Abstract
Aims
Cytokines released by the immune cells at the site of plaque milieu induce smooth muscle cell apoptosis to promote plaque instability. But, neuropeptide Y (NPY), a pleotropic factor, may modulate the effects of cytokines in atherosclerotic plaques of patients with carotid stenosis. Our aim was to investigate the relative expression of NPY-Y1, NPY-Y2 and NPY-Y5 receptors on carotid plaque vascular smooth muscle cells (pVSMCs) of symptomatic (S) and asymptomatic (AS) patients and examine the effect of inflammatory cytokines on the expression of NPY receptors, that may attenuate plaque rupture.
Methods and Results
In healthy carotid artery, there was significantly increased immunopositivity and increased mRNA transcripts of NPY-Y1 and NPY-Y5 receptors in thin sections and isolated VSMCs, respectively, compared to S and AS plaques. However, the NPY-Y2 expression was higher in S and AS pVSMCs than controls. Stimulation of the cells with TNF-α, IL-12 or IFN-γ (50 ng/ml) decreased mRNA transcripts of NPY-Y1 and NPY-Y5 and increased NPY-Y2 mRNAs in VSMCs of healthy carotid artery. The effect of the cytokines on mRNA transcripts of NPY-Y5 and NPY-Y2 in pVSMCs of S and AS patients was similar to healthy VSMCs, but with variable effect on NPY-Y1.
Conclusion
Increased expression of NPY-Y2 receptors in symptomatic pVSMCs than in healthy and asymptomatic subjects suggests a potential role of NPY-Y2 in plaque instability. This is further supported by the pronounced effect of atheroma-associated cytokines to increase NPY-Y2 mRNA transcripts in pVSMCs of patients with carotid stenosis.
Keywords: Atheromatous cytokines, Atherosclerosis, Carotid plaque, Carotid stenosis, Neuropeptide Y, Smooth muscle cells
1. Introduction
Atherosclerosis is an inflammatory disease characterized by endothelial dysfunction, vascular inflammation, and the deposition of lipids, cholesterol, calcium, and cellular debris within the intima of the vessel wall (Vukovic et al., 2006). The plaque deposition and their subsequent rupture may lead to acute and chronic luminal obstruction, abnormalities of blood flow, and diminished oxygen supply to target organs. The cells involved in the atherosclerotic response, including macrophages and T-lymphocytes, secrete inflammatory cytokines such as tumour necrosis factor-alpha (TNF-α) (Branen et al., 2004; Hansson et al., 2002), Interleukin-12 (IL-12) and Interferon- γ (IFN-γ) (Tedgui and Mallat, 2006). Interaction between these cell types, cytokines and the connective tissue appears to determine the development and rupture of plaque.
Many vessels that are occluded by atherosclerotic plaques are richly innervated by sympathetic nerve fibers. The role of the sympathetic nerves in atherosclerosis and restenosis is established but believed to be indirectly involved via vasoconstrictive effects, stimulation of platelet aggregation, or insulin resistance (Li et al., 2005). Neuropeptide Y (NPY), a 36-amino-acid residue peptide, is the most prominent neuropeptide in the sympathetic nervous system, and is usually present in sympathetic neurons innervating blood vessels (Nilsson and Edvinsson, 2000). NPY is critical in the migration and proliferation of various cells in atherosclerotic plaque and in the induction of immune-mediated inflammation. In mammals, NPY acts through a series of G-protein coupled receptors (Balasubramaniam, 2002), Y1, Y2, Y3, Y4, Y5 and Y6, and are modified by the modifying enzyme dipeptidyl transferase IV, which cleaves NPY1-36 to an Y2/Y5 receptor preferring peptide, NPY3-36. Elevated circulating NPY levels were observed in patients with cardiovascular disease such as acute myocardial infarction, angina pectoris, heart failure and hypertension where sympathetic nerve activity is increased (Malmstrom, 2002). Li et al (Li et al., 2003) reported increased expression of Y1, Y2 and Y5 receptors in carotid artery segments 14 days after angioplasty. Moreover, they could effectively inhibit neointima formation and medial thickening in the angioplasty by intravenous treatment of Y1 or Y5 receptor antagonists, indicating the clinical importance of NPY in regulating vessel function.
The cytokine signaling in the presence of NPY can modify their effect on vascular cell function, which may promote lesion expansion or alternatively retard progression, thus regulating the vulnerability of a plaque. The present study aims to investigate the relative expression of NPY-Y1, NPY-Y2 and NPY-Y5 receptors on carotid plaque vascular smooth muscle cells (pVSMCs) of symptomatic (S) and asymptomatic (AS) patients and examine the effect of atheroma-associated inflammatory cytokines on expression of NPY receptors on symptomatic and asymptomatic carotid plaque derived VSMCs. Both ex vivo and in vitro approaches were followed to examine the effect of inflammatory cytokines on the expression and activity of NPY receptors in carotid plaque VSMCs of S and AS patients.
Materials and Methods
Carotid endarterectomy specimen and smooth muscle cell culture
Human carotid plaque specimens were obtained from 12 patients undergoing carotid endarterectomy procedures from Nebraska Heart Hospital, Lincoln, Nebraska. The carotid endarterectomy specimens were categorized as either symptomatic (S) or asymptomatic (AS) according to patients' history and clinical examination. Symptoms included hemispheric transient ischemic attacks, amaurosis fugax or stroke (Jia et al., 2006). Three normal carotid arteries were collected from Nebraska Organ Retrieval System. The specimens were transported to the laboratory within 2-3h in University of Wisconsin solution. Part of the section was fixed in formaldehyde and processed for paraffin sections. For cell culture, VSMCs were isolated from the plaques by a method previously reported from our laboratory(Dhume and Agrawal, 2003). Briefly, endothelial and adventitial layer of the specimens were scraped off and the medial layer was homogenized, washed in serum free DMEM (GibcoBRL, Grand Island, NY, USA) and was treated with Trypsin (0.025%) for 30 minutes at 37°C. The tissue was again washed with serum free medium and was incubated with 0.1% collagenase solution for 3 h. After washing with serum free DMEM, pellet was resuspended in Smooth muscle cell medium (ScienCell, Carlsbad, CA, USA) and filtered using 100μm filter. The cells after washing was seeded on to 25cm2 culture plates and was maintained at 5% CO2 at 37 °C. Nonatherosclerotic carotid artery specimens were obtained from patients (n = 6) were used as negative control samples and were manipulated like the atherosclerotic plaque specimens. The cells from second to fourth passage were used for the studies. The cultured cells showed characteristic hill and valley morphology. The cell purity was identified by positive staining for α-smooth muscle actin and calponin.
Histology and Immunohistochemistry
Sections of 5μm thickness were cut from paraffin blocks using a microtome (Tissue-Tek, Miles Scientific Inc., Bohemia, NY) and mounted on Superfrost/Plus microscope slides (Fisher Scientific, Pittsburg, PA, USA). The sections were stained using NPY-Y1, Y2 and Y5 antibodies (SantaCruz biotechnology, SantaCruz, CA, USA) using the Vectastain ABC reagent (Vector Laboratories, Burlingame, CA, USA). Briefly, the sections were treated with 3% hydrogen peroxidase in methanol for 20 min to quench the endogenous peroxidase activity. The slides were then washed with PBS followed by blocking with normal blocking serum for 20 min. The sections were incubated with primary antibody (1:200 dilution) in blocking buffer overnight at 4°C. After washing with PBS, the tissue was incubated with biotinylated secondary antibody for 30 min. The tissues were washed with PBS and incubated with ABC reagent for 30min. The tissues were further washed with PBS and immunoreactivity was detected using diaminobenzidine/hydrogen peroxide for 2 minutes and a hematoxylin nuclear counter stain. The slides were washed and fixed using mounting medium and was observed using an Olympus inverted microscope using the bright field (Olympus BX51, St. Louis, MO, USA). Negative controls of sections were run without incubation with the primary antibody.
RNA isolation, Reverse Transcription and Real Time PCR
The symptomatic and asymptomatic pVSMCs were stimulated with 50ng/ml TNF-alpha, IL-12 and IFN-γ for 12h. The respective unstimulated cells were used as the control. The total RNA was isolated using the Trizol reagent (Sigma, St. Louis, MO, USA) method. The yield of RNA was quantified using Genequant 1300 (GE Healthcare, Pittsburgh, PA, USA). First-strand cDNA was synthesized using 1 μg total RNA with oligo dT (1μg), 5 X reaction buffer, MgCl2, dNTP mix and Improm II reverse transcriptase as per Improm II reverse transcription kit (Promega, Madison, WI, USA). Following the first strand synthesis, Real time PCR was done using 8μl cDNA, 10 μl SYBR green PCR master mix (Applied biosystems, Carlsbad, CA, USA) and forward and reverse primers (10 pmol/μl) (Integrated DNA Technologies, USA) using a 7500 Real Time PCR system (Applied biosystems, Carlsbad, CA, USA). The primer sequences used were GAPDH Forward – 5′ – GGGAAGGTGAAGGTCGGAGT -3′, GAPDH Reverse - 5′-TTGAGGTCAATGAAGGGGTCA-3′; NPY-Y1 R Forward -5′-TTGCTGTGATTTGGGTCCTTGCTG-3′, NPY-Y1 R Reverse 5′-TGAGGTGGCAGAGCAGGAATAACA-3′; NPY-Y2 R Forward 5′-TCCTGGAGCTGCAAATGACCACTA-3′, NPY-Y2 R Reverse 5′-TAGCCTTGAATGTCACGGACACCT-3′; NPY-Y5 R Forward 5′-TTGCTGGATCAGTGGATGTTTGGC-3′, NPY-Y5 R Reverse 5′-AGTTTCACCTGAGGCCCACTCTTT-3′. The specificity of the primers was analyzed by running a melting curve. The PCR cycling conditions used were 5 min at 95 °C for initial denaturation, followed by 45 cycles of 45sec at 95 °C, 45 s at 55 °C and 45 sec at 72 °C. Each real-time PCR was carried out in triplicates and the threshold cycle values were averaged. Calculations of relative gene expression were based on the differences in the threshold cycles. The fold change in expression between samples was calculated by fold change= 2 −ΔΔCt method. The results were normalised against glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Statistical analysis of real-time quantitative PCR results was performed using Single factor ANOVA.
Results
NPY Receptor immunostaining in normal carotid artery, symptomatic and asymptomatic carotid plaques
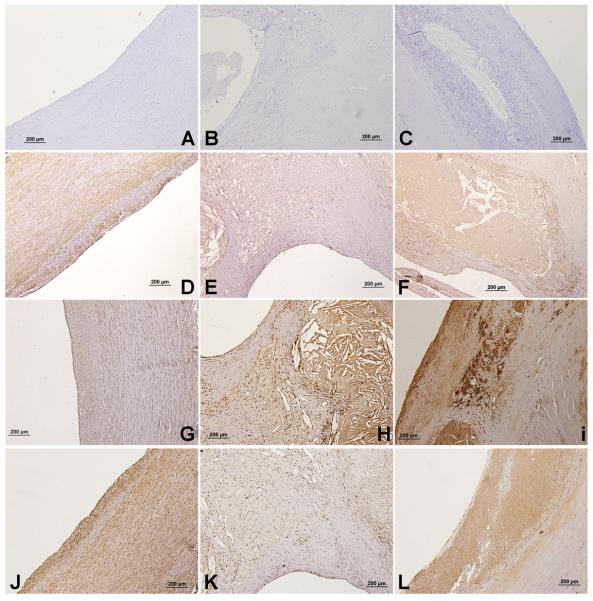
There was expression of NPY-Y1, Y2 and Y5 receptors in normal, S and AS arteries. The NPY-Y1 expression was greater in the SMCs of normal artery (Fig. 1D) compared to AS (Fig. 1E) and S (Fig. 1F) plaques. However, there was increased staining of NPY-Y1 in the lesion areas in the carotid plaques (Fig. 1F) where there are more macrophages. The SMCs in the surrounding tissue to the necrotic core showed less staining for NPY-Y1. A similar expression profile was seen for NPY-Y5. There was an increased expression of NPY-Y5 in the SMCs of normal arteries (Fig. 1J) when compared to AS and S plaques. Symptomatic carotid plaques showed increased staining for Y1 and Y5 (Fig. 1F&L) compared to AS (Fig. 1E&K). NPY-Y2 immunopositivity was significantly higher in S plaques (Fig. 1I) compared to AS (Fig. 1H) and normal arteries (Fig. 1G). Negative controls for the normal artery (Fig. 1A), AS (Fig. 1B) and S (Fig. 1C) plaques showed no background expression.
Figure 1. NPY-Y1, NPY-Y2 and NPY-Y5 immunostaining in sections of normal carotid artery, symptomatic and asymptomatic carotid plaques.
The type of artery and receptor are, A) Negative control – Normal artery, B) Negative control – Asymptomatic, C) Negative control – Symptomatic, D) NPY-Y1- Normal artery, E) NPY-Y1- Asymptomatic, F) NPY-Y1- Symptomatic, G) NPY-Y2 - Normal artery, H) NPY-Y2- Asymptomatic, I) NPY-Y2- Symptomatic, J) NPY-Y5- Normal artery, K) NPY-Y5- Asymptomatic, L) NPY-Y5- Symptomatic.
Basal mRNA expression of NPY receptors in healthy carotid artery and plaque SMCs
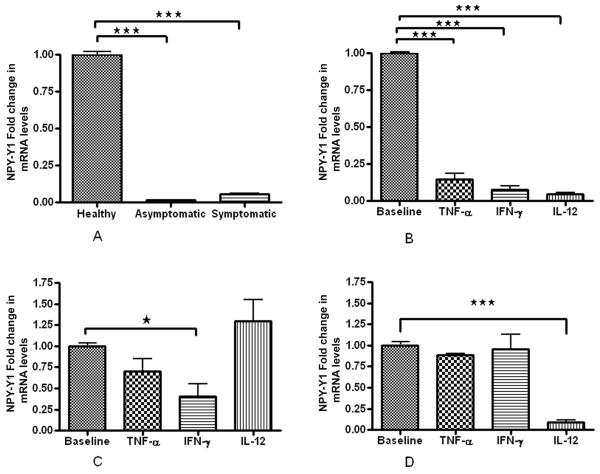
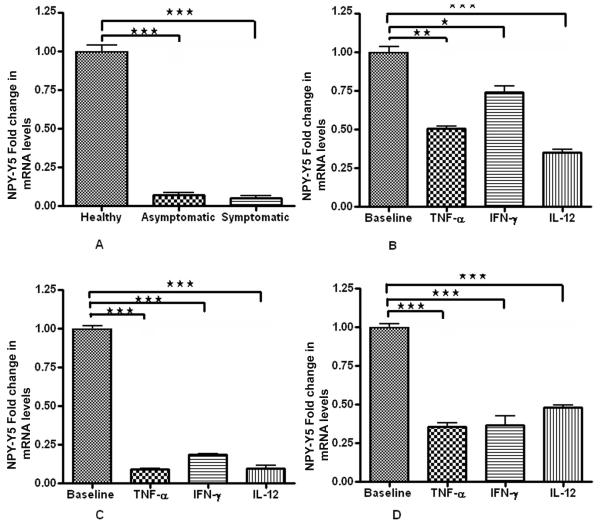
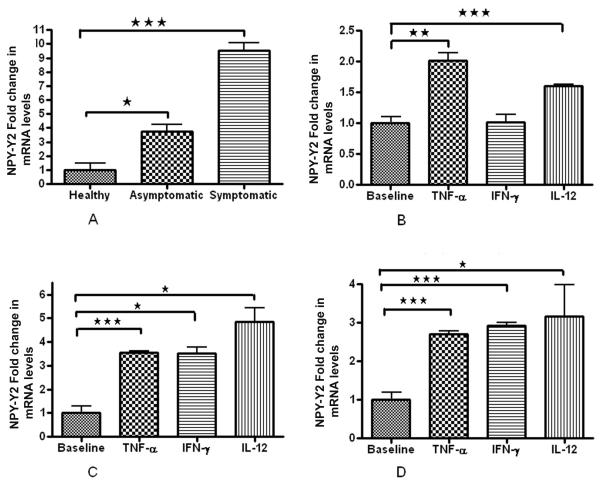
We found significant decrease in mRNA expression of both NPY-Y1 and NPY-Y5 receptors in plaque SMCs than control healthy SMCs. NPY-Y1 expression was higher (2 fold) in S compared to AS (Fig. 2A). There was no significant difference in the NPY-Y5 basal expression levels in AS and S pVSMCs (Fig. 4A). Interestingly, NPY-Y2 mRNA expression was 10 times greater in SMCs of symptomatic plaque than healthy carotid artery. Also there was 3 times more NPY-Y2 mRNA expression in the SMCs of symptomatic than asymptomatic plaque (Fig. 3A).
Figure 2. NPY-Y1 R mRNA expression.
(A) Basal levels of NPY-Y1 mRNA expression in healthy VSMCs, AS and S pVSMCs. (B) Fold change in mRNA expression levels of NPY-Y1 receptor in normal carotid artery vascular smooth muscle cells (VSMCs) (C) asymptomatic and (D) symptomatic carotid plaque derived VSMCswhen stimulated with inflammatory cytokines TNF-α , IL-12 and IFN-γ, compared to the corresponding non-stimulated cells. (*** represents p<0.0001, ** represents p<0.001, n =6).
Figure 4. NPY-Y5 R mRNA expression.
(A) Basal levels of NPY-Y1 mRNA expression in healthy VSMCs, AS and S pVSMCs. (B) Fold change in mRNA expression levels of NPY-Y5 receptor in normal carotid artery vascular smooth muscle cells (VSMCs) (C) asymptomatic and (D) symptomatic carotid plaque derived VSMCs when stimulated with inflammatory cytokines TNF-α, IL-12 and IFN-γ, compared to the corresponding non-stimulated cells. (*** represents p<0.0001, ** represents p<0.001, n=6).
Figure 3. NPY-Y2 R mRNA expression.
(A) Basal levels of NPY-Y1 mRNA expression in healthy VSMCs, AS and S pVSMCs. (B) Fold change in mRNA expression levels of NPY-Y2 receptor in normal carotid artery vascular smooth muscle cells (VSMCs) (C) asymptomatic and (D) symptomatic carotid plaque derived VSMCs when stimulated with inflammatory cytokines TNF-α , IL-12 and IFN-γ, compared to the corresponding non-stimulated cells. (*** represents p<0.0001, ** represents p<0.001, n=6).
Effect of inflammatory cytokines on NPY-Y1 receptors in healthy carotid artery SMCs and pVSMCs
Atheroma associated cytokines, TNF-α, IFN-γ and IL-12 (50ng/ml each), significantly decreased NPY-Y1 receptor mRNA expression in human carotid artery SMCs (Fig. 2B). There was no significant change in the NPY-Y1 expression in AS pVSMCs when the cells were stimulated with TNF-α and IL-12(Fig. 2C). But NPY-Y1 expression decreased after stimulation with IFN-γ (Fig. 2C). In S pVSMCs, IL-12 significantly decreased the mRNA transcripts of NPY-Y1 receptors (Fig. 2D). IL-12 had no significant effect on NPY-Y1 receptors in asymptomatic plaque SMCs.
Effect of inflammatory cytokines on NPY-Y2 receptors in healthy carotid artery SMCs and pVSMCs
In normal VSMCs inflammatory cytokines significantly increased NPY-Y2 receptors TNF-α (2.1 fold) and IL-12 (1.5 fold), but the change was not significant with IFN-γ (Fig. 3B). In both AS (Fig. 3C) and S VSMCs (Fig. 3D) the NPY-Y2 R expression increased significantly when exposed to inflammatory cytokines compared to their respective untreated controls. The cytokine-induced increased NPY-Y2 expression was more elevated in asymptomatic VSMCs compared to symptomatic.
Effect of inflammatory cytokines on NPY-Y5 receptors in healthy carotid artery SMCs and pVSMCs
In normal VSMCs, treatment with inflammatory cytokines decreased the NPY-Y5 R expression significantly (Fig. 4B). In symptomatic and asymptomatic pVSMCs there was a significant fold decrease in NPY-Y5 expression when exposed to TNF- α, IFN- γ and IL-12 (50ng/ml) (Fig. 4C, 4D).
Discussion
The interactions between NPY and the inflammatory cytokines released in an atherosclerotic milieu may play a major role in the regulation of plaque destabilization and rupture. NPY, a sympathetic neurotransmitter involved in the regulation of cardiovascular system, is a vasoconstrictor as well as a potent mitogenic and angiogenic factor (Lee et al., 2003a). NPY is released after more intense and prolonged nerve activation unlike epinephrine (Chronwall and Zukowska, 2004). Majority of the studies have shown that the fibrous cap of symptomatic atherosclerotic plaques is thinner and inflammation is more common, with greater number of macrophages and T-lymphocytes, but lesser amount of SMCs and collagen (Golledge et al., 2000), although the necrotic core of the plaques appears to be similar in both. Earlier investigations from our laboratory showed increased expression of IL-12 and IFN-γ in symptomatic plaques compared to that of asymptomatic plaques (Jia et al., 2007). Hence, it is likely that the VSMCs in the S and AS atherosclerotic milieu may interact differently to the NPY in the presence of atheroma associated cytokines. In the current study we provide evidence that smooth muscle cells in symptomatic and asymptomatic carotid plaques express NPY receptors and show differential expression of NPY receptors upon inflammatory cytokine stimulation which may consequently influence cellularity and rupture of the plaque.
In the present study we observed a lower basal level of NPY-Y1 and NPY-Y5 receptor expression in both AS and S plaques compared to the normal VSMCs. The NPY receptors, Y1 and Y5 mediate NPY's mitogenic effects. Activation of Y1 and Y5 receptors dose-dependently stimulated mitogenesis in primary rat aortic vascular smooth muscle cells in vitro, and an NPY-Y1 and Y5 receptor agonist cocktail could effectively block the mitogenic effects of NPY(Pons et al., 2003). In another study, the treatment with Y1 and/or Y5 receptor antagonists significantly decreased neointima formation by 50% due to angioplasty alone, and completely prevented the occlusive pro-atherosclerotic effects of NPY (Li et al., 2003). Since NPY-Y1 and Y5 receptors are associated with the mitogenesis of VSMCs the decrease expression of these receptors might be due to the lesser number of mitotic smooth muscle cells in the carotid plaque environment. The increased inflammation in the plaque sites decreased the smooth muscle cell proliferation.
The NPY-Y1 expression of healthy VSMCs decreased significantly due to the decrease in proliferation of VSMCs or apoptosis of cells after cytokine stimulation. Although, the stimulation of the cells with TNF-α and IFN-γ did not significantly affect Y1 expression in the S and AS pVSMCs, IL-12 significantly decreased the NPY-Y1 expression in symptomatic carotid plaque VSMCs. This might be due to the decreased presence of IL-12 receptors on the SMCs of asymptomatic plaques, but there are no reported studies on this aspect. Further studies, on the expression of IL-12 receptors in S and AS plaques are warranted to further answer the cause of this differential expression.
Recent studies using the Y2 receptor knockout mice showed that the knockouts have significantly diminished NPY-induced angiogenesis, both in the aortic sprouting and in the ischemic limb model compared with wild-type mice (Lee et al., 2003a), suggesting a major role for the Y2 receptor in NPY-mediated angiogenesis. Previously, Lee et al (Zukowska-Grojec et al., 1998) have found that NPY-Y2 receptor is not expressed or is expressed at lower levels in intact vessels or non growing endothelial cells and vascular smooth muscle cells. In the present study also, we observed a low expression of NPY-Y2 receptors in healthy VSMCs and artery sections. However, when these cells were subjected to NPY, injury or ischemia the expression of Y2 receptors is markedly upregulated (Lee et al., 2003a; Lee et al., 2003b). This data support our findings which showed a higher basal expression level of NPY-Y2 in the plaque sections. The S plaques showed higher expression levels of NPY-Y2 in comparison to asymptomatic plaques. Earlier studies have proved that patients with symptomatic atherosclerosis had a denser network of vasa vasorum than patients with asymptomatic disease. Microvessel density of vasa vasorum (blood vessels per mm2 adventitia) was higher in iliac, carotid, and renal arteries of S patients (Fleiner et al., 2004). Gene expression analysis comparing symptomatic and asymptomatic plaques showed increased abundance of 31 genes known to promote angiogenesis in symptomatic plaques (Tureyen et al., 2006). Hence the increased NPY-Y2 expression might be attributed to the increased angiogenesis in symptomatic plaques compared to asymptomatic plaques. NPY stimulated formation of long, thick sprouts resembling normal vessels in rat aortic rings embedded in collagen (Zukowska-Grojec et al., 1998). NPY also induces the expression of other growth factors such as basic fibroblast growth factor (bFGF) and vascular endothelial growth factor. In the present study, inflammatory cytokines increased the NPY-Y2 expression in healthy, AS and S VSMCs. The change was obvious in AS (3.5 -5 fold increase) and S VSMCs (3 fold increase). Surprisingly, the cytokine stimulation further increased the NPY-Y2 expression in S VSMCs although the basal expression levels were high. This shows that in the presence of cytokines and NPY there is a high chance for increased in vivo angiogenesis in asymptomatic carotid plaques. The fold increase in S VSMCs was lower since the cells have been already exposed to a cytokine stimulated environment in vivo. Hence the effect was more evident in AS smooth muscle cells.
Previous studies have shown that Y5 receptors were not expressed in intact vessels or non growing cells but can be induced in both endothelial and vascular smooth muscle cells following their ischemia or injury. On the contrary, we have observed Y5 receptor expression in normal artery sections as well as healthy VSMCs in culture. Y5 receptors share some properties with Y1 as well as Y2 receptors. Y1 and Y5 receptor antagonists similarly inhibit proliferation of vascular smooth muscle cells in vitro, as well as in vivo by preventing angioplasty-induced neointima formation in the rat carotid artery (Li et al., 2003). Furthermore, in the rat aortic sprouting assay, Y2 and Y5 receptor antagonists equally inhibited sprout formation (Movafagh et al., 2006).
The basal levels of NPY-Y5 expression was less in S and AS VSMCs compared to the healthy VSMCs. Since Y1 and Y5 receptors are both involved in the mitotic activity of NPY, the increased NPY-Y5 expression might be due to the increased VSMC proliferation in normal carotid artery compared to the carotid plaques. In plaque sections also more positivity of NPY-Y5 was seen more towards the cells towards the plaque areas. The inflammatory cytokine stimulation decreased the expression of NPY-Y5 in all the VSMCs. The decrease was more obvious in Asymptomatic VSMCs. Previous investigation have demonstrated increased expression of Bcl-2, a member of the anti-apoptotic protein family, in asymptomatic carotid plaques than in the symptomatic plaques (Artese et al., 2005). The marker of proliferation, proliferating cell nuclear antigen (PCNA), was highly expressed in the fibrous cap, necrotic core and base of the asymptomatic plaque than the symptomatic plaques (Moran and Agrawal, 2007). Symptomatic VSMCs were already subjected to inflammatory cytokine stimulation in in vivo condition. This might be the reason why there is a decrease in the activity of these cytokine in symptomatic VSMCs.
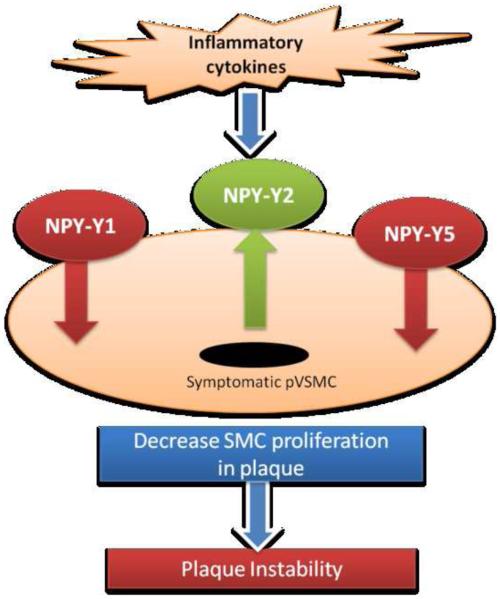
In conclusion, atheroma associated cytokines decreased the density of NPY-Y1 and NPY-Y5 receptors and increased the density of NPY-Y2 receptors in the VSMCs of symptomatic carotid plaques (Fig. 5) and thus inhibit NPY-induced survival and attenuate angiogenesis in symptomatic plaques. Increased expression of NPY-Y2 receptors in symptomatic pVSMCs than in healthy and asymptomatic subjects supports a potential role of NPY-Y2 in plaque instability. Due to NPYs ability to affect vascular smooth muscle cell proliferation as well as in vivo angiogenesis in carotid plaques, application of NPY receptor antagonists would address the issue of atherogenesis by inhibiting further neointimal formation and angiogenesis within the plaque itself regulating the vulnerability of the plaque.
Figure 5.
Schematic representation of our data showing decreased NPY-Y1 and NPY-Y5, and increased NPY-Y2 receptor expression after stimulation with inflammatory cytokines which may result in decreased SMC proliferation and thus plaque instability.
Acknowledgements
This work was supported by the National Institutes of Health Grant R01 HL073349 to D.K.A.
Abbreviations
- AS
Asymptomatic
- ANOVA
Analysis of variance
- IL-12
Interleukin-12
- IFN-γ
Interferon -gamma
- NPY
Neuropeptide Y
- NPY-Y1
Neuropeptide Y1 receptor
- NPY-Y2
Neuropeptide Y2 receptor
- NPY-Y5
Neuropeptide Y5 receptor
- PCR
Polymerase Chain Reaction
- pVSMCs
plaque derived vascular smooth muscle cells
- S
Symptomatic
- TNF-α
Tumor necrosis factor- alpha
- VSMC
Vascular smooth muscle cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artese L, Ucchino S, Piattelli A, Piccirilli M, Perrotti V, Mezzetti A, Cipollone F. Factors associated with apoptosis in symptomatic and asymptomatic carotid atherosclerotic plaques. Int J Immunopathol Pharmacol. 2005;18:645–53. doi: 10.1177/039463200501800405. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam A. Clinical potentials of neuropeptide Y family of hormones. Am J Surg. 2002;183:430–4. doi: 10.1016/s0002-9610(02)00803-6. [DOI] [PubMed] [Google Scholar]
- Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:2137–42. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- Chronwall BM, Zukowska Z. Neuropeptide Y, ubiquitous and elusive. Peptides. 2004;25:359–63. doi: 10.1016/j.peptides.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Dhume AS, Agrawal DK. Inability of vascular smooth muscle cells to proceed beyond S phase of cell cycle, and increased apoptosis in symptomatic carotid artery disease. J Vasc Surg. 2003;38:155–61. doi: 10.1016/s0741-5214(02)75463-3. [DOI] [PubMed] [Google Scholar]
- Fleiner M, Kummer M, Mirlacher M, Sauter G, Cathomas G, Krapf R, Biedermann BC. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation. 2004;110:2843–50. doi: 10.1161/01.CIR.0000146787.16297.E8. [DOI] [PubMed] [Google Scholar]
- Golledge J, Greenhalgh RM, Davies AH. The symptomatic carotid plaque. Stroke. 2000;31:774–81. doi: 10.1161/01.str.31.3.774. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281–91. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- Jia G, Cheng G, Gangahar DM, Agrawal DK. Insulin-like growth factor-1 and TNF-alpha regulate autophagy through c-jun N-terminal kinase and Akt pathways in human atherosclerotic vascular smooth cells. Immunol Cell Biol. 2006;84:448–54. doi: 10.1111/j.1440-1711.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- Jia G, Cheng G, Soundararajan K, Agrawal DK. Insulin-like growth factor-I receptors in atherosclerotic plaques of symptomatic and asymptomatic patients with carotid stenosis: effect of IL-12 and IFN-gamma. Am J Physiol Heart Circ Physiol. 2007;292:H1051–7. doi: 10.1152/ajpheart.00801.2006. [DOI] [PubMed] [Google Scholar]
- Lee EW, Grant DS, Movafagh S, Zukowska Z. Impaired angiogenesis in neuropeptide Y (NPY)-Y2 receptor knockout mice. Peptides. 2003a;24:99–106. doi: 10.1016/s0196-9781(02)00281-4. [DOI] [PubMed] [Google Scholar]
- Lee EW, Michalkiewicz M, Kitlinska J, Kalezic I, Switalska H, Yoo P, Sangkharat A, Ji H, Li L, Michalkiewicz T, Ljubisavljevic M, Johansson H, Grant DS, Zukowska Z. Neuropeptide Y induces ischemic angiogenesis and restores function of ischemic skeletal muscles. J Clin Invest. 2003b;111:1853–62. doi: 10.1172/JCI16929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Jonsson-Rylander AC, Abe K, Zukowska Z. Chronic stress induces rapid occlusion of angioplasty-injured rat carotid artery by activating neuropeptide Y and its Y1 receptors. Arterioscler Thromb Vasc Biol. 2005;25:2075–80. doi: 10.1161/01.ATV.0000179601.19888.19. [DOI] [PubMed] [Google Scholar]
- Li L, Lee EW, Ji H, Zukowska Z. Neuropeptide Y-induced acceleration of postangioplasty occlusion of rat carotid artery. Arterioscler Thromb Vasc Biol. 2003;23:1204–10. doi: 10.1161/01.ATV.0000071349.30914.25. [DOI] [PubMed] [Google Scholar]
- Malmstrom RE. Pharmacology of neuropeptide Y receptor antagonists. Focus on cardiovascular functions. Eur J Pharmacol. 2002;447:11–30. doi: 10.1016/s0014-2999(02)01889-7. [DOI] [PubMed] [Google Scholar]
- Moran EP, Agrawal DK. Increased expression of inhibitor of apoptosis proteins in atherosclerotic plaques of symptomatic patients with carotid stenosis. Exp Mol Pathol. 2007;83:11–6. doi: 10.1016/j.yexmp.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movafagh S, Hobson JP, Spiegel S, Kleinman HK, Zukowska Z. Neuropeptide Y induces migration, proliferation, and tube formation of endothelial cells bimodally via Y1, Y2, and Y5 receptors. Faseb J. 2006;20:1924–6. doi: 10.1096/fj.05-4770fje. [DOI] [PubMed] [Google Scholar]
- Nilsson T, Edvinsson L. Neuropeptide Y stimulates DNA synthesis in human vascular smooth muscle cells through neuropeptide Y Y1 receptors. Can J Physiol Pharmacol. 2000;78:256–9. [PubMed] [Google Scholar]
- Pons J, Kitlinska J, Ji H, Lee EW, Zukowska Z. Mitogenic actions of neuropeptide Y in vascular smooth muscle cells: synergetic interactions with the beta-adrenergic system. Can J Physiol Pharmacol. 2003;81:177–85. doi: 10.1139/y02-166. [DOI] [PubMed] [Google Scholar]
- Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–81. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Vemuganti R, Salamat MS, Dempsey RJ. Increased angiogenesis and angiogenic gene expression in carotid artery plaques from symptomatic stroke patients. Neurosurgery. 2006;58:971–7. doi: 10.1227/01.NEU.0000210246.61817.FE. discussion 971-7. [DOI] [PubMed] [Google Scholar]
- Vukovic I, Arsenijevic N, Lackovic V, Todorovic V. The origin and differentiation potential of smooth muscle cells in coronary atherosclerosis. Exp Clin Cardiol. 2006;11:123–8. [PMC free article] [PubMed] [Google Scholar]
- Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Rose W, Rone J, Movafagh S, Ji H, Yeh Y, Chen WT, Kleinman HK, Grouzmann E, Grant DS. Neuropeptide Y: a novel angiogenic factor from the sympathetic nerves and endothelium. Circ Res. 1998;83:187–95. doi: 10.1161/01.res.83.2.187. [DOI] [PubMed] [Google Scholar]