Abstract
Age-related increases in ectopic fat accumulation are associated with greater risk for metabolic and cardiovascular diseases, and physical disability. Reducing skeletal muscle fat and preserving lean tissue are associated with improved physical function in older adults. PPARγ-agonist treatment decreases abdominal visceral adipose tissue (VAT) and resistance training preserves lean tissue, but their effect on ectopic fat depots in nondiabetic overweight adults is unclear. We examined the influence of pioglitazone and resistance training on body composition in older (65–79 years) nondiabetic overweight/obese men (n = 48, BMI = 32.3 ± 3.8 kg/m2) and women (n = 40, BMI = 33.3 ± 4.9 kg/m2) during weight loss. All participants underwent a 16-week hypocaloric weight-loss program and were randomized to receive pioglitazone (30 mg/day) or no pioglitazone with or without resistance training, following a 2 × 2 factorial design. Regional body composition was measured at baseline and follow-up using computed tomography (CT). Lean mass was measured using dual X-ray absorptiometry. Men lost 6.6% and women lost 6.5% of initial body mass. The percent of fat loss varied across individual compartments. Men who were given pioglitazone lost more visceral abdominal fat than men who were not given pioglitazone (−1,160 vs. −647 cm3, P = 0.007). Women who were given pioglitazone lost less thigh subcutaneous fat (−104 vs. −298 cm3, P = 0.002). Pioglitazone did not affect any other outcomes. Resistance training diminished thigh muscle loss in men and women (resistance training vs. no resistance training men: −43 vs. −88 cm3, P = 0.005; women: −34 vs. −59 cm3, P = 0.04). In overweight/obese older men undergoing weight loss, pioglitazone increased visceral fat loss and resistance training reduced skeletal muscle loss. Additional studies are needed to clarify the observed gender differences and evaluate how these changes in body composition influence functional status.
INTRODUCTION
As the prevalence of obesity among older adults increases, the prevalence of obesity-related disability is anticipated to increase as well (1–3). Older adults with disability are more likely to require long-term health care, leading to a reduction in quality of life and a high economic burden (4). Fat accumulates in and around organs such as skeletal muscle (intermuscular fat) and the heart (pericardial fat). This is associated with greater risk for functional impairment, and for metabolic and cardiovascular diseases, independent of total adiposity (5–7).
Caloric restriction that produces weight loss ameliorates some of the metabolic consequences of obesity and is effective in reducing ectopic fat depots in younger age groups (8–10). Despite the benefits, weight loss is not routinely recommended for older adults since it may lead to skeletal muscle loss, and potentially exacerbate the age-related impairments in muscle function and mobility (11–13). An ideal therapy for changing body composition in older adults would target ectopic fat compartments while preserving muscle mass, thereby providing a metabolic benefit while minimizing the risk of loss of physical function.
Two potentially complementary strategies to achieve these body composition goals are resistance training and PPARγ-agonist treatment. Progressive resistance training has been shown to preserve lean tissue in older adults undergoing weight loss (14). Strength-training and power-training are reported to be equally effective in increasing total lean mass and improving functional performance in older (>65 years old) adults (15). The extent to which resistance training which focuses on improving muscle power, affects lean tissue loss in older men and women during weight loss is less clear. Furthermore, whether a synergy between resistance training and hypocaloric weight loss exists for the reduction of intermuscular fat is unknown.
PPARγ-agonists are insulin sensitizers used to treat type 2 diabetes that redistribute adipose tissue from the abdominal visceral to the subcutaneous compartment, which is considered a more metabolically favorable profile (16). Although diabetic patients treated with PPARγ-agonists lose abdominal visceral fat they often gain weight, in the absence of caloric restriction (17). However, concurrent adherence to a low-calorie diet can prevent overall weight gain (18). It is not known if PPARγ-agonist treatment during hypocaloric weight loss also affects fat depots other than the visceral abdominal depot, such as intermuscular or pericardial fat. Increased intermuscular fat in particular is associated with poorer functional status in older age (7,19); it could therefore be hypothesized that enhancing fat loss in multiple visceral depots would improve physical function in overweight/obese older adults undergoing hypocaloric weight loss. Since changes in body composition differ between older men and women following weight loss (20,21), the effects of pioglitazone and resistance training on body composition in the context of weight-loss merit exploration in men and women separately.
Therefore, the purpose of this study was to test the hypotheses that pioglitazone treatment decreases multiple ectopic fat depots and resistance training reduces lean appendicular tissue loss during weight loss. To do so, we evaluated the independent effects of the PPARγ-agonist pioglitazone and resistance training on fat depots of significance to functional health in older (65–79 years) nondiabetic overweight/obese men and women undergoing weight loss. The overall effect of hypocaloric weight loss on multiple ectopic fat depots and lean tissue was also determined in men and women separately.
METHODS AND PROCEDURES
Design overview
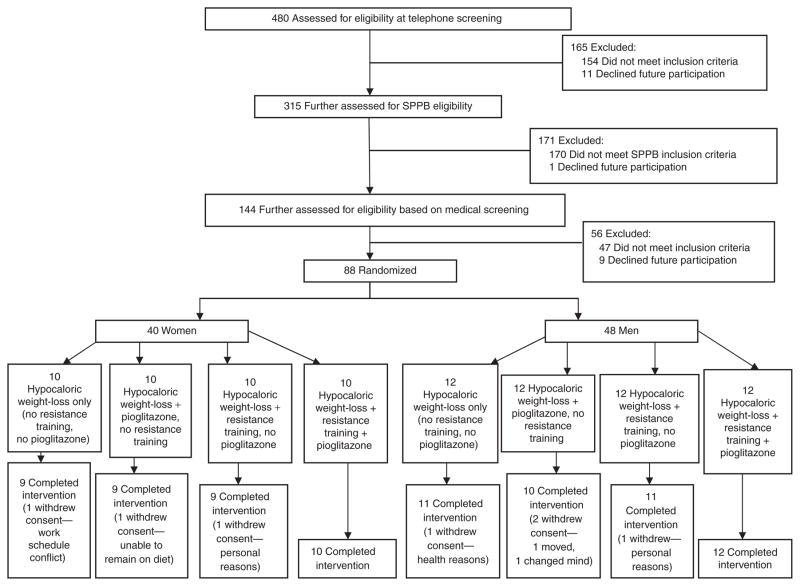
All participants underwent a 16-week caloric restriction program designed to achieve 7% weight loss and were concurrently randomized to one of four intervention groups following a 2 × 2 factorial design: no resistance training/no pioglitazone (hypocaloric weight loss only), resistance training/no pioglitazone, no resistance training/pioglitazone (Actos), or resistance training/pioglitazone (Figure 1).
Figure 1.
Participation and randomization of the optimizing body composition for function in older adults study.
Four hundred and eighty community-dwelling older adults from Forsyth County, NC and the surrounding area were screened by telephone for eligibility. Eligibility criteria were based on National Heart, Lung, and Blood Institute recommendations: (i) BMI ≥ 30 kg/m2 or (ii) a BMI between 25 and 29.9 kg/m2 or a waist circumference >88.9 cm (women) or >101.6 cm (men), plus at least one of the following: clinically manifest coronary disease, other atherosclerotic disease, sleep apnea, osteoarthritis of the knee or hip, hypertension, low-density lipoprotein cholesterol ≥ 160, high-density lipoprotein cholesterol ≤ 35, impaired fasting glucose, or family history of premature coronary heart disease (22). In addition, participants had to have a Short Physical Performance Battery (SPPB) score between 3 and 10. A score <10 is predictive of increased risk for mobility disability (23). All participants were nondiabetic and were weight-stable over the previous 6 months. Medical exclusions included severe arthritis, cancer, lung disease, serious neurological disorder, abnormal kidney or liver function tests, uncontrolled hypertension, hip fracture, hip or knee replacement, spinal surgery in the past 6 months, uncontrolled arrhythmia, myocardial infarction, major heart surgery, stroke, deep vein thrombosis or pulmonary embolus in the past 6 months, undergoing physical therapy, or mini-mental state examination score < 21, current smoking, alcohol or drug abuse, inability to communicate with study staff, enrollment in another randomized trial involving lifestyle or pharmaceutical interventions, anti-inflammatory steroids use, PPARγ-agonist use, clinically evident edema or anemia, and currently involved in high-intensity aerobic or resistance training or a weight loss regimen. After exclusion for not meeting National Heart, Lung, and Blood Institute weight loss (n = 154) or Short Physical Performance Battery (n = 170) inclusion criteria or declining further participation (n = 12), 144 participants underwent a medical screening. After further exclusion (n = 47) and declining to participate (n = 9), 88 participants (48 men, 40 women) were randomized to one of the four treatment groups (Figure 1). Randomization was performed using a centralized web-based system. The study and protocol were approved by the Wake Forest University School of Medicine institutional review board, and has been registered at http://www.clinicaltrials.gov.com/(NCT00315146). All participants provided written informed consent.
Interventions
Weight loss
The goal for all participants was a loss of ~7% of initial body mass over 16 weeks. The intervention incorporated meal replacements, nutrition education, and lifestyle behavior modification. Prior to randomization, each participant met with a study dietitian and were assigned a meal plan to provide a daily energy deficit of 500 kcal calculated from estimated total daily energy requirements for weight maintenance, using formulas established by the Institute of Medicine and the National Academy of Science. Two meal replacements per day (bars and shakes) were provided to all participants (containing ~220 kcal with 7–10 g protein, 33–46 g carbohydrates, and 1.5–5 g fat with 2–5 g of fiber). For the third meal, a weekly menu plan with recipes was given to the participants. This meal was composed of traditional foods, was low in fat, high in vegetables but allowed for individual preferences, and provided 500–750 kcal. In addition, up to three snacks (~100 kcal each) were allowed each day. The macronutrient goal for each individual was ~55–60% carbohydrates, ~15% protein, and ~25% fat. All participants attended weekly group behavioral and educational sessions. The sessions (n = ~10 per group) were conducted by a registered dietitian with expertise working with older adults on diet behavior modification. At each weekly session, all participants turned in food diaries which they were asked to complete daily for self-monitoring, and body weight was measured and recorded. If an individual was not meeting weight loss goals, energy intake was individually modified accordingly to produce the desired rate of weight loss. All participants were also encouraged to incorporate more physical activity into their day.
Pioglitazone (Actos)
Participants randomized to receive pioglitazone (donated by Takeda Pharmaceuticals, Deerfield, IL) were given an initial 15 mg/day dose. Participants were assessed for side effects after 3 weeks, but none were reported. Therefore, after 3 weeks, the dose was increased to 30 mg/day for the remainder of the study for all participants randomized to receive pioglitazone. In order to understand the potential effects of changes in body composition independent of drug effects, a 1-week wash-out period preceded the follow-up outcome assessment.
Resistance training
The goal of the resistance training program was to increase strength and muscle mass in the major muscle groups of the body, while also improving muscle power in the lower extremity. Participants randomized to the resistance training exercised 3 days/week at the Department of Health and Exercise Science Clinical Research Center. There was a 48 h rest period between resistance training sessions consistent with all published guidelines of resistance training in older adults. All resistance training sessions were supervised by two trained interventionists who worked with participants on safety, correct form, and progression of resistance as well as assisting participants with recording individual logs of training volume. Participants warmed-up by walking or cycling for 3–5 min at a slow pace followed by 5 min of large muscle flexibility exercises targeting the major muscle groups of the body. Training sessions ended with a cool-down session of light stretching.
The lower-body resistance training exercises were conducted on Keiser pneumatic resistance machines. The two lower body exercises to improve muscle power included knee extension and lower extremity extension. Participants were instructed to complete the concentric phase of the movement “as fast as possible”, pause briefly at the midpoint of the movement and complete the eccentric phase of the movement in ~2–3 s. After the initial progression, the resistance on the machines was adjusted every 2 weeks by repeating the one repetition maximum (RM) testing. For upper body training, a combination of Nautilus resistance machines and dumbbells was used. Since the resistance for the upper body training was not pneumatic, moving the weights “as fast as possible” was not feasible, so the concentric and eccentric phases of the upper body exercises were completed in 2 and 3 s, respectively. The progression used for all resistance exercises was: week 1—two sets of 8–10 reps at 40–50% of one RM; week 2—three sets of 8–10 reps at 50–60% of one RM; weeks 3–16—three sets of 8–10 reps at 70% of one RM. Participants rested for ~2–3 min between each set. The progression of the exercise program was based on American College of Sports Medicine guidelines (24). Except for participants who dropped out of the study, all participants randomized to receive resistance training followed the protocol as described above.
Outcomes
Body composition
Anthropometrics
Height (cm) and body mass (kg) were measured with shoes and jackets or outer garments removed. BMI was calculated as body mass in kg divided by the square of height in meters (kg/m2).
Dual energy X-ray absorptiometry
Percent body fat, lean mass (total and appendicular) and total mass were measured by dual energy X-ray absorptiometry (Hologic Delphi QDR, Bedford, MA, software Version 12.3). Appendicular lean mass was calculated as the sum of nonbone lean mass in arms and legs. Absolute changes in body composition measures were calculated as end point value (after 16-week intervention) subtracted from the corresponding baseline value.
Computed tomography (CT)
The CT images of the thorax, abdomen and thigh were obtained on a multidetector CT (LightSpeed Pro16, General Electric Medical Systems, Waukesha, WI) to provide measures of pericardial fat, abdominal adiposity (total, subcutaneous and visceral fat), thigh muscle and thigh fat (volume and attenuation), and liver attenuation (steatosis). The volumes of abdominal visceral, subcutaneous, and intermuscular fat were measured in a 15 cm section of the abdomen centered at the junction of the lumbar 4th and 5th as previously described (25). The measure of pericardial fat was made based on a region 15 mm above and 30 mm below the left main coronary artery as previously described and includes both epicardial and paracardial (aka. mediastinal or paracardial) depots both of which have been demonstrated to be metabolically active (26,27). Thigh muscle and adipose tissue was measured using a 5 cm section of the thigh centered at the junction of the proximal and middle third of the femur. Intermuscular adipose tissue was separated from subcutaneous adipose by drawing a line along the deep fascial plane surrounding the thigh muscles. Thigh muscle area was considered the total area of nonadipose and nonbone tissue within the deep fascial plane. Thigh muscle attenuation (Hounsfield units), excluding intermuscular fat, was assessed and used to indicate fat infiltration into the muscle. Measurements were made for each leg independently and measures of thigh muscle volume and intermuscular fat were summed for statistical analyses. Liver attenuation (Hounsfield units) was measured as the average density of three regions (≈1 cm2 each). The primary end points for this study were abdominal visceral fat volume measured by CT and measures of nonskeletal appendicular lean mass measured by dual energy X-ray absorptiometry. The additional measures of body composition were secondary outcomes.
Statistical analysis
This study was designed to focus on the main effects of pioglitazone and resistance training. The sample size of 48 men (40 women) was selected to have 82% (84% for women) power to detect a 1.4 kg (1.2 kg for women) mean difference at follow-up in appendicular nonbone lean mass between groups, assuming a 0.05 level of statistical significance for each test. For women, we had 82% power to detect a difference of 225 cm2 in abdominal visceral adipose tissue (VAT) between groups. We did not have sufficient preliminary data on the variability in abdominal VAT in men to calculate statistical power for that outcome and assumed that the variability in abdominal VAT would be similar for both genders. For primary analyses, the average VAT volume at follow-up was compared between the pioglitazone and no pioglitizone groups, and the average appendicular nonbone lean mass at follow-up was compared between resistance training and no resistance training groups, using two-way analysis of covariance. Each model contained the baseline value for the outcome and the main effect for each randomized group (pioglitazone, resistance training). Main effects were tested at the 0.05 level of significance. The effects of the main interventions on the change in the other measures of body composition were examined secondarily following the same statistical approach. Differences in least-squares means, and associated 95% confidence intervals, were calculated for each main effect on each outcome. P values are reported for the primary outcomes. The overall effect of caloric restriction on the measures of body composition and ectopic fat depots, collapsed over treatment group, was determined using contrasts within analysis of covariance, with adjustment for the main effect of each intervention arm and the interaction term between interventions. Men and women were analyzed separately throughout.
RESULTS
A total of 81 participants (92%, 44 men and 37 women) completed the study (Figure 1). Five of the seven who dropped out did so for reasons unrelated to the study. One was unable to remain on the diet and one withdrew for health reasons unrelated to the study interventions. Of the seven who did not complete the study, four participants did not complete the dietary intervention, two of whom were randomized to resistance training and did not complete that intervention; however, these participants were able to complete the 16-week closeout measurements. Attendance to weekly nutrition sessions was 90% and attendance to the resistance training sessions (for those randomized to resistance training) was 84%. Compliance to pioglitazone, based on pill count, was 96%.
Baseline characteristics of men and women according to intervention group are described in Table 1. Since our analysis determined the main effects of resistance training and pioglitazone treatment, we included baseline characteristics of men and women according to main intervention arms as well. At baseline the men randomized to resistance training weighed more than those not randomized to resistance training (P = 0.03, based on independent samples t-test). Otherwise, there were no differences between groups in baseline characteristics (Table 1). Likewise, there were no differences in baseline characteristics between groups, among those participants who completed the study (n = 44 men, 37 women). On average, men (age = 69.8 ± 3.7 years) and women (age = 70.0 ± 3.3 years) were obese at baseline, with a mean ± s.d. BMI of 32.3 ± 3.8 kg/m2 in men and 33.3 ± 4.9 kg/m2 in women. Overall, 54% of men and 73% of women reported some difficulty with performing daily tasks.
Table 1.
OPTIMA participants baseline characteristics (mean ± SD or N%) by randomization group and main intervention arm
| According to randomized group |
According to main intervention arm |
|||||||
|---|---|---|---|---|---|---|---|---|
| Resistance training/ pioglitazone |
No resistance training/ pioglitazone |
Resistance training/no pioglitazone |
No resistance training/no pioglitazone |
Resistance training |
No resistance training |
Pioglitazone | No pioglitazone | |
| Women | n = 10 | n = 10 | n = 10 | n = 10 | n = 20 | n = 20 | n = 20 | n =20 |
| Age (years) | 69.0 ± 1.7 | 69.8 ± 4.1 | 71.3 ± 4.9 | 70.0 ± 2.6 | 70.2 ± 3.7 | 69.9 ± 3.3 | 69.4 ± 3.1 | 70.7 ± 3.9 |
| Height (cm) | 164.1 ± 4.7 | 161.0 ± 5.6 | 161.9 ± 8.0 | 162.8 ± 8.8 | 163.0 ± 6.5 | 161.9 ± 7.2 | 162.5 ± 5.3 | 162.3 ± 8.2 |
| Body mass (kg) | 91.4 ± 17.4 | 89.3 ± 14.2 | 81.9 ± 12.9 | 89.5 ± 14.2 | 86.6 ± 15.7 | 89.4 ± 13.9 | 90.3 ± 15.5 | 85.7 ± 13.8 |
| BMI (kg/m2) | 33.9 ± 5.9 | 34.5 ± 5.3 | 31.1 ± 3.1 | 33.8 ± 5.4 | 32.5 ± 4.8 | 34.1 ± 5.2 | 34.2 ± 5.4 | 32.5 ± 4.5 |
| Abdominal circumference (cm) | 114.0 ± 15.4 | 103.9 ± 10.5 | 103.3 ± 9.2 | 110.0 ± 15.7 | 108.7 ± 13.5 | 106.8 ± 13.2 | 109.0 ± 13.8 | 106.5 ± 12.8 |
| Race/ethnicity | ||||||||
| African American/Black | 1 (10%) | 2 (20%) | 1 (10%) | 2 (20%) | 2 (10%) | 4 (20%) | 3 (15%) | 3 (15%) |
| White | 9 (90%) | 8 (80%) | 8 (80%) | 7 (70%) | 17 (85%) | 15 (75%) | 17 (85%) | 15 (75%) |
| Latino, Hispanic or Spanish and other/mixed | 0 (0%) | 0 (0%) | 1 (10%) | 1 (10%) | 1 (5%) | 1 (5%) | 0 (0%) | 2 (10%) |
| Any activities of daily living difficulty | 8 (80%) | 5 (50%) | 8 (80%) | 8 (80%) | 16 (80%) | 13 (65%) | 13 (65%) | 16 (80%) |
| Men | n = 12 | n = 12 | n = 12 | n = 12 | n = 24 | n = 24 | n = 24 | n =24 |
| Age (years) | 69.5 ± 3.5 | 69.7 ± 2.8 | 69.5 ± 3.9 | 70.5 ± 4.7 | 69.5 ± 3.6 | 70.1 ± 3.8 | 69.6 ± 3.1 | 70.0 ± 4.2 |
| Height (cm) | 177.8 ± 5.8 | 178.0 ± 4.6 | 179.0 ± 5.6 | 175.4 ± 6.8 | 178.4 ± 5.6 | 176.7 ± 5.8 | 177.9 ± 5.1 | 177.2 ± 6.4 |
| Body mass (kg) | 106.0 ± 14.2 | 98.1 ± 9.8 | 106.1 ± 15.0 | 97.3 ± 13.2 | 106.1 ± 14.3 | 97.7 ± 11.4 | 102.1 ± 12.6 | 101.7 ± 14.5 |
| BMI (kg/m2) | 33.6 ± 4.6 | 31.0 ± 2.8 | 33.2 ± 5.1 | 31.5 ± 2.8 | 33.4 ± 4.7 | 31.2 ± 2.8 | 32.3 ± 4.0 | 32.4 ± 4.1 |
| Abdominal circumference (cm) | 114.4 ± 12.8 | 110.4 ± 7.9 | 116.3 ± 11.4 | 126.0 ± 47.5 | 115.3 ± 11.9 | 117.0 ± 36.1 | 112.5 ± 10.7 | 119.6 ± 35.4 |
| Race/ethnicity | ||||||||
| African American/Black | 1 (8%) | 0 (0%) | 1 (8.3%) | 0 (0%) | 2 (8.3%) | 0 (0%) | 1 (4.2%) | 1 (4.2%) |
| White | 11 (91.7%) | 12 (100%) | 11 (91.7%) | 12 (100%) | 22 (91.7%) | 24 (100%) | 23 (95.8%) | 23 (95.8%) |
| Latino, Hispanic or Spanish and other/mixed | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Any activities of daily living difficulty | 9 (75%) | 5 (41.7%) | 7 (58.3%) | 5 (41.7%) | 16 (66.7%) | 10 (41.7%) | 14 (58.3%) | 12 (50.0%) |
Across treatment groups, men lost 6.6% and women lost 6.5% of initial body weight, over 16 weeks. In both men and women there was a significant loss of both fat mass (−13.9% in men, −9.7% in women, P < 0.05) and lean mass (−3.3% in men, −4.1% in women, P < 0.05). Overall, the volume of all regional fat depots significantly decreased, with the greatest percent loss from the abdominal visceral compartment (−16.3% in women, −18.8% in men), and the lowest percent loss from the pericardial depot (−6.4% in women, −6.8% in men). Measures of body composition at baseline and follow-up, and the adjusted estimates (95% confidence interval) of change (from baseline) are shown for men and women, collapsed over treatment, in Supplementary Table S1 online.
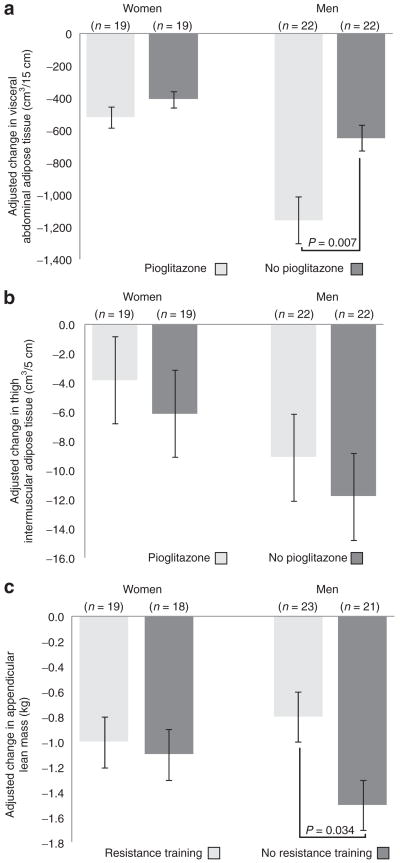
Men receiving pioglitazone lost more abdominal visceral fat compared to men who were not given pioglitazone and men who resistance-trained lost less appendicular lean mass compared to men who did not (Figure 2). Men who were given pioglitazone lost more thigh muscle volume, compared to men who were not given pioglitazone (−82 vs. −47 cm3; P = 0.026). Among women, there was no significant effect of pioglitazone on change in abdominal VAT (P = 0.223) and no effect of resistance training on change in appendicular lean mass (P = 0.829). Women receiving pioglitazone lost less thigh subcutaneous fat compared to women who were not given pioglitazone (−104 vs. −298 cm3; P = 0.002). However, there was no effect of pioglitazone on any other measure of body composition in women. Men and women randomized to resistance training lost less thigh muscle volume compared to those who did not resistance train (men, −43 vs. −88 cm3, P = 0.005; women, −34 vs. −59 cm3, P = 0.040). The baseline and follow-up values (adjusted for baseline) for all measures of body composition in men and women and the adjusted between group differences for the main effects are presented in Table 2.
Figure 2.
Adjusted mean (s.e.m.) 16-week change in (a) visceral abdominal adipose tissue a and (b) thigh intermuscular adipose tissue, according to randomization to pioglitazone treatment a; and (c) appendicular lean mass according to randomization to resistance training.b aAdjusted for resistance training and baseline measure. b Adjusted for pioglitazone treatment and baseline measure.
Table 2.
Baseline and 16-week change in measures of body composition following weight loss in nondiabetic men and women, according to main intervention arm
| Pioglitazone | No Pioglitazone | Resistance Training | No Resistance Training | |
|---|---|---|---|---|
| Women | n = 19 | n = 19 | n = 19 | n = 18 |
| Abdominal visceral adipose tissue (cm3/15 cm) | ||||
| Baseline | 2,875 (246) | 2,829 (246) | 2,885 (278) | 2,819 (208) |
| Adjusted change from baselinea | −521 (64) | −407 (66) | −506 (64) | −424 (66) |
| Between group difference in changea,b (LS mean (95% CI)) | −114 (−301, 73) | −82 (−269, 105) | ||
| Appendicular lean mass (kg) | ||||
| Baseline | 20.8 (0.7) | 20.0 (0.8) | 20.1 (0.7) | 20.7 (0.8) |
| Adjusted change from baselinea | −1.02 (0.2) | −1.04 (0.2) | −1.00 (0.2) | −1.06 (0.2) |
| Between group difference in changea,b (LS mean (95% CI)) | 0.02 (−0.6, 0.7) | 0.06 (−0.6, 0.7) | ||
| Total mass (kg) | ||||
| Baseline | 91.6 (3.5) | 87.0 (3.0) | 88.1 (3.6) | 90.5 (3.0) |
| Adjusted change from baselinea | −5.1 (0.7) | −6.5 (0.7) | −6.1 (0.7) | −5.4 (0.7) |
| Between group difference in changea,b (LS mean (95% CI)) | 1.3 (−0.8, 3.5) | −0.7 (−2.9, 1.4) | ||
| Fat mass (kg) | ||||
| Baseline | 40.6 (2.0) | 37.0 (1.8) | 38.2 (2.2) | 39.4 (1.7) |
| Adjusted change from baselinea | −3.2 (0.5) | −4.4 (0.5) | −4.2 (0.5) | −3.3 (0.5) |
| Between group difference in changea,b (LS mean (95% CI)) | 1.1 (−0.4, 2.7) | −0.8 (−2.4, 0.7) | ||
| Abdominal subcutaneous adipose tissue (cm3/15 cm) | ||||
| Baseline | 5,672 (351) | 5,109 (335) | 5,271 (345) | 5,510 (352) |
| Adjusted change from baselinea | −568 (97) | −765 (100) | −677 (96.4) | −650 (99) |
| Between group difference in changea,b (LS mean (95% CI)) | 196.1 (−90.3, 482.6) | −27.6 (−309.3, 254.1) | ||
| Thigh intermuscular adipose tissue (cm3/5 cm) | ||||
| Baseline | 56.7 (6) | 66.8 (11.6) | 58.6 (8.5) | 65 (9.9) |
| Adjusted change from baselinea | −3.8 (3.3) | −6.1 (3.4) | −6.4 (3.3) | −3.4 (3.4) |
| Between group difference in changea,b (LS mean (95% CI)) | 2.3 (−7.4, 12.0) | −3.0 (−12.6, 6.6) | ||
| Thigh subcutaneous adipose tissue (cm3/5 cm) | ||||
| Baseline | 2,110 (133) | 1,972 (104) | 1,978 (136) | 2,104 (100) |
| Adjusted change from baselinea | −104 (41) | −298 (42) | −230 (41) | −166 (42) |
| Between group difference in changea,b (LS mean (95% CI)) | 194 (75,313) | −65 (−184,55) | ||
| Pericardial adipose tissue (cm3/4.5 cm) | ||||
| Baseline | 101.5 (7.4) | 102.3 (9.1) | 113.1 (9.6) | 90.8 (5.6) |
| Adjusted change from baselinea | −5.6 (1.9) | −7.4 (1.9) | −6.6 (1.9) | −6.4 (2) |
| Between group difference in changea,b (LS mean (95%CI)) | 1.8 (−3.7, 7.3) | −0.2 (−5.9, 5.4) | ||
| Lean mass (kg) | ||||
| Baseline | 48.8 (1.6) | 47.8 (1.6) | 47.7 (1.6) | 48.9 (1.5) |
| Adjusted change from baselinea | −1.9 (0.3) | −2.1 (0.4) | −2.0 (0.3) | −2.0 (0.4) |
| Between group difference in changea,b (LS mean (95% CI)) | 0.1 (−0.9, 1.1) | 0.1 (−1.0, 1.1) | ||
| Thigh muscle volume (cm3/5 cm) | ||||
| Baseline | 1,115 (33) | 1,074 (36) | 1,089 (35) | 1,200 (34) |
| Adjusted change from baselinea | −56 (8) | −35 (9) | −34 (8) | −59 (9) |
| Between group difference in changea,b (LS mean (95%CI)) | −21 (−46, 3) | 25 (1, 49) | ||
| Left Leg muscle attenuation (HU) | ||||
| Baseline | 48.8 (1.2) | 47.6 (2.1) | 48.8 (1.9) | 47.6 (1.6) |
| Adjusted change from baselinea | −2.9 (1.1) | −0.5 (1.2) | −0.9 (1.1) | −2.6 (1.2) |
| Between group difference in changea,b (LS mean (95% CI)) | −2.3 (−5.7, 1) | 1.7 (−1.7, 5.0) | ||
| Right leg muscle attenuation (HU) | ||||
| Baseline | 48.9 (1.2) | 48.5 (1.9) | 49.1 (1.7) | 48.4 (1.5) |
| Adjusted change from baselinea | −2.9 (0.9) | −0.4 (0.9) | −1.1 (0.9) | −2.3 (0.9) |
| Between group difference in changea,b (LS mean (95% CI)) | −2.5 (−5.1, 0.1) | 1.2 (−1.3, 3.8) | ||
| Liver attenuation (HU) | ||||
| Baseline | 56.3 (2.7) | 58.7 (2.8) | 57.8 (2.7) | 57.2 (2.9) |
| Adjusted change from baselinea | 6.4 (1.5) | 2.9 (1.5) | 4.8 (1.5) | 4.6 (1.5) |
| Between group difference in changea,b (LS mean (95% CI)) | 3.6 (−0.8, 8.0) | 0.1 (−4.2, 4.5) | ||
| Men | n = 22 | n = 22 | n = 23 | n = 21 |
| Abdominal visceral adipose tissue (cm3/15 cm) | ||||
| Baseline | 4,856 (264) | 4,758 (206) | 4,901 (270) | 4,713 (197) |
| Adjusted change from baselinea | −1,160 (128) | −647 (128) | −850 (125) | −963 (131) |
| Between group difference in changea,b (LS mean (95% CI)) | −513 (−880, −146) | 112 (−255, 479) | ||
| Appendicular lean mass (kg) | ||||
| Baseline | 30.0 (0.6) | 29.4 (0.8) | 30.1 (0.7) | 29.2 (0.7) |
| Adjusted change from baselinea | −1.1 (0.2) | −1.2 (0.2) | −0.8 (0.2) | −1.5 (0.2) |
| Between group difference in changea,b (LS mean (95% CI)) | 0.2 (−0.4, 0.8) | 0.7 (0.1, 1.3) | ||
| Total mass (kg) | ||||
| Baseline | 103.0 (2.5) | 102.6 (3.0) | 106.6 (2.9) | 99.0 (2.4) |
| Adjusted change from baselinea | −7.2 (0.9) | −6.1 (0.9) | −5.6 (0.9) | −7.9 (1.0) |
| Between group difference in changea,b (LS mean (95% CI)) | −1.1 (−3.8, 1.6) | 2.3 (−5.1, 5.1) | ||
| Fat mass (kg) | ||||
| Baseline | 32.0 (1.8) | 33.0 (2.0) | 35.6 (2.2) | 29.5 (1.2) |
| Adjusted change from baselinea | −5.3 (0.6) | −3.7 (0.6) | −4.2 (0.6) | −4.9 (0.7) |
| Between group difference in changea,b (LS mean (95% CI)) | −1.5 (−3.4, 0.3) | 0.7 (−1.3, 2.6) | ||
| Abdominal subcutaneous adipose tissue (cm3/15 cm) | ||||
| Baseline | 3,952 (308) | 3,911 (364) | 4,369 (387) | 3,495 (247) |
| Adjusted change from baselinea | −546 (101) | −494 (101) | −414 (101) | −636 (105) |
| Between group difference in changea,b (LS mean (95% CI)) | −52 (−341, 237) | 222 (−78, 522) | ||
| Thigh intermuscular adipose tissue (cm3/5 cm) | ||||
| Baseline | 60.4 (5.6) | 71.5 (10.7) | 71.1 (7.2) | 60.9 (9.7) |
| Adjusted change from baselinea | −9.1 (2.7) | −11.8 (2.7) | −8.9 (2.7) | −12.1 (2.8) |
| Between group difference in changea,b (LS mean (95% CI)) | 2.7 (−5.1, 10.5) | 3.1 (−4.7, 11) | ||
| Thigh subcutaneous adipose tissue (cm3/5 cm) | ||||
| Baseline | 1,034 (93) | 1,129 (98) | 1,241 (114) | 922 (57) |
| Adjusted change from baselinea | −110 (26) | −136 (26) | −117 (26) | −130 (27) |
| Between group difference in changea,b (LS mean (95% CI)) | 27 (−47, 100) | 13 (−64, 90) | ||
| Pericardial adipose tissue (cm3/4.5 cm) | ||||
| Baseline | 139.9 (11) | 150.5 (6.7) | 155.6 (11.2) | 134.8 (5.8) |
| Adjusted change from baselinea | −14.1 (4.5) | −5.4 (4.5) | −5.7 (4.4) | −14.3 (4.6) |
| Between group difference in changea,b (LS mean (95% CI)) | −8.7 (−21.6, 4.1) | 8.6 (−4.6, 21.7) | ||
| Lean mass (kg) | ||||
| Baseline | 68.0 (1.3) | 66.9 (1.5) | 68.1 (1.3) | 66.7 (1.5) |
| Adjusted change from baselinea | −2.0 (0.4) | −2.5 (0.4) | −1.5 (0.4) | −2.9 (0.4) |
| Between group difference in changea,b (LS mean (95% CI)) | 0.5 (−0.7, 1.7) | 1.4 (0.2, 2.7) | ||
| Thigh muscle volume (cm3/5 cm) | ||||
| Baseline | 1,607 (40) | 1,546 (41) | 1,570 (40) | 1,583 (42) |
| Adjusted change from baselinea | −82 (11) | −47 (11) | −43 (10) | −88 (11) |
| Between group difference in changea,b (LS mean (95% CI)) | −35 (−66, −5) | 45 (15, 75) | ||
| Left leg muscle attenuation (HU) | ||||
| Baseline | 50.4 (1.2) | 50.4 (1.2) | 50 (1.4) | 50.8 (1.0) |
| Adjusted change from baselinea | 3.3 (1.0) | 0.9 (1) | 2.9 (1.0) | 1.2 (1.0) |
| Between group difference in changea,b (LS mean (95% CI)) | 2.4 (−0.4, 5.3) | 1.7 (−1.1, 4.6) | ||
| Right leg muscle attenuation (HU) | ||||
| Baseline | 49.3 (1.2) | 49.4 (1.1) | 48.7 (1.4) | 50 (0.9) |
| Adjusted change from baselinea | 2.8 (1.0) | 0.2 (1.0) | 2.5 (1.0) | 0.4 (1.0) |
| Between group difference in changea,b (LS mean (95% CI)) | 2.5 (−0.2, 5.3) | 2.1 (−0.7, 4.9) | ||
| Liver attenuation (HU) | ||||
| Baseline | 61.8 (1.4) | 55.7 (2.4) | 60.7 (1.4) | 56.8 (2.5) |
| Adjusted change from baselinea | −1.7 (1.4) | −0.8 (1.4) | −1.3 (1.4) | −1.2 (1.4) |
| Between group difference in changea,b (LS mean (95% CI)) | −0.9 (−5.1, 3.3) | −0.2 (−4.2, 3.8) | ||
CI, confidence interval; HU, Hounsfield units; LS, least squares.
Measures of change and between group differences are adjusted for main-effects and baseline measure.
A negative number indicates greater loss in the treatment group; a positive number indicates less loss in the treatment group.
DISCUSSION
In older overweight and obese nondiabetic men and women, participation in a hypocaloric weight-loss program reduced overall fat mass, as well as adipose tissue volumes across multiple fat compartments. The percent of fat loss was greatest from the abdominal visceral compartment in men and women, but we also observed significant, although proportionally lower, losses from the abdominal subcutaneous, thigh intermuscular, thigh subcutaneous, and pericardial compartments.
Although changes in ectopic fat depots following weight loss are reported, most have studied adults younger than our participants and none have assessed all the depots we did (8,9,25,28). A recent ancillary analysis of the Look AHEAD (Action For Health in Diabetes) trial (mean age = 60 years), determined the effect of a 1-year lifestyle weight-loss intervention on abdominal, thigh, and hepatic adipose tissue in 58 type 2 diabetics and also found the greatest reduction to be from the visceral abdominal compartment in both sexes, accompanied by significant reductions in the abdominal subcutaneous, hepatic, and superficial and subfascial thigh adipose tissue as well (28). The men and women in this study lost more weight than our participants (12.2% of initial body weight in men, 8.2% in women), which may be due to the longer intervention. Santanasto et al. (2010) recently compared the 6-month change in abdominal and skeletal muscle adipose tissue of older adults (mean age 70 years) randomized to a weight-loss intervention to those not randomized to a weight-loss intervention, and found the greatest reduction to be from the abdominal visceral compartment, with significant reductions in abdominal subcutaneous and thigh muscle adipose tissue areas among participants in weight-loss intervention group (29). Neither of these studies (28,29) reported pericardial fat loss; although pericardial fat loss during weight loss is reported (8,9), it has not been so in older men and women specifically. Our results confirm and build upon the available studies because we demonstrated the volume of fat in multiple depots, including pericardial and intermuscular, decreases during weight loss in nondiabetic men and women 65 years and older.
Our data suggest that in older overweight men, a 6.6% weight loss corresponds to an 18.8% loss of visceral abdominal fat and a 6.8% loss of pericardial fat, while in women an overall weight loss of 6.5% corresponds to a 16.3% loss of visceral abdominal fat and a 6.4% loss of pericardial fat. In older adults, a 10–25% loss of visceral abdominal fat has been shown to improve metabolic and cardiovascular outcomes (30–32). In moderately obese middle-aged men, a loss of 8–17% of epicardial fat was associated with improved insulin sensitivity and systolic blood pressure (8,33). Generally, abdominal visceral fat is preferentially lost during weight loss, compared to the abdominal subcutaneous and epicardial depots (8,30). In older men and women, even a modest weight loss can reduce visceral abdominal adiposity to a degree that has been associated with improved cardiometabolic health outcomes. A greater weight loss may need to be achieved to reduce epicardial fat to the amount associated with improved clinical outcomes. However, the therapeutic significance of reducing pericardial or epicardial fat remains obscure (34).
Intermuscular fat is an important determinant of physical function in older men and women (7,19), and it is plausible that an alternate mechanism by which weight loss improves physical performance is through loss of intermuscular fat. In longitudinal analyses of the Health ABC (Health Aging, and Body Composition) study, muscle fat infiltration strongly predicted muscle weakness and limited mobility (19,35). Santanasto et al. (2010) reported a 5–6% reduction in total body weight, a 2.4% reduction in total body fat, a 12.9% reduction in total thigh muscle fat accompanied by significant improvement in physical function in older men and women randomized to the weight-loss intervention compared to those who were not. The 6-month change in thigh intermuscular adipose tissue area was inversely correlated with the change in physical performance as well (29). In this study both the weight-loss and nonweight-loss groups participated in a physical activity intervention comprised of aerobic, strength, balance and flexibility exercises. All of our study participants underwent dietary weight loss and we did not observe an added benefit of resistance training with respect to changes in thigh muscle fat or any other ectopic fat depots. Therefore, our results suggest weight loss is a key determinant of fat loss from intermuscular and other depots, compared to physical activity, as others have also observed (29).
In their weight-loss intervention trial Santanasto et al. also reported a significant loss of thigh muscle mass and knee extensor strength among participants randomized to their weight-loss intervention (in conjunction with a combined physical activity program), which did not appear to affect physical performance as measured by the short physical performance battery (29). Frimel et al. (2008) reported less appendicular muscle and total muscle loss in older adults undergoing weight loss who participated in a resistance training program that progressed up to 85% of one RM (14). Participation in our resistance training program, which progressed to 70% of one RM, reduced the loss of thigh muscle volume in men and women, and men who resistance-trained retained more appendicular lean mass and total lean mass during weight loss. Taken together these outcomes suggest resistance training is important to reducing lean tissue loss, and that less intensive resistance training that focuses on improving lower extremity power is an effective strategy to preserve lean tissue during weight loss in older adults.
During hypocaloric weight loss, treatment with the PPARγ-agonist pioglitazone decreased abdominal visceral fat in nondiabetic men, but did not significantly alter abdominal adipose tissue distribution in women. However, loss of subcutaneous fat in the thigh was attenuated in women taking pioglitazone. Previous studies have shown that PPARγ-agonist treatment decreases abdominal visceral fat but increases abdominal subcutaneous fat in type 2 diabetics (16,36,37). However, most studies assessing the effect of pioglitazone treatment on adipose tissue distribution have not differentiated visceral from subcutaneous fat in the extra-abdominal regions, and have not examined men and women separately. Men and women differ in adipose tissue loss from the visceral and subcutaneous compartments during weight-loss trials, which may partially explain why pioglitazone treatment had a differential effect on men and women in our study. Although others have reported that pioglitazone treatment (45 mg/day) lowers intramyocellular lipid content (38,39); the thigh intermuscular fat volume and leg muscle attenuation did not change in the men and women receiving pioglitazone in our study. This may be due, in part, to the lower dose (30 mg/day) of pioglitazone used in our study. Since the pericardial fat volume did not change in participants receiving pioglitazone, it may indicate that extra-abdominal visceral fat depots are not preferential targets of PPARγ-agonists. However, this is the first known assessment of the influence of a PPARγ-agonist on change in multiple visceral fat depots in nondiabetic men and women, so whether or not the response of adipose tissue to pioglitazone differs by anatomical location and/or by dose merits additional investigation.
The randomized-controlled 2 × 2 factorial design and the simultaneous measurements of abdominal, pericardial, and intermuscular adipose tissue and thigh muscle volumes using CT allowed for the efficient examination of the influences of a PPARγ-agonist and resistance training on region-specific changes in body composition during weight loss in older men and women, which is an important strength of our study. It is plausible that that the PPARγ-agonist and resistance training worked synergistically to reduce visceral abdominal adiposity and reduce muscle loss. Our study was powered to test for main effects and we would have only been able to detect interactions if the interaction effect size was very large.
Our examination of the overall effect of weight loss on region-specific adipose tissue loss was analyzed utilizing a trial designed to determine the effect of two interventions, following a 2 × 2 factorial design. We adjusted our analyses for intervention, and have demonstrated that hypocaloric weight loss reduces adipose tissue volume across multiple ectopic depots, using CT to quantify fat loss. This study was not powered to assess associations between fat loss and metabolic or functional outcomes, so the association between region-specific fat-loss and these outcomes merit additional investigation in future weight-loss intervention trials. Muscle attenuation was used to indicate fat infiltration in the thigh and abdominal muscle, and no direct measures of muscular lipid content were used for these analyses. However, CT attenuation has been shown to correlate with the myocellular lipid content measured from biopsy samples (40). Finally, due to our medical exclusion criteria, applicability of these findings to less healthy populations is limited.
Older adults are particularly vulnerable to the detrimental effects of weight gain, yet are not necessarily advised to lose weight, primarily due to concerns about loss of muscle or physical function. Increased ectopic fat accumulation is adversely associated with physical function (3), and weight-loss trials with functional outcomes are emerging (29). In order to reduce the risk of obesity-related disability and co-morbid diseases among older adults, it is important to identify intervention strategies that promote fat loss and reduce lean tissue loss. Our findings demonstrate weight-loss strategies that target fat-loss and reduce muscle loss in older adults are feasible and well-adhered to. Following hypocaloric weight loss, overall there was significant reduction in fat across multiple ectopic depots, including intermuscular and pericardial fat. Pioglitazone reduced abdominal visceral fat loss in nondiabetic older men, but did not appear to affect other ectopic fat depots or nondiabetic older women. Resistance training during weight loss reduced lean tissue loss, but we were unable to detect an effect on intermuscular fat or any depots measured. Together our findings provide the basis for the development of future trials to determine the benefit of similar interventions on physical function and disability-related outcomes in older adults.
Supplementary Material
Footnotes
Supplementary material is linked to the online version of the paper at http://www.nature.com/oby
DISCLOSURE
This work was supported by the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332) and Takeda Pharmaceuticals North America. The authors declared no conflict of interest.
References
- 1.Alley DE, Chang VW. The changing relationship of obesity and disability, 1988–2004. JAMA. 2007;298:2020–2027. doi: 10.1001/jama.298.17.2020. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Rejeski WJ, Marsh AP, Chmelo E, Rejeski JJ. Obesity, intentional weight loss and physical disability in older adults. Obes Rev. 2010;11:671–685. doi: 10.1111/j.1467-789X.2009.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang T, Streeper T, Cawthon P, et al. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010;21:543–559. doi: 10.1007/s00198-009-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lara-Castro C, Garvey WT. Intracellular lipid accumulation in liver and muscle and the insulin resistance syndrome. Endocrinol Metab Clin North Am. 2008;37:841–856. doi: 10.1016/j.ecl.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–856. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim MK, Tanaka K, Kim MJ, et al. Comparison of epicardial, abdominal and regional fat compartments in response to weight loss. Nutr Metab Cardiovasc Dis. 2009;19:760–766. doi: 10.1016/j.numecd.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Iacobellis G, Singh N, Wharton S, Sharma AM. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity (Silver Spring) 2008;16:1693–1697. doi: 10.1038/oby.2008.251. [DOI] [PubMed] [Google Scholar]
- 10.Ryan AS, Nicklas BJ, Berman DM. Aerobic exercise is necessary to improve glucose utilization with moderate weight loss in women. Obesity (Silver Spring) 2006;14:1064–1072. doi: 10.1038/oby.2006.122. [DOI] [PubMed] [Google Scholar]
- 11.Bales CW, Buhr G. Is obesity bad for older persons? A systematic review of the pros and cons of weight reduction in later life. J Am Med Dir Assoc. 2008;9:302–312. doi: 10.1016/j.jamda.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Morley JE. Weight loss in older persons: new therapeutic approaches. Curr Pharm Des. 2007;13:3637–3647. doi: 10.2174/138161207782794149. [DOI] [PubMed] [Google Scholar]
- 13.Sørensen TI. Weight loss causes increased mortality: pros. Obes Rev. 2003;4:3–7. doi: 10.1046/j.1467-789x.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- 14.Frimel TN, Sinacore DR, Villareal DT. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med Sci Sports Exerc. 2008;40:1213–1219. doi: 10.1249/MSS.0b013e31816a85ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henwood TR, Riek S, Taaffe DR. Strength versus muscle power-specific resistance training in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2008;63:83–91. doi: 10.1093/gerona/63.1.83. [DOI] [PubMed] [Google Scholar]
- 16.Miyazaki Y, Mahankali A, Matsuda M, et al. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2002;87:2784–2791. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]
- 17.Derosa G, Tinelli C, Maffioli P. Effects of pioglitazone and rosiglitazone combined with metformin on body weight in people with diabetes. Diabetes Obes Metab. 2009;11:1091–1099. doi: 10.1111/j.1463-1326.2009.01087.x. [DOI] [PubMed] [Google Scholar]
- 18.Gupta AK, Smith SR, Greenway FL, Bray GA. Pioglitazone treatment in type 2 diabetes mellitus when combined with portion control diet modifies the metabolic syndrome. Diabetes Obes Metab. 2009;11:330–337. doi: 10.1111/j.1463-1326.2008.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 20.Newman AB, Lee JS, Visser M, et al. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82:872–878. doi: 10.1093/ajcn/82.4.872. quiz 915. [DOI] [PubMed] [Google Scholar]
- 21.Visser M, Pahor M, Tylavsky F, et al. One- and two-year change in body composition as measured by DXA in a population-based cohort of older men and women. J Appl Physiol. 2003;94:2368–2374. doi: 10.1152/japplphysiol.00124.2002. [DOI] [PubMed] [Google Scholar]
- 22.National Institute of Health and National Heart Lung and Blood Institute. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. 1998. [Google Scholar]
- 23.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraemer WJ, Adams K, Cafarelli E, et al. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34:364–380. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 25.Nicklas BJ, Wang X, You T, et al. Effect of exercise intensity on abdominal fat loss during calorie restriction in overweight and obese postmenopausal women: a randomized, controlled trial. Am J Clin Nutr. 2009;89:1043–1052. doi: 10.3945/ajcn.2008.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carr JJ, Ding J. Response to “Epicardial and pericardial fat: close but very different”. Obesity (Silver Spring) 2009;17:626–627. doi: 10.1038/oby.2008.575. [DOI] [PubMed] [Google Scholar]
- 27.Iacobellis G. Epicardial and pericardial fat: close, but very different. Obesity (Silver Spring) 2009;17:625–627. doi: 10.1038/oby.2008.575. [DOI] [PubMed] [Google Scholar]
- 28.Albu JB, Heilbronn LK, Kelley DE, et al. Metabolic changes following a 1-year diet and exercise intervention in patients with type 2 diabetes. Diabetes. 2010;59:627–633. doi: 10.2337/db09-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santanasto AJ, Glynn NW, Newman MA, et al. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: a randomized clinical trial. J Obes. 2011 doi: 10.1155/2011/516576. e-pub ahead of print 10 October 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaston TB, Dixon JB. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes (Lond) 2008;32:619–628. doi: 10.1038/sj.ijo.0803761. [DOI] [PubMed] [Google Scholar]
- 31.Nicklas BJ, Dennis KE, Berman DM, et al. Lifestyle intervention of hypocaloric dieting and walking reduces abdominal obesity and improves coronary heart disease risk factors in obese, postmenopausal, African-American and Caucasian women. J Gerontol A Biol Sci Med Sci. 2003;58:181–189. doi: 10.1093/gerona/58.2.m181. [DOI] [PubMed] [Google Scholar]
- 32.Park HS, Lee K. Greater beneficial effects of visceral fat reduction compared with subcutaneous fat reduction on parameters of the metabolic syndrome: a study of weight reduction programmes in subjects with visceral and subcutaneous obesity. Diabet Med. 2005;22:266–272. doi: 10.1111/j.1464-5491.2004.01395.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim MK, Tomita T, Kim MJ, et al. Aerobic exercise training reduces epicardial fat in obese men. J Appl Physiol. 2009;106:5–11. doi: 10.1152/japplphysiol.90756.2008. [DOI] [PubMed] [Google Scholar]
- 34.Sacks HS. Weight loss in obesity reduces epicardial fat thickness; so what? J Appl Physiol. 2009;106:1–2. doi: 10.1152/japplphysiol.91396.2008. [DOI] [PubMed] [Google Scholar]
- 35.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 36.Kelly IE, Han TS, Walsh K, Lean ME. Effects of a thiazolidinedione compound on body fat and fat distribution of patients with type 2 diabetes. Diabetes Care. 1999;22:288–293. doi: 10.2337/diacare.22.2.288. [DOI] [PubMed] [Google Scholar]
- 37.Mori Y, Murakawa Y, Okada K, et al. Effect of troglitazone on body fat distribution in type 2 diabetic patients. Diabetes Care. 1999;22:908–912. doi: 10.2337/diacare.22.6.908. [DOI] [PubMed] [Google Scholar]
- 38.Rasouli N, Raue U, Miles LM, et al. Pioglitazone improves insulin sensitivity through reduction in muscle lipid and redistribution of lipid into adipose tissue. Am J Physiol Endocrinol Metab. 2005;288:E930–E934. doi: 10.1152/ajpendo.00522.2004. [DOI] [PubMed] [Google Scholar]
- 39.Thomas EL, Potter E, Tosi I, et al. Pioglitazone added to conventional lipid-lowering treatment in familial combined hyperlipidaemia improves parameters of metabolic control: relation to liver, muscle and regional body fat content. Atherosclerosis. 2007;195:e181–e190. doi: 10.1016/j.atherosclerosis.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 40.Larson-Meyer DE, Smith SR, Heilbronn LK, et al. Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity (Silver Spring) 2006;14:73–87. doi: 10.1038/oby.2006.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.