Abstract
A new cupric-superoxo complex [LCuII(O2•−)]+, which possesses particularly strong O–O and Cu–O bonding, is capable of intermolecular C-H activation of the NADH analogue 1-benzyl-1,4-dihydronicotinamide (BNAH). Kinetic studies indicate a first-order dependence on both the Cu-complex and BNAH with a deuterium kinetic isotope effect (KIE) of 12.1, similar to that observed for certain copper monooxygenases.
Copper(I) reactions with molecular oxygen play fundamental roles in many chemical and biological processes.1,2 Copper-dependent proteins perform a diverse array of oxidative and oxygenative reactions. This has inspired considerable efforts in the design of novel ligands and copper coordinated complexes as well as the study of ligand-copper(I) dioxygen adducts to elucidate their structures, electronic characteristics and substrate reactivity.2–4 Compared to binuclear copper-dioxygen derived species, mononuclear analogues have been synthetically challenging and hence are less understood.3,5 However, they are fundamentally important and are directly relevant to copper proteins including dopamine-β-monooxygenase (DβM) and peptidlyglycine-α-hydroxylating monooxygenase (PHM).6 These enzymes possess a so-called non-coupled binuclear active site,7 which comprises two Cu centers separated by ~11Å. Dioxygen binding and substrate hydroxylation occur at one of the copper sites (designated CuM). In an important PHM X-ray structure, a dioxygen derived species presumed to be an end-on bound cupric superoxide species (i.e., CuII-O-O•−) resides adjacent to an inhibitory substrate analog.6c Along with biochemical,6a,b,8 chemical and computational studies,5,9 the cupric-superoxo species is thought by many to be the reactive intermediate responsible for initiating oxidation via hydrogen-atom abstraction. However, other species have been considered as important intermediates in enzymatic turnover, either prior to or following substrate attack, including cupric-hydroperoxo (CuII-−OOH)10 and high-valent cupryl (CuII-O• <—> CuIII=O) entities.4b,9c,11
In our own research program, we seek to elucidate the chemical nature of all of these mononuclear species. In this report, we describe the generation and characterization of a new CuII(O2•−) and an example of substrate C-H activation (i.e., oxidative C–H bond cleavage). To this point, cupric-superoxo complexes have been shown to exhibit phenol O–H bond cleavage reactions,10a,12 and in one case, Itoh and coworkers13 provided evidence for a CuII(O2•−) mediated intramolecular benzylic C–H oxygenation. Here, for the first time, an intermolecular C–H substrate oxidation reaction has been achieved, with kinetic data clearly implicating the involvement of the CuII(O2•−) complex in rate-limiting substrate C–H bond cleavage.
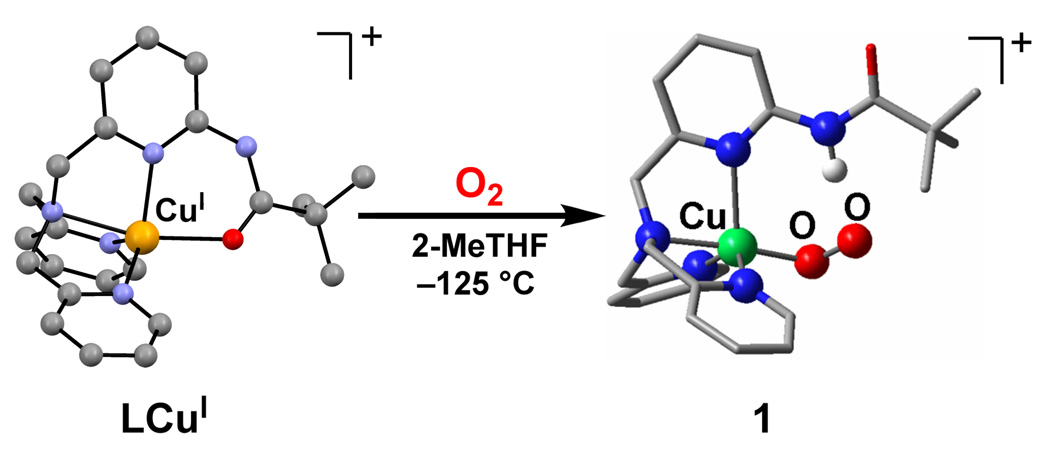
The CuI complex of a ligand previously employed by Masuda and coworkers,14a L [bis(pyrid-2-ylmethyl){[6-(pivalamido) pyrid-2-yl]methyl}amine], [LCuI]+ (as the B(C6F5)4− salt,14 was exposed to O2 (by bubbling via a syringe needle) in 2-methyltetrahydrofuran (MeTHF) solvent at −125 °C to form the adduct [LCuII(O2•−)]+ (1) (Figure 1) with UV-Vis absorptions [λmax = 410 nm (3700 M−1 cm−1), 585 nm (900 M−1 cm−1), 741 nm (1150 M−1 cm−1)]15 characteristic of a mononuclear end-bound CuII-superoxo complex. This well below −80 °C temperature is necessary in order to observe this Cu/O2 = 1:1 adduct. Under these conditions, this green EPR-silent species is quite stable, decaying only very slowly (½ life > 4 hrs) with conversion to [{(LCuII)}2(O22−)]2+ (2), a μ-1,2-peroxodicopper(II) complex [λmax = 541 nm (9,900 M−1cm−1)], that is observed when [LCuI]+ is oxygenated at −80 °C.14b
Figure 1.
Left: Representation of the cationic portion of the X-ray structure of [LCuI](B(C6F5)4−), revealing the N4O(amide) coordination. (Cu-O = 2.190 Å) Right: Calculated structure (see Supporting Information) of [LCuII(O2•−)]+ (1), indicating the H-bonding interaction between the ligand and the superoxo β-oxygen atom. See text for further discussion.
A resonance Raman (rR) spectrum (excitation at 413 nm, 77 K) of 1 in MeTHF frozen solution is shown in Figure 2. The O-O stretch is observed at 1130 cm−1 as a single peak when the complex was formed with 16O2 (Figure 2B, red); but upon 18O2 isotopic substitution, two features are observed (Figure 2B, blue). This behavior is consistent with a Fermi resonance of the 18O2-vibration with a non-enhanced mode at similar energy. Using the energy and intensity of the two observed mixed-modal features, the pre-interaction energy of the resonantly enhanced 18O-18O stretch was calculated to be 1067 cm−1. Thus the O-O stretch shifts down in energy by 63 cm−1 upon 18O2-substitution, consistent with a bound superoxo species.16 For the lower energy region, both the 16O2 and 18O2 data show two peaks with an intensity distribution which changes with isotope. Therefore these are both mixed due to Fermi resonance. Analysis15 of the lower energy region (Figure 2A) leads to a Cu-16O stretch at 482 cm−1, which shifts to 462 cm−1 with 18O.17
Figure 2.
Solvent-subtracted rR spectra of MeTHF solutions of 1 (λex=413 nm). (A) v(Cu-O) region. (B) v(O-O) region. Red, 16O2; blue, 18O2.
Thus the rR spectroscopic data confirm the formulation of [LCuII(O2•−)]+ as an end-on superoxo-containing complex, with O-O and Cu-O stretches of 1130 cm−1 and 482 cm−1, respectively.15 Notably, these values are higher than those found for all cupric superoxo complexes previously described: ν(O-O) for the one structurally defined side-on bound cupric superoxo complex is 1043 cm−1, while those for the end-on bound species range up to 1122 cm−1; ν(Cu-O) varies from 422 to 474 cm−1.15 Using DFT calculations, we have evaluated the Cu-O and O-O vibrational frequencies of 1, those for this structure with the pivalamido group at the para instead of the ortho position, and for a structure with Cu-N bonds constrained but no pivalamido group.15 The comparison between para and ortho substitution eliminates an inductive effect from the pivalamido group as the origin of the higher frequencies. Consistent with the ν(O-O) of 1130 cm−1, 1 is calculated to be an end-on bound Cu(II)-superoxo species with a triplet ground state (the singlet/triplet splitting is calculated to be 1581 cm−1,15 after correction for spin contamination). For L and its close analogs, Masuda and coworkers14a have found that the pivalamido pyridyl substituent forms intramolecular H-bonds to the alpha oxygen atoms of a peroxide and/or hydroperoxide moiety ligated to the copper(II),14a,18 or to the alpha N-atom for an azide coordinated to copper(II).14a Our calculations15 suggest that the superoxo moiety in [LCuII(O2•−)]+ (1) forms an intramolecular H-bond, however with either the alpha- or beta-oxygen. This would contribute to the relative stability of 1, and can account for the higher O-O and Cu-O frequencies. rR data on the analogs [CuII(TMPA)(O2•−)]+ (TMPA = tris(2-pyridylmethyl)amine)) and [CuII(NMe2-TMPA)(O2•−)]+ show a ν(O-O) of 1120 cm−1 and a ν(Cu-O) of 472 cm−1;12 thus ν(O-O) and ν(Cu-O) are both about 10 cm−1 higher in 1. Optimized geometries of [CuIITMPA(O2•−)]+, [CuII(NMe2-TMPA)(O2•−)]+ have also been obtained through DFT calculations and compared to 1. These calculations give an increase in both ν(Cu-O) and ν(O-O) when H bonding is included in 1, particularly to the beta oxygen of the superoxo ligand.15,19 The calculated structure shows that Cu-O bond length decreases by 0.005 Å indicating that a slightly stronger Cu-O bond is associated with the higher ν(Cu-O). From the DFT calculation of the structure with Cu-N bond lengths constrained at the values of the optimized structure of 1 but with no pivalamido group, this appears to reflect a distortion of the Nequatorial ligand system which decreases its donor interaction with the Cu. The donor interaction of the superoxo with the Cu thus increases and this leads to a stronger Cu-O bond. Alternatively, the calculations show that the O-O bond actually increases by 0.005 Å in 1, indicating that the increase in ν(O-O) relative to [CuIITMPA(O2−)]+ does not reflect a stronger O-O bond but derives from structural coupling of vibrations within the ligand system due to the H-bond. This H-bonding stabilizes the superoxo species (relative to the binuclear peroxo species) and allows its reactivity to be studied.
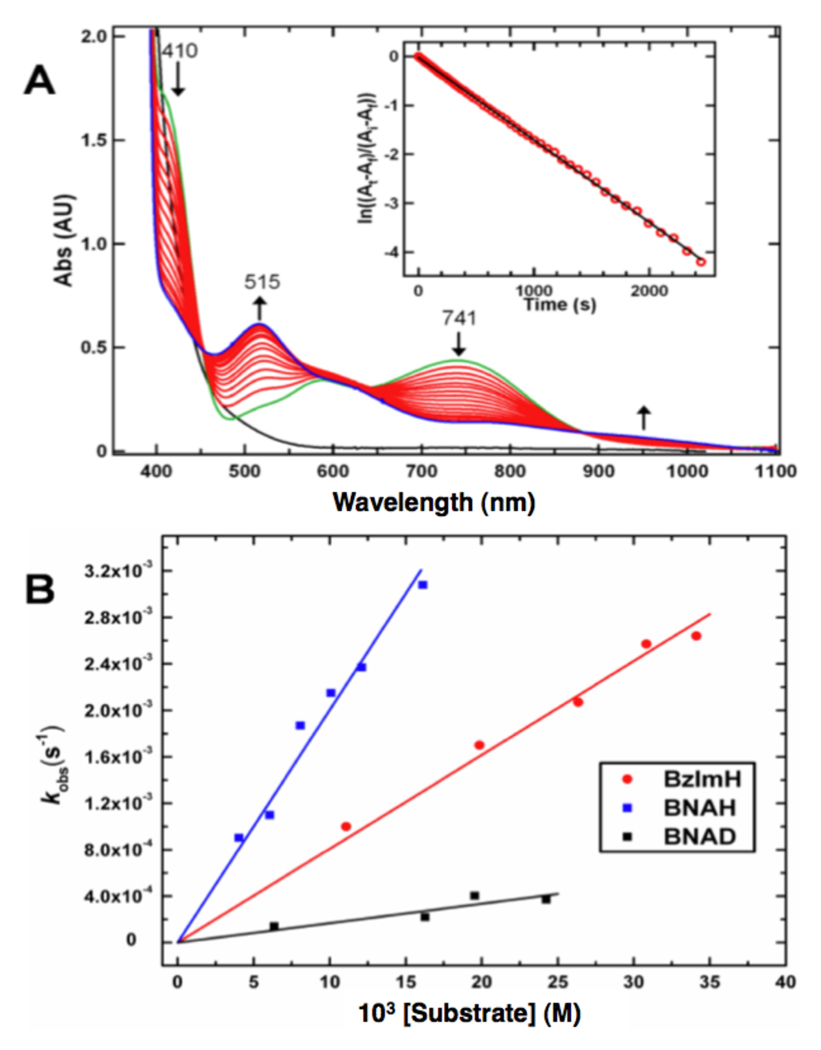
[LCuII(O2•−)]+ (1) is unreactive toward a number of commonly employed C–H substrates, such as dihydroanthracene, xanthene and 10-methyl-9,10-dihydroacridine – substrates possessing C-H bonds significantly weaker than those found for DBM and PHM substrates (dopamine, 85 kcal/mol; hippuric acid, 87 kcal/mol).6b However, the addition of an excess of 1-benzyl-1,4-dihydronicotinamide (BNAH) – an NADH analogue, which is both a strong H-atom (H•) and hydride (H−) donor,19 to solutions of [LCuII(O2•−)]+ leads to the latter’s decay, as observed by UV-Vis spectroscopy (Figure 3a). Kinetic interrogation of this reaction (−125 °C) showed pseudo first-order decay behavior for [LCuII(O2•−)]+ (1) (for 10–40 eq. BNAH). The decay was also first order in [BNAH]. Thus, the cupric superoxo is responsible for promoting the substrate oxidation (vide infra). A second-order rate constant of 0.19 M−1s−1 was obtained (Figure 3b); when the substrate was deuterated in the 4,4’ position, i.e., 1-benzyl-1,4-dihydro[4,4’-2H2]nicotinamide (BNAD), a significant slowing of the reaction occurred, k = 0.016 M−1s−1 (Figure 3b). This gives a kinetic isotope effect (KIE) of 12.1. This KIE value is comparable to that (KIE = 10) reported for C-H bond cleavage of BNAH by a trans-dioxomanganese(V) porphyrin.20 Product analysis of the [LCuII(O2•−)]+/BNAH reaction (following quenching with HCl at −130 °C)15 confirms that BNAH has undergone oxidation by 1. The substrate’s 4’ C-H bond has been oxidatively cleaved to form 1-benzylnicotinamidium ion (BNA+) in 42% yield (1H-NMR), based on the initial copper concentration (Scheme 1). Additionally, upon acidification, liberated hydrogen peroxide is also detected in ~30% yield, approximately corresponding to the amount of peroxo-dicopper(II) complex [{(LCuII)}2(O22−)]2+ (2) that is also a reaction product, identified by its characteristic UV-Vis absorption bands (vide supra).
Figure 3.
(a) Spectral changes of 0.4 mM [LCuII(O2•−)]+ in the presence of 8 mM BNAH at −125°C in MeTHF. The first spectrum recorded (green) is that immediately following O2-bubbling to a solution of [LCuI]+ + BNAH. Inset: pseudo first order kinetics fit from 741 nm data. (b) Plots of kobs as a function of BNAH, BNAD, and BzIMH concentration used to determine second order rate constants.
Scheme 1.
Cupric superoxo-promoted cleavage of the C-H bond likely follows one of two possible pathways (Scheme 1). One is initial H-atom transfer (HAT), in which the C-H bond cleaves homolytically (with the thus-formed BNA-radical rapidly losing a second electron); the other, hydride transfer resulting from heterolytic cleavage. To provide further mechanistic insight into the mode of C-H activation by 1, a second substrate – 1,3-dimethyl-2,3-dihydrobenzimidazole (BzImH)21 – was studied for reactivity. Like BNAH, BzImH possesses a weak C-H bond, but with markedly different bond strengths compared to BNAH (homolytic, BDE = 73.4 kcal/mol, compared to 70.7 kcal/mol for BNAH) and heterolytic (hydride affinity of 49.5 kcal/mol, compared to 64.2 kcal/mol for BNAH)).21 Kinetic studies reveal that the oxidation of BzImH occurs ~2.4 times slower than that of BNAH, with a second order rate constant of 0.078 M−1s−1 (Figure 3b). The slower rate of C-H oxidation of BzImH (stronger H− donor) vs BNAH (stronger H• donor) thus suggests that at least for these substrates, the preferred mode of C-H activation by [LCuII(O2•−)]+ is via rate-limiting homolytic C-H bond cleavage; that is, H-atom transfer (HAT).
Based on extensive studies of the enzymes PHM/DβM, the most accepted mechanistic proposal is that these enzymes initially proceed by (non-classical)6b,22 hydrogen-atom transfer (HAT) chemistry, promoted by a cupric superoxo generated at the CuM site.8a,9a Interestingly, enzyme kinetic studies carried out on DβM and PHM reveal KIE’s of 10–14.6,23 (depending on conditions), thus very similar to that found here for our system. Our studies reported here with a “model” cupric superoxide complex suggest that this mechanism is followed, at least for the relatively weak C-H substrates investigated. In one case, theoretical calculations inspired by results on a model system led to the suggestion that an initial hydride abstraction may occur for PHM/DβM.24 Our findings here suggest this is not likely to be a favorable pathway.
Following C-H activation, the overall mechanism of substrate hydroxylation by the enzymes DβM/PHM is poorly understood, with nearly as many proposals for the subsequent steps as there are researchers in the field.15 The steps to products following the transition state are also difficult to surmise in our chemical systems.26
Despite these uncertainties, the importance of the present studies lies in the observation of intermolecular C-H activation by a cupric superoxo complex. To summarize, a new-ligand supported cupric superoxo complex with reactivity behavior of relevance to the DβM and PHM enzymes has been described. Significant advances are: (a) [LCuII(O2•−)]+ (1) is the first cupric superoxo complex which incorporates a hydrogen-bonding ligand feature; (b) this results in a compound with stronger Cu-O and O-O bonds compared to previously known examples; (c) not necessarily related to the latter properties, the reactions of 1 with BNAH and BzImH provide the first examples of inter-molecular C-H bond activation by a cupric superoxo complex. Note that it is not unexpected that relatively weak C–H bonds are cleaved for an inter-molecular situation; one does not have the advantage of a proximate substrate, as found for the known single example of intramolecular CuII-O2•− mediated C–H oxidation,13 and finally, (d) the relative rates for C-H oxidation of these two substrates support rate-limiting homolytic C-H bond activation. Further corroborating studies with other C-H mechanistic probes will be investigated. The clean kinetic behavior, striking deuterium kinetic isotope effect (KIE), and determination of rate-limiting HAT demonstrate that the reaction of [LCuII(O2•−)]+ (1) with C-H substrates may possess biological significance in direct comparison to the DβM/PHM enzymes reaction mechanism.
Supplementary Material
Acknowledgement
This research was supported by National Institutes of Health (K.D.K GM28962 and E.I.S. DK31450, WCU Program R31-2008-000-10010-0 (K.D.K and S.F.), and the Global COE program from the Ministry of Education, Culture, Sports, Science and Technology, Japan (S.F.)
Footnotes
Supporting Information Available: Experimental procedures, spectra, DFT calculations and explanations and supporting diagrams. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Holm RH, Kennepohl P, Solomon EI. Chem. Rev. 1996;96:2239–2314. doi: 10.1021/cr9500390. [DOI] [PubMed] [Google Scholar]; (b) Solomon EI, Chen P, Metz M, Lee S-K, Palmer AE. Angew. Chem., Int. Ed. 2001;40:4570–4590. doi: 10.1002/1521-3773(20011217)40:24<4570::aid-anie4570>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 2.(a) Mirica LM, Ottenwaelder X, Stack TDP. Chem. Rev. 2004;104:1013–1045. doi: 10.1021/cr020632z. [DOI] [PubMed] [Google Scholar]; (b) Lewis EA, Tolman WB. Chem. Rev. 2004;104:1047–1076. doi: 10.1021/cr020633r. [DOI] [PubMed] [Google Scholar]
- 3.Itoh S. Curr. Opin. Chem. Biol. 2006;10:115–122. doi: 10.1016/j.cbpa.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 4.(a) Quant Hatcher L, Karlin KD. J. Biol. Inorg. Chem. 2004;9:669–683. doi: 10.1007/s00775-004-0578-4. [DOI] [PubMed] [Google Scholar]; (b) Himes RA, Karlin KD. Curr. Opin. Chem. Biol. 2009;13:119–131. doi: 10.1016/j.cbpa.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cramer CJ, Tolman WB. Acc. Chem. Res. 2007;40:601–608. doi: 10.1021/ar700008c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Klinman JP. Chem. Rev. 1996;96:2541–2561. doi: 10.1021/cr950047g. [DOI] [PubMed] [Google Scholar]; (b) Klinman JP. J. Biol. Chem. 2006;281:3013–3016. doi: 10.1074/jbc.R500011200. [DOI] [PubMed] [Google Scholar]; (c) Prigge ST, Eipper BA, Mains RE, Amzel LM. Science. 2004;304:864–867. doi: 10.1126/science.1094583. [DOI] [PubMed] [Google Scholar]
- 7.Chen P, Solomon EI. Proc. Nat. Acad. Sci. 2004;101:13105–13110. doi: 10.1073/pnas.0402114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Evans JP, Ahn K, Klinman JP. J. Biol. Chem. 2003;278:49691–49698. doi: 10.1074/jbc.M300797200. [DOI] [PubMed] [Google Scholar]; (b) Bauman AT, Yukl ET, Alkevich K, McCormack AL, Blackburn NJ. J. Biol. Chem. 2006;281:4190–4198. doi: 10.1074/jbc.M511199200. [DOI] [PubMed] [Google Scholar]
- 9.(a) Chen P, Solomon EI. J. Am. Chem. Soc. 2004;126:4991–5000. doi: 10.1021/ja031564g. [DOI] [PubMed] [Google Scholar]; (b) Chen P, Bell J, Eipper BA, Solomon EI. Biochemistry. 2004;43:5735–5747. doi: 10.1021/bi0362830. [DOI] [PubMed] [Google Scholar]; (c) Comba P, Knoppe S, Martin B, Rajaraman G, Rolli C, Shapiro B, Stork T. Chem.–Eur. J. 2008;14:344–357. doi: 10.1002/chem.200700865. [DOI] [PubMed] [Google Scholar]
- 10.(a) Maiti D, Lee D-H, Gaoutchenova K, Würtele C, Holthausen MC, Narducci Sarjeant AA, Sundermeyer J, Schindler S, Karlin KD. Angew. Chem., Int. Ed. 2008;47:82–85. doi: 10.1002/anie.200704389. [DOI] [PubMed] [Google Scholar]; (b) Maiti D, Narducci Sarjeant AA, Karlin KD. Inorg. Chem. 2008;47:8736–8747. doi: 10.1021/ic800617m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Decker A, Solomon EI. Curr. Opin. Chem. Biol. 2005;9:152–163. doi: 10.1016/j.cbpa.2005.02.012. [DOI] [PubMed] [Google Scholar]; (b) Yoshizawa K, Kihara N, Kamachi T, Shiota Y. Inorg. Chem. 2006;45:3034–3041. doi: 10.1021/ic0521168. [DOI] [PubMed] [Google Scholar]; (c) Crespo A, Martí MA, Roitberg AE, Amzel LM, Estrin DA. J. Am. Chem. Soc. 2006;128:12817–12828. doi: 10.1021/ja062876x. [DOI] [PubMed] [Google Scholar]; (d) Huber SM, Ertem MZ, Aquilante F, Gagliardi L, Tolman WB, Cramer CJ. Chem.–Eur. J. 2009;15:4886–4895. doi: 10.1002/chem.200802338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maiti D, Fry HC, Woertink JS, Vance MA, Solomon EI, Karlin KD. J. Am. Chem. Soc. 2007;129:264–265. doi: 10.1021/ja067411l. [DOI] [PubMed] [Google Scholar]
- 13.Kunishita A, Kubo M, Sugimoto H, Ogura T, Sato K, Takui T, Itoh S. J. Am. Chem. Soc. 2009;131:2788–2789. doi: 10.1021/ja809464e. [DOI] [PubMed] [Google Scholar]
- 14. Yamaguchi S, Wada A, Funahashi Y, Nagatomo S, Kitagawa T, Jitsukawa K, Masuda H. Eur. J. Inorg. Chem. 2003:4378–4386. doi: 10.1021/ic035080x. (b) [{(LCuII)}2(O22−)]2+ (2) possesses distinctive UV-vis absorptions and ν(O-O) = 837 cm−1.
- 15.See Supporting Information.
- 16.The complex formed with 18O2 had a small 16O2 contamination estimated to be 18% from the weak O-O feature at 1130 cm−1 observed in the 18O sample spectrum. The signal of the 16O sample was scaled and subtracted from the 18O spectrum for analyzing the Cu-18O vibrational region.
- 17.A resonance enhanced peak at 485 cm−1 was observed for the complex formed with 16O2, together with a less intense peak at 452 cm−1. For the complex formed with 18O2, two peaks were observed at 468 cm−1 and 448 cm−1, with their relative intensities reversed compared to the spectrum of the 16O sample. This pattern also indicates a Fermi resonance with a non-enhanced mode at similar energy, in this case for both the Cu-16O and Cu-18O spectra. From the analysis (see Supporting Information), the pre-interaction Cu-O stretching frequency for the 16O complex is at 482 cm−1 and that for the 18O complex is at 462 cm−1.
- 18.Wada A, Harata M, Hasegawa K, Jitsukawa K, Masuda H, Mukai M, Kitagawa T, Einaga H. Angew. Chem., Int. Ed. 1998;37:798–799. doi: 10.1002/(SICI)1521-3773(19980403)37:6<798::AID-ANIE798>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.(a) Fukuzumi S, Koumitsu S, Hironaka K, Tanaka T. J. Am. Chem. Soc. 1987;109:305–316. [Google Scholar]; (b) Yuasa J, Fukuzumi S. J. Am. Chem. Soc. 2006;128:14281–14293. doi: 10.1021/ja0604562. [DOI] [PubMed] [Google Scholar]
- 20.Lee JY, Lee Y-M, Kotani H, Nam W, Fukuzumi S. Chem. Commun. 2009:704–706. doi: 10.1039/b814928c. [DOI] [PubMed] [Google Scholar]
- 21.Zhu X-Q, Zhang M-T, Yu A, Wang C-H, Cheng J-P. J. Am. Chem. Soc. 2008;130:2501–2516. doi: 10.1021/ja075523m. [DOI] [PubMed] [Google Scholar]
- 22.Francisco WA, Blackburn NJ, Klinman JP. Biochemistry. 2003;42:1813–1819. doi: 10.1021/bi020592t. [DOI] [PubMed] [Google Scholar]
- 23.Bollinger JM, Jr, Krebs C. Curr. Opin. Chem. Biol. 2007;11:151–158. doi: 10.1016/j.cbpa.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 24.de la Lande A, Parisel O, Gérard H, Moliner V, Reinaud O. Chem. Eur. J. 2008;14:6465–6473. doi: 10.1002/chem.200701595. [DOI] [PubMed] [Google Scholar]
- 25.Nagel ZD, Klinman JP. Chem. Rev. 2006;106:3095–3118. doi: 10.1021/cr050301x. [DOI] [PubMed] [Google Scholar]
- 26.For BNAH, no organic products beside BNA+ are detected in the reaction mixture. The sole inorganic product identified is the trans-peroxo complex (2), though only accounting for approximately 50% of the total copper content in the reaction. Several pathways may be envisioned which lead to (2) following sequential HAT/e− transfer to (1), giving a putative Cu(I)-OOH moiety that could “trap” an equivalent of 1 to give 2 and HO2−. This would lead to consumption of 2 equiv. of 1 per equiv. of substrate and explain the close-to-50% (as opposed to quantitative) yield of BNA+. The trans-peroxo (2) is not reactive toward BNAH at −125°C, and only very slowly decomposes in the presence of large excesses of BzImH. Further incubation of the final reaction mixture does not lead to higher detected yields of BNA+ – i.e., other copper species present following the conclusion of the reaction do not react with substrate at the temperature examined.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.