratory-confirmed cases were obtained from two surveillance networks of general practitioners. During the epidemic, 99.7% of influenza isolates were pandemic A(H1N1). Pandemic and seasonal vaccine uptakes in the population were obtained from the National Health Insurance database and by telephonic surveys, respectively. Effectiveness estimates were adjusted by age and week. The presence of residual biases was explored by calculating vaccine effectiveness after the influenza period. The effectiveness of pandemic vaccines in preventing ILI was 52% (95% confidence interval: 30–69) during the pandemic and 33% (4–55) after. It was 86% (56–98) against confirmed influenza. The effectiveness of seasonal vaccines against ILI was 61% (56–66) during the pandemic and 19% (−10–41) after. It was 60% (41–74) against confirmed influenza.
Conclusions
The effectiveness of pandemic vaccines in preventing confirmed pandemic A(H1N1) influenza on the field was high, consistently with published findings. It was significantly lower against ILI. This is unsurprising since not all ILI cases are caused by influenza. Trivalent 2009-2010 seasonal vaccines had a statistically significant effectiveness in preventing ILI and confirmed pandemic influenza, but were not better in preventing confirmed pandemic influenza than in preventing ILI. This lack of difference might be indicative of selection bias.
Introduction
Estimating the field effectiveness of influenza vaccines (VE) poses specific challenges for the 2009 A(H1N1) pandemic. In particular, both pandemic and seasonal vaccination campaigns took place during the epidemic and, as a consequence, vaccine coverage changed through time, both in influenza cases and in the population as a whole.
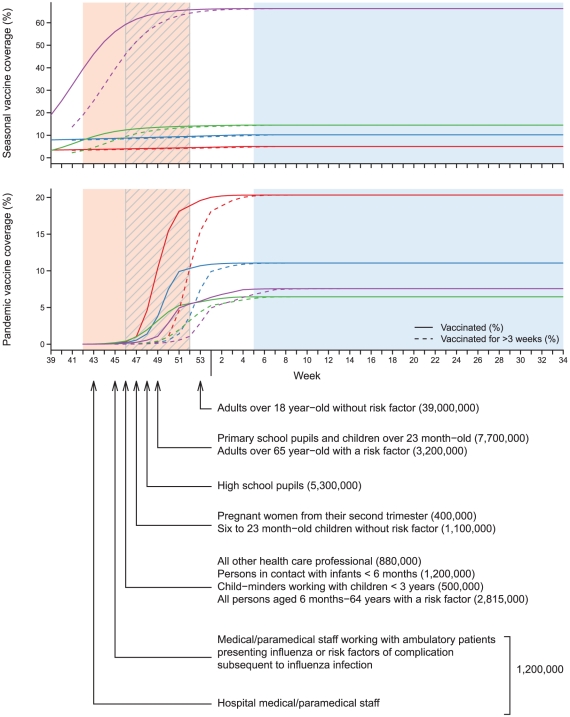
In France, pandemic vaccination conformed to a priority list established by public health authorities based on exposure and/or transmission probability, or on risk of complication subsequent to influenza [1]. The priority allocation of pandemic vaccines is shown in Figure 1, along with the evolution of vaccine coverage over time, by broad age categories. Medical and paramedical staffs working in hospitals were first called, on October 20th (week 43). Individuals working with ambulatory patients presenting with influenza or working with patients at high risk of complication for influenza were called on November 2nd (week 45). Risk factors of complication, stated in a High Committee of Public Health advice, on September 7th 2009, were: pregnancy (in particular from the second trimester), obesity, and chronic conditions such as bronco-pulmonary diseases, heart diseases, diabetes and immunosuppression [2]. On November 12th (week 46) all other health care professional were called (880,000), as well as all persons in contact with infants younger than six month-old (1,200,000), child-minders working with children under three year-old (500,000), and every person between six months and 64 years of age with a risk factor (2,815,000). Pregnant women from their second trimester and 6- to 23 month-old children without risk factor were called on November 20th (week 47). High school pupils were called on November 25th (week 48). People over 65 year-old with a risk factor (3,200,000) and children older than 23 month-old (7,700,000) and were called in week 49. Finally, adults over 18 year-old without a risk factor were called in week 53 (39,000,000). In the end, 63,295,000 persons had been called to vaccination centers to receive a pandemic influenza vaccine: all the French population, except infants younger than 6 month-old.
Figure 1. Weekly coverage of pandemic and seasonal vaccines in the population throughout the study period.
Red curves: vaccine coverage in the 6 month- to 4 year-old age group; Blue: in the 5 to 14 year-old age group; Green: in the 15 to 64 year-old age group; Violet: in the ≥65 year-old age group. Pandemic vaccination targeted different at-risk groups, which were called in turn, according to a calendar established by French public health authorities. The principal steps of this calendar are outlined below the figure. Grey hatched area: epidemic study period for the effectiveness of pandemic vaccines (weeks 46/2009 to 52/2009); Pink area: epidemic study period for seasonal vaccines (weeks 42/2009 to 52/2009); Blue area: post-epidemic study period for pandemic and seasonal vaccines (weeks 05/2010 to 34/2010).
Seasonal vaccines were available to everyone at high risk of seasonal influenza related complications, from September 21st (week 39/2009). For both vaccines, most vaccinations were completed by the end of January 2010.
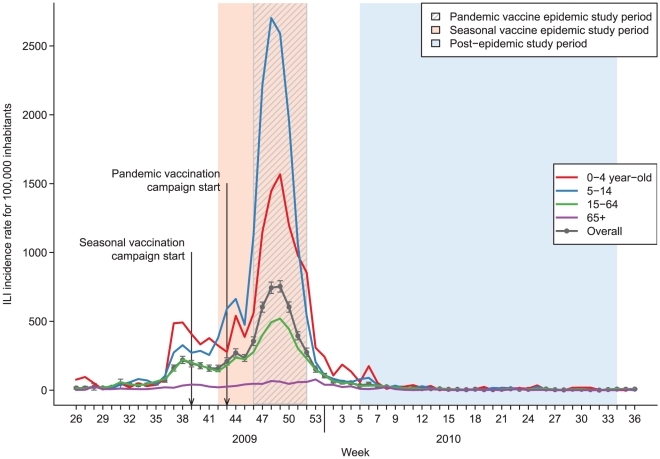
In France, influenza-like illness (ILI) incidence has been monitored since 1984 by the Sentinelles network, a surveillance system based on sentinel general practitioners (GPs) [3]. ILI incidence crossed the epidemic threshold in week 37/2009, while detection of 2009 pandemic A(H1N1) viruses remained sporadic until week 42 (see Figure 2). ILI incidence peaked in week 49 and fell below the epidemic threshold in week 53. During the epidemic, the pandemic strain was dominant (99.7%) among influenza virus isolates [4]. In the first weeks of the pandemic, the sharpest increase in ILI incidence was observed in children under five year-old. After five weeks, 5 to 14 year-old children became the most affected group. The biggest difference between age-specific incidence rates was observed at the peak of the epidemic. It can be seen in Figure 2 that adults over 65 year-old were almost unaffected by the pandemic wave in France.
Figure 2. Weekly ILI incidence rates during the 2009–2010 pandemic in France (including Corsica, excluding overseas territories).
Black curve: national ILI incidence rate and 95% confidence interval. Red curve: national ILI incidence rate in the 0 to 4 year-old age group; Blue: in the 5 to 14 year-old age group; Green: in the 15 to 64 year-old age group; Violet: in the ≥65 year-old age group.
The purpose of this study was to assess how effective the pandemic and 2009-2010 trivalent seasonal vaccines were in preventing ILI on the field during the 2009-2010 season, using surveillance data. To evaluate how using ILI instead of a more specific influenza endpoint biased VE estimates, we conducted a validation study on a sample of laboratory-confirmed A(H1N1) influenza. We also assessed the existence of selection biases by estimating VE outside the influenza-circulating period.
Methods
The Orenstein's screening method was used to calculate vaccine effectiveness with the following formula:
where PCV is the proportion of vaccinated among influenza cases and PPV is the proportion of vaccinated among the population [5], [6]. PPV was obtained from administrative sources. Two influenza datasets were used: one of clinically defined ILI cases, and one of laboratory-confirmed pandemic A(H1N1) influenza cases.
One injection was considered sufficient to confer a vaccinated status [7], [8]. Because influenza vaccines were not given to children under six month-old, they were excluded from the study. Individuals with missing age or vaccination status were also excluded.
Study period
For ILI data, an epidemic and a post-epidemic study period were defined for each vaccine. The epidemic study period began three weeks after the start of the vaccination campaign: in week 42/2009 (October 12th) for seasonal vaccines and in week 46/2009 (November 9th) for pandemic vaccines, and lasted until week 52/2009, end of the epidemic. The post-epidemic study period started in week 05/2010 (February 1st) and lasted until week 34/2010 (August 29th). Weeks 53/2009 to 04/2010 constituted a “washout period” since residual circulation of influenza viruses was observed even though the epidemic itself was over. Study periods are shown in Figure 1 and 2.
For laboratory-confirmed data, a single study period, spanning weeks 46/2009 to 04/2010, was defined.
Case recruitment
ILI cases were reported by Sentinelles GPs in France excluding overseas territories but including Corsica, as part of a surveillance routine using the following definition: “sudden onset of fever >39°C (>102°F) with respiratory signs and myalgia” [3].
Laboratory-confirmed influenza cases in France excluding Corsica and overseas territories were reported by physicians from the Regional Groups of Influenza Observation (GROG) as part of a surveillance routine. GROG is a network of private-practice GP and pediatricians dedicated to the virological surveillance of influenza [9]. Corsican laboratory-confirmed influenza cases were reported by Sentinelles GPs [10]. Nasopharyngeal swabs were collected through a randomized selection routine. Doctors included the first patient of each week, of any age (Sentinelles protocol) or of a personally assigned age group among 0–4, 5–14, 15–64, and ≥65 year-old (GROG protocol). Only patients presenting between 0 and 7 days after symptom onset were swabbed.
GROG doctors swabbed patients presenting with acute respiratory infection, defined as: sudden onset of a respiratory sign (cough, rhinitis, coryza,…) and a systemic sign evoking an infection (fever, asthenia, headache, myalgia, faintness). Corsican Sentinelles GPs swabbed patients presenting with ILI according to the Sentinelles definition. Swabs were analyzed in 11 laboratories, depending on the region of swabbing (two national reference centers and nine laboratories), by real-time polymerase chain reaction and/or culture.
For both ILI and laboratory-confirmed influenza cases, the following information was included: date of consultation, age, status regarding pandemic and seasonal influenza vaccination, and a logical indicating whether the time from vaccination to consultation was ≤3 or >3 weeks. The viral strain was known for laboratory-confirmed influenza cases.
Population data
The pandemic vaccine coverage in the population was calculated from the National Health Insurance database (Caisse Nationale d'Assurance Maladie). The provided database contained weekly counts of all persons vaccinated in France (excluding overseas territories and including Corsica) with a pandemic vaccine, from the start of the vaccination campaign to week 18 of 2010. Detailed coverage was provided for nine age groups: 6 month- to 4 year-old, 5–14, 15–24, 25–34, 35–49, 50–64, 65–69, 70–74, and ≥75 year-old. Counts from weeks 19 to 34 of 2010 were assumed to be equal to those during week 18, since the data show that very few pandemic vaccinations were completed after winter.
Pandemic vaccine coverage at week w, for age group g, 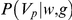 , was calculated as:
, was calculated as:
with 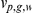 the number of people in age group g vaccinated with the pandemic vaccine before or during week w, and popg the population size of age group g in 2009 in France (with Corsica, without overseas territories).
the number of people in age group g vaccinated with the pandemic vaccine before or during week w, and popg the population size of age group g in 2009 in France (with Corsica, without overseas territories).
This vaccine coverage was monotonically increasing since 1) once vaccinated, people remained as such throughout the season and 2) the database contained all people vaccinated with a pandemic vaccine, so that no random fluctuation was present.
For seasonal vaccines, a similar database was not maintained by the National Health Insurance. Instead, vaccine coverage in the population was estimated each month from September 2009 to April 2010 by the Health Surveillance Institute (Institut de Veille Sanitaire, InVS) using computer-assisted telephone surveys. Each month a random sample from the French population (excluding overseas territories and including Corsica) were interviewed during an, on average, 11 day window. During the first stage of each survey, the sampling frame was the telephone numbers list, stratified by region and town size. At the second stage, the sampling frame was household residents, stratified by age (<5 years, ≥5 years). Each month around 800 questionnaires were filled in. Details of the sample design are provided elsewhere [11]. Pandemic vaccination status was also asked from November 2009, enabling identification of the proportion of people vaccinated with both vaccines, 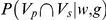 .
.
For the purpose of calculating the weekly seasonal vaccine coverage, we began by attributing the interview data to the weeks that accounted for most days of the interview windows. In other words, using the interview data, we first estimated the proportion of people vaccinated with seasonal vaccines, 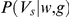 , and co-vaccinated with pandemic and seasonal vaccines,
, and co-vaccinated with pandemic and seasonal vaccines, 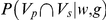 , for weeks 38, 42, 47 and 50 of 2009, and 2, 7, 11 and15 of 2010.
, for weeks 38, 42, 47 and 50 of 2009, and 2, 7, 11 and15 of 2010.
We then fitted logistic functions to these data points to obtain smoothed, monotonically increasing weekly predictions of vaccine coverage for all weeks in the study period: 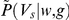 and
and 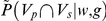 . To account for the end of the vaccination campaigns, the predicted coverage of seasonal vaccines and of co-vaccinations, were constrained to remain stable respectively from week 5 and week 11 of 2010.
. To account for the end of the vaccination campaigns, the predicted coverage of seasonal vaccines and of co-vaccinations, were constrained to remain stable respectively from week 5 and week 11 of 2010.
Finally, the weekly coverage of seasonal vaccines in people that did not get the pandemic vaccine, 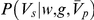 , was calculated as:
, was calculated as:
Estimation of field vaccine effectiveness
We used Farrington's implementation of the screening method to estimate VE adjusted on age and time [12]. In brief, VE was estimated with a logistic regression model allowing a different offset in each age × time strata. Age was divided into nine strata (6 month- to 4 year-old, 5–14, 15–24, 25–34, 35–49, 50–64, 65–69, 70–74 and ≥75 year-old), and time was divided into one week strata.
A consensus view is that a period of at least 14 days is needed to achieve a protective concentration of antibody after influenza vaccination, but this timeline may vary [13]. Herein, for pandemic and seasonal influenza vaccines, individuals were considered vaccinated three weeks after receiving a dose of vaccine, and unvaccinated if they had received no vaccine or if the vaccine had been given in the last three weeks.
In practice, for the calculation of VE in a week t, we compared the proportion of population vaccinated for more than three weeks at week t to the proportion of influenza cases vaccinated for more than three weeks that were seen by sentinel GPs in week t. Figure 1 shows the proportion of vaccinated in the population (plain lines), and the proportion of population that has been vaccinated for more than three weeks (dashed lines).
Since delay from injection was missing for some influenza cases, we ran two analyses. In the first one we assumed that these cases had received influenza vaccination in the three weeks preceding the consultation, and included them as unvaccinated. In the second analysis, we assumed that they had been vaccinated for more than three weeks and we included them as vaccinated. We explored in a third analysis what vaccine effectiveness was when vaccination was considered completed immediately after the injection of one vaccine dose. Because the question related to the interval between consultation and vaccination was dichotomized at three weeks, we could no further explore the protection timeline.
As trivalent 2009–2010 seasonal vaccines were expected to be less specific of 2009 A(H1N1) than pandemic vaccines, effectiveness of the seasonal vaccines was calculated only using those individuals that did not receive pandemic vaccination. As a sensitivity analysis, a second analysis included those cases.
Assessment of bias
The use of ILI, a non-specific influenza outcome, as a primary endpoint for estimating the effectiveness of influenza vaccines can bias VE estimates downward. Indeed, if only 50% of ILI cases are caused by influenza, a hundred percent effective influenza vaccine will have at most 50% effectiveness in preventing ILI: as the specificity of the outcome decreases, so do VE estimates [14]–[16]. To assess this first source of bias, we compared VE estimates obtained using the ILI case sample provided by Sentinelles GPs to VE estimates obtained using the confirmed pandemic A(H1N1) influenza cases provided by GROG and some Sentinelles GPs.
A second source of bias was identified in that we used observational data of patients consulting a GP for an influenza episode, and compared their vaccine coverage to the one in the general population. Patients consulting a GP for an influenza episode might not be comparable to the rest of the population. In particular, if people vaccinated with influenza vaccine consulted differently than the rest of the population for an influenza episode, because of comorbidities or because of a different propensity to seek care for example, this could be a confusion factor when estimating VE. As suggested by several authors, such a selection bias should affect VE estimated on GP data during the influenza-circulation period as well as VE estimated outside this period [17]–[22]. This is particularly interesting when using ILI, as influenza vaccine effectiveness in preventing ILI should be null outside the influenza-circulation period, if no bias was present. We therefore calculated VE against ILI after the influenza epidemic period, as an indication of selection bias in our data. As no vaccine was given before the epidemic start, pre-epidemic VE could not be assessed. We did not calculate VE against laboratory-confirmed A(H1N1) influenza outside the influenza-circulating period.
Results
ILI cases
Pandemic vaccines
During the study period, Sentinelles GPs reported 7586 ILI cases above 6 month-old, with information on age and pandemic vaccine status, which were included in the pandemic vaccines analysis. Among them, 172 (2%) were vaccinated. Detailed numbers by study period and age group are presented in Table 1.
Table 1. Pandemic vaccine uptake among ILI cases included in the pandemic vaccine effectiveness analysis.
| Epidemic study period | Post-epidemic study period | |||||||||
| Vaccinated for | Vaccinated for | |||||||||
| Age group | Total described | Total vaccinated | >3 weeks | Unknown | ≤3 weeks | Total described | Total vaccinated | >3 weeks | Unknown | ≤3 weeks |
| 6 m–4 y | 875 | 43 | 13 | 1 | 29 | 51 | 5 | 4 | 1 | 0 |
| 5–14 | 2740 | 44 | 6 | 4 | 34 | 89 | 9 | 7 | 0 | 2 |
| 15–24 | 1250 | 13 | 1 | 2 | 10 | 55 | 2 | 2 | 0 | 0 |
| 25–34 | 763 | 7 | 1 | 0 | 6 | 80 | 3 | 3 | 0 | 0 |
| 35–49 | 937 | 19 | 2 | 0 | 17 | 135 | 11 | 11 | 0 | 0 |
| 50–64 | 378 | 8 | 1 | 0 | 7 | 68 | 6 | 5 | 0 | 1 |
| 65–69 | 46 | 0 | 0 | 0 | 0 | 11 | 0 | 0 | 0 | 0 |
| 70–74 | 28 | 2 | 0 | 0 | 2 | 10 | 0 | 0 | 0 | 0 |
| ≥75 | 55 | 0 | 0 | 0 | 0 | 15 | 0 | 0 | 0 | 0 |
| Total | 7072 | 136 | 24 | 7 | 105 | 514 | 36 | 32 | 1 | 3 |
Pandemic influenza vaccination had been done in the three weeks preceding the consultation for 108 vaccinated cases (63%), more than three weeks before consultation for 56 cases (33%), or at an unknown date for 8 of them. During the pandemic period, 67% (29/43) of the 6 month- to 4 year-old ILI cases were vaccinated in the three weeks preceding the consultation, versus 77% (34/44) of the 5 to14 year-old and 86% (42/49) of the 15 to 64 year-old (Fisher's test p = 0.12). None of the two vaccinated ILI cases ≥65 year-old reported by Sentinelles GPs during the pandemic had been vaccinated for more than three weeks.
Six month to four year-old children mostly received the non-adjuvanted Panenza® vaccine (21/48 i.e. 44%); four of them (8%) received the adjuvanted Pandemrix® vaccine; trademark was unknown for the remaining 23 ones. On the contrary, 5 to 14 year-old children mostly received the Pandemrix® vaccine (22/53 i.e. 42%); nine of them (17%) received the non-adjuvanted Panenza® vaccine; trademark was unknown for the remaining 22 ones. Most cases aged15 year-old and over received the Pandemrix® vaccine (43/71 i.e. 61%); four had the Panenza®, four the Q-Pan H1N1® and one the Focetria® vaccine; trademark was unknown for 19. Most cases received one dose of vaccine (154/172 i.e. 90%). Eight cases received two doses of vaccines: three children below 15 year-old and five 35 to 64 year-old adults.
Seasonal vaccines
For the purpose of estimating the effectiveness of seasonal influenza vaccines, 9564 cases were eligible. Among them, 540 (6%) were vaccinated. Detailed numbers by study period and age group are presented in Table 2. Vaccination had been done in the three weeks preceding the consultation for 115 cases (21%), more than three weeks before consultation for 401 cases (74%), or at an unknown date for 24 of them. Sixty-one of them (11%) were also vaccinated with the pandemic vaccine, while 465 (86%) were not, the remaining 14 patients having not answered the pandemic vaccination question.
Table 2. Seasonal vaccine uptake among ILI cases included in the seasonal vaccine effectiveness analysis.
| Epidemic study period | Post-epidemic study period | |||||||||
| Vaccinated for | Vaccinated for | |||||||||
| Age group | Total described | Total vaccinated | >3 weeks | Unknown | ≤3 weeks | Total described | Total vaccinated | >3 weeks | Unknown | ≤3 weeks |
| 6 m–4 y | 1123 | 18 | 12 | 1 | 5 | 54 | 3 | 3 | 0 | 0 |
| 5–14 | 3447 | 73 | 43 | 2 | 28 | 100 | 4 | 4 | 0 | 0 |
| 15–24 | 1571 | 48 | 38 | 2 | 8 | 59 | 3 | 3 | 0 | 0 |
| 25–34 | 1020 | 34 | 21 | 4 | 9 | 83 | 6 | 5 | 0 | 1 |
| 35–49 | 1205 | 95 | 69 | 6 | 20 | 145 | 24 | 20 | 1 | 3 |
| 50–64 | 486 | 95 | 72 | 4 | 19 | 76 | 21 | 20 | 0 | 1 |
| 65–69 | 57 | 23 | 18 | 1 | 4 | 11 | 3 | 2 | 1 | 0 |
| 70–74 | 36 | 23 | 14 | 1 | 8 | 11 | 8 | 8 | 0 | 0 |
| ≥75 | 65 | 48 | 40 | 0 | 8 | 15 | 11 | 9 | 1 | 1 |
| Total | 9010 | 457 | 327 | 21 | 109 | 554 | 83 | 74 | 3 | 6 |
Laboratory-confirmed cases
Pandemic vaccines
Together, the GROG and Sentinelles samples provided 838 laboratory-confirmed pandemic A(H1N1) influenza cases ≥6 month-old that were described for pandemic vaccination and age, and were included in the analysis of pandemic vaccines. We did not include in the analysis those influenza isolates that were not typed (18), were of not-subtyped type A (26), or were of subtype A(H3N2) (one). No B strain was present in the sample.
Twenty-five of the included A(H1N1) cases were vaccinated (3%). Detailed numbers by age group are presented in Table 3. It is noteworthy that none of the five confirmed A(H1N1) cases aged ≥65 year-old that were included in the study had received a pandemic vaccine.
Table 3. Pandemic vaccine uptake among laboratory-confirmed cases included in the pandemic vaccine effectiveness analysis.
| Vaccinated for | |||||
| Age group | Total described | Total vaccinated | >3 weeks | Unknown | ≤3 weeks |
| 6 m–4 y | 209 | 13 | 0 | 2 | 11 |
| 5–14 | 339 | 9 | 1 | 0 | 8 |
| 15–24 | 93 | 1 | 1 | 0 | 0 |
| 25–34 | 78 | 0 | 0 | 0 | 0 |
| 35–49 | 85 | 0 | 0 | 0 | 0 |
| 50–64 | 29 | 2 | 0 | 1 | 1 |
| 65–69 | 2 | 0 | 0 | 0 | 0 |
| 70–74 | 0 | 0 | 0 | 0 | 0 |
| ≥75 | 3 | 0 | 0 | 0 | 0 |
| Total | 838 | 25 | 2 | 3 | 20 |
Six month- to four year-old children mostly received the non-adjuvanted Panenza® vaccine (12/13 i.e. 92%); only one of them received the adjuvanted Pandemrix® vaccine. Four of the nine vaccinated children between five and fourteen year-old were given the Panenza® vaccine (44%); three receive the Pandemrix® vaccine (33%); two received a pandemic vaccine of unknown brand. Among the three vaccinated cases above 15 years, two received the Pandemrix® and one the Panenza® vaccine.
Vaccination had been done in the three weeks preceding the consultation for 20 of the 25 vaccinated cases (80%), before that delay for two cases (8%), or at an unknown date for three of them (12%). Eighty-five percent (11/13) of the 6 month- to 4 year-old laboratory-confirmed cases were vaccinated in the three weeks preceding the consultation, versus 89% (8/9) of the 5 to14 year-old and 33% (1/3) of the 15 to 64 year-old (Fisher's test p = 0.16). Twenty-three cases (92%) had received one dose of vaccine, the other two cases did not precise this information. None has received two injections.
Seasonal vaccines
The laboratory-confirmed case sample contained 856 pandemic A(H1N1) cases that were described for age and seasonal vaccine status and could be included in the study. Fifty-one of them (6%) were vaccinated. Detailed numbers by age group are presented in Table 4.
Table 4. Seasonal vaccine uptake among laboratory-confirmed cases included in the seasonal vaccine effectiveness analysis.
| Vaccinated for | |||||
| Age group | Total described | Total vaccinated | >3 weeks | Unknown | ≤3 weeks |
| 6 m–4 y | 213 | 5 | 2 | 0 | 3 |
| 5–14 | 342 | 17 | 11 | 2 | 4 |
| 15–24 | 98 | 4 | 3 | 0 | 1 |
| 25–34 | 82 | 3 | 0 | 2 | 1 |
| 35–49 | 86 | 7 | 4 | 3 | 0 |
| 50–64 | 30 | 10 | 5 | 4 | 1 |
| 65–69 | 2 | 2 | 1 | 1 | 0 |
| 70–74 | 0 | 0 | 0 | 0 | 0 |
| ≥75 | 3 | 3 | 3 | 0 | 0 |
| Total | 856 | 51 | 29 | 12 | 10 |
Vaccination had been done in the three weeks preceding the consultation for 10 of the vaccinated cases (20%), more than three weeks before consultation for 29 cases (57%), or at an unknown date for 12 of them (24%). All confirmed pandemic A(H1N1) cases ≥65 year-old included in the study had received the seasonal vaccine. Five (10%) of the cases vaccinated with a seasonal vaccine had also received a pandemic vaccine, while 43 (84%) had not.
Field vaccine effectiveness
As exposed in the method section, three analyses were carried out. 1) In the first one (primary one), vaccination is considered completed three weeks after injection and all influenza cases with missing injection date are treated as unvaccinated. 2) As a first sensitivity analysis, influenza cases with missing vaccination date are treated as vaccinated. 3) Vaccination is considered completed immediately after the injection of one vaccine dose.
Since, during the pandemic, most reported influenza cases were vaccinated in the three weeks that preceded the consultation, considering missing injection date as belonging to the last three weeks, as was done in the first analysis, might be the more realistic option. The first analysis is thus considered the primary one, and its main results are reported hereafter. Detailed results of the three analyses are provided in Table 5 and Table 6.
Table 5. Field effectiveness of pandemic vaccines, by case definition, age group and study period.
| Age group | FVE (%) | 95% CI | |
| ILI, epidemic period | |||
| Vaccinated for ≤3 weeks or at an unknown date are considered unvaccinated | 6 m–4 y | 12 | (−47–53) |
| 5–14 | 53 | (1–82) | |
| 15–64 | 77 | (51–92) | |
| ≥65 | 100 | – | |
| All ages | 52 | (30–69) | |
| Vaccinated for ≤3 weeks are considered unvaccinated. Vaccinated at an unknown date are considered vaccinated. | 6 m–4 y | 5 | (−57–47) |
| 5–14 | 18 | (−47–60) | |
| 15–64 | 68 | (38–86) | |
| ≥65 | 100 | – | |
| All ages | 38 | (13–58) | |
| All vaccinees are considered vaccinated, regardless of the injection date | 6 m–4 y | 53 | (36–66) |
| 5–14 | 59 | (46–70) | |
| 15–64 | 49 | (33–62) | |
| ≥65 | 30 | (−124–88) | |
| All ages | 54 | (45–61) | |
| ILI, post-epidemic period | |||
| Vaccinated for ≤3 weeks or at an unknown date are considered unvaccinated | 6 m–4 y | 65 | (14–90) |
| 5–14 | 28 | (−45–70) | |
| 15–64 | 11 | (−38–47) | |
| ≥65 | 100 | – | |
| All ages | 33 | (4–55) | |
| Vaccinated for ≤3 weeks are considered unvaccinated. Vaccinated at an unknown date are considered vaccinated. | 6 m–4 y | 56 | (−2–85) |
| 5–14 | 28 | (−45–70) | |
| 15–64 | 11 | (−38–47) | |
| ≥65 | 100 | – | |
| All ages | 31 | (1–53) | |
| All vaccinees are considered vaccinated, regardless of the injection date | 6 m–4 y | 56 | (−2–85) |
| 5–14 | 5 | (−78–56) | |
| 15–64 | 6 | (−44–43) | |
| ≥65 | 100 | – | |
| All ages | 24 | (−7–47) | |
| Laboratory-confirmed influenza | |||
| Vaccinated for ≤3 weeks or at an unknown date are considered unvaccinated | 6 m–4 y | 100 | – |
| 5–14 | 72 | (−28–98) | |
| 15–64 | 74 | (−15–99) | |
| All ages | 86 | (56–98) | |
| Vaccinated for ≤3 weeks are considered unvaccinated. Vaccinated at an unknown date are considered vaccinated. | 6 m–4 y | 69 | (1–95) |
| 5–14 | 72 | (−28–98) | |
| 15–64 | 48 | (−64–91) | |
| All ages | 64 | (21–87) | |
| All vaccinees are considered vaccinated, regardless of the injection date | 6 m–4 y | 44 | (4–70) |
| 5–14 | 44 | (−4–73) | |
| 15–64 | 69 | (19–92) | |
| All ages | 49 | (25–67) | |
Table 6. Field effectiveness of seasonal vaccines, by case definition, age group and study period.
| Excluding pandemic vaccine recipients | Including pandemic vaccine recipients | ||||
| Age group | FVE (%) | 95% CI | FVE (%) | 95% CI | |
| ILI, epidemic period | |||||
| Vaccinated for ≤3 weeks or at an unknown date are considered unvaccinated | 6 m–4 y | 77 | (59–89) | 72 | (54–85) |
| 5–14 | 84 | (78–89) | 87 | (83–91) | |
| 15–64 | 52 | (44–59) | 47 | (38–54) | |
| ≥65 | 19 | (−14–42) | 21 | (−10–44) | |
| All ages | 61 | (56–66) | 63 | (58–67) | |
| Vaccinated for ≤3 weeks are considered unvaccinated. Vaccinated at an unknown date are considered vaccinated. | 6 m–4 y | 75 | (56–87) | 70 | (51–84) |
| 5–14 | 83 | (77–88) | 87 | (82–90) | |
| 15–64 | 47 | (39–55) | 42 | (33–50) | |
| ≥65 | 14 | (−20–38) | 17 | (−16–40) | |
| All ages | 58 | (53–63) | 60 | (55–64) | |
| All vaccinees are considered vaccinated, regardless of the injection date | 6 m–4 y | 67 | (44–82) | 62 | (42–77) |
| 5–14 | 69 | (60–76) | 79 | (73–83) | |
| 15–64 | 41 | (33–49) | 41 | (33–48) | |
| ≥65 | −7 | (−50–23) | −2 | (−43–27) | |
| All ages | 47 | (42–53) | 54 | (49–58) | |
| ILI, post-epidemic period | |||||
| Vaccinated for ≤3 weeks or at an unknown date are considered unvaccinated | 6 m–4 y | 54 | (−112–97) | −18 | (−220–71) |
| 5–14 | 61 | (−22–94) | 61 | (7–88) | |
| 15–64 | 7 | (−32–37) | 7 | (−27–33) | |
| ≥65 | 25 | (−52–62) | 39 | (−23–70) | |
| All ages | 19 | (−10–41) | 19 | (−5–39) | |
| Vaccinated for ≤3 weeks are considered unvaccinated. Vaccinated at an unknown date are considered vaccinated. | 6 m–4 y | 54 | (−112–97) | −18 | (−220–71) |
| 5–14 | 61 | (−22–94) | 61 | (7–88) | |
| 15–64 | 7 | (−32–37) | 4 | (−30–31) | |
| ≥65 | 3 | (−99–52) | 22 | (−62–61) | |
| All ages | 14 | (−16–38) | 15 | (−11–35) | |
| All vaccinees are considered vaccinated, regardless of the injection date | 6 m–4 y | 54 | (−108–97) | −16 | (−216–72) |
| 5–14 | 61 | (−23–94) | 62 | (8–88) | |
| 15–64 | −4 | (−47–28) | −7 | (−44–22) | |
| ≥65 | −6 | (−119–47) | 17 | (−72–59) | |
| All ages | 5 | (−27–31) | 7 | (−21–29) | |
| Laboratory-confirmed influenza | |||||
| Vaccinated for ≤3 weeks or at an unknown date are considered unvaccinated | 6 m–4 y | 74 | (18–96) | 77 | (29–96) |
| 5–14 | 60 | (25–82) | 65 | (40–82) | |
| 15–64 | 64 | (36–82) | 59 | (29–78) | |
| All ages | 60 | (41–74) | 61 | (44–74) | |
| Vaccinated for ≤3 weeks are considered unvaccinated. Vaccinated at an unknown date are considered vaccinated. | 6 m–4 y | 74 | (18–96) | 77 | (29–96) |
| 5–14 | 50 | (11–75) | 59 | (31–78) | |
| 15–64 | 29 | (−11–57) | 24 | (−16–53) | |
| All ages | 38 | (14–57) | 43 | (23–60) | |
| All vaccinees are considered vaccinated, regardless of the injection date | 6 m–4 y | 31 | (−51–75) | 46 | (−18–81) |
| 5–14 | 26 | (−22–59) | 48 | (18–69) | |
| 15–64 | 22 | (−18–51) | 25 | (−12–52) | |
| All ages | 20 | (−7–42) | 35 | (14–52) | |
Effectiveness of pandemic vaccines
When vaccination was considered completed three weeks after the injection of one vaccine dose, the effectiveness of pandemic vaccine in preventing ILI was 52% (95% confidence interval 30–69) in the epidemic period, and 33% (4–55) in the post-epidemic period. When vaccination was considered completed immediately after injection, pandemic vaccine effectiveness in preventing ILI was 54% (45–61) in the epidemic period and 24% (−7–47) in the post-epidemic period.
When vaccination was considered completed three weeks after injection, pandemic vaccine effectiveness in preventing laboratory-confirmed pandemic A(H1N1) influenza was 86% (56–98). When vaccination was considered completed immediately after injection, pandemic vaccine effectiveness in preventing laboratory-confirmed pandemic A(H1N1) influenza was 49% (25–67).
The effectiveness of pandemic vaccines in preventing ILI and confirmed pandemic A(H1N1) influenza in different age groups is presented in Table 5. As no confirmed case above 65 year-old was vaccinated, the age-specific effectiveness of pandemic vaccine in this age group is not presented.
Effectiveness of seasonal vaccines
In the primary analysis of the field effectiveness of trivalent 2009–2010 seasonal vaccine, shown below, we excluded those individuals that received pandemic vaccination. The results of the analysis that included them are shown in the last column of Table 6.
When vaccination was considered completed three weeks after the injection of one vaccine dose, the effectiveness of seasonal vaccine in preventing ILI was 61% (56–66) in the epidemic period, and 19% (−10–41) in the post-epidemic period. When vaccination was considered completed immediately after injection, seasonal vaccine effectiveness in preventing ILI was 47% (42–53) in the epidemic period and 5% (−27–31) in the post-epidemic period.
When vaccination was considered completed three weeks after the injection of one vaccine dose, the effectiveness of seasonal vaccine in preventing laboratory-confirmed pandemic A(H1N1) influenza was 60% (41–74). When vaccination was considered completed immediately after injection, seasonal vaccine effectiveness in preventing laboratory-confirmed pandemic A(H1N1) influenza was 20% (−7–42).
The effectiveness of seasonal vaccines in preventing ILI and confirmed pandemic A(H1N1) influenza by age groups is presented in Table 6. Since only five laboratory-confirmed pandemic A(H1N1) cases older than 65 year-old were included in the analysis, we do not present age-specific VE for this age group.
Discussion
We obtained estimates of vaccine effectiveness by comparing vaccine coverage of influenza cases (clinically defined or biologically confirmed) to vaccine coverage of population samples, using Orenstein's screening method [5],[12]. To allow for a delay between the injection of a vaccine dose and immunization, vaccination was considered completed only three weeks after injection. In other words, in our primary analysis, individuals vaccinated for less than three weeks were considered unvaccinated. As a consequence of the late vaccination campaigns, the number of vaccinations we could consider completed during the pandemic was low.
Pandemic vaccine effectiveness
We found that pandemic vaccines had a field effectiveness of 86% (56–98) in preventing laboratory-confirmed pandemic A(H1N1) influenza, which is in the range of previously reported values [7], [8], [23]-[26]. More specifically, our estimates are slightly above the results of a multicenter case-control study conducted in seven European countries and are above the results of a case-control study conducted in the United Kingdom, which both found VE = 72% [8], [23]. They are also above the VE found by a Korean study, namely 73% [24]. However, they are below the estimates from a Scottish cohort study (95%) [25], a German study based on the screening method (83–97%) [26] and a Canadian test-negative incident case-control study (>90%) [7].
We found that pandemic vaccines had a significantly lower effectiveness in preventing ILI than in preventing confirmed A(H1N1) influenza: 52% (30–69) versus 86% (56–98). This is unsurprising as only a part of the 2009–2010 ILI cases were due to pandemic A(H1N1) influenza and therefore could be prevented by the pandemic vaccine. This part was estimated between 30% and 55%, depending on the country [27], [28].
The effectiveness of pandemic vaccines in preventing ILI and confirmed influenza seemed to vary with age, yet not significantly and in no reproducible order through our different analysis. Since few reported influenza cases were vaccinated, large confidence intervals surround our age-specific VE estimates. In particular, no confirmed A(H1N1) case and only two ILI cases above 65 year-old were vaccinated with the pandemic vaccine. Indeed, elderly people were not a priority target group for pandemic vaccination and were called late to vaccination centers (Figure 1). Estimations of vaccine effectiveness in this subgroup are therefore highly uncertain.
As others, we considered that one dose of pandemic vaccine was sufficient to provide immunization for all age classes [7], [8], [23], [24], [26]. However, it was shown that a single dose of pandemic A(H1N1) vaccine was more immunogenic in children older than three years than in younger children, and that a second dose was needed to reach seroprotection and seroconversion rates of 90–99% in both of these age groups [29].
Influenza vaccines are theoretically expected to be more effective three weeks after their injection than immediately after. We did observe a significantly higher effectiveness of pandemic vaccines in preventing laboratory-confirmed pandemic A(H1N1) influenza when only vaccinations dating back to more than three weeks were considered completed than when treating all vaccinations as completed: 86% (56–98) versus 49% (25–67). Yet, we did not find a significant difference in that respect regarding the effectiveness of pandemic vaccines against ILI: 52% (30–69) versus 54% (45–61), which is a first indication of the presence of biases in the estimation.
Seasonal vaccine effectiveness
We found that the 2009–2010 seasonal vaccines had a significant effectiveness in preventing laboratory-confirmed influenza: VE = 60% (41–74). Unlike pandemic vaccines, seasonal vaccines were not significantly better in preventing laboratory-confirmed pandemic A(H1N1) influenza than in preventing ILI: respectively VE = 60% (41–74) versus VE = 61% (56–66). This result cannot be explained by ILI comprising an appreciable number of seasonal influenza cases against which seasonal vaccine could have proven effective: more than 99% of influenza isolates during the 2009-2010 season were pandemic A(H1N1) [4]. This result might, instead, indicate that our effectiveness estimates of seasonal vaccines are driven by biases, in particular case selection biases. Indeed, the population that yearly vaccinates against seasonal influenza might be distinct from the rest of the population, in particular in its propensity to consult. This could induce a selection bias, since influenza cases are recruited through GPs. Therefore, the absence of adjustments in our study, when most studies adjusted by comorbidities and previous vaccination, could be a reason for the high effectiveness we observed.
We identified a source of bias in our seasonal vaccine study in that we used underestimated seasonal vaccine coverage for the 6 month- to 4 year-old population age group. Indeed, the vaccine coverage data provided by the InVS study concerned the 0–4 year-old age group, even if 0 to 6 month-old children were not concerned by seasonal influenza vaccination. Nevertheless, this should result in under-estimating, not over-estimating, vaccine effectiveness in this age group.
Our results come within a succession of contradictory evidences regarding the effectiveness of seasonal vaccines against pandemic 2009 A(H1N1) influenza. In a literature review by Viboud et al, four studies of the 2008–2009 seasonal vaccines found no protection against 2009 pandemic A(H1N1) influenza; two other found a significant protection; two other found that 2008–2009 seasonal vaccination increased the risk of contracting pandemic 2009 A(H1N1) influenza [30]. A subsequent paper evidenced a moderate effectiveness of the 2008–2009 seasonal vaccines against pandemic A(H1N1) mild outcomes (VE = 42%, 29–53%) [31], while another one put forward an increased risk (odds-ratio = 2.45, 1.34–4.48) [32].
Regarding the effectiveness of the 2009–2010 seasonal vaccines, most works found no protection against confirmed pandemic A(H1N1) [8], [23],[33]–[36], while one found an effectiveness of 50% (40–59) in preventing pandemic A(H1N1) related hospitalizations [37].
Adjustments
We adjusted by age to take into account different vaccine responses with age, and used weekly strata to account for the evolution of vaccine coverage in the population throughout the study period. As noted before, the lack of further adjustment could result in biased VE estimates, both for ILI and confirmed influenza. However, as our study was integrated in an ongoing surveillance system based on GP voluntary reporting of ILI cases, adjustment covariates were not collected in order to keep the questionnaire as short as possible. As a compromise, only essential covariates for VE calculation were asked to GPs: age, vaccine status (pandemic and seasonal), delay since vaccination (dichotomized at three weeks), and vaccine trademark (for pandemic vaccine only). Considering the small number of reported vaccinated influenza cases, pandemic VE was not calculated by vaccine trademark.
We used a three weeks delay between vaccination and consultation to differentiate vaccinated influenza cases fully immunized from those that were still unprotected. This delay might be too astringent: in most papers, a two week delay between vaccination and symptom onset is used [7], [23], [24], [26], as data on seasonal influenza showed that protective antibodies were present in over 90% of persons fourteen days after vaccination [13]. However, as the question used for ILI cases was “Is delay since vaccination ≤3 weeks?”, no data was available to make sensitivity analysis.
Biases
Bias due to the use of a non-specific endpoint
ILI is a non-specific proxy for influenza infection. Disposing of a validation sample of laboratory-confirmed pandemic A(H1N1) influenza cases allowed us to explore the bias in VE inherent to the use of such a non-specific outcome. Regarding the effectiveness of pandemic vaccines, the results are in accordance with the theoretical principle which stipulates that, as the specificity of the outcome decreases, so should the measured vaccine effectiveness [14]–[16]. Effectiveness of seasonal vaccines, however, did not show such a difference.
A few studies have used ILI as an endpoint for evaluating influenza vaccine effectiveness on the field [38]–[41], although laboratory-confirmed influenza is a more common endpoint. In particular, a case-control study based on university students reporting ILI episodes through a web interface evidenced a significant reduction of ILI among vaccinated students during seasonal influenza (adjusted odds ratio: 0.70, 95% confidence interval 0.56–0.89) but not during non-influenza periods (adjusted odds ratio: 0.98, 0.73–1.30) [42].
Herein, we do observe a lower effectiveness of pandemic vaccines in preventing ILI after the epidemic period (VE = 33%, 4–55); however, unlike in Nichol et al, the effectiveness remains significantly above zero. As suggested by previous works, we can attribute this overestimation to the presence of biases, such as selection biases [17]–[22]. Stochastic variations due to our little sample sizes are another possible explanation.
Sensitivity of the outcome “ILI consulting a GP” to track true influenza is also an issue: asymptomatic cases, subclinical cases and presentations different from the chosen ILI definition are missed. Call et al reviewed the probabilities of presence of different symptoms in influenza cases, i.e. the sensitivity of these symptoms for detecting influenza: depending on the age group, they were between 0.3 and 0.9, with lower sensitivities in the elderly [43]. Orenstein et al demonstrated in a simulation study that poorly sensitive outcomes could lead to underestimation of field vaccine effectiveness in three different observational designs [16]. However, our analysis seems more subject to overestimation biases.
Bias due to incomparability of cases and population sample
Another potential source of bias in our study is the uncertain comparability of cases recruited by GPs with samples drawn from the general population. To further assess the possible lack of comparability between cases and population samples, an alternative population sample taken directly among GPs usual patients could be used for validation. To that effect, during 2010–2011 influenza season, a cross-sectional survey at GPs offices will be carried out to assess the vaccine coverage among Sentinelles GPs patients.
Selection biases
As was visible in the results of seasonal vaccine effectiveness, selection biases might be present in our study design and bias result upwards. To try and further evidence these selection biases, vaccine effectiveness against ILI was computed after the influenza circulation period. Effectiveness of pandemic vaccine during this period was significantly above zero (33%, 4–57), although not significant in most age subgroups except in the six to fourteen year-old group. Effectiveness of seasonal vaccines was, as for it, not significant, whether overall (19%, −10–41) or in each age subgroup. In conclusion, for both vaccine types, a moderate vaccine effectiveness was found after the 2009 A(H1N1) influenza virus stopped circulating at appreciable levels, which is indicative of upward biases in our VE estimates, but this post-pandemic effectiveness was mainly not statistically significant.
Acknowledgments
The authors wish to thank all the participating general practitioners, Pierre-Yves Boëlle for his help on statistical methodology aspects, Angie Bone for her help with data management and the two anonymous referees for helping improving the manuscript.
Footnotes
Competing Interests: Several authors have competing interests, unrelated to the submitted study: SVDW has had a conference invitation from GSK; a research grant from GSK on an unrelated subject; joined patent from institution with GSK on an unrelated subject: vaccines against severe acute respiratory syndrome coronavirus (SARS-CoV) infection and their use in the prevention of SARS (patent US 2010/0233250 A1, published September 16 2010); travel grants for meetings from GSK; contributed to a clinical trial financed by Roche; is a member of the advisory committee on influenza of the French ministry of health; is a member of ESWI; is a member of the scientific committee of the GEIG; and is vice-president of the GROG network. BL has had paid consultancy and board membership (Roche, GSK, Novartis, BioCryst, MedImmune); has had research grants from Roche and Sanofi-Pasteur; and has received travel grants and honoraria for speaking or participation at meetings (Roche, Sanofi-Pasteur). TB has had a conference invitation from Roche in 2010 and is a member of the scientific committee of the GEIG. JMC has involvement in some epidemiological studies partially or fully granted by Roche and GSK, and travel grants from Roche for participation in scientific meetings. AM has a membership in the ministry of health advisory board on influenza; involvement in some epidemiological studies partially or fully granted by Roche and GSK; and travel grants from Roche for participation in scientific meetings. FC was a consultant for Novartis and GSK. The other authors have declared that no competing interests exist.
Funding: This study was funded by a European Vaccine Manufacturers grant through the Epiconcept society. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Haut Conseil de la Santé Publique. List of prioritary target groups for pandemic vaccination (updated December 1, 2009). 2009. Available: http://www.sante-sports.gouv.fr/IMG/pdf/Personnes_invitees_a_se_faire_vacciner_par_ordre_de_priorite.pdf. Accessed 2011 Jan 10.
- 2.Haut Conseil de la Santé Publique. Recommendations on the sanitary priorities of influenza A(H1N1)v pandemic vaccines (September 7, 2009). 2009. Available: http://www.sante-sports.gouv.fr/IMG/pdf/Personnes_invitees_a_se_faire_vacciner_par_ordre_de_priorite.pdf. Accessed 2011 Jan 10.
- 3.Valleron AJ, Bouvet E, Garnerin P, Menares J, Heard I, et al. A computer network for the surveillance of communicable diseases: the French experiment. Am J Public Health. 1986;76:1289–1292. doi: 10.2105/ajph.76.11.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rousset D, Bouscambert DM, Enouf V, Valette M, Cohen J, et al. A(H1N1)2009 pandemic in France: epidemiological features based on virological surveillance. Influenza and Other Respiratory Viruses Options VII Conference Proceedings. 2011 In press. [Google Scholar]
- 5.Orenstein WA, Bernier RH, Dondero TJ, Hinman AR, Marks JS, et al. Field evaluation of vaccine efficacy. Bull World Health Organ. 1985;63:1055–1068. [PMC free article] [PubMed] [Google Scholar]
- 6.Orenstein WA, Bernier RH, Hinman AR. Assessing vaccine efficacy in the field. Further observations. Epidemiol Rev. 1988;10:212–241. doi: 10.1093/oxfordjournals.epirev.a036023. [DOI] [PubMed] [Google Scholar]
- 7.Skowronski DM, Janjua NZ, De Serres G, Hottes TS, Dickinson JA, et al. Effectiveness of AS03 adjuvanted pandemic H1N1 vaccine: case-control evaluation based on sentinel surveillance system in Canada, autumn 2009. BMJ. 2011;342:c7297. doi: 10.1136/bmj.c7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valenciano M, Kissling E, Cohen JM, Oroszi B, Barret AS, et al. Estimates of Pandemic Influenza Vaccine Effectiveness in Europe, 2009-2010: Results of Influenza Monitoring Vaccine Effectiveness in Europe (I-MOVE) Multicentre Case-Control Study. PLoS Med. 2011;8:e1000388. doi: 10.1371/journal.pmed.1000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannoun C, Dab W, Cohen JM. A new influenza surveillance system in France: the Ile-de-France "GROG". 1. Principles and methodology. Eur J Epidemiol. 1989;5:285–293. doi: 10.1007/BF00144828. [DOI] [PubMed] [Google Scholar]
- 10.Falchi A, Varesi L, Arena C, Leveque N, Renois F, et al. Co-circulation of two genetically distinct sub-groups of A/H3N2 influenza strains during the 2006-2007 epidemic season in Corsica Island, France. J Clin Virol. 2009;45:265–268. doi: 10.1016/j.jcv.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Vaux S, Van Cauteren D, Guthmann JP, Le Strat Y, Vaillant V, et al. Influenza vaccination coverage against seasonal and pandemic influenza and their determinants in France: a cross-sectional survey. BMC Public Health. 2011;11:30. doi: 10.1186/1471-2458-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrington CP. Estimation of vaccine effectiveness using the screening method. Int J Epidemiol. 1993;22:742–746. doi: 10.1093/ije/22.4.742. [DOI] [PubMed] [Google Scholar]
- 13.Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol. 2004;59:1–15. doi: 10.1111/j.0300-9475.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 14.Halloran ME, Longini IM., Jr Using validation sets for outcomes and exposure to infection in vaccine field studies. Am J Epidemiol. 2001;154:391–398. doi: 10.1093/aje/154.5.391. [DOI] [PubMed] [Google Scholar]
- 15.Scharfstein DO, Halloran ME, Chu H, Daniels MJ. On estimation of vaccine efficacy using validation samples with selection bias. Biostatistics. 2006;7:615–629. doi: 10.1093/biostatistics/kxj031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orenstein EW, De Serres G, Haber MJ, Shay DK, Bridges CB, et al. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol. 2007;36:623–631. doi: 10.1093/ije/dym021. [DOI] [PubMed] [Google Scholar]
- 17.Mangtani P, Cumberland P, Hodgson CR, Roberts JA, Cutts FT, et al. A cohort study of the effectiveness of influenza vaccine in older people, performed using the United Kingdom general practice research database. J Infect Dis. 2004;190:1–10. doi: 10.1086/421274. [DOI] [PubMed] [Google Scholar]
- 18.Vu T, Farish S, Jenkins M, Kelly H. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine. 2002;20:1831–1836. doi: 10.1016/s0264-410x(02)00041-5. [DOI] [PubMed] [Google Scholar]
- 19.Fireman B, Lee J, Lewis N, Bembom O, van der Laan M, et al. Influenza vaccination and mortality: differentiating vaccine effects from bias. Am J Epidemiol. 2009;170:650–656. doi: 10.1093/aje/kwp173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eurich DT, Marrie TJ, Johnstone J, Majumdar SR. Mortality reduction with influenza vaccine in patients with pneumonia outside "flu" season: pleiotropic benefits or residual confounding? Am J Respir Crit Care Med. 2008;178:527–533. doi: 10.1164/rccm.200802-282OC. [DOI] [PubMed] [Google Scholar]
- 21.Rivetti D, Jefferson T, Thomas R, Rudin M, Rivetti A, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2006;3:CD004876. doi: 10.1002/14651858.CD004876.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Baxter R, Ray GT, Fireman BH. Effect of influenza vaccination on hospitalizations in persons aged 50 years and older. Vaccine. 2010 doi: 10.1016/j.vaccine.2010.08.088. [DOI] [PubMed] [Google Scholar]
- 23.Hardelid P, Fleming DM, McMenamin J, Andrews N, Robertson C, et al. Effectiveness of pandemic and seasonal influenza vaccine in preventing pandemic influenza A(H1N1)2009 infection in England and Scotland 2009-2010. Euro Surveill. 2011;16 [PubMed] [Google Scholar]
- 24.Song JY, Cheong HJ, Heo JY, Noh JY, Choi WS, et al. Effectiveness of the pandemic influenza A/H1N1 2009 monovalent vaccine in Korea. Vaccine. 2011;29:1395–1398. doi: 10.1016/j.vaccine.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 25.Simpson CR, Ritchie LD, Robertson C, Sheikh A, McMenamin J. Vaccine effectiveness in pandemic influenza - primary care reporting (VIPER): an observational study to assess the effectiveness of the pandemic influenza A (H1N1)v vaccine. Health Technol Assess. 2010;14:313–346. doi: 10.3310/hta14340-05. [DOI] [PubMed] [Google Scholar]
- 26.Wichmann O, Stocker P, Poggensee G, Altmann D, Walter D, et al. Pandemic influenza A(H1N1) 2009 breakthrough infections and estimates of vaccine effectiveness in Germany 2009-2010. Euro Surveill. 2010;15 [PubMed] [Google Scholar]
- 27.Furuse Y, Suzuki A, Kishi M, Galang HO, Lupisan SP, et al. Detection of novel respiratory viruses from influenza-like illness in the Philippines. J Med Virol. 2004;82:1071–1074. doi: 10.1002/jmv.21763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laguna-Torres VA, Gomez J, Aguilar PV, Ampuero JS, Munayco C, et al. Changes in the viral distribution pattern after the appearance of the novel influenza A H1N1 (pH1N1) virus in influenza-like illness patients in Peru. PLoS One. 2010;5:e11719. doi: 10.1371/journal.pone.0011719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plennevaux E, Blatter M, Cornish MJ, Go K, Kirby D, et al. Influenza A (H1N1) 2009 two-dose immunization of US children: an observer-blinded, randomized, placebo-controlled trial. Vaccine. 2011;29:1569–1575. doi: 10.1016/j.vaccine.2010.12.116. [DOI] [PubMed] [Google Scholar]
- 30.Viboud C, Simonsen L. Does Seasonal Influenza Vaccination Increase the Risk of Illness with the 2009 A/H1N1 Pandemic Virus? Plos medicine. 2009;7:e1000259. doi: 10.1371/journal.pmed.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johns MC, Eick AA, Blazes DL, Lee SE, Perdue CL, et al. Seasonal influenza vaccine and protection against pandemic (H1N1) 2009-associated illness among US military personnel. PLoS One. 2010;5:e10722. doi: 10.1371/journal.pone.0010722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janjua NZ, Skowronski DM, Hottes TS, Osei W, Adams E, et al. Seasonal influenza vaccine and increased risk of pandemic A/H1N1-related illness: first detection of the association in British Columbia, Canada. Clin Infect Dis. 2010;51:1017–1027. doi: 10.1086/656586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carcione D, Giele C, Goggin LS, Kwan KS, Smith DW, et al. Association between 2009 seasonal influenza vaccine and influenza-like illness during the 2009 pandemic: preliminary results of a large household transmission study in Western Australia. Euro Surveill. 2010;15 [PubMed] [Google Scholar]
- 34.Puig-Barbera J, Arnedo-Pena A, Pardo-Serrano F, Tirado-Balaguer MD, Perez-Vilar S, et al. Effectiveness of seasonal 2008-2009, 2009-2010 and pandemic vaccines, to prevent influenza hospitalizations during the autumn 2009 influenza pandemic wave in Castellon, Spain. A test-negative, hospital-based, case-control study. Vaccine. 2010;28:7460–7467. doi: 10.1016/j.vaccine.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 35.Carlson SJ, Durrheim DN, Dalton CB. Flutracking provides a measure of field influenza vaccine effectiveness, Australia, 2007-2009. Vaccine. 2010;28:6809–6810. doi: 10.1016/j.vaccine.2010.08.051. [DOI] [PubMed] [Google Scholar]
- 36.Jefferies S, Earl D, Berry N, Blackmore T, Rooker S, et al. Effectiveness of the 2009 seasonal influenza vaccine against pandemic influenza A(H1N1)2009 in healthcare workers in New Zealand, June-August 2009. Euro Surveill. 2011;16 [PubMed] [Google Scholar]
- 37.Orellano PW, Reynoso JI, Carlino O, Uez O. Protection of trivalent inactivated influenza vaccine against hospitalizations among pandemic influenza A (H1N1) cases in Argentina. Vaccine. 2010;28:5288–5291. doi: 10.1016/j.vaccine.2010.05.051. [DOI] [PubMed] [Google Scholar]
- 38.Oshitani H, Saito R, Seki N, Tanabe N, Yamazaki O, et al. Influenza vaccination levels and influenza-like illness in long-term-care facilities for elderly people in Niigata, Japan, during an influenza A (H3N2) epidemic. Infect Control Hosp Epidemiol. 2000;21:728–730. doi: 10.1086/501725. [DOI] [PubMed] [Google Scholar]
- 39.Kawai N, Ikematsu H, Iwaki N, Satoh I, Kawashima T, et al. A prospective, Internet-based study of the effectiveness and safety of influenza vaccination in the 2001-2002 influenza season. Vaccine. 2003;21:4507–4513. doi: 10.1016/s0264-410x(03)00508-5. [DOI] [PubMed] [Google Scholar]
- 40.Millot JL, Aymard M, Bardol A. Reduced efficiency of influenza vaccine in prevention of influenza-like illness in working adults: a 7 month prospective survey in EDF Gaz de France employees, in Rhone-Alpes, 1996-1997. Occup Med (Lond) 2002;52:281–292. doi: 10.1093/occmed/52.5.281. [DOI] [PubMed] [Google Scholar]
- 41.Ochiai H, Fujieda M, Ohfuji S, Fukushima W, Kondo K, et al. Inactivated influenza vaccine effectiveness against influenza-like illness among young children in Japan--with special reference to minimizing outcome misclassification. Vaccine. 2009;27:7031–7035. doi: 10.1016/j.vaccine.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 42.Nichol KL, D'Heilly S, Ehlinger EP. Influenza vaccination among college and university students: impact on influenzalike illness, health care use, and impaired school performance. Arch Pediatr Adolesc Med. 2008;162:1113–1118. doi: 10.1001/archpedi.162.12.1113. [DOI] [PubMed] [Google Scholar]
- 43.Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza? JAMA. 2005;293:987–997. doi: 10.1001/jama.293.8.987. [DOI] [PubMed] [Google Scholar]