Abstract
Objective
To describe the clinical presentation, laboratory findings and outcome of patients with Pneumocystis pneumonia (PCP) and biopsy-proven giant cell arteritis (GCA) seen at a tertiary referral center.
Methods
Using ICD-9 codes, all patients with GCA and PCP between January 1, 1976 and December 31, 2008 were identified. Medical records were reviewed. PCP was defined by the identification of Pneumocystis jiroveci organism in the clinical setting of pneumonia.
Results
We identified 7 GCA patients (5 women and 2 men) who developed PCP; mean age at diagnosis was 71.6 (±6.1) years. Median time from GCA diagnosis to PCP diagnosis was 3 (range 1–18) months. All patients were on prednisone at diagnosis of PCP; median dose 50 (range 30–80) mg daily. None were on PCP prophylaxis. PCP was diagnosed by positive smear on broncho-alveolar lavage fluid in 6 patients (86%) and positive sputum polymerase chain reaction (PCR) in 1 patient. All patients were hospitalized; median duration 17 (range 12–39) days. Four patients (57%) were admitted to intensive care unit. Three patients (43%) required mechanical ventilation. Two patients (29%) died; both were on mechanical ventilation.
Conclusion
PCP is rare among patients with GCA. However, this preventable infection is associated with significant morbidity and mortality.
Keywords: Giant cell arteritis, Pneumocystis jiroveci, Pneumocystis pneumonia, corticosteroids
INTRODUCTION
Giant cell arteritis (GCA) is a granulomatous vasculitis involving the aorta and its primary and secondary branches. Corticosteroids (CS) remain the mainstay of treatment but are associated with significant morbidity (1). In a population-based incident cohort of 125 patients with GCA, 103 patients (86%) experienced adverse events associated with CS treatment, of which infectious complications were observed in 37 patients (30%) (1). Pneumocystis jiroveci, a fungal organism with tropism for lung parenchyma, can cause opportunistic infection in humans with impaired immunity (2). While Pneumocystis pneumonia (PCP) is well recognized as an acquired immunodeficiency syndrome (AIDS) defining illness, it is also a serious opportunistic infection in immunocompromised patients without human immunodeficiency virus (HIV) infection. CS use is a recognized risk factor for PCP. In a series of PCP patients without AIDS, over 90% had received corticosteroids within 1 month prior to PCP diagnosis (3). More importantly, 25% of these patients were on prednisone at doses of 16 mg or less daily (3). Since patients with GCA typically receive high dose CS treatment and prolonged taper, they are at risk for developing this infection. Only isolated cases of PCP in GCA have been reported in the literature and information on the frequency, clinical presentation and prognosis is lacking (4–7). The objective of this study was to review the clinical findings and outcomes of all cases of PCP occurring in patients with GCA seen at our institution between January 1, 1976 and December 31, 2008.
PATIENTS AND METHODS
This is a retrospective case series of GCA patients diagnosed at Mayo Clinic between January 1, 1976 and December 31, 2008 who developed PCP. The study was approved by the Institutional Review Board at Mayo Clinic. Cases were identified using the International Classification of Diseases, 9th Revision (ICD-9) codes for GCA and PCP. All medical records were reviewed to confirm the diagnosis of GCA (based on 1990 American College of Rheumatology Classification criteria). PCP was defined as clinical findings of pneumonia with identification of Pneumocystis jiroveci organisms by smear or polymerase chain reaction (PCR) in sputum, bronchoalveolar lavage (BAL) fluid, or lung biopsy specimens. Patients in whom Pneumocystis infection could not be confirmed by the methods stated above or patients with a diagnosis of PCP made elsewhere were excluded. Medical records were reviewed and the following data was abstracted: age at diagnosis of GCA, gender, date of diagnosis of GCA, date at diagnosis of PCP, symptoms at diagnosis of PCP, laboratory and radiographic findings at the time of PCP diagnosis, corticosteroid dose, any other immunosuppressive treatment or comorbidities, treatment of PCP and outcome. The data were analyzed using descriptive statistics.
RESULTS
During the study period, 7,543 patients were evaluated at Mayo Clinic, Rochester for possible GCA. A medical retrieval specialist identified 17 cases with ICD-9 codes for GCA and PCP. Of these, 9 patients were excluded after review of the medical record because GCA was not their final diagnosis. One patient with confirmed GCA was excluded because PCP was diagnosed at an outside institution and could not be confirmed. Our final study population included 7 GCA patients (5 women and 2 men). All subjects had a temporal artery biopsy that was positive for vasculitis. Mean age at diagnosis of GCA was 71.9 (±6.1) years. The median time from GCA diagnosis to PCP diagnosis was 4 (range 1–18) months. At PCP diagnosis, all patients were on prednisone, median daily dose 50 (range 30–80) mg. The median duration of corticosteroid treatment at PCP diagnosis was 4 months (0.67–18) months. None of the patients were receiving PCP prophylaxis. Two patients had previously received methotrexate but this had been discontinued 1 month prior to diagnosis in both cases. Other pertinent comorbidities included myelodysplastic syndrome in 1 patient and interstitial lung disease secondary to asbestosis in 1 patient.
The median time interval from the onset of symptoms to diagnosis of PCP was 13 (range 0–28) days. The most common presenting symptom was dyspnea (6 patients, 86%). Pulmonary physical examination was abnormal in 4 cases (57%). Four patients (57%) were hypoxic (by oximetry or arterial blood gas partial pressure oxygen) at diagnosis. The clinical characteristics of the 7 patients are summarized in Table 1.
Table 1.
Clinical features and outcomes in 7 GC patients with PCP
| Patient | Age at PCP (years) |
Sex | Symptoms | CS use at PCP diagnosis |
Use of other agents for GCA |
Laboratory/radiographic findings |
Length of hospital stay (days) |
Length of ICU stay (days) |
Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 65 | F | Dyspnea, cough | Pred 30 mg | None | Anemia, ESR 117 mm/hour, normal CXR, PCP diagnosed by BAL | 17 days | N/A | Alive |
| 2 | 71 | F | Incidentally noted on x-ray | Pred 80 mg | None | ESR 73 mm/hour, abnormal CXR and CT, PCP by BAL | 39 | N/A | Alive |
| 3 | 70 | F | Dyspnea, fever | Pred 50 mg | None | ESR 16 mm/hour, abnormal CXR, PCP by BAL | 23 | 2 | Alive |
| 4 | 69 | F | Dyspnea, fever | Pred 50 mg | MTX | ESR 73 mm/hour, CRP 159.9 mg/L, normal CXR, abnormal CT, PCP by BAL | 13 | N/A | Alive |
| 5* | 78 | F | Dyspnea, cough | Pred 60 mg | MTX | ESR 87 mm/hour, CRP 111.5 mg/L, abnormal CXR and CT, PCP by BAL | 30 | 21 | Alive |
| 6* | 72 | M | Dyspnea, cough, fever | Pred 40 mg | None | ESR 48 mm/hour, abnormal CXR, PCP by BAL | 12 | 7 | Deceased |
| 7* | 82 | M | Dyspnea, cough | Pred 40 mg | None | ESR 49 mm/hour, CRP 35.2 mg/L, abnormal CXR and CT, PCP by sputum PCR | 13 | 13 | Deceased |
Patients 5, 6 and 7 required mechanical ventilation while in the ICU
BAL=bronchoalveolar lavage; CRP=C-reactive protein; CS=corticosteroid; CT=computed tomography; CXR=chest x-ray; ESR=erythrocyte sedimentation rate; GCA=Giant cell arteritis; ICU=intensive care unit; MTX=methotrexate; PCP=pneumocystis pneumonia; PCR=polymerase chain reaction; Pred=prednisone
The erythrocyte sedimentation rate was elevated in 6 cases (86%) (Table 2). The initial chest x-ray was abnormal in 5 patients (71%) and the most common abnormality was presence of bilateral lung infiltrates (Table 2). Computed tomography (CT) imaging was obtained in 4 cases (57%) and the most common finding was diffuse patchy, ground glass opacities bilaterally (Figure 1). Other findings included cavitary mass (1 patient), mediastinal lymphadenopathy (2 patients) and changes of pleural plaques and traction bronchiectasis with honeycombing in the patient with asbestos exposure. All patients underwent bronchoscopy. PCP was diagnosed by positive smear for Pneumocystis jiroveci on BAL fluid in 6 patients (86%). One patient had clinical features consistent with PCP along with positive sputum PCR for Pneumocystis.
Table 2.
Laboratory and radiographic findings in 7 patients with GCA and PCP
| Variable | Value |
|---|---|
| Laboratory findings | |
| Anemia, No (%) | 4 (57) |
| Mean hemoglobin (±SD), g/dL | 11.9 (±2.8) |
| Leukocytosis, No (%) | 1 (14) |
| Mean WBC (±SD) | 8.4 (±3.6) |
| Lymphopenia*, No (%) | 5/5 (100) |
| Mean ESR (±SD), mm/hour | 66 (±32.3) |
| Median Creatinine (range), mg/dL | 0.9 (0.4–3.4) |
| Chest x-ray findings | |
| Abnormal, No (%) | 5 (71%) |
| Bilateral infiltrates | 3 |
| Cavitary mass | 1 |
| Focal infiltrate | 1 |
Differential not available in 2 cases
No=number
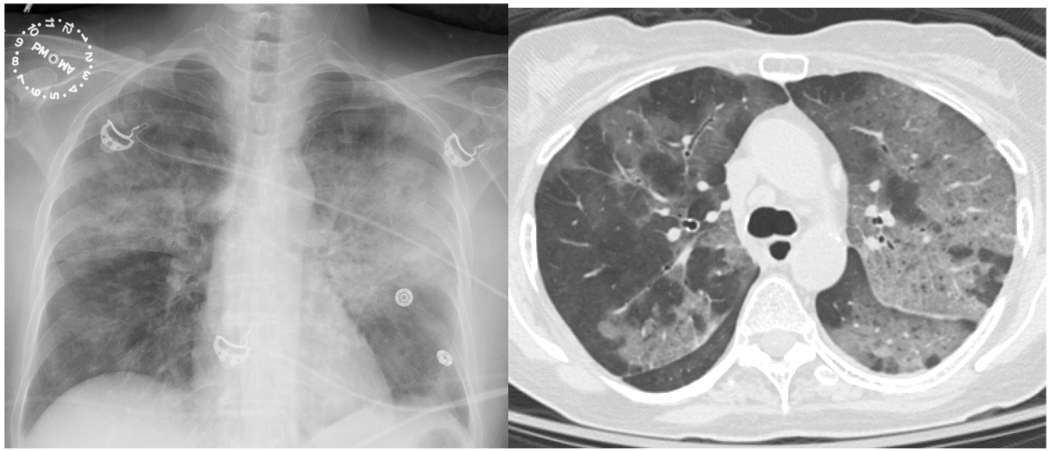
Figure.
Extensive patchy bilateral opacities on chest x-ray (Panel A) and on computed tomography (Panel B) in a GCA patient with pneumocystis pneumonia.
All patients required hospitalization and received appropriate treatment with intravenous trimethoprim/sulfamethoxazole. The median duration of hospital stay was 17 (range 12–39) days. Four patients (57%) were admitted to the intensive care unit (ICU); median duration ICU stay 7.5 (range 2–21) days. Three patients (43%) required mechanical ventilation. There were 2 deaths (29%), both in patients who were on mechanical ventilation.
DISCUSSION
We report the first case series of patients with PCP occurring in the context of treatment for GCA. The clinical features and outcomes of this group of patients appear similar to those of other PCP patients without AIDS.
Overall, PCP appears to be a rare infection in patients with GCA. During the 32-year study period we identified only 7 cases of PCP despite the large volume of patients evaluated for GCA at this tertiary referral center. Reports of PCP in GCA are scarce in the literature and limited to isolated case reports (4–7). In clinical trials of GCA, no cases of PCP have been reported even in the absence of prophylaxis (8). Additionally, we did not identify any cases of PCP in our population-based GCA cohort using the Rochester Epidemiology Project database (data not shown). This cohort includes 204 incident cases of GCA in Olmsted County, MN, over a 55 year period with median follow-up of 7.7 years (9). In contrast, PCP is known to occur more frequently in other forms of vasculitis, particularly Wegener’s granulomatosis (10, 11).
CS use is a risk factor for PCP in non-HIV patients (3). In a study evaluating PCP in patients without AIDS, 90.5% of the patients had received systemic corticosteroids in the month prior to diagnosis (3). Even relatively low doses of CS were associated with PCP, as in 25% patients the daily dose of prednisone was 16 mg or less (3). In the present study, all patients were receiving high dose CS therapy (≥30 mg) at PCP diagnosis and none were on PCP prophylaxis. Of note, 4 of the 7 subjects were still on high doses of CS (≥30 mg) 4–18 months after GCA diagnosis, which is higher than would be expected for the duration of disease. The remaining 3 subjects were diagnosed with PCP within 2.5 months of GCA diagnosis. In reported cases of GCA and PCP in the literature, corticosteroid doses were between 30–80 mg at the time of PCP diagnosis (4–6). Two of the patients in our series were also on methotrexate as a steroid-sparing agent prior to PCP diagnosis. In both cases methotrexate was discontinued approximately 1 month prior to PCP diagnosis due to pulmonary symptoms and concern for methotrexate pneumonitis. Therefore, it is possible that PCP developed in the context of methotrexate and glucocorticoid use in both cases. In one prior case report of PCP in GCA, the patient was both on prednisone and methotrexate (5). Whether adjunctive immunosuppression like methotrexate increases risk of PCP in GCA is unknown. In ANCA-associate vasculitis (AAV), the risk of PCP is especially high during the induction of remission phase and may be related to use of high dose corticosteroid therapy in addition to cyclophosphamide (8).
Dyspnea was the most common presenting symptom and chest examination was abnormal in most patients. Laboratory findings were non-specific with elevated sedimentation rate in 6 patients at the time of PCP diagnosis. Five patients in the present study had white cell count differential available and lymphopenia present in all cases. The significance of this finding is unclear and is likely related to CS therapy. Lymphopenia has been associated with PCP in WG and was likely related to immunosuppressive treatment (10, 12).
PCP infection in GCA was associated with significant morbidity and mortality. All patients in this series were hospitalized and median duration of hospitalization was 2.5 weeks. Furthermore, 3 patients required mechanical ventilation. As evident in Table 1, the patients who required mechanical ventilation tended to be older. We did not observe any other prominent clinical differences between patients requiring mechanical ventilation and those who did not. One of the three patients in the mechanical ventilation group had pre-existing interstitial lung disease from asbestosis. There were 2 deaths in this study. In the 4 cases reported in the literature, there were 3 deaths from PCP in GCA patients (5–7). Several studies have found that mortality in PCP is greater in non-HIV patients compared to those with HIV infection (3, 13–15). An in-hospital mortality of 46% was reported in one large study of PCP occurring in patients with autoimmune diseases (16).
None of the patients in this series had received prophylaxis. In a recent survey of nearly 730 rheumatologists, 30% reported that they never prescribed PCP prophylaxis (17). In this study, rheumatologists were less likely to offer PCP prophylaxis to patients on prednisone monotherapy (12.5%) regardless of the dose. However, 75.6% of rheumatologists responded they would recommend PCP prophylaxis to patients treated with cyclophosphamide and 49.1% would recommend prophylaxis for a combination therapy that included prednisone. Rheumatologists from the United States, those in academic centers and those early in their careers were more likely to prescribe PCP prophylaxis (17). The variability of use of PCP prophylaxis in GCA is at least in part related to the absence of any consensus guidelines on use. PCP prophylaxis has been shown to be cost-effective in WG (18). However, PCP occurs more frequently in WG and has been reported to be as high as 20% in one clinical trial (11). Calculating the cost-effectiveness of PCP prophylaxis in GCA would be challenging given the rarity of this complication.
Our study has several limitations which need to be considered when interpreting our findings. The small number of patients in this study allowed us to make only descriptive observations. The true incidence of PCP in GCA remains unknown and could not be determined based on our series which is derived from a referral population. Given the small numbers, we were unable to design a meaningful study to evaluate the risk factors (clinical or treatment-related) which may be associated with PCP in GCA. It is also possible that our study underestimates the true frequency of PCP in GCA as most patients seen for this diagnosis in recent years have been prescribed PCP prophylaxis. Due to the retrospective design, our data was limited to information already present in the medical records. While our data imply that CS therapy was the main risk factor for PCP in this series, we cannot exclude that other concomitant causes of immunocompromise may have contributed to the development of PCP.
In summary, high-dose CS use in GCA may be associated with development of PCP, a potentially preventable and serious infection. It remains unclear whether use of methotrexate for GCA increases the risk of PCP in these patients. PCP should be considered in the differential diagnosis of GCA patients who present with new pulmonary symptoms. While PCP is a rare infection in GCA, in this series, it was associated with significant morbidity including hospitalization, mechanical ventilation, and, mortality in 29% cases. Further study would help raise awareness of this infection and could further our understanding of other disease-specific factors that may predispose GCA patients to PCP.
Acknowledgments
Grant Supports: Dr. Kermani has received a Fellowship Award from the Vasculitis Clinical Research Consortium (NIH National Center for Research Resources; Grant Number U54-RR-019497).
REFERENCES
- 1.Proven A, Gabriel SE, Orces C, O'Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum. 2003;49:703–708. doi: 10.1002/art.11388. [DOI] [PubMed] [Google Scholar]
- 2.Thomas CF, Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487–2498. doi: 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 3.Yale SH, Limper AH. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo Clin Proc. 1996;71:5–13. doi: 10.4065/71.1.5. [DOI] [PubMed] [Google Scholar]
- 4.Crayton HE, Sundstrom WR. Pneumocystis carinii pneumonia following corticosteroid therapy for giant cell arteritis. Wis Med J. 1991;90:170–171. [PubMed] [Google Scholar]
- 5.Krebs S, Gibbons RB. Low-dose methotrexate as a risk factor for Pneumocystis carinii pneumonia. Mil Med. 1996;161:58–60. [PubMed] [Google Scholar]
- 6.Hedderwick SA, Bonilla HF, Bradley SF, Kauffman CA. Opportunistic infections in patients with temporal arteritis treated with corticosteroids. J Am Geriatr Soc. 1997;45:334–337. doi: 10.1111/j.1532-5415.1997.tb00949.x. [DOI] [PubMed] [Google Scholar]
- 7.Sendi P, Wolf A, Graber P, Zimmerli W. Multiple opportunistic infections after high-dose steroid therapy for giant cell arteritis in a patient previously treated with a purine analog. Scand J Infect Dis. 2006;38:922–924. doi: 10.1080/00365540500540475. [DOI] [PubMed] [Google Scholar]
- 8.Moosig F, Holle JU, Gross WL. Value of anti-infective chemoprophylaxis in primary systemic vasculitis: what is the evidence? Arthritis Res Ther. 2009;11:253. doi: 10.1186/ar2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kermani TA, Schafer VS, Crowson CS, Hunder GG, Gabriel SE, Matteson EL, et al. Increase in age at onset of giant cell arteritis: a population-based study. Ann Rheum Dis. 2010;69:780–781. doi: 10.1136/ard.2009.111005. [DOI] [PubMed] [Google Scholar]
- 10.Ognibene FP, Shelhamer JH, Hoffman GS, Kerr GS, Reda D, Fauci AS, et al. Pneumocystis carinii pneumonia: a major complication of immunosuppressive therapy in patients with Wegener's granulomatosis. Am J Respir Crit Care Med. 1995;151:795–799. doi: 10.1164/ajrccm/151.3_Pt_1.795. [DOI] [PubMed] [Google Scholar]
- 11.Guillevin L, Cordier JF, Lhote F, Cohen P, Jarrousse B, Royer I, et al. A prospective, multicenter, randomized trial comparing steroids and pulse cyclophosphamide versus steroids and oral cyclophosphamide in the treatment of generalized Wegener's granulomatosis. Arthritis Rheum. 1997;40:2187–2198. doi: 10.1002/art.1780401213. [DOI] [PubMed] [Google Scholar]
- 12.Godeau B, Mainardi JL, Roudot-Thoraval F, Hachulla E, Guillevin L, Huong Du LT, et al. Factors associated with Pneumocystis carinii pneumonia in Wegener's granulomatosis. Ann Rheum Dis. 1995;54:991–994. doi: 10.1136/ard.54.12.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arend SM, Kroon FP, van't Wout JW. Pneumocystis carinii pneumonia in patients without AIDS, 1980 through 1993. An analysis of 78 cases. Arch Intern Med. 1995;155:2436–2441. [PubMed] [Google Scholar]
- 14.Monnet X, Vidal-Petiot E, Osman D, Hamzaoui O, Durrbach A, Goujard C, et al. Critical care management and outcome of severe Pneumocystis pneumonia in patients with and without HIV. Crit Care. 2008;12:R28. doi: 10.1186/cc6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enomoto T, Azuma A, Kohno A, Kaneko K, Saito H, Kametaka M, et al. Differences in the clinical characteristics of Pneumocystis jirovecii pneumonia in immunocompromized patients with and without HIV infection. Respirology. 2010;15:126–131. doi: 10.1111/j.1440-1843.2009.01660.x. [DOI] [PubMed] [Google Scholar]
- 16.Ward MM, Donald F. Pneumocystis carinii pneumonia in patients with connective tissue diseases: the role of hospital experience in diagnosis and mortality. Arthritis Rheum. 1999;42:780–789. doi: 10.1002/1529-0131(199904)42:4<780::AID-ANR23>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 17.Cettomai D, Gelber AC, Christopher-Stine L. A survey of rheumatologists' practice for prescribing pneumocystis prophylaxis. J Rheumatol. 2010;37:792–799. doi: 10.3899/jrheum.090843. [DOI] [PubMed] [Google Scholar]
- 18.Chung JB, Armstrong K, Schwartz JS, Albert D. Cost-effectiveness of prophylaxis against Pneumocystis carinii pneumonia in patients with Wegner's granulomatosis undergoing immunosuppressive therapy. Arthritis Rheum. 2000 43;:1841–1848. doi: 10.1002/1529-0131(200008)43:8<1841::AID-ANR21>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]