Abstract
Left ventricular hypertrophy due to hypertension represents a major risk factor for adverse cardiovascular events and death. In recent years, the prevalence of cardiac hypertrophy has increased due to obesity and an aging population. Notably, a significant number of individuals have persistent cardiac hypertrophy in the face of blood pressure that is normalized by drug treatment. Thus, a better understanding of the processes underlying the cardiac remodeling events that are set into play by hypertension is needed. At the level of the cardiac myocytes, hypertrophic growth is often described as physiological, as occurs with exercise, or pathological, as seen with hypertension. Here we discuss recent developments in three areas that are fundamental to pathological hypertrophic growth of cardiac myocytes. These areas are the transient receptor potential canonical (TRPC) channels, mammalian target of rapamycin (mTOR) complexes, and histone deacetylase (HDAC) enzymes. In the last several years, studies in each of these areas have yielded new and exciting discoveries into the genesis of pathological growth of cardiac myocytes. The phosphoinositide 3-kinase – Akt signaling network may be the common denominator that links these areas together. Defining the interrelationship among TRPC channels, mTOR signaling, and HDAC enzymes is a promising, but challenging area of research. Such knowledge will undoubtedly lead to new drugs that better prevent or reverse left ventricular hypertension.
Keywords: left ventricular hypertrophy, cardiac remodeling, mechanosensitive channels, cardiac myocyte, histone deacetylases, hypertension
1. Introduction
Hypertrophy of the left ventricle (LV) due to hypertension is an important, independent predictor of morbidity and mortality. Indeed, LV hypertrophy is a stronger risk factor for adverse cardiovascular events than blood pressure itself [1]. As such, hypertensive cardiac hypertrophy is pathological and much progress has been made since the 1990’s in elucidating the adverse cardiac remodeling events responsible for that designation and their cellular and molecular underpinnings. The neuroendocrine factors and their associated tangled web of intracellular signaling cascades that contribute to pathological cardiac hypertrophy have been known for some time [2]. In the last several years though great progress has been made in identifying new cellular processes that are arguably more fundamental in initiating and sustaining pathologic LV hypertrophy. Here we review developments in three such areas - transient receptor potential canonical (TRPC) channels, mammalian target of rapamycin (mTOR) complexes, and histone deacetylase (HDAC) enzymes. Besides providing novel insights into the pathogenesis of cardiac hypertrophy, we propose that these molecules may be linked together mechanistically via the phosphoinositide 3-kinase (PI3K) – Akt signaling network as discussed later in this article.
2. TRPC Channels
Relatively modest but sustained increases in intracellular calcium within cardiac myocytes, manifested as increases in the frequency or amplitude of Ca2+ transients, due to stretch or neuroendocrine stimulation, have been linked to pathological cardiac hypertrophy and gene expression through activation of the calcineurin-NFAT (nuclear factor of activated T-cell) signaling cascade (Fig. 1) [3,4]. Recent evidence indicates that TRPC channels are the major route for this Ca2+ entry [5–8].
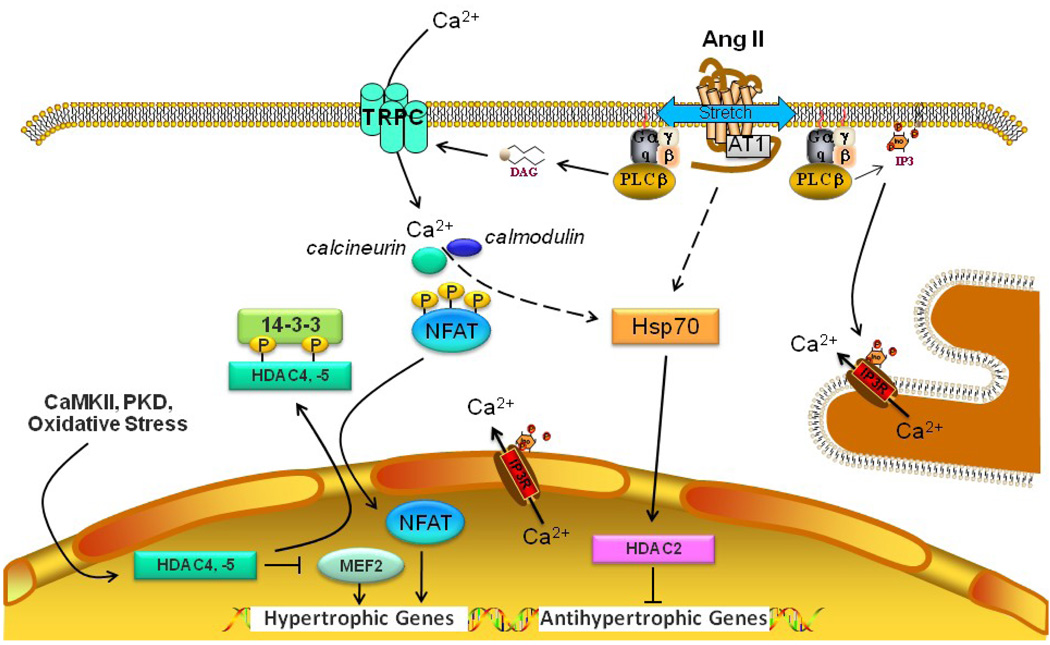
Figure 1.
The role of transient receptor potential canonical (TRPC) channels and histone deacetylases (HDACs) in pathological cardiac hypertrophy. Membrane stretch and/or Ang II activate the AT1 receptor, which in turn activates phospholipase C (PLC) to produce diacylglycerol (DAG) and inositol trisphosphate (IP3). DAG directly activates TRPC3/6/7 leading to an influx of Ca2+. IP3 can increase cytosolic Ca2+ by opening IP3 receptors in the sarcoplasmic reticulum or nuclear envelope. Calcineurin is activated by Ca2+-calmodulin and dephosphorylates NFAT, which translocates to the nucleus and induces hypertrophic genes. Kinases such CaMKII and protein kinase D (PKD) that are activated by hypertrophic stimuli phosphorylate Class IIa HDACs (HDAC4,-5) leading to their association with the 14-3-3 intracellular chaperone protein and nuclear export. This in turn leads to the derepression of pathological cardiac gene expression, for instance by freeing up the myocyte enhancer factor (MEF)2 transcription factor. The Class I HDACs, HDAC1 and -2, play a dominant role in pathological cardiac hypertrophy by blocking the expression of antihypertrophic genes. HDAC2 is activated by heat shock protein 70 (Hsp70), the expression of which is increased by hypertrophic stimuli.
The TRPC proteins constitute a subfamily of the TRP cation channel superfamily of proteins and were identified in the mid-1990s as candidate mammalian mechanosensitive cation channels that carry Na+, K+, Ca2+ and Mg2+ fluxes across the cell membrane in response to cell stretch or swelling. The 7 TRPC proteins are thought to assemble as tetramers to form nonselective, Ca2+ permeable cation channels with Ca2+/Na+ permeability ratios that range from 1 to 9 [6]. TRPC proteins have 6 membrane-spanning helices (TM1–6), cytoplasmic N- and C-termini, and an extended region linking TM5 and TM6 that is thought to contribute one side of the pore region in the tetramer [5]. The human heart expresses TRPC1, TRPC4, TRPC5, and TRPC6 proteins, while mouse and rat cardiac myocytes express all 7 TRPC proteins [5]. Recent studies implicating TRPC1, 4, 5, and 6 in pressure-overload cardiac hypertrophy are listed in Table 1. In general, TRPC1/4/5 are activated by mechanical stretch or depletion of intracellular Ca2+ stores, while TRPC3/6/7 are directly activated by diacylglycerol (DAG) that arises from phospholipase C activation by Gq/11-coupled receptors, such as the angiotensin II (Ang II) AT1 and endothelin 1 (ET-1) ETA receptors [6,22]. Based on this distinction, TRPC1/4/5 were originally considered store-operated ion channels (SOCs), although other proteins are now known to contribute to SOCs, and TRPC3/6/7 were classified as receptor-operated channels (ROCs), although SOC functions have been described for both TRPC3, which directly interacts with the inositol trisphosphate and ryanodine receptors, and TRPC7 [6,22]. Functional TRPC channels are commonly comprised of homo- or heterotetramers among members of the same group, but functional associations among members of the two different groups appear to occur in the presence of TRPC1 [22]. This would explain reports implicating TRPC1 in agonist-induced cardiac myocyte hypertrophy [9,11,22]; additionally, cardiac myocytes of mice resulting from crossing dominant negative TRPC4 with TRPC3 transgenic mice were found to have attenuated TRPC3-dependent Ca2+ influx under store-depleted conditions [14].
Table 1.
Evidence linking human heart expressed TRPC channels to cardiac hypertrophy
| SUBTYPE | FINDING | REFERENCES |
|---|---|---|
| TRPC1 | • Only TRPC1 expression increased in hearts of abdominal aortic-banded rats for 4 weeks. | [9] |
| • Silencing of TRPC1 gene in cultured cardiac myocytes reduced store-operated Ca2+ entry and prevented ET-1-, Ang-II-, and PE-induced hypertrophy. |
[9] | |
| • TRPC1 expression increased in older mdx mice with dilated cardiomyopathy, a model of Duchenne’s muscular dystrophy. Resting Ca2+ elevated in isolated myocytes from old mdx animals. |
[10] | |
| • Cardiac myocytes of TRPC1 KO mice do not exhibit PO-induced upregulation of nonselective cation (TRPC) current. KO mice subjected to hemodynamic and neurohormonal stress maintained cardiac function and do not exhibit maladaptive cardiac hypertrophy. Mechanosensitive signaling through calcineurin/NFAT, mTOR and Akt altered in KO mice. |
[11] | |
| • Reduced SERCA2 expression associated with up-regulation of TRPC4 and TRPC5 and Na+/Ca2+ exchanger due to calcineurin activation. |
[12] | |
| TRPC4, | • Only TRPC5 expression induced in failing hearts from patients with end-stage idiopathic dilated cardiomyopathy. | [13] |
| TRPC5 | • PO- and neuroendocrine-induced cardiac hypertrophy attenuated in cardiac-specific TG mice expressing dnTRPC3, dnTRPC6, or dnTRPC4. Cardiac myocytes of TG mice lack unique stress-induced store-operated Ca2+ entry current. TRPC channel inhibition reduced NFAT activity. |
[14] |
| TRPC6 | • TRPC6 (and TRPC3) activity correlated with Ang II-induced NFAT activation and hypertrophy in rat neonatal cardiomyocytes. |
[15] |
| • TRPC6 upregulated in hearts from cardiac myocyte-targeted calcineurin TG mice and PO-subjected wild type mice, as well as failing human hearts (dilated cardiomyopathy). Two conserved NFAT consensus sites in TRPC6 gene promoter identified. Cardiac-targeted TRPC6 TG mice had enhanced sensitivity to PO and tendency for lethal cardiac growth and heart failure. |
[16] | |
| • PO-induced TRPC6 expression, cardiac hypertrophy, cardiac dysfunction and mortality attenuated/prevented in DGKε-TG mice. |
[17] | |
| • TRPC6 expression increased by PO in vivo and by Ang II or ET-1 in neonatal and adult cardiac myocytes in vitro; Ang II- or ET-1induced NFAT activity and protein blocked by 8Br-cGMP or sildenafil. Expression of T70A or S322Q TRPC6 mutants blocked inhibition; phospho-mimetic mutants suppressed NFAT activation. |
[18] | |
| • Inhibition of cGMP-selective PDE5 in rat neonatal cardiac myocytes inhibited ET-1-, DAG analog-, and mechanical stretch-induced Ca2+ influx and hypertrophy. Associated with TRPC6 T69 phosphorylation and not seen in TRPC6 S69A overexpressing cells. RGS2 and RGS4 knockdown did not affect inhibition of receptor- activated Ca2+ influx by PDE5 inhibition. |
[19] | |
| • dnTRPC6 blocked cardiac hypertrophy in vivo (see above). | [14] | |
| • ANP inhibited Ang II Ca2+currents and transients in adult mouse ventricular myocytes, but not those of ISO, due to cGMP production (lost in myocytes lacking GC-A, PKG I, or PKG I-target RGS 2). Ang II-(but not ISO-) induced cardiac hypertrophy enhanced in cardiac myocyte-restricted GC-A KO mice and associated with further enhancement of phosphorylation. |
[20] | |
| • ANP induced TRPC6 phosphorylation at PKG phosphorylation site T69 in neonatal rat ventricular myocytes and inhibited ET-induced Ca2+ influx and NFAT activation; TRPC6 overexpression in GC-A KO mice worsened cardiac hypertrophy. |
[21] | |
Abbreviations: Ang II, angiotensin II; CaMKII, Ca2+/calmodulin-dependent kinase II; DAG, diacylglycerol; DGK, diacylglycerol kinase-ε; dn, dominant-negative; ET-1, endothelin 1; GC-A, guanylyl cyclase-A; ISO, isoproterenol; KO, knockout; PE, phenylephrine; PDE5, phosphodiesterase 5; PKG, protein kinase G; PKG I, cyclic GMP-dependent protein kinase I; PO, pressure overload; RGS, regulator of G protein signaling; TG, transgenic.
Unexpectedly, reconstitution studies indicate that neither TRPC subgroup exhibits intrinsic mechanosensitivity [8]. TRPC3/6/7 proteins have been shown to respond to membrane stretch or cell swelling when co-expressed with a Gq/11-coupled receptor, but these receptors themselves are activated by mechanical forces independent of ligand [7,8]. Mechanoactivation is phospholipase C-dependent and inhibited by inverse agonists. No mechanosensitivity is observed by co-expressing Gs-coupled or tyrosine kinase receptors, which do not undergo a similar conformational change when the membrane is subjected to mechanical forces. However, as pointed out elsewhere in order for TRPC3/6/7 channels to respond to mechanical forces in these studies, the Gq/11-coupled receptor needed to be overexpressed [8]. This obviously raises a question as to whether Gq/11-coupled receptor - TRPC3/6/7 coupling could have pathophysiological relevance in the heart. A possible resolution to this issue is provided by a recent study suggesting that mechanical forces may potentiate agonist-driven activation of vascular TRPC6 channels via formation of the phospholipase A2 and P450 CYP4A metabolite of arachidonic acid, 20-HETE (20-hydroxyeicosatetraenoic acid) [23]. How TRPC1/4/5 proteins respond to stretch in situ is not known, but could be through association with TRPC3/6/7 proteins, some other membrane stretch sensor, or perhaps a Gq/11-coupled receptor. Members of both TRPC subgroups have been shown to associate with a diverse group of scaffolding and signaling proteins (See the Human Protein Reference Database for lists of interactions -http://www.hprd.org/index_html) [24].
Cardiac-derived atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) exert antihypertrophic actions on cardiac myocytes through cell membrane receptors that have intrinsic guanylyl cyclase activity (GC-A) [25]. Evidence indicates that the rapid increase in cytosolic cGMP and subsequent activation of cGMP-dependent protein kinase type I (PKG I) acts to inhibit the hypertrophic calcineurin-NFAT signaling pathway [26]. Several recent studies have provided evidence that the ANP/BNP – GC-A – cGMP – PKG I signaling axis inhibits Ca2+-induced calcineurin-NFAT signaling by targeting TRPC6 channel activity [18,19,21,26]. In neonatal rat ventricular myocytes, ANP inhibited ET-1-induced promoter activity linked to calcineurin-NFAT signaling [21]. The inhibitory effects of ANP or a membrane-permeable cGMP analogue, 8Br-cGMP were not seen with overexpressed TRPC6 mutants having an alanine replace either a serine or threonine residue that are phosphorylated by PKG [18,21]. 8Br-cGMP was also able to block ET-1 induced calcineurin-NFAT activation [21]. Moreover, ANP was shown to block ET-1-stimulated TRPC6 channel activity and Ca2+ influx. Of note, the human and murine TRPC6 promotors have several NFAT consensus sites, making TRPC6 part of a positive feedback loop for cardiac hypertrophy. Consequently, GC-A knockout mice, which exhibit salt-resistant hypertension and cardiac hypertrophy, have increased cardiac expression of TRPC6. In these mice, a selective TRPC inhibitor reduced cardiac hypertrophy and reduced TRPC6 levels without affecting hypertension or heart rate [21]. In contrast, the Ca2+ channel inhibitor nitrendipine modestly reduced blood pressure with no effect on cardiac hypertrophy. Two studies reported that increasing cyclic GMP by treating neonatal rat or adult mouse ventricular myocytes with the phosphodiesterase 5 (PDE5) inhibitor, sildenafil, inhibited Ang II-or ET-1-induced Ca2+ influx, NFAT activation, and hypertrophic response [18,19]. The actions of sildenafil were dependent upon PKG-mediated phosphorylation of TRPC6. Yet there is also evidence that other routes for activating calcineurin-NFAT and cardiac hypertrophy exist for G protein coupled receptors in general, including L-type Ca2+ channels (LTCC) and IP3 receptors [26–28]. While LTCC contribute to pressure overload-induced cardiac hypertrophy, current evidence indicates that IP3 receptors do not [28]. Using knockin mice heterozygous for an R176Q mutation in ryanodine receptor 2 (RyR2), others recently demonstrated that Ca2+ leak from the sarcoplasmic reticulum enhances pressure overload-induced calcineurin-NFAT activity and hypertrophy, consistent with recent clinical studies indicating that defects in the RyR2 gene are associated with hypertrophic cardiomyopathy [29]. In any case, the spatial constraints that link localized Ca2+ fluxes to calcineurin-NFAT activation and hypertrophic gene expression are not well understood. But on this subject, two recent studies have reported on the importance of a novel Z-disc associated LIM protein, Lmcd1/Dyxin, in coupling pressure overload to calcineurin activity, NFAT activation, and subsequent cardiac hypertrophy [30,31].
Regulator of G protein signaling 2 (RGS2) and RGS4 are also phosphorylated by PKG with a consequent increase in their activity and suppression of G protein coupled receptor signaling. Conflicting evidence has been reported concerning the involvement of RGS2 and RGS4 in the actions of ANP or sildenafil on agonist-induced Ca2+-mediated hypertrophic signaling in cardiac myocytes. One study reported that knockdown of both RGS2 and RGS4 in neonatal rat ventricular myocytes had no effect on ET-1-induced Ca2+ influx and increased NFAT activity nor in the blocking actions of PDE5 inhibition on these ET-1 responses [19]. In contrast, another study found that a dominant negative RGS4 attenuated the inhibitory actions of ANP on ET-1-stimulated hypertrophic events in neonatal rat ventricular myocytes [32]. In addition, the same group reported that cardiac RGS4 overexpression suppressed the enhanced expression of hypertrophy-related genes in GC-A knockout mice [32]. RGS2 was reported to contribute to the inhibitory actions of ANP on Ang II-induced Ca2+ influx in adult mouse ventricular myocytes and to mediate the antihypertrophic and cardioprotective effects of PDE5 inhibitors in a pressure overload mouse model [20,33].
3. mTOR Complexes
The serine/threonine kinase mTOR forms two known multiprotein complexes with only the adaptor protein mLST8 in common [34]. Both complexes are downstream targets for activation by the serine/threonine protein kinase, Akt. Association of mTOR with Rictor and several other proteins constitutes mTOR complex 2 (mTORC2), which exerts cardiac protective actions through further Akt phosphorylation and activation [34]. mTORC2 survival signaling may include activation of autophagy and removal of proapoptotic factors [35]; alternatively, mTORC2 inhibition may enhance autophagy by eliminating the negative constraint of Akt on Fox03, a transcription factor involved in autophagy-related gene expression [36].
Raptor complexes with mTOR and mLST8 to form mTORC1, which is inhibited specifically by the rapamycin-FKBP12 complex [34], although prolonged treatment with rapamycin can inhibit mTORC2 as well in a cell-specific manner. mTORC1 plays a central role in regulating cell growth/size by stimulating protein synthesis and inhibiting autophagy. mTORC1 inhibits autophagy by phosphorylating autophagy-related gene 13 (ATG13) and ULK1 [37]. In addition, a recent study suggests that mTORC1 may tonically suppress an apoptotic response in cardiac myocytes that is linked to an accumulation of eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) by phosphorylating 4E–BP1, thereby leading to its ubiquitination and degradation [38].
In the GTP-bound form, the GTPase Rheb positively stimulates mTORC1 activity; however, the positive influence of Rheb is tonically inhibited by the GTPase activity of tuberous sclerosis complex 1/2 (TSC1/TSC2). A number of stimuli, including insulin, growth factors, hypertrophic agonists, amino acids, and integrins regulate mTORC1 activity through a number of signaling molecules that act at the level of TSC1/TSC2 and/or mTORC1 [34]. Akt, PKA and PKC activate protein synthesis, while low intracellular ATP inhibits protein synthesis through AMP-activated protein kinase (AMPK). Class 1A and 1B PI3Ks activate mTORC1 via a pathway involving Akt-mediated inhibition of TSC1/TSC2, but the mechanism used by Class III PI3Ks is not as clearly defined [34]. mTORC1 has been implicated in both agonist- and pressure overload-induced cardiac hypertrophy, with integrins playing a role in the latter [39–42]. A recent study showed that mTORC1 is required for the development of cardiac hypertrophy that occurs with rising blood pressure in spontaneously hypertensive rats (SHR) [43].
mTORC1 has been implicated in both translation initiation and the ribosomal biogenesis associated with cardiac hypertrophy, but the downstream signaling events remain obscure. Known targets of mTORC1 include protein phospatase 2A (PP2A), 4E–BP1, ribosomal protein S6 kinases (S6Ks), and eukaryotic elongation factor 2 (eEF2). The role of 4E–BP, S6Ks, and eEF2 in regulating protein synthesis are described elsewhere [44]. Studies to date do not support a role for 4E-BP or S6Ks in regulating the rapid increase in protein synthesis downstream of mTORC1 in cardiac myocytes associated with cardiac hypertrophy [34,43,45,46] although a case for the related kinase p90rsk acting in lieu of S6Ks could be made [34]. An attractive possibility is that mTORC1 stimulates the elongation step of protein synthesis by phosphorylating and inhibiting eEF2 kinase, a highly specific Ca2+/calmodulin-dependent kinase that negatively regulates eEF2 binding to the ribosome. eEF2 is critical for the transfer of peptidyl-tRNA from the ribosomal A to P site in peptide-chain elongation. Of note, eEF2 kinase is also a key regulator of stress-induced autophagy [47]. Thus, conceivably the contribution of mTORC1 to hypertrophic growth may also involve the inhibition of protein degradation, in addition to the stimulation of protein synthesis; although evidence supporting this possibility has not been reported. Finally, mTOR was recently shown to be recruited to promoters of tRNA and 5S rRNA genes via interaction with TFIIIC, a transcription factor that binds to polymerase III promoters, and to stimulate polymerase III transcription by inactivating a repressor protein Maf1 [48].
Understanding the mechanisms that normally control mTORC1 activity could potentially lead to therapeutic strategies to prevent or reverse cardiac hypertrophy. Recent evidence was found that extracellular adenosine lessens pressure overload-induced cardiac hypertrophy by attenuating mTORC1 activity [49]. This study did not define the mechanism for adenosine’s actions, but a subsequent study by another group reported that adenosine blocked phenylephrine-induced hypertrophy of neonatal rat ventricular myocytes by activating AMPK through phosphorylation of a specific threonine residue [50]. AMPK is highly expressed in cardiac myocytes and functions as a trimer of the α catalytic subunit with the β and γ regulatory subunits. 5-Aminoimidazole-4-carboxamide riboside (AICAR), an AMPK activator that previously was shown to attenuate pressure overload-induced cardiac hypertrophy in vivo [50], also blocked the prohypertrophic actions of phenylephrine. Conversely, mice deficient in the major α catalytic subunit in cardiac muscle, AMPKα2, exhibited enhanced myocardial hypertrophy and cardiac remodeling in response to pressure overload, which was associated with activation of mTOR/p70S6K signaling [51].
AMPK inhibits mTORC1 activity and protein synthesis by targeting TSC1/TSC2 and raptor. This serine/threonine kinase is activated by various stresses associated with increases in the AMP/ATP ratio and plays a pivotal role in cellular energy homoeostasis regulation [52]. Although not definitive, evidence suggests that AMPK may have a role in attenuating cardiac hypertrophy [53]. AMPK activity is enhanced through phosphorylation on a specific threonine residue by a number of upstream kinases, including Ca2+/calmodulin-dependent protein kinase II (CaMKII) and LKB1. Thus, activation of CaMKII and LKB1 would be predicted to be associated with reduced mTORC1 activity.
CaMKII activity is reported to be increased in the heart by hypertrophy, with the major isoform being CaMKIIδ. The splice variant of CaMKIIδ that is localized to the cytoplasm was recently shown to inhibit NFAT nuclear translocation by inhibiting the phosphatase activity of calcineurin [54]. NFAT activation is linked not only to hypertrophy but to a survival response in cardiac myocytes, both of which would be attenuated by the enhanced activity of cytoplasmic CaMKIIδ [54]. Thus, cytoplasmic CaMKIIδ may act as a brake on cardiac hypertrophy by inhibiting NFAT activation, as well as by enhancing AMPK activity, but at the price of greater susceptibility to cell death. This possibility might have particular significance in the case of heart failure for which CaMKIIδ-mediated phosphorylation of calcium handling proteins has been linked to abnormal calcium signaling [54].
The serine/threonine kinase LKB1 is the major upstream regulator of AMPK. Cardiac-specific deletion of LKB1 in mice led to cardiac hypertrophy and dysfunction, and early death within 6 months [55]. Of note, LKB1 knockout mice exhibit reduced VEGF expression and capillary density in atria and ventricles. A related study showed that AMPK inhibition causes attenuated myocardial capillary density and decreased VEGF expression along with cardiac dysfunction in response to pressure overload [56]. Inhibition of LKB1 by oxidative stress, specifically the lipid peroxidation byproduct 4-hydroxy-2-nonenal, was shown also to contribute to hypertension-associated cardiac hypertrophy in the SHR and was reversible by administering the antioxidant resveratrol [57]. LKB1 activity and anti-hypertrophic actions against certain agonists are also susceptible to enhancement by exogenous addition of NAD both in vitro and in vivo [58]. Both pressure overload- and agonist-induced cardiac hypertrophy are associated with depletion of intracellular NAD, possibly through increased oxidative stress [58,59]. Knockout and transgenic mouse models showed the antihypertrophic actions of exogenous NAD were due to activation of class III histone deacetylase SIRT3, which was shown to activate LKB1 activity through deacetylation, thereby enhancing the LKB1-AMPK pathway [58]. SIRT3 likely attenuates cardiac hypertrophy as well by inducing expression of several antioxidant proteins [58]. Another input signal which would seem to tonically attenuate mTORC1 activation and enhanced protein synthesis is the protein tyrosine phosphatase Shp2 [60]. In this case, Shp2 was shown to associate with and deactivate focal adhesion kinase (FAK). Active FAK can stimulate mTORC1 by inhibiting TSC2 either through direct association or indirectly by activating Akt.
4. Histone Deacetylases (HDACs)
The Class IIa HDACs HDAC4 and -5 shuttle between the nucleus and cytoplasm and function as endogenous repressors of genes involved in pathological cardiac hypertrophy (Fig. 1) [61,62]. The ability of Class IIa HDACs to repress cardiac hypertrophy occurs via their direct binding to prohypertrophic myocyte enhancer factor-2 (MEF2) transcription factor or indirect association with other hypertrophic signaling transcription factors [61]. Hypertrophic stimuli overcome this repression by inducing the nuclear export of these Class II HDACs by their phosphorylation, or in the case of reactive oxygen, by oxidation of cysteine residues [61–63]. In general, a number of kinases phosphorylate Class IIa HDACs, including PKCδ and G protein- coupled receptor kinases [61,64]. In cardiac myocytes, evidence was reported that in response to pressure overload (the nuclear localized splice variant of) CaMKIIδ induces HDAC4 nuclear export through phosphorylation, thereby freeing up MEF2 [65,66]. CaMKIIδ seems also to induce HDAC5 export, though not by direct phosphorylation [65]. In adult ventricular myocytes, the hypertrophic agonist endothelin-1 was shown to induce HDAC5 phosphorylation and nuclear export by triggering nuclear envelope Ca 2+ release via inositol 1–4,5-trisphosphate receptor activation [67]. Protein kinase D1 has been identified as a major Class II HDAC kinase important to pressure overload-induced cardiac hypertrophy as well, suggesting that more than one signal is needed for MEF2 activation [65,68]. A potential strategy to repress cardiac hypertrophy could be based either on inhibiting a particular HDAC kinase or by inhibiting the CRM1 nuclear export receptor, which mediates the cytoplasmic translocation of Class IIa HDACs. The latter approach was employed by Monovich et al. who used a novel high throughput assay to identify two compounds that potently inhibited phenylephrine-induced HDAC5 nuclear export in neonatal rat ventricular myocytes [69]. This maneuver was associated with inhibition of phenylephrine-induced MEF2 target gene expression, increase in ANP protein levels, and increased cell size.
Pharmacological inhibitors of HDAC catalytic activity, such as sodium butyrate and trichostatin A (TSA), have been shown to block both agonist and pressure overload-induced cardiac hypertrophy [70,71]. Like other standard HDAC inhibitors, sodium butyrate and TSA act on both Class I and Class II HDACs, but the pro-hypertrophic actions of the former are dominant in cardiac myocytes. Genetic evidence has revealed that the basis for this observation is the prohypertrophic actions of the Class I HDACs, HDAC1 and -2 [61,70]. A full picture of how these HDACs promote pathological cardiac hypertrophy is not available; however, evidence has been obtained indicating that HDAC1 and -2 repress cardiac protective and anti-hypertrophic genes [61,70]. Class I HDACs repress expression of Krüppel-like factor (Klf)4 transcription factor, which in turn suppresses expression of hypertrophic genes [72]. HDAC2 was shown to regulate a fetal gene expression profile associated with pathological cardiac myocytes hypertrophy and to repress expression of inositol polyphosphate-5-phosphatase f (Inpp5f), which was associated with increased Akt activity (due to increased PIP3 levels) [73]. Increased Akt activity in turn led to reduced glycogen synthase kinase 3 beta (GSK3β) activity due to its phosphorylation, which would be expected to result in cardiac hypertrophy.
Knowledge of the input signals that activate Class I HDACs is rudimentary. Recently, evidence was presented that a number of hypertrophic stimuli both in vivo and in vitro selectively activate HDAC2 among all HDACs in cardiac myocytes, through the induction of heat shock protein 70 (Hsp70) [74]. Stimuli were those that induce pathological cardiac hypertrophy (aortic banding, phenylephrine, isoproterenol, and Ang II), as well as swimming, which induces physiological hypertrophy. HDAC2 activation occurred through its physical association with Hsp70. Although recent evidence has shown that the lipid sphingosine-1-phosphate (S1P) inhibits the activity of HDAC1 and HDAC2 [75], S1P is reported to induce cardiac myocyte hypertrophy in vitro [76]. Since HDAC1 and HDAC2 are reported to form a complex in the nucleus with S1P and the enzyme that produces it, sphingosine kinase, the explanation for this discrepancy may be differences in sites of action, as well as chronic vs. acute effects, and the levels of S1P. Finally, Class I HDACs have non-histone substrates that likely contribute to their pro-hypertrophic actions. Using neonatal rat ventricular myocytes, Glenn et al. recently showed that the importance of HDAC2 activity in the regulation of human B-type natriuretic peptide (BNP) gene promoter activity by the transcription factor YY1 [77]. In response to ET-1, YY1 was shown to associate with HDAC2, which directly associated with the BNP promoter, and the reduction in YY1 acetylation correlated with BNP promoter transcriptional activity. This observation has significance as hypertrophy of cardiac myocytes is associated with increased BNP gene expression.
5. Tying Things Together
Besides being monikers with 4 letters and being linked to cardiac hypertrophy, what connects TRPC, mTOR, and HDAC? In other words, what relationship exists among these factors short of 6 degrees of separation? As outlined in Figure 2, one common denominator would seem to be PI3K–initiated signaling. Class I PI3Ks produce phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which recruits Akt to the membrane to be activated via phosphorylation by phosphoinositide dependent kinase 1 (PDPK1) and mTORC2 (or an alternative kinase such as integrin-linked kinase (ILK)) [34]. PI3K–Akt signaling has been linked to cardiac hypertrophy through both the inhibition of Gsk3β, which removes an inhibitory constraint on various pro-hypertrophic pathways (for example, Gsk3β-mediated NFAT phosphorylation induces its nuclear export), as well as the activation of mTORC1 [78]. PIP3 also prolongs TRPC channel activity by disrupting the association of Ca 2+ -calmodulin to a negative feedback inhibitory site [79,80]. PIP3 cellular levels are reduced by activity of the polyphosphoinositide phosphatase, Inpp5f, the gene expression of which is negatively regulated by HDAC2 [73,81]. Both Inpp5f knockout and HDAC2 transgenic mice exhibit an enhanced cardiac hypertrophy response that is associated with increased Akt and Gsk3β phosphorylation, indicative of their respective activation and inhibition [73,82]. Conversely, HDAC2 deficient mice, which are resistant to cardiac hypertrophy, exhibit increased expression of Inpp5f, inactivation of Akt, and constitutive activation of Gsk3β [73]. Yet to be explained in this scenario is the role of PI(3,4)P2, which may enhance ROS generation by NADPH oxidase and ought to exert some positive effect on Akt activity as well [82]. The scaffolding protein Homer 1a, which is thought to play a role in positioning TRPC channels at specific membrane sites and in proximity to ryanodine receptors, may also act to recruit NFAT transcription factors to the vicinity of TRPC channels [83–85]. NFAT transcription factors play a key role in cardiac hypertrophy and may also induce the expression of some TRPC channel isoforms thereby creating a positive feed-forward mechanism to enhance cardiac hypertrophy [21]. mTORC2 can also participate in the activation of Akt, in which case Akt activation has been linked to cytoprotection as well as activation of autophagy [35]. Based on what has been shown in podocytes, mTORC2 may positively affect the protein translation of TRPC channels or other proteins involved in the TRPC/PI3K network [86]. Of course, other interrelationships among TRPC channels, mTOR and HDAC are likely to occur as well and await discovery.
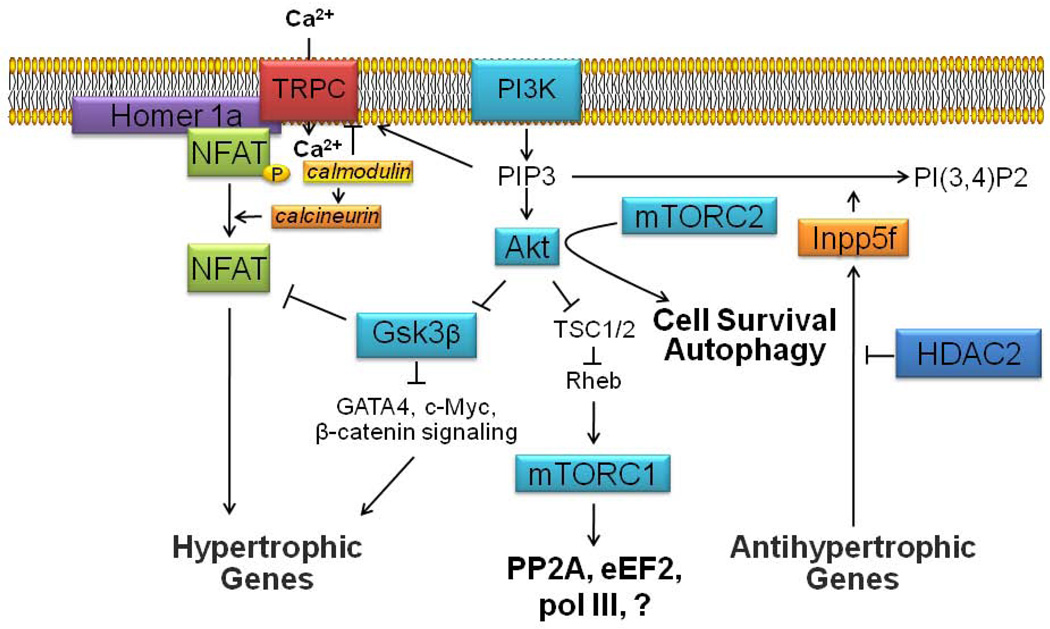
Figure 2.
Scheme showing proposed intracellular signaling network that links TRPC channels, mTOR complexes, and HDACs together mechanistically in subserving pathological cardiac hypertrophy. Calcium influx through TRPC channels is induced by mechanical stretch and/or diacylglycerol (DAG) that is generated by activation of G protein-coupled receptors. NFAT transcription factors in close proximity to TRPC channels underdo dephosphorylation with subsequent transclocation to the nucleus, due to Ca2+-calmodulin-induced activation of calcineurin. Nuclear NFAT induces the transcription of hypertrophic genes. The activity of TRPC channels may be prolonged by phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which also leads to the activation of the serine/threonine protein kinase Akt, which in turn leads to cardiac hypertrophy in part by (1) removing the inhibitory constraints resulting from glycogen synthase kinase 3 beta (Gsk3β) activity, such as NFAT phosphorylation, and (2) increasing mTORC1 activity by lessening the inhibitory constraints on this complex. Enhanced mTORC1 activity is linked to increased ribosomal biogenesis and cardiac hypertrophy through several targets including polymerase III (pol III), elongation factor 2 (eEF2), and protein phosphatase 2A (PP2A). mTORC2-induced enhancement of Akt activity has been linked to cell survival and autophagy, as well as TRPC channel protein expression. PIP3 cellular levels and Akt activity are reduced by the polyphosphoinositide phosphatase, Inpp5f, the gene expression of which is negatively regulated by HDAC2. Thus, enhanced HDAC2 activity is associated with enhanced PI3K-Akt signaling and cardiac hypertrophy.
6. Conclusion and Perspectives
Population-based studies and meta-analysis have shown that the risk for cardiovascular events, cardiovascular deaths, and total mortality increases with the extent of left ventricular cardiac hypertrophy [1,87]. This predictive ability of cardiac hypertrophy holds even in the absence of coronary heart disease. But cardiac hypertrophy is a reversible process, and therapy-based reductions in left ventricular mass reduce the risk for cardiovascular events irrespective of blood pressure reductions [87,88]. However, left ventricular hypertrophy may persist in as many as 20% of those individuals who attain target blood pressure, and left ventricular hypertrophy confers the same risk irrespective of blood pressure [88,90]. Thus, novel strategies to reduce cardiac hypertrophy are needed and a better understanding of the fundamental processes discussed here that underlie cardiac hypertrophy ought to help achieve that goal. Defining the interactions among these fundamental processes ought to aid in developing comprehensive treatments for preventing or regressing cardiac hypertrophy based on a concerted multi-pronged attack. Not discussed here is the exciting and fast developing area of microRNAs. For instance, reciprocal repression recently demonstrated between miR-133 and calcineurin that is important in regulating cardiac hypertrophy might be exploitable therapeutically [91]. Undoubtedly new avenues yet to be discovered exist as well.
Acknowledgments
This work was supported by grants from the National Heart, Lung, and Blood Institute to G. W. Booz (R01HL088101-04 and R01HL088101-02S1) and grants from the Lebanese University, the Lebanese National Council for Scientific Research (CNRS) and the COMSTECH-TWAS (09-122 RG/PHA/AF/AC_C) to M. Kurdi.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Booz GW. Left ventricular physiology in hypertension. In: Lip YH, Hall JE, editors. Comprehensive Hypertension. Philadelphia, PA: Mosby Elsevier; 2007. pp. 113–121. [Google Scholar]
- 2.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signaling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 4.Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. 2004;63:467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe H, Murakami M, Ohba T, Ono K, Ito H. The pathological role of transient receptor potential channels in heart disease. Circ J. 2009;73:419–427. doi: 10.1253/circj.cj-08-1153. [DOI] [PubMed] [Google Scholar]
- 6.Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23:297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Delmas P, Patel A, et al. Sensing pressure in the cardiovascular system: Gq-coupled mechanoreceptors and TRP channels. J Mol Cell Cardiol. 2010;48:83–89. doi: 10.1016/j.yjmcc.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Patel A, Sharif-Naeini R, Folgering JR, Bichet D, Duprat F, Honoré E. Canonical TRP channels and mechanotransduction: from physiology to disease states. Pflugers Arch. 2010;460:571–581. doi: 10.1007/s00424-010-0847-8. [DOI] [PubMed] [Google Scholar]
- 9.Ohba T, Watanabe H, Murakami M, Takahashi Y, Iino K, Kuromitsu S, et al. Upregulation of TRPC1 in the development of cardiac hypertrophy. J Mol Cell Cardiol. 2007;42:498–507. doi: 10.1016/j.yjmcc.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Ward ML, Williams IA, Chu Y, Cooper PJ, Ju YK, Allen DG. Stretch-activated channels in the heart: contributions to length-dependence and to cardiomyopathy. Prog Biophys Mol Biol. 2008;97:232–249. doi: 10.1016/j.pbiomolbio.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Seth M, Zhang ZS, Mao L, Graham V, Burch J, Stiber J, et al. TRPC1 channels are critical for hypertrophic signaling in the heart. Circ Res. 2009;105:1023–1030. doi: 10.1161/CIRCRESAHA.109.206581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seth M, Sumbilla C, Mullen SP, Lewis D, Klein MG, Hussain A, et al. Sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) gene silencing and remodeling of the Ca2+ signaling mechanism in cardiac myocytes. Proc Natl Acad Sci U S A. 2004;101:16683–16688. doi: 10.1073/pnas.0407537101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bush EW, Hood DB, Papst PJ, Chapo JA, Minobe W, Bristow MR, et al. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J Biol Chem. 2006;281:33487–33496. doi: 10.1074/jbc.M605536200. [DOI] [PubMed] [Google Scholar]
- 14.Wu X, Eder P, Chang B, Molkentin JD. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc Natl Acad Sci U S A. 2010;107:7000–7005. doi: 10.1073/pnas.1001825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onohara N, Nishida M, Inoue R, Kobayashi H, Sumimoto H, Sato Y, et al. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 2006;25:5305–5316. doi: 10.1038/sj.emboj.7601417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, et al. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116:3114–3126. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niizeki T, Takeishi Y, Kitahara T, Arimoto T, Ishino M, Bilim O, et al. Diacylglycerol kinase-ε restores cardiac dysfunction under chronic pressure overload: a new specific regulator of Gαq signaling cascade. Am J Physiol Heart Circ Physiol. 2008;295:H245–H255. doi: 10.1152/ajpheart.00066.2008. [DOI] [PubMed] [Google Scholar]
- 18.Koitabashi N, Aiba T, Hesketh GG, Rowell J, Zhang M, Takimoto E, et al. Cyclic GMP/PKG-dependent inhibition of TRPC6 channel activity and expression negatively regulates cardiomyocyte NFAT activation Novel mechanism of cardiac stress modulation by PDE5 inhibition. J Mol Cell Cardiol. 2010;48:713–724. doi: 10.1016/j.yjmcc.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishida M, Watanabe K, Sato Y, Nakaya M, Kitajima N, Ide T, et al. Phosphorylation of TRPC6 channels at Thr69 is required for anti-hypertrophic effects of phosphodiesterase 5 inhibition. J Biol Chem. 2010;285:13244–13253. doi: 10.1074/jbc.M109.074104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klaiber M, Kruse M, Völker K, Schröter J, Feil R, Freichel M, et al. Novel insights into the mechanisms mediating the local antihypertrophic effects of cardiac atrial natriuretic peptide: role of cGMP-dependent protein kinase and RGS2. Basic Res Cardiol. 2010;105:583–595. doi: 10.1007/s00395-010-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinoshita H, Kuwahara K, Nishida M, Jian Z, Rong X, Kiyonaka S, et al. Inhibition of TRPC6 channel activity contributes to the antihypertrophic effects of natriuretic peptides-guanylyl cyclase-A signaling in the heart. Circ Res. 2010;106:1849–1860. doi: 10.1161/CIRCRESAHA.109.208314. [DOI] [PubMed] [Google Scholar]
- 22.Nishida M, Kurose H. Roles of TRP channels in the development of cardiac hypertrophy. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:395–406. doi: 10.1007/s00210-008-0321-8. [DOI] [PubMed] [Google Scholar]
- 23.Inoue R, Jensen LJ, Jian Z, Shi J, Hai L, Lurie AI, et al. Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase C/diacylglycerol and phospholipase A2/ω-hydroxylase/20-HETE pathways. Circ Res. 2009;104:1399–1409. doi: 10.1161/CIRCRESAHA.108.193227. [DOI] [PubMed] [Google Scholar]
- 24.Keshava Prasad TS, Goel R, Kandasamy K, Keerthikumar S, Kumar S, Mathivanan S, et al. Human Protein Reference Database--2009 update. Nucleic Acids Res. 2009;37:fD767–fD772. doi: 10.1093/nar/gkn892. (Database issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Booz GW. Putting the brakes on cardiac hypertrophy: exploiting the NO-cGMP counter-regulatory system. Hypertension. 2005;45:341–336. doi: 10.1161/01.HYP.0000156878.17006.02. [DOI] [PubMed] [Google Scholar]
- 26.Fiedler B, Lohmann SM, Smolenski A, Linnemuller S, Pieske B, Schroder F, et al. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc Natl Acad Sci U S A. 2002;99:11363–11368. doi: 10.1073/pnas.162100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tandan S, Wang Y, Wang TT, Jiang N, Hall DD, Hell JW, et al. Physical and functional interaction between calcineurin and the cardiac L-type Ca2+ channel. Circ Res. 2009;105:51–60. doi: 10.1161/CIRCRESAHA.109.199828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakayama H, Bodi I, Maillet M, Desantiago J, Domeier TL, Mikoshiba K, et al. The IP3 Receptor Regulates Cardiac Hypertrophy in Response to Select Stimuli. Circ Res. 2010;107:659–666. doi: 10.1161/CIRCRESAHA.110.220038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Oort RJ, Respress JL, Li N, Reynolds C, De Almeida AC, Skapura DG, et al. Accelerated development of pressure overload-induced cardiac hypertrophy and dysfunction in an RyR2-R176Q knockin mouse model. Hypertension. 2010;55:932–938. doi: 10.1161/HYPERTENSIONAHA.109.146449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frank D, Frauen R, Hanselmann C, Kuhn C, Will R, Gantenberg J, et al. Lmcd1/Dyxin, a novel Z-disc associated LIM protein, mediates cardiac hypertrophy in vitro and in vivo. J Mol Cell Cardiol. 2010;49:673–682. doi: 10.1016/j.yjmcc.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Bian ZY, Huang H, Jiang H, Shen DF, Yan L, Zhu LH, et al. LIM and cysteine-rich domains 1 regulates cardiac hypertrophy by targeting calcineurin/nuclear factor of activated T cells signaling. Hypertension. 2010;55:257–263. doi: 10.1161/HYPERTENSIONAHA.109.135665. [DOI] [PubMed] [Google Scholar]
- 32.Tokudome T, Kishimoto I, Horio T, Arai Y, Schwenke DO, Hino J, et al. Regulator of G-protein signaling subtype 4 mediates antihypertrophic effect of locally secreted natriuretic peptides in the heart. Circulation. 2008;117:2329–2339. doi: 10.1161/CIRCULATIONAHA.107.732990. [DOI] [PubMed] [Google Scholar]
- 33.Takimoto E, Koitabashi N, Hsu S, Ketner EA, Zhang M, Nagayama T, et al. Regulator of G protein signaling 2 mediates cardiac compensation to pressure overload and antihypertrophic effects of PDE5 inhibition in mice. J Clin Invest. 2009;119:408–420. doi: 10.1172/JCI35620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balasubramanian S, Johnston RK, Moschella PC, Mani SK, Tuxworth WJ, Jr, Kuppuswamy D. mTOR in growth and protection of hypertrophying myocardium. Cardiovasc Hematol Agents Med Chem. 2009;7:52–63. doi: 10.2174/187152509787047603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gurusamy N, Lekli I, Mukherjee S, Ray D, Ahsan MK, Gherghiceanu M, Popescu LM, Das DK. Cardioprotection by resveratrol: a novel mechanism via autophagy involving the mTORC2 pathway. Cardiovasc Res. 2010;86:103–112. doi: 10.1093/cvr/cvp384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganley IG, Lam du H, Wang J, Ding X, Chen S, Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009;284:12297–12305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang D, Contu R, Latronico MV, Zhang J, Rizzi R, Catalucci D, Miyamoto S, Huang K, Ceci M, Gu Y, Dalton ND, Peterson KL, Guan KL, Brown JH, Chen J, Sonenberg N, Condorelli G. MTORC1 regulates cardiac function and myocyte survival through 4E–BP1 inhibition in mice. J Clin Invest. 2010;120:2805–2816. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Umar S, van der Valk EJ, Schalij MJ, van der Wall EE, Atsma DE, van der Laarse A. Integrin stimulation-induced hypertrophy in neonatal rat cardiomyocytes is NO-dependent. Mol Cell Biochem. 2009;320:75–84. doi: 10.1007/s11010-008-9900-8. [DOI] [PubMed] [Google Scholar]
- 40.Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, et al. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 41.Balasubramanian S, Kuppuswamy D. RGD-containing peptides activate S6K1 through β3 integrin in adult cardiac muscle cells. J Biol Chem. 2003;278:42214–42224. doi: 10.1074/jbc.M303428200. [DOI] [PubMed] [Google Scholar]
- 42.Moschella PC, Rao VU, McDermott PJ, Kuppuswamy D. Regulation of mTOR and S6K1 activation by the nPKC isoforms, PKCε and PKCδ, in adult cardiac muscle cells. J Mol Cell Cardiol. 2007;43:754–766. doi: 10.1016/j.yjmcc.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soesanto W, Lin HY, Hu E, Lefler S, Litwin SE, Sena S, et al. Mammalian target of rapamycin is a critical regulator of cardiac hypertrophy in spontaneously hypertensive rats. Hypertension. 2009;54:1321–1327. doi: 10.1161/HYPERTENSIONAHA.109.138818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Booz GW, Baker KM. Protein phosphorylation. In: Izzo JL, Black HR sr, editors. Hypertension Primer: The Essentials of High Blood Pressure. 4th. Baltimore, Maryland: Lippincott Williams & Wilkins; 2008. pp. 16–21. [Google Scholar]
- 45.Huang BP, Wang Y, Wang X, Wang Z, Proud CG. Blocking eukaryotic initiation factor 4F complex formation does not inhibit the mTORC1-dependent activation of protein synthesis in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2009;296:H505–H514. doi: 10.1152/ajpheart.01105.2008. [DOI] [PubMed] [Google Scholar]
- 46.Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E–BPs. Science. 2010;328:1172–1176. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng Y, Li H, Ren X, Niu T, Hait WN, Yang J.Cytoprotective effect of the elongation factor-2 kinase-mediated autophagy in breast cancer cells subjected to growth factor inhibition PLoS One 20105:pe9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kantidakis T, Ramsbottom BA, Birch JL, Dowding SN, White RJ. mTOR associates with TFIIIC, is found at tRNA and 5S rRNA genes, and targets their repressor Maf1. Proc Natl Acad Sci U S A. 2010;107:11823–11828. doi: 10.1073/pnas.1005188107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu X, Fassett J, Hu X, Zhu G, Lu Z, Li Y, et al. Ecto-5’-nucleotidase deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction. Hypertension. 2008;51:1557–1564. doi: 10.1161/HYPERTENSIONAHA.108.110833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li HL, Yin R, Chen D, Liu D, Wang D, Yang Q, et al. Long-term activation of adenosine monophosphate-activated protein kinase attenuates pressure-overload-induced cardiac hypertrophy. J Cell Biochem. 2007;100:1086–1099. doi: 10.1002/jcb.21197. [DOI] [PubMed] [Google Scholar]
- 51.Zhang P, Hu X, Xu X, Fassett J, Zhu G, Viollet B, et al. AMP activated protein kinase-α2 deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction in mice. Hypertension. 2008;52:918–924. doi: 10.1161/HYPERTENSIONAHA.108.114702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong AK, Howie J, Petrie JR, Lang CC. AMP-activated protein kinase pathway: a potential therapeutic target in cardiometabolic disease. Clin Sci (Lond) 2009;116:607–620. doi: 10.1042/CS20080066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim AS, Miller EJ, Young LH. AMP-activated protein kinase: a core signalling pathway in the heart. Acta Physiol (Oxf) 2009;196:37–53. doi: 10.1111/j.1748-1716.2009.01978.x. [DOI] [PubMed] [Google Scholar]
- 54.MacDonnell SM, Weisser-Thomas J, Kubo H, Hanscome M, Liu Q, Jaleel N, et al. CaMKII negatively regulates calcineurin-NFAT signaling in cardiac myocytes. Circ Res. 2009;105:316–325. doi: 10.1161/CIRCRESAHA.109.194035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ikeda Y, Sato K, Pimentel DR, Sam F, Shaw RJ, Dyck JR, et al. Cardiac-specific deletion of LKB1 leads to hypertrophy and dysfunction. J Biol Chem. 2009;284:35839–35849. doi: 10.1074/jbc.M109.057273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shimano M, Ouchi N, Shibata R, Ohashi K, Pimentel DR, Murohara T, et al. Adiponectin deficiency exacerbates cardiac dysfunction following pressure overload through disruption of an AMPK-dependent angiogenic response. J Mol Cell Cardiol. 2010;49:210–220. doi: 10.1016/j.yjmcc.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dolinsky VW, Chan AY, Robillard Frayne I, Light PE, Des Rosiers C, Dyck JR. Resveratrol prevents the prohypertrophic effects of oxidative stress on LKB1. Circulation. 2009;119:1643–1652. doi: 10.1161/CIRCULATIONAHA.108.787440. [DOI] [PubMed] [Google Scholar]
- 58.Pillai VB, Sundaresan NR, Kim G, Gupta M, Rajamohan SB, Pillai JB, et al. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J Biol Chem. 2010;28:3133–3144. doi: 10.1074/jbc.M109.077271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2α deacetylase activity. J Biol Chem. 2005;280:43121–43130. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]
- 60.Marin TM, Clemente CF, Santos AM, Picardi PK, Pascoal VD, Lopes-Cendes I, et al. Shp2 negatively regulates growth in cardiomyocytes by controlling focal adhesion kinase/Src and mTOR pathways. Circ Res. 2008;103:813–824. doi: 10.1161/CIRCRESAHA.108.179754. [DOI] [PubMed] [Google Scholar]
- 61.Bush EW, McKinsey TA. Targeting histone deacetylases for heart failure. Expert Opin Ther Targets. 2009;13:767–784. doi: 10.1517/14728220902939161. [DOI] [PubMed] [Google Scholar]
- 62.Verdin E, Dequiedt F, Kasler HG. Class II histone deacetylases: versatile regulators. Trends Genet. 2003;19:286–293. doi: 10.1016/S0168-9525(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 63.Oka S, Ago T, Kitazono T, Zablocki D, Sadoshima J. The role of redox modulation of class II histone deacetylases in mediating pathological cardiac hypertrophy. J Mol Med. 2009;87:785–791. doi: 10.1007/s00109-009-0471-2. [DOI] [PubMed] [Google Scholar]
- 64.Martini JS, Raake P, Vinge LE, DeGeorge BR, Jr, Chuprun JK, Harris DM, et al. Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. Proc Natl Acad Sci U S A. 2008;105:12457–12462. doi: 10.1073/pnas.0803153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, Patrick DM, et al. The δ isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci U S A. 2009;106:2342–2347. doi: 10.1073/pnas.0813013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Little GH, Bai Y, Williams T, Poizat C. Nuclear calcium/calmodulin-dependent protein kinase IIδ preferentially transmits signals to histone deacetylase 4 in cardiac cells. J Biol Chem. 2007;282:7219–7231. doi: 10.1074/jbc.M604281200. [DOI] [PubMed] [Google Scholar]
- 67.Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest. 2006;116:675–682. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Monovich L, Vega RB, Meredith E, Miranda K, Rao C, Capparelli M, et al. A novel kinase inhibitor establishes a predominant role for protein kinase D as a cardiac class IIa histone deacetylase kinase. FEBS Lett. 2010;584:631–637. doi: 10.1016/j.febslet.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 69.Monovich L, Koch KA, Burgis R, Osimboni E, Mann T, Wall D, et al. Suppression of HDAC nuclear export and cardiomyocyte hypertrophy by novel irreversible inhibitors of CRM1. Biochim Biophys Acta. 2009;1789:422–431. doi: 10.1016/j.bbagrm.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 70.Bush EW, McKinsey TA. Protein acetylation in the cardiorenal axis: the promise of histone deacetylase inhibitors. Circ Res. 2010;106:272–284. doi: 10.1161/CIRCRESAHA.109.209338. [DOI] [PubMed] [Google Scholar]
- 71.Kong Y, Tannous P, Lu G, Berenji K, Rothermel BA, Olson EN, et al. Suppression of class I and II histone deacetylases blunts pressure-overload cardiac hypertrophy. Circulation. 2006;113:2579–2588. doi: 10.1161/CIRCULATIONAHA.106.625467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kee HJ, Kook H. Krüppel-like factor 4 mediates histone deacetylase inhibitor-induced prevention of cardiac hypertrophy. J Mol Cell Cardiol. 2009;47:770–780. doi: 10.1016/j.yjmcc.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 73.Trivedi CM, Luo Y, Yin Z, Zhang M, Zhu W, Wang T, et al. Hdac2 regulates the cardiac hypertrophic response by modulating Gsk3 beta activity. Nat Med. 2007;13:324–331. doi: 10.1038/nm1552. [DOI] [PubMed] [Google Scholar]
- 74.Kee HJ, Eom GH, Joung H, Shin S, Kim JR, Cho YK, et al. Activation of histone deacetylase 2 by inducible heat shock protein 70 in cardiac hypertrophy. Circ Res. 2008;103:1259–1269. doi: 10.1161/01.RES.0000338570.27156.84. [DOI] [PubMed] [Google Scholar]
- 75.Riccio A.New endogenous regulators of class I histone deacetylases Sci Signal 20103:pe1. [DOI] [PubMed] [Google Scholar]
- 76.Karliner JS. Lysophospholipids and the cardiovascular system. Biochim Biophys Acta. 2002;1582:216–221. doi: 10.1016/s1388-1981(02)00174-9. [DOI] [PubMed] [Google Scholar]
- 77.Glenn DJ, Wang F, Chen S, Nishimoto M, Gardner DG. Endothelin-stimulated human B-type natriuretic peptide gene expression is mediated by Yin Yang 1 in association with histone deacetylase 2. Hypertension. 2009;53:549–555. doi: 10.1161/HYPERTENSIONAHA.108.125088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oudit GY, Penninger JM. Cardiac regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc Res. 2009;82:250–260. doi: 10.1093/cvr/cvp014. [DOI] [PubMed] [Google Scholar]
- 79.Kwon Y, Hofmann T, Montell C. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol Cell. 2007;25:491–503. doi: 10.1016/j.molcel.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saleh SN, Albert AP, Large WA. Activation of native TRPC1/C5/C6 channels by endothelin-1 is mediated by both PIP3 and PIP2 in rabbit coronary artery myocytes. J Physiol. 2009;587:5361–5375. doi: 10.1113/jphysiol.2009.180331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kee HJ, Kook H. Roles and targets of class I and IIa histone deacetylases in cardiac hypertrophy. J Biomed Biotechnol. 2011;2011:928326. doi: 10.1155/2011/928326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu W, Trivedi CM, Zhou D, Yuan L, Lu MM, Epstein JA. Inpp5f is a polyphosphoinositide phosphatase that regulates cardiac hypertrophic responsiveness. Circ Res. 2009;105:1240–1247. doi: 10.1161/CIRCRESAHA.109.208785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sabourin J, Cognard C, Constantin B. Regulation by scaffolding proteins of canonical transient receptor potential channels in striated muscle. J Muscle Res Cell Motil. 2009;30:289–297. doi: 10.1007/s10974-010-9206-9. [DOI] [PubMed] [Google Scholar]
- 84.Pouliquin P, Dulhunty AF. Homer and the ryanodine receptor. Eur Biophys J. 2009;39:91–102. doi: 10.1007/s00249-009-0494-1. [DOI] [PubMed] [Google Scholar]
- 85.Stiber JA, Tabatabaei N, Hawkins AF, Hawke T, Worley PF, Williams RS, Rosenberg P. Homer modulates NFAT-dependent signaling during muscle differentiation. Dev Biol. 2005;287:213–224. doi: 10.1016/j.ydbio.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 86.Vollenbröker B, George B, Wolfgart M, Saleem MA, Pavenstädt H, Weide T. mTOR regulates expression of slit diaphragm proteins and cytoskeleton structure in podocytes. Am J Physiol Renal Physiol. 2009;296:F418–F426. doi: 10.1152/ajprenal.90319.2008. [DOI] [PubMed] [Google Scholar]
- 87.Artham SM, Lavie CJ, Milani RV, Patel DA, Verma A, Ventura HO. Clinical impact of left ventricular hypertrophy and implications for regression. Prog Cardiovasc Dis. 2009;52:153–167. doi: 10.1016/j.pcad.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 88.Koren MJ, Ulin RJ, Koren AT, Laragh JH, Devereux RB. Left ventricular mass change during treatment and outcome in patients with essential hypertension. Am J Hypertens. 2002;15:1021–1028. doi: 10.1016/s0895-7061(02)03061-3. [DOI] [PubMed] [Google Scholar]
- 89.Mancia G, Carugo S, Grassi G, Lanzarotti A, Schiavina R, Cesana G, et al. Prevalence of left ventricular hypertrophy in hypertensive patients without, with blood pressure control: data from the PAMELA population Pressioni Arteriose Monitorate E Loro Associazioni. Hypertension. 2002;39:744–749. doi: 10.1161/hy0302.104669. [DOI] [PubMed] [Google Scholar]
- 90.Brown DW, Giles WH, Croft JB. Left ventricular hypertrophy as a predictor of coronary heart disease mortality and the effect of hypertension. Am Heart J. 2000;140:848–856. doi: 10.1067/mhj.2000.111112. [DOI] [PubMed] [Google Scholar]
- 91.Dong DL, Chen C, Huo R, Wang N, Li Z, Tu YJ, et al. Reciprocal repression between microRNA-133 and calcineurin regulates cardiac hypertrophy: a novel mechanism for progressive cardiac hypertrophy. Hypertension. 2010;55:946–952. doi: 10.1161/HYPERTENSIONAHA.109.139519. [DOI] [PubMed] [Google Scholar]