Abstract
Background
More than one million new cases of sexually transmitted diseases (STDs) occur each day. The immune responses and inflammation induced by STDs and other frequent non-STD microbial colonizations (i.e. Candida and bacterial vaginosis) can have serious pathologic consequences in women including adverse pregnancy outcomes, infertility and increased susceptibility to infection by other pathogens. Understanding the types of immune mediators that are elicited in the lower genital tract by these infections/colonizations can give important insights into the innate and adaptive immune pathways that are activated and lead to strategies for preventing pathologic effects.
Methodology/Principal Findings
32 immune mediators were measured by multiplexed immunoassays to assess the immune environment of the lower genital tract mucosa in 84 women attending an urban STD clinic. IL-3, IL-1ß, VEGF, angiogenin, IL-8, ß2Defensin and ß3Defensin were detected in all subjects, Interferon-α was detected in none, while the remaining mediators were detected in 40% to 93% of subjects. Angiogenin, VEGF, FGF, IL-9, IL-7, lymphotoxin-α and IL-3 had not been previously reported in genital mucosal fluid from women. Strong correlations were observed between levels of TNF-α, IL-1ß and IL-6, between chemokines IP-10 and MIG and between myeloperoxidase, IL-8 and G-CSF. Samples from women with any STD/colonization had significantly higher levels of IL-8, IL-3, IL-7, IL-1ß, lactoferrin and myeloperoxidase. IL-1ß and lactoferrin were significantly increased in gonorrhea, Chlamydia, cervicitis, bacterial vaginosis and trichomoniasis.
Conclusions/Significance
These studies show that mucosal fluid in general appears to be an environment that is rich in immune mediators. Importantly, IL-1ß and lactoferrin are biomarkers for STDs/colonizations providing insights into immune responses and pathogenesis at this mucosal site.
Introduction
It is estimated that more than one million new cases of sexually transmitted diseases (STDs) occur each day worldwide [1]. STDs in women induce innate and/or adaptive immune responses that in most cases do not lead to clearance of the microbial infection, but can cause inflammation and/or influx of immune cells into the lower genital tract [2]. While not STDs, bacterial vaginosis and vaginal candidiasis are frequent microbial colonizations encountered in the setting of the STD clinic that also induce immune responses in the lower genital tract of women [3], [4]. The combination of the infecting/colonizing microorganisms along with the induced immune responses and inflammation can have serious pathologic consequences in women including infertility, adverse pregnancy outcomes, and increased susceptibility to infection by other pathogens [1], [5].
Identification of the immune mediators that are elicited in the lower genital tract by STDs and other microbial colonizations can potentially give important insights into the innate and adaptive immune pathways that are activated in response to the microorganisms, how the organisms are able to avoid clearance by these immune pathways as well as their pathologic mechanism. For example, some cytokines and chemokines are induced in the genital tract by STDs and this induction has been associated with increased susceptibility to infection with HIV suggesting a possible role for those immune mediators in increasing HIV susceptibility (Reviewed in [6]). Some genital immune mediators have been reported to be increased in trichomoniasis, Chlamydia trachomatis infection and in bacterial vaginosis [7], [8], [9]. However, most previous studies of STDs measured limited types of mediators (e.g. only proinflammatory mediators or chemokines) and the relationships between mediators and STDs have not been well defined.
In this study we tested genital mucosal fluid from women attending an STD clinic for 32 distinct immune mediators. The mediators measured in this study included pro-inflammatory cytokines (IL-1ß, TNF-α, IL-6), anti-inflammatory cytokines (IL-4, IL-10, IL-13), chemokines (MCP-1, RANTES, IL-8, IP-10, MIG, MIP-1α, MIP-1ß, eotaxin), anti-microbial proteins (ß2defensin, ß3defensin, lactoferrin), a marker for neutrophils (myeloperoxidase, MPO), interferons (interferon-α and interferon-γ), and other cytokines and growth factors (GM-CSF, G-CSF, IL-3, IL-12, IL-7, IL-5, vascular endothelial growth factor (VEGF), angiogenin, fibroblast growth factor (FGF), IL-9 and lymphotoxin-α). The goals of this study were to determine which of the immune mediators were detectable at this mucosal site, to determine if there were any strong associations between the different mediators, and to investigate the hypothesis that specific types or patterns of immune mediators would be broadly changed over all STDs or changed with particular pathogens. Such patterns could provide biomarkers predictive of pathogenesis and help identify immune responses important for immune control of pathogens.
Methods
Subjects
All studies were approved by the Institutional review boards of Rush University Medical Center and Cook County Stroger Hospital. The study population was recruited at the Ruth M. Rothstein CORE Center STD Screening Clinic of Cook County Stroger Hospital. Symptomatic, female patients presenting for an STD evaluation were approached for study participation and written informed consent was obtained from all subjects. Standard of care genital exams were performed and swabs taken for diagnosis. Cervical-vaginal lavage samples were then obtained by irrigation of the cervix with 10 mL of non-bacteriostatic sterile saline, followed by aspiration from the posterior fornix. Urine was also obtained for a pregnancy test. None of the subjects were pregnant. The following tests were performed; BD Probetec ET (Becton Dickinson, Franklin Lakes, NJ) for Chlamydia trachomatis and Neisseria gonorrhoeae; culture for herpes (serology was not run); for Syphilis a rapid plasma reagin test (Arlington Scientific, Utah) was performed and confirmed using passive particle agglutination (Fujirebio, Malvern, PA); for HIV, an enzyme immunoassay (Biorad Laboratories, Hercules, CA) was performed and confirmed by western blot; for Trichomonas, wet mount examination and ELISA for the p65 protein (HyTest, Turku, Finland) were both performed; for bacterial vaginosis, wet mount for clue cells, vaginal pH, whiff test, and Nugent gram stain were performed; for Candida a KOH preparation was examined microscopically; Pelvic Inflammatory Disease and Warts were diagnosed by clinical exam.
Immunoassays
All immunoassays, except interferon-α, lactoferrin, myeloperoxidase (MPO), human ßdefensin2 (HßD2) and human ßdefensin3 (HßD3) utilized Cytometric Bead Arrays (BD Biosciences, San Jose, CA). The commercial arrays had lower limits of detection of 1–3 pg/ml. Custom cytometric bead arrays were made to detect interferon-α, lactoferrin and myeloperoxidase by coupling blank beads (BD CBA Functional Beads) with either rabbit antibody to interferon-α (US Biological, Swampscott, MA), rabbit antibody to lactoferrin (Biodesign International, Saco, ME) or rabbit antibody to myeloperoxidase (ICL Inc., Newberg, OR). Standards for the custom bead arrays were recombinant human interferon-α2 (Cell Sciences, Canton, MA), milk lactoferrin (Sigma Chem. Co, St. Louis, MO) and myeloperoxidase (Calbiochem EMD Chemicals, Gibbstown, NJ). Sensitivities for the interferon-a , lactoferrin and myeloperoxidase assays were 15 pg/ml, 30 pg/ml and 30 pg/ml respectively. All bead arrays were assayed on a FACS Calibur flow cytometer and levels of cytokines calculated using BD CBA software. HßD2 and HßD3 were measured by ELISA using previously described methods [10].
Statistical Analysis
We first performed a summary analysis of the counts of STDs/conditions and the immune mediators on the 84 subjects. Correlation coefficients between pairs of the mediators were computed and the null hypothesis of no linear association was tested. Univariate logistic regression analyses were performed in examining the association between having STDs/conditions and the immune mediators or the clinical factors. Multivariate logistic regression with stepwise variable selection was then performed on the significant variables found in the univariate analysis. Subjects with a particular STD or condition were compared with those without any STD/condition by logistic regression when the number of cases was relatively large and by the Fisher exact test when the number of cases was small. Such analyses were performed both on subjects having the STD/condition alone and for all subjects having the STD/condition. No multivariate analysis was performed for individual STD/condition because of the sample size limitation.
Results
Subject characteristics
The study population consisted of 84 women, 18 years of age or older, attending a clinic for STD screening. The largest proportion of subjects were between 18 and 24 years old (40%), while 33% were between 25 and 34, 18% were between 35 and 44 and 8% were between 45 and 54. The subjects were 90% African American, 7% Latino, and 3% Caucasian.
Of the 84 subjects, 13 had no STD or condition (conditions included bacterial vaginosis and Candida that are technically not STDs), 49 subjects had one STD or condition, 20 subjects had 2 STDs or conditions while 3 subjects had 3 STDs or conditions. The numbers and types of STDs and conditions are shown in Table 1. The most common diagnosis was bacterial vaginosis followed by Candida, Chlamydia infection and trichomoniasis.
Table 1. Frequency of STDs and/or Conditions.
| Only | All | |
| Bacterial Vaginosis | 12 | 23 |
| Yeast/Candida | 12 | 22 |
| Trichomoniasis | 6 | 12 |
| Chlamydia Trachomatis | 5 | 13 |
| Cervicitis | 3 | 8 |
| Gonorrhea Cervicitis | 3 | 8 |
| Lesions | 2 | 2 |
| Vaginitis | 1 | 2 |
| HSV | 0 | 4 |
| Warts | 0 | 3 |
| PID* | 0 | 2 |
| No Condition | 13 | 13 |
*Pelvic Inflammatory Disease.
In the 48 hours prior to cervical-vaginal lavage (CVL) donation, one woman reported use of a vaginal tampon, five reported douching, nine reported use of vaginal medications (either suppositories, creams, jellies, foam, sponge, perfume or lubricant), 23 reported having vaginal sex with a male partner while 2 reported menstrual blood flow (although blood was not evident at the time of CVL donation). All CVL was tested for semen using the Abacard test for prostate specific antigen (PSA). Fourteen were PSA positive, three were PSA +/− while 67 were PSA negative.
Detection of immune mediators
Table 2 shows the median, mean and range for each of the 32 immune mediators listed from lowest to highest median concentration in CVL samples. Six mediators were detected in all CVL (IL-1ß, VEGF, angiogenin, IL-8, HßD2 and HßD3) while interferon-α was the only substance not detected in any of the CVL samples. Lactoferrin, MPO and GCSF were the three most frequently detected of the remaining mediators (detected in 88%, 90% and 93% of subjects respectively) while lymphotoxin-α, IL-9 and eotaxin were the three least frequently detected (40% 45% and 46% respectively). Lymphotoxin-α, IL-13, IL-9, eotaxin, RANTES, IL-2, IFN-γ, IL-5 and MIP-1α were detected too infrequently to obtain reliable associations with other mediators or with disease status and therefore were excluded from the further analysis described below.
Table 2. Levels of Immune Mediators in CVL.
| Mediator* | Missing | ND | Min | Median | Max | Detectable | Mean | SD |
| IFN-α | 0 | 84 | ND | ND | ND | 0 | ||
| LT-α | 0 | 51 | ND | ND | 14 | 33 | 3 | 2 |
| IL-13 | 0 | 50 | ND | ND | 50 | 34 | 10 | 8.1 |
| IL-9 | 0 | 47 | ND | ND | 74 | 37 | 15 | 12 |
| IFN-γ | 8 | 38 | ND | ND | 92 | 38 | 25 | 21 |
| Eotaxin | 3 | 46 | ND | ND | 573 | 35 | 84 | 100 |
| IL-4 | 8 | 26 | ND | 1.8 | 20 | 50 | 4 | 4 |
| IL-10 | 8 | 24 | ND | 2 | 23 | 52 | 5 | 5 |
| RANTES | 5 | 39 | ND | 3 | 178 | 40 | 17 | 30 |
| IL-2 | 8 | 24 | ND | 3 | 20 | 52 | 5 | 4 |
| TNF-α | 8 | 24 | ND | 3 | 190 | 52 | 13 | 28 |
| MIP-1 α | 0 | 38 | ND | 3 | 162 | 46 | 17 | 32 |
| IL-5 | 9 | 2 | ND | 3 | 14 | 73 | 4 | 2 |
| IL-3 | 3 | 36 | ND | 3 | 89 | 45 | 10 | 13 |
| G-CSF | 3 | 6 | ND | 4 | 52 | 75 | 438 | 879 |
| GM-CSF | 3 | 17 | 4 | 52 | 64 | 7 | 7 | |
| IL-6 | 8 | 13 | ND | 5 | 1736 | 63 | 93 | 312 |
| IL-12 | 7 | 8 | ND | 8 | 81 | 69 | 12 | 12 |
| MCP-1 | 5 | 20 | ND | 12 | 2500 | 59 | 80 | 327 |
| MIP-1ß | 0 | 16 | ND | 21 | 5714 | 68 | 183 | 729 |
| FGF | 0 | 41 | ND | 28 | 86 | 43 | 41 | 12 |
| IL-7 | 9 | 18 | ND | 44 | 285 | 57 | 90 | 68 |
| IP-10 | 5 | 17 | ND | 54 | 2500 | 62 | 328 | 590 |
| MIG | 5 | 5 | ND | 64 | 2500 | 74 | 391 | 661 |
| IL-1ß | 7 | 0 | 2.2 | 184 | 5082 | 77 | 610 | 1050 |
| VEGF | 0 | 0 | 33 | 246 | 15742 | 84 | 627 | 1813 |
| Angiogenin | 0 | 0 | 18 | 459 | 151800 | 84 | 3258 | 16644 |
| IL-8 | 7 | 0 | 31 | 3729 | 5046 | 77 | 2909 | 2085 |
| HBD2 | 0 | 0 | 1.6 | 7 | 35 | 84 | 9 | 6 |
| MPO | 0 | 8 | ND | 369 | 6400 | 76 | 904 | 1145 |
| HBD3 | 0 | 0 | 1.1 | 1069 | 3156 | 84 | 1127 | 839 |
| Lactoferrin | 0 | 10 | ND | 1741 | 5198 | 74 | 1669 | 917 |
Table is arranged from lowest to highest median values. ND: Not Detectable; Mean and SD (Standard Deviation) are computed for the detectable.
The total sample size: N0+N1+N2 = 84.
*Levels in pg/ml except that HBD2, MPO, HBD3 and Lactoferrin are in ng/ml.
Associations between mediators
A number of significant positive associations between the immune mediators were observed. Associations with Pearson correlation coefficients >0.7 (strong correlations) are shown in Table 3. The strongest correlation was between MIG and IP-10, two IFN-γ-induced, CXCR3-binding chemokines that also have direct anti-bacterial activity [11]. Strong correlations were also observed between each of the three pro-inflammatory cytokines TNF-α, IL-1ß and IL-6. MPO, a marker for the presence of neutrophils, was strongly correlated with IL-8 and G-CSF, mediators that induce neutrophil migration and support neutrophil function, respectively.
Table 3. Associations Between Mediators with Pearson Correlation Coefficients >0.7.
| TNF-α | IL-3 | IL-6 | IL-7 | IL-8 | IL-10 | IL -1β | GM-CSF | IP-10 | MIG | G-CSF | MPO | |
| TNF-α | 1.00 | 0.70 | 0.81 | 0.70 | ||||||||
| IL-3 | 1.00 | 0.81 | ||||||||||
| IL-6 | 1.00 | 0.73 | 0.74 | 0.83 | ||||||||
| IL-7 | 1.00 | |||||||||||
| IL-8 | 1.00 | 0.83 | 0.71 | 0.84 | ||||||||
| IL-10 | 1.00 | 0.77 | ||||||||||
| IL -1β | 1.00 | 0.77 | ||||||||||
| GM-CSF | 1.00 | |||||||||||
| IP-10 | 1.00 | |||||||||||
| MIG | 1.00 | |||||||||||
| G-CSF | 1.00 |
The statistic is the correlation coefficient. Correlation coefficients smaller than 0.7 are suppressed. All displayed correlation coefficients have p-values smaller than 0.0001 in testing the hypothesis of no correlation.
No significant strong negative correlations (Pearson correlation coefficients between −1.0 and −0.7) were observed between any of the immune mediators possibly reflecting the fact that only a few of the mediators tested had known activity for down-regulating immune responses. However, significant (p<0.05), but relatively weak, negative correlations between HßD2 were observed with IL-8, IL-1ß, GM-CSF, IP-10, MIG, G-CSF and MPO (Pearson coefficients ranging from −0.24 to −0.34). Similarly, HßD3 had negative correlations with TNF-α, IL-4, IL-6, IL-10, IL-1ß, IL-12, GM-CSF, MCP-1, G-CSF, VEGF and lactoferrin (Pearson coefficients ranging from −0.24 to −0.42).
Association of mediators with STD/colonization
The subjects that had no STDs or colonization were used as a control group and compared with all other subjects to determine if having any STDs/colonization was associated with changes in levels of immune mediators. When compared to controls, women with any STD/colonization had significantly higher levels of IL-3, IL-4, IL-6, IL-8, IL-10, IL-1ß, MIG, G-CSF, VEGF, Angiogenin, lactoferrin and MPO (Table 4). In a multivariate analysis however, only MIG and lactoferrin were significantly different between the two groups (Table 4).
Table 4. Relationships between STDs/Conditions and Immune Mediators.
| Mediator* | Control (SD) | Any STD (SD) | Univariate Analysis | Multivariate Analysis | ||||
| OR | 95% CI | P | OR | 95% CI | P | |||
| TNF-α (8) | 3 (1) | 10 (26) | ||||||
| IL-4 (9) | 2 (1) | 3 (4) | 2.0 | 1.1, 3.7 | 0.02 | |||
| IL-6 (7) | 5 (5) | 92 (308) | 1.9 | 1.1, 3.3 | 0.02 | |||
| IL-10 (9) | 2 (2) | 4 (5) | 2.1 | 1.1, 4.4 | 0.03 | |||
| IP-10 (5) | 75 (27) | 294 (573) | ||||||
| MCP-1 (5) | 24 (5) | 67 (305) | ||||||
| MIG (5) | 87 (23) | 421 (682) | 1.5 | 1.1, 2.1 | 0.02 | 1.5 | 1.0, 2.1 | 0.04 |
| IL-8 (7) | 679 (219) | 679 (219) | 1.7 | 1.1, 2.6 | 0.01 | |||
| GM-CSF (3) | 3 (2) | 7 (8) | ||||||
| G-CSF (3) | 35 (12) | 476 (902) | 1.5 | 1.1, 2.1 | 0.008 | |||
| IL-3 (9) | 12 (10) | 24 (21) | 7.4 | 1.5, 38 | 0.02 | |||
| IL-7 (9) | 31 (22) | 76 (74) | ||||||
| IL-12 (7) | 7 (7) | 12 (13) | ||||||
| IL-1ß (7) | 72 (12) | 719 (1103) | 1.8 | 1.2, 2.6 | 0.003 | |||
| VEGF (0) | 185 (164) | 708 (1937) | 2.4 | 1.1, 5.4 | 0.03 | |||
| Angiog. (0) | 605 (201) | 3744 (17829) | 1.8 | 1.1, 3.1 | 0.03 | |||
| FGF (0) | 27 (35) | 20 (22) | ||||||
| MIP-1ß (0) | 29 (18) | 170 (706) | ||||||
| HBD2 (0) | 10 (18) | 9 (14) | ||||||
| MPO (0) | 217 (92) | 928 (1183) | 1.3 | 1.1, 1.7 | 0.02 | |||
| Lactof. (0) | 370 (176) | 1672 (959) | 1.4 | 1.1, 1.7 | 0.002 | 1.3 | 1.1, 1.6 | 0.008 |
| HBD3 (0) | 1446 (1822) | 1069 (1585) | ||||||
Levels in pg/ml except that HBD2, MPO, HBD3 and Lactoferrin are in ng/ml. Any STD includes BV and Candida. Only variables with p-value<0.05 have OR, 95% confidence interval and p-value shown. The p-values are calculated based on Fisher exact test and the odds ratios are calculated with correction, i.e., by adding 0.5 to counts if there were zero counts. Only two variables are selected in the final model.
*Number missing in parenthesis.
Subjects were further sub-categorized as having either no STD or colonization, only one STD/colonization or several STD/colonizations (Table 5). Compared to the control group, women with only GC, Chlamydia, cervicitis, bacterial vaginosis or Trichomoniasis had significantly different levels of 7, 17, 4, 11 and 9 different immune mediators respectively (Table 5). There were too few women with HSV, non-specific lesions, warts or PID to obtain meaningful comparisons with the control group. In general, when more than one STD/colonization was present (“All”, Table 5), more mediators were significantly different than controls (13, 17, 8, 15 and 5 for GC, Chlamydia, cervicitis, bacterial vaginosis (BV) and trichomoniasis repectively). Most of the significant differences were increases in mediators when compared to control. However, MIP-1ß was decreased in trichomoniasis, while HßD2 was decreased in Chlamydia infection. Strikingly, IL-1ß and lactoferrin were both significantly increased in all five of the STDs/colonizations. A varying pattern of significant changes in other mediators was seen. For example MIP-1α, VEGF and MPO levels were significantly different than controls in Chlamydia and trichomoniasis but not BV while in contrast, IL-6, IL-10, GM-CSF, G-CSF, IL-3, IL-7 and IL-12 were significantly changed in Chlamydia and BV but not trichomoniasis.
Table 5. P-values in testing association between specific STDs/Conditions and Immune Mediators.
| Gonorrhea | Chlamydia | Cervicitis | B. Vaginosis | Trichomonas | ||||||
| Only* | All* | Only | All | Only | All | Only | All | Only | All | |
| TNF-α | 0.02 | 0.03 | 0.008 | 0.006 | ||||||
| IL-4 | 0.02 | 0.01 | 0.02 | 0.01 | 0.04 | |||||
| IL-6 | 0.007 | 0.004 | 0.001 | 0.01 | 0.03 | 0.03 | 0.004 | |||
| IL-10 | 0.02 | 0.01 | 0.001 | 0.001 | 0.01 | 0.01 | ||||
| IP-10 | 0.01 | 0.04 | 0.03 | |||||||
| MIG | 0.04 | 0.003 | 0.03 | 0.03 | 0.01 | 0.01 | ||||
| IL-8 | 0.02 | 0.01 | 0.001 | 0.01 | 0.03 | 0.002 | 0.04 | |||
| GM-CSF | 0.02 | 0.02 | 0.02 | 0.007 | 0.006 | |||||
| G-CSF | 0.01 | 0.001 | 0.0001 | 0.0001 | 0.02 | 0.009 | ||||
| IL-3 | 0.05 | 0.04 | 0.003 | 0.004 | ||||||
| IL-7 | 0.01 | 0.002 | 0.02 | 0.006 | 0.02 | 0.002 | ||||
| IL-12 | 0.02 | 0.02 | 0.004 | 0.001 | 0.0001 | |||||
| IL-1ß | 0.007 | 0.002 | 0.002 | 0.0001 | 0.02 | 0.005 | 0.001 | 0.0001 | 0.01 | 0.02 |
| VEGF | 0.03 | 0.02 | 0.002 | 0.003 | 0.004 | 0.001 | 0.03 | |||
| Angiog. | 0.03 | 0.01 | 0.01 | 0.004 | ||||||
| MIP-1ß | 0.02 | 0.04 | 0.006 | 0.05 | ||||||
| Lacto. | 0.002 | 0.0003 | 0.004 | 0.0001 | 0.003 | 0.001 | 0.001 | 0.001 | 0.03 | |
| MPO | 0.02 | 0.003 | 0.002 | 0.04 | ||||||
| HBD2 | 0.02 | 0.003 | 0.002 | 0.04 | ||||||
Only those p-values<0.05 are shown. Tests are significant after the adjustment of column-wise multiple tests if p-value<0.05/32 = 0.0016, where 32 is the number of tests performed, including those mediators tested but not shown.
*Only -subjects that had only one STD/colonization; All –all subjects with that STD/colonization.
MCP-1, FGF and HßD3 were not significantly changed in any of the STDs/colonizations, while MIG and IP-10 were not significantly different in any of the “only” STDs/colonizations.
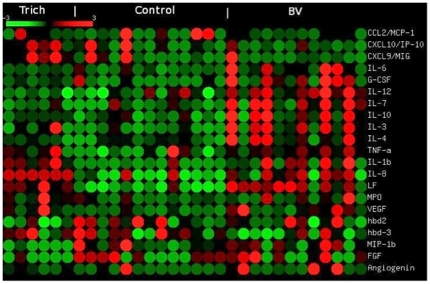
When viewed in a heat map format, differential patterns of increases in cytokines in relation to disease were apparent when comparing levels from subjects with trichomoniasis, bacterial vaginosis and controls (Fig. 1). Thus, IL-1ß, lactoferrin and IL-8 were increased in both trichomoniasis and BV when compared to controls. In contrast, IL-12, IL-7, IL-10, IL-3, and IL-4 were increased in BV when compared to control samples but not in trichomoniasis.
Figure 1. Heat map of cytokine levels.
The heat map was generated using the web-based program Matrix2png [34] that displays microarray data visually (http://www.bioinformatics.ubc.ca/matrix2png/). The mean for each row of cytokine values is 0 with red representing values greater than 0, green lower than 0 and black 0.
Interestingly, women with only yeast infection (N = 12) did not have a significant change in any of the immune mediators (not shown).
Discussion
This study provides several novel observations concerning immune mediators in genital mucosal fluids of women. First, IL-1ß and lactoferrin levels were significantly increased when comparing controls with all infected subjects and when comparing controls with each of the STDs/colonizations except Candida. Thus, increased levels of IL-1ß and lactoferrin were the most robust changes found in this clinic population with IL-1ß levels increased 10-fold and lactoferrin increased 4.5 fold compared to controls. IL-1ß is a strong inducer of inflammation at mucosal sites and IL1ß release from cells is mediated by the inflammasome, an intracellular molecular complex that is the subject of recent intense interest [12]. The inflammasome is activated not only by microbial products including peptidoglycans and toxins, but also by host-derived danger signals such as changes in potassium levels, extracellular ATP and reactive oxygen species suggesting that these endogenous and less-specific inducers could be the commonality that is present in all STDs/colonizations that leads to IL-1ß production [12]. Lactoferrin is an iron-binding protein synthesized by neutrophils and epithelial cells and is implicated in the host defense response against bacterial, fungal and viral pathogens [13], [14], [15]. Lactoferrin and IL-1ß are both associated with increased HIV-1 genital tract shedding in women [16]. Both lactoferrin and IL-1ß have been reported to be elevated in bacterial vaginosis and increased IL-1ß was found in chlamydia infection while increased lactoferrin was reported in cervicitis and trichomoniasis [7], [17], [18], [19], [20]. However, elevations of lactoferrin in Chlamydia infection and Gonorrhea have not been previously reported and elevated IL-1ß in trichomoniasis, Gonorrhea or cervicitis have not been previously reported. One study reported that IL-1ß was not elevated in Trichomonas vaginalis, Chlamydia trachomatis or Neisseria gonorrhoeae infections [21]. Lactoferrin also is recognized to be anti-inflammatory and in fact has been shown to down-regulate IL-1ß-induced inflammation in the skin [22]. Therefore, lactoferrin in the genital tract may counteract the pro-inflammatory environment induced by IL-1ß and other cytokines. Lactoferrin was previously found to be associated with leukocyte levels in CVL samples [23] and in the current study, lactoferrin was significantly associated with MPO levels (p<0.0001) although the correlation coefficient of 0.47 did not achieve the level of the strong correlations shown in Table 3. In microbicide studies, IL-1ß is considered a marker for inflammation/damage to the epithelium [24].
Other potentially important findings of this study were that mucosal fluid in general appears to be an environment that is rich in immune mediators, since all of the 32 mediators that were tested, except one, were detected in at least 40% of the subjects and several (IL-3, IL-1ß, VEGF, Angiogenin, IL-8, ß-Defensins 2 and 3), were detected in all of the subjects. To our knowledge, some of the mediators, including VEGF, angiogenin, FGF, IL-9, IL-7, lymphotoxin-α and IL-3, had not been previously reported to be present in female genital secretions. While the role that these previously-unrecognized mediators play in genital immunity is not known, several have reported functions that could be important in immune responses to STDs. For example, angiogenin has anti-microbial activity against Candida albicans and certain bacteria in vitro [25], but has not yet been tested for a possible role in immunity to genital yeast or other infections in women. VEGF has been postulated to play a role during inflammation and infections by increasing vascularity and promoting adhesion of leukocytes [26], [27]. IL-7 is a crucial survival and expansion factor for T cells [28] and could therefore play a role in mucosal T cell responses to STDs.
This study also showed strong correlations between several groups of immune mediators, suggesting possible linkages of inducing pathways or common producing cell types. IP-10 and MIG had the strongest association and these two chemokines have many previously described parallels. They are both induced by interferons, bind to CXCR3, have direct anti-bacterial activity and are antagonists for CCR3 [11], [29]. Strong correlations were also observed between each of the three pro-inflammatory cytokines TNF-α, IL-1ß and IL-6 (Table 2). IL-8, which is in some cases considered a pro-inflammatory mediator, was also strongly associated with IL-1ß, but not with TNF-α and IL-6. Several previous studies have noted parallels between several of these inflammatory mediators in genital fluid from different groups of women. For example, levels of IL-1ß and IL-6, but not TNF-α, were significantly elevated in subjects in labor when compared to those not in labor [30]. In contrast, several studies have shown that while IL-1ß is increased in bacterial vaginosis, IL-6 and TNF-α are not [7], [31]. In this study, IL-1ß, IL-6 and IL-8 were significantly increased in bacterial vaginosis while TNF-α was not.
This study had several limitations. Several conditions in the subjects were not known at the time of sample collection such as smoking and exact stage of the menstrual cycle which have been shown to be associated with changes in levels of certain cytokines [32]. Also, the women used as a control group were those visiting the STD clinic. Thus it is possible that a group of women with no risk factors may have had differing levels of immunologic mediators than the group assessed here. Another limitation of this study was that samples were collected by cervical vaginal lavage and therefore the initial concentration of mediators in undiluted genital fluid is not known. However, collection of samples by this method would not affect the associations between mediators that were observed. Further, the numbers of women infected with herpes are likely to be underestimated in this group of women since culture was used to determine herpes infection instead of the more sensitive PCR-based methods [33].
In conclusion this study shows that IL-1ß and lactoferrin are increased in a broad array of STDs and other genital conditions potentially providing insights into pathogenesis and immune responses at this mucosal site. These studies also show that genital mucosal fluids in women have a wider range of immune mediators than previously demonstrated.
Acknowledgments
The authors thank Carrie Pierce for excellent technical work and Joyce Li for help in organizing data and data analysis.
Footnotes
Competing Interests: Author RB was funded by BD Biosciences which could be perceived as a competing interest. This competing interest does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: Financial support for this work was provided by a Rush University Translational Science Consortium Pilot award and by NIH Grant P01HD40539. BD Biosciences is also a funder since it provided the salary of author RB. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript, except that author RB had a role in the conceiving and designing of the experiments, and in the interpretation of the results.
References
- 1.Mayaud P, Mabey D. Approaches to the control of sexually transmitted infections in developing countries: old problems and modern challenges. Sex Transm Infect. 2004;80:174–182. doi: 10.1136/sti.2002.004101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wira CR, Fahey JV, Ghosh M, Patel MV, Hickey DK, et al. Sex Hormone Regulation of Innate Immunity in the Female Reproductive Tract: The Role of Epithelial Cells in Balancing Reproductive Potential with Protection against Sexually Transmitted Pathogens. Am J Reprod Immunol. 2010 doi: 10.1111/j.1600-0897.2010.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koumans EH, Sternberg M, Bruce C, McQuillan G, Kendrick J, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34:864–869. doi: 10.1097/OLQ.0b013e318074e565. [DOI] [PubMed] [Google Scholar]
- 4.Sobel JD, Faro S, Force RW, Foxman B, Ledger WJ, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol. 1998;178:203–211. doi: 10.1016/s0002-9378(98)80001-x. [DOI] [PubMed] [Google Scholar]
- 5.Sulak PJ. Sexually transmitted diseases. Semin Reprod Med. 2003;21:399–413. doi: 10.1055/s-2004-815595. [DOI] [PubMed] [Google Scholar]
- 6.Keller MJ, Herold BC. Impact of microbicides and sexually transmitted infections on mucosal immunity in the female genital tract. Am J Reprod Immunol. 2006;56:356–363. doi: 10.1111/j.1600-0897.2006.00436.x. [DOI] [PubMed] [Google Scholar]
- 7.St John E, Mares D, Spear GT. Bacterial vaginosis and host immunity. Curr HIV/AIDS Rep. 2007;4:22–28. doi: 10.1007/s11904-007-0004-y. [DOI] [PubMed] [Google Scholar]
- 8.Simhan HN, Anderson BL, Krohn MA, Heine RP, Martinez de Tejada B, et al. Host immune consequences of asymptomatic Trichomonas vaginalis infection in pregnancy. Am J Obstet Gynecol. 2007;196:59 e51–55. doi: 10.1016/j.ajog.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 9.Cauci S, Culhane JF. Modulation of vaginal immune response among pregnant women with bacterial vaginosis by Trichomonas vaginalis, Chlamydia trachomatis, Neisseria gonorrhoeae, and yeast. Am J Obstet Gynecol. 2007;196:133 e131–137. doi: 10.1016/j.ajog.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh SK, Gerken TA, Schneider KM, Feng Z, McCormick TS, et al. Quantification of human beta-defensin-2 and -3 in body fluids: application for studies of innate immunity. Clin Chem. 2007;53:757–765. doi: 10.1373/clinchem.2006.081430. [DOI] [PubMed] [Google Scholar]
- 11.Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, et al. Cutting edge: IFN-inducible ELR- CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001;167:623–627. doi: 10.4049/jimmunol.167.2.623. [DOI] [PubMed] [Google Scholar]
- 12.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 13.Farnaud S, Evans RW. Lactoferrin–a multifunctional protein with antimicrobial properties. Mol Immunol. 2003;40:395–405. doi: 10.1016/s0161-5890(03)00152-4. [DOI] [PubMed] [Google Scholar]
- 14.Soukka T, Tenovuo J, Lenander-Lumikari M. Fungicidal effect of human lactoferrin against Candida albicans. FEMS Microbiol Lett. 1992;69:223–228. doi: 10.1016/0378-1097(92)90650-d. [DOI] [PubMed] [Google Scholar]
- 15.van der Strate BW, Beljaars L, Molema G, Harmsen MC, Meijer DK. Antiviral activities of lactoferrin. Antiviral Res. 2001;52:225–239. doi: 10.1016/s0166-3542(01)00195-4. [DOI] [PubMed] [Google Scholar]
- 16.Cummins JE, Christensen L, Lennox JL, Bush TJ, Wu Z, et al. Mucosal innate immune factors in the female genital tract are associated with vaginal HIV-1 shedding independent of plasma viral load. AIDS Res Hum Retroviruses. 2006;22:788–795. doi: 10.1089/aid.2006.22.788. [DOI] [PubMed] [Google Scholar]
- 17.Rein MF, Shih LM, Miller JR, Guerrant RL. Use of a lactoferrin assay in the differential diagnosis of female genital tract infections and implications for the pathophysiology of bacterial vaginosis. Sex Transm Dis. 1996;23:517–521. doi: 10.1097/00007435-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Goldenberg RL, Andrews WW, Guerrant RL, Newman M, Mercer B, et al. The preterm prediction study: cervical lactoferrin concentration, other markers of lower genital tract infection, and preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 2000;182:631–635. doi: 10.1067/mob.2000.104211. [DOI] [PubMed] [Google Scholar]
- 19.Agrawal T, Vats V, Wallace PK, Singh A, Salhan S, et al. Recruitment of myeloid and plasmacytoid dendritic cells in cervical mucosa during Chlamydia trachomatis infection. Clin Microbiol Infect. 2009;15:50–59. doi: 10.1111/j.1469-0691.2008.02113.x. [DOI] [PubMed] [Google Scholar]
- 20.Sawada M, Otsuki K, Mitsukawa K, Yakuwa K, Nagatsuka M, et al. Cervical inflammatory cytokines and other markers in the cervical mucus of pregnant women with lower genital tract infection. Int J Gynaecol Obstet. 2006;92:117–121. doi: 10.1016/j.ijgo.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Hedges SR, Sibley DA, Mayo MS, Hook EW, 3rd, Russell MW. Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: effects of concomitant infections. J Infect Dis. 1998;178:742–751. doi: 10.1086/515372. [DOI] [PubMed] [Google Scholar]
- 22.Ward PP, Paz E, Conneely OM. Multifunctional roles of lactoferrin: a critical overview. Cell Mol Life Sci. 2005;62:2540–2548. doi: 10.1007/s00018-005-5369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novak RM, Donoval BA, Graham PJ, Boksa LA, Spear G, et al. Cervicovaginal Levels of Lactoferrin, Secretory Leukocyte Protease Inhibitor, and RANTES and the Effects of Coexisting Vaginoses in Human Immunodeficiency Virus (HIV)-Seronegative Women with a High Risk of Heterosexual Acquisition of HIV Infection. Clin Vaccine Immunol. 2007;14:1102–1107. doi: 10.1128/CVI.00386-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trifonova RT, Bajpai M, Pasicznyk JM, Chandra N, Doncel GF, et al. Biomarkers of leukocyte traffic and activation in the vaginal mucosa. Biomarkers. 2007;12:608–622. doi: 10.1080/13547500701600670. [DOI] [PubMed] [Google Scholar]
- 25.Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4:269–273. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 26.Frantz S, Vincent KA, Feron O, Kelly RA. Innate immunity and angiogenesis. Circ Res. 2005;96:15–26. doi: 10.1161/01.RES.0000153188.68898.ac. [DOI] [PubMed] [Google Scholar]
- 27.Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, et al. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001;276:7614–7620. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]
- 28.Capitini CM, Chisti AA, Mackall CL. Modulating T-cell homeostasis with IL-7: preclinical and clinical studies. J Intern Med. 2009;266:141–153. doi: 10.1111/j.1365-2796.2009.02085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loetscher P, Pellegrino A, Gong JH, Mattioli I, Loetscher M, et al. The ligands of CXC chemokine receptor 3, I-TAC, Mig, and IP10, are natural antagonists for CCR3. J Biol Chem. 2001;276:2986–2991. doi: 10.1074/jbc.M005652200. [DOI] [PubMed] [Google Scholar]
- 30.Imseis HM, Greig PC, Livengood CH, 3rd, Shunior E, Durda P, et al. Characterization of the inflammatory cytokines in the vagina during pregnancy and labor and with bacterial vaginosis. J Soc Gynecol Investig. 1997;4:90–94. [PubMed] [Google Scholar]
- 31.Mattsby-Baltzer I, Platz-Christensen JJ, Hosseini N, Rosen P. IL-1beta, IL-6, TNFalpha, fetal fibronectin, and endotoxin in the lower genital tract of pregnant women with bacterial vaginosis. Acta Obstet Gynecol Scand. 1998;77:701–706. [PubMed] [Google Scholar]
- 32.Lieberman JA, Moscicki AB, Sumerel JL, Ma Y, Scott ME. Determination of cytokine protein levels in cervical mucus samples from young women by a multiplex immunoassay method and assessment of correlates. Clin Vaccine Immunol. 2008;15:49–54. doi: 10.1128/CVI.00216-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wald A, Huang ML, Carrell D, Selke S, Corey L. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis. 2003;188:1345–1351. doi: 10.1086/379043. [DOI] [PubMed] [Google Scholar]
- 34.Pavlidis P, Noble WS. Matrix2png: a utility for visualizing matrix data. Bioinformatics. 2003;19:295–296. doi: 10.1093/bioinformatics/19.2.295. [DOI] [PubMed] [Google Scholar]