Abstract
Based on in vitro rat and human hepatocyte uptake studies showing inhibition of warfarin uptake in the presence of the non-specific organic anion transporting polypeptide (OATP) inhibitor rifampin, a clinical study was conducted in 10 healthy volunteers. In a randomized, single-dose, two-period, crossover design, subjects received a 7.5 mg dose of warfarin alone or immediately following a 600 mg intravenous dose of rifampin. Rifampin did not significantly alter R- or S- warfarin area under the concentration-time curve (AUC) from 0–12 hours (period of hepatic OATP inhibition by rifampin) or Cmax (maximum plasma concentration). AUC0–∞ was decreased on rifampin days for both R- (25% reduction; p < 0.001) and S-warfarin (15% reduction; p < 0.05). No differences were seen on the area under the INR-time curve. Our study suggests hepatic uptake via OATPs may not be clinically important in the pharmacokinetics of warfarin.
Keywords: warfarin, pharmacokinetics, drug transporters, drug interaction, healthy volunteers
INTRODUCTION
Warfarin is a commonly prescribed anticoagulant for the prevention of venous thromboembolism, systemic embolism, and other thromboembolic events. But, successful use of warfarin has been challenged by its large inter-individual variation in required warfarin dose and narrow therapeutic index.(1) Doses can differ >10-fold among patients to achieve similar levels of anticoagulation. In attempts to explain the variability in warfarin dose, formal pharmacogenetic techniques have been applied to try and identify potential common genetic polymorphisms in genes that may be important. It is now well-recognized that Single Nucleotide Polymorphisms (SNPs) in warfarin's major metabolizing enzyme (cytochrome P-450 (CYP) 2C9) and its pharmacodynamic target (vitamin K epoxide reductase; VKORC1) have a significant impact on warfarin dose requirement.(2–5) Multiple investigators have incorporated patients' genetic status within these genes along with demographic factors (i.e. age, weight, gender, ethnicity, interacting drugs, etc.) into dosing algorithms for improved dose prediction.(6–9) Yet, these regression models can only account for at most 55–60% of the variability in warfarin dose.
The Biopharmaceutics Drug Disposition Classification System (BDDCS), which classifies drugs based on the characteristics of solubility and extent of metabolism, allows investigators to predict whether intestinal and hepatic transporters can potentially affect disposition of a drug.(10) Specifically, BDDCS predicts that following oral dosing of poorly soluble, extensively metabolized drugs (i.e. Class 2 drugs), efflux transporter effects may predominate in the intestine, but both uptake and efflux transporter effects can be important in the liver. In those organs where the relevant metabolizing enzymes are located, extensive transporter-enzyme interplay will also occur. Recent human pharmacokinetic studies from our laboratory have demonstrated in vivo the importance of hepatic uptake via organic anion transporting polypeptides (OATPs) in the disposition of two BDDCS Class 2 drugs: atorvastatin(11) and glyburide.(12) Using concomitant administration of single dose intravenous rifampin as a non-specific OATP inhibitor, hepatic uptake inhibition resulted in a 1.3 and 6.8 fold increase in glyburide and atorvastin exposure, respectively, as measured by the total area under the concentration-time curve (AUC). The importance of uptake transporters has been shown for a number of other Class 2 drugs as well.(13)
Warfarin is a racemic mixture of S- and R- enatiomers with S-warfarin being 2–5 times more potent and conferring the majority of its anticoagulant activity.(14) Both the S- and R-enatiomers are eliminated almost exclusively by CYP metabolism, i.e., S-warfarin principally by CYP2C9 metabolism to form the primary metabolite 7-hydroxywarfarin (14, 15); R-warfarin principally by CYP3A4 metabolism to form 10-hydroxywarfarin.(15)
Since warfarin is classified as a BDDCS Class 2 drug(10), the potential for hepatic uptake transporters to play a role in its pharmacokinetics should be considered, a proposition not previously studied. By inhibiting the uptake of warfarin into the liver, warfarin would have decreased access to its major metabolizing CYP enzymes resulting in an increased AUC. Based on initial in vitro rat and human hepatocyte data showing decreased uptake of warfarin in the presence of rifampin, a clinical trial in healthy volunteers was conducted to examine the impact of hepatic uptake inhibition on warfarin pharmacokinetics.
RESULTS
In Vitro Study – Rat and Human Hepatocyte Uptake
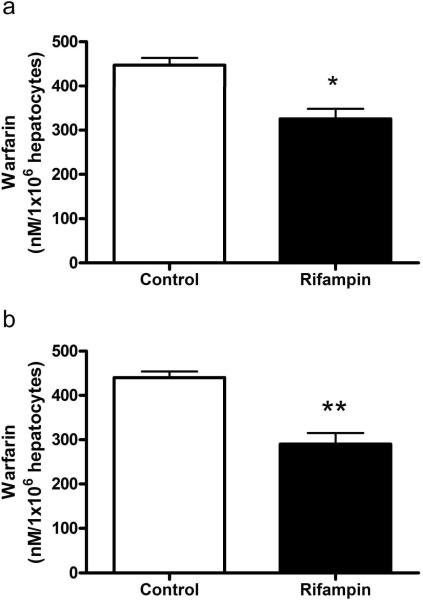
In the presence of 100 μM rifampin, the uptake of 1 μM racemic warfarin was decreased by 23% in rat hepatocytes (p < 0.05; Figure 1A) and 34% in human hepatocytes (p< 0.001; Figure 1B) compared to control.
Figure 1.
Inhibition of the uptake of warfarin (1μM) by rifampin (100 μM) into (a) rat and (b) human hepatocytes. Values are mean ± SD (rat, n = 9 per group; human, n = 6 per group). * p<0.05; ** p < 0.001 compared to control.
Clinical Study
Pharmacokinetics of S- and R-warfarin
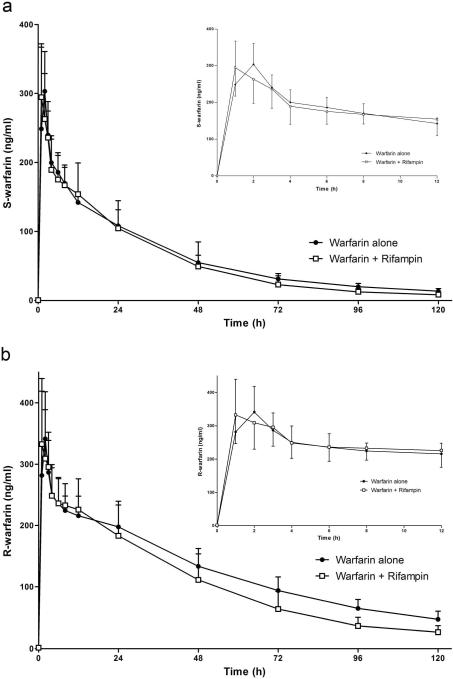
Figure 2 displays the concentration-time profiles of S- and R-warfarin after a single oral dose of 7.5 mg warfarin alone or with a single intravenous dose of rifampin. Data from a previous study by our laboratory and information provided in the package insert, predict relevant concentrations of rifampin for up to 12 hours after a single 600 mg intravenous dose(11, 16). Therefore, in the current study we focused on S- and R-warfarin concentration-time data from 0–12 hours (Figure 2 inset) that would represent the time period of hepatic OATP inhibition by rifampin and when any uptake effects on warfarin pharmacokinetics, if any, would be seen. Rifampin had no effect on the AUC0–12h of S-warfarin or R-warfarin compared to control days when warfarin was given alone (Table 1), as well as Cmax (maximum plasma concentration) and Tmax (time of maximum concentration).
Figure 2.
The effect of a single dose of intravenous rifampin on the mean plasma concentrations of (a) S-warfarin and (b) R-warfarin in 10 healthy volunteers after a single oral dose of 7.5 mg racemic warfarin. Inset: depicts 0 to 12 h data only. Error bars show SD.
Table 1.
Pharmacokinetic parametersa of R- and S-warfarin in 10 healthy volunteers following a single oral dose of 7.5 mg warfarin alone or in combination with a single dose of intravenous rifampin.
| Parameter | Warfarin alone | Rifampin phase | Geometric Mean Ratio (rifampin to control) |
|
|---|---|---|---|---|
| Mean | 95% C.I. | |||
| S-warfarin | ||||
| Cmax (ng/ml) | 329 ± 70 | 303 ± 82 | 0.91 | 0.78 – 1.05 |
| Tmaxb (hr) | 1.5 (1–2) | 1 (1–2) | -- | -- |
| t1/2 (hr) | 39.4 ± 6.1 | 34.6 ± 7.4 | 0.87 | 0.72 – 1.05 |
| AUC0–12 h (ng/ml · hr) | 2,250 ± 340 | 2,230 ± 460 | 0.98 | 0.89 – 1.08 |
| AUC0–120 h (ng/ml · hr) | 7,600 ± 950 | 6,990 ± 2490 | 0.89 | 0.77 – 1.02 |
| AUC0–∞ (ng/ml · hr) | 8,420 ± 1090 | 7,410 ± 2500* | 0.85 | 0.74 – 0.99 |
| CL/F (mL/hr/kg) | 5.9 ± 1.1 | 6.9 ± 1.2* | 1.17 | 1.01 – 1.36 |
| Vss/F (mL/kg) | 263 ± 30 | 257 ± 66 | 0.95 | 0.80 – 1.13 |
| R-warfarin | ||||
| Cmax (ng/ml) | 375 ± 116 | 351 ± 85 | 0.95 | 0.82 – 1.12 |
| Tmaxb (hr) | 2 (1–2) | 2 (1–3) | -- | -- |
| t1/2 (hr) | 49.0 ± 8.7 | 36.8 ± 7.2** | 0.75 | 0.66 – 0.85 |
| AUC0–12 h (ng/ml · hr) | 2,850 ± 460 | 2,930 ± 510 | 1.02 | 0.96 – 1.09 |
| AUC0–120 h (ng/ml · hr) | 15,200 ± 2500 | 12,700 ± 3700** | 0.82 | 0.75 – 0.90 |
| AUC0–∞ (ng/ml · hr) | 18,600 ± 3400 | 14,100 ± 4100** | 0.75 | 0.68 – 0.82 |
| CL/F (mL/hr/kg) | 2.7 ± 0.6 | 3.6 ± 0.8** | 1.34 | 1.22 – 1.47 |
| Vss/F (mL/kg) | 183 ± 28 | 175 ± 33 | 0.95 | 0.87 – 1.04 |
AUC, area under the plasma concentration-time curve; C.I., confidence interval; CL/F, oral clearance; t1/2, terminal half-life; Tmax, time of observed maximal concentration; Vss/F, oral steady-state volume of distribution.
Values are shown as mean ± SD unless otherwise stated.
Tmax data are given as median and range.
p < 0.05
p < 0.001 significantly different from warfarin alone control phase.
Analysis of all concentration-time data showed rifampin significantly decreased the AUC0–∞ of S-warfarin by 15% (p < 0.04) and R-warfarin by 25% (p < 0.001) (Figure 2; Table 1). The apparent terminal half-life (t1/2) of S-warfarin was not significantly different on rifampin days (p = 0.13). For R-warfarin, a 25% shortening of the terminal t1/2 was seen on rifampin days (p < 0.001). The steady-state volume of distribution (Vss/F) was unaffected by the presence of rifampin for both S- and R-warfarin.
Pharmacokinetics of warfarin metabolites
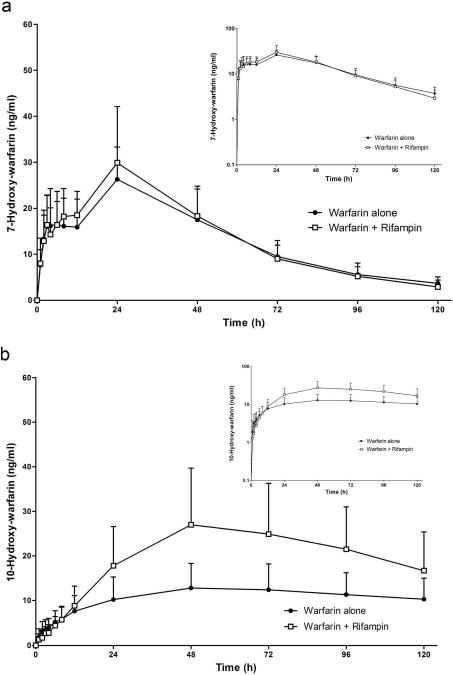
The concentration-time profiles of 7-hydroxywarfarin (S-warfarin metabolite) and 10-hydroxywarfarin (R-warfarin metabolite) after a single oral dose of 7.5 mg warfarin alone or with a single intravenous dose of rifampin are shown in Figure 3. Rifampin had no significant effect on 7-hydroxywarfarin AUC0–12h or any other its pharmacokinetic parameters (Table 2). For 10-hydroxywarfarin, rifampin also had no effect on the AUC0–12h(Table 2). But, after 12 hours the concentration-time profile of 10-hydroxywarfarin between treatments began to diverge with higher concentrations on rifampin days (Figure 3b). The Cmax of 10-hydroxywarfarin was increased 111% on rifampin days (p < 0.001) but no change in Tmax was seen. Rifampin treatment also resulted in an 84% increase in the AUC0–120h (p < 0.001) and 16% increase in the AUC0–∞ (p < 0.05) of 10-hydroxywarfarin. Terminal t1/2 decreased 50% on rifampin days (p < 0.001). Because of its extremely long terminal t1/2, a large portion of 10-hydroxywarfarin's concentration-time profile was not captured within our sampling time-period (0–120 hour), and therefore, a significant proportion of AUC0–∞ was extrapolated (warfarin alone, 64 +/− 11%; warfarin plus rifampin, 44% +/− 12%,). For the same reason, our terminal t1/2 estimate for 10-hydroxywarfarin may not exclusively represent the terminal phase.
Figure 3.
The effect of a single dose of intravenous rifampin on the mean plasma concentrations of (a) 7-hydroxywarfarin and (b) 10-hydroxywarfarin in 10 healthy volunteers after a single oral dose of 7.5 mg racemic warfarin. Inset: depicts the same data on a semilogarithmic scale. Error bars show SD.
Table 2.
Pharmacokinetic parametersa of warfarin metabolites in 10 healthy volunteers following a single oral dose of 7.5 mg warfarin alone or in combination with a single dose of intravenous rifampin.
| Parameter | Warfarin alone | Rifampin phase | Geometric Mean Ratio (rifampin to control) |
|
|---|---|---|---|---|
| Mean | 95% C.I. | |||
| 7-Hydroxywarfarin | ||||
| Cmax (ng/ml) | 26.9 ± 6.0 | 30.9 ± 11.3 | 1.11 | 0.90 – 1.36 |
| Tmaxb (h) | 24 (24–48) | 24 (12–48) | -- | -- |
| t1/2 (h) | 33.1 ± 7.2 | 31.3 ± 12.3 | 0.91 | 0.70 – 1.18 |
| AUC0–12 h (ng/ml · hr) | 175 ± 67 | 183 ± 59 | 1.06 | 0.93 – 1.22 |
| AUC0–120 h (ng/ml · hr) | 1570 ± 420 | 1650 ± 480 | 1.04 | 0.87 – 1.24 |
| AUC0–∞ (ng/ml · hr) | 1760 ± 480 | 1790 ± 490 | 1.01 | 0.86 – 1.20 |
| 10-Hydroxywarfarin | ||||
| Cmax (ng/ml) | 13.2 ± 5.8 | 28.2 ± 12.7** | 2.11 | 1.80 – 2.48 |
| Tmaxb (h) | 48 (48–96) | 48 (48–96) | -- | -- |
| t1/2 (h) | 176 ± 75 | 87.8 ± 32.3** | 0.50 | 0.43 – 0.59 |
| AUC0–12h (ng/ml · hr) | 57.2 ± 42.5 | 53.4 ± 26.7 | 0.93 | 0.76 – 1.15 |
| AUC0–120h (ng/ml · hr) | 1280 ± 570 | 2390 ± 1060** | 1.84 | 1.60 – 2.12 |
| AUC0–∞ (ng/ml · hr) | 3840 ± 1780 | 4580 ± 2310* | 1.16 | 1.06 – 1.28 |
AUC, area under the plasma concentration-time curve; C.I., confidence interval; Cl/F, oral clearance; t1/2, terminal half-life; Tmax, time of observed maximal concentration; Vss/F, oral steady-state volume of distribution.
Values are shown as mean ± SD unless otherwise stated.
Tmax data are given as median and range.
p < 0.05
p < 0.001 significantly different from warfarin alone control phase.
AUC ratios of metabolite to parent
The AUC0–∞ ratio of 7-hydroxywarfarin to S-warfarin and 10-hydroxywarfarin to R-warfarin increased 20% and 58% respectively with rifampin treatment (p < 0.01 for both; Table 3).
Table 3.
AUC0–∞ ratios of wafarin metabolite to parent during each treatment period.
| AUCm/AUCpa | Warfarin aloneb | Rifampin phaseb |
|---|---|---|
| 7-Hydroxy-warfarin/S-warfarin | 0.21 ± 0.06 | 0.25 ± 0.07* |
| 10-Hydroxy-warfarin/R-warfarin | 0.21 ± 0.09 | 0.33 ± 0.17* |
AUC0–∞ ratio of hydroxywarfarin metabolite to parent warfarin.
Values are shown as mean ± SD
p < 0.01 significantly different from warfarin alone phase.
Pharmacodynamics
A single 7.5 mg dose of oral warfarin caused a small, transient increase in INR from baseline during each treatment period as reflected in the maximum INR (INRmax) vs. baseline INR (INRbaseline; p < 0.01 for both), but rifampin caused no differences in INR measures compared to warfarin alone (Table 4).
Table 4.
Mean pharmacodynamic parameters in 10 healthy volunteers following a single oral dose of 7.5 mg warfarin alone or in combination with a single dose of intravenous rifampin.
| Parameter | Warfarin alone | Rifampin phase |
|---|---|---|
| INRbaseline | 1.1 ± 0.1 | 1.1 ± 0.1 |
| INRmax | 1.2 ± 0.1 | 1.2 ± 0.1 |
| AUC0–96h, INR (INR · h) | 106 ± 3 | 108 ± 7 |
INRbaseline, International Normalized Ratio at baseline; INRmax, maximum INR; AUC0–96h, INR, area under the INR versus time curve from 0 to 96 hours. Values are shown as mean ± SD. There were no statistically significant differences between the two treatments.
Safety
All 10 subjects who were enrolled completed the study. No significant adverse events were observed and no bleeding complications occurred.
DISCUSSION
In this study, we attempted to determine the importance of hepatic uptake transporters for the disposition of the widely-used, narrow therapeutic index, anticoagulant warfarin. The results of our clinical study demonstrated that co-administration of the potent OATP inhibitor rifampin, given as a single intravenous dose, had no effect on the AUC0–12h and Cmax values of S- and R- warfarin or S-warfarin's hydroxyl metabolite. Accordingly, OATP uptake transport is likely not clinically significant for the disposition of warfarin and will provide no further insight into mechanisms of warfarin drug-drug interactions or genetic variation important in warfarin dose selection.
Rifampin was chosen as a model OATP inhibitor to block the in vivo OATP-mediated hepatic uptake of warfarin(11, 17, 18). Because of rifampin's shorter t1/2 (2.9 ±0.9 h(19)) relative to S- and R- warfarin (39h and 49h respectively, as determined in our clinical study) and because we could not give rifampin as multiple doses dues to its metabolic enzyme induction effect, rifampin was only present in plasma for a portion of warfarin's concentration-time curve.OATP inhibition and any potential effect on warfarin pharmacokinetics occurred at most during the time of rifampin presence, and therefore, outcome measures for our study needed to be suitably selected to capture this period of OATP inhibition. Previous reports have shown that after a single 600 mg intravenous dose of rifampin to healthy volunteers, rifampin was present for up to 12–24 hours in plasma. (11, 16) Assuming a similar time of exposure for rifampin in our clinical study, the best parameters to capture the potential effects of OATP inhibition on warfarin pharmacokinetics were the AUC0–12h and Cmax. No effect on either of these pharmacokinetic parameters for S- or R-warfarin were seen in the presence of rifampin suggesting OATP transport of warfarin is not clinically important.
Two previous clinical studies by our laboratory using a similar study design as used here demonstrated significant changes in the pharmacokinetics of atorvastatin and glyburide with hepatic OATP inhibition by rifampin(11, 12). The AUC of atorvastatin was 680% higher and the AUC of glyburide 130% higher in rifampin vs. control phases. In those clinical studies rifampin also produced a large decrease in the Vss/F for both drugs (atorvastatin, 93% decrease; glyburide, 60% decrease). This decrease in Vss/F likely demonstrated inhibition of uptake transporter mechanisms important for drug distribution in tissues. In our current study we did not see a change in Vss/F for S- or R-warfarin on rifampin days (Table 1), which we would have predicted if OATP transport processes were important for warfarin distribution.
In contrast to our clinical study, our in vitro data showed that warfarin uptake into hepatocytes was inhibited by rifampin in both rats and humans (Figure 1) presumably via OATP uptake transporter inhibition. The magnitude of warfarin uptake inhibition (23% in rat hepatocytes and 34% in human hepatocytes) was modest at the concentration of rifampin (100 μM) used for the hepatocyte uptake studies. The 100 μM concentration of rifampin used in our hepatocyte uptake studies is significantly higher than peak plasma concentrations that were likely achieved in our healthy volunteers after a single IV dose of 600 mg rifampin (17–20μM (11, 16)) and could explain the disagreement between our in vitro and in vivo results, but the in vitro effects were similar to what we observed in vitro with glyburide. We cannot rule-out the possibility that higher plasma concentrations of rifampin would result in additional OATP inhibition and therefore alter warfarin pharmcokinetics, although the clinical relevance would be questionable. The discordance between our in vitro and in vivo data highlights the importance of translational research in drug transporter research and the need to verify the clinical relevance of all preclinical findings using appropriately designed clinical trials.
Our healthy volunteer clinical data are in line with the numerous genome wide association studies (GWAS) undertaken to identify polymorphisms in the human genome that may be important in warfarin dose selection. In these GWAS studies, no common variants in hepatic uptake transporters or any other drug transporters were found to be significant in determining the warfarin dose requirements of patients.(2–5) Genetic variants of a transporter will not produce a significant signal in a GWAS if the drug is not a substrate for the transporter of interest since changes in transporter function will not alter the drug's pharmacokinetics and thus dose requirements. This negative predictive value has now been demonstrated nicely for warfarin and OATP uptake transporters through the results of our clinical study and the above mentioned GWASs. In contrast, for drugs that are known substrates of uptake or efflux transporters including OTAP1B1 and OATP1B3, established polymorphisms in the corresponding transporter have been shown to alter drug pharmacokinetics in human clinical studies.(13, 20, 21)
Intravenous administration of rifampin conceivably minimized any potential confounding interactions in the intestine at either the enzyme or transporter level although the lack of an interaction cannot be fully confirmed. By giving rifampin as a single dose, we attempted to avoid the well-described and clinically relevant CYP induction effect seen with multiple dosing.(16, 22, 23) Induction of both CYP2C9(24, 25) and CYP3A4 (25–28), the major metabolizing enzymes for S- and R-warfarin respectively, has been described after multiple dose rifampin. The effect of multiple dose rifampin on warfarin pharmacokinetics was highlighted in a clinical trial of four healthy men.(24) After 3 days of pretreatment with 300 mg oral rifampin twice per day, a three-fold increase in R-warfarin clearance and a two-fold increase in S-warfarin clearance were seen. Interestingly, our clinical study using only single dose rifampin immediately prior to warfarin likely demonstrates a mild enzyme inductive effect indicated by the small but significant decrease in AUC0–∞ for S- and R-warfarin (15% decrease and 25% decrease respectively) and terminal t1/2 for R-warfarin (25% decrease) with rifampin treatment. The increase in Cmax and AUC for the R-warfarin metabolite 10-hydroxywarfarin and the increase in the AUC ratio of 10-hydroxywarfarin to R-warfarin further supports induction of R-warfarin metabolism via CYP3A4. The pharmacokinetics of the specific S-warfarin metabolite 7-hydroxywarfarin did not change with rifampin treatment and therefore is not congruent with induction of S-warfarin metabolism via CYP2C9.
The pharmacokinetic effect of enzyme induction appeared to be delayed since early in the time course S- and R-warfarin concentration-time curves, AUC0–12h, and Cmax were similar with and without rifampin. Such a delay is expected based on the mechanism of rifampin enzyme induction through activation of the nuclear transcription factor pregnane X receptor (PXR).(23) A previous clinical study examining the time-course of CYP enzyme induction with multiple-dose rifampin using verapamil as a probe substrate reported a half-life of 1 day for the increase in CYP enzyme activity.(29) CYP induction after single dose rifampin has previously been shown with a single dose of 1200 mg oral rifampin given 12 hours before oral nifedipine, resulting in a 64% decrease in the AUC0–∞ of nifedipine.(30) Nonetheless, the potential induction of warfarin metabolism by single-dose rifampin in our study does not alter the interpretation of the primary objective of this study as any induction was small (especially for the more biologically active S-warfarin) and it occurred late in the time-course after the primary endpoints (0–12 hours).
We purposely selected a warfarin dose for this study (7.5mg) that would provide adequate drug levels for pharmacokinetic analysis, yet in favor of subject safety produce minimal anticoagulation. Our nominal increase in anticoagulation was similar to a previous healthy volunteer study that also used a single 7.5mg dose of oral warfarin.(31) The addition of rifampin had no effect on the warfarin pharmacodynamic response as measured by INRmax and AUC0–96h, INR (Table 3) and is in general agreement with our pharmacokinetic data. However, the study was neither designed nor powered to detect a difference in pharmacodynamics.
In conclusion, our single dose clinical study of the effect of rifampin on warfarin pharmacokinetics in healthy volunteers suggeststhat hepatic uptake via OATP may not be clinicallyimportant in the disposition for this highly metabolized, narrow therapeutic index, anticoagulant. Single-dose rifampin may induce metabolizing enzymes such as CYP2C9 and CYP3A4.
METHODS
In Vitro Study
Hepatocyte Uptake Studies
Rat hepatocytes were isolated from Male Sprague-Dawley rats (250–300g, Charles River Laboratories, Wilmington, MA) as previously described by our laboratory(32) and suspended in oxygenated hepatocyte wash buffer with 1% bovine serum albumin to yield a concentration of 1 million cells/ml. Cryopreserved human hepatocytes pooled from 5 female and 5 male donors were used according to the provider's instructions (CellzDirect™; Durham, NC). In brief, hepatocytes were thawed quickly in a 37°C water bath and washed twice with ice-cold hepatocyte wash buffer to remove cryopreservation solution. Cells were suspended in oxygenated hepatocyte wash buffer to yield a concentration of 0.5 million cells/mL.
Suspended hepatocytes were then pre-incubated for 5 minute in a 37°C water bath with either control (0.1% DMSO) or 100 μM rifampin. The 100 μM concentration of rifampin was chosen to maximize OATP inhibition and any effect on warfarin uptake Rifampin is a known inhibitor of the 3 major human OATPs, being a more potent inhibitor of OATP1B1 and OATP1B3 than OATP2B1.(18,33) The uptake study was initiated by addition of 1 μM racemic warfarin (Sigma-Aldrich; St. Louis, MO). After 2 minutes of uptake, a 0.5 mL aliquot was transferred to a tube containing 700 μL of silicone:mineral oil mixture (83.3:16.7 v/v). The tube was then centrifuged for 10 sec at 13,000 rpm to isolate the hepatocyte pellet. The oil mixture was removed and the pellet was lysed by sonication in the presence of 200 μL H2O. The samples were then prepared for HPLC-MS/MS analysis by liquid-liquid extraction. Student's t-test was used to analyze differences between the two groups (rat, n = 9 per group; human, n =6 per group).
Clinical Study
Subjects
Subjects were 6 male and 4 female paid, non-smoking, healthy volunteers 23 to 55 years of age (mean age 30 ± 10 years). Their mean BMI was 25.6 ± 3.5 kg/m2. Participants included four Caucasians, four Asians/Pacific Islanders and two Hispanics/Latinos. After written informed consent was obtained, subjects underwent a screening visit consisting of a medical history, physical examination, and standard clinical laboratory tests (general hematology, blood chemistry, coagulation studies, urinalysis, and urine pregnancy) to confirm eligibility. In addition, CYP2C9 genotyping was performed and only subjects who were CYP2C9 *1/*1 were included. All subjects were medication free throughout study enrollment. The study was approved by the Committee on Human Research of the University of California, San Francisco.
Study design
A randomized, open-labeled, single-dose, two-period, crossover clinical pharmacokinetic study was conducted at the Clinical Research Center at the University of California, San Francisco. The study followed the protocol used previously in our laboratory to document the importance of OATP hepatic uptake in healthy voluneteers for atorvastatin(11) and glyburide(12). Subjects received on Day 1 of each study period either (A) 7.5 mg warfarin (one 7.5 mg warfarin tab; Bristol-Myers Squibb, Princeton, NJ) or (B) 7.5 mg warfarin immediately following a 30-minute intravenous infusion of 600 mg rifampin (600 mg rifampin powder for injection reconstituted in 10 ml sterile water; Bedford Laboratories, Bedford, OH). Each subject received both treatments separated by a minimum of 14 days, and the treatment order was determined by randomization. Subjects fasted starting the night before until 3 hours after a warfarin dose, abstained from caffeinated beverages, alcoholic beverages and orange juice starting the night before until 24 hours after a warfarin dose. Grapefruit and grapefruit juice were not allowed to be consumed from 7 days before the first study day until completion of the entire study.
Venous blood samples (8ml) for drug level measurements were collected at the following times relative to warfarin dosing: predose, 1, 2, 3, 4, 6, 8, 12, 24, 48, 72, 96, 120 hours. The blood samples were centrifuged within 30 minutes, and plasma was separated and stored at −80°C until analysis. In addition, venous blood samples (5ml) were collected for INR determination at 0, 12, 24, 48, 72 and 96 hours after warfarin dosing.
Pharmacokinetic analysis
Pharmacokinetic parameters for R- and S-warfarin and their major metabolites were estimated from plasma concentration data via non-compartmental analysis using WinNonlin Professional software (version 5.2.1; Pharsight Corporation, Mountain View, CA). The Cmax and corresponding Tmax were obtained directly from the observed data. The terminal rate constant (λz) was determined by linear regression analysis of the terminal portion of the log plasma concentration–time curve. We were most interested in rifampin's inhibitory effect on hepatic OATPs and the anticipated changes in R- and S-warfarin pharmacokinetics (i.e. AUC). Given S-warfarin's much longer half-life(31, 34–41) compared to rifampin(41), rifampin was expected to be present in plasma for only portion of warfarin's concentration time profile. Data from a previous study by our laboratory and information provided in the package insert, predict relevant plasma concentrations of rifampin for up to 12 hours after a single 600 mg intravenous dose.(11, 16) Accordingly, our primary outcome was the AUC0–12h of S- and R-warfarin, which represents the time a potential inhibitory effect on hepatic OATPs by rifampin would occur.
AUC0–12h and AUC0–120h were found using the linear/logarithmic trapezoidal method. Summation of AUC0–120h and the concentration at the last measured point divided by λz yielded AUC0–∞. The terminal half-life (t1/2) was calculated as ln 2/λz. CL/F was calculated as dose/AUC0–∞. The mean residence time (MRT) was calculated as the ratio of the area under the first moment curve (AUMC0–∞) divided by AUC0–∞ minus the estimated mean absorption time (MAT) (i.e., MRT=(AUMC0–∞ / AUC0–∞) − MAT). MAT was the reciprocal of the first-order absorption rate (ka) constant estimated when fitting warfarin data to a two-compartmental model with first-order absorption. Vss/F was calculated as MRT × CL/F. The values of CL/F and Vss/F were normalized by body weight.
Pharmacodynamic analysis
Pharmacodynamic parameters were estimated from the INR data on each treatment day by non-compartmental methods using WinNonlin. Baseline INR (INRbaseline) and maximum INR (INRmax) were obtained directly from the observed data. Area under the INR-time curve from 0 to 96 hours (AUC0–96h, INR) was calculated using the linear/logarithmic trapezoidal method.
Statistical analysis
Using previously reported warfarin pharmacokinetic data in healthy volunteers(31), a sample size of 10 subjects would provide > 80% power to detect a 30% difference in S-warfarin AUC0–∞ between the two treatments. Logarithmic transformation of Cmax, t1/2, Vss/F, CL/F, AUC0–12h, AUC0–∞ was performed prior to statistical analysis. Pharmacokinetic and pharmacodynamic parameters were compared between the two treatments by use of the paired t-test, except for Tmax in which the Wilcoxon's signed rank test was used. Data are presented as mean ± standard deviation. In addition, the geometric mean ratios of the rifampin treatment values relative to control values (warfarin alone) and their 95% confidence intervals were calculated for all parameters. The data were analyzed using STATA 10 (StataCorp LP, College Station, TX). Differences were considered statistically significant at p < 0.05.
Analytic Methods
The concentrations of S- and R-warfarin and their main metabolites were measured using high pressure liquid chromatography/tandem mass spectrometry system consisting of an Applied Biosystems AB4000 triple quadrupole mass spectrometer (Foster City, CA) with electrospray negative ionization mode. The multiple reaction monitor was set at 307–161 m/z for R- and S-warfarin, 323–177 m/z for 7-hydroxy-warfarin, 323–250 m/z for 10-hydroxy-warfarin, 312–166 m/z for d5-warfarin (internal standard for S- and R-warfarin) and 312–117 m/z for d5-warfarin (internal standard for metabolites).
The samples and standards for S- and R-warfarin (Sigma-Aldrich, St. Louis, MO) were prepared by liquid-liquid extraction of 0.5 mL plasma with 2.5 mL methyl t-butyl ether containing 1μM d5-warfarin as internal standard. After being vortexed for one minute, the sample was centrifuged at 3,000 rpm for 10 min. The bottom aqueous layer was frozen via a dry-ice/methanol bath. The top organic layer was collected, dried under nitrogen, and then reconstituted in acetonitrile for analysis.
The samples and standards for warfarin metabolites were prepared by liquid-liquid extraction of 100μL plasma with 500μL of methanol containing 1μM d5-warfarin as internal standard. Samples were vortexed for one minute followed by centrifugation at 13,000 rpm for 10 min. The supernatant was collected and dried under nitrogen. The residue was reconstituted in 50:50 methanol:H2O then centrifuged at 13,000 rpm for 5 min. The supernatant was collected for analysis.
Chromatographic conditions for S- and R-warfarin were as described by Naidong and colleagues(43). In brief, R-warfarin and S-warfarin were separated isocratically on a Cyclobond™ I 2000 β-cyclodextrin 250 × 4.6mm, 5μm column with a Cyclobond™ I 2000 50 × 4.6, 5μm guard column (Sigma-Aldrich, St. Louis, MO). R-d5-warfarin and S-d5-warfarin (racemic d5-warfarin from Toronto Research Chemicals, Inc., Ontario, Canada) enantiomers were also separated by the β-cyclodextrin column and co-eluted with R-warfarin and S-warfarin, respectively. The mobile phase consisted of acetonitrile:acetic acid:triethylamine (1000:3:2.5 v:v:v) and the flow rate was 1mL/min. The injection volume was 100μL. Retention times were 7.5 min for S-warfarin and 8.3 min for R-warfarin.
Chromatography for warfarin metabolites was performed on an Agilent Eclipse XDB C18 column (150 × 4.6 mm, 5μm particle size, Agilent Technologies, Santa Clara, CA). The mobile phase A was 0.1% acetic acid in H2O and mobile phase B was 0.1% acetic acid in methanol. The mobile phase gradient was as follows: 0–0.5 min 50% B; 5.5 min 90% B; 5.6–9.0 min 100% B, 9.5–10.0 min 50% B at a flow rate of 1mL/min. The run time was 10 min.
The methods for S- and R-warfarin were validated from 1.5 to 300 ng/ml. The 1 h and 2 h samples were diluted 1-fold to ensure concentrations within the validated range. The methods for 7-hydroxywarfarin and 10-hydroxywarfarin were validated from 1.5 to 150 ng/ml. The curves were linear over the validated ranges and 1/x2 weighting was used.
ACKNOWLEDGEMENTS
This study was supported in part by NIH grants GM61390 and GM75900. It was carried out in part in the Clinical Research Center, Moffitt Hospital, University of California, San Francisco, which is supported by the National Institutes of Health (NIH) / National Center for Research Resources (NCRR) UCSF-CTSI Grant Number UL1 RR024131. A.F. is supported by Grant T32 GM07546 from the National Institute of General Medical Sciences. S.S. was supported during a portion of these studies as an Amgen fellow. The authors thank the nurses and staff at the Clinical Research Center, Moffitt Hospital, University of California, San Francisco for their help with the clinical study and Andrew Smith for his help with subject genotyping. This work was presented at the 2010 American Society for Clinical Pharmacology and Therapeutics (ASCPT) Annual Meeting, March 17, Atlanta, GA by AF as an ASCPT Presidential Trainee Award Winner.
Footnotes
DISCLOSURE The authors have no conflict of interests to declare.
REFERENCES
- (1).Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:204S–233S. doi: 10.1378/chest.126.3_suppl.204S. [DOI] [PubMed] [Google Scholar]
- (2).Caldwell MD, et al. CYP4F2 genetic variant alters required warfarin dose. Blood. 2008;111:4106–4112. doi: 10.1182/blood-2007-11-122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Cooper GM, et al. A genome-wide scan for common genetic variants with a large influence on warfarin maintenance dose. Blood. 2008;112:1022–1027. doi: 10.1182/blood-2008-01-134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Takeuchi F, et al. A genome-wide association study confirms VKORC1, CYP2C9, and CYP4F2 as principal genetic determinants of warfarin dose. PLoS Genet. 2009;5:e1000433. doi: 10.1371/journal.pgen.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Wadelius M, et al. Association of warfarin dose with genes involved in its action and metabolism. Hum. Genet. 2007;121:23–34. doi: 10.1007/s00439-006-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Gage BF, et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin. Pharmacol. Ther. 2008;84:326–331. doi: 10.1038/clpt.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Wadelius M, et al. The largest prospective warfarin-treated cohort supports genetic forecasting. Blood. 2009;113:784–792. doi: 10.1182/blood-2008-04-149070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Wu AH, et al. Dosing algorithm for warfarin using CYP2C9 and VKORC1 genotyping from a multi-ethnic population: comparison with other equations. Pharmacogenomics. 2008;9:169–178. doi: 10.2217/14622416.9.2.169. [DOI] [PubMed] [Google Scholar]
- (9).Schelleman H, et al. Dosing algorithms to predict warfarin maintenance dose in Caucasians and African Americans. Clin. Pharmacol. Ther. 2008;84:332–339. doi: 10.1038/clpt.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm. Res. 2005;22:11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- (11).Lau YY, Huang Y, Frassetto L, Benet LZ. Effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin. Pharmacol. Ther. 2007;81:194–204. doi: 10.1038/sj.clpt.6100038. [DOI] [PubMed] [Google Scholar]
- (12).Zheng HX, Huang Y, Frassetto LA, Benet LZ. Elucidating rifampin's inducing and inhibiting effects on glyburide pharmacokinetics and blood glucose in healthy volunteers: unmasking the differential effects of enzyme induction and transporter inhibition for a drug and its primary metabolite. Clin. Pharmacol. Ther. 2009;85:78–85. doi: 10.1038/clpt.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Shugarts S, Benet LZ. The role of transporters in the pharmacokinetics of orally administered drugs. Pharm. Res. 2009;26:2039–2054. doi: 10.1007/s11095-009-9924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Coumadin(R) Tablets. Bristol-Meyers Squibb Company; Princeton, NJ: 2007. package insert. [Google Scholar]
- (15).Kaminsky LS, Zhang ZY. Human P450 metabolism of warfarin. Pharmacol. Ther. 1997;73:67–74. doi: 10.1016/s0163-7258(96)00140-4. [DOI] [PubMed] [Google Scholar]
- (16).Rifampin for injection USP. Bedford Laboratories; Bedford, OH: 2004. package insert. [Google Scholar]
- (17).Baldes C, Koenig P, Neumann D, Lenhof HP, Kohlbacher O, Lehr CM. Development of a fluorescence-based assay for screening of modulators of human organic anion transporter 1B3 (OATP1B3) Eur. J. Pharm. Biopharm. 2006;62:39–43. doi: 10.1016/j.ejpb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- (18).Vavricka SR, Van Montfoort J, Ha HR, Meier PJ, Fattinger K. Interactions of rifamycin SV and rifampicin with organic anion uptake systems of human liver. Hepatology. 2002;36:164–172. doi: 10.1053/jhep.2002.34133. [DOI] [PubMed] [Google Scholar]
- (19).Israili ZH, Rogers CM, el-Attar H. Pharmacokinetics of antituberculosis drugs in patients. J. Clin. Pharmacol. 1987;27:78–83. doi: 10.1177/009127008702700113. [DOI] [PubMed] [Google Scholar]
- (20).Niemi M. Role of OATP transporters in the disposition of drugs. Pharmacogenomics. 2007;8:787–802. doi: 10.2217/14622416.8.7.787. [DOI] [PubMed] [Google Scholar]
- (21).Zair ZM, Eloranta JJ, Stieger B, Kullak-Ublick GA. Pharmacogenetics of OATP (SLC21/SLCO), OAT and OCT (SLC22) and PEPT (SLC15) transporters in the intestine, liver and kidney. Pharmacogenomics. 2008;9:597–624. doi: 10.2217/14622416.9.5.597. [DOI] [PubMed] [Google Scholar]
- (22).Finch CK, Chrisman CR, Baciewicz AM, Self TH. Rifampin and rifabutin drug interactions: an update. Arch. Intern. Med. 2002;162:985–992. doi: 10.1001/archinte.162.9.985. [DOI] [PubMed] [Google Scholar]
- (23).Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivisto KT. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin. Pharmacokinet. 2003;42:819–850. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- (24).Heimark LD, Gibaldi M, Trager WF, O'Reilly RA, Goulart DA. The mechanism of the warfarin-rifampin drug interaction in humans. Clin. Pharmacol. Ther. 1987;42:388–394. doi: 10.1038/clpt.1987.168. [DOI] [PubMed] [Google Scholar]
- (25).Kanebratt KP, et al. Cytochrome P450 induction by rifampicin in healthy subjects: determination using the Karolinska cocktail and the endogenous CYP3A4 marker 4beta-hydroxycholesterol. Clin. Pharmacol. Ther. 2008;84:589–594. doi: 10.1038/clpt.2008.132. [DOI] [PubMed] [Google Scholar]
- (26).Backman JT, Kivisto KT, Olkkola KT, Neuvonen PJ. The area under the plasma concentration-time curve for oral midazolam is 400-fold larger during treatment with itraconazole than with rifampicin. Eur. J. Clin. Pharmacol. 1998;54:53–58. doi: 10.1007/s002280050420. [DOI] [PubMed] [Google Scholar]
- (27).Bidstrup TB, Stilling N, Damkier P, Scharling B, Thomsen MS, Brosen K. Rifampicin seems to act as both an inducer and an inhibitor of the metabolism of repaglinide. Eur. J. Clin. Pharmacol. 2004;60:109–114. doi: 10.1007/s00228-004-0746-z. [DOI] [PubMed] [Google Scholar]
- (28).Kyrklund C, Backman JT, Neuvonen M, Neuvonen PJ. Effect of rifampicin on pravastatin pharmacokinetics in healthy subjects. Br. J. Clin. Pharmacol. 2004;57:181–187. doi: 10.1046/j.1365-2125.2003.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Fromm MF, Busse D, Kroemer HK, Eichelbaum M. Differential induction of prehepatic and hepatic metabolism of verapamil by rifampin. Hepatology. 1996;24:796–801. doi: 10.1002/hep.510240407. [DOI] [PubMed] [Google Scholar]
- (30).Ndanusa BU, Mustapha A, Abdu-Aguye I. The effect of single does of rifampicin on the pharmacokinetics of oral nifedipine. J. Pharm. Biomed. Anal. 1997;15:1571–1575. doi: 10.1016/s0731-7085(97)00044-7. [DOI] [PubMed] [Google Scholar]
- (31).Washington C, Hou SY, Hughes NC, Campanella C, Berner B. Ciprofloxacin prolonged-release tablets do not affect warfarin pharmacokinetics and pharmacodynamics. J. Clin. Pharmacol. 2007;47:1320–1326. doi: 10.1177/0091270007305504. [DOI] [PubMed] [Google Scholar]
- (32).Lam JL, Okochi H, Huang Y, Benet LZ. In vitro and in vivo correlation of hepatic transporter effects on erythromycin metabolism: characterizing the importance of transporter-enzyme interplay. Drug Metab. Dispos. 2006;34:1336–1344. doi: 10.1124/dmd.106.009258. [DOI] [PubMed] [Google Scholar]
- (33).Treiber A, Schneiter R, Häusler S, Stieger B. Bosentan is a substrate of human OATP1B1 and OATP1B3: inhibition of hepatic uptake as the common mechanism of its interactions with cyclosporin A, rifampicin, and sildenafil. Drug Metab Dispos. 2007;35:1400–1407. doi: 10.1124/dmd.106.013615. [DOI] [PubMed] [Google Scholar]
- (34).Jiang X, et al. Effect of St John's wort and ginseng on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br. J. Clin. Pharmacol. 2004;57:592–599. doi: 10.1111/j.1365-2125.2003.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Jiang X, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br. J. Clin. Pharmacol. 2005;59:425–432. doi: 10.1111/j.1365-2125.2005.02322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Kim JS, Nafziger AN, Gaedigk A, Dickmann LJ, Rettie AE, Bertino JS., Jr. Effects of oral vitamin K on S- and R-warfarin pharmacokinetics and pharmacodynamics: enhanced safety of warfarin as a CYP2C9 probe. J. Clin. Pharmacol. 2001;41:715–722. doi: 10.1177/00912700122010618. [DOI] [PubMed] [Google Scholar]
- (37).Kong AN, et al. Losartan does not affect the pharmacokinetics and pharmacodynamics of warfarin. J. Clin. Pharmacol. 1995;35:1008–1015. doi: 10.1002/j.1552-4604.1995.tb04018.x. [DOI] [PubMed] [Google Scholar]
- (38).Lilja JJ, Backman JT, Neuvonen PJ. Effects of daily ingestion of cranberry juice on the pharmacokinetics of warfarin, tizanidine, and midazolam--probes of CYP2C9, CYP1A2, and CYP3A4. Clin. Pharmacol. Ther. 2007;81:833–839. doi: 10.1038/sj.clpt.6100149. [DOI] [PubMed] [Google Scholar]
- (39).Mohammed Abdul MI, et al. Pharmacodynamic interaction of warfarin with cranberry but not with garlic in healthy subjects. Br. J. Pharmacol. 2008;154:1691–1700. doi: 10.1038/bjp.2008.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Soon D, et al. Effect of exenatide on the pharmacokinetics and pharmacodynamics of warfarin in healthy Asian men. J. Clin. Pharmacol. 2006;46:1179–1187. doi: 10.1177/0091270006291622. [DOI] [PubMed] [Google Scholar]
- (41).Uno T, Sugimoto K, Sugawara K, Tateishi T. The effect of CYP2C19 genotypes on the pharmacokinetics of warfarin enantiomers. J. Clin. Pharm. Ther. 2008;33:67–73. doi: 10.1111/j.1365-2710.2008.00887.x. [DOI] [PubMed] [Google Scholar]
- (42).Loos U, Musch E, Jensen JC, Mikus G, Schwabe HK, Eichelbaum M. Pharmacokinetics of oral and intravenous rifampicin during chronic administration. Klin. Wochenschr. 1985;63:1205–11. doi: 10.1007/BF01733779. [DOI] [PubMed] [Google Scholar]
- (43).Naidong W, Ring PR, Midtlien C, Jiang X. Development and validation of a sensitive and robust LC-tandem MS method for the analysis of warfarin enantiomers in human plasma. J. Pharm. Biomed. Anal. 2001;25:219–26. doi: 10.1016/s0731-7085(00)00476-3. [DOI] [PubMed] [Google Scholar]