Abstract
Recently it has been shown that the expression of the immunoproteasome increased in proportion to the degree of chronic inflammation in both the liver cell cytoplasm and nuclei in liver biopsies from patients who had chronic active hepatitis or cirrhosis. In the present study, biopsies from patients with steatohepatitis, with or without Mallory-Denk body (MDB) formation, were studied by immunofluorescent staining. Normal liver showed colocalization of FAT10, LMP2, LMP7, and MECL-1 at the mitochondria. Only LMP2 and LMP7 were found in the cell nuclei. Liver biopsies from patients with steatohepatitis and MDB formation, and a case of hepatocellular carcinoma forming MDBs in the tumor cells, showed colocalization of FAT10 and ubiquitin with LMP2, LMP7 and MECL-1 within the MDB. This indicates involvement of the immunoproteasome in MDB formation in steatohepatitis cases and in a case of HCC forming MDBs. Prior studies have shown that the immunoproteasome was involved in drug-induced MDB formation using the same immunofluorescent colocalization approach as was used on these human liver biopsies. The increase in the immunoproteasome subunit proteins was made at the expense of the 26S proteasome. This indicates that the shift from the 26S to the immunoproteasome had occurred in the MDB positive hepatocytes.
Keywords: Mallory-Denk bodies (MDB), Immunoproteasome, FAT10, LMP2, LMP7, MECL-1, mitochondria
INTRODUCTION
Steatohepatitis frequently includes Mallory-Denk body (MDB) formation. In the case of alcoholic steatohepatitis, MDB formation occurs in 80% of biopsies (French, 1981a and b). Recently, using a drug-induced mouse model where MDB formation occurred, it was shown that the MDB formation was associated with an up regulation of the immunoproteasome catalytic subunits and a down regulation of the β5 26S proteasome subunit (Bardag-Gorce et al., 2010a). There was a loss of activity in the 26S proteasome causing accumulation of undigested proteins by the liver cells (Bardag-Gorce et al., 2010a). The undigested proteins aggregated and MDBs formed. The switch from the 26S proteasome to the immunoproteasome was associated with the up regulation of the TLR 2/4 pathway, the increase of the catalytic subunits of the immunoproteasome and the expression of TNFa and IFNg receptors (Bardag-Gorce et al., 2010a) (Bardag-Gorce et al., 2010b). The increase in the expression of the TLR 2/4 pathway was associated with the up regulation of TNFα and IFNγ receptors and TNFα expression (Bardag-Gorce et al., 2010b). The process of MDB formation is driven by a synergistic activation of genes localized in the MHC1 locus (Oliva et al., 2010). An interferon sequence responsive element (ISRE) on the Fat10 promoter is activated synergistically by the combination of IFNγ and TNFα as shown in vitro (Oliva et al., 2010). The genes activated i.e. TNFα, IFNγ, LMP2, LMP7 and MECL-1 cause the formation of the immunoproteasome, and consequently decrease the 26S proteasome activity (Bardag-Gorce et al., 2010a). The whole sequence of events involved in experimental MDB formation was prevented by feeding S-adenosylmethionine (SAMe) due to a gene silencing mechanism. This occurred through epigenetic events, which resulted from SAMe-initiated methylation of histones, including H3K27 (Bardag-Gorce et al., 2007, Bardag-Gorce et al., 2008, Bardag-Gorce et al., 2010a and 2010b; Oliva et al., 2008). This phenomenon has recently been reviewed (French et al., 2010a), as well as the relationship of MDB formation to the development of hepatocellular carcinoma through the proinflammatory mechanism (French et al., 2010b).
Recently, the expression of the immunoproteasome subunits in hepatocytes of liver biopsies from controls and patients with chronic liver disease and cirrhosis has been described (Vasuri et al., 2010). The expression of LMP2, LMP7, MECL-1 and PA28α/β has been shown to increase in proportion to the degree of chronic inflammation, in both controls and chronic liver disease. Using immunoperoxidase localization, it was shown that the immunoproteasome subunits were found in the cytoplasm and the nuclei of hepatocytes. The increase of LMP2 and PA28α in the total liver was also shown by Western blot. There was also an increase in the subunits LMP2 and 7 and PA28α in the nuclear extracts. Their immunoperoxidase results were confirmed in the present study. Here, we focused on changes observed in MDB forming hepatocytes.
METHODS
Human tissues
Archived liver biopsies were studied from patients with alcoholic liver disease where Mallory-Denk bodies (MDBs) were found. Different stages from fatty liver, to alcoholic hepatitis, to cirrhosis to hepatocellular carcinoma (HCC) were stained with either FAT10 or ubiquitin antibodies and double stained for LMP2, LMP7, MECL-1 and PA28α and β. A total of 14 biopsies from ALD patients, one with NASH and one autopsy liver from advanced alcoholic cirrhosis were stained, together with one normal liver biopsy obtained in a failed attempt to find a liver metastasis.
Immunofluorescence staining
Antibodies used were obtained as follows: ubiquitin Dako (Carpenteria, CA) or Chemicon (Tamecula, CA); FAT10, Enzo (Plymouth, Meeting, PA) or Santa Cruz Biotech (Santa Cruz, CA); LMP7, ABCAM (Cambridge, MA) or Santa Cruz Biotech (Santa Cruz, CA); MECL-1, Santa Cruz Biotech (Santa Cruz, CA); PSME 1 and 2, ABCAM (Cambridge, MA). Double stains were done using antibodies from two different species against 2 different human antigens. The immunostains were examined using a Nikon 400 microscope with filters for FITC, Texas red and Tricolor. Confocal microscopy was performed using a Leica microscope with computer software.
RESULTS
A control liver biopsy from a patient suspected of having liver metastasis showed cytoplasmic staining of liver cytoplasm by antibodies for FAT10, MECL-1, LMP2 and LMP7. The cytoplasm showed colocalization of LMP7 and FAT10 (Fig 1,). Hepatocytes in areas of normal liver in 3 out of 4 alcoholic hepatitis cases showed co-localization of one or more antibodies to FAT10, with MECL-1, LMP2 or LMP7 around mitochondria (Fig 2). Lysosomal localization of PA28A, LMP7, and MECL-1 occurred in the non-neoplastic part of a liver removed for hepatocellular carcinoma (Fig 3).
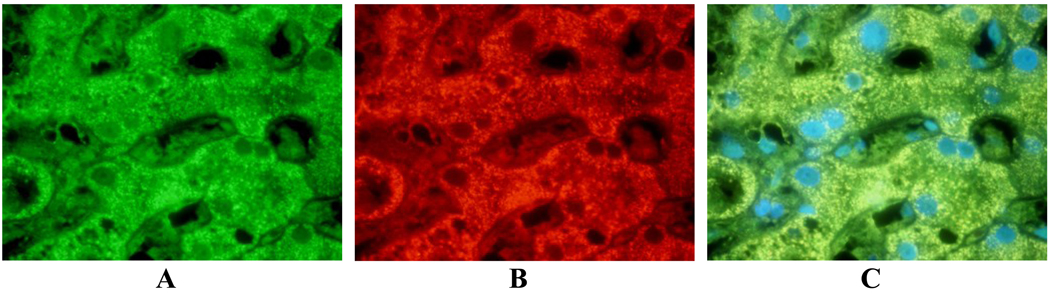
Fig 1.
Control liver double stained with FAT10 and LMP7 antibodies: A- FITC forLMP7; B- Texas Red for Fat10; C- Tricolor blue for DAP1. Note that FAT10 and LMP7 antibodies co-localize around the mitochondria. X654.
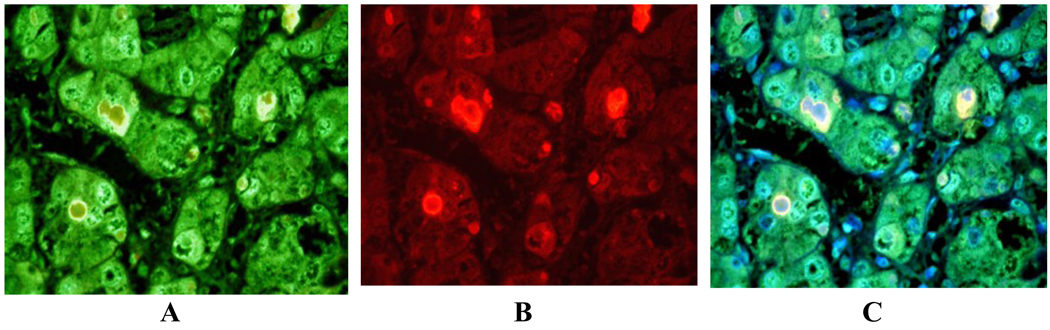
Fig 2.
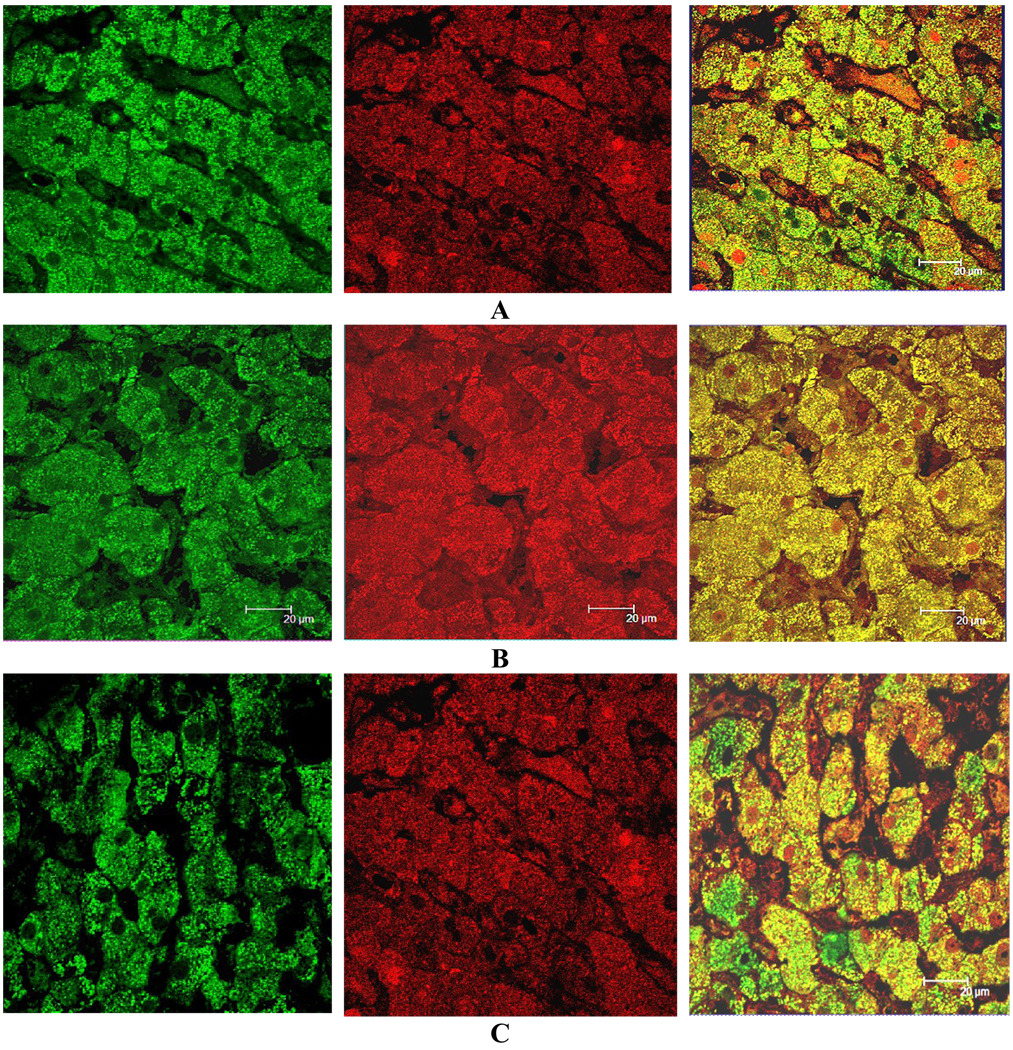
Normal area of a liver biopsy from a patient with NASH showing co-localization of A- LMP2, B- MECL-1, and C- LMP7 around the mitochondria (green) and FAT10 (Texas red) merged (yellow). Magnification bar 20 µm.
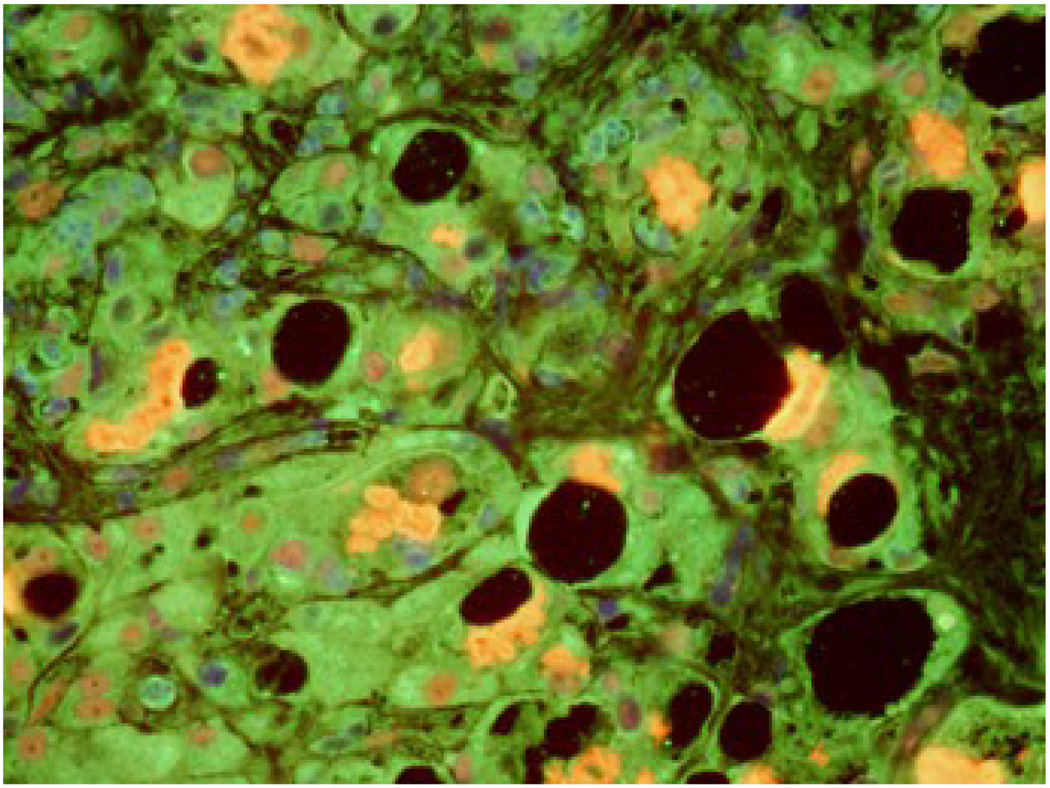
Fig 3.
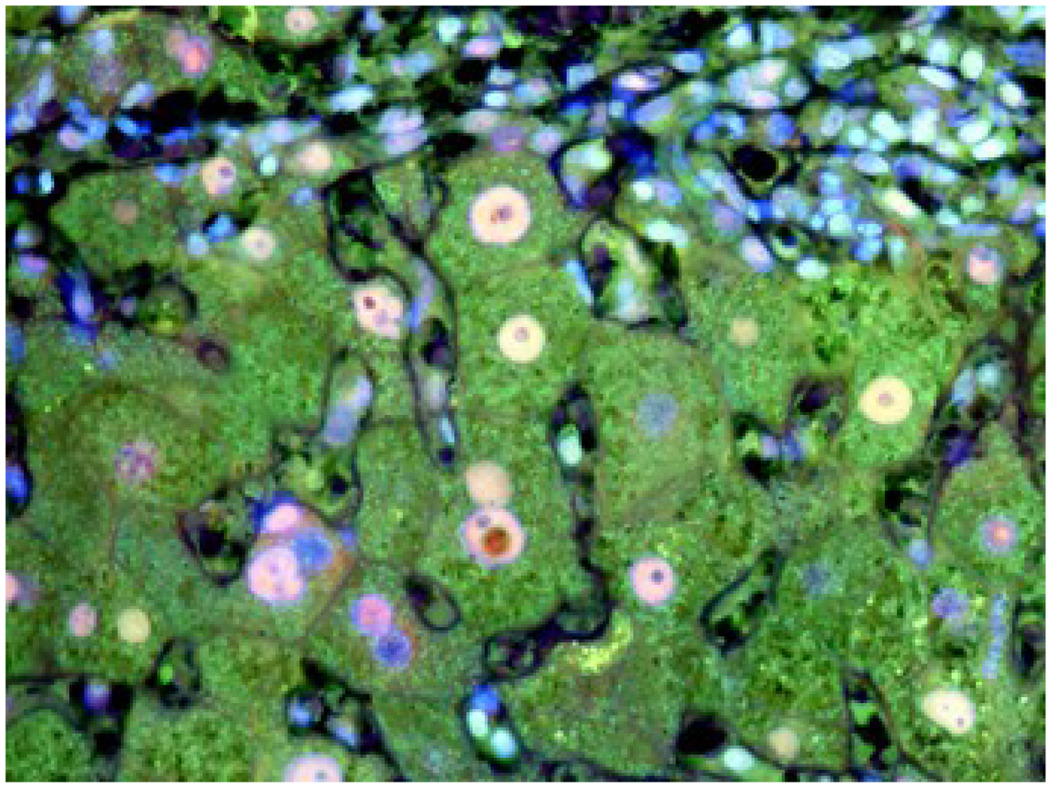
Liver biopsy from a post alcoholic patient with cirrhosis showing staining of lysosomes with antibodies to A- PA281 (PA28 alpha (green), B- FAT10 (red), and C- merged (Tricolor) The lysosomes stained positive with PA28A, not FAT10. X436.
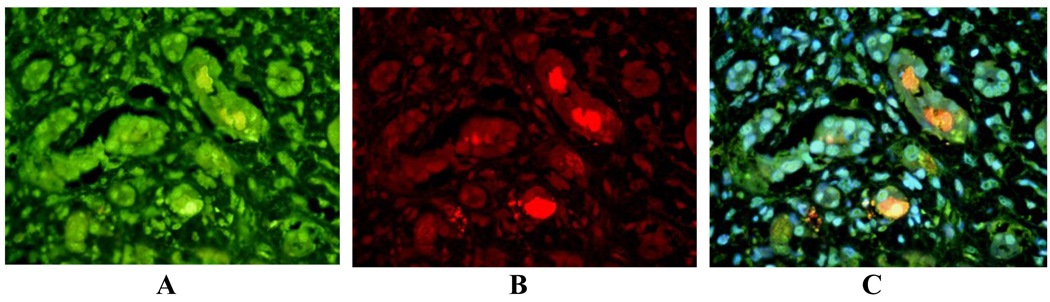
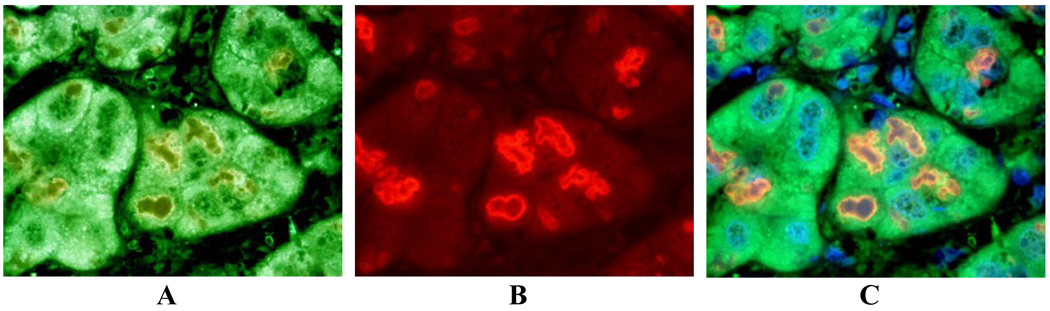
In the 9 cases with ALD where MDBs were found, the MDBs were double stained by either FAT10 or ubiquitin with LMP2, LMP7, MECL-1 or PA28β. There was co-localization in MDBs in one or more immunoproteasome subunits in 7 cases. LMP2 co-localized with ubiquitin, at the periphery of the MDBs. LMP2 was the most common subunit to be found in MDBs (Fig 4). LMP7 co-localized in MDBs with ubiquitin (Fig 5). In one case, MDBs were present in duct metaplastic cells where the MDBs stained positive for LMP2 (fig.6). In liver tumor cells in a case of hepatocellular carcinoma, where MDBs had formed in most of the tumor cells, the MDBs that were positive for ubiquitin were double stained for LMP2, LMP7 and MECL-1 (Fig 7). This carcinoma was from an alcoholic patient who had stopped drinking but had residual early cirrhosis in the non-neoplastic liver. Three cases of alcoholic liver disease with fatty liver with fibrosis did not show mitochondrial localization of immunoproteasome subunits (data not shown). Scattered nuclear staining by ubiquitin and LMP2 (Fig 8), LMP7, and MECL-1 (not shown) was also noted.
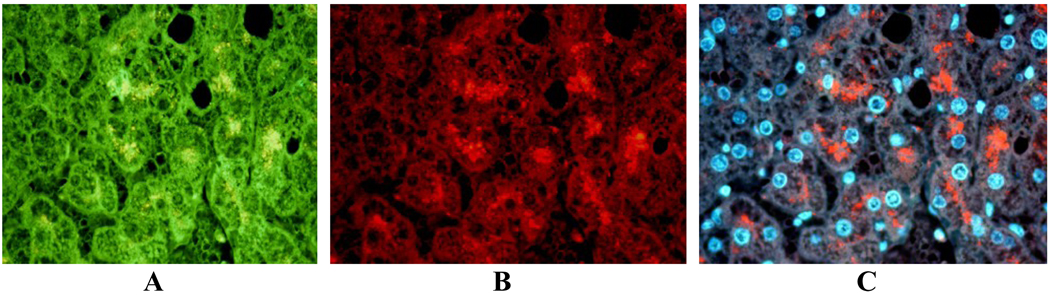
Fig 4.
Liver biopsy from a patient with alcoholic hepatitis where MDBs were double stained for A- LMP2 (green); B- ubiquitin (red); C- MDBs show co-localization, yellow with a Tricolor filter. X436.
Fig 5.
Liver biopsy from a patient with alcoholic hepatitis double stained for LMP7 (green) and ubiquitin (red).The MDBs (yellow) stained positive with both antibodies (Tricolor filter). X872.
Fig 6.
Liver from the autopsy of a patient with far advanced ALD where MDBs were very numerous and some MDBs were present in metaplastic ducts derived from former hepatocytes. The MDBs were double stained with antibodies to A- LMP2 (green); B- ubiquitin (red) and C- co-localized (yellow). Tricolor filter. X436.
Fig 7.
Liver removed for hepatocellular carcinoma in an alcoholic patient who had stopped drinking but had cirrhosis. The liver was double stained for A- MECL-L (green), B- ubiquitin (red), and C- co-localized (yellow) with a Tricolor filter. X436.
Fig 8.
Liver biopsy from a patient with alcoholic cirrhosis double stained for LMP2 (green) and ubiquitin (red). Many nuclei stained positive with both antibodies (Tricolor filter). X520.
DISCUSSION
Vasuri first described localization of the immunoproteasome subunits in the cytoplasm and nuclei of hepatocytes using immunoperoxidase labeling (Vasuri, et al., 2010). The concentration of these subunits increased in chronic liver disease. In the present study, we also found some subunits localized to the cytoplasm and nuclei in hepatocytes in various stages of alcoholic liver disease, including cirrhosis. The catalytic subunits LMP2, LMP7, MECL-1, and the regulatory subunits PA28, were found in the cytoplasm of hepatocytes. In normal liver, LMP2, LMP7 and MECL-1 were also found around the mitochondria. The concentration of subunits was reduced in liver cells showing globular fat storage or (MDB) formation. The LMP2 and LMP7 proteins co-localized with FAT10 and ubiquitin at the periphery of the MDBs, indicating that the subunits, like FAT10 and ubiquitin, were involved in aggresome formation. Prior studies have implicated the conversion of the 26S proteasome to form the immunoproteasome at the expense of the 26S proteasome activated by ATP. (Bardag-Gorce et al., 2010a, Boes et al 1994, French et al., 2010a and 2010b, Groettrup et al, 2010). MDBs formed as a consequence of the loss of 26S proteasome turnover of short lived proteins, such as the cytokeratins and tubulin.
One unanticipated finding in this study was the localization of the immunoproteasome subunits and FAT10 around the mitochondria. Until now, only the 26S proteasome subunits and ubiquitin ligands have been localized around the mitochondria.. Neutzner et al showed that the proteasome was involved in the degradation of mitochondrial outer membrane proteins damaged by oxidative stress (Neutzner et al, 2007; Neutzner et al, 2008). The oxidative stress was generated by the mitochondria in a way that is similar to the ER associated degradation (ERAD) where p97 is involved in removing unfolded proteins produced by ER stress and mitophagy (Tanaka et al., 2010). The relationship between mitochondria, membrane-confined organelles and the cytosolic ubiquitin-proteasome system (UPS) for the removal of mitochondrial proteins has recently been reviewed (Livnat-Levanson and Glickman 2010). UPS is involved in mitochondrial protein recycling of damaged or misfolded proteins, which require a responsive quality control system. Thus, changes in proteasome location seen in chronic liver disease may be a response to mitochondrial damage, as well as the quality control of the cytoskeletal system where MDBs form in hepatocytes.
Acknowledgement
The authors thank Adriana Flores for typing the paper. Supported by an NIH/NIAAA Alcohol Center Grant PA50-11999, Morphology Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bardag-Gorce F, Dedes J, French BA, Oliva J, Li J, French SW. Mallory body formation is associated with epigenetic phenotypic change in hepatocytes in vivo. Exp Mol Pathol. 2007;83:160–168. doi: 10.1016/j.yexmp.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardag-Gorce F, Oliva J, French BA, Li J, Dedes J, French SW. SAMe prevents the induction of immunoproteasome and preserves the 26s proteasomes in the DDC induced MDB. Mouse Model Exp Mol Pathol. 2010a;88:352–362. doi: 10.1016/j.yexmp.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardag-Gorce F, Oliva J, Lin A, Li J, French BA, French SW. SAMe prevents the up regulation of toll-like receptor signaling in Mallory-Denk body forming hepatocytes. Exp Mol Pathol. 2010b;88:376–379. doi: 10.1016/j.yexmp.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardag-Gorce F, Oliva J, Villegas J, Fraley S, Amidi F, Li J, Dedes J, French BA, French SW. Epigenetic mechanisms regulate Mallory Denk body formation in the livers of drug-primed mice. Exp Mol Pathol. 2008;94:113–121. doi: 10.1016/j.yexmp.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes B, Hengel H, Ruppert T, Multhaup G, Koszinowski UH, Kloetzel PM. Interferon gamma stimulation modulates the proteolytic activity and cleavage site preference of 20S mouse proteasomes. J Exp Med. 1994 Mar 1;179(3):901–909. doi: 10.1084/jem.179.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SW, Bardag-Gorce F, Li J, French BA, Oliva J. Mallory-Denk body pathogenesis revisited. World J Hepatology. 2010a;2:295–301. doi: 10.4254/wjh.v2.i8.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SW, Oliva J, French BA, Bardag-Gorce F. Alcohol Nutrition and Liver Cancer. Roll of toll-like receptor signaling. World J Gastroenterol. 2010b;16:1344–1348. doi: 10.3748/wjg.v16.i11.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SW. Nature, pathogenesis, and significance of the Mallory body. Seminars in Liver Disease. 1981a;1:217–231. doi: 10.1055/s-2008-1041749. [DOI] [PubMed] [Google Scholar]
- French SW. The Mallory body structure composition and pathogenesis. Hepatology. 1981b;1:776–783. doi: 10.1002/hep.1840010113. [DOI] [PubMed] [Google Scholar]
- Groettrup M, Kirk CJ, Basler M. 2010 Proteasomes in immune cells: more than peptide producers? Nat Rev Immunol. 2010 Jan;10(1):73–78. doi: 10.1038/nri2687. Epub 2009 Dec 11. [DOI] [PubMed] [Google Scholar]
- Li J, Bardag-Gorce F, Dedes J, French BA, Amidi F, Oliva J, French SW. S-adenosylmethionine prevents Mallory Denk body formation in drug-primed mice by inhibiting the epigenetics memory. Hepatology. 2008;47:613–624. doi: 10.1002/hep.22029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livnat-Levanon A, Glickman MH. Ubiquitin-proteasome system and mitochondria-reciprocity. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbagrm.2010.07.005. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Neutzner A, Benard G, Youle BG, Karbouwski M. Role of the ubiquitin conjugation system in the maintenance of mitochondrial homeostasis. Ann Ny Acad Sci. 2008;1147:242–253. doi: 10.1196/annals.1427.012. [DOI] [PubMed] [Google Scholar]
- Neutzner A, Youle RJ, Karbowski M. Outer mitochondrial membrane protein degradation by the proteasome. Novartis Found Symp. 2007;287:4–14. Discussion 14–20. [PubMed] [Google Scholar]
- Oliva J, Bardag-Gorce F, French BA, Li J, McPhaul L, Amidi F, Dedes J, Habibi A, Nguyen S, French SW. FAT10 is an epigenetic marker for liver preneoplasia in a drug-primed mouse model of tumorigenesis. Exp Mol Pathol. 2008;94:101–112. doi: 10.1016/j.yexmp.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva J, Bardag-Gorce F, Lin A, French BA, French SW. Role of cytokines in UBD promoter regulation and Mallory-Denk body-like aggresomes. Exp Mol Pathol. 2010:1–8. doi: 10.1016/j.yexmp.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada A, Cleland HM, Xu S, Neredra DP, Karbowski M, Youle RJ. Proteasome and p97 mediated mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol. 2010 doi: 10.1083/jcb.201007013. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasuri F, Capizzi E, Bellavista E, Mishto M, Santoro A, Fiorentiono M, Capri M, Cescon M, Grazi GL, Grigioni WF, D’Errico-Grigioni A, Franceschi C. Studies on immunoproteasome in human livers. Part I: Absence in fetuses, presence in normal subjects, and increased levels in chronic active hepatitis and cirrhosis. Biochem Biophys Res Commun. 2010;397:301–306. doi: 10.1016/j.bbrc.2010.05.104. [DOI] [PubMed] [Google Scholar]