Abstract
BACKGROUND
Patients with rheumatoid arthritis (RA) are at increased risk for heart failure and left ventricular diastolic dysfunction (LVDD). BNP may be useful to screen for LVDD in the general population.
OBJECTIVE
We compared the effectiveness of B-type natriuretic peptide (BNP) as a screening tool for LVDD in RA and non-RA subjects without cardiovascular disease (CVD).
METHODS
Study subjects were recruited from population-based samples with and without RA, excluding subjects with CVD. LVDD was assessed by 2D/Doppler echocardiography and categorized as none, mild, moderate/severe or indeterminate. Linear regression and proportional odds models evaluated the association between LVDD and BNP adjusting for age, sex, and body mass index.
RESULTS
Among 231 RA and 1730 non-RA subjects without CVD, BNP was significantly higher in subjects with moderate/severe LVDD compared to those with no or mild LVDD (p= 0.02 for RA and p<0.001 for non-RA subjects). More RA subjects had elevated BNP than non-RA subjects (16% vs. 9%, p<0.001). Positive predictive value (25% in RA and 18% in non-RA) and sensitivity (40% in RA and 26% in non-RA) were similarly low in both cohorts, but specificity was significantly lower in RA than in non-RA (89% vs. 94%, p=0.02).
CONCLUSIONS
While RA subjects were more likely to have elevated BNP, few RA patients with elevated BNP actually have LVDD. Also, normal BNP levels are less likely to rule out LVDD in RA than in non-RA subjects. Thus, BNP may be less effective for screening in RA subjects compared to the general population.
Rheumatoid arthritis (RA) is a chronic, debilitating disease affecting ~1% of the population.(1) Patients with RA have an increased risk of cardiovascular disease (CVD), including a two-fold increased risk of heart failure (HF) compared to the subjects without RA.(2) In addition, patients with RA and HF are more likely to have preserved ejection fraction compared to HF patients without RA, and the HF in RA patients is less likely to be preceded by CVD.(3)Furthermore, RA patients have a higher prevalence (31%) of any left ventricular diastolic dysfunction (LVDD) compared to non-RA subjects (26%; age and sex adjusted).(4) Nonetheless, the prevalence of moderate/severe LVDD was similar in both groups (8% in RA compared to 11% in non-RA). Despite this, routine screening for LVDD in RA patients remains controversial due to expense and the lack of accurate screening tools, as well as efficacious therapies for LVDD.
B-type natriuretic peptide (BNP) is cardiovascular biomarker that is released primarily from the ventricles and the atrium in response to increases in volume or pressure.(5, 6) BNP is a useful adjunct to clinical assessment of patients with HF and has been suggested as a potential screening tool for LVDD in the general population.(7) However, the high false positive rate may lead to a high percentage of unnecessary echocardiograms. Indeed, using BNP to screen for LVDD would require echocardiograms in 12% of those screened, resulting in mostly negative echocardiograms (75%) and missing the majority (59%) of patients with LVDD. Further, whether it operates similarly in patients with RA is unknown. Indeed, few studies have examined the association between BNP and LVDD in patients with rheumatic disease. (8–10) The objective of this study was to compare the effectiveness of BNP as a screening tool for LVDD in RA patients and non-RA subjects without CVD.
METHODS
Study Subjects and Design
This community population-based study of residents of Olmsted County, Minnesota was conducted using the resources of the Rochester Epidemiology Project, a population-based medical records linkage system that allows ready access to the complete medical records from all community medical providers.(11) An incidence cohort of all residents of Olmsted County, Minnesota aged ≥18 years who first fulfilled 1987 American College of Rheumatology classification criteria for RA between 1/1/1980 and 12/31/2007 was identified.(1, 12) From among this incident RA cohort, we identified eligible RA subjects, namely those alive and living in Olmsted County. For this study, we recruited 231 (58%) of the 401 eligible RA subjects without CVD.
A cross-sectional study comparing these RA subjects to a previously identified cohort of subjects without RA who were randomly selected from the population of Olmsted County was performed.(13) From this random sample of the Olmsted County population, a total of 2042 subjects participated (47% of the eligible residents invited). Of these, 312 subjects were excluded due to prior CVD or RA. The institutional review boards of the Mayo Foundation and the Olmsted Medical Center approved this study. All subjects provided written informed consent prior to participation.
Data Collection
Study participation for subjects in both the RA and non-RA cohorts was identical except that RA subjects were asked additional questions pertaining to their RA disease. Subjects in both cohorts completed a cardiovascular risk factor and medication usage questionnaire, underwent an echocardiogram and a physical exam (including measurement of blood pressure, waist circumference, body mass index [BMI]) and provided a blood sample. Medical records were reviewed to ascertain diagnoses of CVD and hypertension. History of CVD was defined as presence of any of the following: angina pectoris, coronary artery disease, myocardial infarction, coronary revascularization procedures (i.e. bypass grafting, percutaneous coronary intervention), or heart failure.
In both cohorts, BNP and C-reactive protein were measured. BNP testing was performed using the fluorescence immunoassay by Biosite Diagnostics (San Diego, California). Age- and sex-specific reference values were used to define elevated BNP, as previously described.(14). For women these reference values range from 64 pg/mL at age 45 years to 167 pg/mL at age 84 years, and for men the reference values range from 35 pg/mL at age 45 years to 93 pg/mL at age 84 years. C-reactive protein testing was performed by immunoturbidimetric assay (Roche CRPLX reagent, Indianapolis, IN). For individuals in the RA cohort, rheumatoid factor, tumor necrosis factor alpha (TNF-alpha), interleukin 6 (IL-6) and creatinine levels were measured. Rheumatoid factor testing was performed by nephelometry (latex enhanced assay; Behring Nephelometer II, Dade Behring, Inc., Newark, DE). IL-6 and TNF-alpha tests were performed by enzyme immunoassay from R & D Systems (Minneapolis, MN). Creatinine testing was performed by modified Trinder reaction using a colorimetric indicator (Roche Diagnostics, Indianapolis, IN).
Two-dimensional and Doppler echocardiograms were performed on all subjects in the RA cohort following protocols identical to those used in the non-RA cohort, as previously described.(13) All echocardiograms were performed by registered diagnostic cardiac sonographers following standardized procedures and interpreted in the Mayo Clinic Echocardiographic Laboratory (by BLK and DDB). The following echocardiographic parameters were measured and/or estimated in each subject: pulmonary artery pressure, LV mass index, both end diastolic and end systolic LV diameters, and both septum and posterior LV wall thicknesses. Diastolic dysfunction was categorized as none (normal diastolic function); mild, defined as impaired relaxation without evidence of increased filling pressures; moderate, defined as impaired relaxation associated with moderate elevation of filling pressures or pseudonormal filling; and severe, defined as advanced reduction in compliance or reversible or fixed restrictive filling.(13) Diastolic function was classified as indeterminate when these criteria could not be fully assessed, such as in cases of dysrhythmias or immeasurable Doppler data, or (per protocol) when borderline Doppler parameters did not meet cutoff values.
Statistical Methods
Descriptive statistics were used to summarize the demographics of the RA and non-RA cohorts. Differences between the two cohorts were tested using Chi-square and rank sum tests. Linear regression models were used to assess differences in log-transformed BNP for RA compared to non-RA cohort adjusting for age, sex and BMI. Proportional odds models, a variation of logistic regression for ordinal outcomes, were used to evaluate the association between LVDD and BNP in the RA subjects. Receiver operator characteristic (ROC) analyses were used to assess the effectiveness of BNP as a screening tool for moderate/severe LVDD in the RA cohort. Subjects with indeterminate LVDD were excluded from these analyses. Tests between measures of effectiveness were performed assuming the proportions for the RA and non-RA cohorts were binomially distributed.
The optimal cutpoint was defined as the value that resulted in sensitivity and specificity closest in distance to the point of the perfect marker (i.e. sensitivity and specificity of 100%). Since BNP has age and sex specific reference values, the residual BNP value defined as the difference between each patient’s BNP value and the appropriate reference value were calculated, and the optimal cutpoint for the residual values was estimated. This allows estimation of an optimal cutpoint value that would maintain the age and sex specific structure of the reference values.
RESULTS
The study included 231 RA subjects without CVD, who were compared to 1730 non-RA subjects without CVD. Baseline characteristics for both cohorts are reported in Table 1. The RA subjects (mean age [SD] 58.6 [13.0] years) were significantly younger than the non-RA subjects (mean age [SD] 61.3 [10.1] years), and a higher percentage of the RA cohort were women compared to the non-RA cohort (75% vs. 55%, respectively, p<0.001). In addition, a higher percentage of the RA cohort was non-white compared to the non-RA cohort (p<0.001). BMI, smoking and C-reactive protein were similar in both cohorts. RA patients were more likely to be hypertensive or using antihypertensive medications, and their pulmonary artery pressures were higher compared to non-RA subjects (p<0.001 for both). Few patients had abnormal ejection fraction in either cohort (1% in RA vs. 3% in non-RA; p=0.11). Among the RA subjects, 68% were rheumatoid factor positive and the mean RA disease duration was 8.8 (SD 6.2) years. BNP was higher in the RA cohort compared to the non-RA cohort. More RA subjects had elevated BNP (above the age- and sex-specific upper limit of normal values) than non-RA subjects (16% vs. 9%, respectively, p<0.001).
Table 1.
Characteristics of 231 rheumatoid arthritis (RA) and 1730 non-RA subjects without cardiovascular disease.
| Characteristic | RA (n = 231) | Non-RA (n = 1,730) | p-value |
|---|---|---|---|
| Age, years, mean ± SD | 58.6 ± 13.0 | 61.3 ± 10.1 | 0.005 |
| Female | 173 (75) | 947 (55) | <0.001 |
| White race | 215 (93) | 1690 (98) | <0.001 |
| Smoking (current or former) | 104 (46) | 547 (44) | 0.50 |
| BMI, kg/m2, mean ± SD | 28.6 ± 5.8 | 28.3 ± 5.1 | 0.62 |
| C-reactive protein, mg/L, median (IQR) | 2.1 (0.8, 4.6) | 1.9 (0.8, 4.2) | 0.46 |
| BNP, pg/mL, median (IQR) | 33 (17, 58) | 19 (7, 42) | <0.001 |
| Elevated (age- and sex-specific) | 38 (16) | 154 (9) | <0.001 |
| Diagnosis of hypertension/use of anti-hypertensive medication | 138 (60) | 648 (37) | <0.001 |
| Pulmonary artery pressure, mmHg, mean ± SD | 29.1 ± 5.7 | 22.4 ± 4.8 | <0.001 |
| Ejection fraction < 50% | 2 (1) | 45 (3) | 0.11 |
| Rheumatoid factor positivity | 156 (68) | -- | -- |
| RA disease duration, years, mean ± SD | 8.8 ± 6.2 | -- | -- |
Values are n (%) unless otherwise specified. BMI = body mass index; SD = standard deviation; IQR = interquartile range.
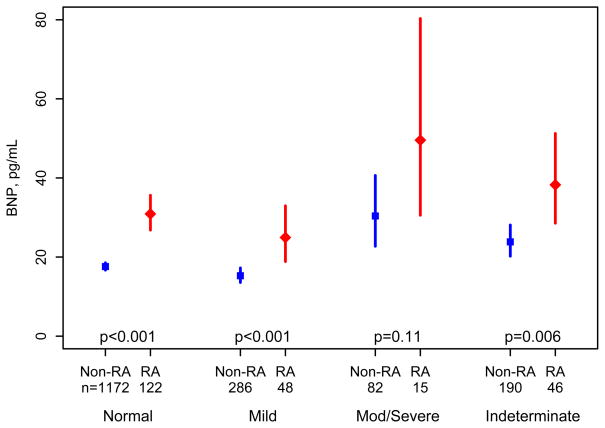
Among the RA subjects, 122 (53%) had normal, 48 (21%) mild, 15 (6.5%) moderate/severe LVDD and 46 were indeterminate. Among the non-RA subjects, 1172 (68%) had normal, 286 (17%) mild, 82 (4.7%) moderate/severe LVDD and 190 were indeterminate. BNP was significantly higher in subjects with moderate/severe LVDD compared to those with no or mild LVDD (p= 0.02 for RA and p<0.001 for non RA subjects). Figure 1 compares the BNP levels in RA subjects and non-RA subjects within each category of LVDD (none, mild, moderate/severe or indeterminate) adjusted for age, sex and BMI. Displayed values in Figure 1 are means and 95% confidence intervals obtained from linear regression models of log-transformed BNP values. RA subjects without LVDD had higher BNP than non-RA subjects without LVDD (fold change 1.9; p<0.001). Similarly, BNP was higher in RA compared to non-RA subjects with mild LVDD (fold change 1.9; p<0.001) and with moderate/severe LVDD (fold change 1.9; p = 0.11).
Figure 1.
Comparison of B-type Natriuretic Peptide (BNP) in patients with rheumatoid arthritis (RA) compared to non-RA subjects according to categories of left ventricular diastolic dysfunction (none, mild, moderate/severe or indeterminate) adjusted for age, sex and body mass index. Displayed values are means and 95% confidence intervals obtained from linear regression models of log-transformed BNP values.
Among RA subjects, the association between BNP and LVDD was examined. RA subjects with elevated BNP were more likely to have LVDD (odds ratio [OR] 2.5; 95% confidence interval [CI]: 1.02, 6.0 adjusted for age, sex, race and BMI) as compared to those with normal BNP. This association persisted after adjustment for C-reactive protein, TNF-alpha, IL-6, rheumatoid factor positivity, creatinine, RA disease duration, and hypertension/use of antihypertensive medications (OR 2.6; 95% CI: 1.01, 6.7).
The effectiveness of BNP as a screening tool for moderate/severe diastolic dysfunction was examined (Table 2). The sensitivity was low in both cohorts (40% in RA and 26% in non-RA), which means many of the subjects with high BNP did not have LVDD. The specificity was significantly lower in the RA cohort compared to the non-RA cohort (89% vs. 94%, respectively; p=0.02). This means fewer RA subjects with normal BNP have normal diastolic function. The low positive predictive value (25%) indicates use of BNP as a screening tool for LVDD would result in a large number of normal echocardiograms.
Table 2.
Implications for use of B-type Natriuretic Peptide (BNP) to screen for moderate/severe preclinical diastolic dysfunction in rheumatoid arthritis (RA) and non-RA subjects
| Measure | RA | Non-RA | p-value |
|---|---|---|---|
| Sensitivity, % | 40 | 26 | 0.26 |
| Specificity, % | 89 | 94 | 0.02 |
| Positive predictive value, % | 25 | 18 | 0.52 |
| Negative predictive value, % | 94 | 96 | 0.43 |
The optimal cutpoint for BNP was estimated in the RA subjects and was found to be identical to the age and sex specific reference values for BNP in the general population. Furthermore, while the positive predictive value increased with higher levels of BNP for non-RA subjects (13% for BNP values 1–2 times the reference value and 38% for BNP values > 2 times the reference value), it appears to rise sooner and plateau in the RA subjects (24% for BNP value 1–2 times the reference value and 25% for BNP values > 2 times the reference value). Therefore, increasing the BNP reference values for RA subjects will not improve the effectiveness of this test as a screening tool for predicting LVDD.
The associations between BNP and LV diameters/wall thicknesses were also examined. LV mass index was lower among RA subjects compared to non-RA subjects (p<0.001). Both the end diastolic LV diameter (mean 47 [sd 4] mm in RA vs. 49 [5] mm in non-RA) and the LV septal wall thickness (mean 9 [sd 2] mm in RA vs. 11 [2] mm in non-RA) were smaller among RA subjects than non-RA subjects (p<0.001 for both). There were no differences in LV end systolic diameter (mean 29 [sd 4] mm in RA and in non-RA; p=0.89) or LV posterior wall thickness (mean 9 [sd 2] mm in RA and 10 [1] mm in non-RA; p=0.15) among RA subjects compared to no-RA subjects. While BNP was positively correlated with LV mass index among non-RA subjects (p=0.043), no association between LV mass index and BNP was noted among RA subjects (p=0.99). In other words, BNP was more strongly associated with LV mass index in non-RA patients compared to RA subjects (interaction p=0.033). BNP was weakly correlated with both LV diameters and both LV wall thicknesses (Spearman correlation coefficients ranged from −0.06 to −0.14). These correlations did not differ for RA subjects compared to non-RA subjects (interaction p>0.7 for all).
Due to the higher percentage of non-white subjects in the RA cohort, the analyses were repeated using only white subjects. However, no differences in results were found.
DISCUSSION
Among subjects without clinical CVD, RA subjects were more likely to have elevated BNP than non-RA subjects. RA subjects with abnormal BNP were 2.5 times more likely to have LVDD compared to those with normal BNP, even after adjustment for RA characteristics, including inflammatory markers (OR: 2.6; 95% CI: 1.01; 6.7). This suggests BNP may be a useful marker of LVDD in RA subjects without CVD. However, the positive predictive value of BNP to detect LVDD was poor and the specificity was statistically significantly lower among RA subjects than among non-RA subjects. Thus, few RA patients with elevated BNP levels actually have LVDD and normal BNP levels are less likely to rule out LVDD in RA compared to non RA subjects.
While no other studies have compared the effectiveness of BNP for prediction of LVDD in subjects with and without RA, other studies have shown findings similar to ours for BNP and NT-proBNP in subjects with RA. Note that BNP and NT-proBNP are closely correlated (r=0.9; p<0.001).(15) Two other studies compared BNP/NT-proBNP levels in RA subjects and controls and found higher BNP/NT-proBNP levels in RA subjects than in controls. (9, 10) In addition, these study and others found significant correlations of NT-proBNP levels in RA subjects with clinical and laboratory measures of RA disease activity, even after adjustment for CVD and cardiovascular risk factors.(8, 16) These findings are in contrast to our study, where BNP levels in RA subjects were significantly higher than those in non-RA subjects, but C-reactive protein levels were similar in both groups (as the majority of RA subjects have established RA with low disease activity).
Two studies also included echocardiographic assessment and reported BNP/NT-proBNP levels were significantly higher in RA subjects with measures indicative of preclinical CVD. (8, 10) Several letters suggested BNP/NT-proBNP may be useful for screening RA patients for CVD prior to initiating treatment with Cox-2 inhibitors. (10, 17, 18) In addition, a recent study suggests NT-proBNP levels decrease in RA patients following initiation of TNF-alpha blockade treatment, and these changes correlated with changes in erythrocyte sedimentation rate.(19)
Because BNP is primarily produced in atrial and ventricular myocytes, it is thought to be almost exclusively a cardiac biomarker and is not commonly regarded as an inflammatory marker.(20) Studies in other patient populations have suggested that inflammation, as well as infections, sepsis, chronic obstructive pulmonary disease, renal failure and other clinical conditions, influence BNP levels in the absence of myocardial dysfunction.(21–25) Thus, elevated BNP in RA patients may not indicate CVD. In fact, the elevated BNP in patients with RA could result from right atrial dilatation from pulmonary hypertension, which is more common in patients with RA. This agrees with our findings that the screening effectiveness of BNP for prediction of LVDD in RA patients is worse than in the general population. In addition to assessing cardiac function in RA patients with elevated BNP, physicians should consider the possibility of alternative explanations for elevated BNP.
Furthermore, natriuretic peptides may play a significant role in modulating the immune and endocrine systems.(26–28) Natriuretic peptide receptors are expressed by immune cells, such as macrophages, dendritic cells and T lymphocytes. Changes in multiple subsystems, including the immune and endocrine systems, have been implicated in the aging process.(29) These findings along with the unexplained higher levels of BNP in women and increases in BNP with age, lead to conjecture that the higher BNP levels in RA patients may be associated with accelerated aging.(14, 30)
We previously investigated the hypothesis of accelerated aging in patients with RA and found the mortality experience of patients with RA was consistent with an accelerated aging of 2 years at the incidence of RA and a rate of 11.4 years for each 10 years thereafter.(30) Using these acceleration rates, we examined whether accelerated aging could explain the elevation in BNP among RA subjects compared to non-RA subjects. The difference in mean BNP between cohorts adjusting for age and sex was estimated using actual ages and effective ages based on estimated acceleration rates for the RA cohort. Comparing these differences revealed accelerated aging could only explain 16% of the increase in BNP in the RA cohort. Thus, the elevation in BNP among RA patients is largely due to other, as yet unknown, factors.
Strengths of our study include its population-based design with a sizable RA cohort (>200 subjects) and a large population-based comparison cohort (>1000 subjects). In addition, comprehensive review of all inpatient and outpatient medical records from the community ensured accurate assessment of CVD without recall bias. A limitation of this study is that only 58% of eligible subjects agreed to participate in the study. However, the participation rate among RA subjects was similar to that in the non-RA cohort, as were comparisons of participation biases which revealed that participants were less likely to be smokers and were better educated than non-participants, so participation bias is unlikely to have had a substantial effect on the comparisons between the cohorts.(31) Also, a higher percentage of the RA cohort was non-white compared to the non-RA cohort. However, analyses restricted to white subjects revealed the same results as the full cohort. Finally, the population of Olmsted County, Minnesota is predominantly white, so the results of this study may not be generalizable to other more diverse populations.
In conclusion, BNP levels were higher in RA patients compared to non-RA patients and abnormal BNP was associated with LVDD in RA subjects, suggesting BNP may be a useful biomarker for LVDD in RA patients. However, BNP is less useful as a screening test in RA patients when compared to the general population, largely because of its significantly lower specificity and poor positive predictive value. While this suggests BNP will likely be of limited clinical utility for the detection of LVDD in RA, further research is needed to determine whether BNP could be useful for risk stratification as part of a multi-marker risk tool. Finally, due to the poor performance of BNP in RA patients, physicians should consider alternate possibilities to explain elevated BNP in RA patients and should not rely solely on normal BNP to rule out CVD in these patients.
Acknowledgments
The authors acknowledge Cynthia Stoppel and Konnie Bicknese for recruiting patients and Margaret Donohue, RN, Julie Gingras, RN, Denise Herman, RN, Constance Neuman, RN, and Diane Wilke, RN for performing data abstraction.
Funding: This work was partially funded by grants from the National Institutes of Health, NIAMS (R01 AR46849) and NHLBI (R01 HL55502) and made possible by the Rochester Epidemiology Project (R01 AG034676 from the National Institute on Aging).
References
- 1.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the Incidence of Rheumatoid Arthritis Rising? Results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62(6):1576–82. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicola PJ, Maradit-Kremers H, Roger VL, Jacobsen SJ, Crowson CS, Ballman KV, et al. The risk of congestive heart failure in rheumatoid arthritis: A population-based study over 46 years. Arthritis Rheum. 2005;52(2):412–420. doi: 10.1002/art.20855. [DOI] [PubMed] [Google Scholar]
- 3.Davis JM, 3rd, Roger VL, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum. 2008;58(9):2603–11. doi: 10.1002/art.23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang KP, Myasoedova E, Crowson CS, Davis JM, 3rd, Roger VL, Karon BL, et al. Increased Prevalence of Diastolic Dysfunction in Rheumatoid Arthritis. Ann Rheum Dis. 2010 doi: 10.1136/ard.2009.124362:NIHMS202134. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munagala VK, Burnett JC, Jr, Redfield MM. The natriuretic peptides in cardiovascular medicine. Curr Probl Cardiol. 2004;29(12):707–69. doi: 10.1016/j.cpcardiol.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Kinnunen P, Vuolteenaho O, Ruskoaho H. Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: effect of stretching. Endocrinology. 1993;132(5):1961–70. doi: 10.1210/endo.132.5.8477647. [DOI] [PubMed] [Google Scholar]
- 7.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide to detect preclinical ventricular systolic or diastolic dysfunction: a community-based study. Circulation. 2004;109(25):3176–81. doi: 10.1161/01.CIR.0000130845.38133.8F. [DOI] [PubMed] [Google Scholar]
- 8.Giannoni A, Tani C, Clerico A, Passino C, Tavoni A, d’Ascanio A, et al. When the heart is burning: Amino-terminal pro-brain natriuretic peptide as an early marker of cardiac involvement in active autoimmune rheumatic disease. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 9.Solus J, Chung CP, Oeser A, Avalos I, Gebretsadik T, Shintani A, et al. Amino-terminal fragment of the prohormone brain-type natriuretic peptide in rheumatoid arthritis. Arthritis Rheum. 2008;58(9):2662–9. doi: 10.1002/art.23796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harney SM, Timperley J, Daly C, Harin A, James T, Brown MA, et al. Brain natriuretic peptide is a potentially useful screening tool for the detection of cardiovascular disease in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65(1):136. doi: 10.1136/ard.2005.040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maradit Kremers H, Crowson CS, Gabriel SE. Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases. Rheum Dis Clin North Am. 2004;30(4):819–34. vii. doi: 10.1016/j.rdc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis & Rheumatism. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 13.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of Systolic and Diastolic Ventricular Dysfunction in the Community: Appreciating the Scope of the Heart Failure Epidemic. JAMA. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 14.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide concentration: impact of age and gender. Journal of the American College of Cardiology. 2002;40(5):976–82. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 15.Richards M, Nicholls MG, Espiner EA, Lainchbury JG, Troughton RW, Elliott J, et al. Comparison of B-type natriuretic peptides for assessment of cardiac function and prognosis in stable ischemic heart disease. J Am Coll Cardiol. 2006;47(1):52–60. doi: 10.1016/j.jacc.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 16.Provan SA, Angel K, Odegard S, Mowinckel P, Atar D, Kvien TK. The association between disease activity and NT-proBNP in 238 patients with rheumatoid arthritis: a 10-year longitudinal study. Arthritis Res Ther. 2008;10(3):R70. doi: 10.1186/ar2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haupl T, Burmester GR, Giannitsis E, Rohrlach T, Spanuth E, Parsch H, et al. N-terminal prohormone brain natriuretic peptide: a biomarker for detecting cardiovascular risks in patients with rheumatoid arthritis or osteoarthritis? Ann Rheum Dis. 2007;66(6):838–9. doi: 10.1136/ard.2006.066522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giannitsis E. Rationale for testing the cardiovascular risk for patients with COX-2 inhibitors on the basis of biomarker NT-proBNP. Clin Lab. 2005;51(1–2):63–83. [PubMed] [Google Scholar]
- 19.Peters MJ, Welsh P, McInnes IB, Wolbink GJ, Dijkmans BA, Sattar N, et al. TNF{alpha} blockade therapy reduces circulating NT-proBNP levels in RA patients with active disease: results from prospective cohort study. Ann Rheum Dis. 2009 doi: 10.1136/ard.2009.119412. [DOI] [PubMed] [Google Scholar]
- 20.Corson MA. Emerging inflammatory markers for assessing coronary heart disease risk. Curr Cardiol Rep. 2009;11(6):452–9. doi: 10.1007/s11886-009-0065-1. [DOI] [PubMed] [Google Scholar]
- 21.Brueckmann M, Huhle G, Lang S, Haase KK, Bertsch T, Weiss C, et al. Prognostic value of plasma N-terminal pro-brain natriuretic peptide in patients with severe sepsis. Circulation. 2005;112(4):527–34. doi: 10.1161/CIRCULATIONAHA.104.472050. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Li C. Prognostic significance of brain natriuretic peptide obtained in the ED in patients with SIRS or sepsis. Am J Emerg Med. 2009;27(6):701–6. doi: 10.1016/j.ajem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Vela-Zarate P, Varon J. BNP this, BNP that... Now in sepsis? Am J Emerg Med. 2009;27(6):707–8. doi: 10.1016/j.ajem.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 24.Piechota M, Barylski M, Hannam S, Mikhailidis DP, Rysz J, Banach M. Natriuretic peptides in septic patients. Curr Med Chem. 2009;16(30):4020–31. doi: 10.2174/092986709789352330. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs LH, van de Kerkhof JJ, Mingels AM, Passos VL, Kleijnen VW, Mazairac AH, et al. Inflammation, overhydration and cardiac biomarkers in haemodialysis patients: a longitudinal study. Nephrol Dial Transplant. 25(1):243–8. doi: 10.1093/ndt/gfp417. [DOI] [PubMed] [Google Scholar]
- 26.Casserly BP, Sears EH, Gartman EJ. The Role of Natriuretic Peptides in Inflammation and Immunity. Recent Pat Inflamm Allergy Drug Discov. doi: 10.2174/187221310791163125. [DOI] [PubMed] [Google Scholar]
- 27.Shaw SM, Fildes JE, Puchalka CM, Basith M, Yonan N, Williams SG. BNP directly immunoregulates the innate immune system of cardiac transplant recipients in vitro. Transpl Immunol. 2009;20(3):199–202. doi: 10.1016/j.trim.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Omland T, Hagve TA. Natriuretic peptides: physiologic and analytic considerations. Heart Fail Clin. 2009;5(4):471–87. doi: 10.1016/j.hfc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Paganelli R, Di Iorio A, Cherubini A, Lauretani F, Mussi C, Volpato S, et al. Frailty of older age: the role of the endocrine--immune interaction. Curr Pharm Des. 2006;12(24):3147–59. doi: 10.2174/138161206777947533. [DOI] [PubMed] [Google Scholar]
- 30.Crowson CS, Liang KP, Therneau TM, Kremers HM, Gabriel SE. Could accelerated aging explain the excess mortality in patients with seropositive rheumatoid arthritis? Arthritis Rheum. 2010;62(2):378–82. doi: 10.1002/art.27194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobsen SJ, Mahoney DW, Redfield MM, Bailey KR, Burnett JC, Jr, Rodeheffer RJ. Participation bias in a population-based echocardiography study. Ann Epidemiol. 2004;14(8):579–84. doi: 10.1016/j.annepidem.2003.11.001. [DOI] [PubMed] [Google Scholar]