Abstract
Toll-like receptors (TLR) play a role in mediating the proinflammatory response, fibrogenesis and carcinogenesis in chronic liver diseases such as alcoholic liver disease, non-alcoholic liver disease, hepatitis C and hepatocellular carcinoma. This is true in experimental models of these diseases. For this reason, we investigated the TLR proinflammatory response in the chronic intragastric tube feeding rat model of alcohol liver disease. The methyl donor S-adenosylmethionine was also fed to prevent the gene expression changes induced by ethanol. Ethanol feeding tended to increase the up regulation of the gene expression of TLR2 and TLR4. SAMe feeding prevented this. TLR4 and MyD88 protein levels were significantly increased by ethanol and this was prevented by SAMe. This is the first report where ethanol feeding induced TLR2 and SAMe prevented the induction by ethanol. CD34, FOS, interferon responsive factor 1 (IRF-1), Jun, TLR 1,2,3,4,6 and 7 and Traf-6 were found to be up regulated as seen by microarray analysis where rats were sacrified at high blood alcohol levels compared to pair fed controls. Il-6, IL-10 and IFNγ were also up regulated by high blood levels of ethanol. The gene expression of CD14, MyD88 and TNFR1SF1 were not up regulated by ethanol but were down regulated by SAMe. The gene expression of IL-1R1 and IRF1 tended to be up regulated by ethanol and this was prevented by feeding SAMe. The results suggest that SAMe, fed chronically prevents activation of TLR pathways caused by ethanol. In this way the proinflammatory response, fibrogenesis, cirrhosis and hepatocellular carcinoma formation due to alcohol liver disease could be prevented by SAMe.
Keywords: Toll-like receptor (TLR), S-adenosylmethionine (SAMe), alcoholic liver disease (ASH)
INTRODUCTION
What is the mechanism by which alcohol abuse increases the risk of hepatocellular carcinoma in patients with hepatitis C, hepatitis B, non-alcoholic and alcoholic liver disease, hemachromatosis and α1-antitrypsin deficiency? Evidence is emerging that the synergism is due to the activation of a common pathway in which Toll-like receptor (TLR) signaling induces proinflammatory cytokine production through NFκB up regulation of growth factors by activation of activator protein 1 (Mandrekar et al., 2009; Rodrigues et al., 2008). The presence of toxins in the body (like lipopolysacharrides and alcohol) induces an inflammatory reaction in the liver. LPS recognition by Toll-like receptor 4 (TLR4) on macrophages and other cell types in the liver and activation of downstream signaling pathways culminating in activation of transcription factors such as NFkB and AP-1 lead to increase inflammatory cytokine production in alcoholic liver diseases. In the model of chronic ethanol feeding by intragastric cannula, we observed mRNA expression increase of the TLR genes and the TLR signaling pathway, in rat livers obtained after one month of ethanol feeding. Increased activation of the TLR4 signaling pathway by ethanol, which was dependent on Trif and not the MyD88 pathway, had been reported earlier (Hansen et al., 1994; Hritz et al., 2008; Uesugi et al., 2001).
In the refeeding drug-primed mouse model, we observed that the activation of the TLR pathway, when used with the drug DDC, was prevented by SAMe (Bardag-Gorce et al., 2010c). Based on that previous observation, we postulated that SAMe fed with ethanol would prevent TLR up regulation (Bardag-Gorce et al., 2010c). In a prior study with ethanol using the intragastric cannula feeding model, we observed that the expression of a large number of genes were induced by high blood ethanol levels but were barely changed at low blood alcohol levels (BAL) trough of the BAL cycle (Bardag-Gorce et al., 2006). Similar results were observed when rats were sacrificed after being fed SAMe (Bardag-Gorce et al., 2010a). However, from microarray gene mining, it was observed that numerous changes in the expression of cytokines and TLR were prevented when SAMe was fed with ethanol. Specifically, IL-1-2, Tnfrsf6, IL-1R1, CxC14, Ccl6, TLR4, TLR2, CxC12, Tgfβr3 and Tnf were all up regulated by ethanol when sacrified at low blood ethanol levels. This was prevented when SAMe was added to the diet.
To further investigate this phenomenon, PCR arrays (SA Biosciences) on mRNA of the same rat livers studied above were done to further explore changes in cytokines and TLR pathways. The changes noted were confirmed partially by qRT-PCR. The results of this present study have been reported in part in an abstract (Oliva et al., 2010).
METHODS
Animals
Male rats were fed ethanol with or without SAMe intragastrically for 1 month. Results from the animals used have been reported previously. A portion of the fast frozen livers stored at −80°C were used in the present study to further characterize the effects of ethanol and SAMe on the expression of cytokines and the TLR pathway using PCR arrays (SA Biosciences) n=1. The treatment of the animals has been published (Bardag-Gorce et al., 2010a). One male rat fed ethanol intragastrically for 1 month was sacrificed at the peak blood alcohol level, in order to generate hypoxia and select proteins for gene expression analysis. A pair-fed control was also sacrificed at the same time. The livers from both animals were subjected to microplate array specific for the TLR signaling pathway (SA Bioscience a Qiagen company).
Quantitative Real-time RT-PCR Assay
Total liver RNAs were extracted with Trizol Plus RNA Purification kit (Invitrogen, Carlsbad, CA). Synthesis of cDNAs was performed with 5 μg total RNA, and 50 ng random hexamer primers using SuperSriptIII RNase H− Reverse Transcriptase (Invitrogen, Carlsbad, CA). PCR primers were designed with the Primer Express software (Applied Biosystems, Foster City, CA).
| Gene | Identification | Forward | Reverse |
|---|---|---|---|
| CD 14 | NM_021744.1 | CCGGGAACTGACTCTTGAAAAC | CATCCAGAAGCGGCGAAA |
| Keap-1 | NM_057152.1 | CAGAACAAGCCATGCCTTCTT | TCTGGTCTTCCACAAGGTCCTT |
| TLR2 | NM_198769.2 | GCAGTGAGTGGTGCAAGTATGAA | CGCGTCATTGTTCTCGTCAA |
| TLR3 | NM_198791.1 | GCACTTTCTCCGGGCTGAA | GTCGCGGAGGCTGTTGTAG |
| TLR4 | NM_019178.1 | GGTGTGAAATTGAGACAATTGAAGAC | GTITCCTGTCAGTACCAAGGTTGA |
| TLR9 | NM_198131.1 | TCCTCCAGAAACTGGATGTCAGT | TCTACCGCCAGAGCAAAGAAG |
| IL1R1 | NM_013123.3 | CCCATATCAGCGGACAAGGA | TGGCGGGAACAAACCAAA |
| IRF1 | M34253.1 | CCCAAGACTTGGAAGGCAAA | TGGTCCTTCACTTCCTCGATGT |
| Myd88 | NM_198130.1 | GCCAGCGAGCTCATTGAGA | TITGCAGGTAATCGTCAGAAACA |
| TNRF1SF1 | NM_153629.1 | TGCTGCTGACATGACAAAGGTA | GC AGGTTC AGCTTACTATTCATCCA |
Western Blot Analysis
Proteins (50 μg) from liquid nitrogen frozen stored livers and nuclear and histone extracts were separated by SDS-PAGE gels and transferred to a PVDF membrane (Bio-Rad, Hercules, CA) for 1 h in 25 mM Tris-HCl (pH 8.3), 192 mM glycine and 20% methanol. The membranes were stained using primary antibodies against TLR4 (BioVision, Mountain View, CA) and Myd88 (Thermo Scientific, Worcester MA). Appropriate species polyclonal and monoclonal HRP-conjugated antibodies were used as the secondary antibodies. The membranes were subjected to chemiluminescence detection using luminol, according to the manufacturer’s instructions (Amersham Pharmacia Biotech, Piscataway, NJ).
Statistics
Data were obtained from at least three separate experiments. Bars represent mean values ± SEM. P values were determined by one-way ANOVA and Student-Newman Keuls for multiple group comparisons (Sigma-Stat software, San Francisco, CA). Statistical significance is set at p= < 0.05.
RESULTS
PCR microplate array analysis specific for the rat TLR signaling pathway was performed (SA Biosciences) on a rat fed ethanol for 1 month intragastrically and sacrificed at the peak blood alcohol level. It was compared with a pair fed control fed isocaloric glucose. The results are shown in Table 1 (n=1). This PCR array analysis led to the study of the key player genes in the TLR signaling pathway such as TLR 2, 4, 3, 9 and the associated proteins CD14, MyD88. The levels of proinflammatory cytokines and chemokine mRNA levels were also investigated. To determine whether s-adenosylmethionine (SAMe) supplementation would prevent these changes in the TLR signaling pathway caused by ethanol, qRT-PCR was performed on the 4 groups of rats fed ethanol for 1 month. The expression of a number genes, which play an important role in the pathogenesis of alcoholic liver disease, were found to be up regulated, e.g. TLR1,2,4,5,6,7, CD34, FOS, Jun oncogene, IRF-1 and Traf-6 (Table 1).
Table 1.
Data mining analysis of TLR signaling pathway using specific PCR microarray (BAL Peak vs. Control)
| Description | Gene Name | FC |
|---|---|---|
| CD14 molecule | Cd14 | 2.82 |
| Cd80 molecule | Cd80 | 1.9 |
| CD86 molecule | Cd86 | 1.86 |
| C-type lectin domain family 4, member e | Clec4e | 26.53 |
| Eukaryotic translation initiation factor 2-alpha kinase 2 | Eif2ak2 | 1.68 |
| FBJ osteosarcoma oncogene | Fos | 17.38 |
| Hypoxanthine phosphoribosyltransferase 1 | Hprt 1 | 1.22 |
| Heat shock 70kD protein 1A | Hspa 1a | 1.98 |
| Interferon-alpha 1 | Ifna 1 | 2.49 |
| Interferon gamma | Ifng | 2.02 |
| Interferon regulatory factor 1 | Irf 1 | 2.53 |
| Inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase beta | Ikbkb | 2.28 |
| Interleukin 1D | II 10 | 4.08 |
| Interleukin 1 receptor, type I | II1r1 | 3.22 |
| Interleukin 2 | II2 | 2.18 |
| Interleukin 6 | II6 | 5.09 |
| Interleukin-1 receptor-associated kinase 1 | Irak 1 | 1.21 |
| Interleukin-1 receptor-associated kinase 2 | Irak 2 | 1.94 |
| Jun oncogene | Jun | 3.86 |
| Lymphotoxin alpha (TNF superfamily, member 1) | Lta | 1.31 |
| Lymphocyte antigen 96 | Ly96 | 1.54 |
| Mitogen activated protein kinase kinase 3 | Map2k3 | 1.79 |
| Mitogen activated protein kinase kinase 4 | Map2k4 | 1.5 |
| Mitogen activated protein kinase kinase kinase 7 | Map3k7 | 2.12 |
| Mitogen-activated protein kinase 8 interacting protein 3 | Mapk8ip3 | 1.86 |
| Mitogen-activated protein kinase 9 | Mapk9 | 2.15 |
| Myeloid differentiation primary response gene 88 | Myd88 | 1.24 |
| Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha | Nfkbia | 1.67 |
| Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, beta | Nfkbib | 1.8 |
| Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor-like 1 | Nfkbil 1 | 3.09 |
| Nuclear factor related to kappa B binding protein | Nfrkb | 1.8 |
| Nuclear receptor subfamily 2, group C, member 2 | Nr2c2 | 1.69 |
| Pellino 1 | Peli 1 | 1.93 |
| Peptidoglycan recognition protein 1 | Pglyrp 1 | 3.25 |
| Peroxisome proliferator activated receptor alpha | Ppara | 1.6 |
| Prostaglandin-endoperoxide synthase 2 | Ptgs2 | 2.18 |
| V-rel reticuloendotheliosis viral oncogene homolog (avian) | Rel | 1.63 |
| V-rel reticuloendotheliosis viral oncogene homolog A (avian) | Rela | 2.09 |
| Receptor-interacting serine-threonine kinase 2 | Ripk2 | 1.31 |
| Ring finger protein 138 | Rnf138 | 1.64 |
| Sterile alpha and TIR motif containing 1 | Sarm 1 | 5.31 |
| Toll-like receptor adaptor molecule 2 | Ticam2 | 3.53 |
| Toll-like receptor 1 | Tlr1 | 3.73 |
| Toll-like receptor 2 | Tlr2 | 2.8 |
| Toll-like receptor 3 | Tlr3 | 3.27 |
| Toll-like receptor 4 | Tlr4 | 1.4 |
| Toll-like receptor 5 | Tlr5 | 1.45 |
| Toll-like receptor 6 | Tlr6 | 4.85 |
| Toll-like receptor 7 | Tlr7 | 1.85 |
| Toll-like receptor 9 | Tlr9 | 1.33 |
| Toll interacting protein | Tollip | 1.8 |
| Tumor necrosis factor (TNF superfamily, member 2) | Tnf | 1.2 |
| Tumor necrosis factor receptor superfamily, member 1a | Tnfrsf 1a | 2.47 |
| TNFAIP3 interacting protein 2 | Tnip2 | 1.86 |
| TNFRSF 1A-associated via death domain | Tradd | 1.53 |
| Tnf receptor-associated factor 6 | Traf6 | 2.26 |
Quantitated RT-PCR Results
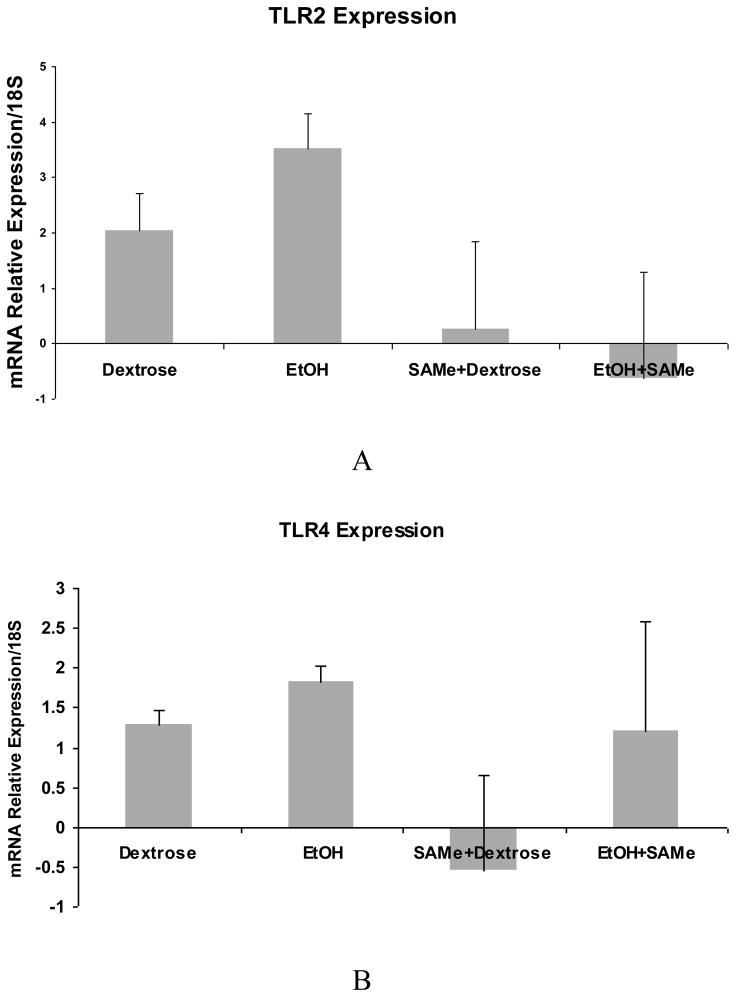
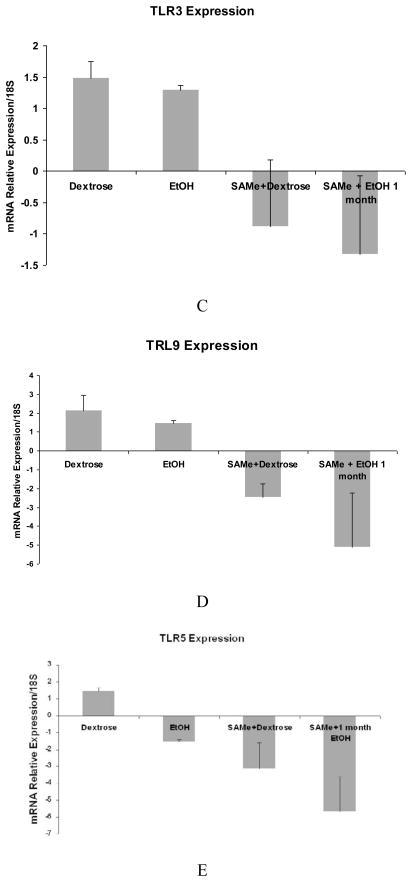
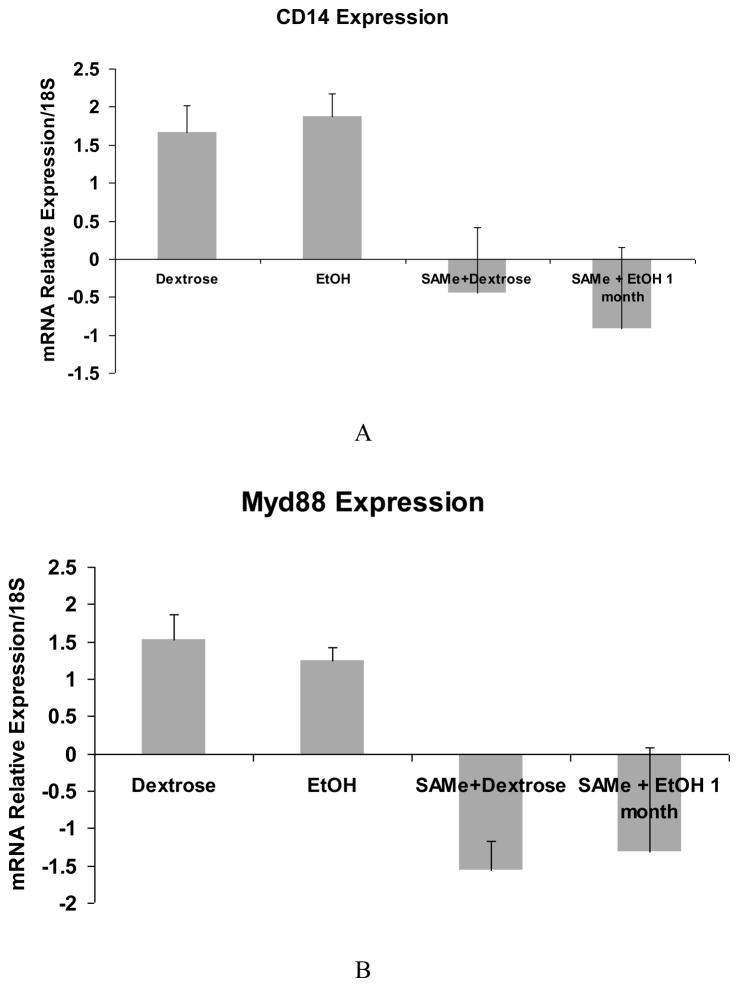
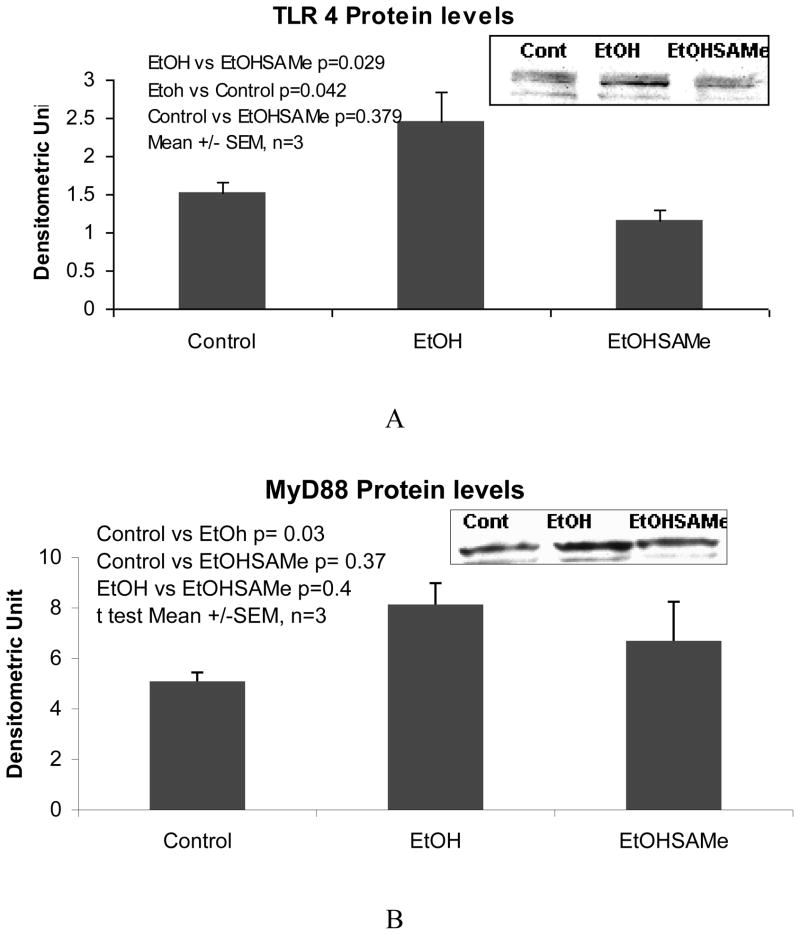
A trend in the up regulation of mRNA of TLR2 and TLR4 expression by ethanol was found. SAMe feeding prevented this increase (Fig 1A and B). However, TLR3 and TLR9 were not up regulated by ethanol but were down regulated by SAMe (Fig 1C and D). The expression of TLR 5 was down regulated and SAMe amplified this decrease (Fig 1E). However TLR5 expression was up regulated when the blood alcohol level was at its peak (Table 1). The expression of CD14 was not changed by ethanol feeding in this group of rats and MyD88 tended to be decreased by ethanol feeding. SAMe decreased both these gene expressions (Fig 2A and B). However, the protein expression of TLR4 and MyD88 levels were increased by ethanol feeding and SAMe supplementation prevented this increase (Fig 3A and B).
Fig 1.
qRT-PCR analysis of TLR2 (A) and TLR4 (B), gene expression tended to be increased by ethanol, but not TLR3 (C), TLR9 (D) and TLR5 (E). This increase or the expression of mRNA was down regulated when SAMe was supplemented with ethanol. (Mean ± SEM, n=3). (C: Dex vs. SAMe+EtOH p=0.049) (D: Dex vs. SAMe+EtOH p=0.04; EtOH vs. SAMe+dex p=0.006).
Figure 2.
qRT-PCR analysis of CD14 (A) and MyD88 (B) gene expression. Note that there was no change in CD14 expression in the liver of rats fed ethanol but there was a small decrease in MyD88 expression in the liver of ethanol fed rats. SAMe down regulated the expression of MyD88 with or without ethanol. (Mean ± SEM, n=3) (B: Dex vs. SAMe+Dex p=0.002; EtOH vs. SAMe+Dex p=0.003)
Figure 3.
Protein levels analyzed by Western blot showed that TLR4 and MyD88 were increased by ethanol feeding. SAMe supplementation prevented this increase.
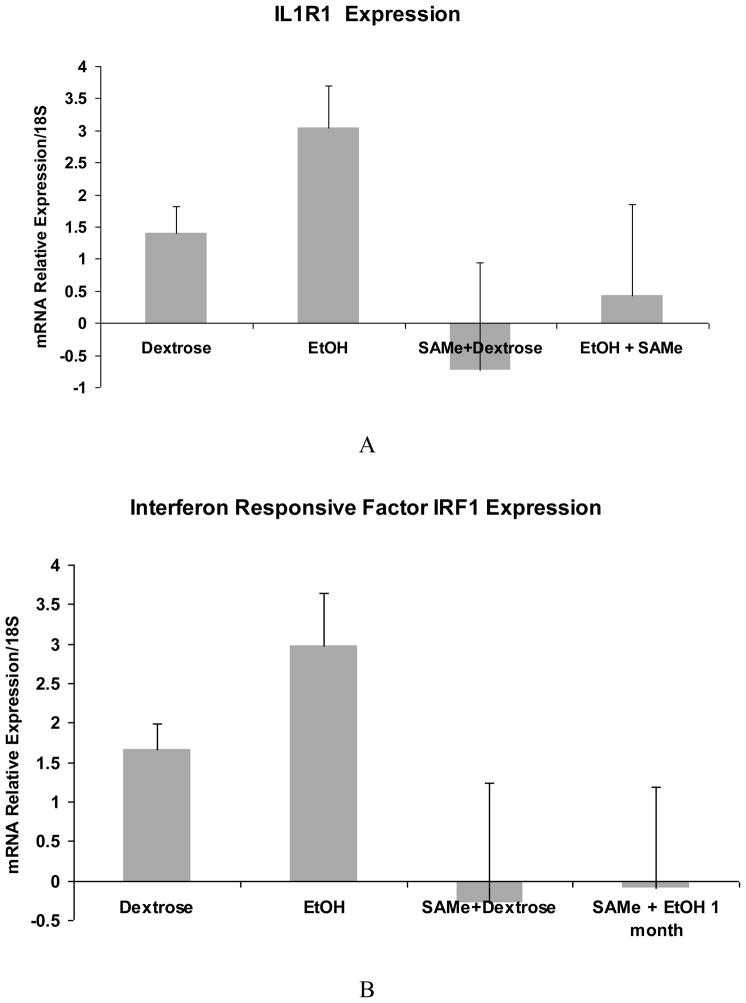
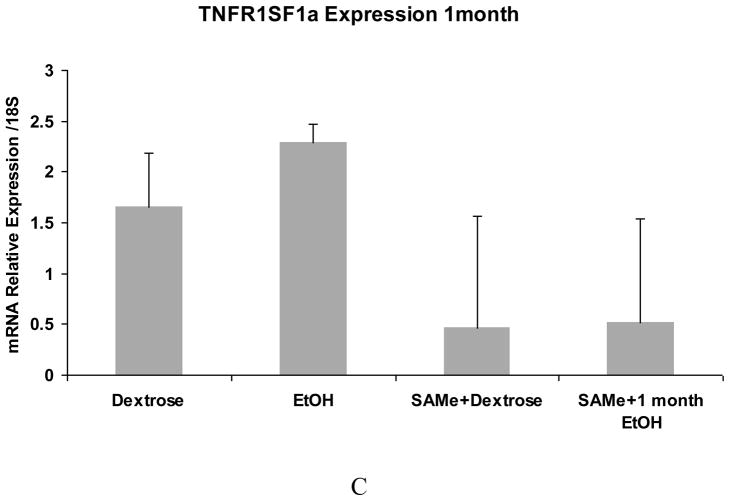
The expression of IL-1R1 and interferon response factor 1 (IRF1) tended to be up regulated by ethanol and this up regulation was prevented by SAMe (Fig 4A and B). TNFR1SF1a (Fig 4C) up regulation, found in the PCR arrays, was not confirmed by qRT-PCR although the expression was down regulated by SAMe. There was a trend in TNFR1SF1a to be increased by ethanol but not significantly. The large variation between the samples could be caused by different levels of blood alcohol at the time that the rats were sacrificed.
Fig 4.
qRT-PCR analysis of IL-1R1 (A), IRF1 (B) and TNFR1SF1a (C) gene expression. Note that ethanol feeding tended to increase the mRNA levels of IL-R1 and IRF-1. SAMe supplementation tended to decrease this up regulation. TNFR1SF1a expression was not significantly increased by ethanol feeding although SAMe supplementation tended to decrease the expression of TNFRISF1a.. (Mean ±SEM, n=3)
DISCUSSION
Toll-like receptors are involved in an adaptive immune response. Ten Toll—like receptors have been identified in humans and 13 in mice (Kawai and Akira, 2006)). The activation of the Toll-like receptors induce the recruitment of adapter molecules such as myeloid differentiation factor-88 (MyD88), TIR-associated protein (TIRAP), Toll receptor-associated-activator of interferon (TRIF) and/or Toll-receptor-associated molecule (TRAM) (Adams, 2009). Toll-like receptors in the liver mediate a proinflammatory response to the presence of their respective ligands (Kawai and Akira, 2006). TLRs are activated mainly by pathogen-associated molecular patterns (PAMP) but they can also be activated by endogenous molecules that are associated with cell damage or wound-healing responses that promote inflammation. These are termed damage-associated molecular patterns (DAMPS) (Pradere et al., 2010). Some DAMPS include high mobility group protein b-1 (HMGB1), S-100 proteins, heat shock proteins, hyaluronan and fibronectin. Most of these ligands have been postulated to be direct agonists of TLR2 or TLR4 or both receptors (Pradere et al., 2010). Saturated free fatty acids may also activate TLR2 and 4. Both HMGB1 and hyaluronan are increased in liver disease. It has been suggested that HMGB signals the presence of necrosis and subsequently triggers inflammation (Pradere et al., 2010). It facilitates and amplifies the inflammatory response to different cytokines and TLR4/2 agonists. Hyaluronan can activate a TLR2/4 inflammatory response by macrophages. Hepatocytes express TLR4 but their response is fairly weak compared to the Kupffer cell response.
Lipopolysaccharide (LPS) from gram-negative bacterial products, derived from the gut due to ethanol ingested, alter the intestinal permeability to LPS and activate TLR4 (Pradere et al., 2010). LPS is transferred to CD14 by a lipid transferase LPS-binding protein (Ulevitch and Tobias, 1995). LPS/CD14 stimulates TLR4/MD-2 on the cell surface (Shimazu et al., 1999). This usually involves the TLR4-MD2 receptor complex after binding to CD14 and lipopolysaccharide binding protein (LBP). This leads to activation of the MyD88-induced signaling and activation of IRAK 1, IRAK4 and IRF5 to induce the expression of the proinflammatory cytokines. In parallel, using the MyD88-independent pathway, LPS activates TRIF, IRF3 to induce the expression of Type I Interferon (Lu et al., 2008). When LBP and CD14 are not involved, the activated pathways are restricted to the MyD88 pathway leading to IRF-5. The qRT-PCR results in the present study indicate that CD14, MyD88, and TLR4 genes were not significantly over expressed. However, the MyD88 and TLR4 proteins were increased by ethanol and the induction of TLR4 was prevented by SAMe, indicating a potential activation of these proteins by alcohol. EtOH feeding activates the MyD88 dependant pathway inducing the production of proinflammatory cytokines. The other TLR receptors such as TLR3, TLR5 and TLR9 are not induced by alcohol and don’t seem to be involved in the proinflammatory response unless the blood alcohol level (BAL) is at its peak level in the BAL cycle. We found that TLR5 expression is induced by high blood alcohol levels (500mg %) and not by medium blood alcohol levels ((200–300mg%) (Bardag-Gorce et al., 2010b).
Indication of TLR2 expression in respiratory epithelium in vitro by ethanol has been reported where it was shown to be up regulated through a NO/cGMP dependent pathway (Bailey et al., 2010; Bailey et al., 2009). Heavy alcohol use is associated with severe bronchitis. This is related to inflammation in the airway of alcohol abusers. TLR2 is an important mediator of inflammation in the airway epithelium. TLR2 initiates an inflammatory response to gram-positive bacteria in response to its ligand and peptidoglycan derived from streptococcus pneumonia. Alcohol down regulates TLR2 mediated inflammatory signaling in macrophages (Bailey et al., 2010). This is the first report showing a tendency for the induction of the expression of TLR2 in the liver of rats fed ethanol chronically. It was elevated at the high BAL. We found that TLR2 was up regulated in the liver of mice that had developed chronic cholestasis when fed a carcinogen DDC (Bardag-Gorce et al., 2010c). As in the current study, feeding of SAMe with the drug DDC prevented the up regulation of TLR2, accompanied by a decrease in the liver pathology (Bardag-Gorce et al., 2010c).
Previous reports from different laboratories showed that LTR activation induced the activation of MAPK pathways to activate ultimately AP-1 (Li et al., 2010)). Based on the PCR array, the expression of different MAPKs were up regulated by high blood alcohol levels and could be involved in the transduction signal downstream of the TLRs, in our model (Li et al., 2010).
In order to balance the activation of TLR4, inhibitory pathways are necessary to protect the organism against pro-inflammation-induced damage. Different inhibitors were identified: RP105, IL1R1 and SIGIRR (Brint et al., 2004; Divanovic et al., 2005; Mansell et al., 2006). In our study, the expression of IL1R1 tended to be up regulated by alcohol to balance the over expression of TLR2 and TLR4. This result indicates that SAMe could mimic an over activation of IL1R1 and other inhibitors of the TLR4 activation to prevent the inflammatory response induced by the alcohol.
TLRs are thought to play a role in chronic liver diseases, causing inflammation and fibrogenesis (Pradere et al., 2010) and ultimately liver cancer formation (French et al., 2010; Machida et al., 2009). The down regulation of TLR signaling by SAMe feeding described here suggests that SAMe prophylaxis in chronic liver disease could abort the procirrhosogenic and procarcinogenic outcomes of chronic liver disease.
Acknowledgments
Res Funded by NIH/NIAAA Grant R01-8116 and P50-11999 Morphology Core
The authors thank Adriana Flores for typing the manuscript. This study was supported by a grant from NIH/NIAAA 8116.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams S. Toll-like receptor agonists in cancer therapy. Immunotherapy. 2009;1:949–64. doi: 10.2217/imt.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KL, et al. Alcohol up-regulates TLR2 through a NO/cGMP dependent pathway. Alcohol Clin Exp Res. 2010;34:51–6. doi: 10.1111/j.1530-0277.2009.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KL, et al. Alcohol functionally upregulates Toll-like receptor 2 in airway epithelial cells. Alcohol Clin Exp Res. 2009;33:499–504. doi: 10.1111/j.1530-0277.2008.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardag-Gorce F, et al. Gene expression patterns of the liver in response to alcohol: in vivo and in vitro models compared. Exp Mol Pathol. 2006;80:241–51. doi: 10.1016/j.yexmp.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Bardag-Gorce F, et al. The cyclic pattern of blood alcohol levels during continuous ethanol feeding in rats: the effect of feeding S-adenosylmethionine. Exp Mol Pathol. 2010a;88:380–7. doi: 10.1016/j.yexmp.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardag-Gorce F, et al. The effects of high vs. low blood alcohol levels (BAL) on toll-like receptors during the BAL cycle. ASCB Meeting; Philadelphia. 2010b. [Google Scholar]
- Bardag-Gorce F, et al. SAMe prevents the up regulation of toll-like receptor signaling in Mallory-Denk body forming hepatocytes. Exp Mol Pathol. 2010c;88:376–9. doi: 10.1016/j.yexmp.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brint EK, et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5:373–9. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- Divanovic S, et al. Negative regulation of Toll-like receptor 4 signaling by the Toll-like receptor homolog RP105. Nat Immunol. 2005;6:571–8. doi: 10.1038/ni1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SW, et al. Mallory-Denk Body Pathogenesis Revisited. World J Hepathology. 2010;2:295–301. doi: 10.4254/wjh.v2.i8.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, et al. The role of tumor necrosis factor-alpha in acute endotoxin-induced hepatotoxicity in ethanol-fed rats. Hepatology. 1994;20:461–74. [PubMed] [Google Scholar]
- Hritz I, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–31. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–25. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Li X, et al. Toll-like receptor signaling in cell proliferation and survival. Cytokine. 2010;49:1–9. doi: 10.1016/j.cyto.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YC, et al. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–51. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Machida K, et al. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl Acad Sci U S A. 2009;106:1548–53. doi: 10.1073/pnas.0807390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar P, et al. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J Immunol. 2009;183:1320–7. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansell A, et al. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol. 2006;7:148–55. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- Oliva J, et al. SAMe Blocks the Up Regulation of Toll-like Receptor Signaling by Alcohol. Vol. 34. ISBRA, ACER; Paris: 2010. p. 152A. [Google Scholar]
- Pradere JP, et al. Toll-like receptor 4 and hepatic fibrogenesis. Semin Liver Dis. 2010;30:232–44. doi: 10.1055/s-0030-1255353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues A, et al. Activation of toll-like receptor 4 (TLR4) by in vivo and in vitro exposure of rat epididymis to lipopolysaccharide from Escherichia Coli. Biol Reprod. 2008;79:1135–47. doi: 10.1095/biolreprod.108.069930. [DOI] [PubMed] [Google Scholar]
- Shimazu R, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–82. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesugi T, et al. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–8. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–57. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]