Abstract
Objective
The health and longevity effects of body weight reduction resulting from exercise and caloric restriction in rodents are well known, but less is known about whether similar effects occur with weight reduction from the use of a pharmaceutical agent such as sibutramine, a serotonin-norepinephrine reuptake inhibitor.
Results & Conclusion
Using data from a two-year toxicology study of sibutramine in CD rats and CD-1 mice, despite a dose-dependent reduction in food intake and body weight in rats compared to controls, and a body weight reduction in mice at the highest dose, there was no compelling evidence for reductions in mortality rate.
Keywords: Sibutramine, Meridia, anorexigens, obesity, weight loss, survival, lifespan, longevity, mortality, caloric restriction
Introduction
Obesity represents a major public health concern by virtue of both its high prevalence and its deleterious consequences (1). Weight loss achieved through decreased calorie intake is associated with prolonged life among obese rodents (2), and we have fairly convincing evidence that surgically induced weight loss among severely obese persons leads to similar gains in health and mortality reduction (3;4). However, to date there has never been a study showing that weight loss induced by pharmaceutical agents leads to reduced mortality rate in humans. Given the current controversy about potential links between sibutramine and cardiovascular disorders (5–8), this gap in our knowledge is both surprising and troubling. With the exception of two studies of drugs never approved for use as obesity treatments in the United States (ephedrine sulfate (9) and rimonabant (10)) we are aware of no published studies evaluating whether a drug marketed for weight loss has a beneficial effect on mortality rate among animals.
Two large-scale human trials were designed to study whether weight loss drugs prolonged life. One, the Crescendo study (11), was discontinued after rimonabant was withdrawn from the market. The other is the SCOUT (Sibutramine Cardiovascular Outcome Trial) study investigating sibutramine (7;8;12). Recently, preliminary data were released from the data safety and monitoring board of SCOUT that raised concerns about an increased rate of serious adverse cardiovascular events including “heart attack, stroke, resuscitated cardiac arrest, or death” (13). In response, on January 21, 2010, the “FDA notified healthcare professionals that the review of additional data indicates an increased risk of heart attack and stroke in patients with a history of cardiovascular disease using sibutramine. Based on the serious nature of the review findings, [the] FDA requested and the manufacturer agreed to add a new contraindication to the sibutramine drug label stating that sibutramine is not to be used in patients with a history of cardiovascular disease” (14).
Given this background and the fact that sibutramine represents one of only two FDA approved drugs for long-term weight loss, with increasing usage even in persons under age 18 (15), it seems timely to investigate the effects of sibutramine on weight loss and longevity in an animal model. Using a Freedom of Information Act request, we obtained the raw data that the manufacturer supplied to the FDA from their long-term toxicity studies which we used for this purpose.
Methods
Study Description
Raw data were obtained from the FDA under the Freedom of Information Act. All animal work was performed at Boots Pharmaceuticals (Nottingham, England) between January 1987 and February 1992 in compliance with Good Laboratory Practices (GLP). Data from two studies titled “Sibutramine Hydrochloride: Effects on Tumour Incidence in the Rat (RC 0078)”, Report #:TX92021 and “Sibutramine Hydrochloride: Effects on Tumour Incidence in the Mouse (MC 0031)”, Report #: TX92006 were analyzed. Both study reports were inspected/audited by the Boots Quality Assurance Unit and considered to be an accurate presentation of the raw data.
Animals and Husbandry
A total of 520 (n=260/sex) Charles River Laboratory Sprague-Dawley CD (cesarean derived) rats (Portage, Michigan, USA) and 520 (n=260/sex) Charles River Laboratory CD-1 mice (Margate, Kent, England) were studied. Barrier maintained CD rats were acquired at 28±1 days of age and randomly allocated to treatment groups (CON: n=104/sex and n=52/group/sex in 3 dosage groups: SIB LO 1, SIB MED 3 or SIB HI 9 mg/kg/day) within 6 days of arrival such that the mean body weight was comparable within each sex across groups. All rats were uniquely ear marked and housed in polypropylene cages with stainless steel grids and lids, with 4 rats/cage of the same sex and dosage. Aggressive rats were separated to individual cages of similar form. Males and females were housed in separate temperature (21±2°C) and humidity (50±15%) controlled rooms with a 12:12 light:dark cycle (commencing 0700 GMT). Barrier maintained CD-1 mice were acquired at similar ages (28±1 days) and randomly allocated to treatment groups similar to rats (CON: n=104/sex or n=52/sex/group in 3 dosage groups: SIB LO 1.25, SIB MED 5 or SIB HI 20 mg/kg/day sibutramine) within 4 days of arrival and housed in solid bottom polyproprylene cages with stainless steel lids, under similar conditions (temperature: 22±2°C; humidity: 50±15%; 12:12 light:dark cycle commencing 0700 GMT). Sibutramine hydrochloride (BTS 54 524, N-1-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutyl-N,N-dimethylamine hydrochloride monohydrate) was administered by incorporation in the diet (Labsure CRM Diet (Poole, England): 56.9 carbohydrate, 2.4 fat and 18.1g protein/100g; powdered and irradiated) at the specified dosage with adjustments for intake differences within groups to maintain nominal dosages. All animals had ad libitum access to the diet and tap water throughout the study.
Outcome Variables
Bodyweight and food consumption were recorded weekly for the duration of the study. Surviving male mice were euthanized after 95 weeks’ treatment, when survival in one control group and in the group at 20 mg/kg approached 25%. Surviving female mice, as well as male and female rats, were euthanized after 104 weeks’ treatment. Moribund rats and mice, and all surviving animals at study completion, were euthanized by carbon dioxide inhalation.
Data Analysis
Separate analyses were performed for each species. ANOVA models were fitted at multiple time points (baseline, and 3 week averages at week 26, 52, and 78) to assess the effect of treatment on body weight and food consumption. Within each species, Cox regressions were performed combined to test for effects on mortality rate. Sex by treatment interactions were tested and found to be non-significant; therefore, a single Cox regression was fitted to males and females combined while including a main effect term for sex. The assumption of proportional hazards was checked by testing time-dependent effects, and no treatment groups violated the assumption [data not shown]. All analyses were performed using SAS 9.1 statistical software (SAS Institute, Cary, NC, USA).
Results
Effects on Body Weight and Food Intake
At one week prior to treatment, the body weight of rats did not differ significantly between groups (Fig. 1, Supp. Table 1). Upon initiation of sibutramine treatment and throughout the course of the study, a significant dose-dependent reduction of food intake was observed with rats, concomitant with a dose-dependent reduction in body weight gain (Fig. 1, Supp. Table 1). In contrast, only mice at the highest dose had significantly reduced body weight, that is, there was no monotonic dose-response relation for mice in weight or food intake (Fig. 1, Supp. Table 1).
Figure 1. Changes in body weight and food intake over time.
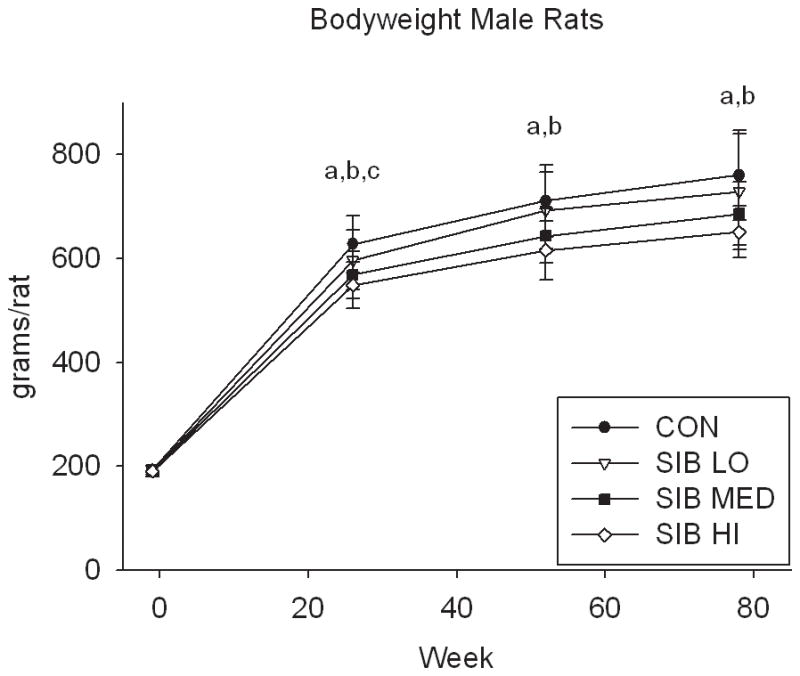
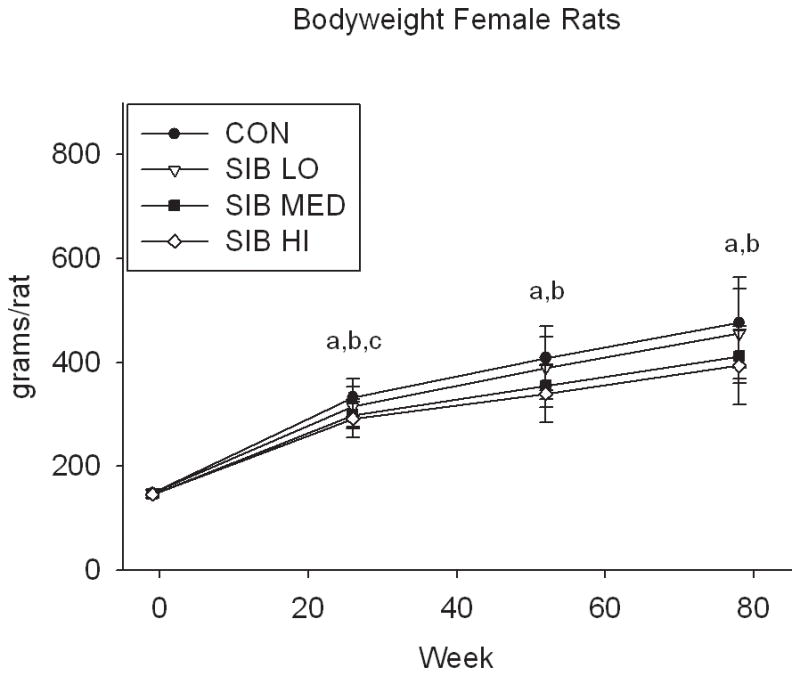
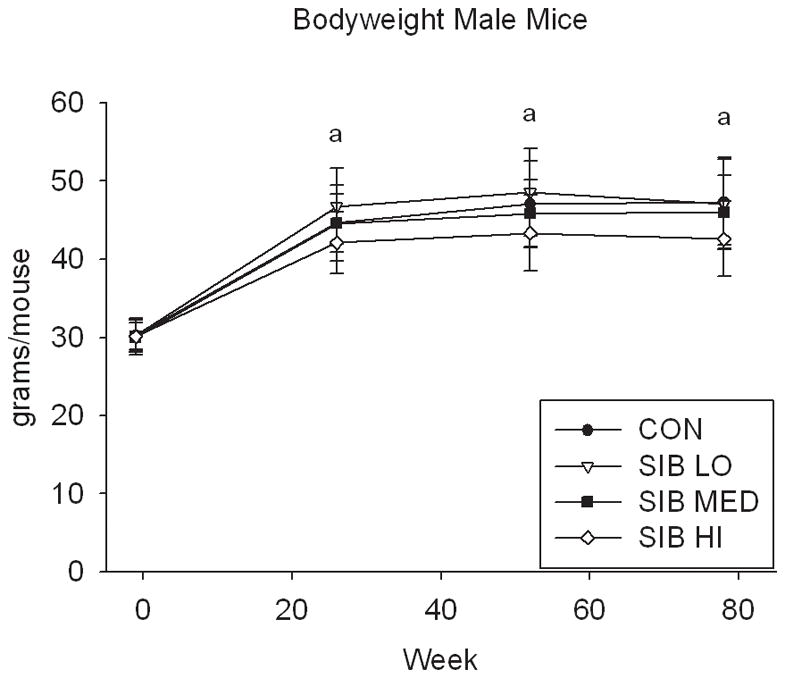
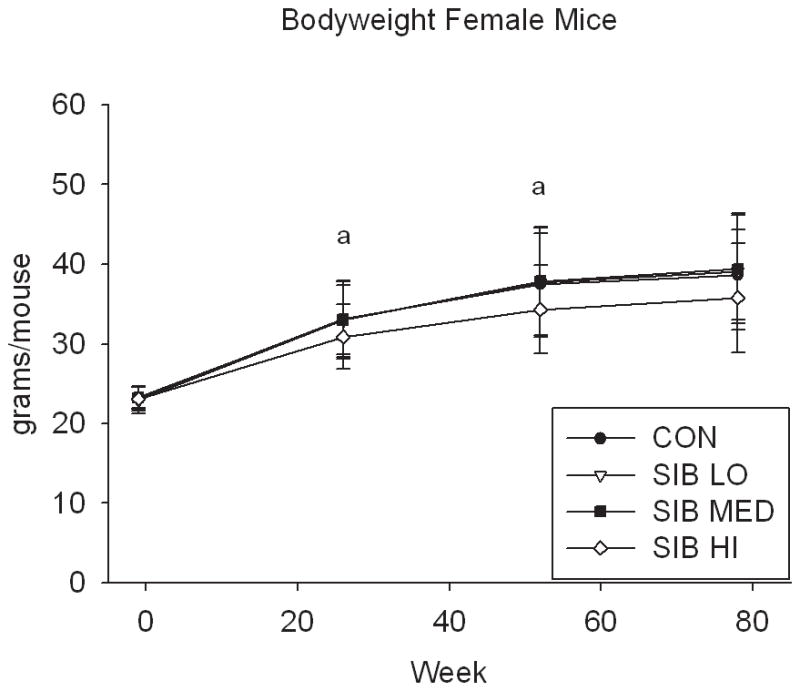
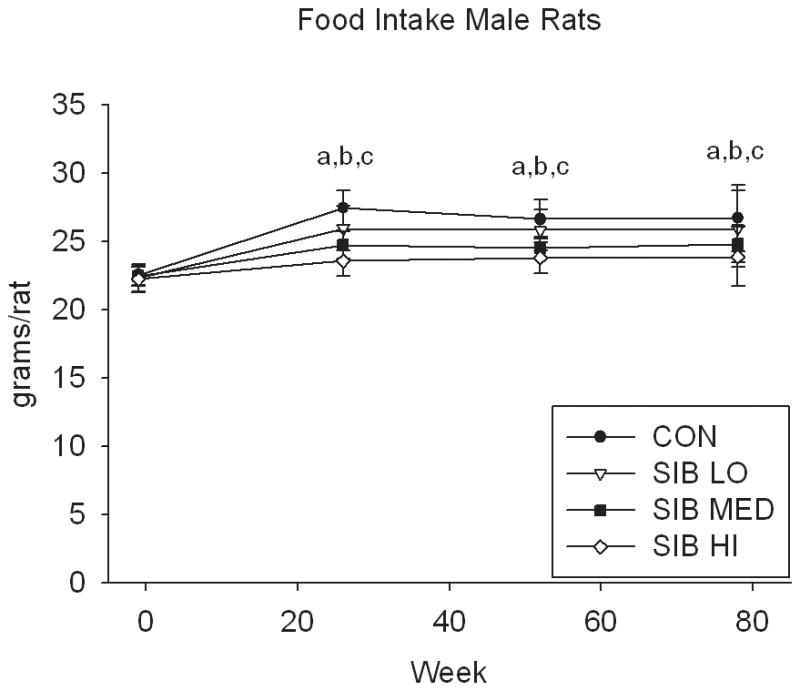
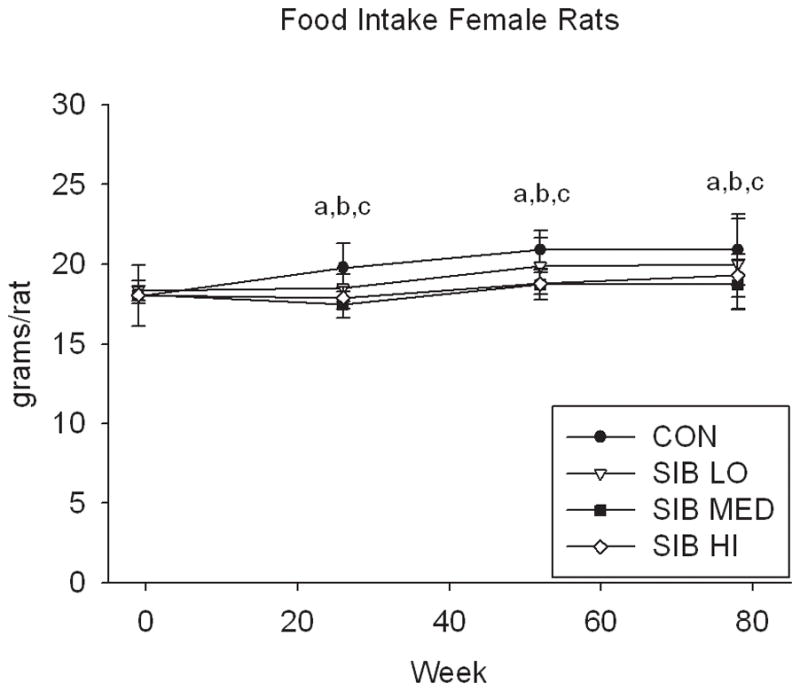
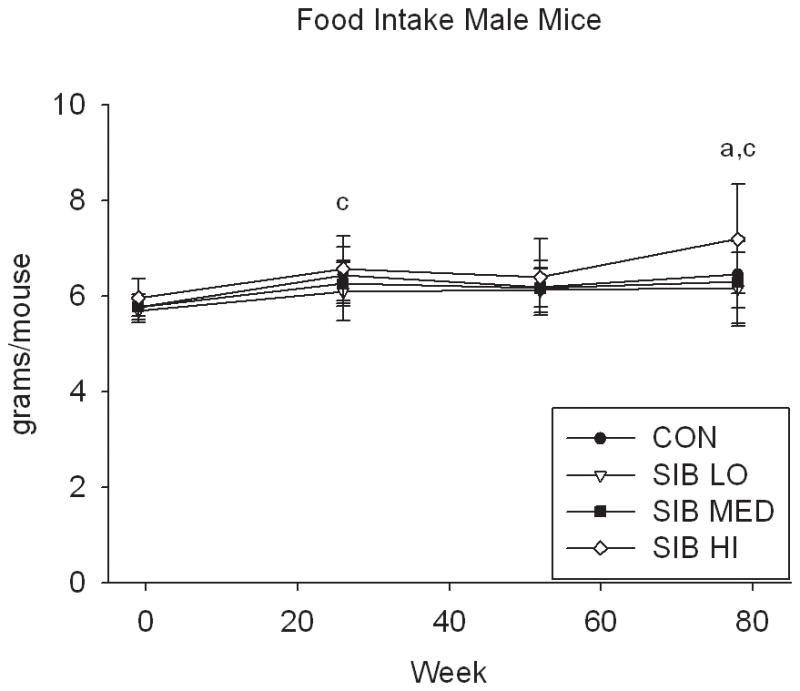
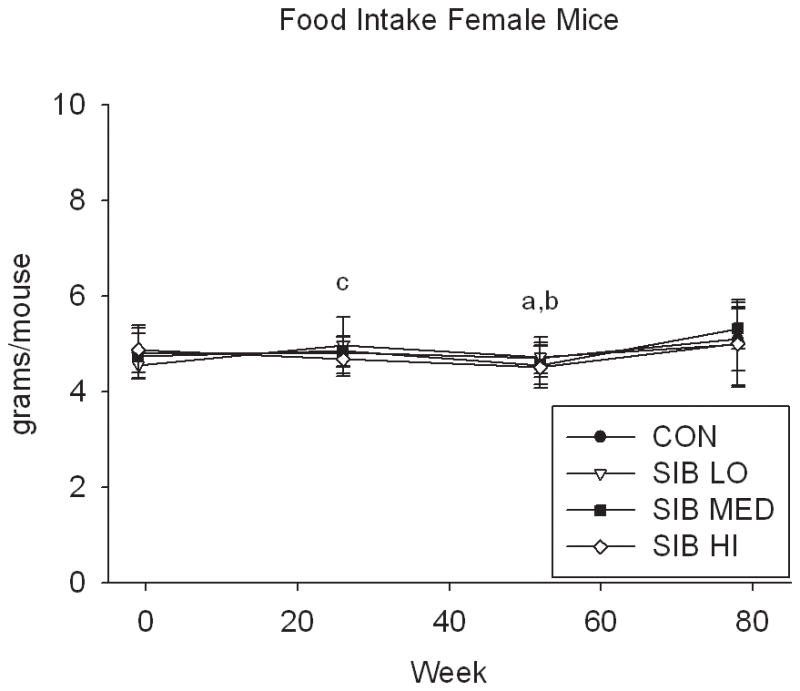
Group average body weight body weight (mean±s.d.) for Control and Sibutramine treated A) male rats, B) female rats, C) male mice and D) female mice at baseline and weeks 26, 52 and 78. Group average food intake (mean±s.d.) for Control and Sibutramine treated E) male rats, F) female rats, G) male mice and H) female mice at baseline and weeks 26, 52 and 78. Letters on graphs represent significance of p<0.01 between respective groups (aSIB HI vs. CON, bSIB MED vs. CON, and cSIB LO vs. CON).
Effects on Survival
With respect to mortality rate, there were no significant sex by treatment interactions (p=0.49 for rats and 0.25 for mice); therefore, males and females were combined and sex was included as a covariate. With males and females combined, the omnibus 3 degrees of freedom (df) likelihood ratio test (LRT) of the overall effect of group assignment on mortality rate was not significant (p=0.09 for rats and p=0.79 for mice). A more focused 1 df LRT in which placebo and low, medium, and high doses of sibutramine were coded as 0, 1, 2, and 3 respectively, showed p=0.07 for rats and p=0.91 for mice.
Thus, despite the dose-dependent reduction in body weight and food intake in rats, sibutramine treatment did not affect the longevity (either positively or negatively) in a dose-dependent manner (Fig. 2, Table 1 & Supp. Table 2). Although rats treated at the medium dose had a statistically significant increase in lifespan at the unadjusted nominal 0.05 level, (p = 0.01 for both sexes combined; male (p = 0.04), female (p = 0.17), Table 1 & Supp. Table 2), the overall test of significance for differences among male rats in all dosage groups was non-significant (p=0.16), suggesting the possibility of a type I error due in part to multiple testing (these values would not be significant were a Bonferroni correction applied). There were no significant differences in lifespan among groups in mice, even in the highest dosage group (Fig. 2, Table 1).
Figure 2. Kaplan-Meier Survival plots for Control and Sibutramine treated animals.
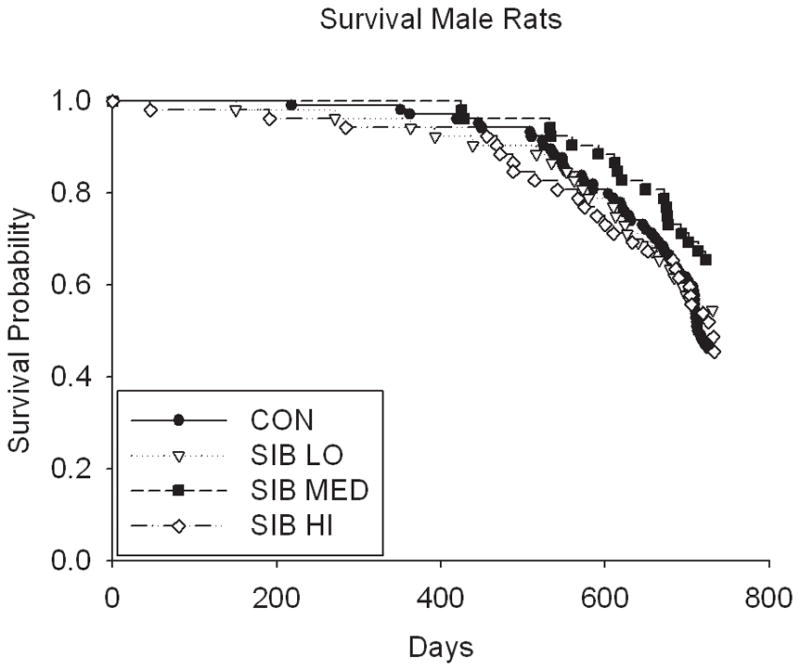
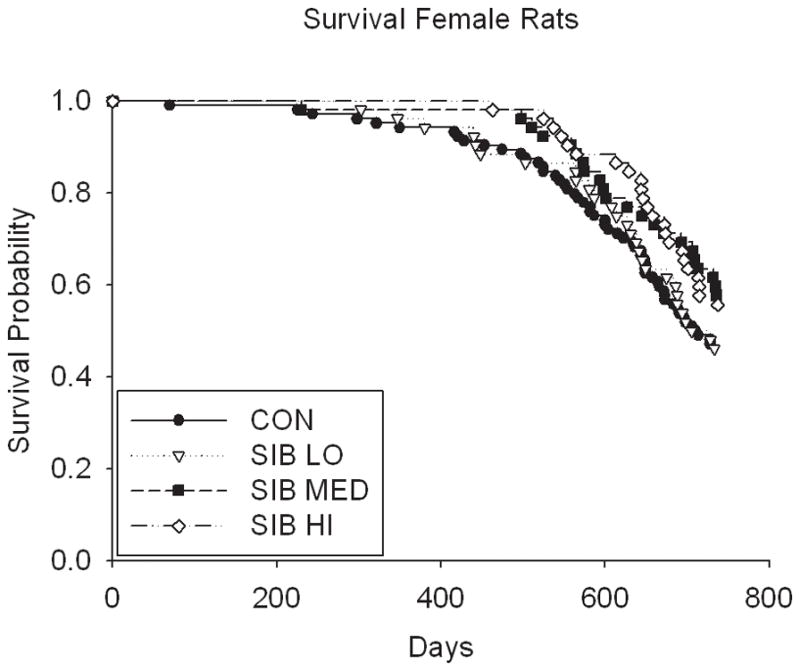
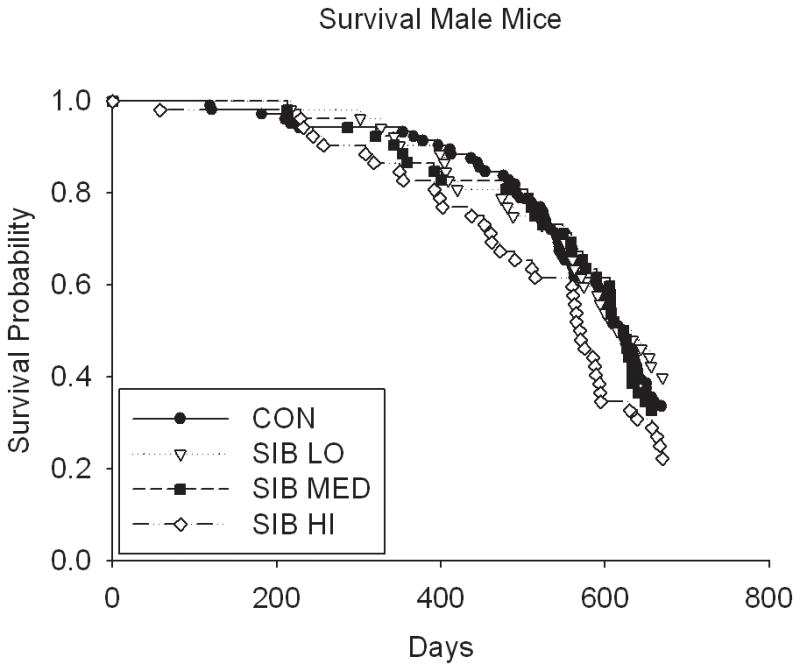
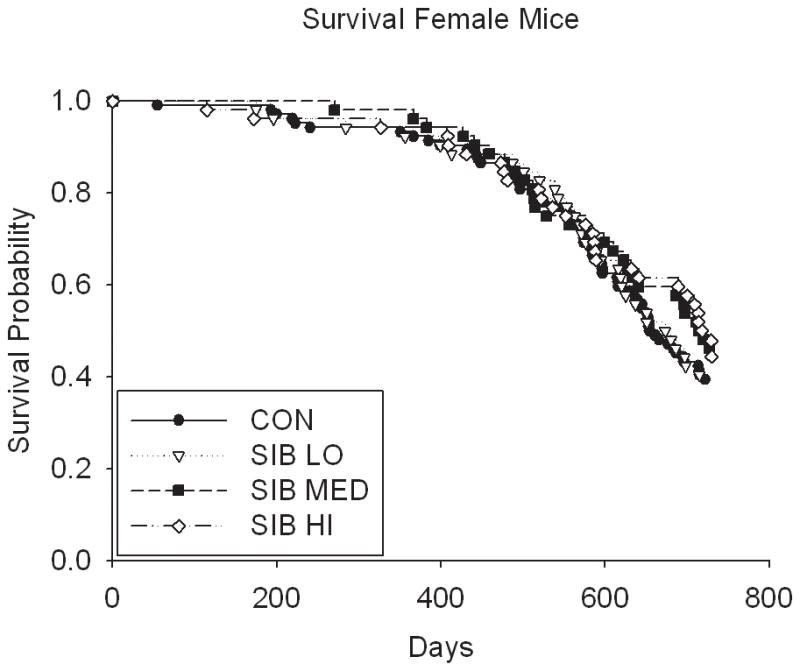
A) male rats, B) female rats, C) male mice and D) female mice.
Table 1.
Cox Regression survival analysis.*
| Species | Sex | Parameter | DF | Parameter Estimate | Standard Error | Chi-Square | Pr > ChiSq† | Hazard Ratio |
|---|---|---|---|---|---|---|---|---|
| Rats | Male | SIB LO | 1 | −0.19043 | 0.24775 | 0.5909 | 0.44 | 0.827 |
| SIB MED | 1 | −0.56049 | 0.27111 | 4.274 | 0.04 | 0.571 | ||
| SIB HI | 1 | −0.00839 | 0.23437 | 0.0013 | 0.97 | 0.992 | ||
| Likelihood ratio test for the overall model yielded a p-value of 0.16. | ||||||||
| Female | SIB LO | 1 | −0.00655 | 0.23218 | 0.0008 | 0.98 | 0.993 | |
| SIB MED | 1 | −0.34371 | 0.24916 | 1.903 | 0.17 | 0.709 | ||
| SIB HI | 1 | −0.35648 | 0.24885 | 2.0521 | 0.15 | 0.7 | ||
| Likelihood ratio test for the overall model yielded a p-value of 0.30. | ||||||||
| Mice | Male | SIB LO | 1 | −0.13364 | 0.21591 | 0.3831 | 0.54 | 0.875 |
| SIB MED | 1 | 0.01037 | 0.20717 | 0.0025 | 0.96 | 1.01 | ||
| SIB HI | 1 | 0.32853 | 0.19862 | 2.7359 | 0.10 | 1.389 | ||
| Likelihood ratio test for the overall model yielded a p-value of 0.25. | ||||||||
| Female | SIB LO | 1 | −0.03368 | 0.2194 | 0.0236 | 0.88 | 0.967 | |
| SIB MED | 1 | −0.19407 | 0.22723 | 0.7294 | 0.39 | 0.824 | ||
| SIB HI | 1 | −0.19963 | 0.22729 | 0.7714 | 0.38 | 0.819 | ||
| Likelihood ratio test for the overall model yielded a p-value of 0.74. | ||||||||
Separate analyses were performed for each species-sex combination.
p value
Discussion
Based on these results, sibutramine associated reductions in body weight gain and food intake were not associated with improvements in lifespan. However, as with most studies performed in rodents a few points merit consideration. First, the study was undertaken to assess the effect of sibutramine treatment on tumor incidence and not specifically designed as a longevity study. As such, many animals survived beyond the 2-year period of the study (Supp. Table 3), which offers lesser power to detect effects on mortality rate than does a study with all animals followed until death. Nevertheless, power was actually quite high (see below), given the sample size and anticipated effects. Second, although rodent models are commonly used for toxicology and translation research, they are not always optimal models of cardiovascular disease (16;17). Therefore, the interpretation of mortality effects using rodent models must be cautiously applied to other species, including humans, where weight loss-associated benefits on diabetes and cardiovascular disease may be realized as longevity gains. This also implies rodent models may not be ideal to reveal drug-related cardiovascular complications which have been discovered in human trials. Third, this study was performed with a single dietary composition, which may not be equivalent to the obesity-inducing diets associated with human subjects receiving sibutramine for weight loss. Since sibutramine treatment is currently prescribed for weight loss after the establishment of obesity (while this study represents long-term sibutramine treatment prior to any established obese state), the question of whether sibutramine-associated weight loss can alleviate the morbidity and mortality associated with the obese state is not directly answered. Finally, while group housing reduces study costs, it also prevents a definitive assignment of dosage received by individual animals since food intake was measured per cage.
Despite these limitations, the comprehensive nature of the study presents several strengths. First, the large sample size and duration of study provides roughly 91% power within each species to detect a 10% difference in mortality compared to controls, even when considering the limited two-year duration of the study (see appendix). This suggests the lack of an observed lifespan benefit with sibutramine treatment is not simply due to low power. Additionally, it is clear that sibutramine was efficacious for weight control, particularly for rats. While restriction amounts from 30–40% are commonly applied in rodent longevity studies, alternative methods of dietary restriction like intermittent fasting or every other day feeding have resulted in health and longevity benefits concomitant with milder amounts of food restriction (10–15%) or body weight reduction (18–20). The observed relative bodyweight and food intake reductions (~10%) of both male and female treated rats compared with controls might be expected to result in an approximately 7.3–8.4% increase in lifespan based on the relationship observed in many CR studies (with an expected relative increase of 8.6–13.3% in the SIB HI dose group) (21). Therefore, the lack of a significant effect on lifespan in rats cannot be attributed to lack of weight-loss efficacy of sibutramine. Since there were no pair-fed groups included in the study, it is not possible to determine if an equivalent food intake reduction independent of sibutramine treatment would have resulted in lifespan extension. Had such extension been observed, as might be expected, the lack of lifespan benefit with sibutramine treatment would imply sibutramine treatment offset the potential lifespan gains. Finally, the consistent animal husbandry and care applied to toxicology studies conforming to good GLP should provide a relevant backdrop to investigate treatment-related changes in lifespan.
Suggestions for Future Research
To our knowledge, the manufacturers of sibutramine have never made a specific claim regarding longevity. Nevertheless, we think it noteworthy that we do not yet have evidence, even in an animal model, that anti-obesity drugs, and sibutramine in particular, will prolong lifespan. Whether this is indicative of a fundamental difference between the mechanisms of action of pharmacologically induced weight loss and calorie restriction might be assumed, but has not been directly tested. The hypothesized reduction in mortality rate with weight loss is a rationale for the use of anti-obesity drugs, but at present remains to be demonstrated.
Our results suggest several possibilities for future research. First, we would propose that companies marketing anti-obesity drugs also conduct full longevity studies in rodents to evaluate the effects of their drugs on lifespan. We are not suggesting that such studies be deterministic as to whether or not a drug is appropriate for marketing or use, but we do suggest they add to the available information and will be helpful in decision-making. Second, given that companies conduct two-year toxicology studies of the type we have analyzed here, the raw data from those extant studies on existing drugs should be made publicly available for analysis of the type we have done and the results published. Third, given the well known finding that caloric restriction (CR) results in increased lifespan among rodents (22;23) including among rodents that have been allowed to become obese and subsequently placed on weight-loss-inducing CR (2), further mechanistic studies investigating why a drug that causes a decrease in food intake and body weight does not cause a compelling and dose-response reduction in mortality rate seem warranted.
Implications
From a basic science perspective, these data raise an interesting question. That CR, which leads to weight loss or less weight gain, decreases mortality rate has been shown repeatedly in many studies and many species. However, the mechanism(s) of action remain unclear. It has been speculated that one mechanism may be the neurobiological cascade that underlies or follows the phenomenological experience of hunger (24). If this were a primary mechanism, it might explain why sibutramine did not reduce mortality rate despite causing a dose-dependent reduction in food intake and body weight. That is, sibutramine reduces food intake by reducing hunger, whereas CR appears to increase hunger (24;25). However, methionine restriction has also been reported to increase lifespan without requiring forced food intake reductions, with animals actually eating more, particularly per gram body weight, in many cases (26–29). Whether a hunger drive is present and contributes to any longevity benefits in the methionine restriction model would also be of interest. Future research might further explore these possibilities.
With respect to clinical and public health implications, in our opinion, these data do not prove that sibutramine-induced weight loss will not improve mortality rates in humans, nor constitute evidence that sibutramine should not be used. Yet, they do temper enthusiasm for its long-term projected benefits on hard endpoints, until such benefits can be shown to accrue in other studies.
Supplementary Material
Acknowledgments
We thank Michelle Feese, Chasity Bender and Elizabeth Eadie for assistance with data entry and Dr. Kyle Grimes for editorial assistance in manuscript preparation. Supported in part by NIH grants P30DK056336, P60DK079626, R01AG033682, T32DK062710, and T32HL079888. The opinions expressed herein are those of the authors and not necessarily those of the NIH or any other organization with which the authors are affiliated.
Footnotes
Disclosures
DBA has received book royalties, grants, consulting fees, and donations from multiple profit and non-profit entities with interests in obesity, including pharmaceutical companies which compete with the manufacturers of sibutramine and from the manufacturers of sibutramine.
TRN has received grants and consulting fees from profit entities with interests in obesity and owns common stock in other profit entities with interests in obesity, including companies that compete with the manufacturers of sibutramine.
DLS, RAD and HTR: None
Reference List
- 1.Allison DB, Downey M, Atkinson RL, Billington CJ, Bray GA, Eckel RH, et al. Obesity as a disease: a white paper on evidence and arguments commissioned by the Council of the Obesity Society. Obesity (Silver Spring) 2008 Jun;16(6):1161–77. doi: 10.1038/oby.2008.231. [DOI] [PubMed] [Google Scholar]
- 2.Vasselli JR, Weindruch R, Heymsfield SB, Pi-Sunyer FX, Boozer CN, Yi N, et al. Intentional weight loss reduces mortality rate in a rodent model of dietary obesity. Obes Res. 2005 Apr;13(4):693–702. doi: 10.1038/oby.2005.78. [DOI] [PubMed] [Google Scholar]
- 3.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007 Aug 23;357(8):753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 4.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007 Aug 23;357(8):741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 5.von HS, Lainscak M, Anker SD. Sibutramine in cardiovascular disease: is SCOUT the new STORM on the horizon? Eur Heart J. 2007 Dec;28(23):2830–1. doi: 10.1093/eurheartj/ehm493. [DOI] [PubMed] [Google Scholar]
- 6.Arterburn DE, Crane PK, Veenstra DL. The efficacy and safety of sibutramine for weight loss: a systematic review. Arch Intern Med. 2004 May 10;164(9):994–1003. doi: 10.1001/archinte.164.9.994. [DOI] [PubMed] [Google Scholar]
- 7.Caterson I, Coutinho W, Finer N, Van GL, Maggioni A, Torp-Pedersen C, et al. Early Response to Sibutramine in Patients Not Meeting Current Label Criteria: Preliminary Analysis of SCOUT Lead-in Period. Obesity (Silver Spring) 2009 Oct 8; doi: 10.1038/oby.2009.327. [DOI] [PubMed] [Google Scholar]
- 8.Torp-Pedersen C, Caterson I, Coutinho W, Finer N, Van GL, Maggioni A, et al. Cardiovascular responses to weight management and sibutramine in high-risk subjects: an analysis from the SCOUT trial. Eur Heart J. 2007 Dec;28(23):2915–23. doi: 10.1093/eurheartj/ehm217. [DOI] [PubMed] [Google Scholar]
- 9.Toxicology and Carcinogenesis Studies of Ephedrine Sulfate (CAS No. 134-72-5) in F344/N Rats and B6C3F1 Mice (Feed Studies) National Toxicology Program: Technical Report Series 2010. 307:1–188. Available from: URL: http://ntp.niehs.nih.gov/ntp/htdocs/LT_rpts/tr307.pdf. [PubMed] [Google Scholar]
- 10.Janiak P, Poirier B, Bidouard JP, Cadrouvele C, Pierre F, Gouraud L, et al. Blockade of cannabinoid CB1 receptors improves renal function, metabolic profile, and increased survival of obese Zucker rats. Kidney Int. 2007 Dec;72(11):1345–57. doi: 10.1038/sj.ki.5002540. [DOI] [PubMed] [Google Scholar]
- 11.Comprehensive Rimonabant Evaluation Study of Cardiovascular ENDpoints and Outcomes (CRESCENDO) ClinicalTrials gov. 2009. Aug 27, [cited 2010 Apr 14];Available from: URL: http://www.clinicaltrials.gov/ct/show/NCT00263042.
- 12.Van Gaal LF, Caterson ID, Coutinho W, Finer N, Maggioni AP, Sharma AM, et al. Weight and blood pressure response to weight management and sibutramine in diabetic and non-diabetic high-risk patients: an analysis from the 6-week lead-in period of the sibutramine cardiovascular outcomes (SCOUT) trial. Diabetes Obes Metab. 2010 Jan;12(1):26–34. doi: 10.1111/j.1463-1326.2009.01090.x. [DOI] [PubMed] [Google Scholar]
- 13.FDA; 2009. Nov 20, Early Communication about an Ongoing Safety Review of Meridia (sibutramine hydrochloride) [cited 2010 Apr 14];Available from: URL: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm191650.htm. [Google Scholar]
- 14.FDA; 2010. Jan 21, Follow-Up to the November 2009 Early Communication about an Ongoing Safety Review of Sibutramine, Marketed as Meridia. [cited 2010 Apr 14];Available from: URL: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm198206.htm. [Google Scholar]
- 15.Viner RM, Hsia Y, Neubert A, Wong IC. Rise in antiobesity drug prescribing for children and adolescents in the UK: a population-based study. Br J Clin Pharmacol. 2009 Dec;68(6):844–51. doi: 10.1111/j.1365-2125.2009.03528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell JC, Proctor SD. Small animal models of cardiovascular disease: tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis. Cardiovasc Pathol. 2006 Nov;15(6):318–30. doi: 10.1016/j.carpath.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Russell JC. Of mice and men, rats and atherosclerosis. Cardiovasc Res. 2003 Oct 1;59(4):810–1. doi: 10.1016/s0008-6363(03)00530-3. [DOI] [PubMed] [Google Scholar]
- 18.Arum O, Bonkowski MS, Rocha JS, Bartke A. The growth hormone receptor gene-disrupted mouse fails to respond to an intermittent fasting diet. Aging Cell. 2009 Dec;8(6):756–60. doi: 10.1111/j.1474-9726.2009.00520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonkowski MS, Dominici FP, Arum O, Rocha JS, Al Regaiey KA, Westbrook R, et al. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS One. 2009;4(2):e4567. doi: 10.1371/journal.pone.0004567. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Carlson AJ, Hoelzel F. J Nutr. 1946;31:363–75. doi: 10.1093/jn/31.3.363. [DOI] [PubMed] [Google Scholar]
- 21.Merry BJ. Molecular mechanisms linking calorie restriction and longevity. Int J Biochem Cell Biol. 2002 Nov;34(11):1340–54. doi: 10.1016/s1357-2725(02)00038-9. [DOI] [PubMed] [Google Scholar]
- 22.McCay CM, Crowell MF. The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr. 1935;10:63–79. Ref Type: Generic. [PubMed] [Google Scholar]
- 23.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005 Sep;126(9):913–22. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Minor RK, Chang JW, de CR. Hungry for life: How the arcuate nucleus and neuropeptide Y may play a critical role in mediating the benefits of calorie restriction. Mol Cell Endocrinol. 2009 Feb 5;299(1):79–88. doi: 10.1016/j.mce.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Speakman JR, Hambly C. Starving for life: what animal studies can and cannot tell us about the use of caloric restriction to prolong human lifespan. J Nutr. 2007 Apr;137(4):1078–86. doi: 10.1093/jn/137.4.1078. [DOI] [PubMed] [Google Scholar]
- 26.Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr. 1993 Feb;123(2):269–74. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- 27.Zimmerman JA, Malloy V, Krajcik R, Orentreich N. Nutritional control of aging. Exp Gerontol. 2003 Jan;38(1–2):47–52. doi: 10.1016/s0531-5565(02)00149-3. [DOI] [PubMed] [Google Scholar]
- 28.Hasek BE, Stewart LK, Henagan TM, Boudreau A, Lenard NR, Black C, et al. Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. Am J Physiol Regul Integr Comp Physiol. 2010 Jun 10; doi: 10.1152/ajpregu.00837.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plaisance EP, Henagan TM, Echlin H, Boudreau A, Hill KL, Lenard NR, et al. Role of {beta}-adrenergic receptors in the hyperphagic and hypermetabolic responses to dietary methionine restriction. Am J Physiol Regul Integr Comp Physiol. 2010 Jun 16; doi: 10.1152/ajpregu.00838.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


