Abstract
We examined whether chronic systemic treatment with agonists for peroxisome proliferator-activated receptor alpha (PPARα) influences neuroinflammation induced by lipopolysaccharide (LPS) injection into the somatosensory cortex in adult mice. Mice were pretreated with Wy-14643 or fenofibrate, both at 30 mg/kg, for 7 days. These treatment protocols increased the amount of PPARα mRNA and active form of PPARα protein in the brain. LPS injection reduced the PPARα mRNA level in the brain. On the contrary, TNFα, IL-1β, IL-6, iNOS, COX-2, ICAM-1, VCAM-1, and PECAM-1 were elevated at 6 hours after LPS. Wy-14643 and fenofibrate inhibited the elevations of TNFα, IL-1β, IL-6, COX-2, ICAM-1, and VCAM-1. Wy-14643, but not fenofibrate, also attenuated the iNOS elevation. At 3 days after LPS, Wy-14643 and fenofibrate showed similar inhibitions in these molecules. LPS injection also elevated IL-6 protein levels in the brain and serum at 6 hours, which was inhibited by fenofibrate. Histological analyses showed that Wy-14643 and fenofibrate profoundly attenuated microglia/macrophage activation, neutrophil recruitment, and neuronal injury at 3 days after LPS. These findings suggest that activation of PPARα attenuates neuroinflammation in the adult mouse brain, implicating that PPARα may be a potential therapeutic target for CNS diseases in which neuroinflammation plays a substantial role.
Keywords: innate immune response, stroke, microglia, lipopolysaccharide, cytokine, adhesion molecule, PPAR, fibrate
1. Introduction
Peroxisome proliferator-activated receptor (PPAR) is a ligand-dependent transcription factor (Chinetti et al., 2000). Currently, three subtypes α, γ, and β/δ are identified. PPARs regulate lipid/glucose metabolism and adipocyte differentiation; therefore, PPARs have gained a great deal of attention as a potential therapeutic target for metabolic syndrome (Kersten et al., 2000).
PPARα has been implicated in inflammation (Cunard et al., 2002; Staels et al., 1998). PPARα null mice showed a prolonged inflammation in response to leukotriene B4 (Devchand et al., 1996). PPARα agonists exert anti-inflammatory actions in a variety of inflammatory diseases (Okamoto et al., 2005; Tanaka et al., 2001), including CNS diseases (reviewed in Drew et al., 2006; Heneka and Landreth, 2007; Bright et al., 2008). For instance, gemfibrozil and fenofibrate improved neurological signs in a mouse model of multiple sclerosis (Lovett-Racke et al., 2004). These effects of PPARα agonists required PPARα expression (Gocke et al., 2009).
PPARα activation has been shown to suppress glial inflammatory responses to lipopolysaccharide (LPS), an endotoxin which is expressed on the outer membrane of bacteria. LPS-induced innate immune response in the brain is mediated through Toll-like receptor 4 (Rivest, 2009). In vitro studies using dissociated glial cultures have provided a substantial amount of data concerning the effects of PPARα activation on the glial response to LPS (Lee et al., 2005; Paintlia et al., 2008a,b; Xu et al., 2005, 2006, 2007); however, in vitro studies often lack other crucial cellular components, such as endothelial cells and peripheral blood cells, for inflammation. One study recently reported that maternal LPS exposure depleted developing oligodendrocyte in the fetal brain, which was prevented by Wy-14643, a PPARα-specific agonist (Paintlia et al., 2008b). Less is known about the in vivo effects of PPARα activation on LPS-induced neuroinflammation in adult brain.
Gemfibrozil and fenofibrate are prescribed for dyslipidemia. Most of dyslipidemic cases are seen in adults. In addition, little is known about the effects of chronic treatment with PPARα agonists on neuroinflammation. Therefore, we examined the influence of chronic treatment with PPARα agonists on neuroinflammation in adult mice. In the current study, we induced neuroinflammation by injecting LPS directly into the somatosensory cortex so that potential direct interactions between the systemically administered PPARα agonists and LPS were minimized. This model could also minimize the potential influence of LPS on peripheral organs.
2. Materials and methods
2.1. Animals and drug treatments
Male C57BL/6J mice (6–8 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME) and were housed with a 12 hour daily light/dark cycle. Food and water were provided ad libitum. Experimental procedures performed on animals were in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Morehouse School of Medicine. The animals were randomly divided into three treatment groups: Wy-14643 (30 mg/kg, Sigma-Aldrich, St. Louis, MO), fenofibrate (30 mg/kg, Sigma-Aldrich), or vehicle (10 ml/kg of 0.5% carboxymethyl cellulose, Sigma-Aldrich). The drugs were given through a feeding needle once per day for 7 consecutive days. The dosages of Wy-14643 and fenofibrate were selected from our previous studies in mouse ischemic stroke models (Inoue et al., 2003; Guo et al., 2010). Neuroinflammation is a significant component of ischemic stroke. For examining the influence of Wy-14643 and fenofibrate in non-LPS injected brain, 5 animals were analyzed in each treatment group. Ten LPS-injected animals for each treatment were randomly subdivided into two groups with a survival period of 6 hours or 3 days after LPS. In the 3 days survival group, the treatment with PPARα agonists was continued for 3 days after LPS. In each LPS-injected animal, the injection was made on both sides at 1 hour after the seventh drug administration. Additional 10 animals that received intracerebral injection with saline but without PPARα agonist treatment served as control (n = 5 for both 6 hours and 3 days). To test the effect of post-LPS treatment with fenofibrate, vehicle or fenofibrate (30 mg/kg) was given 15 minutes after LPS injection, followed by daily administration until brain was collected at 3 days after LPS injection (n = 6 and 7 for vehicle and fenofibrate, respectively).
2.2. Intracerebral LPS injection
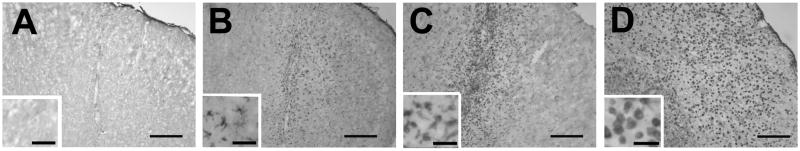
Animals were anesthetized with 1.5% isoflurane, and placed in a David Kopf stereotaxic apparatus (Kopf, Tujunga, CA). Burr holes centered at +0.14 mm anterior to the bregma and 2.0 mm lateral to the sagittal suture were made bilaterally on the skull. LPS (Escherichia coli, serotype 055:B5, Sigma-Aldrich) was dissolved in 5 μl sterile pyrogen-free saline and was delivered through a Hamilton syringe/needle (33 gauge) over 5 minutes and left for 5 minutes. The delivery of LPS was aimed at 1.2 mm deep from the cortical surface. In preliminary experiments, we tested 5, 20, 50, and 100 ng of LPS (n = 3–5 for each dose) and analyzed brain sections by staining with Isolectin B4 (40 ng/ml, Griffonia simplicifolia lectin I, Sigma Aldrich) at 3 days after LPS injection (Fig. 1). Isolectin B4 was visualized by Vectastain ABC kit (Vector Laboratories, Burlingame, CA) with 0.02% 3,3-diaminobenzidine (DAB) tetrahydrochloride and 0.003% hydrogen peroxide. The other hemisphere received 5 μl pyrogen-free saline in the same manner, as a sham injection. Five nanograms LPS did not show positive cells (data not shown) as seen after saline injection (Fig. 1A). Twenty nanograms showed positive cells in only one animal (Fig. 1B). All animals that received 50 ng survived 3 days and they showed consistent Isolectin B4 staining localized in the somatosensory cortex with a minor involvement in the corpus callosum (Fig. 1C). Animals receiving 100 ng showed a mortality rate of 66% before 3 days and the surviving animal showed strong staining in the cortex and corpus callosum with a partial involvement of the striatum (Fig. 1D). Thus, we selected 50 ng for the subsequent experiments.
Fig. 1.
Photomicrographs showing Isolectin B4 staining at 3 days after LPS injection in the somatosensory cortex. LPS was at different doses: (A) 0 ng, (B) 20 ng, (C) 50 ng, and (D) 100 ng. At 20 ng, ramified microglia were mainly seen (B) while round-shape phagocytic cells were the majority at 100 ng (D). Scale bar, 250 μm (40 μm for the inlets).
2.3. Tissue sampling
Blood and brains were collected at either 6 hours or 3 days after LPS injection. The brains were split into two hemispheres. One hemisphere was quickly frozen and kept at −80°C until further biochemical and mRNA analyses. The other hemisphere was fixed with 4% paraformaldehyde solution. Coronal brain sections (20 μm thick) were made in a cryostat and eight equidistant sections with 0.5 mm intervals mounted on a glass slide for histological analyses.
2.4. Electrophoretic mobility shift assay (EMSA)
For measuring the level of active form of PPARα protein, a non-radioactive eletrophoretic mobility shift assay kit was used according to the manufacturer’s instructions (Panomics, Redwood, CA). Nuclear protein (4 μg) that was extracted from the brain tissue was incubated with poly d(I–C) in binding buffer for 5 minutes at room temperature, and then at 15°C for 30 minutes with biotinylated oligonucleotide containing the PPARα-binding site. The samples were separated by gel eletrophoresis using 6% polyacrylamide gel in 50 mM Tris buffer containing 45 mM boric acid and 0.5 mM EDTA at 4°C, blotted onto a Biodyne B (0.45μm) positively charged nylon membrane (Pall Corporation, Ann Arbor, MI), and then cross-linked for 3 minutes with an ultraviolet light. The biotinylated oligonucleotides were detected with streptavidin-horseradish peroxidase conjugate and the membranes were exposed to autoradiography film (Denville, Metuchen, NJ).
2.5. Real-time quantitative polymerase chain reaction (PCR)
Total RNA was isolated with Trizol (Invitrogen, Carsbad, CA) according to the manufacturer’s instructions. Real-time quantitative polymerase chain reaction (PCR) was done with iQ SYBR Green Supermix kit (BioRad, Richmond, CA) by following the manufacturer’s instructions. Primer sequences for the analyzed genes are listed in Table 1. The PCR was carried out in a iCycler iQ at 95°C for 10 minutes, and 35 cycles of 95°C for 10 seconds, 55°C for 10 seconds, and 72°C for 10 seconds. The fold expression of each gene was calculated with the comparative cross-threshold method (Livak and Schmittgen, 2001) using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the reference gene.
Table 1.
Primer sequence for PCR analysis
| Target gene | Forward | Reverse |
|---|---|---|
| PPARα | 5′-gcagctcgtacaggtcatca-3′ | 5′-ctcttcatccccaagcgtag-3′ |
| TNFα | 5′-agcccccagtctgtatcctt-3′ | 5′-ctccctttgcagaactcagg-3′ |
| IL-1β | 5′-gcccatcctctgtgactcat-3′ | 5′-aggccacaggtattttgtcg-3′ |
| IL-6 | 5′-agttgccttcttgggactga-3′ | 5′-cagaattgccattgcacaac-3′ |
| iNOS | 5′-ctcactgggacagcacagaa-3′ | 5′-gcttgtctctgggtcctctg-3′ |
| COX-2 | 5′-acgaaatcaacaaccccgta-3′ | 5′-ggcagaacgactcggttatc-3′ |
| ICAM-1 | 5′-ttcacactgaatgccagctc-3′ | 5′-gtctgctgagacccctcttg-3′ |
| VCAM-1 | 5′-taccagctcccaaaatcctg-3′ | 5′-tctgctaattccagcctcgt-3′ |
| PECAM-1 | 5′-tgcaggagtccttctccact-3′ | 5′-acggtttgattccactttgc-3′ |
| GAPDH | 5′-aactttggcattgtggaagg-3′ | 5′-acacattgggggtaggaaca-3′ |
2.6. IL-6 protein enzyme-linked immunosorbent assay
Brain tissues were homogenized with lysis buffer using a tightly fitting homogenizer. The homogenates were centrifuged at 12,000×g for 20 minutes, and the protein concentration of the supernatants was determined using the BioRad protein assay. IL-6 protein levels in the brain and serum were measured by enzyme-linked immunosorbent assay (ELISA), according to the manufacture’s instructions (Pierce, Rockford, IL).
2.7. Histological assessment of neuroinflammation
Immunostaining was conducted using primary antibodies against Iba1 (1 μg/ml; Wako Chemicals USA, Richmond, VA), neutrophil elastase (NE) (1:50; Santa Cruz Biotechnology, Santa Cruz, CA), IL-6 (15 μg/ml; R & D Systems, Minneapolis, MN), and iNOS (2 μg/ml; Millipore, Billerica, MA). Brain sections were incubated with phosphate buffer (pH 7.4) containing 2% bovine serum albumin and respective primary antibodies overnight at 4°C. Immunostaining was visualized by either Alexa Fluor (Invitrogen)-conjugated or horseradish peroxidase (HRP)-conjugated secondary antibodies against appropriate immunoglobulin. HRP-labeled immune-complex was visualized by Vectastain ABC kit with the DAB reaction described above. Cellular and tissue architectural changes were determined by cresyl violet staining. Injured neurons were determined by Fluoro-Jade B staining by following the manufacture’s instructions (Millipore). The slides were immersed in distilled water for 2 minutes; then, transferred to 0.06% potassium permanganate for 10 minutes on a shaker table. After rinsing with distilled water, the slides were incubated for 20 minutes in 0.001% Fluoro-Jade B dissolved in 0.1% acetic acid. Slides were rinsed in water, dried at 37°C, dehydrated in xylene, and coverslipped.
2.8. Computerized automatic quantitative cell counting
For quantitative analysis of the cresyl violet stained sections, images of whole hemisphere were acquired using a 1.25× objective lens with a CCD camera. Injured areal size in eight serial sections with 0.5 mm intervals was determined with a MCID computerized image analyzer (Imaging Research, Cambridge, UK) and the injured volume was calculated by summing the areal size. For counting Iba1, NE, Isolectin B4, or Fluoro-Jade B positive cells, images that covered the entire hemisphere were acquired with an epifluorence microscope (Carl Zeiss Axioskop 2) under the same exposure condition. The number of positive cells in each of eight sections was automatically determined with the computer assisted MCID image analyzer and integrated to obtain total positive cell number.
2.9. Statistical analysis
Data are presented as mean ± SD and the differences among the groups were analyzed by one way analysis of variance (ANOVA) followed by Scheffe post-hoc analysis using SPSS 15.0 software (SPSS, Chicago, IL). p<0.05 was considered statistically significant.
3. Results
3.1. Systemic treatment with PPARα activators activates PPARα in brain
We first examined whether systemically administered Wy-14643 and fenofibrate activate PPARα at the protein level in brains. EMSA showed that both PPARα activators increased the amount of active PPARα protein in the nuclear fraction that was obtained from the brains without LPS injection (Fig. 2).
Fig. 2.
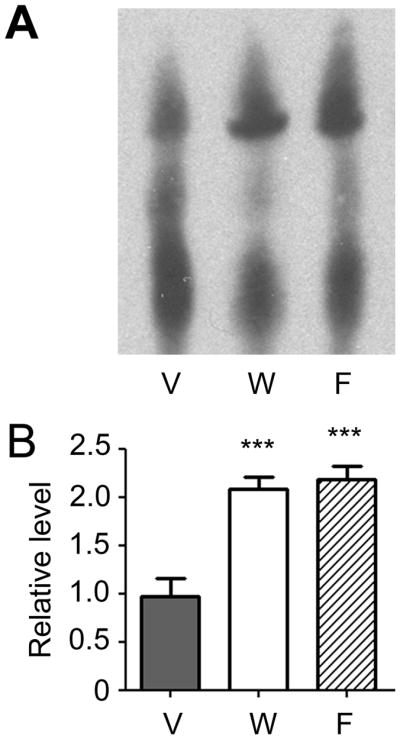
Effects of Wy-14643 and fenofibrate on PPARα protein activation in non-LPS injected brains. PPARα protein activation was measured by electrophoretic mobility shift assay using nuclear protein from the brain. V, vehicle; W, Wy-14643 at 30 mg/kg; F, fenofibrate at 30 mg/kg. Experiments were done in 5 animals in each group. Data are shown mean + SD. ***P<0.001 compared to vehicle group. Statistical analysis was made by one-way ANOVA followed by Scheffe post-hoc test.
3.2. PPARα activators elevate PPARα mRNA in brain
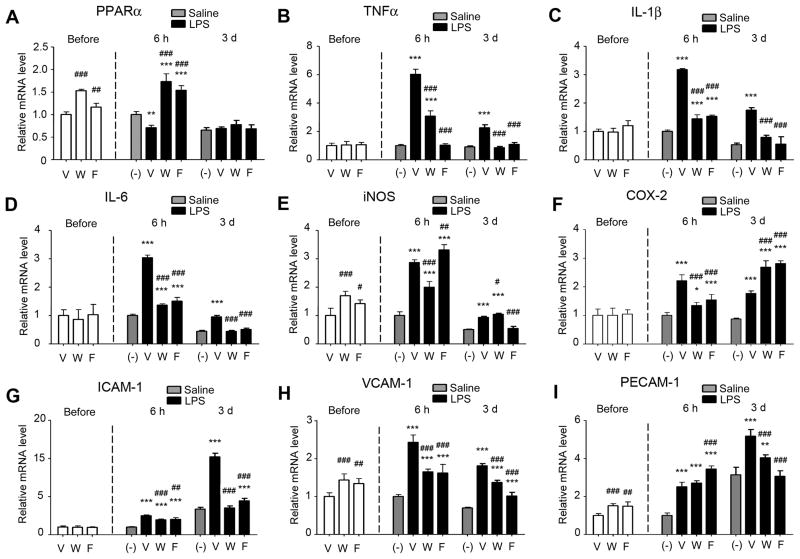
We measured the influence of Wy-14643 and fenofibrate on mRNA levels of PPARα and several pro-inflammatory molecules in the brains that were not injected with LPS. PPARα was significantly elevated by Wy-14643 and fenofibrate, both at 30 mg/kg (Fig. 3A).
Fig. 3.
Effects of Wy-14643 or fenofibrate on the mRNA levels of PPARα and pro-inflammatory molecules in brains before LPS injection and at 6 hours and 3 days after LPS. For the groups before LPS, the mean value from the vehicle-treated group was considered as one, and then relative expression levels in other treatment groups were calculated for each molecule. For the groups after injection, the mean value from the saline-injected group at 6 hours was considered as one. V, vehicle; W, Wy-14643 at 30 mg/kg; F, fenofibrate at 30 mg/kg. Experiments were done in 5 animals in each group. Data are shown mean + SD. #P<0.05, ##P<0.01, ###P<0.001 compared to vehicle-treated group; *P<0.05, **P<0.01, and ***P<0.001 compared to saline-injected group. Statistical analysis was made by one-way ANOVA followed by Scheffe post-hoc test.
Regarding pro-inflammatory genes, tissue necrosis factor α (TNFα), interleukin (IL)-1β, IL-6, cyclooxygenase 2 (COX-2), and intercellular adhesion molecule (ICAM)-1 were not affected by Wy-14643 and fenofibrate. However, inducible nitric oxide synthase (iNOS), vascular cell adhesion molecule (VCAM)-1, and platelet/endothelial cell adhesion molecule (PECAM)-1, showed a similar pattern of influence by Wy-14643 and fenofibrate to that on PPARα (Fig. 3B–I).
3.3. PPARα activators influence mRNA levels of pro-inflammatory molecules after LPS injection
Since glial PPARα expression has been reported to be influenced by inflammatory stimuli including LPS (Paintlia et al., 2008a,b; Gocke et al., 2009), we also measured brain mRNA level of PPARα after LPS injection. Compared with saline injection, LPS significantly reduced PPARα at 6 hours compared to saline injection (Fig. 3A). Pretreatment with Wy-14643 and fenofibrate significantly increased PPARα after LPS injection compared to vehicle pretreatment. The levels were even higher than those in the saline-injected brains. At 3 days after LPS, there was no difference in the PPARα levels among the animal groups (Fig. 3A).
We next measured mRNA levels of the pro-inflammatory molecules in the brains after LPS injection. LPS elevated TNFα, IL-1β, IL-6, iNOS, and COX-2 at 6 hours (Figs. 3B–F). The levels of TNFα, IL-1β, IL-6, and iNOS were returned toward the basal at 3 days after LPS (Figs. 3B–E); however, they remained significantly higher compared to saline injection. Wy-14643 and fenofibrate inhibited the LPS-induced elevations of TNFα, IL-1β, IL-6, and COX-2. Wy-14643, but not fenofibrate, also attenuated iNOS at 6 hours (Fig. 3E). At 3 days after LPS, Wy-14643 and fenofibrate showed similar inhibitory patterns in TNFα, IL-1β, and IL-6 (Figs. 3B–D). iNOS was attenuated by fenofibrate but not by Wy-14643 (Fig. 3E). Both drugs rather elevated COX-2 (Fig. 3F).
We also measured mRNA levels of cell adhesion molecules: ICAM-1, VCAM-1, and PECAM-1. LPS elevated all of the three molecules at 6 hours and the elevations of ICAM-1 and PECAM-1 were more pronounced at 3 days (Figs. 3G–I). Again, Wy-14643 and fenofibrate significantly inhibited these elevations.
3.4. Fenofibrate inhibits LPS-induced elevation of IL-6 in brain and blood
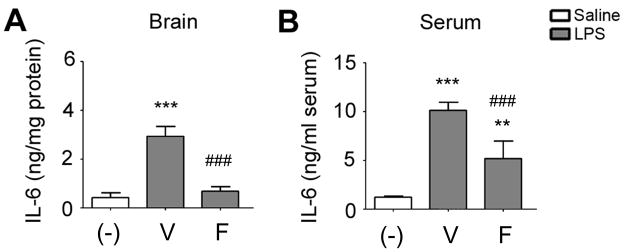
We next examined whether the LPS injection into the somatosensory cortex induces systemic immune responses. For this purpose, we measured IL-6 protein levels in the brains and sera at 6 hours after LPS. LPS strongly elevated IL-6 protein in both, which was dramatically inhibited by fenofibrate (Fig. 4). Although a previous study reported splenic atrophy at 4 days after ischemic stroke in mice (Offner et al., 2006), there was no detectable difference in spleen size at 3 days between saline- and LPS-injected groups (data not shown).
Fig. 4.
Effects of fenofibrate on IL-6 protein levels in brain (A) and serum (B) measured by ELISA at 6 hours after LPS injection. V, vehicle; W, Wy-14643 at 30 mg/kg; F, fenofibrate at 30 mg/kg. Experiments were done in 5 animals in each group. Data are shown as mean + SD. *P<0.05, **P<0.01 and ***P<0.001 compared to saline-injected group; ##P<0.01 compared to vehicle-treated LPS-injected group. Statistical analysis was made by one-way ANOVA followed by Scheffe post-hoc test.
3.5. Pretreatment with PPARα activators attenuates microglial/macrophage activation, leukocyte recruitment, and neuronal injury after LPS
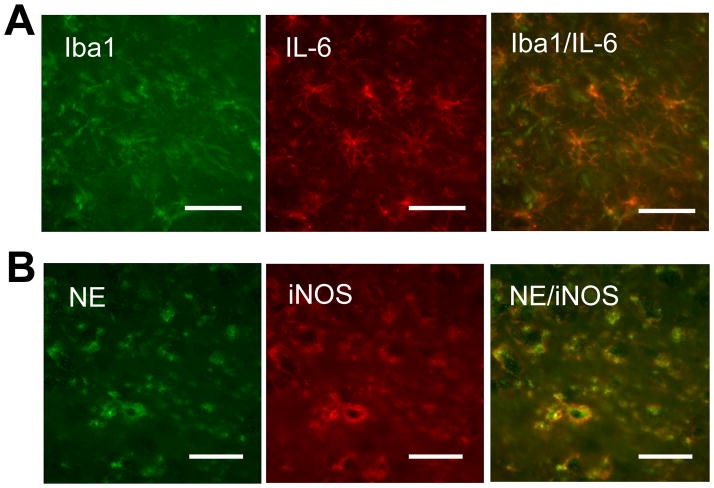
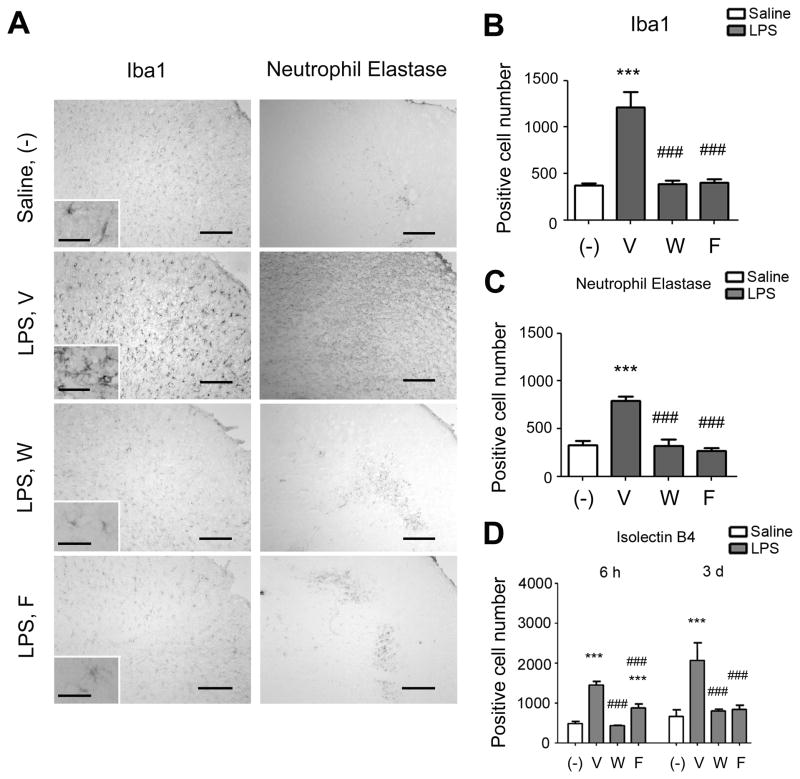
Iba1 immunostaining (microglia/macrophage marker) and NE immunostaining (neutrophil marker) were detected at 6 hours in the LPS-injected brains (Fig. 5). Iba1 positive cells showed the typical shape of microglia and almost of them exhibited IL-6 immunostaining (Fig. 5A). On the contrary, ~80% of IL-6 positive cells were Iba1 positive. NE immunoreactivity coincided with iNOS immunoreactivity (Fig. 5B). Iba1-positive cells and NE-positive cells were seen more pronouncedly at 3 days after LPS injection (Fig. 6A). Wy-14643 and fenofibrate dramatically decreased the numbers of these positive cells (Figs. 6A–C). Similar effects by Wy-14643 and fenofibrate were seen on the number of Isolectin B4 positive cells (Fig. 6D).
Fig. 5.
LPS injection into the cortex activates microglia/macrophages and recruits neutrophils to the injection site. Fluorescent double-immunostaining showing Iba1 and IL-6 (A) and neutrophil elastase (NE) and iNOS (B) in the somatosensory cortex at 6 hours after LPS injection. Representative images taken from at least 3 animals are shown. Scale bar, 30 μm.
Fig. 6.
Effects of Wy-14643 and fenofibrate on histological outcomes of neuroinflammation. (A) Photomicrographs showing Iba1 immunostaining (left column) and neutrophil elastase immunostaining (right column) in the somatosensory cortex at 3 days after LPS injection. Scale bar, 200 μm (40 μm for the inlets). (B) Numbers of Ib1a immunoreactive cells at 3 days after LPS injection. (C) Numbers of neutrophil elastase immunoreactive cells at 3 days after LPS injection. (D) Numbers of Isolectin B4 stained cells at 6 hours and 3 days after LPS injection. V, vehicle; W, Wy-14643 at 30 mg/kg; F, fenofibrate at 30 mg/kg. Experiments were done in 5 animals in each group. Data are shown as mean + SD. ***P<0.001 compared to saline-injected group; #P<0.05, ###P<0.001 compared to vehicle-treated LPS-injected group. Statistical analysis was made by one-way ANOVA followed by Scheffe post-hoc test.
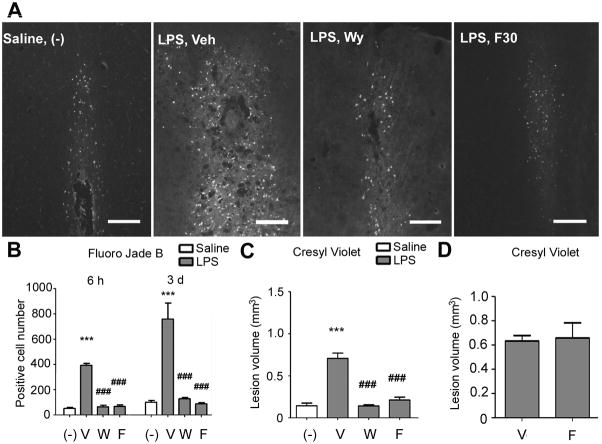
Fluoro-Jade B positive cells were clearly detected in the LPS injected brains (Fig. 7A). Wy-14643 and fenofibrate dramatically decreased the number of Fluoro-Jade B positive cells (Figs. 7A and B). Cortical lesion volume determined by cresyl violet staining at 3 days was also profoundly reduced by these drugs (Fig. 7C).
Fig. 7.
Effects of Wy-14643 and fenofibrate on histological outcomes of neuronal injury. (A) Fluorescent photomicrographs showing Fluoro-Jade B staining at 3 days after LPS injection. Animals were treated with either Wy-14643 or fenofibrate for 7 days before LPS and continued until brain was collected. Scale bar, 200 μm. (B) Numbers of Fluoro-Jade B stained cells at 6 hours and 3 days after LPS injection. (C) Brain tissue injury volume determined by cresyl violet staining at 3 days after LPS. (D) Graph showing effects of post-LPS treatment with fenofibrate on brain tissue injury volume at 3 days after LPS. Veh and V, vehicle; Wy and W, Wy-14643 at 30 mg/kg; F30 and F, fenofibrate at 30 mg/kg. Experiments were done in 5–7 animals in each group. Data are shown as mean + SD. ***P<0.001 compared to saline-injected group; ##P<0.01 and ###P<0.001 compared to vehicle-treated LPS-injected group. Statistical analysis was made by one-way ANOVA followed by Scheffe post-hoc test.
3.6. Fenofibrate treatment initiated after LPS injection does not attenuate neuronal injury
To examine the effect of post-LPS treatment with fenofibrate, fenofibrate treatment was initiated at 15 minutes after completing LPS injection and followed by daily administration until the brain was collected. No influence by fenofibrate was observed on lesion volume at 3 days after LPS (Fig. 7D).
4. Discussion
We showed that the current systemic treatment protocols with Wy-14643 or fenofibrate activated PPARα protein in the brain without LPS injection. In parallel, Wy-14643 and fenofibrate also elevated mRNA level of PPARα. LPS injection into the somatosensory cortex decreased the mRNA level of PPARα and elevated many pro-inflammatory genes in the brain. Wy-14643 and fenofibrate inhibited the concomitant LPS-induced changes in the level of PPARα and pro-inflammatory molecules. Furthermore, these PPARα agonists attenuated subsequent microglia/macrophage activation, leukocyte recruitment, and neuronal injury. These findings suggest that PPARα activation attenuates neuroinflammation induced by LPS. This notion is consistent with a previous report that LPS-induced inflammation was augmented in PPARα null mice (Kono et al., 2009). Whether or not and how activated PPARα directly influences the gene expression of a variety of pro-inflammatory molecules is a research area that is being extensively studied (Daynes and Jones, 2002; Glass and Saijo, 2010).
Among the pro-inflammatory molecules measured in the current study, COX-2 showed distinct responses to the PPARα agonists. Wy-14643 and fenofibrate attenuated the LPS-induced elevation of COX-2 at 6 hours after LPS but both drugs rather augmented the elevation at 3 days. Collino et al. (2006) reported irresponsiveness of COX-2 to Wy-14643 (6 mg/kg intra-venous injection at 30 minutes before ischemia) in the rat hippocampus after forebrain ischemia/reperfusion. Since the cellular type that was responsible for COX-2 elevation might be different at 6 hours and 3 days, more detailed cell-type specific analyses are required for understanding the unique response of COX-2 to the PPARα agonists.
The current experimental model with local injection of LPS into the somatosensory cortex enabled to show that the systemic immune system acutely responds to the centrally initiated neuroinflammation. Focal ischemic stroke has been shown to induce a similar activation of systemic immune system (Offner et al., 2008). In our current study, the cortical LPS injection increased IL-6 protein in the brain, which was detected in microglia. Fenofibrate showed a nearly complete block in the increase of IL-6 in the brain whereas the IL-6 inhibition in the serum was by ~50%, suggesting that the LPS-induced IL-6 increase in the serum was not merely a release from the brain. The systemic immune response could in turn influence the neuroinflammation at the primary site of LPS injection. As Wy-14643 and fenofibrate were systemically administered, these drugs might affect the secondary phase of immune response in the peripheral organs. Future studies are needed for understanding how the primary site in the brain sends signal to the peripheral immune organs.
The current study showed that Wy-14643 and fenofibrate dramatically inhibited elevations of ICAM-1, VCAM-1, and PECAM-1 in the brain after LPS. Since these cellular adhesion molecules are important in leukocyte recruitment to the inflammation focus in the brain, and since recruited leukocytes could release substances that harm neurons, Wy-14643 and fenofibrate might protect neurons by inhibiting the leukocyte recruitment. Consistent with this possibility, Wy-14643 and fenofibrate dramatically decreased the number of NE positive cells around the LPS injection site.
We previously reported that PPARα activators improved blood circulation in the cerebral cortex after focal ischemia/reperfusion (Guo et al., 2009; 2010). The cortical area into which LPS was injected corresponds to the so-called ischemic penumbra in middle cerebral artery occlusion models which are frequently used to mimic ischemic stroke in humans. In the penumbra, brain inflammation is pronounced. In addition, therapeutic improvement in microcirculation is more pronounced in the penumbra. Therefore, Wy-14643 and fenofibrate may improve penumbral circulation by preventing adhesion of leukocytes and platelets to the endothelial surface, at least in part. Consistent with this notion, fenofibrate has been shown to inhibit ICAM-1 immunoreactivity in the brain after ischemic stroke (Deplanque et al., 2003; Ouk et al., 2009).
In summary, we reported that chronic pretreatment with Wy-14643 or fenofibrate in adult mice attenuated neuroinflammation induced by intracerebral injection of LPS. Since fibrates have been prescribed as drugs for many years, more studies are needed to investigate their effects on CNS diseases such as meningitis, stroke, Alzheimer’s disease, cerebrovascular dementia, and epilepsy in which neuroinflammation plays a significant role.
Acknowledgments
This work was supported in part by NS048532 and NS060659 from NIH/NINDS and S21MD000101 from NIH/NCMHD. The study was conducted in a facility constructed with support from Research Facilities Improvement Grant 1 C06 RR-07571 from NIH/NCRR. We thank Dr. Qingmin Guo of Department of Neurobiology at Morehouse School of Medicine for her help in image analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bright JJ, Kanakasabai S, Chearwae W, Chakraborty S. PPAR regulation of inflammatory signaling in CNS diseases. PPAR Res. 2008;2008:658520. doi: 10.1155/2008/658520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000;49:497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- Collino M, Aragno M, Mastrocola R, Benetti E, Gallicchio M, Dianzani C, Danni O, Thiemermann C, Fantozzi R. Oxidative stress and inflammatory response evoked by transient cerebral ischemia/reperfusion: effects of the PPAR-alpha agonist WY14643. Free Radic Biol Med. 2006;41:579–589. doi: 10.1016/j.freeradbiomed.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Cunard R, DiCampli D, Archer DC, Stevenson JL, Ricote M, Glass CK, Kelly CJ. WY14,643, a PPAR alpha ligand, has profound effects on immune responses in vivo. J Immunol. 2002;169:6806–6812. doi: 10.4049/jimmunol.169.12.6806. [DOI] [PubMed] [Google Scholar]
- Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2:748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- Deplanque D, Gele P, Petrault O, Six I, Furman C, Bouly M, Nion S, Dupuis B, Leys D, Fruchart JC, Cecchelli R, Staels B, Duriez P, Bordet R. Peroxisome proliferator-activated receptor-alpha activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J Neurosci. 2003;23:6264–6271. doi: 10.1523/JNEUROSCI.23-15-06264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W. The PPARα-leukotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- Drew PD, Xu J, Storer PD, Chavis JA, Racke MK. Peroxisome proliferator-activated receptor agonist regulation of glial activation: relevance to CNS inflammatory disorders. Neurochem Int. 2006;49:183–189. doi: 10.1016/j.neuint.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat Rev Immunol. 2010;10:365–376. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- Gocke AR, Hussain RZ, Yang Y, Peng H, Weiner J, Ben LH, Drew PD, Stuve O, Lovett-Racke AE, Racke MK. Transcriptional modulation of the immune response by peroxisome proliferator-activated receptor-α agonists in autoimmune disease. J Immunol. 2009;182:4479–4487. doi: 10.4049/jimmunol.0713927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Wang G, Liu X, Namura S. Effects of gemfibrozil on outcome after permanent middle cerebral artery occlusion in mice. Brain Res. 2009;1279:121–130. doi: 10.1016/j.brainres.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Wang G, Namura S. Fenofibrate improves cerebral blood flow after middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2010;30:70–78. doi: 10.1038/jcbfm.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Landreth GE. PPARs in the brain. Biochim Biophys Acta. 2007;1771:1031–1045. doi: 10.1016/j.bbalip.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Inoue H, Jiang XF, Katayama T, Osada S, Umesono K, Namura S. Brain protection by resveratrol and fenofibrate against stroke requires peroxisome proliferator-activated receptor alpha in mice. Neurosci Lett. 2003;352:203–206. doi: 10.1016/j.neulet.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- Kono K, Kamijo Y, Hora K, Takahashi K, Higuchi M, Kiyosawa K, Shigematsu H, Gonzalez FJ, Aoyama T. PPARα attenuates the proinflammatory response in activated mesangial cells. Am J Physiol Renal Physiol. 2009;296:F328–336. doi: 10.1152/ajprenal.00484.2007. [DOI] [PubMed] [Google Scholar]
- Lee JH, Joe EH, Jou I. PPAR-alpha activators suppress STAT1 inflammatory signaling in lipopolysaccharide-activated rat glia. Neuroreport. 2005;16:829–833. doi: 10.1097/00001756-200505310-00010. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lovett-Racke AE, Hussain RZ, Northrop S, Choy J, Rocchini A, Matthes L, Chavis JA, Diab A, Drew PD, Racke MK. Peroxisome proliferator-activated receptor alpha agonists as therapy for autoimmune disease. J Immunol. 2004;172:5790–5798. doi: 10.4049/jimmunol.172.9.5790. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2008;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Iwamoto T, Kotake S, Momohara S, Yamanaka H, Kamatani N. Inhibition of NF-kappa B signaling by fenofibrate, a peroxisome proliferator activated receptor-alpha ligand, presents a therapeutic strategy for rheumatoid arthritis. Clin Exp Rheumatol. 2005;23:323–330. [PubMed] [Google Scholar]
- Ouk T, Laprais M, Bastide M, Mostafa K, Gautier S, Bordet R. Withdrawal of fenofibrate treatment partially abrogates preventive neuroprotection in stroke via loss of vascular protection. Vascul Pharmacol. 2009;51:323–330. doi: 10.1016/j.vph.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Paintlia MK, Paintlia AS, Contreras MA, Singh I, Singh AK. Lipopolysaccharide-induced peroxisomal dysfunction exacerbates cerebral white matter injury: attenuation by N-acetyl cysteine. Exp Neurol. 2008;210:560–576. doi: 10.1016/j.expneurol.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintlia MK, Paintlia AS, Khan M, Singh I, Singh AK. Modulation of peroxisome proliferator-activated receptor-alpha activity by N-acetyl cysteine attenuates inhibition of oligodendrocyte development in lipopolysaccharide stimulated mixed glial cultures. J Neurochem. 2008;105:956–970. doi: 10.1111/j.1471-4159.2007.05199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- Staels B, Koenig W, Habib A, Merval R, Lebret M, Torra IP, Delerive P, Fadel A, Chinetti G, Fruchart JC, Najib J, Maclouf J, Tedgui A. Activation of human aortic smooth-muscle cells is inhibited by PPARalpha but not by PPARgamma activators. Nature. 1998;393:790–793. doi: 10.1038/31701. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Kohno H, Yoshitani S, Okumura A, Murakami A, Hosokawa M. Ligands for peroxisome proliferator activated receptors alpha and gamma inhibit chemically induced colitis and formation of aberrant crypt foci in rats. Cancer Res. 2001;61:2424–2428. [PubMed] [Google Scholar]
- Xu J, Storer PD, Chavis JA, Racke MK, Drew PD. Agonists for the peroxisome proliferator-activated receptor-alpha and the retinoid X receptor inhibit inflammatory responses of microglia. J Neurosci Res. 2005;81:403–411. doi: 10.1002/jnr.20518. [DOI] [PubMed] [Google Scholar]
- Xu J, Chavis JA, Racke MK, Drew PD. Peroxisome proliferator-activated receptor-alpha and retinoid X receptor agonists inhibit inflammatory responses of astrocytes. J Neuroimmunol. 2006;176:95–105. doi: 10.1016/j.jneuroim.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Xu J, Racke MK, Drew PD. Peroxisome proliferator-activated receptor-alpha agonist fenofibrate regulates IL-12 family cytokine expression in the CNS: relevance to multiple sclerosis. J Neurochem. 2007;103:1801–1810. doi: 10.1111/j.1471-4159.2007.04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]