Abstract
Bcl-2 proteins represent a rheostat that controls cellular viability. Obatoclax, a BH3-mimetic, has been designed to specifically target and counteract anti-apoptotic Bcl-2 proteins. We evaluated the biological effects of obatoclax on the anti-tumour activity of rituximab and chemotherapy agents. Obatoclax induced cell death of rituximab/chemotherapy-sensitive (RSCL), -resistant cell lines (RRCL) and primary tumour-cells derived from patients with B-cell lymphomas (N=39). Obatoclax also enhanced the activity of rituximab and had synergistic activity when combined with chemotherapy agents. The ability of Obatoclax to induce PARP cleavage varied between patient samples and was not observed in some RRCL. Inhibition of caspase activity did not affect obatoclax activity, suggesting the existence of caspase-independent death pathways. Autophagy was detected by LC3 conversion and/or electron microscopy in RRCL and in patient-derived tumour cells. Moreover, obatoclax activity was inhibited by Beclin-1 knockdown. In summary, obatoclax is an active Bcl-2 inhibitor that potentiates the activity of chemotherapy agents and, to a lesser degree, rituximab. Defining the molecular events triggered by obatoclax is necessary to further its clinical development and identify potential biomarkers that are predictive of response.
Keywords: Obatoclax, Rituximab, Lymphoma
Introduction
The use of rituximab in combination with systemic chemotherapy has not only improved the clinical outcome of patients with B-cell lymphoma, but appears to be changing the biology of relapsed/refractory disease.(Coiffier, et al 2002, Czuczman, et al 1999, Hagberg and Gisselbrecht 2006, Pfreundschuh, et al 2008, Pfreundschuh, et al 2006) Martin et. al. (2008) reported that patients with diffuse large B-cell lymphoma (DLBCL) failing upfront rituximab-based immunochemotherapy had lower response rates to salvage chemotherapy, as well as inferior progression-free survival (PFS) and overall survival (OS) rates after high dose chemotherapy and autologous stem cell support (HDC-ASCS) when compared to historical controls. In order to address the challenge of managing relapsed/refractory B-cell lymphoma in the rituximab era, it is important to evaluate novel therapeutic strategies targeting key regulatory pathways associated with rituximab-chemotherapy resistance.
Bcl-2 was the first protein involved in regulating apoptosis that was determined to be an oncogene.(Cleary, et al 1986, Tsujimoto, et al 1985) Aberrant expression of Bcl-2 family members may lead to an increase in the apoptotic threshold of cancer cells and association with chemotherapy resistance resulting in poor clinical outcomes seen in subsets of refractory non-Hodgkin lymphoma (NHL).(Bannerji, et al 2003, Cory and Adams 2002, Gascoyne, et al 1997, Sohn, et al 2003)
Functionally, Bcl-2 family members can promote or prevent program cell death and can be subdivided into three groups: 1) anti-apoptotic (Bcl-2, Mcl-1, A1, Bcl-XL and Bcl-w), 2) pro-apoptotic (Bak, Bax), and 3) BH3 single domain pro-apoptotic proteins (Bim, Puma, Noxa, Bid, Bik, Hrk, Bad and Bmf) that potentiate the effects of Bax and Bak upon activation following cytotoxic signals.(Adams, et al 2005)
It is postulated that tissue homeostasis is regulated largely by the balance between BH3-only proteins and Bcl-2 pro-survival family members. Upon cell damage (e.g. exposure to chemotherapy agents) BH3-only proteins are activated and inactivate Bcl-2 anti-apoptotic proteins by inserting their shared BH3 domain into a hydrophobic groove on the surface of Bcl-2 pro-survival proteins.(Kim, et al 2006) The neutralization of Bcl-2 pro-survival proteins by BH3-only proteins favours the activation and oligomerization of Bax/Bak that leads to changes in mitochondrial potential and apoptosis.(Cheng, et al 2001) The activation of BH3-only proteins is complex and varies between the different members of this subfamily of proteins. For example, Bim and Bmf are regulated in part by sequestration to the cytoskeleton while Puma and Noxa are regulated at the transcriptional level by p53.(Puthalakath, et al 1999, Shibue, et al 2006, Shibue, et al 2003) As recently discovered, the members of the Bcl-2 family proteins interact with other cellular proteins involved in cell cycle and autophagy and thereby influence other cellular functions.
In order to study the mechanisms of rituximab resistance, our group of investigators developed and characterized several rituximab-resistant cell lines. (Czuczman, et al 2008, Olejniczak, et al 2008) Repeated exposure to rituximab resulted in deregulation of several members of the Bcl-2 family proteins and downregulation of surface CD20, leading to a phenotype resistant to both chemotherapy agents and rituximab. (Olejniczak, et al 2008) These findings suggest the existence of common shared resistance pathways between chemotherapy agents and monoclonal antibodies targeting CD20, and strongly suggest the importance of Bcl-2 family members in the biology of relapsed/refractory B-cell lymphoma.
The use of BCL-2 oligonucleotides targeting endogenous Bcl-2, G3139 (i.e. oblimersen sodium) was explored in pre-clinical lymphoma models.(Cotter, et al 1999, Ramanarayanan, et al 2004) Subsequently, results from G3139 clinical trials in patients with relapsed/refractory chronic lymphocytic leukaemia (CLL) or melanoma were not as robust as predicted and lead scientists to develop alternative ways to target Bcl-2 family members.(Bedikian, et al 2006, O’Brien, et al 2007) Several compounds that mimic the binding site of the BH3 single domain proteins have been developed and are currently in pre-clinical and clinical stages of evaluation(Labi, et al 2008).
Obatoclax (GX15-070), is BH3-mimetic capable of binding and inactivating Mcl-1, Bcl-XL and Bcl-w with minimal to no interaction with Bcl-2.(Nguyen, et al 2007) Based on its binding affinity for Mcl-1, various investigators studied the anti-tumour activity of obatoclax in mantle-cell lymphoma (MCL) in pre-clinical and clinical models with modest results.(Perez-Galan, et al 2007) In the present report we demonstrate that obatoclax had potent anti-tumour activity in a panel of rituximab-sensitive and resistant cell lines and in tumour samples derived from patients with various subtypes of de novo or refractory/relapsed B-cell lymphoma. We evaluated the biological interactions between obatoclax and rituximab and three chemotherapeutic agents utilized in the treatment of B-cell lymphoma. Finally, we found that depending on the status of the apoptotic machinery, obatoclax can execute apoptosis or autophagy, suggesting the existence of a dual mechanism of action for this novel targeted agent.
Materials and Methods
Antibodies, Drugs, and Reagents
Obatoclax, GX15-070 was provided by Gemin X Pharmaceuticals (Montreal, ON, Canada). Cisplatin was purchased from American Pharmaceutical Partners (Schaumburg, IL), doxorubicin obtained from Bedford Labs (Bedford, OH), and vincristine was provided by the Roswell Park Cancer Institute (RPCI) Pharmacy.
Therapeutic antibodies, rituximab (anti-CD20) and trastuzumab (anti-Her-2/neu; used as isotype control) were obtained from Genentech, Inc. (San Francisco, CA) and, unless otherwise specified, were used at a final concentration of 10 μg/ml.
Primary mouse anti-human antibodies raised against Bak and actin were obtained from Sigma Chemicals (St. Louis, MO), Bik from Santa Cruz Biotechnology (Santa Barbara, CA), Moesin (Ab-1) from Lab Vision (Fremont, CA), PARP-1 from BD Pharmigen™ (San Jose, CA), Noxa from Calbiochem (San Diego, CA), p53 and puma from BD Bioscience (San Jose, CA) and LC3 from MBL International (Woburn, MA). Alkaline phosphatase (AP) or horseradish peroxidase (HRP) conjugated anti-mouse secondary antibodies were purchased from Jackson Immuno Research (West Grove, PA).
Ficoll-Hypaque was purchased from Sigma Chemical, St. Louis, MO. Sodium chromate51 (51Cr) and [3H] thymidine radioisotopes (Perkin-Elmer Life Inc., Boston MA) were utilized in functional assays assessing antibody-associated cytotoxicity and cell proliferation, respectively. Triton X-100, trypan blue and histopaque-1077 were obtained from Sigma-Aldrich Inc. (St Louis, MO). Cell Titer-Glo Luminescent Viability Assay reagent was purchased from Promega (Madison, WI). Three-methyladenine was obtained from Sigma Chemicals, zVAD-fmk and Q-VD-OPh from MBL International.
Cell Lines
A panel of rituximab-sensitive (RSCL) or resistant (RRCL) cell lines was used for the experiments. The parental, RSCL Raji, and RL were purchased from American Type Culture Collection (ATCC, Manassas, VA). The SU-DHL-4 cell line was a kind gift from Steven Treon (Dana Farber Cancer Institute, Boston, MA). RRCL were created from each RSCL by repeat exposure of cells to escalating doses of rituximab in the presence (4RH) or absence (2R) of human complement. Individual clones were isolated and expanded. These RRCL have been previously described and characterized.(Czuczman, et al 2008, Olejniczak, et al 2008)
Patient’s specimens
Neoplastic B-cells were isolated from pre-treatment biopsy tissue obtained from 45 patients with B-cell NHL or lymphocyte predominant Hodgkin lymphoma (LPHL) treated at RPCI. Samples from patient biopsy specimens were procured under Institutional Review Board (IRB) RPCI protocols I42804 and I42904. Tissue specimens were placed in phosphate-buffered saline-containing collagenase type IV (1 mg/ml; Sigma-Aldrich, St. Louis, MO) and incubated for 15 min at 37°C, including manual agitation for five minutes. Next, samples were diluted with RPMI 1640 medium containing 10% fetal bovine serum (FBS) and the cell suspension was filtered through a 100μm cell strainer to remove large clumps. Subsequently, lymphocytes were enriched for by density centrifugation. B-cells were then isolated from enriched lymphocytes by magnetis-activated cell sorting (MACS) separation using a human B-cell Isolation Kit II (Miltenyi Biotec, Gladbach, Germany). B-cell purity was assessed by flow cytometry using antibodies to CD19 and CD20 (Becton Dickenson, San Jose, CA). Greater than 95% pure CD19+ cells were obtained from the first 8 specimens processed.
In vitro effects of obatoclax on viability of NHL cell lines
In order to determine the biologically active dose of obatoclax, RRCL or RSCL were exposed to escalating doses of GX15-070 (2–20μM) or vehicle control (dimethyl sulfoxide; DMSO) for 24 or 48 h. All cells were plated at a density of 0.5 × 106 cells/ml. After each time period, samples were collected and cell viability was determined by 0.1% trypan blue staining or alamar blue reduction. Experiments were performed in triplicate and read by two independent investigators. To confirm effects in cell viability, changes in ATP content following obtatoclax exposure were evaluated using the Cell Titer-Glo Luminescent Viability Assay reagent (Promega, Madison, WI).
In vitro effects of obatoclax with and without chemotherapy or rituximab on DNA synthesis and cell proliferation of NHL cell lines
RSCL and RRCL were placed in 96-well plates (1 × 105 cells/well, cell density of 0.5 × 106 cells/ml) and exposed to obatoclax (1–10 μM) and/or cisplatin (0.1–10 μM), doxorubicin (4–400 μM), vincristine (0.1–10 nM). Cells were then incubated at 37°C and 5% CO2 for 24 and 48 h. Then, 37 kBq/well of [3H]-thymidine was added and cells were incubated for an additional 18 h. Cells were then harvested using the Tomac Cell Harvester (Tomtec, Hamden, CT) into 96 well glass filters (Wallac Inc, Turku, Finland) and [3H]-thymidine uptake was measured using an automated scintillation counter (Wallace, Gaithersburg, MD).
Synergistic anti-tumour activity between obatoclax and each chemotherapy agent used was evaluated using a pharmacodynamic (PD) model. The inhibitory Emax model [Effect = Eo-(Imax × Cγ)/(IC50γ + Cγ)], WinNonlin (Pharsight Corporation Version 5.3) was first used to fit individual cell viability (PD)-concentration curves following 48-h exposure of obatoclax, cisplatin, doxorubicin, and vincristine. The individual PD parameters, including IC50, Imax, and Hill’s constant (γ) (a measure of the steepness of the slope), for single agent exposure to RSCL and RRCL cells were obtained from the model (Table 1). To quantify the synergy of obatoclax with cytotoxic agents (cisplatin, doxorubicin, and vincristine), a simplified PD model was developed using ADAPT 5(BMSR)(D’Argenio, et al 2009). A modified version of the PD interaction model that was originally developed by Ariuens and Simonis (Ariuens and Simonis 1964) and more recently adapted by.Chakraborty and Jusko (2002) was used to fit the data upon exposure to the combination of obatoclax with either, cisplatin, doxorubicin, or vincristine as follows:
Table 1.
Summary of Estimated Interaction Parameter (Ψ) upon exposure of Cisplatin, Doxorubicin, and Vincristine in combination with Obatoclax in both rituximab-sensitive (RSCL) and –resistant (RRCL) cell lines. Two-tailed student T-test evaluated the difference in Ψ between cell lines for each cytotoxic agent combined with Obatoclax, expressed with a level of significance set at P<0.05
| Drug combination | Cell Line | Ψ | P value |
|---|---|---|---|
| Cisplatin + Obatoclax | RSCL (RL) | 0.391 (0.017) | }0.129 |
| Cisplatin + Obatoclax | RRCL (RL-4RH) | 0.444 (0.045) | |
|
| |||
| Doxorubicin + Obatoclax | RSCL (RL) | 0.432 (0.085) | }0.336 |
| Doxorubicin + Obatoclax | RRCL (RL-4RH) | 0.492 (0.043) | |
|
| |||
| Vincristine + Obatoclax | RSCL (RL) | 0.712 (0.045) | }0.069 |
| Vincristine + Obatoclax | RRCL (RL-4RH) | 0.640 (0.023) | |
RSCL = rituximab-sensitive cell lines; RRCL = rituximab-resistant cell line.
For the purpose of modelling two drugs simultaneously, obatoclax is represented as drug A and cytotoxic agents (cisplatin, doxorubicin, and vincristine) are represented by drug B in each model run. ImaxA or ImaxB are the maximal inhibitory responses achieved for drug A or B, B1 and B2 are the drug concentrations for each drug representing the response, IC50A or B represent the concentration producing 50% inhibitory response. An interaction symbol, termed psi (ψ), was introduced into the Hill function to characterize the interaction of obatoclax with the cytotoxic agents (see above formula). Individual PD parameters in equation 2 are all fixed to the estimated values determined upon single agent exposure in equation 1 and the ψ is estimated. The degree of interaction between the two drugs of interest was determined by the following criteria: When ψ equals 1, there is no interaction or the interaction between the two drugs may be additive, a ψ less than 1 is representative of synergy, and a ψ greater than 1 indicates antagonism.
51Cr release assay for the assessment of the impact that obatoclax exposure has on rituximab-mediated complement-mediated cytotoxicity (CMC) and antibody-dependent cellular cytotoxicity (ADCC)
RSCL or RRCL were exposed in vitro to obatoclax (2–10μM) or DMSO (0.001%) an incubated at 37°C and 5% CO2 for 24 h. Subsequently, 2 ×106 viable cells were labelled with 51Cr at 37°C, 5% CO2 for 2 h. 51Cr-labelled RSCL or RRCL were then placed in 96-well plates at a cell concentration of 1 × 105 cells/well (CMC assay) or 1 × 104 cells/well (ADCC assay). Cells were then exposed to rituximab or isotype and human serum (CMC, 1:4 dilution) or peripheral blood mononuclear cells (PBMC) (ADCC, 40:1 effector: target ratio) for 6 h at 37°C and 5% CO2. 51Cr release was measured from the supernatant by standard gamma counter and the percentage of lysis was calculated as following: % Lysis = [Test cpm – background cpm]/[Maximum cpm – background cpm]. PBMC were obtained from healthy donors (IRB-approved protocol CIC-016). Pooled human serum as source of complement for CMC assays.
In vitro effects of obatoclax on rituximab direct anti-tumour effects
To determine the effects of obatoclax on rituximab direct anti-tumour activity, standard [3H]-thymidine incorporation assays were performed. NHL cells were exposed to obatoclax and/or rituximab (10μg/ml) or isotype control (tratuzumab) for 48 h without cross-linking. Effects of cell proliferation were determined by [3H]-thymidine incorporation as described above.
In vitro effects of obatoclax on ATP content of lymphoma cell lines and primary tumour cells derived from patients with B-cell lymphoma
To further confirm the biological activity of obatoclax as a single agent and to study its effects in a more clinically relevant model we studied changes in ATP following obatoclax exposure in RSCL, RRCL and in 45 tumour specimens collected from patients with various types of B-cell lymphoproliferative disorders. Briefly, RSCL, RRCL and primary neoplastic B-cells were exposed to obatoclax (0.1–5 μM) or DMSO control (0.0001%) at a cell-density of 0.5 × 106 cells/ml. Following a period of 24 or 48 h incubation at 37°C and 5% CO2, changes in ATP were determined using the Cell Titer-Glo Luminescent Viability Assay.
Effect of caspase inhibition on obatoclax anti-tumour activity
RSCL and RRCL were incubated with obatoclax (20μM) or vehicle control for 24 or 48 h with or without the pan-caspase inhibitors zVAD-fmk (50μM) and Q-VD-OPh (5μM). Differences in cell viability following caspase inhibition were determined using the Cell Titer-Glow Luminescent Viability Assay reagent.
Changes in the expression of several pro-apoptotic or autophagy proteins in RSCL or RRCL after exposure to obatoclax
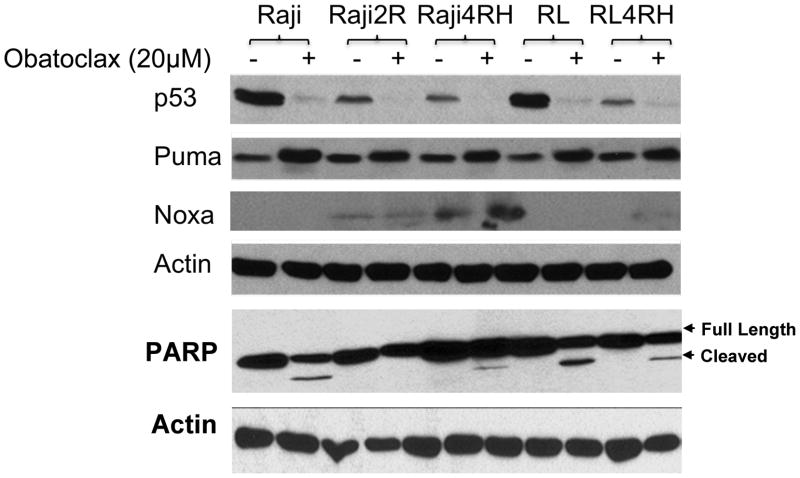
In order to study the mechanisms responsible for obatoclax anti-tumour activity in B-cell lymphoma, RSCL or RRCL were exposed to obatoclax (20μM) or control in vitro for 24 or 48 h. Subsequently, changes in the expression of puma, noxa, p53, PARP cleavage, bak, bax and LC3 were determined by Western blotting.
Morphological changes in RSCL or RRCL following obatoclax exposure
In order to further evaluate if obatoclax induced autophagy in RRCL with low levels of Bak/Bax, RSCL and RRCL were exposed in vitro to obatoclax (20μM) or vehicle control for 48 h. Subsequently, cells were fixed in ultrapure gluteraldehyde and stored at 4°C. Fixed cells were then sent to the RPCI imaging facility for analysis by transition electron microscopy (TEM). Experiments were performed in triplicates on three separate occasions. Representative photographs of the results are presented. Pathological interpretation of the morphologic changes observed was performed by a pathologist who was blinded to experimental conditions.
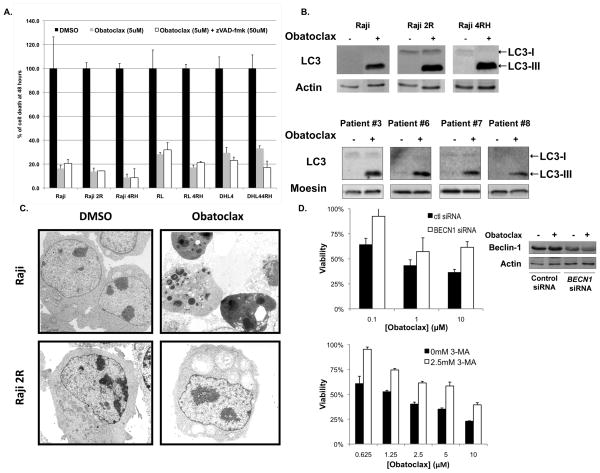
The role of autophagy on obatoclax anti-tumour activity in RRCL
In order to characterize the role of autophagy execution following therapy with obatoclax, we inhibited the autophagy pathway in RRCL. First, RRCL were incubated with or without 3-methyladenine (2.5 mM) and then exposed to obatoclax (0–10 μM) for 24 or 48 h. Cell viability was determined by changes in the ATP content using the Cell Titer-Glow Luminescent Assay. Subsequently, siRNA-mediated knockdown of Beclin-1 was performed. Knockdown of BECN1 was achieved using ON-TARGET plus SMART pool siRNA containing a mixture of 4 siRNAs designed to specifically target BECN1 (Dharmacon, Lafayette, CO). Non-targeting SMART pool siRNA (Dharmacon) was used as a negative control. Efficient knockdown of Beclin-1 was confirmed by Western blotting. Once conditions were optimized, RRCL were transfected with 2μg of siRNA targeting BECN1 or control using an Amaxa Nucleofector (Lonza Laboratories, Anaheim CA) and cells were incubated with obatoclax (0–10μM) for an additional 24 h. Differences in cell viability were determined by ATP quantification as previously described.
Results
Obatoclax induces cell death in RSCL and RRCL regardless of baseline Bak/Bax levels
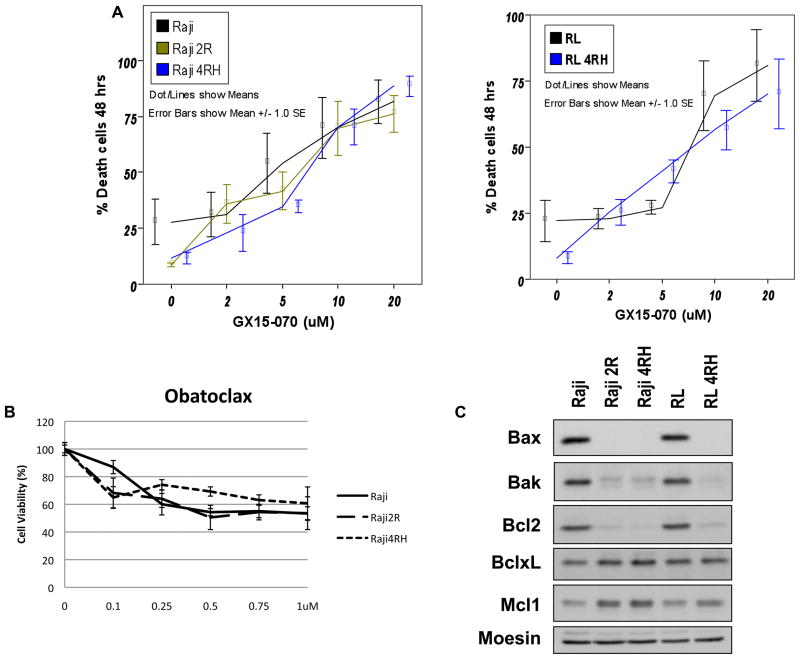
While the expression of Bcl-2 members differs between RSCL and RRCL, obatoclax induced cell death in a dose-dependent fashion in all the cell lines tested (Figure 1). RRCL are characterized as having higher levels of MCL-1/Bcl-XL and lower levels of Bak/Bax as compared to RSCL (Olejniczak, et al 2008). The dose of obatoclax that resulted in cell death of 50% of the RSCL or RRCL was 5μM following a 48 h period of incubation (Figure 1) and was independent of rituximab-chemotherapy sensitivity or Bak/Bax levels (Figure 1).
Figure 1.
In vitro exposure of B-cell lymphoma cells lines to Obataclox results in dose-dependent cell death and altered expression of Bcl-2 family proteins in Rituximab sensitive (Raji, and RL) and resistant (Raji 2R, Raji 4RH, and RL 4RH). RRSL or RRCL were exposed to escalating doses of obataclox. Cell death was determined by trypan exclusion (A) or Cell Titer-Glow Luminescence Assay (B). Western blot analysis and quantification of Bcl-2 family proteins from total cell lysates from Raji or RL cells vs. Raji-derived or RL-derived RRCL revealed decreased Bax, Bak and Bcl-2 expression and increased Bcl-xL and Mcl-1 expression (C).
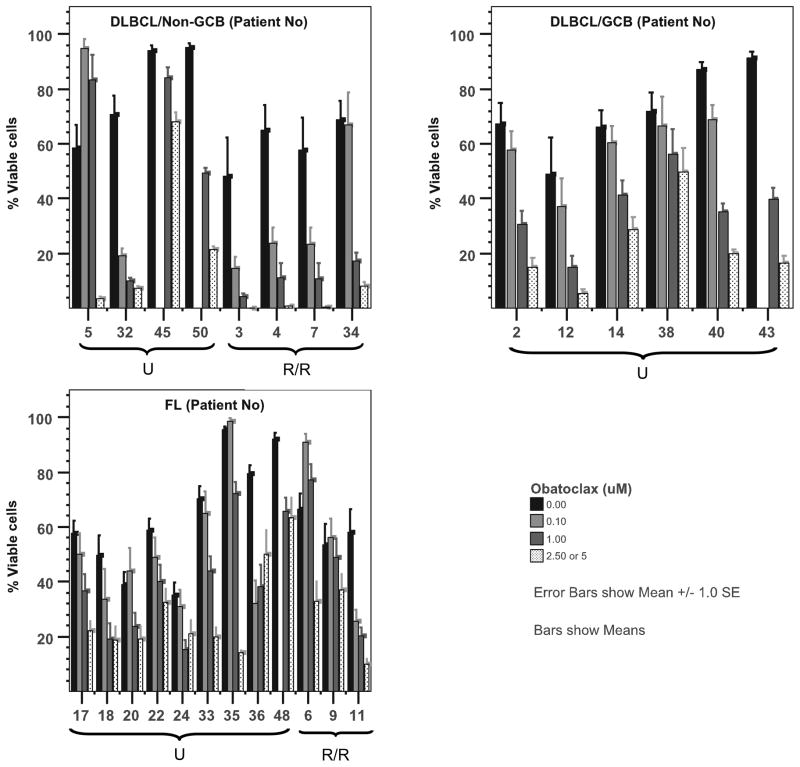
Obatoclax has potent anti-tumour activity in tumour cells derived from patients with diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL)
Subsequently, we studied the anti-tumour activity of obatoclax against tumour cells derived from patients with B-cell lymphoma. A total of 39 tumour samples were tested. The demographic characteristics of the patients whose tumour cells were evaluated are summarized in Table 2. The majority of the patients had DLBCL (N=15) or FL (N=12), and 44% had relapsed/refractory disease. Of interest, obatoclax activity was observed in both de novo and relapsed/refractory lymphoma. Cell death was observed at all doses tested (0.1 – 5μM) and was more pronounced at 48 h (Figure 2). When analysing DLBCL using the Han’s algorithm, activity was observed in both germinal centre B-cell (GCB) (5/6, 83%) and in non-GCB lymphomas (9/9, 100%). In addition, obatoclax decreased cell viability in 90% and 75% of the FL (Figure 2) or LPHL (data not shown) samples tested respectively. Although only evaluated in 2 patient samples of each, obatoclax had a minimal to modest (up to 25% cell death at 48 h) anti-tumour activity in small lymphocytic lymphoma (SLL), marginal zone lymphoma (MZL), and mantle cell lymphomas (MCL) (Supplemental Figure 1). No biological effects were found in 4 samples obtained from patients with benign lymphoid hyperplasia (data not shown).
Table 2.
Demographic characteristics of the patients whose primary tumour cells were derived and exposed in vitro to obatoclax
| Number of cases | 39 |
|---|---|
| Median Age, years (range) | 62 (24–83) |
|
| |
| Sex: | |
| Male | 25 (64%) |
| Female | 14 (36%) |
|
| |
| Diagnosis: | |
| DLBCL | 15 |
| GCB DLBCL | 6 |
| Non-GCB DLBCL | 9 |
| FL | 12 |
| LPHL | 2 |
| SLL/MZL/MCL | 6 |
| Lymphoid Hyperplasia | 4 |
|
| |
| Disease Status: | |
| De Novo | 22 (56%) |
| Relapsed/Refractory | 17 (44%) |
|
| |
| Prior rituximab therapy | 35% |
DLBCL = Diffuse Large B-cell Lymphoma
GCB = Germinal Centre B-cell
FL = Follicular Lymphoma
LPHL = Lymphocyte predominant Hodgkin lymphoma
SLL = Small Lymphocytic Lymphoma
MZL = Marginal Zone Lymphoma
MCL = Mantle Cell Lymphoma
Figure 2.
Ex vivo exposure of lymphoma cells derived from patients with untreated (U) or relapsed/refractory (R/R) germinal centre B-cell (GCB) or Non-GCB diffuse large B-cell lymphoma (DLBCL), or follicular lymphoma (FL) to obatoclax resulted in dose-dependent cell death. Production of ATP was used as a surrogate of viability after 48 h of incubation. *Obatoclax was utilized at 0.1, 1 and 2.5 μM for most of the samples obtained. Only cells obtained from Patient 3 were exposed to obatoclax at higher dose (i.e. 5 μM)
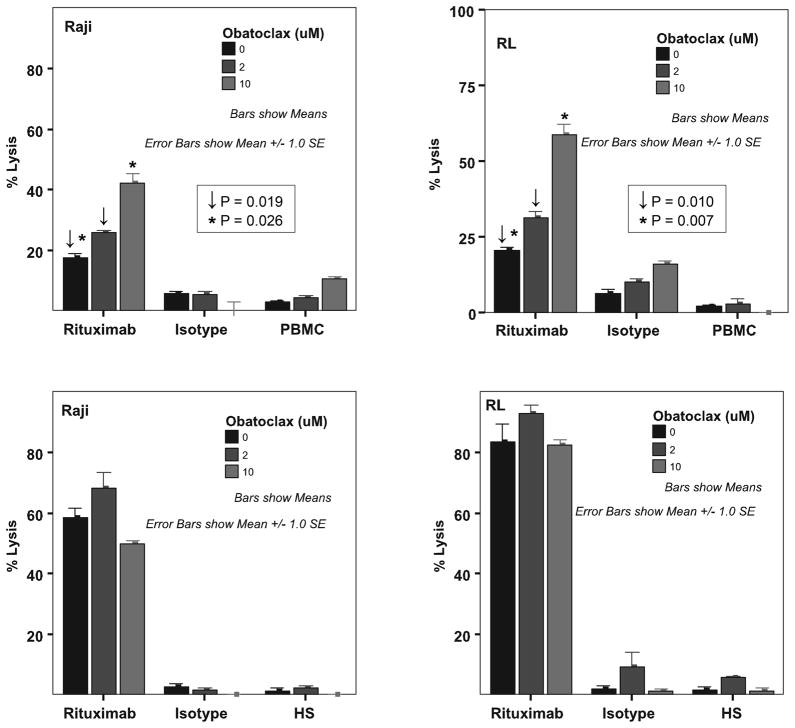
Obatoclax enhances rituximab-medicated ADCC in vitro
Previous investigators had demonstrated that granzyme-B released by effector cells following cytokine activation of ADCC could activate tBid, a member of the BH3 single domain subfamily proteins favouring apoptosis of target cells(Waterhouse, et al 2005). ADCC is a key mechanism of action of rituximab in B-cell lymphoma and therefore, we evaluated the effects of pre-incubating RSCL or RRCL with obatoclax prior to rituximab exposure. In RSCL (RL and Raji cells), in vitro exposure to obatoclax for 24 h prior to the exposure of rituximab, resulted in a dose-dependent increase in rituximab-mediated ADCC. The mean percentage of rituximab-associated ADCC on DMSO (vehicle) pre-treated RL and Raji cells was 20.3% +/−1.14% standard error of the mean (sem) and 17.6% +/−1.07%sem, respectively. Exposure of RL cells to obatoclax (5μM) for 24 h prior to rituximab exposure lead to a statistically significant increase in rituximab-mediated ADCC [mean % lysis 58.56% +/− 3.6%sem, P= 0.007]. Similar results were seen in Raji cells [mean % lysis 42.6% +/−3.03%sem, P=0.026] (Figure 3a).
Figure 3.
Obataclox (GX15-070) enhances rituximab-mediated antibody-dependent cellular cytotoxicity (ADCC) but not complement-mediated cytotoxicity (CMC) in rituximab sensitive cell lines. Raji or RL cells were exposed to Obataclox (2 and 10 μM) for 24 h and subsequently labelled with 51Cr. Labelled cells were then exposed to rituximab or isotype and peripheral blood mononuclear cells at an effector: target ratio of 40:1(A) (ADCC) or 20% human serum pooled from healthy volunteers (B) (CMC) and incubated at 37°C, 5% CO2 for 6 h. 51Cr-release was measured and the percentage of lysis calculated.
No significant improvement in rituximab-mediated ADCC was observed in RRCL (data not shown). In addition, obatoclax did not impact rituximab-associated CMC in RSCL or RRCL or direct anti-proliferative effects (Figure 3b and supplemental figure 2). No significant changes in CD20 surface antigen expression were observed in lymphoma cells exposed to obatoclax (data not shown). Still, the ability of obatoclax to improve rituximab-associated ADCC in vitro warrants further pre-clinical studies that may lead to evaluate obatoclax in combination with rituximab in lymphoma clinical trials.
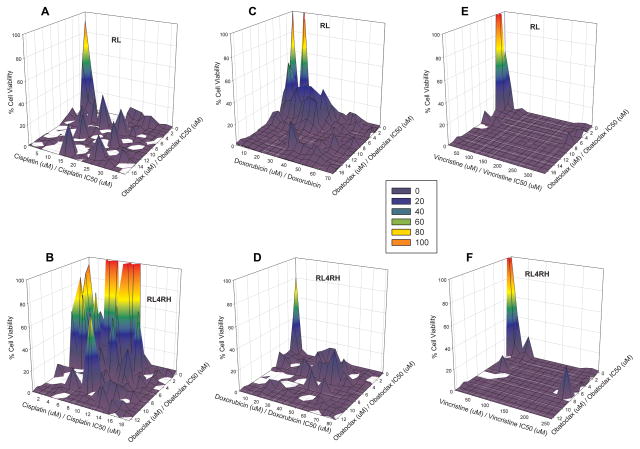
Obatoclax potentiates chemotherapy effects in both RSCL and RRCL
Increasing concentrations of the combination of Obatoclax and a cytotoxic agent results in enhanced cell kill. A graphical representation of the interaction of obatoclax with cytotoxic agents (cisplatin, doxorubicin, and vincristine) on cell kill, normalized by single agent IC50 values, demonstrated that when used in combination, obatoclax was synergistic with the tested cytotoxic agents (Figure 4). Viability is represented by a colour scheme, where red and orange indicate greater cell viability; the dark blue regions represents 100% inhibition of cell growth. A clear synergism was observed for doxorubicin, cisplatin, and vincristine in combination with obatoclax in both RSCL (RL cells) and RRCL (RL-4RH), as estimated Ψ values were all well below 1 (Table 1). A greater degree of synergy, as represented by the lowest Ψ values, was apparent with cisplatin and doxorubicin in combination with obatoclax in RSCL (RL) cells relative to the vincristine-obatoclax combination. The surface representing the complete inhibition of cell growth generated for the vincristine-obatoclax combination is most likely to be due to predominantly vincristine, as it was the most potent cytotoxic single agent tested in these cell lines (Figure 4E,F). As a result, this may warrant additional experiments using lower concentrations of vincristine to further characterize contribution of obatoclax on cell inhibition with this drug combination. There was no difference in the estimated Ψ between cell lines for each combination tested (p>0.05) suggesting that obatoclax effects on each chemotherapy agents was similar in both RSCL and RRCL (Table 1). These results demonstrate that when used in combination with cytotoxic agents, obatoclax has enhanced killing in both cell lines and may support its use in combination with such agents.
Figure 4.
In vitro exposure of rituximab sensitive (RL, a–c) and resistant cell lines (RL-4RH, d–f) to Obatoclax potentiates the anti-proliferative effects of chemotherapy agents. NHL cells were exposed to obatoclax with or without escalating doses of cisplatin, doxorubicin, or vincristine. Following drug exposure for 48 h, changes in DNA synthesis were performed using [3H]-thymidine incorporation assays. Cell viability response is represented as a surface for combination of obatoclax with cytotoxic agents, cisplatin (A, B), doxorubicin (C, D), and vincristine (E,F) normalized by estimated single agent IC50 in both RSCL (A, C, E) and RRCL (B, D, F); a colour scheme is used to reflect cell kill, 100% cell viability is represented in orange progressing to 0% represented by dark blue.
In vitro exposure of RSCL and RRCL to obatoclax results in the up-regulation of p53-regulated BH3 single domain proteins Puma and Noxa
In order to characterize the mechanisms by which obatoclax kills lymphoma cells and potentiates the effects of rituximab and chemotherapy agents, we studied its effects on the expression of other regulatory proteins of the apoptotic pathway. In vitro exposure of RSCL and RRCL to obatoclax for 48 h resulted in decreased p53 protein and the upregulation of p53-regulated Puma, and to a lesser degree Noxa (Figure 5). Of interest, PARP cleavage was only detected in RSCL exposed to obatoclax that are known to have adequate levels of Bax and Bak. While cell death and upregulation of Puma and Noxa was observed in RRCL exposed to obatoclax; PARP cleavage was not observed (Figure 5). This finding strongly suggests the existence of additional pathways of cell death being executed by obatoclax in cells with low levels of Bak/Bax and therefore a higher apoptotic threshold.
Figure 5.
Changes in key regulatory proteins of apoptosis and cell cycle in rituximab-sensitive or resistant cell lines following in vitro exposure to obatoclax. In vitro exposure of lymphoma cell lines to obatoclax for 48 h resulted in the down-regulation of p53 and the up-regulation of p53 regulated puma and noxa. In addition, PARP cleavage following obatoclax exposure was observed in rituximab-sensitve cell lines (Raji and RL cells) and in some rituximab-resistant cell lines (Raji4RH and RL-4RH), but not in Raji 2R cells.
Obatoclax induces caspase-independent cell death in lymphoma cell lines
To further define the mechanisms of cell death responsible for obatoclax activity in B-cell lymphoma, we studied the consequences of caspase inhibition on obatoclax activity. In vitro exposure of RSCL or RRCL to zVAD-fmk (50μM) did not affect the anti-tumour activity of obatoclax (Figure 6a). Similar effects were observed with 5μM Q-VD-OPh (data not shown).
Figure 6.
Obatoclax induces cell death independently from caspase activation (A) primarily autophagy (B–D). Various rituximab-sensitive and -resistant cell lines were exposed in vitro to obatoclax (5 μM) with or without a pan-caspase inhibitor (zVAD-fmk, 50 μM). Cell viability was detected by ATP generation using the Cell glow luminescent assay after 48 h. (B) Obatoclax induces autophagy in rituximab-sensitive or resistant cell lines and in lymphoma cells isolated from patients with diffuse large B-cell lymphoma (DLBCL). Cell lines and patient-derived DLBCL cells (N=4) were exposed in vitro exposure to obatoclax (5μM) or DMSO. Autophagy was determined by detection of LC3 conversion (C) and was confirmed by electron microscopy. In vitro obatoclax exposure of Raji cells expressing Bak/Bax induced morphological changes corresponding to apoptosis. Autophagy was observed by electron microscopy in rituximab-chemotherapy resistant cells (Raji 2R shown here) that are known to have low to absent levels of Bax/Bak. (d) Knock-down of Beclin-1 using siRNA specific for BECN1 inhibited obatoclax killing of rituximab-resistant cell lines (Raji 4RH shown here). Data normalized to untreated control- or BECN1-siRNA transfected cells. Western blot confirmed knockdown of Beclin-1. Co-incubation of obatoclax with 3-methyladenine inhibited obatoclax-mediated killing of rituximab-resistant cell lines (Raji 2R)
Obatoclax induces autophagy in RSCL, RRCL, and in primary tumour cells derived from patients with DLBCL
Other investigators demonstrated that in certain pre-clinical models, other BH3 mimetics induced autophagy, a caspase-independent cell death pathway.(Hetschko, et al 2008, Maiuri, et al 2007) To further study caspase-independent mechanisms to explain obtatoclax’s activity, we investigated whether autophagy was triggered following in vitro exposure to this novel BH3 mimetic. LC3-II conversion from LC3-1, a surrogate marker of autophagy, was observed in RSCL, RRCL and in primary DLBCL tumour cells following in vitro exposure to obatoclax for 48h (Figure 6b). To confirm the execution of autophagy following obatoclax exposure, TEM was performed in RSCL and RRCL after a 48-h period of incubation with vehicle or obatoclax. Of interest, different cellular effects were observed between RSCL and RRCL exposed to obatoclax. Cellular changes consistent with apoptosis were seen in RSCL (Figure 6c). In contrast, TEM confirmed cellular changes consistent with the induction of autophagy following exposure of rituximab chemotherapy-resistant cells to obatoclax (Figure 6c). Moreover, the cell death induced by obatoclax could be inhibited by transient siRNA knockdown of Beclin-1 or by treatment with 3-methyladenine, an inhibitor of autophagy in RRCL (Figure 6d). Altogether, these data support the premise that obatoclax has a dual mechanism of action and is capable of inducing apoptosis or autophagy in B-cell NHL cells depending on their apoptotic threshold (e.g. Bak/Bax levels).
Discussion
Prior to rituximab, the overexpression of Bcl-2 was found to be a negative prognostic factor with respect to chemotherapy response, PFS, and OS in patients with various subtypes of NHL and other cancers. The addition of rituximab to systemic chemotherapy for B-cell neoplasms has counteracted the negative impact of the over-expression of some Bcl-2 family members in the clinical settings(Mounier, et al 2003, Shivakumar and Armitage 2006, Wilson, et al 2007). Alternatively, based on two rituximab-resistance pre-clinical models and emerging clinical data, de-regulation of Bcl-2 family members will probably have a significant impact in the biology of relapsed/refractory lymphoma.(Jazirehi, et al 2007, Olejniczak, et al 2008)
BH3 mimetics, including obatoclax, are emerging as potentially valuable anti-cancer agents. Obatoclax has been studied in pre-clinical models of haematological and non-haematological malignancies (Konopleva, et al 2008, Li, et al 2008, Perez-Galan, et al 2008, Perez-Galan, et al 2007, Trudel, et al 2007). Significant anti-tumour activity has been observed in models that utilized cancer cell lines. In our current work we demonstrated that obatoclax had anti-tumour activity against RSCL, RRCL, and in primary tumour cells derived from 39 patients with various subtypes of B-cell neoplasms (i.e. DLBCL and FL and LPHL), enhanced the anti-tumour effects of rituximab and had synergistic activity when combined with chemotherapy agents. Our findings are similar to those described by other investigators, who demonstrated that obatoclax has synergistic activity when combined with platinum compounds in lung cancer models.(Li, et al 2008)
Obatoclax has been studied in two clinical studies in patients with haematological malignancies. Schimmer et. al. (2008) conducted the first phase II study of obatoclax in patients with advanced haematological malignancies. The study included 44 patients with refractory acute myeloid leukaemia (AML) or myelodysplastic syndrome (MDS) and received escalating doses of obatoclax (7–40 mg/m2 every 1–2 weeks) as a continuous intravenous infusion (CIVI). Grade 1 to 2 central nervous system (CNS) toxicity was the most common adverse events observed. Anti-tumour activity was observed in one patient with AML and three patients with MDS.(Schimmer, et al 2008) O’Brien et al (2009) evaluated obatoclax in 26 patients with advanced CLL. Obatoclax was administered as a 1-h (3.5 to 14 mg/m2) or 3-h (20 to 40 mg/m2) infusion. Modest anti-tumour activity was observed with reversible grade 1–3 CNS toxicity.(O’Brien, et al 2009) The maximum tolerated dose (MTD) was found to be 28 mg/m2(O’Brien, et al 2009, Schimmer, et al 2008).
Pharmacokinetic studies performed in the context of these two clinical trials demonstrated that maximum plasma concentration (Cmax) and the area under the curve (AUC) of obatoclax were dose-related.(O’Brien, et al 2009, Schimmer, et al 2008) In our experiments, especially those conducted in primary tumour cells, obatoclax was used at doses ranging from 0.1 to 10μM, which are equivalent to 40 to 4000 ng/ml and therefore within the range suitable for the clinical setting. In our experiments we observed anti-tumour activity in cell lines and in primary tumour cells obtained from patients with B-cell lymphomas at clinically relevant doses.
When used at low doses, obatoclax potentiates the anti-tumour activity of rituximab and chemotherapy agents, most likely by affecting the net balance of anti- to pro-apoptotic proteins in NHL cells. The biological interactions between obatoclax and the chemotherapy agents tested could be a result of the direct effects of obatoclax, cisplatin, doxorubicin and vincristine on the net balance of the pro- and anti-apoptotic Bcl-2 family members. In vitro exposure of cancer cells to cisplatin or doxorubicin is known to affect the expression of p53-regulated BH3 single domain proteins Puma and Noxa (Jiang, et al 2006, Middelburg, et al 2005). In addition, microtubules sequestrate Bim and in vitro exposure of cells to vincristine or paclitaxel (i.e. anti-mitotic agents) release Bim to interact with other Bcl-2 family members and induce cell death.(Czernick, et al 2009)
The mechanisms by which pre-incubation of RSCL to obatoclax potentiate the rituximab-associated ADCC in a dose-dependent fashion are not yet determined. However, it is possible that the interactions of granzyme-B released during rituximab-associated ADCC and/or the calcium influx following CD20 binding leads to Bim release from the microtubules play role in this phenomena. Ongoing studies plan to further delineate the role of granzyme B and Bim in the biological interactions between rituximab and obatoclax. The lack of effect of obatoclax in rituximab-associated ADCC in RRCL could be explained by the significant decrease in surface CD20 antigen exhibited by these cell lines.(Czuczman, et al 2008)
In order to optimize the further clinical evaluation of obatoclax, it is important to understand the mechanisms responsible for its anti-tumour activity. Our data suggest that obatoclax: 1) induces the expression of p53-regulated pro-apoptotic BH3 single domain proteins Puma and Noxa and may amplify death signalling; 2) possesses a dual mechanism of action depending on the apoptotic threshold of the cancer cell targeted. In our experiments, caspase inhibition did not affect the anti-tumour activity of obatoclax suggesting the existence of alternative pathways of cell death executed by this BH3 mimetic. PARP cleavage and nucleoli chromatin condensation, were observed following obatoclax exposure in rituximab-sensitive cells lines with intact levels of Bak/Bax. In contrast, cellular changers compatible with autophagy, such as vacuole formation by TEM and LC3-III conversion were observed in RRCL with low levels of Bak/Bax. Moreover, inhibition of autophagy by 3-MA or by siRNA knockout of BECN1 diminish obatoclax anti-tumour activity in RRCL, further stressing the significant role that autophagy plays in the biological activity of obatoclax.
Our findings suggest that Bcl-2 family proteins may influence other cellular processes, such as cell cycle or autophagy. Other investigators have demonstrated that Bcl-2 interacts with p53, preventing its translocation into the nucleus and therefore affecting the cell cycle.(Day, et al 2008, Knoops and de Jong 2008, Paquet, et al 2004) Similarly, there is growing evidence that Bcl-XL interacts with Beclin-1 and may potentially play a role in regulating autophagy under stress conditions. (Oberstein, et al 2007, Pattingre and Levine 2006, Swerdlow and Distelhorst 2007)
In summary, BH3 mimetics, such as obatoclax are emerging as potentially valuable therapeutic agents in the treatment of solid and haematological neoplasms. The multiple cellular events observed following obatoclax exposure suggests that Bcl-2 family members probably play a role in autophagy and/or cell cycle regulation, in addition to apoptosis. Obatoclax has potent anti-tumour activity in rituximab-sensitive and -resistant cell lines, primary NHL cells, as well as clinical activity observed early trials in patients with AML, MDS or CLL. In addition, obatoclax lowers the chemotherapy sensitivity threshold in rituximab-sensitive or -resistant lymphoma cell lines. Our pre-clinical data supports the further evaluation of obatoclax as single agent or in combination with rituximab +/− systemic chemotherapy in the context of future clinical trials in patients with B-cell lymphoma.
Supplementary Material
Acknowledgments
Supported in part by a USPHS grant PO1-CA103985-02 awarded to the Garden Cancer Center, NIH R01 grant CA136907-01A1 awarded to RPCI and the Eugene and Connie Corasanti Lymphoma Research Fund.
Footnotes
Author contributions: E.B., S.K., S.O., P.T., W.R., J.G., P.H., A.I., R.C., J.K., C.M., and F.H.I. performed the research; M.C. and F.H.I. designed the research study, G.D. and J.G. provided with tissue material, G.D., K.T., J.F., M.C. and F.H.I. analysed the data; and E.B., K.T.; M.C.; J.G.; and F.H.I. wrote the paper
References
- Adams JM, Huang DC, Strasser A, Willis S, Chen L, Wei A, van Delft M, Fletcher JI, Puthalakath H, Kuroda J, Michalak EM, Kelly PN, Bouillet P, Villunger A, O’Reilly L, Bath ML, Smith DP, Egle A, Harris AW, Hinds M, Colman P, Cory S. Subversion of the Bcl-2 life/death switch in cancer development and therapy. Cold Spring Harb Symp Quant Biol. 2005;70:469–477. doi: 10.1101/sqb.2005.70.009. [DOI] [PubMed] [Google Scholar]
- Ariuens EJ, Simonis AM. A Molecular Basis for Drug Action. J Pharm Pharmacol. 1964;16:137–157. doi: 10.1111/j.2042-7158.1964.tb07437.x. [DOI] [PubMed] [Google Scholar]
- Bannerji R, Kitada S, Flinn IW, Pearson M, Young D, Reed JC, Byrd JC. Apoptotic-regulatory and complement-protecting protein expression in chronic lymphocytic leukemia: relationship to in vivo rituximab resistance. J Clin Oncol. 2003;21:1466–1471. doi: 10.1200/JCO.2003.06.012. [DOI] [PubMed] [Google Scholar]
- Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, Pavlick AC, DeConti R, Hersh EM, Hersey P, Kirkwood JM, Haluska FG. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24:4738–4745. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- Chakraborty A, Jusko WJ. Pharmacodynamic interaction of recombinant human interleukin-10 and prednisolone using in vitro whole blood lymphocyte proliferation. J Pharm Sci. 2002;91:1334–1342. doi: 10.1002/jps.3000. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Cleary ML, Smith SD, Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986;47:19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- Cotter FE, Waters J, Cunningham D. Human Bcl-2 antisense therapy for lymphomas. Biochim Biophys Acta. 1999;1489:97–106. doi: 10.1016/s0167-4781(99)00139-6. [DOI] [PubMed] [Google Scholar]
- Czernick M, Rieger A, Goping IS. Bim is reversibly phosphorylated but plays a limited role in paclitaxel cytotoxicity of breast cancer cell lines. Biochem Biophys Res Commun. 2009;379:145–150. doi: 10.1016/j.bbrc.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Czuczman MS, Grillo-Lopez AJ, White CA, Saleh M, Gordon L, LoBuglio AF, Jonas C, Klippenstein D, Dallaire B, Varns C. Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol. 1999;17:268–276. doi: 10.1200/JCO.1999.17.1.268. [DOI] [PubMed] [Google Scholar]
- Czuczman MS, Olejniczak S, Gowda A, Kotowski A, Binder A, Kaur H, Knight J, Starostik P, Deans J, Hernandez-Ilizaliturri FJ. Acquirement of rituximab resistance in lymphoma cell lines is associated with both global CD20 gene and protein down-regulation regulated at the pretranscriptional and posttranscriptional levels. Clin Cancer Res. 2008;14:1561–1570. doi: 10.1158/1078-0432.CCR-07-1254. [DOI] [PubMed] [Google Scholar]
- D’Argenio D, Schumitzky A, Wang X. Biomedical Simulations Resource. Los Angeles, CA: 2009. ADAPT 5 User’s Guide: Pharmacokinetic/Pharmacodynamic System Analysis Software. [Google Scholar]
- Day CL, Smits C, Fan FC, Lee EF, Fairlie WD, Hinds MG. Structure of the BH3 domains from the p53-inducible BH3-only proteins Noxa and Puma in complex with Mcl-1. J Mol Biol. 2008;380:958–971. doi: 10.1016/j.jmb.2008.05.071. [DOI] [PubMed] [Google Scholar]
- Gascoyne RD, Adomat SA, Krajewski S, Krajewska M, Horsman DE, Tolcher AW, O’Reilly SE, Hoskins P, Coldman AJ, Reed JC, Connors JM. Prognostic significance of Bcl-2 protein expression and Bcl-2 gene rearrangement in diffuse aggressive non-Hodgkin’s lymphoma. Blood. 1997;90:244–251. [PubMed] [Google Scholar]
- Hagberg H, Gisselbrecht C. Randomised phase III study of R-ICE versus R-DHAP in relapsed patients with CD20 diffuse large B-cell lymphoma (DLBCL) followed by high-dose therapy and a second randomisation to maintenance treatment with rituximab or not: an update of the CORAL study. Ann Oncol. 2006;17(Suppl 4):iv31–32. doi: 10.1093/annonc/mdj996. [DOI] [PubMed] [Google Scholar]
- Hetschko H, Voss V, Senft C, Seifert V, Prehn JH, Kogel D. BH3 mimetics reactivate autophagic cell death in anoxia-resistant malignant glioma cells. Neoplasia. 2008;10:873–885. doi: 10.1593/neo.07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazirehi AR, Vega MI, Bonavida B. Development of rituximab-resistant lymphoma clones with altered cell signaling and cross-resistance to chemotherapy. Cancer Res. 2007;67:1270–1281. doi: 10.1158/0008-5472.CAN-06-2184. [DOI] [PubMed] [Google Scholar]
- Jiang M, Wei Q, Wang J, Du Q, Yu J, Zhang L, Dong Z. Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene. 2006;25:4056–4066. doi: 10.1038/sj.onc.1209440. [DOI] [PubMed] [Google Scholar]
- Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- Knoops L, de Jong D. The role of the p53 pathway in the treatment of follicular lymphoma. Cell Cycle. 2008;7:436–439. doi: 10.4161/cc.7.4.5441. [DOI] [PubMed] [Google Scholar]
- Konopleva M, Watt J, Contractor R, Tsao T, Harris D, Estrov Z, Bornmann W, Kantarjian H, Viallet J, Samudio I, Andreeff M. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15–070 (obatoclax) Cancer Res. 2008;68:3413–3420. doi: 10.1158/0008-5472.CAN-07-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labi V, Grespi F, Baumgartner F, Villunger A. Targeting the Bcl-2-regulated apoptosis pathway by BH3 mimetics: a breakthrough in anticancer therapy? Cell Death Differ. 2008;15:977–987. doi: 10.1038/cdd.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Viallet J, Haura EB. A small molecule pan-Bcl-2 family inhibitor, GX15–070, induces apoptosis and enhances cisplatin-induced apoptosis in non-small cell lung cancer cells. Cancer Chemother Pharmacol. 2008;61:525–534. doi: 10.1007/s00280-007-0499-3. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA, Geneste O, Kroemer G. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Conde E, Arnan M, Canales MA, Deben G, Sancho JM, Andreu R, Salar A, Garcia-Sanchez P, Vazquez L, Nistal S, Requena MJ, Donato EM, Gonzalez JA, Leon A, Ruiz C, Grande C, Gonzalez-Barca E, Caballero MD. R-ESHAP as salvage therapy for patients with relapsed or refractory diffuse large B-cell lymphoma: the influence of prior exposure to rituximab on outcome. A GEL/TAMO study. Haematologica. 2008;93:1829–1836. doi: 10.3324/haematol.13440. [DOI] [PubMed] [Google Scholar]
- Middelburg R, de Haas RR, Dekker H, Kerkhoven RM, Pohlmann PR, Fuentes-Alburo A, Mohar A, Pinedo HM, Lankelma J. Induction of p53 up-regulated modulator of apoptosis messenger RNA by chemotherapeutic treatment of locally advanced breast cancer. Clin Cancer Res. 2005;11:1863–1869. doi: 10.1158/1078-0432.CCR-04-1372. [DOI] [PubMed] [Google Scholar]
- Mounier N, Briere J, Gisselbrecht C, Emile JF, Lederlin P, Sebban C, Berger F, Bosly A, Morel P, Tilly H, Bouabdallah R, Reyes F, Gaulard P, Coiffier B. Rituximab plus CHOP (R-CHOP) overcomes bcl-2--associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL) Blood. 2003;101:4279–4284. doi: 10.1182/blood-2002-11-3442. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, Goulet D, Viallet J, Belec L, Billot X, Acoca S, Purisima E, Wiegmans A, Cluse L, Johnstone RW, Beauparlant P, Shore GC. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien S, Moore JO, Boyd TE, Larratt LM, Skotnicki A, Koziner B, Chanan-Khan AA, Seymour JF, Bociek RG, Pavletic S, Rai KR. Randomized phase III trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2007;25:1114–1120. doi: 10.1200/JCO.2006.07.1191. [DOI] [PubMed] [Google Scholar]
- O’Brien SM, Claxton DF, Crump M, Faderl S, Kipps T, Keating MJ, Viallet J, Cheson BD. Phase I study of obatoclax mesylate (GX15–070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113:299–305. doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J Biol Chem. 2007;282:13123–13132. doi: 10.1074/jbc.M700492200. [DOI] [PubMed] [Google Scholar]
- Olejniczak SH, Hernandez-Ilizaliturri FJ, Clements JL, Czuczman MS. Acquired resistance to rituximab is associated with chemotherapy resistance resulting from decreased Bax and Bak expression. Clin Cancer Res. 2008;14:1550–1560. doi: 10.1158/1078-0432.CCR-07-1255. [DOI] [PubMed] [Google Scholar]
- Paquet C, Schmitt E, Beauchemin M, Bertrand R. Activation of multidomain and BH3-only pro-apoptotic Bcl-2 family members in p53-defective cells. Apoptosis. 2004;9:815–831. doi: 10.1023/B:APPT.0000045791.55282.91. [DOI] [PubMed] [Google Scholar]
- Pattingre S, Levine B. Bcl-2 inhibition of autophagy: a new route to cancer? Cancer Res. 2006;66:2885–2888. doi: 10.1158/0008-5472.CAN-05-4412. [DOI] [PubMed] [Google Scholar]
- Perez-Galan P, Roue G, Villamor N, Campo E, Colomer D. The BH3-mimetic GX15–070 synergizes with bortezomib in mantle cell lymphoma by enhancing Noxa-mediated activation of Bak. Blood. 2007;109:4441–4449. doi: 10.1182/blood-2006-07-034173. [DOI] [PubMed] [Google Scholar]
- Perez-Galan P, Roue G, Lopez-Guerra M, Nguyen M, Villamor N, Montserrat E, Shore GC, Campo E, Colomer D. BCL-2 phosphorylation modulates sensitivity to the BH3 mimetic GX15–070 (Obatoclax) and reduces its synergistic interaction with bortezomib in chronic lymphocytic leukemia cells. Leukemia. 2008;22:1712–1720. doi: 10.1038/leu.2008.175. [DOI] [PubMed] [Google Scholar]
- Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, Ma D, Gill D, Walewski J, Zinzani PL, Stahel R, Kvaloy S, Shpilberg O, Jaeger U, Hansen M, Lehtinen T, Lopez-Guillermo A, Corrado C, Scheliga A, Milpied N, Mendila M, Rashford M, Kuhnt E, Loeffler M. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, Reiser M, Nickenig C, Clemens M, Peter N, Bokemeyer C, Eimermacher H, Ho A, Hoffmann M, Mertelsmann R, Trumper L, Balleisen L, Liersch R, Metzner B, Hartmann F, Glass B, Poeschel V, Schmitz N, Ruebe C, Feller AC, Loeffler M. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60) Lancet Oncol. 2008;9:105–116. doi: 10.1016/S1470-2045(08)70002-0. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Huang DC, O’Reilly LA, King SM, Strasser A. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- Ramanarayanan J, Hernandez-Ilizaliturri FJ, Chanan-Khan A, Czuczman MS. Pro-apoptotic therapy with the oligonucleotide Genasense (oblimersen sodium) targeting Bcl-2 protein expression enhances the biological anti-tumour activity of rituximab. Br J Haematol. 2004;127:519–530. doi: 10.1111/j.1365-2141.2004.05239.x. [DOI] [PubMed] [Google Scholar]
- Schimmer AD, O’Brien S, Kantarjian H, Brandwein J, Cheson BD, Minden MD, Yee K, Ravandi F, Giles F, Schuh A, Gupta V, Andreeff M, Koller C, Chang H, Kamel-Reid S, Berger M, Viallet J, Borthakur G. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:8295–8301. doi: 10.1158/1078-0432.CCR-08-0999. [DOI] [PubMed] [Google Scholar]
- Shibue T, Takeda K, Oda E, Tanaka H, Murasawa H, Takaoka A, Morishita Y, Akira S, Taniguchi T, Tanaka N. Integral role of Noxa in p53-mediated apoptotic response. Genes Dev. 2003;17:2233–2238. doi: 10.1101/gad.1103603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T, Suzuki S, Okamoto H, Yoshida H, Ohba Y, Takaoka A, Taniguchi T. Differential contribution of Puma and Noxa in dual regulation of p53-mediated apoptotic pathways. EMBO J. 2006;25:4952–4962. doi: 10.1038/sj.emboj.7601359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakumar L, Armitage JO. Bcl-2 gene expression as a predictor of outcome in diffuse large B-cell lymphoma. Clin Lymphoma Myeloma. 2006;6:455–457. doi: 10.3816/CLM.2006.n.025. [DOI] [PubMed] [Google Scholar]
- Sohn SK, Jung JT, Kim DH, Kim JG, Kwak EK, Park T, Shin DG, Sohn KR, Lee KB. Prognostic significance of bcl-2, bax, and p53 expression in diffuse large B-cell lymphoma. Am J Hematol. 2003;73:101–107. doi: 10.1002/ajh.10333. [DOI] [PubMed] [Google Scholar]
- Swerdlow S, Distelhorst CW. Bcl-2-regulated calcium signals as common mediators of both apoptosis and autophagy. Dev Cell. 2007;12:178–179. doi: 10.1016/j.devcel.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Trudel S, Li ZH, Rauw J, Tiedemann RE, Wen XY, Stewart AK. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015–070) in multiple myeloma. Blood. 2007;109:5430–5438. doi: 10.1182/blood-2006-10-047951. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228:1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- Waterhouse NJ, Sedelies KA, Browne KA, Wowk ME, Newbold A, Sutton VR, Clarke CJ, Oliaro J, Lindemann RK, Bird PI, Johnstone RW, Trapani JA. A central role for Bid in granzyme B-induced apoptosis. J Biol Chem. 2005;280:4476–4482. doi: 10.1074/jbc.M410985200. [DOI] [PubMed] [Google Scholar]
- Wilson KS, Sehn LH, Berry B, Chhanabhai M, Fitzgerald CA, Gill KK, Klasa R, Skinnider B, Sutherland J, Connors JM, Gascoyne RD. CHOP-R therapy overcomes the adverse prognostic influence of BCL-2 expression in diffuse large B-cell lymphoma. Leuk Lymphoma. 2007;48:1102–1109. doi: 10.1080/10428190701344881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.